Background: The guanine exchange factor Epac is a cAMP sensor.
Results: We have identified a tetrahydroquinoline analog named CE3F4 that blocks Epac activation in response to cAMP in vitro and in living cultured cells.
Conclusion: CE3F4 behaves as an uncompetitive antagonist of Epac with respect to cAMP.
Significance: CE3F4 may serve as a basis for the development of new therapeutic drugs.
Keywords: Cyclic AMP (cAMP), G Protein-coupled Receptors (GPCR), Guanine Nucleotide Exchange Factor (GEF), Molecular Pharmacology, Small GTPases, CE3F4, Epac
Abstract
The cAMP-binding protein Epac is a therapeutic target for the treatment of various diseases such as cardiac hypertrophy and tumor invasion. This points out the importance to develop Epac inhibitors to better understand the involvement of these cAMP sensors in physiology and pathophysiology. Here, we have developed a functional fluorescence-based high-throughput assay with a Z′ value around 0.7 for screening Epac-specific antagonists. We identified an Epac1 inhibitor compound named CE3F4 that blocked Epac1 guanine nucleotide exchange activity toward its effector Rap1 both in cell-free systems and in intact cells. CE3F4 is a tetrahydroquinoline analog that fails to influence protein kinase A holoenzyme activity. CE3F4 inhibited neither the interaction of Rap1 with Epac1 nor directly the GDP exchange on Rap1. The kinetics of inhibition by CE3F4 indicated that this compound did not compete for binding of agonists to Epac1 and suggested an uncompetitive inhibition mechanism with respect to Epac1 agonists. A structure-activity study showed that the formyl group on position 1 and the bromine atom on position 5 of the tetrahydroquinoline skeleton were important for CE3F4 to exert its inhibitory activity. Finally, CE3F4 inhibited Rap1 activation in living cultured cells, following Epac activation by either 8-(4-chlorophenylthio)-2′-O-methyl-cAMP, an Epac-selective agonist, or isoprenaline, a non-selective β-adrenergic receptor agonist. Our study shows that CE3F4 and related compounds may serve as a basis for the development of new therapeutic drugs.
Introduction
cAMP is a universal second messenger that plays a crucial role in the intracellular signal transduction of various stimuli controlling a wide variety of cellular events including secretion, cell proliferation and differentiation, migration, and apoptosis. Besides PKA,4 the guanine exchange factor (GEF) Epac (exchange protein directly activated by cyclic AMP) has been shown to contribute to cAMP signaling in many cellular processes (1, 2). There are two isoforms of Epac, Epac1 and Epac2, both consisting of a regulatory region binding directly cAMP, and a catalytic region that promotes the exchange of GDP for GTP on the Ras-like small GTPases Rap1 and Rap2 isoforms (1, 3–5). The regulatory region of Epac proteins contains a Dishevelled, Egl-10, and pleckstrin domain followed by an evolutionally conserved cyclic nucleotide binding domain to the cyclic nucleotide binding domain of PKA and the bacterial transcriptional factor cAMP receptor protein (4). In the absence of cAMP, the regulatory region containing the cAMP-binding domain directly interacts with the catalytic region and inhibits its GEF activity. Binding of cAMP to Epac induces large conformational changes within the protein and releases the autoinhibitory effect of the N-terminal region, leading to Rap activation (5).
Data from the use of Epac agonists such as the cAMP analog, 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (referred here as 007) has revealed that these cAMP-sensitive GEFs play critical roles in various physiological and pathophysiological processes. These include memory formation, inflammation, and cardiac remodeling, thus identifying Epac as physiologically important and as a possible pharmacological target for the treatment of human disorders (1, 6). Previous studies reported the existence of potential Epac ligands different from cAMP. Firstly, the ADP ribosylation factor-GEF inhibitor brefeldin A was shown to be an inhibitor of Epac function (7), but evidence that it directly attenuates Epac activity is still lacking. Recently, Tsalkova et al. (8) developed a screening assay for identifying antagonists that directly compete with a fluorescent cAMP derivative in binding to Epac proteins. This assay was then used to identify a series of 5-cyano-6-oxo-1,6-dihydro-pyrimidine derivatives that inhibited Epac1- and Epac2-mediated Rap exchange activity (9). Given the importance of Epac in cAMP signaling and to better understand its pathophysiological roles, it is crucial to further develop Epac inhibitors and to test their functional effects in living cultured cells. To this end, we established a high throughput screening assay to isolate Epac inhibitor compounds from chemical libraries. We identified an Epac inhibitor compound named CE3F4 that blocked Epac guanine nucleotide exchange activity toward its effector Rap1 in vitro. Although the Epac1 regulatory domain was necessary for CE3F4 to inhibit Epac1 activity, the compound did not competitively inhibit agonist activation of Epac1. Importantly, CE3F4 inhibited Epac downstream effector Rap1 following Epac activation by either an Epac-selective agonist, 007, or isoprenaline (Iso), a non-selective β-adrenergic receptor agonist in living cultured cells. Our study shows that CE3F4 and related compounds may serve as a basis for the development of new therapeutic drugs.
EXPERIMENTAL PROCEDURES
Compound Library
The screening library is a collection of 640 chemically diverse compounds called “Chimiothèque Essentielle” (CE) that was developed as a representative part of the French National Chemical Library, the latter containing more than 50,000 compounds. The CE library was provided on the basis of 80 compounds per 96-well plate at a concentration of 1 mg/ml in 100% dimethyl sulfoxide. The compounds were then diluted to 0.1 μg/ml with H2O, and the “daughter” plates were kept at −20 °C until use.
Other Reagents
Phenylephrine and Iso were obtained from Sigma-Aldrich. 007 and 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate (referred to as “009” for convenience in this work) were from Biolog, Bremen, Germany. Guanosine 5′-diphosphate, BODIPY FL 2′-(or-3′)-O-(N-(2-aminoethyl)urethane), bis(triethylammonium) salt (bGDP) was from Invitrogen. Compound CE3F4 and its derivatives were synthesized according to methods published previously (10). Antibodies and their suppliers were as follows: anti-GAPDH, anti-Rap1, and anti-Epac1 (Cell Signaling Technology) and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology).
Protein Expression and Purification
Part of the coding sequence of human Epac1 (residues 149–881, i.e. Epac1 deleted of its Dishevelled, Egl-10, and Pleckstrin domain) was PCR-amplified using the plasmid pSC-A-HA-hEpac1 as a template and inserted in pET41a (Novagen). The coding sequence of the catalytic domain of Epac1 (residues 321–881) was PCR-amplified from the same plasmid. The full-length coding sequence of human Rap1A was excised from the plasmid pRK5-HA-hRap1A and inserted in pET41a. All constructs were verified by sequencing and expressed in Escherichia coli Rosetta 2(DE3) (Novagen) as recombinant proteins fused with GST and a His tag at their N terminus according to standard protocol. After lysis of the bacteria, the soluble proteins were purified by glutathione-agarose beads (GE Healthcare) for Rap1A and by nickel-nitrilotriacetic acid beads (Qiagen) for Epac1 and Epac1-Cat. Thrombin was used to cleave GST from Rap1 (11). All proteins used in this study were at least 80% pure as judged by SDS-PAGE.
Measurement of in Vitro Activation of Epac1
GST-Rap1 was preloaded with a fluorescent derivative of GDP, and the Epac1-catalyzed nucleotide exchange was measured using a large excess of non-fluorescent GDP by taking advantage of the spectroscopic difference between free and Rap1-bound fluorescently labeled GDP. The principle of the method is similar to that reported in particular by van den Berghe et al. (12), except that Bodipy FL-labeled GDP (bGDP) was used here rather than 2′,3′-bis(O)-N-methylanthraniloyl-GDP. Indeed, the change in fluorescence between free and Rap1-bound GDP is broadly 2-fold higher using bGDP as a ligand rather than 2′,3′-bis(O)-N-methylanthraniloyl-GDP. Furthermore, replacing the blue fluorescent 2′,3′-bis(O)-N-methylanthraniloyl-GDP with green fluorescent bGDP results in a strong decrease of autofluorescence background generated by the medium and by some compounds under study (data not shown).
To determine Epac1 exchange activity, 200 nm of purified GST-Rap1A preloaded with bGDP were incubated at 22 °C in exchange buffer (50 mm Tris-HCl (pH 7.5), 50 mm NaCl, 5 mm MgCl2, 5 mm 1,4-dithioerythritol, 5% glycerol, 0.01% Nonidet P-40) in the presence of 100 nm purified GST-Epac1 or GST-Epac1-Cat; 20 μm unlabeled GDP; and defined concentrations of cAMP, cyclic nucleotide analogs, and test compounds. Experiments were performed in black 384-well plates (Corning, Inc., Reference no. 3573) in a final volume of 30 μl. bGDP fluorescence (excitation, 480 nm and emission, 535 nm) was measured using a multilabel plate reader (Envision Xcite, PerkinElmer Life Sciences).
Concerning detailed kinetic studies, a “multipoint” (time course) method was used. All components except the agonist (007 or cAMP) were mixed in a well, and the exchange activity of Epac1 was initiated by injection of the agonist. The release of bGDP was measured in real time as the decay of fluorescence. A single exponential was fit to the data using the Graphpad Prism program. The initial velocity (Vi) of GDP exchange was calculated as K(Y0-P), where K, Y0, and P are the best fits for, respectively, the first order rate constant, the fluorescence value at t = 0, and the fluorescence plateau value at infinite times.
A “two points” method was used to screen the CE library. Each 384-well plate received 320 test compounds (final concentration, 6.67 μg/ml). On one side of the plate, 32 wells received no compound addition (as negative inhibition controls), whereas, on the other side of the plate, 32 wells received 009 at 50 μm final concentration (as positive inhibition controls). In all cases, the final dimethyl sulfoxide concentration was 0.67%. All the liquid handlings and plate operations were performed using a robotic work station Biomek FX (Beckman Coulter) coupled to the multilabel plate reader. The fluorescence of bGDP was measured just before (t = 0) the injection in each well of a 007 solution (2 μm final concentration) and 4 min after the injection. The variation in fluorescence (ΔF) was directly considered as a measurement of the exchange activity of Epac1. The inhibition of the exchange activity induced by the test compounds was expressed as the percentage of the exchange activity promoted by 50 μm 009: % control = (ΔFmax − ΔFobs)/(ΔFmax − ΔFmin) × 100, where ΔFmax = mean response (n = 32) obtained with 007 alone, ΔFmin = mean response (n = 32) obtained with 007 plus 009, and ΔFobs = observed response obtained with 007 plus test compound. The two points method allowed reading the 384 wells of a plate in less than 10 min.
Epac1-based Intramolecular BRET Assay
The pcDNA3-EBS eukaryotic expression vector was derived from the pQE30-CAMYEL prokaryotic expression vector (a gift from Dr L. I. Jiang). The Epac1-BRET sensor (EBS) CAMYEL utilizes a circularly permuted versions of citrine (citrine cp229), an enhanced variant of YFP, and Renilla luciferase as the BRET pair, with human Epac1 (amino acids 149–881) inserted in between (13). The CAMYEL construct was transferred to the pcDNA3 vector by usual restriction-ligation procedures. In the original CAMYEL construct, Epac1 contains two point mutations, T781A and F782A, that eliminate the guanine nucleotide exchange activity of Epac1. These mutations were reversed in pcDNA3-EBS by replacing the coding sequence between amino acids 750 and 881 by that of wild-type Epac1 using conventional restriction-ligation procedures at the BamH1 and EcoRI restriction sites. BRET signals were studied essentially as described by Jiang et al. (13). Briefly, the pcDNA3-EBS plasmid was transiently expressed in HEK293 cells. Cells were seeded in 6-well plates (600 000 cells/well) and transfected (3 μg DNA/well) using FuGENE HD (Roche Applied Science) as a transfection reagent. Cells were lysed 24 h post-transfection in 400 μl of buffer/well (20 mm Hepes (pH 7.4), 50 mm KCl, 50 mm NaCl, 2.5 mm MgCl2, 0.2% Nonidet P-40, 5 mm DTT, and protease inhibitor mixture), and a supernatant was collected after centrifugation at 16,000 × g for 15 min at 4 °C. Test compounds and coelenterazine-h (2 μm final concentration) were added 7 min before injection of Epac1 agonists in wells of a white 384-well plate (50 μl final volume). The emission signals from Renilla luciferase and citrine-cp229 were recorded over time in a PerkinElmer Life Sciences Envision Xcite multilabel plate reader. The BRET ratio (mean ± S.E., n = 3) was calculated as the ratio between the signal emitted by citrine-cp and that emitted by Renilla luciferase. The binding of agonists to the EBS was measured as a reduction in the BRET ratio.
Cell Culture
All procedures for cardiac myocyte isolation were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and the Veterinary Committee was informed of the myocyte isolation protocol used. Cardiac myocytes were isolated as described previously by Wollert and colleagues (14). HEK293 cells stably expressing the β1 adrenergic receptor were a gift from Dr. Shenoy (Duke University, North Carolina). HEK293 cells were maintained in minimal essential medium with FBS (10%) and penicillin-streptomycin (1%). All media, sera, and antibiotics used in cell culture were purchased from Invitrogen.
Plasmid and Transfection
Epac1 and Rap1A plasmid constructs (Epac1WT) were generously provided by Drs. J. L. Bos and J. de Gunzburg, respectively. The pQE30-CAMYEL plasmid was a gift from Dr Lily I. Jiang. Transient transfection experiments of HEK cells and primary cardiac myocytes were performed with, respectively, X-treme GENE 9 reagent (Roche Applied Science) and Lipofectamine 2000 (Invitrogen) in the presence of various amounts of plasmid constructs according to the instructions of the manufacturer.
Western Blot Analysis
Cells were rinsed once in cold PBS, scraped and lysed in radioimmune precipitation assay buffer (PBS 1×, 1% Igepal CA-630, 0.5% sodium deoxycholate, protease inhibitors). Cell lysates were analyzed by one-dimensional electrophoresis on 15% SDS-polyacrylamide gels. The proteins were transferred to Immobilon membranes (Millipore). The membranes were blocked for 1 h at room temperature in Tween Tris buffer saline (TTBS) supplemented with either 5% of nonfat milk or 3% of BSA and then probed with primary antibodies raised against Rap1 or Epac1. The second antibody was coupled to horseradish peroxidase (Santa Cruz Biotechnology). Membranes were revealed with a Dura kit (Pierce).
PKA Activity Assay
A PKA kinase activity assay (Biaffin GmbH, reference no. PKA-COOK) was performed according to the instructions of the manufacturer. Briefly, type I and II PKA holoenzyme (Biaffin GmbH) was added at 5 nm with or without CE3F4 (20 μm), H89 (20 μm, Sigma-Aldrich), or cAMP (0.5 μm, Biolog). The reaction was initiated by the addition of the Kemptide peptide, and absorbance was measured at 340 nm every 30 s during 10 min on a spectrophotometer (Bio-Rad). PKA activity was determined in three steps. Decreasing absorbance was plotted against time (min), then a linear regression of the plot was performed, and the absolute slot value was used to calculate PKA activity.
Pull-down Assay
Rap1 pull-down experiments were performed using a GST fusion protein containing the Rap1 binding domain of Ral-GDS as described previously (15). Cells were starved for 1 h before stimulation in serum-free minimal essential medium containing penicillin-streptomycin (1%). After stimulation, cells were lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl (pH 7.5), 500 mm NaCl, 20 mm MgCl2, 0.5% deoxycholic acid, 0.1% SDS, 1% Triton X-100, 1 mm PMSF, protease and phosphatase inhibitors), and 500 μg of protein was incubated with Ral-GDS coupled to glutathione-Sepharose beads (Amersham Biosciences) for 1 h at 4 °C. The beads were then washed three times in washing buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 20 mm MgCl2, 1% Triton X-100, 0.1 mm PMSF, protease and phosphatase inhibitors). Rap1-GTP samples and corresponding total lysates were separated on SDS-PAGE gels and transferred onto a PVDF membrane (Amersham Biosciences). Membranes were revealed with a Dura kit (Pierce).
Determination of the Z′ Factor
Z′ = (ΔM − 3SDmax − 3SDmin)/ΔM, where ΔM = (mean max value − mean min value), and SDmax and SDmin are the respective S.D.
Statistical Analysis
All data are expressed as mean ± S.E. Differences in quantitative variables were examined by one-way analysis of variance or paired two-tailed Student's t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. All analyses were performed using GraphPad Prism.
RESULTS AND DISCUSSION
In Vitro Assay of Epac Guanine Nucleotide Exchange Activity toward Rap1
To search for Epac-specific inhibitors, we developed a variant of the assay described by van den Berghe et al. (12) that is on the basis of the ability of Epac to catalyze the nucleotide exchange activity of Rap1 (see “Experimental Procedures” for details). Rap1 was preloaded with bGDP, a fluorescent derivative of GDP, and the exchange for non-fluorescent GDP, present in a large excess in the incubation medium, was measured because of the spectroscopic difference between free and Rap1-bound labeled GDP.
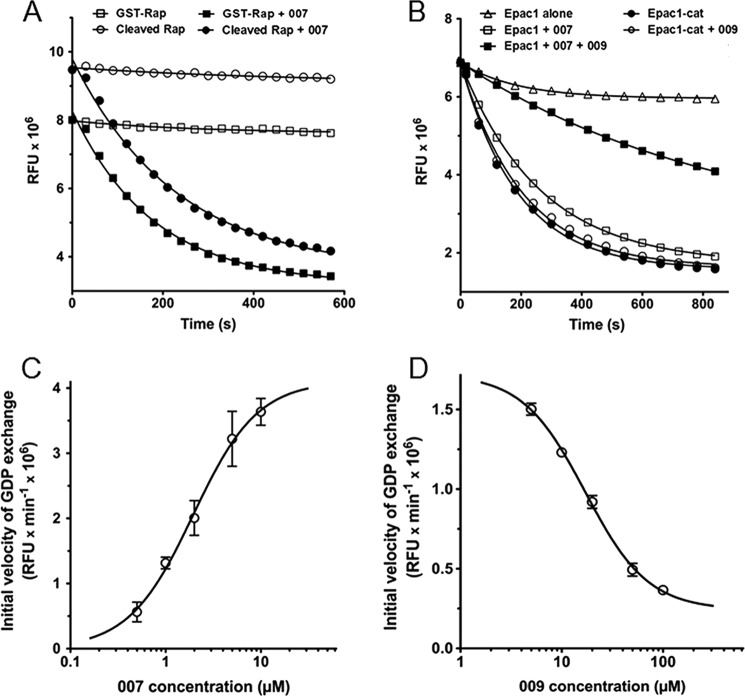
The exchange of guanine nucleotide bound to Rap1 was measured in real time as a decrease in fluorescence (Fig. 1). Epac1 induced a rapid decrease in fluorescence in the presence of 2 μm 007, a strong Epac agonist (6), whereas it had no effect in the absence of 007. When we compared the GDP exchange properties of thrombin-cleaved and uncleaved GST-Rap1 (Fig. 1A), the calculated initial velocity (Vi) of exchange induced by Epac1 and 007 was very similar using either preparations of Rap1 (2.8 × 104 relative luciferase units/second with GST-Rap1 versus 2.7 × 104 relative luciferase units/second with cleaved Rap1). The EC50 of 007 was also measured using thrombin-cleaved Rap1 (not shown) and found to be 2 μm. This EC50 is in agreement with the value of 1.8 μm reported by Rehmann et al. (11). This observation showed that the GST tag did not impair the exchange reaction, and therefore, experiments were performed with uncleaved GST-Rap1 (referred to as Rap1).
FIGURE 1.
Kinetic characterization of GDP exchange catalyzed by the wild-type form of Epac1 and Epac1-catalytic domain (Epac1-Cat). The relative fluorescence units (RFU) were monitored in real time as described under “Experimental Procedures.” Release of bGDP from 200 nm preloaded Rap1 was assayed at 22 °C in triplicate wells of 384-well plates in the presence of 20 μm unlabeled GDP. A, the exchange activity of 100 nm Epac1 (mean ± S.E.; error bars are masked by the symbols) was measured after injection of 007 (■ and ●) or buffer (□ and ○) in the presence of GST-Rap1 (■ and □) or cleaved Rap1 (● and ○). The curves and first-order rate constants were obtained by fitting single exponentials to the data. B, the exchange activity of 100 nm Epac1 was measured in the absence (▵, □, and ○) or presence (■ and ●) of 50 μm 009, a cGMP analog. The exchange activity (mean ± S.E.; error bars are masked by the symbols) of 100 nm Epac1 (■ and □) was measured following injection of 2 μm 007 and preloaded Rap1, and that of 100 nm Epac1-Cat (● and ○) was measured following injection of preloaded Rap1. Release of bGDP from Rap1 was also measured in the absence of Epac1 and Epac1-Cat (▵). C and D, initial velocities of GDP exchange on 200 nm preloaded Rap1 were measured in triplicate in the presence of 100 nm Epac1 by fitting the RFU data to single exponentials with Graphpad Prism. Exchange was induced by increasing concentrations of the agonist 007 (C), or a fixed concentration of 007 (2 μm) and increasing concentrations of the in vitro antagonist 009 (D). Initial velocities (mean ± S.E.; some error bars are masked by the symbols) were plotted against the concentration of each effector on a log scale. The EC50 of 007 and IC50 of 009 were calculated by fitting the data with Graphpad Prism.
The cGMP analog 009 has been reported previously to behave as an inhibitor of cAMP-induced Epac activation in vitro (16, 17). Fifty micromolar 009 strongly reduced the release of Rap1-bound fluorescent bGDP induced by 2 μm 007 (Fig. 1B). The recombinant catalytic domain of Epac1 (Epac1-Cat), lacking the Epac1 cAMP-binding domain, is known to behave as a constitutive activated form of Epac1 (18). Fig. 1B shows that Epac1-Cat had constitutive exchange activity in the absence of 007 and that 009 (50 μm) had no inhibitory effect on Epac1-Cat, as expected for an inhibitor that would compete with agonists at the cAMP binding site of Epac1. Therefore, the combined use of recombinant Epac1 and Epac1-Cat allows discrimination of in vitro Epac1 inhibitors that would exert their effect on the Epac1 regulatory domain from those that would inhibit the interaction of Epac1 with Rap1 and/or directly inhibit nucleotide exchange on Rap1.
Dose-Response Analysis of Cyclic Nucleotide Analogs on Epac1 Activation
To further define the experimental conditions allowing screening for Epac1 pharmacological inhibitors, the kinetic characteristics of the in vitro reconstituted Epac1-Rap1 system were studied in more detail. Measurement of initial velocities of exchange at increasing concentrations of 007 (Fig. 1C) gave an EC50 value of about 2 μm. This concentration of 007 was therefore used to study the antagonism promoted by increasing concentrations of 009 (Fig. 1D). The IC50 of 009 for inhibition of 2 μm 007-stimulated Epac1 activity was approximately 17 μm, suggesting that 009 had a 7- to 8-fold lower affinity for the cAMP binding site of Epac1 compared with 007. Using Graphpad Prism, the IC80 (i.e. the concentration of 009 that promoted 80% inhibition of Epac1 activity) was found to be around 50 μm. Finally, the inhibitory effect of 25 μm 009 was completely suppressed by 100 μm excess of 007 (data not shown), indicating the competitive nature of Epac1 inhibition by 009. A subsaturating concentration of 007 (2 μm) was therefore chosen for subsequent screening experiments to avoid that an excess of this strong Epac1 agonist could prevent inhibition by relatively weak competitive inhibitory compounds.
Validation of the Assay in the 384-well Plate Format
These studies were performed using a two points robotized method (see “Experimental Procedures” for details). In brief, the variation in fluorescence of bGDP in the presence of Epac1 was measured 4 min after the injection of a 007 solution and was directly considered as a measurement of the exchange activity of Epac1. In the context of this inhibition assay, the “max” value was obtained with 2 μm (EC50) of the agonist 007, and the “min” value was obtained with the same concentration of 007 plus 50 μm (the IC80) of the standard in vitro antagonist 009. A mean max value and a mean min value were determined from 64 wells of a 384-well plate in each condition (not shown), allowing the calculation of a Z′-factor. The calculated Z′-factor was approximately 0.7, fulfilling the accepted criterion of a value ≥ 0.5 (19) and indicating that the assay was appropriate for screening.
Screening for Inhibitors of Epac1 Activity Using the CE Sublibrary from the French National Chemical Library
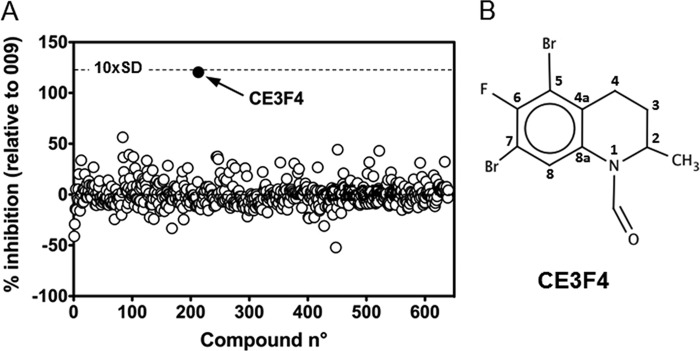
The two point robotized assay validated in the 384-well format was used to screen the 640 compounds of the CE library for their potency to inhibit Epac1 exchange activity. Compounds were blind-screened; the properties, structures, and molar concentrations of the compounds being unknown at this stage. Fig. 2A shows the scatter plot analysis of single-concentration (6.67 μg/ml) screening data. The potency of each library compound was expressed as the percent of inhibition promoted by 50 μm 009, and the positive inhibition control was set at 100%. One compound, identified as CE3F4, strongly deviated from the mean response of the other compounds, showing > 100% of the inhibition promoted by 50 μm 009 (Fig. 2A). The inhibition promoted by CE3F4 differed by approximately 10 standard deviations from the mean inhibition promoted by all of the 640 compounds (i.e. the Z score was approximately 10). Its formula was obtained from the French National Chemical Library and is shown in Fig. 2B. The name of this tetrahydroquinoline analog is 5,7-dibromo-6-fluoro-2-methyl-1,2,3,4-tetrahydroquinoline-1-carbaldehyde, and its molecular mass is 351.01 Da, indicating that CE3F4 was tested at approximately 19 μm in the screening assay. To exclude a possible false positive response, the activity of CE3F4 was confirmed using the multipoint (time course) assay (see “Experimental Procedures”) in triplicate and at the same concentration that was used in the screening process, first from a daughter library plate and then from a new batch of the crystallized compound provided by the French National Chemical Library (data not shown).
FIGURE 2.
Identification of CE3F4 as an EPAC1 antagonist by HTS screening. A, scatter plot of the percentage inhibition of each well from 640 compounds of the CE library tested at 6.67 μg/ml. The exchange activity was induced by 2 μm 007, and the percentage inhibition was calculated on the basis of the maximum inhibition promoted by 50 μm 009 (n = 32) and minimum inhibition measured in the absence of 009 (n = 32). One compound, CE3F4 (●), yielded both > 100% inhibition of the exchange activity and approximately 10 standard deviations (10×SD, dashed line) above the mean of the population of compounds. B, chemical structure of CE3F4. Numbering of the positions in CE3F4 formula corresponds to that of the tetrahydroquinoline skeleton.
Kinetic Characteristics of Epac1 Inhibition by CE3F4
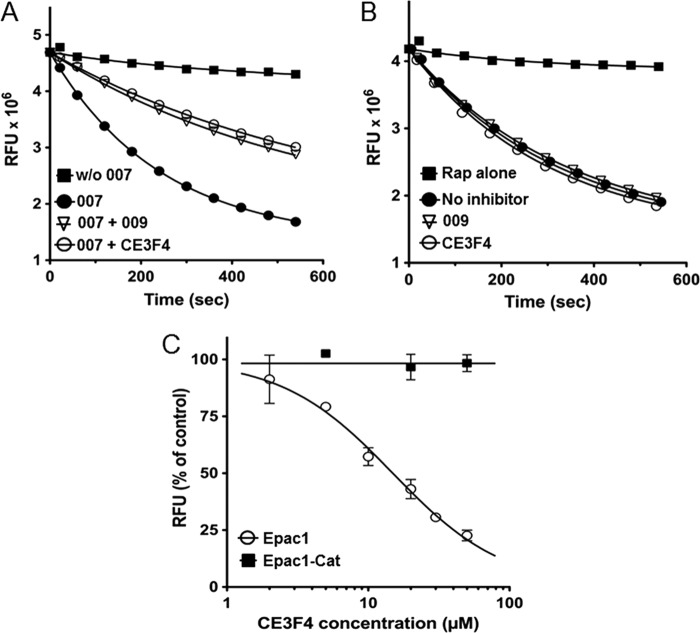
The effects of CE3F4 on the exchange activity of Epac1 and Epac1-Cat were studied manually using the multipoint assay. Fig. 3A shows that the exchange reaction induced by Epac1 activated by 007 (2 μm) was inhibited to a similar extent by CE3F4 (20 μm, close to the concentration used in the screening) and by 009 (25 μm). In contrast, neither CE3F4 nor 009 had any inhibitory effect on the constitutive exchange activity of Epac1-Cat (Fig. 3B). The exchange activity of Epac1 induced by 007 (2 μm) was reduced by CE3F4 in a concentration-dependent manner (IC50 = 23 ± 3 μm), whereas the dose-response study confirmed the lack of inhibition of Epac1-Cat by CE3F4 (Fig. 3C). This result indicates that CE3F4 inhibits neither the interaction of Epac1 with Rap1 nor directly the GDP exchange on Rap1.
FIGURE 3.
Characterization of CE3F4-mediated inhibition of 007-induced Epac1 activity and of constitutive Epac1-Cat activity. Variations of relative fluorescence units (RFU) were studied as a function of time and fitted to single exponentials. Reported values are mean ± S.E. (n = 3; some error bars are masked by the symbols). A, Epac1 nucleotide exchange activity was measured in the absence of 007 (■) or in the presence of 2 μm 007, either alone (●) or with 25 μm 009 (▿) or with 20 μm CE3F4 (○). B, Epac1-Cat exchange activity was measured in the absence of inhibitor (●) or in the presence of 25 μm 009 (▿) or 20 μm CE3F4 (○). Fluorescence was also recorded in the absence of Epac1-Cat (■). C, initial velocities of nucleotide exchange induced by Epac1 together with 2 μm 007 (○) or by Epac1-Cat (■) were measured in the presence of increasing concentrations of CE3F4. The IC50 was calculated using Graphpad Prism.
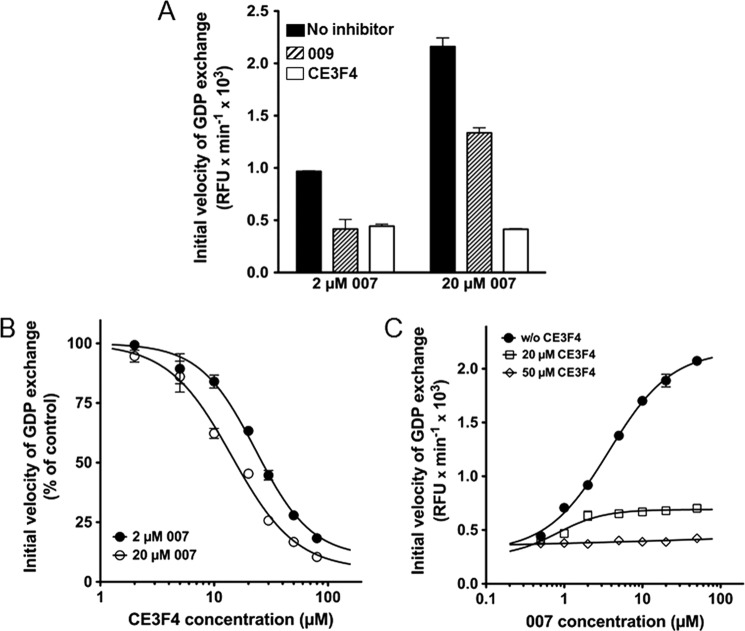
The Epac1 regulatory domain was therefore necessary for CE3F4 to inhibit Epac1 activity. One may speculate that CE3F4 could impair the binding of 007 to the regulatory domain of Epac1 either by direct competition or by an allosteric mechanism. Alternatively, it might impede the agonist-induced conformational changes that are necessary to relieve the autoinhibitory mechanisms that allow Epac1 to adopt its active conformation (11, 16) without interference with agonist binding. This latter hypothesis is supported by the results reported in Fig. 4A, showing that, in contrast to 009, the residual exchange activity of Epac1 in the presence of 20 μm CE3F4 was independent of the concentration of 007 (2 μm or 20 μm) used to activate Epac1. In other words, an excess of agonist did not relieve the inhibition promoted by CE3F4. CE3F4 had also a similar inhibitory effect on the activation of Epac1 by 50 μm cAMP (not shown), even if cAMP at this concentration had only half the potency of 2 μm 007 to activate Epac1. Fig. 4B shows that increasing 007 concentration from 2 to 20 μm resulted in a decrease of CE3F4 IC50 from 22.5 ± 1 μm to 15 ± 2 μm (means ± S.E. of three independent experiments). This suggests that 007 binding was required to stabilize CE3F4 binding to Epac1, a property analogous to uncompetitive inhibition that takes place when an enzyme inhibitor binds only to the complex formed between the enzyme and the substrate. Because both Vmax and Km are reduced in enzymatic uncompetitive inhibition (20), we studied the kinetic characteristics of Epac1 activation by 007 in the absence and presence of CE3F4 (Fig. 4C). CE3F4 (20 μm) decreased both the Vmax of GDP exchange and the EC50 for 007 by ∼80%. At 50 μm CE3F4, Vmax and EC50 were too small to be computed. Taken together, these data suggest that CE3F4 likely acted noncompetitively with respect to the Epac1 agonist 007.
FIGURE 4.
Kinetics of inhibition of Epac1 exchange activity by CE3F4. Initial velocities of GDP exchange were measured in triplicate from time course studies and are shown as mean ± S.E. (some error bars are masked by the symbols). A, Epac1 was activated by either 2 μm or 20 μm 007, and the exchange activity was measured without inhibitor (black bars), with 25 μm 009 (hatched bars), or with 20 μm CE3F4 (empty bars). B, initial velocities of nucleotide exchange induced by Epac1 were measured in triplicate in the presence of 2 μm (●) or 20 μm (○) 007 together with increasing concentrations of CE3F4. The IC50s were calculated using Graphpad Prism. C, initial velocities of nucleotide exchange were measured in the presence of increasing concentrations of 007 and either in the absence (●) or presence of 20 μm (□) or 50 μm (♢) CE3F4. The EC50s and maximum velocities of exchange (Vmax) were calculated using Graphpad Prism.
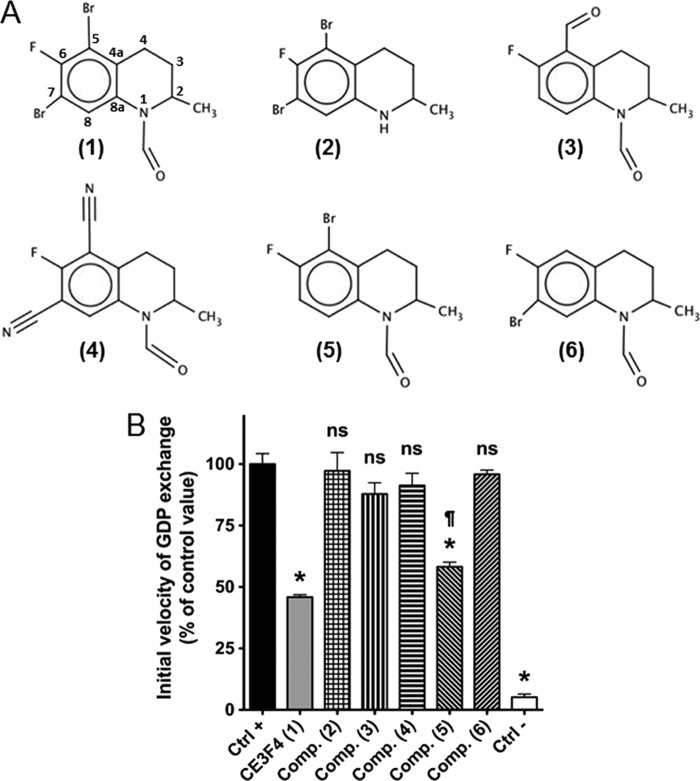
Chemical Specificity of Epac1 toward CE3F4 Analogs
Several analogs or derivatives of CE3F4, whose formulas are shown in Fig. 5A, were studied for their potential to prevent Epac1 activation. Epac1 was stimulated by 20 μm 007 in the absence or presence of 20 μm of each compound, and the initial velocity of GDP exchange on Rap1 was measured (Fig. 5B). CE3F4 (compound 1) showed the strongest inhibitory effect. Compound 2, which lacks the formyl group on position 1, had no inhibitory effect, as it was the case for compound 3 and compound 4, which lack the bromine atoms on both positions 5 and 7. Compound 3 is substituted by a formyl group on carbon 5, whereas compound 4 has two cyano groups on positions 5 and 7. Finally, the removal of only one bromine atom on position 7 (compound 5) significantly reduced but did not suppress inhibition compared with CE3F4, whereas the selective removal of the bromine atom on position 5 (compound 6) completely hampered the inhibitory potential of the original molecule. This preliminary structure-activity study shows that compounds having the formyl group on position 1 and the bromine atom on position 5 seem to be the most efficient molecules to block Epac activation. This does not exclude that other derivatives at these positions may influence Epac1-GEF activity. CE3F4 and its derivatives are tetrahydroquinoline analogs that are chemically unrelated to the series of pyrimidine analogs identified by Chen et al. (9) on the basis of their direct competition with a fluorescent cAMP derivative in binding to Epac1 and Epac2.
FIGURE 5.
Chemical specificity of CE3F4 analogs for Epac1 inhibition. A, structures of the Epac1 antagonist CE3F4 and of its analogs or derivatives. CE3F4 (compound 1) is an antagonist of 007-induced Epac1 activation identified by high throughput screening, whereas compounds 2–6 are analogs or derivatives tested for their potential antagonistic properties. B, the initial velocity of Epac1-catalyzed GDP exchange was measured in the absence (Ctrl-) or presence (Ctrl+) of 20 μm 007, and the latter value was set at 100% exchange activity. CE3F4 and compounds 2–6 were added just before the agonist 007, and the resulting exchange activity was expressed as the percentage of that measured under the Ctrl+ condition. Values are mean ± S.E. (n = 3). Student's t tests: *, p < 1% versus Ctrl+; ns, p > 5% versus Ctrl+; ¶, p < 1% versus CE3F4.
Effect of Agonists and Antagonists on an Epac1-based BRET Sensor
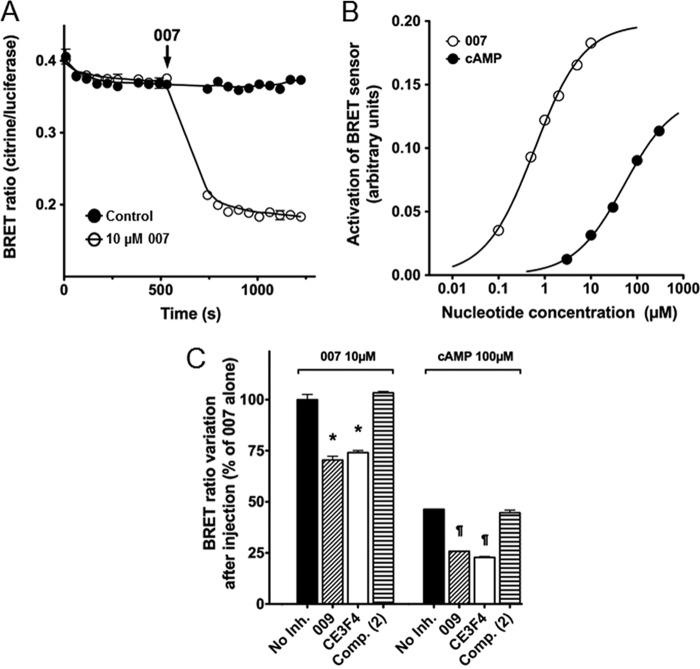
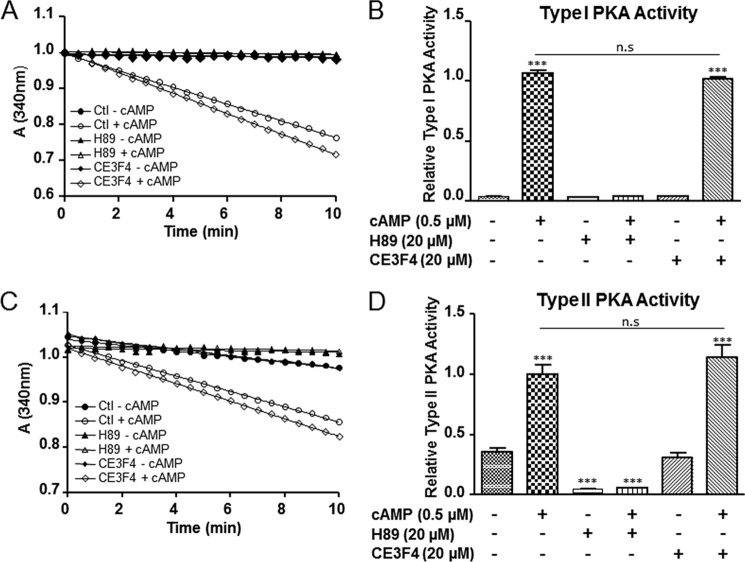
Next we investigated the inhibitory effect of CE3F4 on an EBS consisting of human Epac1 inserted between citrine and Renilla luciferase as the BRET pair (see “Experimental Procedures” for details). Binding of agonists to the EBS induces a conformational change in the Epac1 part, resulting in a decrease of energy transfer from the luciferase to citrine. HEK293 cells were transfected with an EBS-expressing construct, and the changes of BRET ratios were then measured in cell extracts upon binding of Epac1 agonists and antagonists. Fig. 6A shows that the BRET ratio abruptly dropped by approximately 2-fold after addition of 10 μm 007. To further test whether the EBS assay was a valuable tool to evaluate conformational changes undergone by Epac1 upon binding of agonists, BRET ratios were measured in cell extracts in response to increasing concentrations of either 007 or cAMP (Fig. 6B). Both nucleotides induced remodeling of Epac1 conformation, reflected by dose-dependent variations in the measured BRET ratio. The estimated EC50 for 007 and cAMP were 0.6 μm and 50 μm, respectively. Both 009 (50 μm) and CE4F4 (40 μm) inhibited the BRET variations induced by 10 μm 007 (approximately 25% decrease) or 100 μm cAMP (approximately 50% decrease). Compound 2, a CE3F4 analog devoid of antagonistic activity toward Epac1 guanine nucleotide exchange activity (Fig. 6B), had no inhibitory effect on conformational changes induced by either 007 or cAMP (C). These results constitute an independent confirmation that CE3F4 specifically inhibits Epac1 guanine nucleotide exchange activity without interference with Rap1 activity or Epac1-Rap1 interaction. Finally, we found that CE3F4 did not influence PKA activity in the presence or absence of cAMP (Fig. 7) indicating that this compound was specific of the cAMP-binding protein Epac.
FIGURE 6.
Effect of Epac1 antagonists on Epac1-BRET sensor activation by 007 and cAMP. HEK293 cells, transfected with the pcDNA3-EPS expression vector, were lysed, and the soluble fraction was used for BRET measurements. 0.4 μl of cell extract was mixed with the compounds under study in a well of a 384-well plate. Coelenterazine-h (2 μm final concentration) was injected into the well, and the emission signals from Renilla luciferase and citrine-cp were recorded as a function of time before and after injection of a solution of Epac1 agonist (007 or cAMP). The BRET ratio (mean ± S.E. from 3 wells; some error bars are masked by the symbols) was calculated as the ratio of the citrine-cp emission signal to that of Renilla luciferase. A, at the time indicated by the arrow, a 007 solution (10 μm final concentration (○) or vehicle (●) were injected into the well, and the BRET ratios were recorded for an additional period of time. B, the BRET readings (mean ± S.E., n = 3) were obtained 10–12 min after injection of increasing concentrations of 007 (○) or cAMP (●). The variation in the BRET ratio is plotted against the concentration of 007 on a log scale. C, 50 μm 009, 40 μm CE3F4, 40 μm compound 2, or vehicle (No Inh.) were added to the cell extract before injection of 007 (10 μm) or cAMP (100 μm), and BRET ratios (mean ± S.E., n = 3) were measured as in B and plotted as percent variations in BRET ratios relative to each no-inhibitor control value. *, p < 1% versus control value without 007; ¶, *, p < 1% versus control value without cAMP.
FIGURE 7.
CE3F4 does not influence PKA activity. A and C, time course decrease of NADH at 340 nm in the presence of type I and II PKA holoenzymes and the indicated compounds. H89 is a pharmacological inhibitor of PKA and was used as a positive control in the experiments. Similar results were obtained from four independent experiments. n.s., not significant. B and D, relative type I and type II PKA holoenzymes activity. The bar graph represents the mean ± S.E. of four independent experiments. ***, p < 0.001 versus indicated control condition, one-way analysis of variance, Bonferroni comparison test. Results are expressed as the percentage of control (Ctl).
Effects of CE3F4 on the Epac1 Downstream Effector in Living Cultured Cells
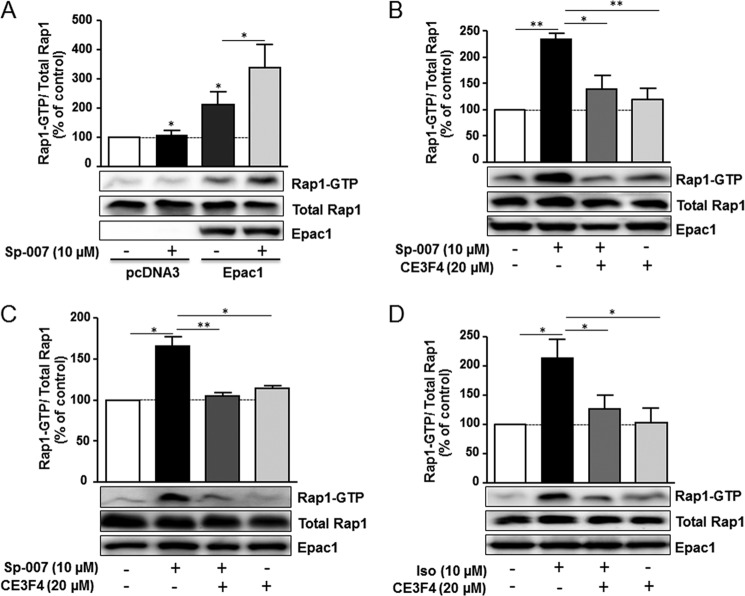
As a next step, we tested the ability of CE3F4 to block Epac1-induced Rap1 activation in living cultured HEK293 cells. For these experiments, we used a membrane-permeant Epac agonist, Sp-8-pCPT-2′-O-Me-cAMPS (Sp-007) (21). We found that Sp-007 (10 μm) had almost no effect on Rap1 activation because of low expression level of Epac1 in this cellular system (Fig. 8A). However, Sp-007 induced a robust activation of Rap1 in cells overexpressing Epac1 compared with control cells transfected with the empty vector (Fig. 8A). Consistent with the data obtained in vitro on Epac exchange reaction, CE3F4 (20 μm) prevented the increase in the amount of Rap1-GTP following 10 μm Sp-007 treatment (Fig. 8B). Similar findings were observed in primary cardiac myocytes as Epac-induced Rap1 activation was blocked by CE3F4 (20 μm) (Fig. 8C). Finally, as β-adrenergic receptors activate Epac in cardiac myocytes and other cell types (15, 22), we tested the effects of CE3F4 on Epac1-induced Rap1 activation following stimulation of β1-adrenergic receptor by Iso (10 μm), a non-selective β-adrenergic receptor agonist. As shown in Fig. 8D, we found that CE3F4 decreased Epac1-induced Rap1 activation in β1-adrenergic receptor-overexpressing HEK293 cells treated with Iso. Altogether, these data show that CE3F4 is efficient in preventing Epac1-induced Rap1 activation in cultured cells.
FIGURE 8.
CE3F4 inhibits Epac-induced Rap1 activation in living cultured cells. HEK293 cells (A and B) and rat neonatal cardiac myocytes (C) were transfected with Epac1 expression vector. HEK293 cells were also transfected with pcDNA3 control vector. 24 h after transfection, cells were preincubated or not preincubated with CE3F4 for 30 min and were then treated or not treated with Sp-007 (10 μm) for 10 min. D, HEK293 cells overexpressing β1AR and transfected with Epac1 were pretreated or not pretreated with CE3F4 as in B and stimulated or not stimulated with Iso (10 μm) for 10 min. Amounts of Rap1-GTP were determined by pull-down assays. A control for total Rap expression is shown. Expression of Epac1 was measured in cell lysates. The bar graph represents the mean ± S.E. of five (A and B) or three (C and D) independent experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05 between indicated conditions, paired two-tailed Student's t test. Results are expressed as the percentage of unstimulated control cells.
In conclusion, using a validated fluorescence-based high throughput screening assay, we have identified a pharmacological tetrahydroquinoline analog, named CE3F4, that displays Epac1 antagonistic activity. CE3F4 behaves as an uncompetitive antagonist of 007 and cAMP with inhibition, working best when 007 (or cAMP) concentration is high. CE3F4 blocks Epac1 guanine nucleotide exchange reaction on Rap1 in intact cells. Because CE3F4 did not exert its antagonism by directly competing with cAMP for binding to Epac1, it is difficult to predict whether it could also inhibit Epac2. More work is necessary to explore this possibility. At the moment, our data show that CE3F4 and related compounds may provide valuable pharmacological tools for determining the biological functions of Epac1 and for better understanding the involvement of Epac1 in the manifestation of diseases such as cardiac hypertrophy, tumor invasion, and inflammation.
Acknowledgments
We thank Dr. Lily I. Jiang for providing the pQE30-CAMYEL plasmid.
This work was supported by grants from the Agence Nationale de la Recherche (“HyperEpac” Genopath09), Région Midi-Pyrénées, and Association Française contre les Myopathies and Fondation pour la Recherche Médicale (Programme Cardiovasculaire) (to F. L.). This work was also supported by a grant from the Centre National de la Recherche Scientifique (Programme Interdisciplinaire de Recherche Innovation Thérapeutique 2010) (to J. P. B.).
- PKA
- protein kinase A
- GEF
- guanine nucleotide exchange factor
- Epac1
- exchange protein directly activated by cAMP, isoform 1
- 007
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- CE
- Chimiothèque Essentielle
- 009
- 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate
- bGDP
- 4,4-difluoro-5,7-dimethyl-4-bora-3 a,4 a-diaza-s-indacene-3-propionic acid 2′-(or-3′)-O-(N-(2-aminoethyl)urethane), bis(triethylammonium) salt
- BRET
- bioluminescence resonance energy transfer
- EBS
- Epac1-BRET sensor
- Sp-007
- 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′, 5′-cyclic monophosphorothioate, Sp-isomer (Sp-8-pCPT-2′-O-Me-cAMPS)
- AR
- adrenergic receptor
- Iso
- isoprenaline
- CAMYEL
- cAMP sensor using YFP-Epac-RLuc.
REFERENCES
- 1. Breckler M., Berthouze M., Laurent A. C., Crozatier B., Morel E., Lezoualc'h F. (2011) Rap-linked cAMP signaling Epac proteins. Compartmentation, functioning and disease implications. Cell. Signal. 23, 1257–1266 [DOI] [PubMed] [Google Scholar]
- 2. Métrich M., Berthouze M., Morel E., Crozatier B., Gomez A. M., Lezoualc'h F. (2010) Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 459, 535–546 [DOI] [PubMed] [Google Scholar]
- 3. Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 [DOI] [PubMed] [Google Scholar]
- 4. de Rooij J., Zwartkruis F. J., Verheijen M. H., Cool R. H., Nijman S. M., Wittinghofer A., Bos J. L. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 [DOI] [PubMed] [Google Scholar]
- 5. Gloerich M., Bos J. L. (2010) Epac. Defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 [DOI] [PubMed] [Google Scholar]
- 6. Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 7. Zhong N., Zucker R. S. (2005) cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J. Neurosci. 25, 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsalkova T., Mei F. C., Cheng X. (2012) A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS ONE 7, e30441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H., Tsalkova T., Mei F. C., Hu Y., Cheng X., Zhou J. (2012) 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 22, 4038–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouyssou P., Le Goff C., Chenault J. (1992) Synthesis of 7- and 5,7-substituted-6-fluoro-2-methyl-1,2,3,4-tetrahydroquinolines. Convenient precursors of quinolone antibacterial agents. J. Heterocyclic. Chem. 29, 895–898 [Google Scholar]
- 11. Rehmann H. (2006) Characterization of the activation of the Rap-specific exchange factor Epac by cyclic nucleotides. Methods Enzymol. 407, 159–173 [DOI] [PubMed] [Google Scholar]
- 12. van den Berghe N., Cool R. H., Horn G., Wittinghofer A. (1997) Biochemical characterization of C3G. An exchange factor that discriminates between Rap1 and Rap2 and is not inhibited by Rap1A(S17N). Oncogene 15, 845–850 [DOI] [PubMed] [Google Scholar]
- 13. Jiang L. I., Collins J., Davis R., Lin K. M., DeCamp D., Roach T., Hsueh R., Rebres R. A., Ross E. M., Taussig R., Fraser I., Sternweis P. C. (2007) Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J. Biol. Chem. 282, 10576–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wollert K. C., Taga T., Saito M., Narazaki M., Kishimoto T., Glembotski C. C., Vernallis A. B., Heath J. K., Pennica D., Wood W. I., Chien K. R. (1996) Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J. Biol. Chem. 271, 9535–9545 [DOI] [PubMed] [Google Scholar]
- 15. Métrich M., Lucas A., Gastineau M., Samuel J. L., Heymes C., Morel E., Lezoualc'h F. (2008) Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 102, 959–965 [DOI] [PubMed] [Google Scholar]
- 16. Rehmann H., Schwede F., Døskeland S. O., Wittinghofer A., Bos J. L. (2003) Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J. Biol. Chem. 278, 38548–38556 [DOI] [PubMed] [Google Scholar]
- 17. Das R., Chowdhury S., Mazhab-Jafari M. T., Sildas S., Selvaratnam R., Melacini G. (2009) Dynamically driven ligand selectivity in cyclic nucleotide binding domains. J. Biol. Chem. 284, 23682–23696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Rooij J., Rehmann H., van Triest M., Cool R. H., Wittinghofer A., Bos J. L. (2000) Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol. Chem. 275, 20829–20836 [DOI] [PubMed] [Google Scholar]
- 19. Zhang J. H., Chung T. D., Oldenburg K. R. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4, 67–73 [DOI] [PubMed] [Google Scholar]
- 20. Cornish-Bowden A. (1974) A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 137, 143–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen A. E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K. K., Martinez A., Maenhaut C., Bos J. L., Genieser H. G., Døskeland S. O. (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 278, 35394–35402 [DOI] [PubMed] [Google Scholar]
- 22. Rangarajan S., Enserink J. M., Kuiperij H. B., de Rooij J., Price L. S., Schwede F., Bos J. L. (2003) Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β 2-adrenergic receptor. J. Cell Biol. 160, 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]