Abstract
Herpes simplex virus 1 (HSV-1) replication in cancer cells leads to their destruction (viral oncolysis) and has been under investigation as an experimental cancer therapy in clinical trials as single agents, and as combinations with chemotherapy. Cellular responses to chemotherapy modulate viral replication, but these interactions are poorly understood. To investigate the effect of chemotherapy on HSV-1 oncolysis, viral replication in cells exposed to 5-fluorouracil (5-FU), irinotecan (CPT-11), methotrexate (MTX) or a cytokine (TNF-α) was examined. Exposure of colon and pancreatic cancer cells to 5-FU, CPT-11, or MTX in vitro significantly antagonizes both HSV-1 replication and lytic oncolysis. Nuclear factor-kappa B (NF-κB) activation is required for efficient viral replication, and experimental inhibition of this response with an IκBα dominant-negative repressor significantly antagonizes HSV-1 replication. Nonetheless cells exposed to 5-FU, CPT-11, TNF-α or HSV-1 activate NF-κB. Cells exposed to MTX do not activate NF-κB, suggesting a possible role for NF-κB inhibition in the decreased viral replication observed following exposure to MTX. The role of eukaryotic initiation factor 2 alpha (eIF-2α) dephosphorylation was examined; HSV-1 mediated eIF-2α dephosphorylation proceeds normally in HT29 cells exposed to 5-FU-, CPT-11-, or MTX. This report demonstrates that cellular responses to chemotherapeutic agents provide an unfavorable environment for HSV-1-mediated oncolysis, and these observations are relevant to the design of both preclinical and clinical studies of HSV-1 oncolysis.
INTRODUCTION
Given the limitations of current treatment options for solid tumor metastases, there remains strong rationale to develop novel therapies such as oncolysis by replicating viruses. We have previously examined regulation of HSV-1 at the molecular level, and used this information to [i] genetically engineer HSV-1 mutants that when introduced intravascularly into the liver replicate preferentially in liver tumors rather than normal cells;1–3 [ii] express therapeutic transgenes such as prodrug activation genes and anti-angiogenesis genes;4, 5 [iii] enhance survival of animals with diffuse liver tumors following intravascular HSV-1 administration.4–6 Clinical trials of HSV-1 oncolytic mutants to treat unresectable and refractory malignancies are currently being conducted and involve administration of HSV-1 before, during, or after administration of chemotherapy agents.7–9 Little has been published regarding chemotherapy-induced modulation of cellular pathways that are intimately linked to viral replication, and we therefore embarked on studies to examine the interactions.
Viruses have evolved numerous mechanisms to achieve robust viral replication by evasion or compensation for host antiviral strategies, such as those involved in induction of apoptosis, cytokine production, and antiviral pathways activated by double-stranded RNA (dsRNA). For example, host cell PKR is a central enzyme involved in antiviral activity, and has been shown to modulate replication of many viruses.10 The importance of PKR in controlling viral infections is underscored by the observation that many viruses use strategies to bypass PKR responses, such as inhibition of PKR activation, sequestration of dsRNA, inhibition of PKR responses, synthesis of PKR pseudosubstrates, activation of antagonist phosphatases, and PKR degradation.10–12 PKR induces NF-κB activation and independently triggers a translational block through phosphorylation of eIF-2α.11 This could markedly attenuate HSV-1 replication; however, the product of an HSV-1 gene γ134.5 interacts with the cellular protein phosphatase-1α to dephosphorylation eIF-2α and enable translation.11, 13–15 To improve clinical studies of chemotherapy and HSV-1, it is important to understand how modulation of PKR by chemotherapy affects HSV-1 replication and oncolysis.
As another example of the interactions between chemotherapy and HSV-1 oncolysis, the dimeric transcription factor NF-κB regulates expression of genes involved in immune responses, inflammatory responses, and apoptotic responses.16, 17 Activation of NF-κB occurs in response to stresses including radiation and chemotherapy; by agents such as tumor necrosis factor-alpha (TNF-α), interleukin-1, lipopolysaccharide, and phorbol ester; and by inducers of endoplasmic reticulum overload.18–21 And viruses have evolved mechanisms to modulate NF-κB activity to enhance expression of viral genes.22, 23 As such, cell modulation of NF -κB in response to chemotherapy may alter cell susceptibility to lytic viral replication. In this study we investigated the effect of chemotherapy on HSV-1 oncolysis using agents that are commonly used to treat gastrointestinal malignancies.
MATERIALS AND METHODS
Cells and viruses
Vero (African Monkey kidney), HT29 and SW620 (human colon carcinoma), Capan2 (human pancreatic cancer) and 293 (human kidney) were obtained from American Type Culture Collection (Rockville, MD). 0–28 [ICP (infected cell protein) 0-transformed Vero cells] and E5 (ICP4-transformed Vero cells) were kindly provided by E. Antonio Chiocca (Massachusetts General Hospital, Boston, MA). V27 (ICP27-transformed Vero cells) were kindly provided by David Knipe (Harvard Medical School, Boston, MA). Cells were propagated in DMEM with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 mg/ml streptomycin. hrR3 (ICP6-defective HSV-1 mutant)24 was kindly provided by Sandra Weller (University of Connecticut, Farmington, CT). 7134 (ICP0-defective HSV-1 mutant)19 and gal4 (ICP4-defective HSV-1 mutant)23 were kindly provided by E. Antonio Chiocca (Massachusetts General Hospital). d27 (ICP27-defective HSV-1 mutant)25 were kindly provided by David Knipe. Wild-type HSV-1 strain KOS26 was kindly provided by Donald Coen (Harvard Medical School). R3616 (γ134.5- defective HSV-1 mutant)13 was kindly provided by Bernard Roizman (University of Chicago, Chicago, IL). Heat-inactivation of viruses was performed as described.27 The drugs used in cell cultures studies were tumor necrosis factor-alpha (TNF-α, SIGMA, St. Louis, MO), methotrexate (MTX, Ben Vanue Laboratories, Inc., Bedford, OH), 5-fluorouracil (5-FU, Pharmacia & Upjohn Co., Kalamazoo, MI) and irinotecan (CPT-11, Pharmacia & Upjohn Co., Kalamazoo, MI).
The recombinant adenovirus vectors used in this study were replication-defective Ad5-based vectors constructed with the transgene expression driven by the CMV early/intermediate promoter/enhancer. All vectors were expanded in 293 cells and purified and titered as described previously28. The vector Ad.CMV.IκBα expresses the super-repressor form of IκBα that is mutated at serine residues 32 and 36 and functions as a potent and specific repressor of NF-κB-mediated events.28, 29 The control vector Ad.CMV3, generously provided by J. A. Roth (University of Texas M.D. Anderson Cancer Center, Houston, TX), contains a CMV promoter similar to Ad.CMV.IκBBα but lacks a transgene insert.
In vitro viral cytotoxicity and replication assays
Viral replication assays were performed by infecting 106 cells with HSV-1 for 2 h, at which time unabsorbed virus was removed by washing with a glycine-saline solution (pH = 3.0). At 0, 8, 16, 24, 32 and 40 h after infection, the supernatant and cells were exposed to three freeze/thaw cycles to release virions and titered on Vero cells or 0–28 cells. Viral cytotoxicity was determined as described previously.1 Briefly, 5000 cells/well were plated onto 96-well plates and grown for 36 h. Cells were then infected with either KOS or HSV-1 mutants using multiplicity of infection (MOI) values ranging from 0.001 to 10. The number of viable cells was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. All experiments were performed in quadruplicate.
Electrophoretic mobility shift assay for NF -κB activation
Activation of NF-κB in response to infection with HSV-1 or treatment with chemotherapeutic drugs was determined by the electrophoretic mobility shift assay (EMSA). HT29 cells (5×106 cells/ plate) were cultured in 100 mm dishes. Twenty-four hours later, cells were infected with HSV-1 or treated with chemotherapeutic drugs. Cells were then harvested at times 0, 3, 6, 12, 24 and 36 h after treatment. For experiments using the adenoviral-mediated delivery of the IκBα supper-repressor (IκBα-SR) gene, HT29 cells were infected with Ad.CMV.IκBα or Ad.CMV3 (MOI = 20) for 1 h and then washed with PBS. Twelve hours after adenovirus infection, cells were infected with HSV-1 (KOS, 7134 and hrR3) for 1 h and then washed with PBS. Cells were then harvested 0, 8, 16, 24, 32 and 40 h after HSV-1 infections and resuspended in 3 volumes of C.E. Buffer [10 mM Hepes (pH = 7.6), 60 mM KCl, 1 mM EDTA, 10 mM 1,4-Dithio-L-threitol (DTT)] with 0.1% NP-40 and proteinase inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 2.5 µg/ml aprotinin, 2.5 µg/ml pepstain, and 2.5 µg/ml leupeptin). Nuclear pellets were collected at 3000 rpm (4° C) to which 100µl of C.E. buffer was added. After centrifugation at 3000 rpm (4° C), the pellet was resuspended in 2 pellet volumes of N.E. Buffer [20 mM Tris (pH = 8.0), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 25% glycerol] with proteinase inhibitors, and then added 60 µl of 5M NaCl. The soluble protein was released by centrifugation (20 min at 14,000 rpm), and stored at −80° C. The amount of protein in the supernatant was quantified using a BCA protein assay kit (Pierce Chemical Co., Rockford, IL). The DNA probe used contains an NF-κB site (underlined) from the H-2κb gene (5’-CAG GGC TGGGGA TTC CCA TCT CCA CAG TTT CAC TTC-3’)28 Ten µg of nuclear extracts were preincubated with 1 µg of poly deoxyinosinic-deoxycytidylic acid [poly (dI-dC)•poly (dI-dC); Amersham Pharmacia Biotech Inc., Piscataway, NJ] in binding buffer (10 mM Tris, 50 mM NaCl, 20% glycerol, 0.5 mM EDTA, and 1 mM DTT) for 10 min at room temperature. Approximately 20,000 cpm of 32P-labeled DNA probe was then added and allowed to bind for 15 min. The complexes were then separated on a 1% agarose/0.25% Synergel (Diversified Biotech, Boston, MA) in a 0.5× TBE buffer and autoradiographed.30
Purification of bacteria-expressed PKR and eIF-2α
Production of PKR and eIF-2α were performed as described previously.6 In brief, Escherichia coli BL21 cells harboring the pGEX-PKR and pQE-eIF-2α expression vectors (kindly provided by Bryan R.G. Williams, Lerner Research Institute, Cleveland, OH) were grown overnight in 50 ml Luria-Bertani (LB) broth containing 50 µg/ml ampicillin. Following 1:10 dilution in fresh LB broth, cells were grown for 3 h to an optical density of 0.8, at which time isopropyl-1-thio-β-D-galactopyranoside (IPTG) was added to a concentration of 1 mM for an additional 4 h. Bacteria were pelleted and used for the fusion protein purification. To purify the His-tagged eIF-2α protein, a His•Bind purification Kit (Novagen Inc., Madison, WI) was used following the manufacturer’s instructions. To purify the GST-tagged PKR, a GST•Bind purification Kit (Novagen Inc.) was used according to the manufacturer’s instructions.
In vitro phosphorylation assays
The PKR autophosphorylation assay was performed as follows. HT29 cells were harvested at 24 h after treatment with 5-FU (100 µM), CPT-11 (1 µM), MTX (25 µM), and TNF-α (10 ng/ml). The cells were rinsed with phosphate-buffered saline (PBS), resuspended in lysis buffer containing 10 mM HEPES (pH = 7.6), 150 mM NaCl, 10 mM MgCl2, 0.2% Triton X-100, 10% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, and 2 mM benzamide, placed on ice for 30 min and subjected centrifugation to remove nuclei. The cell lysates were reacted with [γ32P] ATP (100 µCi/sample) for 30 min at 32° C, precleared with protein A-agarose (Roche Diagnostics Corp., Indianapolis, IL) and reacted with 1 µg of antibody to PKR (K-17) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or without the PKR-antibody for negative control. These complexes were precipitated using protein-A agarose, rinsed with RIPA buffer (PBS containing 0.25% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate), solubilized in buffer C [20 mM Tris-HCl (pH = 7.0), 50 mM KCl and 2 mM MgCl2], electrophoretically separated in a 10% SDS-PAGE gels, and subjected to autoradiography. For eIF-2α phosphorylation assay, 2 µl of purified eIF-2α protein (0.32 µg/µl) was added to the kinase reaction [2 µl of cell lysate (0.32 µg/ l), 7 µl of buffer C, and 1 µl of [γ32P]ATP (10 µCi/µl)]. These reactions were incubated at 32° C for 40 min before the addition of 2 × SDS loading buffer (12 µl) was added to each sample before boiling, separation by 10% SDS-PAGE, and autoradiography. The PKR-32P or eIF-2α-32P was quantified by an image analyzer (LabWorks 4.0; UVP Inc., Upland, CA).
In vitro eIF-2α dephosphorylation assays
Confluent 100 mm plate of HT29 cells were harvested 24 h after treatment with chemotherapeutic drugs [5-FU (100 µM), CPT-11 (1 µM), MTX (25 µM), and TNF-α (10 ng/ml)] in the presence or absence of HSV-1 KOS (MOI = 1) infection. Cells were once rinsed with PBS and added to 100 µl of cell lysate buffer and placed on ice for 30 min and then centrifuged. The amount of protein in supernatant was quantified using a BCA protein assay kit (Pierce Chemical Co.). Purified eIF-2α protein (12.5 µl; 0.32 µg/µl) was reacted with GST-PKR protein (12.5 µl; 0.32 µg/µl) in 38 µl of buffer C and 7 µl of [γ32P]ATP (10 µCi/µl) in a final volume of 70 µl for 40 min at 32° C to yield phosphorylated eIF-2α. After 40 min at 32° C, 8 µl of phosphorylated eIF-2α (eIF-2α-32P) was mixed with 10 µl of the lysates and 7 µl of buffer C. Following incubation at room temperature for different time intervals, 10 µl aliquots were removed at specific time point, mixed with 2 × SDS loading buffer (10 µl), boiled for 3 min and analyzed by SDS-PAGE. The 32P remaining in eIF-2α-32P was quantified by an image analyzer (LabWorks 4.0).
Statistical analysis
The mean values of the cell survival rates from the cytotoxicity assay, viral yields from the viral replication assay and optical density from the EMSA of NF-κB, PKR/eIF-2α phosphorylation assay or eIF-2α dephosphorylation assay were compared by two sided Student’s t test. Statistical analysis and calculation of the regression coefficients were performed using StatView software (SAS Institute Inc., Gary, NC).
RESULTS
Chemotherapy-induced cytotoxicity inhibits viral replication
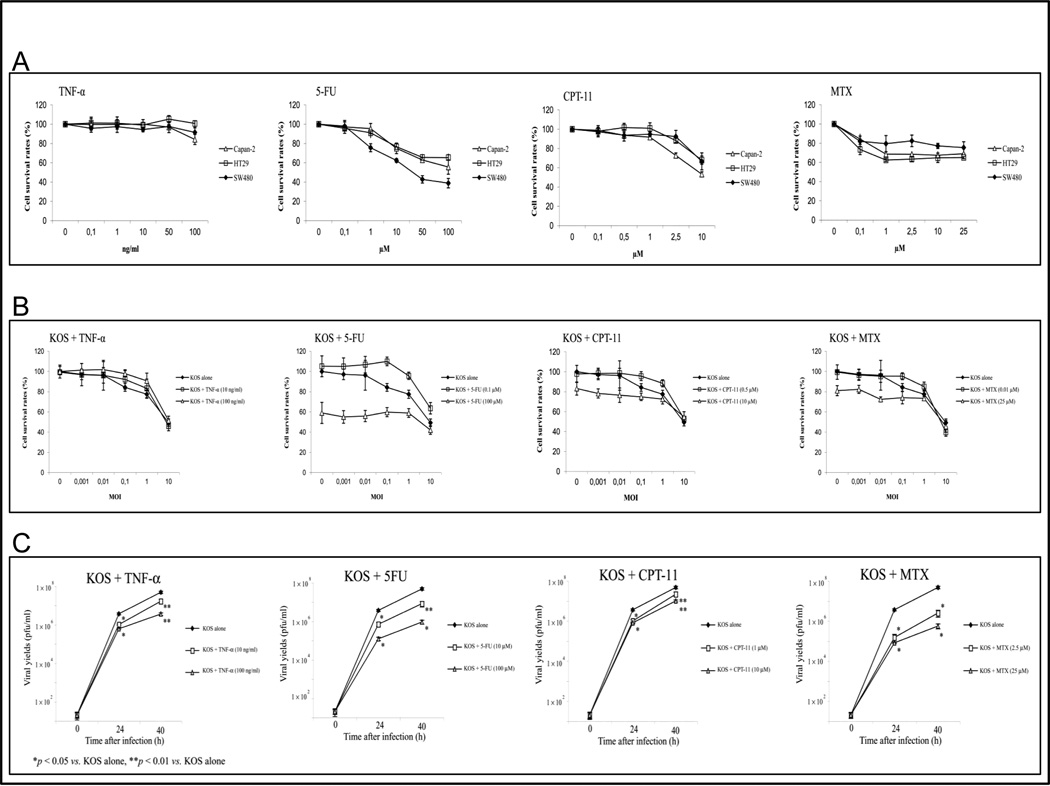
We initially determined the relationship between concentration of chemotherapy agents and cytoxicity in colon and pancreatic carcinoma cells. We included TNF-α in these studies because of the known effect of TNF-α on viral replication, NF-κB, and PKR. As expected, increasing drug concentrations were associated with increasing cytotoxicity for all cell lines (Fig. 1A). We then infected these cells with HSV-1 in three conditions: no chemotherapy, a low concentration that produced no cytotoxicity alone, and a higher concentration that induced cytotoxicity alone. In all three cell lines, cytotoxicity induced by HSV-1 alone was no different than cyotoxicity induced by HSV-1 combined with a low concentration of chemotherapy or TNF-α (Fig 1B). Exposure to a higher concentration of chemotherapy combined with HSV-1 infection did not produce more cytotoxicity that either HSV-1 alone or drug alone, suggesting that chemotherapy agents antagonized viral oncolysis. We observed identical results with other HSV-1 mutants, including 7134 (ICP0-defective) and hrR3 (ICP6-defective) (data not shown). To investigate the relationship between viral replication and cellular responses to chemotherapy, we used a burst assay to measure HSV-1 replication in cells treated with TNF-α, 5-FU, CPT-11, and MTX. KOS replication is significantly inhibited by all of the drugs (Fig. 1C). Similar results were observed with HSV- strains 7134 and hrR3 (data not shown).
Fig. 1. Chemotherapy-induced cytotoxicity inhibits viral replication.
(A) Capan-2, SW480 and HT29 cells were treated with different concentration of TNF-α, 5-FU, CPT-11, or MTX for 3 days. (B) The cell survival rates of HT29 cells were measured at 3 days following KOS infection, (MOI values ranging from 0.001 to 10) in the presence or absence of TNF-α (10 or 100 ng/ml), 5-FU (10 or 100 µM), CPT-11 (1 or 10 µM), or MTX (2.5 or 25 µM). The number of viable cells was determined using a MTT assay. (C) Viral titers were determined at 0, 24, 40 h following infection of HT29 cells with KOS at MOI = 0.1 in the presence or absence of TNF-α (10 or 100 ng/ml), 5-FU (10 or 100 µM), CPT-11 (1 or 10 µM), or MTX (2.5 or 25 µM). Results are shown as mean ± SD.
NF-κB activation is required for efficient HSV-1 replication
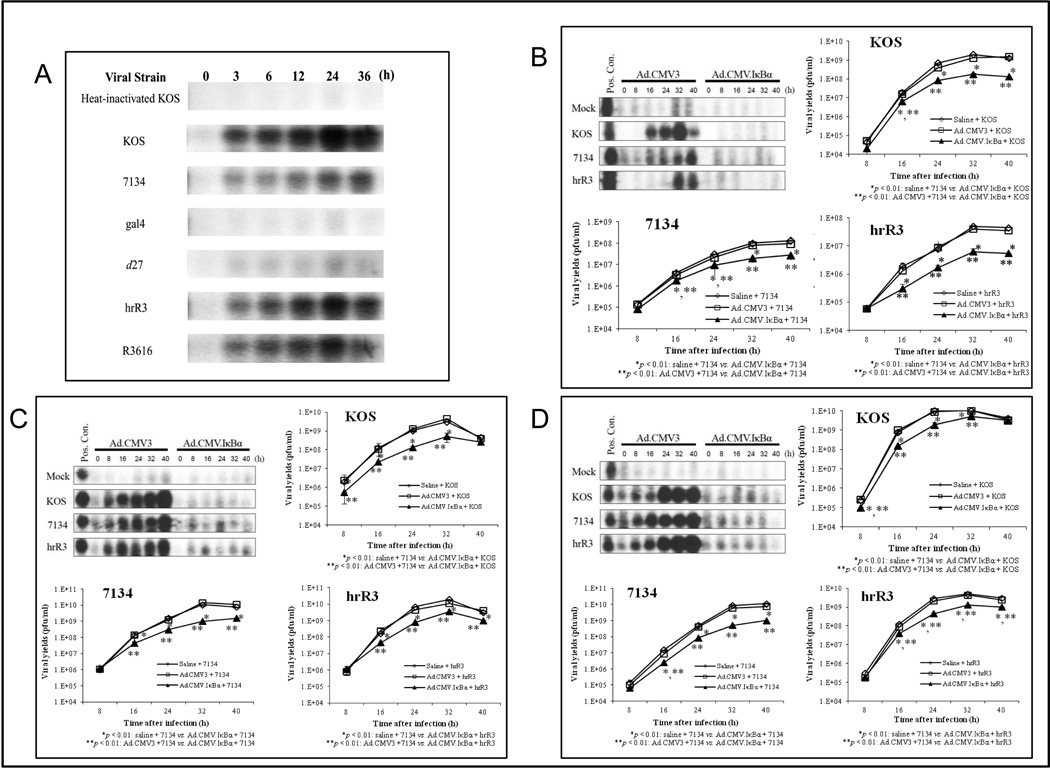
The NF-κB pathway provides an attractive target to viral pathogens; many viruses exploit the anti-apoptotic properties of NF-κB to evade host defense mechanisms.16 Beginning at around 3 h after infection, KOS-infected HT29 cells demonstrate inducible NF-κB activation compared to mock-infected cells (Fig. 2A). NF-κB nuclear translocation increases over time, leading a maximum at 24 h. HT29 cells infected with HSV-1 mutants that are defective in ICP0, ICP4, and ICP27 (7134, gal4, and d27, respectively) do not activate NF-κB as strongly as cells infected with KOS. In contrast, the ICP6-defective HSV-1 mutant (hrR3) and γ134.5-defective HSV-1 mutant (R3616) induce NF-κB activation as effectively as cells infected with KOS.
Fig. 2. NF-κB activation is required for efficient HSV-1 replication.
(A) HT29 cells were either mock infected or infected with HSV-1 (KOS, 7134, gal4, d27, hrR3, and R3616) at MOI = 1 for up to 36 h, and equal amounts (10 µg) of nuclear extracts were prepared and analyzed by EMSA. (B) HT29 cells were infected with the IκBα-SR (Ad.CMV.IκBα) or control (empty vector) adenovirus (Ad.CMV3) at MOI = 20 for 12 h. These cells were infected with KOS, 7134, and hrR3 at MOI = 0.1 for up to 40 h, and the equal amounts (10 µg) of nuclear extracts were prepared and analyzed by EMSA. Positive control: nuclear extracts from HT29 that were treated with TNF-α for 24 h. Mock: Heat-inactivated KOS. After 0 to 40 h following HSV-1 infection, the cells that were initially infected with the Ad.CMV.IκBα, Ad.CMV3 or saline were harvested and supernatants from these cells were titered on Vero cells or 0-28 cells. (C) Same experiment in Capan-2 cells. (D) Same experiment in SW480 cells. Results are shown as mean ± S.D.
To further examine the relationship between HSV-1 replication and NF-κB activation, we used a serine-mutated form of IκBα, which does not undergo phosphorylation at key sites and is therefore not targeted for polyubiquitination and degradation by the proteosomes.17, 29 Adenoviral-mediated delivery of a gene encoding IκBαα supper-repressor (IκBα-SR) effectively blocks NF-κB.17, 29 We first confirmed that infection of HT29 cells with a replication-defective adenovirus expressing IκBα-SR (Ad.CMV.IκBα) 12 h prior to HSV-1 infection blocks NF-κB activation (Fig. 4A). To determine whether inhibition of NF-κB activation affects virus replication, we compared viral replication in IκBα-SR-expressing HT29 cells relative to control cells. HSV-1 replication in the presence of the control adenovirus (Ad.CMV3) is no different than replication observed in saline-treated control cells. In contrast, HSV-1 replication in the presence of the adenovirus expressing the IκBα-SR (Ad.CMV.IκBα) is significantly attenuated for each HSV-1 virus (Fig. 4B). NF-κB activation appears critical for robust viral replication, because inhibition of NF-κB activation markedly attenuates HSV-1 replication. This effect was apparent in all three cell lines examined, with only minor variability observed between KOS, 7134, and hrR3 (Fig. 4C, D).
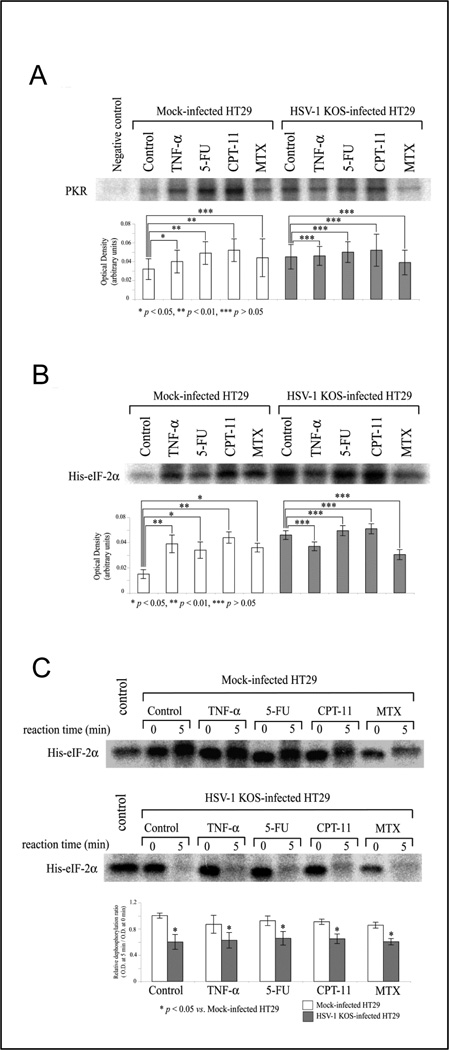
Fig. 4. Chemotherapy induces in vitro PKR/eIF-2α phosphorylation activity.
Protein lysates from HT29 cell that were treated with TNF-α, 5-FU, CPT-11 or MTX for 24 h were examined for their ability to (A) phosphorylate PKR, (B) phosphorylate eIF-2α and (C) dephosphorylate eIF-2α, in the presence or absence of HSV-1 KOS infection. Cells were treated with TNF-α (10 ng/ml), 5-FU (100 µM), CPT-11 (1 µM), and MTX (25 µM) for 24 h and then harvested to assess kinase or phosphatase activity. Control: medium alone (without drug). Mock-infected HT29: HT29 cells incubated with heat-inactivated KOS. Negative control for (A): precipitated cell lysate with protein A-agarose alone (without PKR-antibody). Control for (C): Purified phosphorylated eIF-2α by GST-PKR (without cell lysates). The eIF-2α dephosphorylation assay (C) examines protein lysates from drug-treated (or mock-treated) cells for dephosphorylation of radiolabeled His-tagged eIF-2α bacterial fusion protein over a time interval of 5 minutes (by comparison of His-eIF-2α phosphorylatation after 0 versus 5 minutes of incubation with cell lysate). The optical density of all autoradiography bands was determined using UVP image software, and results are shown as the mean ratio of optical density ± S.D. in graphs beneath each autoradiograph.
Differential effects of chemotherapeutic agents on NF-κB activation
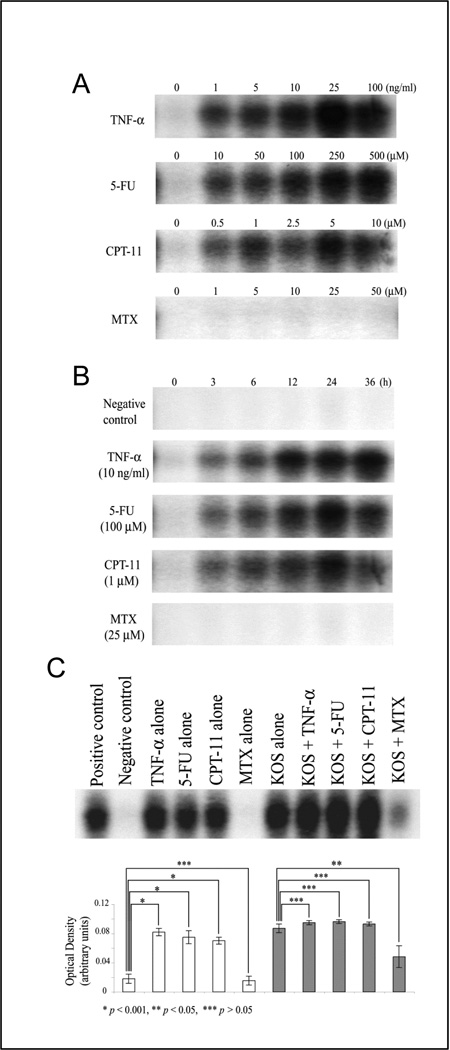
Exposure of colon cancer cells to 5-FU, CPT-11, and TNF-α induces NF-κB activation in a manner that is both time-dependent and dose-dependent (Figs. 3A and 3B). In contrast, exposure to MTX does not activate NF-κB. We examined the effect of chemotherapeutic agents on HSV-1-induced NF-κB activation. Treatment with TNF-α, 5-FU or CPT-11 during KOS infection does not alter NF-κB activation compared to KOS infection alone (Fig. 3C). This finding suggests that the mechanism by which 5-FU and CPT-11 inhibits HSV-1 replication is independent of their effects on NF-κB activation. The mechanism by which MTX inhibits viral replication may be through its ability to inhibit of NF-κB activation.
Fig. 3. Differential effects of chemotherapeutic agents on NF-κB activation.
(A) HT29 cells were treated with different concentration of TNF-α, 5-FU, CPT-11, or MTX for 24 h, after which time equal amounts (10 µg) of nuclear extracts from the cells were prepared and analyzed by EMSA. (B) HT29 cells were treated with 10 ng/ml TNF-α, 100 µM 5-FU, 1 µM CPT-11, and 25 µM MTX for up to 36 h, and nuclear extracts were prepared and analyzed by EMSA. Negative control: medium (DMEM with 10 % FBS and antibiotics) alone. (C) HT29 cells were treated with 10 ng/ml TNF-α, 100 µM 5-FU, 1 µM CPT-11, and 25 µM MTX in the presence or absence of HSV-1 KOS infection for 24 h, and nuclear extracts were prepared and analyzed by EMSA. Negative control: medium (DMEM with 10 % FBS and antibiotics) alone. Positive control: nuclear extracts from HT29 that were treated with 10 ng/ml TNF-α for 24 h. The density of each band was determined using UVP image software, and results are shown as mean ± S.D. in graph.
Chemotherapeutic agents induce PKR/eIF-2α phosphorylation
Eukaryotic viruses and their multicellular hosts have coevolved complex interrelationships to permit virus reproduction without destruction of the host.10, 11 A central enzyme involved in antiviral activity is the PKR.10, 11 One of the major biological functions of PKR is to regulate protein translation through phosphorylation of eIF-2α11 HSV-1 γ134.5 expression results in eIF-2α dephosphorylation, which aids efficient HSV-1 replication,13 and we have previously demonstrated the importance of eIF-2α dephosphorylation for viral oncolysis.6 Accordingly, we measured eIF-2α phosphorylation induced by PKR in response to TNF-α, 5-FU, CPT-11 or MTX. Protein lysates of HT29 cells treated with these agents were examined for their ability to phosphorylate PKR, phosphorylate eIF-2α and dephosphorylate eIF-2α in the presence or absence of HSV-1 infection (KOS). KOS stimulates PKR phosphorylation, as do each of the agents; however, the PKR phosphorylation by KOS infection is unchanged by TNF-α, 5-FU, CPT-11 or MTX (Fig. 4A). Similarly, eIF-2α phosphorylation observed in response to each of the agents is unchanged by (Fig. 4B). CPT-11 and MTX induce some eIF-2α dephosphorylation activity, but overall dephosphorylation activity in response to HSV-1 infection is not altered by any of the agents (Fig. 4C). Thus, chemotherapy induces PKR and eIF-2α phosphorylation, but HSV-1-mediated dephosphorylation of these regulatory proteins remains active. Overall, these results suggest that HSV-1 induced PKR phosphorylation and eIF-2α dephosphorylation remain robust in chemotherapy treated cells, yet viral replication is attenuated in the presence of some of these agents.
DISCUSSION
Oncolytic HSV-1 mutants are now under evaluation in clinical trials in patients with advanced malignancy including gastrointestinal malignancies.31–34 Although mechanisms of interaction between chemotherapy and viral replication are poorly understood, NF-κB activation is required for robust HSV-1 replication, and moreover, various chemotherapeutic agents and HSV-1 infection independently induce NF-κB activation. Thus, we were initially buoyed with the possibility that the combination of chemotherapy and HSV-1 oncolysis could act synergistically through augmented viral replication and oncolysis. At a minimum, we reasoned that cells in which NF-κB is activated in response to chemotherapy would be more susceptible to HSV-1 oncolysis. We thus found it of significance and interest that pre-treatment of cells with specific chemotherapeutic agents simultaneously reduces viral replication and antagonizes viral oncolysis.
Herpesvirus, adenovirus, measelsvirus, vaccinavirus or reovirus based oncolytic vectors have previously been evaluated in combination with various chemotherapeutic agents against different human cancers.35–49 Different interactions have been observed in these studies, and appear to depend on the mechanism of action of the specific chemotherapeutic agent, the type of cancer, and the specific oncolytic virus. In some preclinical studies the combination of an oncolytic virus with chemotherapeutic agents resulted in an enhanced oncolytic effect. Some combinations have already been translated into clinical trials.43, 50, 51 However, Cheema et al demonstrated that CPT-11 inhibits G47Δ (a γ134.5, ICP6 and α47 defective HSV-1 mutant) replication, in U87 glioma cells even at low doses.52 Similarly, doxorubicin, etoposid, and cisplatin were shown to inhibit the replication of G47Δ in LNCaP prostate cancer cells.53 Other studies have shown that the replication of the HSV-1 mutants R3616 and hrR3 in CAPAN-1, PaCa-2, and SW1990 pancreatic cancer cells is decreasedafter treatment with gemcitabine.49 Gutermann et al observed a reduced replication of HSV-1 mutant NV1020 (UL24 and UL56 defective) in WiDr colon carcinoma cells in combination with 5-FU, CPT-11 and oxaliplatin.38 In contrast to our study, Eisenberg et al demonstrated that 5-FU potentiates the efficacy of herpes virus oncolysis mediated by NV1066 (single copy of ICP0, ICP4 and γ134.5 deleted) in human pancreatic cancer cells.54 However, in the study of Eisenberg et al, the cells were treated with a different HSV-1 mutant, were exposed to 5-FU only for 6 hours, with a 5-FU dose up to 100 fold less compared to our study.
Although chemotherapy agents simultaneously induced NF-κB activation and inhibited viral replication, the mechanism by which MTX-treatment inhibits viral replication may be via inhibition of NF-κB activation. Majumdar et al demonstrated in a series of experiments, that MTX suppresses NF-κB activation through the release of adenosine.18 This concept is supported by our observation that inhibition of NF-κB activation by the super-repressor IκBα inhibits viral replication. But other mechanisms may also be operant. MTX is an antifolate and its primary cellular target is dihydrofolate reductase, which catalyzes the reduction of folate and 7,8 dihydrofolate to 5,6,7,8 tetrahydrofolate.55 An essential requirement for both cellular and viral DNA synthesis is a large expansion in the pools of deoxyribonucleoside triphosphates (dNTPs).55, 56 It is conceivable that a lowering of intracellular nucleotide pools observed in response to MTX is partially responsible for inhibition of viral replication.57, 58 Similarly, the pyrimidine antimetabolit 5-FU interferes with DNA synthesis and causes an imbalance of the intracellular deoxynucleotide triphosphate pools59 and modulates cell cycle progression.60 Furthermore, CPT-11 and its active metabolite SN-38 bind to and inhibits irreversibly the enzyme DNA-topoisomerase I and causes double-strand DNA breaks during DNA replication.61, 62 These imbalances in cellular function following treatment of 5-FU and CPT-11 may interfere with and negatively affect HSV-1 replication.
NF-κB induction is critical for expression of not only multiple genes critical for cell survival, but also for expression of specific viral genes. Many viruses have evolved mechanisms to target the NF-κB pathway to facilitate cell survival, viral replication, and evasion of immune responses.16, 20 In addition, some viruses use the NF-κB pathway either for its anti-apoptotic properties to evade the host defense mechanisms or to trigger apoptosis as a mechanism of virus spread.16 HSV-1 modulates the host cell environment to promote its own propagation.6, 63 We observed that wild-type HSV-1 induces NF-κB activation whereas some HSV-1 mutants do not induce NF-κB activation, and this observation is consistent with earlier reports.63–65 The persistent nuclear translocation of NF-κB in HSV-1 infected cells coincides with increased binding of NF-κB to specific DNA response elements.64 Whether the increased DNA-bound NF-κB reflects increased activity and subsequent transcription of target genes is not clear. Patel et al. 64 reported decreased expression of a NF-κB-inducible reporter gene following virus-induced NF-κB activation, while others report increased NF-κB-dependent gene transcription.63, 65 Here we present our observations that HSV-1 induces a persistent activation of NF-κB in HT29 colon carcinoma cells, beginning at around 3 h post-infection, and that regulatory functions of ICP0, ICP4, or ICP27 are required for this robust NF- κB nuclear translocation. Replication of mutants defective in these immediate-early genes is markedly attenuated compared to wild-type KOS replication. But even replication of KOS is attenuated with experimental inhibition of NF- κB activation. NF- κB activation appears to be critically important for robust HSV-1 replication.
The importance of PKR as a host antiviral strategy is highlighted by the observation that many viruses possess mechanisms to counteract its action.10, 11 eIF-2α is normally phosphorylated by PKR in response to HSV-1 infection. Phosphorylated eIF-2α leads to attenuated viral replication as a result of inhibition of protein translation. HSV-1 γ134.5 interacts with protein phosphatase-lα to dephosphorylate eIF-2α to block the shutoff of protein synthesis and permit robust viral replication.11 We and others have previously demonstrated that reduced eIF-2α dephosphorylation during HSV-1 infections is associated with attenuated viral replication.6, 14 In the current study we observed that eIF-2α dephosphorylation in response to HSV-1 infection proceeds regardless of exposure to chemotherapeutic agents.
While NF-κB activation appears to be an indispensable cellular response for robust HSV-1 replication, and chemotherapeutic agents such as 5-FU and CPT-11 induce NF-κB activation, these agents inhibit HSV-1 replication. Cellular responses to chemotherapy provide an unfavorable environment for HSV-1-mediated oncolysis. These observations are relevant to the design of both preclinical and clinical studies of HSV-1 oncolysis.
Acknowledgments
Grant support: NIH Grants CA64454 and CA76183 (K. K. Tanabe), DK43352 (core facilities), CA71345 (J. M. Donahue), and CA077278 (J.C. Cusack) and DFG Grant KU 1989/1-1 (Y. Kulu).
Abbreviations
- NF-κB
nuclear factor-kappa B
- dsRNA
double stranded RNA
- PKR
dsRNA-activated protein kinase
- HSV-1
Herpes simplex virus 1
- eIF-2α
alpha subunit of eukaryotic initiation factor 2
- TNF-α
tumor necrosis factor-alpha
- MTX
methotrexate
- 5-FU
5-fluorouracil
- CPT-11
irinotecan
Footnotes
CONFLICT OF INTEREST STATEMENT
We declare that we have no conflict of interest.
REFERENCES
- 1.Carroll NM, Chiocca EA, Takahashi K, Tanabe KK. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann.Surg. 1996;224(3):323–329. doi: 10.1097/00000658-199609000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlik TM, Nakamura H, Yoon SS, Mullen JT, Chandrasekhar S, Chiocca EA, et al. Oncolysis of diffuse hepatocellular carcinoma by intravascular administration of a replication-competent, genetically engineered herpesvirus. Cancer Res. 2000;60(11):2790–2795. [PubMed] [Google Scholar]
- 3.Yoon SS, Nakamura H, Carroll NM, Bode BP, Chiocca EA, Tanabe KK. An oncolytic herpes simplex virus type 1 selectively destroys diffuse liver metastases from colon carcinoma. FASEB J. 2000;14(2):301–311. [PubMed] [Google Scholar]
- 4.Mullen JT, Donahue JM, Chandrasekhar S, Yoon SS, Liu W, Ellis LM, et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer. 2004;101(4):869–877. doi: 10.1002/cncr.20434. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H, Mullen JT, Chandrasekhar S, Pawlik TM, Yoon SS, Tanabe KK. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 2001;61(14):5447–5452. [PubMed] [Google Scholar]
- 6.Nakamura H, Kasuya H, Mullen JT, Yoon SS, Pawlik TM, Chandrasekhar S, et al. Regulation of herpes simplex virus gamma(1)34.5 expression and oncolysis of diffuse liver metastases by Myb34.5. J.Clin.Invest. 2002;109(7):871–882. doi: 10.1172/JCI10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geevarghese SK, Geller DA, de Haan HA, Horer M, Knoll AE, Mescheder A, et al. Phase I/II Study of Oncolytic Herpes Simplex Virus NV1020 in Patients with Extensively Pretreated Refractory Colorectal Cancer Metastatic to the Liver. Hum.Gene Ther. 2010;21(9):1119–1128. doi: 10.1089/hum.2010.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin.Cancer Res. 2010;16(15):4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future.Oncol. 2010;6(6):941–949. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 10.Gale M, Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol.Ther. 1998;78(1):29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 11.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc.Natl.Acad.Sci.U.S.A. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs BL, Langland JO. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219(2):339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 13.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 14.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc.Natl.Acad.Sci.U.S.A. 1992;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Gross M, Roizman B. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J.Biol.Chem. 1998;273(33):20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 16.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J.Clin.Invest. 2001;107(2):143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274(5288):784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 18.Majumdar S, Aggarwal BB. Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. J Immunol. 2001;167(5):2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 19.Cai WZ, Schaffer PA. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J.Virol. 1989;63(11):4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZQ, Kunimatsu M, Yang JP, Ozaki Y, Sasaki M, Okamoto T. Proteolytic processing of nuclear factor kappa B by calpain in vitro. FEBS Lett. 1996;385(1–2):109–113. doi: 10.1016/0014-5793(96)00360-2. [DOI] [PubMed] [Google Scholar]
- 21.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat.Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KL, Baines JD. UL31 of herpes simplex virus 1 is necessary for optimal NF-kappaB activation and expression of viral gene products. J.Virol. 2011;85(10):4947–4953. doi: 10.1128/JVI.00068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepard AA, Imbalzano AN, DeLuca NA. Separation of primary structural components conferring autoregulation, transactivation, and DNA-binding properties to the herpes simplex virus transcriptional regulatory protein ICP4. J.Virol. 1989;63(9):3714–3728. doi: 10.1128/jvi.63.9.3714-3728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 25.Rice SA, Knipe DM. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J.Virol. 1990;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldin AL, Sandri-Goldin RM, Levine M, Glorioso JC. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J.Virol. 1981;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelie PN, Reesink HW, Lucas CJ. Inactivation of 12 viruses by heating steps applied during manufacture of a hepatitis B vaccine. J.Med.Virol. 1987;23(3):297–301. doi: 10.1002/jmv.1890230313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cusack JC, Jr, Liu R, Baldwin AS., Jr Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-kappaB activation. Cancer Res. 2000;60(9):2323–2330. [PubMed] [Google Scholar]
- 29.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat.Med. 1999;5(4):412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekhar S, Souba WW, Abcouwer SF. Use of modified agarose gel electrophoresis to resolve protein-DNA complexes for electrophoretic mobility shift assay. Biotechniques. 1998;24(2):216–218. doi: 10.2144/98242bm09. [DOI] [PubMed] [Google Scholar]
- 31.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357(9255):525–526. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- 32.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 33.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 34.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 35.AbouEl Hassan MA, Braam SR, Kruyt FA. Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer gene therapy. 2006;13(12):1105–1114. doi: 10.1038/sj.cgt.7700984. [DOI] [PubMed] [Google Scholar]
- 36.Bennett JJ, Adusumilli P, Petrowsky H, Burt BM, Roberts G, Delman KA, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(9):1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 37.Cinatl J, Jr, Cinatl J, Michaelis M, Kabickova H, Kotchetkov R, Vogel JU, et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003;63(7):1508–1514. [PubMed] [Google Scholar]
- 38.Gutermann A, Mayer E, von Dehn-Rothfelser K, Breidenstein C, Weber M, Muench M, et al. Efficacy of oncolytic herpesvirus NV1020 can be enhanced by combination with chemotherapeutics in colon carcinoma cells. Human gene therapy. 2006;17(12):1241–1253. doi: 10.1089/hum.2006.17.1241. [DOI] [PubMed] [Google Scholar]
- 39.Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann D, Bangen JM, Bayer W, Wildner O. Synergy between expression of fusogenic membrane proteins, chemotherapy and facultative virotherapy in colorectal cancer. Gene therapy. 2006;13(21):1534–1544. doi: 10.1038/sj.gt.3302806. [DOI] [PubMed] [Google Scholar]
- 41.Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene. 2010;29(45):6051–6063. doi: 10.1038/onc.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. Journal of the National Cancer Institute. 2012;104(1):42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(7):2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roulstone V, Twigger K, Zaidi S, Pencavel T, Kyula JN, White C, et al. Synergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapy. Gene therapy. 2012 doi: 10.1038/gt.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Molecular cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 47.Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR, Molnar-Kimber KL. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Human gene therapy. 1999;10(18):3013–3029. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- 48.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB, et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67(22):10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe I, Kasuya H, Nomura N, Shikano T, Shirota T, Kanazumi N, et al. Effects of tumor selective replication-competent herpes viruses in combination with gemcitabine on pancreatic cancer. Cancer chemotherapy and pharmacology. 2008;61(5):875–882. doi: 10.1007/s00280-007-0567-8. [DOI] [PubMed] [Google Scholar]
- 50.Opyrchal M, Aderca I, Galanis E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. Methods in molecular biology (Clifton, N.J.) 2009;542:705–717. doi: 10.1007/978-1-59745-561-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(22):5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheema TA, Kanai R, Kim GW, Wakimoto H, Passer B, Rabkin SD, et al. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(23):7383–7393. doi: 10.1158/1078-0432.CCR-11-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer gene therapy. 2009;16(7):551–560. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenberg DP, Adusumilli PS, Hendershott KJ, Yu Z, Mullerad M, Chan MK, et al. 5-fluorouracil and gemcitabine potentiate the efficacy of oncolytic herpes viral gene therapy in the treatment of pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2005;9(8):1068–1077. doi: 10.1016/j.gassur.2005.06.024. discussion 1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blakley RL. Dihydrofolate reductase in folate and pterins. New York: Whiley; 1984. pp. 191–251. [Google Scholar]
- 56.Daikoku T, Yamamoto N, Maeno K, Nishiyama Y. Role of viral ribonucleotide reductase in the increase of dTTP pool size in herpes simplex virus-infected Vero cells. J.Gen.Virol. 1991;72(Pt 6):1441–1444. doi: 10.1099/0022-1317-72-6-1441. [DOI] [PubMed] [Google Scholar]
- 57.Kasahara Y, Nakai Y, Miura D, Kanatani H, Yagi K, Hirabayashi K, et al. Decrease in deoxyribonucleotide triphosphate pools and induction of alkaline-labile sites in mouse bone marrow cells by multiple treatments with methotrexate. Mutat.Res. 1993;319(2):143–149. doi: 10.1016/0165-1218(93)90073-m. [DOI] [PubMed] [Google Scholar]
- 58.Pancheva SN. Methotrexate potentiates anti-herpes simplex virus type 1 activity of E-5-(2-bromovinyl)-2'-deoxyuridine. Acta Virol. 1995;39(2):117–119. [PubMed] [Google Scholar]
- 59.Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, et al. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. The Journal of biological chemistry. 1987;262(17):8235–8241. [PubMed] [Google Scholar]
- 60.Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, et al. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61(3):1029–1037. [PubMed] [Google Scholar]
- 61.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51(16):4187–4191. [PubMed] [Google Scholar]
- 62.Holm C, Covey JM, Kerrigan D, Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989;49(22):6365–6368. [PubMed] [Google Scholar]
- 63.Amici C, Belardo G, Rossi A, Santoro MG. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J.Biol.Chem. 2001;276(31):28759–28766. doi: 10.1074/jbc.M103408200. [DOI] [PubMed] [Google Scholar]
- 64.Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, et al. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology. 1998;247(2):212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 65.Rong BL, Libermann TA, Kogawa K, Ghosh S, Cao LX, Pavan-Langston D, et al. HSV-1-inducible proteins bind to NF-kappa B-like sites in the HSV-1 genome. Virology. 1992;189(2):750–756. doi: 10.1016/0042-6822(92)90599-k. [DOI] [PubMed] [Google Scholar]