Abstract
A previous study showed that the EphA7 receptor regulates apoptotic cell death during early brain development. In this study, we provide evidence that the EphA7 receptor interacts with death receptors such as tumor necrosis factor receptor 1 (TNFR1) to decrease cell viability. We showed that ephrinA5 stimulates EphA7 to activate the TNFR1-mediated apoptotic signaling pathway. In addition, a pull-down assay using biotinylated ephrinA5-Fc revealed that ephrinA5-EphA7 complexes recruit TNFR1 to form a multi-protein complex. Immunocytochemical staining analysis showed that EphA7 was co-localized with TNFR1 on the cell surface when cells were incubated with ephrinA5 at low temperatures. Finally, both the internalization motif and death domain of TNFR1 was important for interacting with an intracytoplasmic region of EphA7; this interaction was essential for inducing the apoptotic signaling cascade. This result suggests that a distinct multi-protein complex comprising ephrinA5, EphA7, and TNFR1 may constitute a platform for inducing caspase-dependent apoptotic cell death.
Keywords: apoptosis, ephrinA5, EphA7, TNFR1
INTRODUCTION
Early elimination of neural stem cells is significantly high during early brain development and has been implicated in determining the appropriate size of the neural progenitor population (de la Rosa and de Pablo, 2000; Kuan et al., 2000; Yeo and Gautier, 2004). Interestingly, evidence suggests that EphA7 or other Eph receptors may be key regulators of the apoptosis of proliferating neural progenitors (Depaepe et al., 2005). A recent study also indicated that a small portion of neuroepithelial cells display apoptotic cell death in the brain region where EphA7 and ephrinA5 are co-expressed (Park et al., 2013). In this region, a highly possible scenario is that EphAs cluster with neighboring ephrinAs via cell-cell contact, thereby increasing region-specific apoptosis. However, it remains to be determined how EphA-ephrinA signaling is biochemically linked to the caspase signaling cascade in inducing region-specific apoptosis.
Cell death receptors are a subset of the tumor necrosis factor receptor (TNFR) superfamily that harbors a death domain (DD) in their intracytoplasmic regions (Locksley et al., 2001; Wajant et al., 2003). This DD enables recruitment of additional proteins and the consequent assembly of a multi-protein complex platform, which permits the activation of caspases and leads to cell disassembly and death (Schutze and Schneider-Brachert, 2009; Schutze et al., 2008). When stimulated by appropriate ligands, death receptors recruit various adaptor molecules, depending on their receptor types. For example, TNFR1 initially recruits the adaptor molecule TRADD, which in turn recruits other proteins such as RIP1, TRAF2, and cIAP1/2 to form TNFR1 complex I (Hsu et al., 1996a; 1996b). This membrane-associated complex is shown to activate the nuclear factor-κB signaling pathway, which is critical for cell survival (Lee et al., 1997). However, when the alternative TNFR1 complex II is formed following receptor internalization, TRADD enables the recruitment of FADD and procaspase-8 to form TNFR1 complex II, which in turn initiates the caspase-dependent apoptotic signaling pathway (Schneider-Brachert et al., 2004). Like apoptosis, necroptosis, programmed necrosis, involves the formation of multi-protein complexes that initiate signaling pathways, ultimately resulting in cellular demise. Since cell death receptor protein complexes play crucial roles in cell death and survival, it is essential to examine these protein complexes to understand how cell death versus cell survival is regulated.
In this report, we found that TNFR1 activation might be triggered by the formation of the EphA7-ephrinA5 complex and that this distinct multi-protein complex induces caspase apoptotic cell death. We propose that this result may underlie the possible mechanism by which Eph-ephrin signaling regulates apoptotic cell death in neural stem cells.
MATERIALS AND METHODS
Expression constructs
Murine EphA7 (GenBank accession no. BC026153) and TNFR1 (GenBank accession no. BC004599) cDNAs were obtained from Thermo Scientific (USA). To construct EphA7-T1 containing 11 unique amino acids (aa) (SLVTME-HLSVL) at their carboxyl-termini instead of a region corresponding to aa 611–994 of full-length EphA7 (aa 611–994), a 275-bp polymerase chain reaction (PCR) product was amplified using a forward primer matching nucleotides (nt) 1600–1626 (5′-GCCACACTTGAG GAAGCTTCAGGTAAA-3′) of EphA7 cDNA with a reverse primer matching nt 1775–1798 (5′-GGCCAAGCTTCTCGAGTTATAAAACTGACAGATGCTCATTTGTTACTAAAGAATGAAAGTAGAGTTCTTCATCCC-3′). The underlined nucleotides represent the sequence matching the specific nt number. Next, the 275-bp PCR product was digested with HindIII and subcloned into the corresponding region of full-length EphA7 cDNA. To construct TNFR1-ΔDD (with a deletion of aa 353–454 of mouse TNFR1), a 311-bp PCR product using primers matching nt 749–785 (5′-GGGATCCCGTGCCTGTCAAAGAGGAGAAGGCTGGAA-3′) and nt 1039–1060 (5′-CATTGTCAGGACGTTGCG GGTG-3′) of TNFR1 cDNA, as well as a 504-bp PCR product using primers matching nt 1048–1060, 1363–1383 (5′-CGTCCTGACAATGTAAAGCCACACCCACAACCTT-3′), and 1835–1855 (5′-CATTTTTAGA CGTTTAGTGT-3′) of TNFR1 cDNA. The resulting partially complementary PCR fragments were annealed and used as templates in another PCR performed with primers matching nt 749–785 and 1835–1855 of TNFR1 cDNA. Next, the 815-bp PCR product was digested with BamHI/NotI and subcloned into the corresponding region of full-length TNFR1 cDNA. The same procedures were used to generate TNFR1-AXXA (with substitution of tyrosine to alanine (Y237A) and tryptophan to alanine (W240A); we amplified a 642-bp PCR product using primers matching nt 81–113 (5′-CACTGGACTAGTCCCTTCT CTTGGTGACCGGGA-3′) and 691–723 (5′-CCTGGCTCGAGGTGCTCGGCACATTAAACTGAT-3′) of TNFR1 cDNA, as well as a 513-bp PCR product using primers matching nt 703–735 (5′-TGCCGAGCACCTCGAGCCAGGCCCGAAGTCTAC-3′) and 1184–1216 (5′-CTTCCAGCATGCTGTACTGAGCCTCGCGCAGGC-3′) of the TNFR1 cDNA. The resulting two partially complementary PCR fragments were annealed and used as templates in another PCR performed with primers matching nt 81–113 and 1184–1216 of TNFR1 cDNA. Next, the 1155-bp PCR product was digested with SpeI/SphI and subcloned into the corresponding region of full-length TNFR1 cDNA.
Cell culture, transfection, and cell viability assay
HEK293 cells were cultured as described previously (Gu et al., 2005). Transient transfection procedures were performed using Metafectin (Biontax) according to the manufacturer’s instructions. For the cell viability assay, cells (1.0 × 105) were seeded in 24-well plates and transfected with total 1 μg plasmid DNA. After 30 hr, cells were briefly washed with phosphate-buffered saline (PBS) and treated with a solution of XTT-PMS (0.3 mg/ml, Sigma-Aldrich) for 8 h at 37°C. The absorbance at 450 nm was measured by spectrofluorometry. The absorbance at 600 nm was also measured as background.
Immunoprecipitation and Western blot
Precipitation and Western blotting were performed essentially as described previously (Shin et al., 2007). Briefly, cells were grown to 70% confluence on 10-cm dishes and transiently transfected with 6 μg plasmid DNA. Fourteen hours post-transfection, cells were incubated with biotinylated ephrinA5-Fc (R&D Systems) for 1 h at 4°C. Labeled cells were lysed with PLC lysis buffer as previously described and bound to streptavidin-agarose beads (Thermo Scientific). Bound material was eluted by boiling the beads and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Immunocytochemical staining
For immunocytochemical staining, cells were plated at a density of 1.0 × 106 cells per 35-mm dish and transiently transfected. Fourteen hours post-transfection, cells were incubated with Fc or eA5-Fc on ice (Noh and Park, 2010; Yoo et al., 2012). Cells were washed twice with PBS, fixed with 4% paraformaldehyde-2% sucrose in PBS on ice for 30 min, rinsed with PBS, and blocked for 30 min at room temperature with 3% bovine serum albumin, 5% horse serum, and 0.1% Triton X-100 in PBS. For apoptotic cell staining, rabbit anti-cleaved caspase-3 antibody (1:750, Cell Signaling) was applied for overnight at 4°C and visualized by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG. The data were quantified by counting the number of cells labeled with antibody in five random microscopic fields (× 200); three independent experiments were performed. For the co-localization study of EphA7 and TNFR1, cells were incubated with eA5-Fc on ice and shifted to a 37°C incubator for the indicated time. Cells were processed for immunofluorescence staining as described above. EphA7 was detected by staining of bound ephrinA5-Fc with rhodamine-conjugated goat anti-human IgG, whereas TNFR1 was detected using FITC-conjugated anti-goat IgG.
Antibodies
Anti-cleaved caspase-3 antibodies were purchased from Cell Signaling. Anti-EphA7 and anti-TNFR1 antibodies were purchased from Santa Cruz Biotechnology. Rhodamine-conjugated goat anti-human IgG, FITC-conjugated anti-rabbit IgG, and FITC-conjugated anti-goat IgG antibodies were purchased from Invitrogen. Horseradish peroxidase-conjugated anti-rat IgG and anti-goat IgG antibodies were acquired from Zymed.
RESULTS
Identification of cell death receptors interacting with the EphA7 receptor
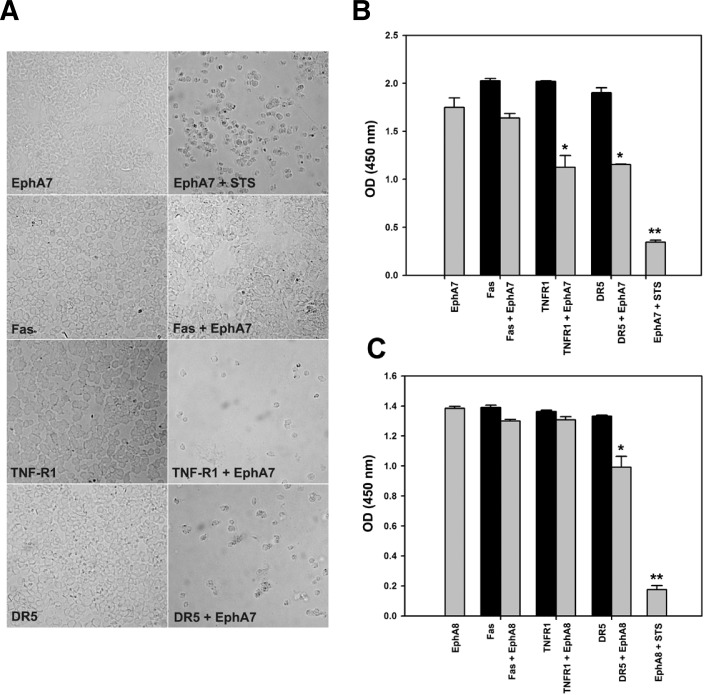
Since the EphA7 receptor was shown to induce apoptotic cell death during early brain development (Depaepe et al., 2005; Park et al., 2013), we postulated that it might interact with cell death receptors to trigger the extrinsic apoptotic signaling pathway. For this purpose, various cell death receptors were co-expressed with EphA7 in HEK293 cells. After a 48-h culture, cells were examined under microscopy (Fig. 1A) or subjected to a cell viability assay (Fig. 1B). Interestingly, cell viability was significantly reduced in cells expressing EphA7 together with TNFR1 or DR5 (Fig. 1A, third and fourth panels; Fig. 1B, lanes 4–7). However, a similar effect was barely observed in cells co-expressing EphA7 and Fas (Fig. 1A, second panel; Fig. 1B, lanes 3 and 4). In addition, when EphA8 was co-expressed with cell death receptors in HEK293 cells, only DR5 was significantly effective in decreasing cell viability together with EphA8 (Fig. 1C, lanes 6 and 7). These results suggest that each mem-ber of the Eph receptor subfamily may interact with different types of cell death receptors to control the extrinsic cell death program.
Fig. 1.
Screening of death receptors interacting with EphA7 using cell viability assay. (A) The indicated death receptor expression constructs were co-transfected with EphA7 expression vector into HEK293 cells; at 30 h post-transfection, dead cells were briefly washed away with culture medium before attached cells were photographed as DIC images. Staurosporine (2 μM) was used as a positive control for inducing apoptotic cell death. (B, C) Transfected cells were subjected to an XTT assay, which measures absorbance at 450 nm to detect cell viability. Data represent the means ± standard error (SE). *p < 0.001.
EphrinA5 stimulates EphA7 to activate the TNFR1-mediated apoptotic signaling pathway
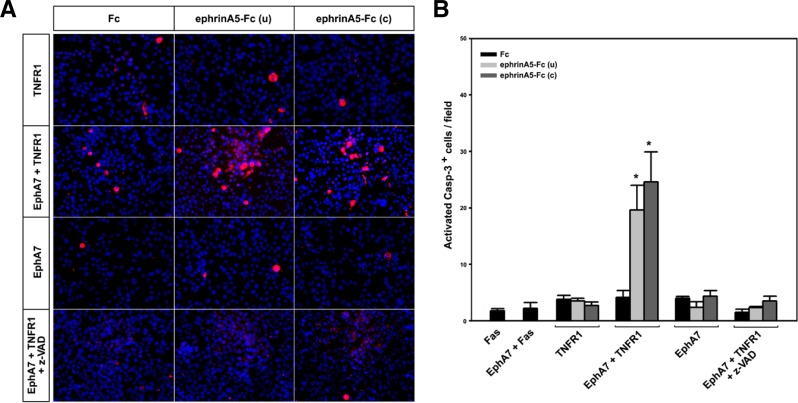
To determine whether TNFR1 is activated by EphA7 upon ephrinA5 binding, co-transfected cells were treated with ephrinA5-Fc for 30 min and subjected to immunostaining with anticleaved caspase 3 antibody for detection of apoptotic cells (Fig. 2). For this experiment, either clustered ephrinA5-Fc or unclustered ephrinA5-Fc was used to compare their effects on activation of the EphA7 receptor. In addition, ephrinA5-Fc was added to the cells under culture conditions where apoptotic cell death minimally occurred after DNA transfection. As a result, ephrinA5-Fc treatment had little effect on the number of apoptotic cells when Fas and EphA7 were co-expressed (Fig. 2B, lane 2; data not shown). Similar results were observed in cells expressing TNFR1 or EphA7 alone (Fig. 2A, first and third panels; Fig. 2B, lanes 3–5 and 9–11). Importantly, when cells were co-transfected with EphA7 and TNFR1, unclustered ephrinA5-Fc treatment increased the number of apoptotic cells approximately 5-fold; this effect was slightly increased by the treatment of clustered ephrinA5-Fc (Fig. 2A, second panel; Fig. 2B, lanes 6–8). Although clustered ephrinA5-Fc was known to be more potent in activating EphA receptors, it was observed that its treatment resulted in a slight increase in tyrosine phosphorylation of the EphA7 receptor in HEK293 cells (data not shown). As expected, this increased apoptotic effect was significantly reduced by treatment with a caspase inhibitor (Fig. 2A, fourth panel; Fig. 2B, lanes 12–14). These results suggest that ephrinA5 binds to EphA7 and that the ephrinA5-EphA7 complex activates TNFR1 to induce the extrinsic apoptotic signaling cascade.
Fig. 2.
The activated EphA7 receptor interacts with TNFR1 to enhance apoptotic signaling. (A) HEK 293 cells were transfected as described in Fig. 1; at 14 h post-transfection, cells were treated with Fc or ephrinA5-Fc for 1 h at 37°C. For clustered ephrinA5-Fc, ephrinA5-Fc (1 μg) was incubated with goat anti-human IgG antibody for 30 min on ice. The cells were washed, fixed, and subjected to immunocytochemical staining using anti-cleaved caspase-3 antibody to detect apoptotic cells (red). Nuclear staining was performed using DAPI (blue). (B) Apoptotic cells in each microscopic field (× 200) were counted; cell counting was repeated for five different fields per dish for quantification. Z-VAD was used as a caspase inhibitor. u, unclustered; c, clustered. Data were obtained from three independent experiments and shown as the means ± SE. *p < 0.001.
Physical association between EphA7 and TNFR1
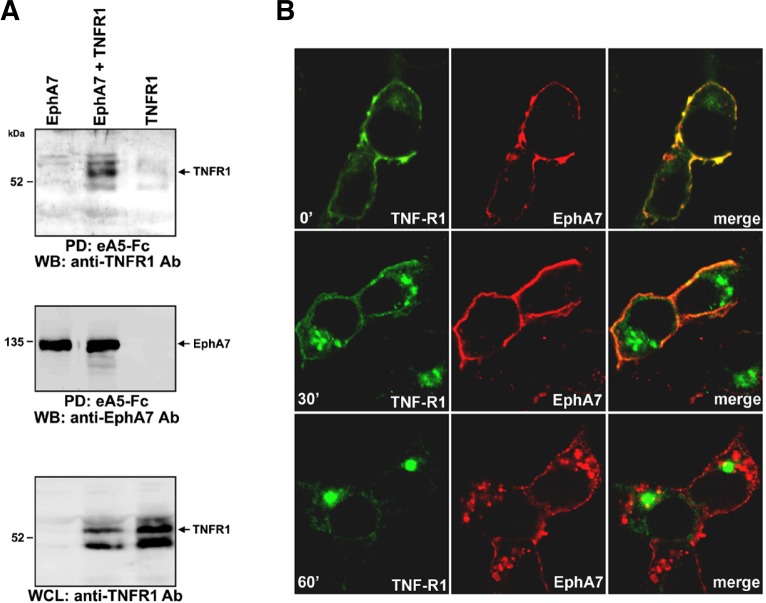
To further assess whether EphA7 is associated with TNFR1 in co-transfected cells, cell lysates were incubated with biotinylated ephrinA5-Fc on ice and bound EphA7 receptor complexes were precipitated using streptavidin-agarose beads. The resulting protein complexes were analyzed by Western blotting using anti-TNFR1 antibody or EphA7 antibody (Fig. 3A). It was evident that EphA7 was co-precipitated with ephrinA5-Fc from cell lysates containing EphA7 but not from cell lysates containing TNFR1 alone (Fig. 3A, second panel, lanes 1 and 3). Importantly, a significant level of TNFR1 was detected in the EphA7 protein complexes pulled-down from cell lysates containing both EphA7 and TNFR1 (Fig. 3A, first panel, lane 2). This result suggests that ephrinA5-EphA7 complexes tightly associate with TNFR1 in HEK293 cells. To further confirm the physical association between TNFR1 and EphA7, we performed a co-localization study. For this, cells co-transfected with EphA7 and TNFR1 were incubated on ice with ephrinA5-Fc for 1 h and shifted to 37°C to induce endocytosis of the receptor complexes. When endocytosis was blocked (0′), it was evident that EphA7 was very well co-localized with TNFR1 on the cell membrane (Fig. 3B, first panel). Thirty minutes after inducing endocytosis of the receptor complexes, a fraction of TNFR1 was internalized and detected as large receptosomal bodies. Interestingly, these endocytosed TNFR1 complexes were not co-localized with EphA7, which appeared to remain at the cell membrane (Fig. 3B, second panel). Sixty minutes after endocytosis, TNFR1 was detected mostly as internalized receptosomal complexes, which were completely separated from the internalized EphA7 complexes. These results suggest that EphA7 is co-localized with TNFR1 on the cell surface and that endocytosis of ephrinA5-EphA7 complexes occurs independent of that of TNFR1 on the cell surface.
Fig. 3.
EphrinA5 induces formation of multi-protein complex containing EphA7 and TNFR1. (A) HEK 293 cells were transfected as described in Fig. 1; at 14 h post-transfection, cells were treated with biotinylated ephrinA5-Fc on ice for 1 h. Cell lysates were further incubated with streptavidin-agarose beads prior to precipitation. Protein complexes were resolved by SDS-PAGE and subjected to Western blot analysis using anti-TNFR1 antibody (top panel) or EphA7 antibody (middle panel). Whole cell lysates were also analyzed by Western blot using anti-TNFR1 antibody (bottom panel). (B) Transfected cells were incubated with ephrinA5-Fc on ice for 1 h. Cells were transferred into a 37°C incubator. At the indicated time, cells were briefly washed, fixed, and subjected to immunocytochemical staining using goat anti-human IgG antibody (conjugated with rhodamine) and anti-TNFR1 antibody (pre-incubated with FITC-conjugated secondary antibody). Note that anti-human IgG antibody specifically stains ephrinA5-Fc bound to EphA7.
Intracytoplasmic region of EphA7 is critical for interaction with TNFR1
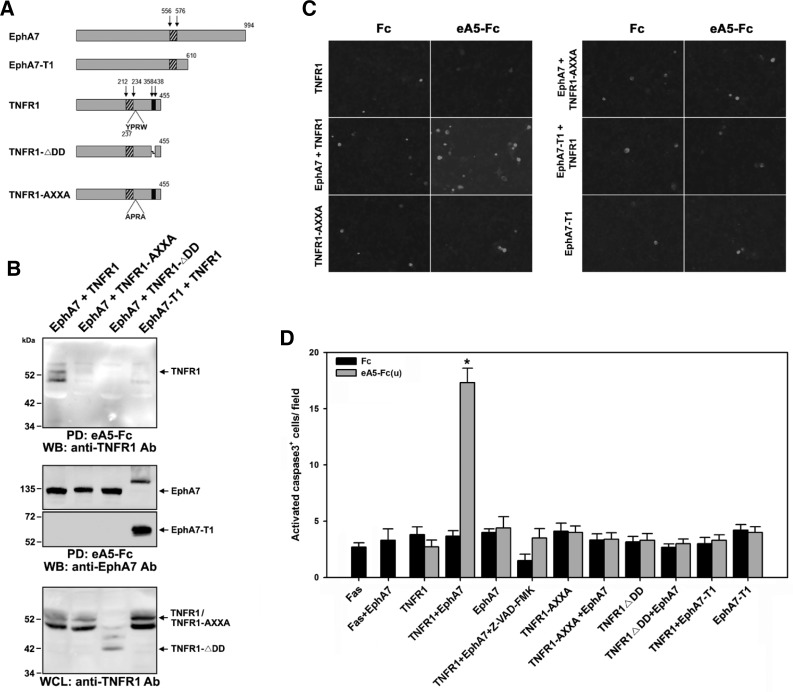
To determine which region of EphA7 is important for interacting with TNFR1, an EphA7-T1 mutant was co-expressed with TNFR1 in HEK293 cells. EphA7-T1 lacks an intracytoplasmic region, spanning amino acids 599 to 994 of mouse EphA7, and this deleted region is replaced by a unique peptide sequence comprised of 11 amino acids (Fig. 4A) (Ciossek et al., 1995). It was evident that this truncated EphA7 mutant was defective for associating with TNFR1 (Fig. 4B, lane 4). In addition, ephrinA5-Fc treatment had little effect on the number of apoptotic cells when EphA7-T1 was co-expressed with TNFR1 (Fig. 4C, right and third panels; Fig. 4D, lanes 21 and 22). This result suggests that an intracytoplasmic region of EphA7 is critical for interacting with TNFR1.
Fig. 4.
Internalization motif or death domain of TNFR1 is critical for interacting with the cytoplasmic region of EphA7. (A) Schematic diagrams showing EphA7 or TNFR1 mutants used for expression in HEK293 cells. EphA7-T1 comprises a unique peptide sequence (SLVTMEHLSVL) at its carboxyl-terminus, which replaces the intracytoplasmic region spanning amino acids 599 to 994 of mouse EphA7. The TNFR1 internalization motif (amino acids 237 to 240) is critical for receptor endocytosis. DD represents death domain as marked by the black bar. The hatched bar indicates the transmembrane domain for each protein. (B, C) Experiments were performed essentially as described in the legends for Figs. 2 and 3. (D) Data represent the means ± SE. *p < 0.001.
It was previously shown that a small peptide sequence corresponding to amino acids 237–240 in human TNFR1 is critical for TNFR1 internalization (Fig. 4A) (Schneider-Brachert et al., 2004). In addition, the cytoplasmic DD of TNFR1 recruits the adaptor protein TRADD and serves as a platform for the death-inducing signaling complex. Therefore, we wanted to analyze whether an internalization-defective or DD-deleted TNFR1 mutant was able to form a complex with EphA7. However, neither the TNFR1-AXXA nor the TNFR1-ΔDD mutant was co-precipitated with EphA7 (Fig. 4B, lanes 2 and 3). Consistently, none of these TNFR1 mutants was able to increase the number of apoptotic cells when EphA7 was stimulated with ephrinA5-Fc (Fig. 4C, left third and right first panels; Fig. 4D, lanes 11–18). These results suggest that either an internalization motif or the DD of TNFR1 is important for interacting with EphA7 and that this interaction is essential for inducing the apoptotic signaling cascade.
DISCUSSION
Although Eph receptors have been implicated in regulating apoptotic cell death in neuroepithelial cells, the molecular mechanism by which Eph signaling is linked with the caspase-dependent apoptotic signaling cascade is poorly understood. The results presented in this study provide biochemical evidence for the possibility that EphA7 recruits TNFR1 to trigger the apoptotic signaling pathway in response to ephrinA5. In our model, after ephrinA5 specifically binds to EphA7, TNFR1 is recruited to the activated EphA7 receptor, and this receptor complex in turn recruits other adapter proteins such as TRADD. This multi-protein complex initiates TNFR1 internalization, which is essential for recruiting FADD and caspase-8 (Schutze et al., 2008). During the endocytosis of TNFR1, it seems that the ephrinA5-EphA7 complex remains at the plasma membrane and that its internalization begins far later after the endocytosis of TNFR1. Thus, our model suggests that the ephrinA5-EphA7 signaling complex may act as a novel ligand for TNFR1, just like TNF, and its role at the plasma membrane is to induce internalization followed by the apoptotic signaling cascade.
Evidence suggests that Eph/ephrin-triggered apoptotic cell death is a phenomenon unique to certain neuroepithelial cells and that it provides a key mechanism of regulating their population size during development of a specific brain region (Depaepe et al., 2005). A key issue is whether TNFR1 or other related death receptors are co-expressed with Eph receptors to trigger apoptotic signaling in neuroepithelial cells. Our results presented here show that TNFR1 tightly associates with EphA7 in HEK293 cells and that this interaction is critical for activating caspase-3 in response to ephrinA5-Fc treatment. Therefore, we have intensively studied whether TNFR1 expression is temporally and spatially regulated in neuroepithelial cells during early brain development. However, our results revealed that TNFR1 is not expressed in neuroepithelial cells during embryonic brain development (HL’s unpublished observation). However, it is possible that certain cell death receptors such as DR5 or DR6 may be expressed in the developing brain and that these death receptors may interact with Eph receptors to trigger apoptotic cell death. It is well known that Eph receptors cross-talk with other cell surface receptors such as ErbB2 (Brantley-Sieders et al., 2008) and TrkB (Marler et al., 2008). More importantly, a recent study indicated that ephrinA5 interacts with p75NTR in the brain (Lim et al., 2008). These reports strongly suggest that these cross-interactions with various other receptor tyrosine kinases or death receptors may provide a great advantage for more sophisticated modulation of apoptotic cell death versus cell proliferation during early brain development.
Acknowledgments
This work was supported by the SRC Research Center for Women’s Diseases of Sookmyung Women’s University (no. 3-1103-0011).
REFERENCES
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciossek T, Millauer B, Ullrich A. Identification of alternatively spliced mRNAs encoding variants of MDK1, a novel receptor tyrosine kinase expressed in the murine nervous system. Oncogene. 1995;10:97–108. [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Gu C, Shim S, Shin J, Kim J, Park J, Han K, Park S. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene. 2005;24:4243–4256. doi: 10.1038/sj.onc.1208584. [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996a;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996b;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Park S. Ectopic expression of Ephrin-A5 under the EphA8 promoter at the anterior region of the superior colliculus. Exp Neurobiol. 2010;19:49–53. doi: 10.5607/en.2010.19.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Kim Y, Noh H, Lee H, Yoo S, Park S. EphA/ephrin-A signaling is critically involved in region-specific apoptosis during early brain development. Cell Death Differ. 2013;20:169–180. doi: 10.1038/cdd.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schutze S, Schneider-Brachert W. Impact of TNF-R1 and CD95 internalization on apoptotic and antiapoptotic signaling. Results Probl Cell Differ. 2009;49:63–85. doi: 10.1007/400_2008_23. [DOI] [PubMed] [Google Scholar]
- Schutze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- Shin J, Gu C, Park E, Park S. Identification of phospho- tyrosine binding domain-containing proteins as novel downstream targets of the EphA8 signaling function. Mol Cell Biol. 2007;27:8113–8126. doi: 10.1128/MCB.00794-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274:233–244. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Yoo S, Kim Y, Lee H, Park S. A gene trap knockout of the Tiam-1 protein results in malformation of the early embryonic brain. Mol Cells. 2012;34:103–108. doi: 10.1007/s10059-012-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]