Abstract
Background
Although 3,4-dihydroxyphenylalanine (L-dopa) is the gold-standard treatment for Parkinson’s disease, it can lead to disabling dyskinesias. Previous work showed that nicotine reduces L-dopa-induced dyskinesias (LIDs) in several parkinsonian animal models. The goal of this study was to determine if the time of nicotine administration affected its ability to reduce LIDs in L-dopa-primed and L-dopa naïve monkeys, and also to test if tolerance developed to nicotine’s beneficial effects.
Methods
Monkeys were injected with MPTP (1.9–2.0 mg/kg sc) over 3–5 months until parkinsonian. Nicotine (300 μg/ml) was administered in drinking water (4–6 months) to L-dopa-primed or L-dopa-naïve monkeys, with L-dopa/carbidopa (10/2.5 mg/kg) gavaged twice daily.
Results
One set of MPTP-lesioned monkeys (n=23) was first gavaged with L-dopa and subsequently given nicotine 4 wk later when dyskinesias have plateaud or 8 wk later when dyskinesias are established. A 60–70% decrease in LIDs was observed after several weeks of nicotine treatment in both groups. A second set of monkeys (n=26) was given nicotine 8 or 2 weeks before L-dopa. In the 8 week nicotine pretreatment group, there was an immediate reduction in LIDs, which plateaud at 60–70%. In the 2 week nicotine pretreatment group, there were initial small decreases in LIDs, which plateaud at 60–70% several weeks later. Thus, nicotine pre- and post-treatment was similarly efficacious in reducing LIDs. The beneficial effect of nicotine persisted throughout the study (17–23 weeks). Nicotine did not worsen parkinsonism.
Conclusion
These data suggest that nicotine treatment has potential as a successful antidyskinetic therapy for Parkinson’s disease patients.
Keywords: dyskinesia, L-dopa, nicotine, nicotinic, nonhuman primate, Parkinson’s disease
Introduction
L-dopa-induced dyskinesias (LIDs) are a debilitating side effect of L-dopa administration for which there is currently little treatment1–8. An extensive pre-clinical literature has implicated numerous neurotransmitters in the etiology of LIDs. However, there are few approved drug therapies for LIDs in Parkinson’s disease patients other than amantadine, which is of only limited usefulness. There is thus a continued search for better treatments to attenuate LIDs. One approach may involve administration of nicotine, a drug that interacts with nicotinic acetylcholine receptors (nAChRs) 9, as accumulating evidence now indicates that nicotine administration reduces LIDs in different parkinsonian animal models 10–12.
Experimental work in rodents shows that nicotine attenuates L-dopa-induced abnormal involuntary movements (AIMs) in both 6-hydroxydopamine-lesioned mice and rats 10, 11, 13. The nicotine-mediated reduction in L-dopa-induced AIMs was dose dependent and not observed with nicotine removal, demonstrating a direct effect of nicotine. In rodents, nicotine decreased AIMs when administered either before L-dopa treatment was initiated or after it had been started 10, 11, 13. The decline in L-dopa-induced AIMs was observed with several different modes of nicotine administration, including its inclusion in the drinking water, release from minipumps and injection 10, 11, 13.
Further studies have shown that nicotine also reduced LIDs in parkinsonian nonhuman primates, which more closely exhibit the deficits observed in Parkinson’s disease. Nicotine administration in the drinking water decreased LIDs by 50–75% when given 8 weeks before L-dopa administration was initiated 12, 14. Nicotine withdrawal led to a return of LIDs to control levels while its subsequent re-administration reduced LIDs, indicating the improvement was nicotine-dependent. These studies in nonhuman primates provide compelling evidence that nicotine reduces LIDs when given before L-dopa.
The objective of the present study was to determine if nicotine reduces LIDs in nonhuman primates when it is given after L-dopa treatment has started. This is an important consideration for optimal treatment of LIDs in Parkinson’s disease patients. To approach this, we tested the effect of nicotine given 4 and 8 weeks after the start of L-dopa treatment, and compared the effects to animals pretreated with nicotine. We selected a time point when the effect of the drug has just plateaud and a longer time point when drug effects are maximal. This thus extends earlier work in which monkeys were given nicotine for 8 wk before L-dopa treatment was started, with some of the monkeys being L-dopa naïve and others L-dopa-primed 12, 14. The present results show that nicotine decreased LIDs to a similar extent when given before or after L-dopa administration was initiated. Because patients also generally require life-long treatment with L-dopa, another objective was to determine whether nicotine’s effects persisted with continued treatment or whether tolerance developed. Results show that the nicotine-mediated improvement in LIDs is maintained during the entire course of treatment, which lasted several months. These results suggest that nicotine treatment may be a useful antidyskinetic strategy for Parkinson’s disease.
Materials and Methods
Animals
Squirrel monkeys (Saimiri sciureus) of both sexes were obtained from World Wide Primates (Miami, FL). Immediately after arrival, they underwent a one-month quarantine, according to California state regulations. The monkeys were maintained in a temperature-controlled room with a 12 h light/dark cycle. They were fed a diet of monkey chow, fruits and vegetables and given water ad libitum. All studies were done as mandated by the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the SRI Institutional Animal Care and Use Committee.
Parkinsonian Ratings and MPTP Administration
Monkeys were individually housed and first trained to perform different motor tasks for measurement of parkinsonian behaviors after MPTP treatment. Seven motor features were measured including spatial hypokinesia (use of available cage space), body bradykinesia (slowness in body movement), left and right hand manual dexterity, balance (ability to hold on to the cage bars), freezing, and action tremor, as described 12. The scale ranged from 0 (normal) to 4 (severely parkinsonian) for each category, with a maximum possible score of 28. After the initial training period, the monkeys were injected subcutaneously with 2.0 mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Sigma-Aldrich, St. Louis, MO) dissolved in saline 12. MPTP injection (1.9 mg/kg) was repeated as necessary at 3 to 4 wk intervals. Parkinsonism was scored once per week on Fridays 1–2 h after L-dopa administration throughout the study.
Drug Treatments and Dyskinesia Ratings
One month after the monkeys were stably parkinsonian, the animals in Group 1 and Group 2 (Fig. 1) were gavaged for 4 and 8 wk, respectively, twice daily at 3.5 h intervals with L-dopa/carbidopa for 5 d per wk 12. L-dopa was started at a lower dose (5.0–7.5 mg/kg) for the first two weeks and increased to 10.0 mg/kg when the animals had accommodated to the drug. Monkeys were given only fruits and vegetables in the morning to allow for optimal L-dopa absorption. Monkey chow, plus fruits and vegetables, were then given in the afternoon 4 h after the second dose of L-dopa. Dyskinesias were rated from videotapes by a blinded rater, with taping done Tuesday, Wednesday and Thursday from 8:00–9:00 AM before the first dose of L-dopa (baseline period) and from 9:00AM–4:30 PM, during L-dopa dosing. Dyskinesias were scored from the afternoon tapings for a 1 min period at 30 min intervals, with the scores shown depicting the average values over several days 15. Scoring was done on a scale of 0 (no dyskinesias) to 4 (severe dyskinesias) as follows: 1 = subtle dyskinesias that were not sustained (< 3 trunk movements in a row); 2 = sustained dyskinesias (≥ 3 trunk movements in a row); 3 = moderate dyskinesias that impaired the ability to remain stationary; and 4 = severe dyskinesias that were generalized and incapacitating 15.
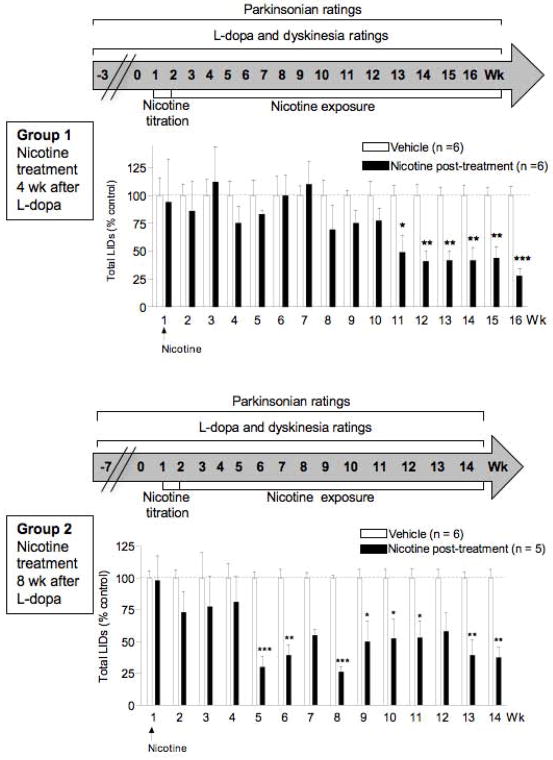
FIG. 1.
Nicotine treatment reduces established LIDs when administered 4 wk (Group 1, top panels) or 8 wk (Group 2, bottom panels) after the start of L-dopa treatment. Values are the mean ± SEM of 5–6 monkeys. Significance of difference from vehicle, *P < 0.05, **P < 0.01, ***P < 0.001 using ANOVA followed by a Bonferroni post hoc test.
After 4 wk (Group 1) or 8 wk (Group 2) on L-dopa, the monkeys were next given 50% Gatorade in the drinking water (Fig. 1). Gatorade was used to hide the bitter taste of nicotine. After 5 d on Gatorade only, the animals were assigned to Gatorade only (vehicle-treated) or Gatorade plus nicotine (free base; Sigma-Aldrich, St. Louis, MO). Nicotine was initially given in Gatorade at 50 μg/ml for 2 d and was then increased to 150 μg/ml for a further 3 d. The monkeys were maintained at a final concentration of 300 μg/ml nicotine for the remainder of the study, with nicotine provided in the drinking solution on a constant basis (Fig. 1). Twenty-five ml of Gatorade plus nicotine or Gatorade only was also added to the monkey chow. Nicotine treatment did not affect fluid intake, body weight or general behavior such as sedation.
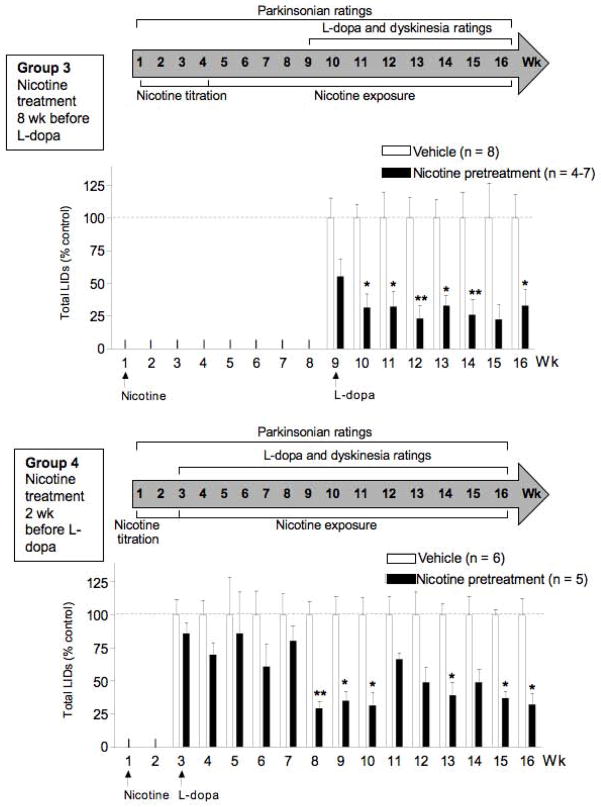
In addition to Groups 1 and 2 (Fig. 1), we tested if nicotine given before L-dopa resulted in a more pronounced reduction in LIDs (Groups 3 and 4, Fig. 2). For these studies, we first gave Gatorade alone (vehicle) or nicotine in Gatorade in escalating doses (titration phase) one month or more after MPTP lesioning. The monkeys were next maintained at the final dose of nicotine (300 μg/ml) for 8 wk (Group 3) or 2 wk (Group 4). Twenty-five ml of Gatorade plus nicotine or Gatorade only was also added to the monkey chow. The Group 3 and 4 monkeys were subsequently gavaged with L-dopa using a similar dosing regimen as for Groups 1 and 2. Nicotine treatment did not affect fluid intake, body weight or general behavior such as sedation.
FIG. 2.
Nicotine treatment reduces LIDs when administered 8 wk (Group 3, top panels) or 2 wk (Group 4, bottom panels) before L-dopa. Values are the mean ± SEM of 4–8 monkeys. Significance of difference from vehicle, *P < 0.05, **P < 0.01 using ANOVA followed by a Bonferroni post hoc test.
Plasma Cotinine Levels
The primary nicotine metabolite cotinine was used as an index of nicotine intake. It has a long half-life (18 h) and thus provides a stable measure of nicotine intake 16. A blood sample was taken from the femoral vein 2 h after the afternoon dose of L-dopa under ketamine anesthesia (15–20 mg/kg im) on Fridays. Plasma cotinine levels were measured at monthly to bi-monthly intervals throughout the study. Plasma was prepared and cotinine levels determined using an ELISA (Orasure Technologies, Bethlehem, PA). Plasma cotinine levels were as follows: 764 ± 28 ng/ml for Group 1; 474 ± 80 ng/ml for Group 2; 371 ± 111 ng/ml for Group 3; 467 ± 63 ng/ml for Group 4. These levels are similar to those in smokers 16.
Data Analyses
L-dopa was given on a 5-day-on/2-day-off schedule with parkinsonian ratings done weekly on Friday. Dyskinesia ratings were determined by averaging the scores on Wednesday and Thursday of each week. Total dyskinesias were determined by calculating area under the curve (AUC) and the data analyzed using two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Differences in parkinsonian or dyskinesia rating scores between groups were analyzed using nonparametric tests (Mann-Whitney test). The data are the mean ± SEM of the indicated number of animals. P ≤ 0.05 was used for statistical significance.
Results
Nicotine Treatment Reduces Established LIDs
In Fig. 1, we tested if nicotine reduced LIDs in monkeys primed with L-dopa and then given nicotine 4 wk (Group 1) or 8 wk (Group 2) after the start of L-dopa treatment. These time points were selected because dyskinesias have plateaud between 2–4 wk and are firmly established after 8 wk, as previously reported 12, 14. In Group 1, monkeys were first gavaged with L-dopa twice daily 5 d per wk. Four wk later, Group 1 was given 50% diluted Gatorade with nicotine or without (vehicle) (Fig. 1 top). The monkeys were initially given 5.0–7.5 mg/kg L-dopa, which was increased to 10 mg/kg once the animals had acclimated to the drug. LIDs were fully developed by 2 wk of L-dopa treatment. The data are expressed as % control, that is, of a vehicle-treated monkey group receiving no nicotine. There was a significant main effect of nicotine treatment (F1,160 = 28.93, P < 0.0001) with no significant interaction by two-way ANOVA (Fig. 1). Declines in LIDs were observed about 8 wk (~25%) after the start of the nicotine treatment, which were significant by 11 wk. The improvement in LIDs persisted until the end of the study.
A second group of MPTP-lesioned monkeys (Group 2) was first gavaged with L-dopa twice daily for 8 wk. L-dopa was started at a dose of 5.0–7.5 mg/kg and then increased to 10 mg/kg when the monkeys had acclimated to the drug. After 8 wk of L-dopa, Group 2 was given 50% diluted Gatorade with nicotine or without (vehicle) (Fig. 1 bottom). LIDs were fully developed by 2 wk of L-dopa treatment in vehicle-treated monkeys. There was a significant main effect of nicotine treatment (F1,126 = 115.3, P < 0.0001) and no significant interaction by two-way ANOVA. Nicotine decreased LIDs as early as 2 wk (~25%) after the start of treatment. The decline in LIDs was significant by 5 wk and persisted until the end of the study.
There was no effect of nicotine treatment on parkinsonism (Table 1), assessed in the presence of L-dopa, in Group 1 (vehicle, 5.3 ± 0.6; nicotine, 4.7 ± 0.5) or Group 2 (vehicle, 3.7 ± 0.5; nicotine, 4.6 ± 0.7), with ratings done once weekly. The data show that the monkeys were moderately impaired. The results shown were done immediately before nicotine treatment was started and at the end of the nicotine treatment regimen, with similar values throughout the study.
TABLE 1.
Nicotine treatment did not affect parkinsonism
| Group | # monkeys | Nicotine treatment | Parkinsonian ratings
|
|||
|---|---|---|---|---|---|---|
| Before nicotine treatment
|
Last wk of nicotine treatment
|
|||||
| Vehicle | Pre-nicotine | Vehicle | Nicotine | |||
| 1 | 6 | 4 wk after L-dopa | 4.9 ± 0.6 | 5.3 ± 0.6 | 5.3 ± 0.6 | 4.7 ± 0.5 |
| 2 | 5 | 8 wk after L-dopa | 5.7 ± 0.5 | 6.9 ± 0.9 | 3.7 ± 0.5 | 4.6 ± 0.7 |
| 3 | 4–7 | 8 wk before L-dopa | 4.0 ± 0.3 | 4.4 ± 0.7 | 3.0 ± 0.1 | 3.0 ± 0.6 |
| 4 | 5 | 2 wk before L-dopa | 4.9 ± 0.6 | 4.7 ± 0.6 | 5.3 ± 0.6 | 4.0 ± 0.3 |
Nicotine Pretreatment Reduced LIDs
In Fig. 2, we tested if nicotine given before L-dopa decreased LIDs to a similar extent as nicotine administered after the start of L-dopa (Fig. 1). For the studies in Fig. 2, MPTP-lesioned monkeys were first exposed to nicotine in the drinking water for 8 wk (Group 3) or 2 wk (Group 4). These time points were chosen to evaluate if there were differential effects with varying lengths of nicotine pretreatment. At the 2 wk time point, nicotine-mediated changes in molecular measures such as receptor upregulation are just at their maximal, while at 8 wk most nicotine-induced alteration are well established.
The monkeys in Group 3 were then gavaged with L-dopa (5–10 mg/kg) twice daily for 5 d a week (Fig. 2 top). The data are expressed as % control, that is, of a vehicle-treated monkey group receiving no nicotine. There was a significant main effect of nicotine (F1,97 = 79.15, P < 0.0001) with no significant interaction using two-way ANOVA, with 50–80% declines in LIDs starting with the first L-dopa dosing. The decline in LIDs was consistently observed throughout the study.
Another set of monkeys (Group 4, Fig. 2 bottom) were exposed to nicotine 2 wk before the start of L-dopa. There was a significant main effect of nicotine treatment (F1,126 = 76.43, P < 0.0001) with no significant interaction by two-way ANOVA. There were small declines in LIDs in the first few weeks (~25%) that were significant by 8 wk (~70% decrease). The nicotine-induced decline in LIDs continued until the end of the study.
There was no effect of nicotine treatment on parkinsonism (Table 1), assessed in the presence of L-dopa, in Group 3 (vehicle, 3.0 ± 0.1; nicotine, 3.0 ± 0.6) or Group 4 (vehicle, 5.3 ± 0.6; nicotine, 4.0 ± 0.3), with ratings done once weekly. The data show that the monkeys were moderately impaired. The results shown were done immediately before nicotine treatment was started and at the end of the nicotine treatment regimen, with similar values throughout the study.
Nicotine Pre- And Post-Treatments Similarly Reduce The Daily Time Course Of LIDs
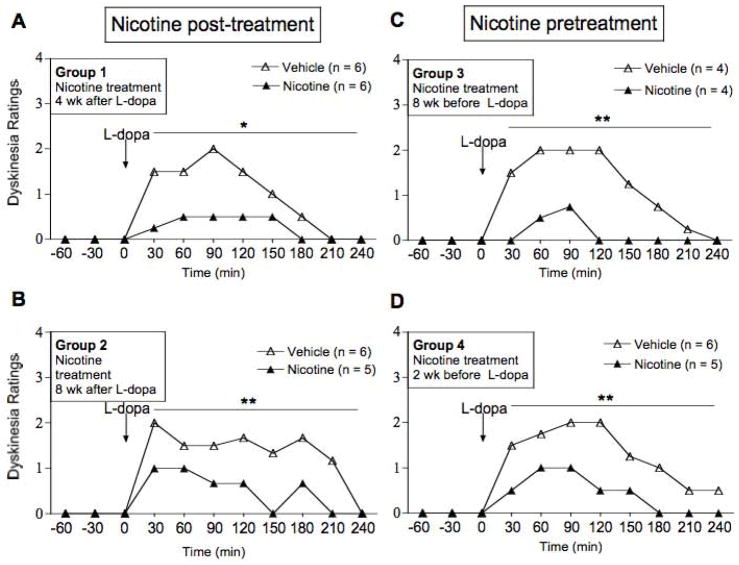
To determine whether pre- or post-treatment differentially improved LIDs, we examined nicotine’s effects on the hourly time course (Fig. 3). The time courses shown represent the effect of nicotine during the last week of the pre- and post-treatment regimens (Figs. 1 and 2). In vehicle-treated monkeys, LIDs maximally developed by 30–90 min after L-dopa administration. LIDs subsequently plateaud and diminished to control levels ~4 h after L-dopa administration. In Groups 1 and 2, in which the monkeys were first treated with L-dopa, subsequent nicotine treatment significantly suppressed established LIDs (Fig. 3A, B). Nicotine treatment suppressed LIDs with a similar time course in the monkeys in Groups 3 and 4 that were first treated with nicotine and then L-dopa (Fig. 3C, D).
FIG. 3.
Nicotine pre- and post-treatments similarly reduce the daily time course of LIDs. The panels show the effect of nicotine on LIDs at wk 15 or 16 of treatment (see timeline in Fig. 1). Values represent the median of 4–6 monkeys. Significance of difference from vehicle treatment, *P < 0.05, **P < 0.01 using a Mann-Whitney test.
The Reduction In LIDs Persists With Continued Nicotine Treatment
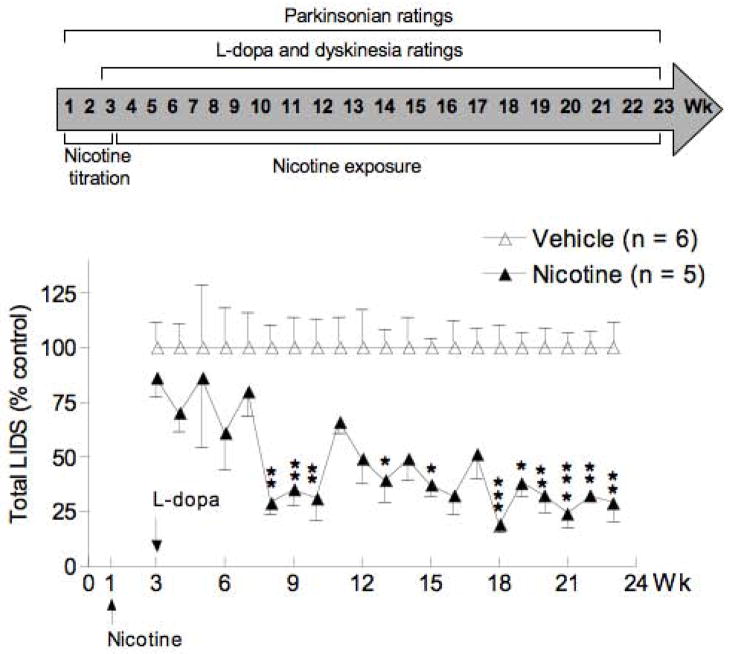
To investigate whether the ability of nicotine to attenuate LIDs persisted with continued treatment, the study with the Group 4 monkeys was continued up to wk 23 after the start of nicotine treatment (Fig. 4). The data are expressed as % control, that is, of a vehicle-treated monkey group receiving no nicotine. There were significant declines in LIDs at most time points between 8 to 23 wk, which consistently remained until the end of the study.
FIG. 4.
The reduction in LIDs persists with continued nicotine treatment. Values represent the mean ± SEM of 5–6 monkeys. Significance of difference from vehicle, *P < 0.05, **P < 0.01, ***P < 0.001 using ANOVA followed by a Bonferroni post hoc test.
Loss Of The Nicotine-Mediated Improvement In LIDs With Nicotine Removal
Experiments were done to determine if the nicotine-mediated decline in LIDs persisted with nicotine removal. The results in Table 2 show a significant decrease in LIDs the last wk of nicotine treatment (On nicotine). Removal of nicotine (Off nicotine) increased LID scores by 4–8 wk of washout, with LIDs similar to that of vehicle-treated monkeys.
TABLE 2.
Loss of the nicotine-mediated improvement in LIDs with nicotine removal
| Nicotine treatment | Total LID scores
|
||||
|---|---|---|---|---|---|
| Last wk of nicotine treatment
|
Last wk of washout
|
||||
| Vehicle | On nicotine | Vehicle | Off nicotine | ||
| Group 1 | 4 wk after L-dopa | 5.8 ± 0.7 | 2.4 ± 0.5** | 8.2 ± 0.3 | 7.7 ± 0.3 |
| Group 2 | 8 wk after L-dopa | 10.5 ± 0.7 | 3.8 ± 0.9** | nd | nd |
| Group 3 | 8 wk before L-dopa | 7.5 ± 0.9 | 1.4 ± 0.7** | 11.1 ± 1.5 | 8.1 ± 1.2 |
| Group 4 | 2 wk before L-dopa | 5.8 ± 0.7 | 1.7 ± 0.5** | 8.2 ± 0.3 | 7.6 ± 0.8 |
MPTP-lesioned monkeys were gavaged with L-dopa and given nicotine/vehicle. Nicotine removal led to a return of LIDs to control values by 4–8 wk after washout. Values are mean ± SEM of the number of monkeys, as in Figs. 1 and 2. Significance of difference from vehicle, **P < 0.01 using ANOVA followed by a Bonferroni post hoc test. nd, not done.
Discussion
The present results show that nicotine administration reduces LIDs in nonhuman primates when administered either before they develop or after they are established. There was a maximal reduction (60–70% decrease) in LIDs as early as 5 wk of treatment, although it generally required a somewhat longer treatment period. Importantly, there was no tolerance, with nicotine’s beneficial effects maintained throughout the course of the different studies (up to 23 wk). Nicotine most likely acts via long term molecular mechanisms since the improvement in LIDs required several weeks to fully develop and also to revert back to control after nicotine removal. Nicotine treatment did not affect parkinsonism under any treatment paradigm. These observations suggest that nicotine treatment may be useful in reducing established LIDs in Parkinson’s disease patients.
The finding that the nicotine-mediated improvement in LIDs occurred over several weeks of treatment bears resemblances to the fairly long time courses required to observe beneficial effects of other drugs used in the treatment of CNS disorders. For example, the different classes of antidepressants including selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors, atypical antidepressants, tricyclic antidepressants and monoamine oxidase inhibitors require weeks for their antidepressant effects to develop, with the positive effects of the medication not fully evident until 6–8 weeks after the start of treatment 17–19. A similar situation exists with antipsychotics used for the treatment of schizophrenia and other psychiatric disorders. Patients generally begin to improve within six weeks of the start of antipsychotic medication, with the full benefit requiring several months 20.
With respect to mechanism of action, studies in parkinsonian rodent models suggest that nicotine reduces LIDs by acting at nAChRs. Evidence for this possibility stems from the use of nAChR null mutant mice lacking the β2, α6 or α4 nAChR subunits, which comprise the primary nAChRs in the striatum 9, 21. Nicotine failed to reduce L-dopa-induced AIMs in β2, α6 and α4 nAChR subunit knockout mice with a 6-hydroxydopamine lesion 10, indicating an essential role for α4β2* and/or α6β2* nAChRs in the antidyskinetic effect of nicotine.
This idea is further supported by experiments in which β2* nAChR drugs were administered to unilateral 6-hydroxydopamine-lesioned rats. Varenicline, an agonist that interacts with multiple nAChRs 22–28, reduced L-dopa-induced AIMs by 40–50% in lesioned rats, consistent with the idea that the effect is nAChR-mediated 29. 5-iodo-A-85380 (A-85380), a drug that acts more selectively at α4β2* and α6β2* nAChRs 29,30, 31, also reduced L-dopa-induced AIMs 29. Further evidence for an involvement of nAChRs stems from studies with the β2* nAChR antagonist mecamylamine, which also suggested that the nicotine-mediated improvement in L-dopa-induced AIMs involves a receptor desensitization block 13. Overall, these combined data indicate that drugs targeting α4β2* and α6β2* nAChRs may be optimal in reducing LIDs.
This interaction at nAChRs may initiate long term adaptive changes that underlie the nAChR-mediated improvement in LIDs. These include alterations in nAChR-mediated intracellular signalling mechanisms that overlap with dopaminergic functions such as alterations in protein kinase A, extracellular signal-regulated mitogen-activated protein kinase, the calcium effector protein calmodulin and phosphatidylinositol 3-kinase/Akt-or protein kinase C-dependent signaling 32. Activation of these diverse signalling cascades has been reported to modulate CREB, tyrosine hydroxylase and other molecular components linked to the development of L-dopa-induced dyskinesias 33–39. Long term alterations in serotonergic mechanisms may also contribute to the nicotine-mediated improvement in LIDs, since the serotonergic system has been implicated in the etiology of LIDs 5, 40 and interacts with the nicotinic cholinergic system at a functional level 41–45.
A question that arises is whether nicotine treatment affects Parkinson’s disease motor symptoms or L-dopa-induced dyskinesias in a clinical setting. Several reports and small clinical trials have been done which show that nicotine improves parkinsonian symptoms but not consistently. Thus, of the studies currently available, five reported an improvement with nicotine treatment, four identified no beneficial effect and one obtained a worsening 46–55. The reason for these differences is not clear but may relate to variations in administration of nicotine (patch, gum, intravenous), dosing, timing/duration (days to weeks) of treatment, the degree of parkinsonism and/or type of trial (open-label versus double-blinded) 47–55. Overall, current data would indicate that nicotine does not worsen and possibly improves Parkinson’s disease symptoms. There are currently no published studies investigating the effect of nicotine on LIDs, although a clinical trial with 50 Parkinson’s disease patients showed that nicotine (designated NP002) significantly reduced a variety of outcome measures related to L-dopa-induced dyskinesias (http://www.neuraltus.com/pages/news.html). Continued work is thus necessary to ascertain nicotine’s effects both on Parkinson’s disease motor symptoms and L-dopa-induced dyskinesias.
A potential interaction between nicotine and L-dopa is an important question since this could affect parkinsonism and LIDs. A recent report suggests that the nicotine patch reduces L-dopa absorption in human controls; however, effects were minimal with significance in only one measure 56. By contrast, nicotine treatment improved or did not alter motor symptoms in Parkinson’s disease patients, as mentioned above 47–55. As well, nicotine did not affect parkinsonism in L-dopa-treated monkeys in this or previous studies 12, 14. The finding that L-dopa does not worsen parkinsonism would suggest that it does not improve LIDs by altering L-dopa kinetics.
In summary, the present results show that nicotine effectively reduces LIDs in both L-dopa-naïve and L-dopa-primed nonhuman primates with no tolerance to its beneficial effects with several months of treatment. These data suggest that such a therapy may prove useful in Parkinson’s disease patients.
Acknowledgments
Funding agencies: This study was supported by NIH grant NS59910.
We thank Dr. Tanuja Bordia for helpful comments regarding the manuscript.
Footnotes
Relevant conflict of interest/financial disclosures: There are no conflicts of interest or disclosures.
Author Roles:
1) Research project: A. Conception, M. Quik
B. Organization, M. Quik
C. Execution; A. Mallela, J. Ly, D. Zhang
2) Statistical Analysis: M. Quik, A. Mallela, J. Ly, D. Zhang
C. Review and Critique;
3) Manuscript: A. Writing of the first draft, M. Quik, A. Mallela
B. Review and Critique, M. Quik
References
- 1.Poewe W, Mahlknecht P, Jankovic J. Emerging therapies for Parkinson’s disease. Curr Opin Neurol. 2012;25(4):448–459. doi: 10.1097/WCO.0b013e3283542fde. [DOI] [PubMed] [Google Scholar]
- 2.Brotchie J, Jenner P. New approaches to therapy. Int Rev Neurobiol. 2011;98:123–150. doi: 10.1016/B978-0-12-381328-2.00005-5. [DOI] [PubMed] [Google Scholar]
- 3.Carta M, Bezard E. Contribution of pre-synaptic mechanisms to l-DOPA-induced dyskinesia. Neuroscience. 2011;198:245–251. doi: 10.1016/j.neuroscience.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Fisone G, Bezard E. Molecular mechanisms of l-DOPA-induced dyskinesia. Int Rev Neurobiol. 2011;98:95–122. doi: 10.1016/B978-0-12-381328-2.00004-3. [DOI] [PubMed] [Google Scholar]
- 5.Iravani MM, Jenner P. Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm. 2011;118(12):1661–1690. doi: 10.1007/s00702-011-0698-2. [DOI] [PubMed] [Google Scholar]
- 6.Cenci MA, Konradi C. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Progress in brain research. 2010;183C:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prashanth LK, Fox S, Meissner WG. l-Dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol. 2011;98:31–54. doi: 10.1016/B978-0-12-381328-2.00002-X. [DOI] [PubMed] [Google Scholar]
- 8.Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson’s disease therapeutics. Mov Disord. 2011;26(6):1072–1082. doi: 10.1002/mds.23714. [DOI] [PubMed] [Google Scholar]
- 9.Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson’s Disease. Pharmacol Rev. 2011;63(4):938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Grady SR, Quik M. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327(1):239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 12.Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 13.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in l-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013;50:30–41. doi: 10.1016/j.nbd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan LC, Protell PH, Langston JW, Togasaki DM. The hyperkinetic abnormal movements scale: a tool for measuring levodopa-induced abnormal movements in squirrel monkeys. Mov Disord. 2002;17(5):902–909. doi: 10.1002/mds.10183. [DOI] [PubMed] [Google Scholar]
- 16.Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 17.Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug Discov Today. 2005;10(8):578–585. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- 18.Slattery DA, Hudson AL, Nutt DJ. Invited review: the evolution of antidepressant mechanisms. Fundam Clin Pharmacol. 2004;18(1):1–21. doi: 10.1111/j.1472-8206.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 19.Thompson C. Onset of action of antidepressants: results of different analyses. Hum Psychopharmacol. 2002;17 (Suppl 1):S27–32. doi: 10.1002/hup.386. [DOI] [PubMed] [Google Scholar]
- 20.Kuhar MJ, Joyce AR. Slow onset of CNS drugs: can changes in protein concentration account for the delay? Trends Pharmacol Sci. 2001;22(9):450–456. doi: 10.1016/s0165-6147(00)01776-4. [DOI] [PubMed] [Google Scholar]
- 21.Gotti C, Clementi F, Fornari A, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78(7):703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of medicinal chemistry. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 23.Rollema H, Coe JW, Chambers LK, et al. Rationale, pharmacology and clinical efficacy of partial agonists of alpha(4)beta(2) nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28(7):316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular pharmacology. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 28.Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at alpha6beta2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342(2):327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang LZ, Campos C, Ly J, Carroll FI, Quik M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in moderately lesioned parkinsonian rats. Neuropharmacology. 2011;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhin AG, Gundisch D, Horti AG, et al. 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57(3):642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- 31.Kulak JM, Sum J, Musachio JL, McIntosh JM, Quik M. 5-Iodo-A-85380 binds to alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors (nAChRs) as well as alpha4beta2* subtypes. J Neurochem. 2002;81(2):403–406. doi: 10.1046/j.1471-4159.2002.00868.x. [DOI] [PubMed] [Google Scholar]
- 32.Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+ FASEB J. 2011;25:2563–2573. doi: 10.1096/fj.11-182824. [DOI] [PubMed] [Google Scholar]
- 33.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25(6):317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm. 2007;114:135–147. doi: 10.1007/s00702-006-0561-z. [DOI] [PubMed] [Google Scholar]
- 36.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 37.Ward RJ, Lallemand F, de Witte P, Dexter DT. Neurochemical pathways involved in the protective effects of nicotine and ethanol in preventing the development of Parkinson’s disease: Potential targets for the development of new therapeutic agents. Prog Neurobiol. 2008;85(2):135–147. doi: 10.1016/j.pneurobio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2011;24 (Suppl 2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- 39.Shimohama S. Nicotinic receptor-mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull. 2009;32(3):332–336. doi: 10.1248/bpb.32.332. [DOI] [PubMed] [Google Scholar]
- 40.Carta M, Carlsson T, Munoz A, Kirik D, Bjorklund A. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Progress in brain research. 2008;172:465–478. doi: 10.1016/S0079-6123(08)00922-9. [DOI] [PubMed] [Google Scholar]
- 41.Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology. 2011;36(7):1319–1331. doi: 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher PJ, Le AD, Higgins GA. Serotonin receptors as potential targets for modulation of nicotine use and dependence. Progress in brain research. 2008;172:361–383. doi: 10.1016/S0079-6123(08)00918-7. [DOI] [PubMed] [Google Scholar]
- 43.Levin ED, Slade S, Johnson M, et al. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. European journal of pharmacology. 2008;600(1–3):93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaniewska M, McCreary AC, Wydra K, Filip M. Differential effects of serotonin (5-HT)2 receptor-targeting ligands on locomotor responses to nicotine-repeated treatment. Synapse (New York, NY. 2010;64(7):511–519. doi: 10.1002/syn.20756. [DOI] [PubMed] [Google Scholar]
- 45.Zaniewska M, McCreary AC, Filip M. Interactions of serotonin (5-HT)2 receptor-targeting ligands and nicotine: locomotor activity studies in rats. Synapse (New York, NY. 2009;63(8):653–661. doi: 10.1002/syn.20645. [DOI] [PubMed] [Google Scholar]
- 46.Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23(12):1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993;117(1–2):28–32. doi: 10.1016/0022-510x(93)90150-w. [DOI] [PubMed] [Google Scholar]
- 48.Fagerstrom KO, Pomerleau O, Giordani B, Stelson F. Nicotine may relieve symptoms of Parkinson’s disease. Psychopharmacology (Berl) 1994;116(1):117–119. doi: 10.1007/BF02244882. [DOI] [PubMed] [Google Scholar]
- 49.Clemens P, Baron JA, Coffey D, Reeves A. The short-term effect of nicotine chewing gum in patients with Parkinson’s disease. Psychopharmacology (Berl) 1995;117(2):253–256. doi: 10.1007/BF02245195. [DOI] [PubMed] [Google Scholar]
- 50.Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord. 1999;14(6):1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 51.Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43(1–3):274–282. [PubMed] [Google Scholar]
- 52.Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57(6):1032–1035. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- 53.Villafane G, Cesaro P, Rialland A, et al. Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol. 2007;14:1313–1316. doi: 10.1111/j.1468-1331.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 54.Shoulson I. Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease. Neurology. 2006;66(3):408–410. doi: 10.1212/01.wnl.0000196466.99381.5c. [DOI] [PubMed] [Google Scholar]
- 55.Lemay S, Chouinard S, Blanchet P, et al. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):31–39. doi: 10.1016/S0278-5846(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 56.Kyaw WT, Nagai M, Kaneta M, et al. Effect of nicotine on the pharmacokinetics of levodopa. Clin Neuropharmacol. 2013;36(2):46–51. doi: 10.1097/WNF.0b013e31827fd9cd. [DOI] [PubMed] [Google Scholar]