Summary
Aims
The aim of this clinical trial was to compare clinical and biochemical healing outcomes following ultrasonic mechanical instrumentation versus ultrasonic mechanical instrumentation associated with topical subgingival application of amino acids and sodium hyaluronate gel.
Methods
Eleven systemically healthy subjects with moderate-severe chronic periodontitis, who had four sites with pocket probing depth and clinical attachment level greater than or equal to 5 mm were randomly assigned to two different types of treatment: two pockets were treated with ultrasonic debridement (Control Group) and two pockets with ultrasonic mechanical instrumentation associated with 0,5 ml of amino acids and sodium hyaluronate gel (Test Group). Probing depth, clinical attachment level, plaque index and bleeding on probing were recorded at baseline, 45 and 90 days. Levels of calprotectin and myeloperoxidase activity in gingival crevicular fluid were assessed at baseline and on day 7 and 45.
Results
Statistical significance was found between baseline and day 45 in relation to probing depth reduction and bleeding on probing between groups for both of the tested treatments. Significant reductions in μg/sample of calprotectin and myeloperoxidase were found after 1-week and an increase at 45 days in both groups. There were no statistically significant differences between other variables evaluated in this study.
Conclusions
These data suggest that subgingival application of hyaluronic acid following ultrasonic mechanical instrumentation is beneficial for improving periodontal parameters.
Keywords: hyaluronic acid, periodontitis, calprotectin, myeloperoxidase
Introduction
A successful treatment of periodontal disease is based on an adequate maintaining of infection control in the subgingival area. According to recent systematic reviews (1,2), there is no major difference in the efficacy of debridement techniques of plaque and calculus from root and tooth surfaces using manual or ultrasonic instrumentation. Ultrasonic mechanical instrumentation combined with effective self-performed supragingival plaque control measure, allows infection control by altering the subgingival ecological environment through disruption of the microbial biofilm and suppression of the inflammation (3).
This goal is frequently not completely attainable; it is nearly impossible to expect to set the root completely free from calculus specially in deeper pockets. Probing of the root surface for detection of remaining deposits is an unreliable method to determine whether adequate debridement has been achieved, while clinical signs of resolution of the inflammatory lesion would indicate sufficient removal of biofilm/calculus (4).
Several recent studies have demonstrated additional improvements in clinical parameters using local or systemic antimicrobial agents (5–7).
Nevertheless, antibacterial agents locally or systemically applied in periodontal pockets, proved to be more effective only when they were used in combination with ultrasonic mechanical instrumentation. Physical disruption of the biofilm is fundamental for the control of periodontal diseases (8). The use of systemic antibiotics should be limited because of the development of resistant organisms, allergic reactions and drug interactions.
Some articles proposed topical applications of hyaluronic acid (HA) to complement mechanical debridement (9,10). HA is a non-sulfated glycosaminoglycan with a high molecular weight. It is one of the components of the extracellular matrices of the connective tissue and was detected in the gingiva (11,12). It has an anti-inflammatory and anti-edematous effect (13) and promotes wound healing (14). Hyaluronan gel application demonstrated a better reduction of the clinical parameters and inflammatory infiltrate in the treatment of plaque-induced gingivitis (15,16). Recently, Pistorius and colleagues confirmed a reduction in the sulcus bleeding index and in the gingival crevicular fluid (GCF), but not in the plaque values after topical hyaluronic acid application in gingivitis therapy (17).
Currently, researchers are establishing the potential benefits of local subgingival application of HA adjunctive to scaling and root planing for the treatment of periodontitis owing to its tissue healing and regenerative properties (14). Research is also under way to establish that topical applications of HA in alveolar bony defects accelerate periodontal wound healing (18).
In 2001 Engstrom’s clinical, immunological and microbiological responses to hyaluronan were studied to evaluate the anti-inflammatory effect on surgical and non-surgical periodontal treatment (9). Parameters considered were periodontal probing depth, gingival crevicular fluid immunoglobulin G (IgG), C3, prostaglandin E2 (PGE2) and presence of plaque.
The anti-inflammatory effect of hyaluronan could not be verified in this study in relation to the immune responses (IgG, C3 and PGE2). In another study by Xu and colleagues it was found that no clinical or microbiological improvement was achieved by the adjunctive use of HA gel compared to scaling and root planing alone in the treatment of periodontitis. Only sulcus flow rate was affected by the use of HA gel in terms of a more rapid reduction in the test sites (10).
In 2009 Johannsen asserted that the application of hyaluronan gel in conjunction with scaling and root planing may have a beneficial effect on periodontal health in patients with chronic periodontitis (19).
Recent reports showed that a gel containing HA promoted wound healing in post-surgical wounds (20–22).
In order to determine the amount of HA needed to lead to clinically significant periodontal healing we planned to evaluate the clinical and the putative anti-inflammatory effects of application of amino acids and sodium hyaluronate gel as an adjunct to ultrasonic mechanical instrumentation in patients with chronic periodontitis. To this purpose as clinical parameters we evaluated PPD, CAL, plaque index (PI) and bleeding on probing (BOP). As inflammatory parameters we evaluated calprotectin concentration and myeloperoxidase activity in gingival crevicular fluid, since these are directly related with both the extent of polymorphonuclear neutrophils (PMNs) infiltration and the severity of periodontal disease (8,23).
Materials and methods
Patient selection and pocket selection
The study design was a split-mouth clinical trial of 12-weeks duration.
11 healthy patients with moderately advanced chronic periodontitis, 7 males and 4 females (mean age was 51 years SD ± 9,8), were recruited for the study following a screening examination including full-mouth probing and radiographic evaluation. The following criteria were used:
Inclusion criteria:
age 40 to 70 years,
a minimum of 18 teeth,
at least two sites per quadrant with probing depth (PPD) and clinical attachment level (CAL)≥ 35 mm.
Exclusion criteria:
use of locally or systemic antimicrobial agents 6 months before the study,
subgingival instrumentation within 6 months before the baseline examination,
previous periodontal therapy during the last 6 months,
pregnancy and lactation period,
smokers,
systemic diseases that could possibly influence the condition of the periodontal tissue and the subgingival microflora.
Power calculation based on the detection of a difference in the mean PPD reduction of 0,5 mm between different treatment, standard deviation (SD) 0,035, a error defined to 0.05 and b error defined to 0.20, revealed that at least 18 sites in each treatment were required (PS 2.1.31 for windows) (24).
Approval of the study protocol by the Ethics Committee at Azienda Ospedaliero-Universitaria “Ospedali Riuniti” of Trieste (n. 27/2008) was obtained, and all participating subjects provided informed consent before the start of the study.
Assessment baseline
The following variables were recorded for each site:
Plaque index (PI) (25): presence/absence of plaque at the cervical area of the tooth.
Bleeding on probing (BOP) (26): presence/absence of bleeding within 15s following pocket probing.
Clinical attachment level (CAL): distance between a fixed reference point on the tooth (cemento enamel junction, CEJ, or the margin of a restoration) to the bottom of the clinical pocket, measured with a manual periodontal probe (UNC15 Hu-Friedy®, Chicago, IL, USA).
Probing depth (PPD): distance from gingival margin to the bottom of the clinical pocket, measured with a manual periodontal probe (UNC15 Hu-Friedy®, Chicago, IL, USA).
In another session were recorded in the same site:
Level calprotectin and myeloperoxidase (MPO): The selected area was isolated with cotton rolls and gently air-dried. Then, a perio paper strip (PerioCol Collection Strip, Oraflow, Plainview, NY, USA) was inserted into the sulcus at PPD less than 1 mm and removed after 10s. Strips visibly contaminated with blood were discarded. After the measurements, samples were stored separately in 200 μl sterile phosphate-buffered saline at −80°C until further processing.
Gingival crevicular fluid volume (GCF): after 10 minutes of the quantitative evaluated of calprotectin and MPO, sites were reisolated with new cotton rolls and gently airdried. Then new perio paper strip (PerioCol Collection Strip, Oraflow, Plainview, NY, USA) was inserted into the sulcus and removed after 10s. The gingival crevicular fluid volume was determined using a calibrated moisture meter (Periotron, Siemens, Bensheim, Germany) and calculated in μl from a standard curve. The Periotron was calibrated by pipetting known volumes of four different liquids (phosphate-buffered saline, human saliva, human serum, PBS phosphate buffered saline) on perio paper strips (27). Calibration to baseline was performed with a dry perio paper.
Treatment procedures
After initial measurements and collection had been done, the selected patients have been treated with full-mouth subgingival debridement using a piezoceramic ultrasonic instrument (Piezosteril 5, Castellini, MI, Italy). The instrumentation was considered completed when the root surface was clinically judged to be clean and smooth. Then, a split mouth method design was applied. After ultrasonic debridement, four sites, two per quadrant, were randomly allocated (computer generated randomization list, Random Generator) to test group or control group. The additional treatment at the test sites consisted of subgingival administration of 0,5 ml of amino acids and sodium hyaluronate gel (Aminogam Ò A, lotto 190308A, Errekappa Euroterapici Spa, MI, Italy) by a syringe with blunt needle placed 3 mm to bottom of the clinical pocket; in the same way 0,5 ml of placebo gel (Aminogam Ò B, lotto 190308B, Errekappa Euroterapici Spa, MI, Italy) was applied in two control sites. Operator did not know the contents of the gel. Aminogam A and Aminogam B were made at the sites following the same procedure at 7, 15, 30 and 45 days.
The patient was instructed not to drink or eat or toothbrush for 1 hour after device administration. All patients were included in a personalized hygiene program.
Clinical and biochemical assessment after treatment
At day 45 and day 90, a dental hygienist registered new values of PI, PPD, CAL and BOP for all of the treated sites and oral hygiene motivation was given when indicated.
The quantity of calprotectin, MPO and gingival crevicular fluid volume was evaluated and recorded at test and control sites at 7 and 45 days.
Biochemical analyses
To extract the GCF from the perio paper strip, the samples were thawed, vortexed for 60 s by a vortex-mixer (Maxi Mixer 714, Asal Srl., MI, Italy) and eluted by centrifugation at 3000 cycles/min for 5min (MSE, Sanyo, Singapore).
The crevicular protein content was quantified with the Bradford method (28) using bovine - serum - albumin (BSA) as standard.
Calprotectin
Crevicular calprotectin levels were measured by enzyme- linked-immunosorbent assay using a commercial kit (Calprest, Eurospital, TS, Italy). The assays were performed according to the manufacturer’s instructions, and the results referred to a calibration curve expressed in μg/μl. Samples were diluted using assay buffer to 1:100 and assayed in triplicate.
Salivary myeloperoxidase (MPO)
Crevicular peroxidase (MPO) activity was calculated from the rate of H202-dependent oxidation of TMB (29). Briefly the activity of crevicular MPO was determined at room temperature in 96 wells microtiter plate using a reaction mixture containing 1mM (final concentration) tetramethylbenzidine (TMB) (29) [added from a 25 mM stock solution in dimethylsulphoxide (DMSO)] as substrate, 0,02% cetyltrimethylammonium bromide (CTAB). The reaction was started by adding 0,30 mM hydrogen peroxide (final concentration), stopped after 2 minutes by adding 0,4N H2SO4 (final concentration) and the reaction product was quantitated spectrophotometrically at 413nm. Since PMN are the only source of peroxidase activity in the crevicular fluid (30) the concentration of MPO in crevicular fluid was calculated by referring to a calibration curve obtained with pure human MPO (Rz 0.8) prepared from human neutrophils (31) as previously described (32).
Calprotectin and peroxidase data were presented in μg/μl for concentrations and in μg/sample for total amounts in the selected sites.
Statistical analysis
Statistics were performed with the site as the unit of analysis. The mean of all the analysed periodontal parameters was calculated. The c2 test was used to determine the differences in dichotomous variables (PI and BOP). The distribution of continuous variables was initially analysed with Kolmogorov-Smirnov and Shapiro-Wilks. Differences between mean values for gingival crevicular fluid volume were statistically analysed by the use of the repeated measurements analysis of variance. Differences in PPD and CAL between the groups at baseline, day 45 and day 90 were tested by the use of Mann-Whitney U-test with exact test Monte Carlo Sig (1-talied) based on 10000 sampled tables. Changes of concentrations of calprotectin and MPO were analysed by non-parametric test. Statistical significance was defined as p<0.05.
A software program (SpSS 16.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for all calculations.
Results
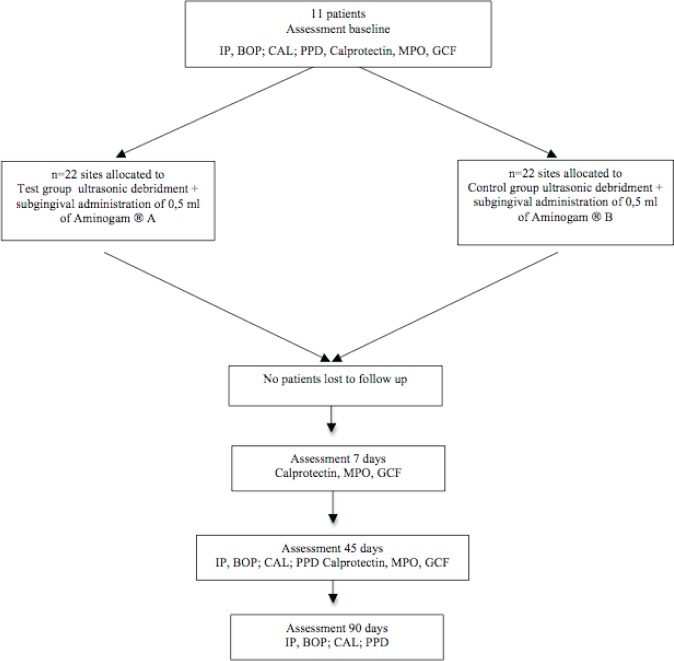
All eleven participants completed the study and there were not protocol deviations. No adverse effects were reported (Fig. 1).
Figure 1.
Flow diagram of the trial.
Clinical findings
The clinical characteristics of the sites included in the study are reported in Table 1.
Table 1.
Clinical group characteristics; mean values (95% CI).
| Group Test | Group Control | |
|---|---|---|
| Plaque index score PI (%) | 63,6 | 81,8 |
| 45 days | 31,8 | 36,4 |
| 90 days | 13,6 | 22,7 |
| BoP score (%) | 72,7 | 72,7 |
| 45 days | 9,1 | 27,3 |
| 90 days | 4,5 | 18,2 |
| Mean CAL (mm) | 5,91 (5–6,84) | 5,91 (5–6,83) |
| 45 days | 5,27 (4,28–6,26) | 5,36 (4,46–6,27) |
| 90 days | 4,86 (3,86–5,86) | 5,05 (4,09–6) |
| Mean PPD (mm) | 6,14 (5,70–6,58) | 6,36 (5,86–6,87) |
| 45 days | 5,18 (4,74–5,63) | 5,82 (5,29–6,34) |
| 90 days | 4,64 (4,10–5,18) | 5,36 (4,79–5,94) |
Plaque index score (PI)
The plaque index score in the two study groups improved during the study period. The PI was reduced by 31,8% in the test group, by 36,4% in the control group. No statistically significant difference between the two treatment groups was observed at any of the examination intervals.
Bleeding on probing (BOP)
The number of bleeding sites was markedly reduced following both treatments. At 45 days, the BOP was reduced from 72,7% to 27,3% in sites treated with only ultrasonic instruments and from 72,7% to 9,1% in sites treated with ultrasonic instruments and amino acids and sodium hyaluronate gel. At re-evaluation at 90 days, bleeding sites were reduced in both groups but those treated only with non-surgery mechanical periodontal therapy obtained higher BoP scores (test group=4,5%; control group =18,2%).
There was statistically significant difference in BOP between the two treatment groups (p<0.05).
Clinical attachment level (CAL)
At re-evaluation at 45 days a CAL gain amounted 0.64 mm (test group) and 0,55 mm (control group) was obtained. At 90 days, CAL mean was reduced from 5,91 mm to 4,86 mm in test group, from 5,91 mm to 5,05 mm in control group.
There was no statistically significant difference in CAL gain between baseline and re-examination intervals (45 and 90 days) for both groups (p>0.05). There was no statistically significant difference in CAL gain between sites treated with ultrasonic instruments and those treated with mechanical instruments combined with amino acids and sodium hyaluronate gel (p>0.05).
Probing pocket depth (PPD)
A marked reduction of probing pocket depth was observed in both groups. At the 45 days re-examination, mean PPD reduction was 0,96 mm for test group and 0,54 mm for control group. At 90 days, PPD was reduced from 6,36 mm to 5,36 mm in sites treated with ultrasonic instruments only and from 6,14 mm to 4,64 mm in sites treated with ultrasonic instruments and amino acids and sodium hyaluronate gel. Mann-Whitney U-test demonstrated significant differences in pocket depth reduction at 45 days (p<0.03) and 90 days (p<0.02) between test group and control group.
Biochemical finding
Gingival crevicular fluid markers
Biochemical characteristics of the sites included in the study are reported in Table 2.
Table 2.
Biochemical group characteristics; mean values (95% CI).
| Group Test | Group Control | |
|---|---|---|
| Gingival crevicular fluid volume (GCF) (μl) | 74,09 (56,93–91,26) | 70,95 (52,57–89,34) |
| 7 days | 32,82 (23,93–40,71) | 34,45 (27,68–41,23) |
| 45 days | 33,38 (27,58–39,18) | 38,36 (30,56–46,17) |
| Calprotectin (μg)* | 7,00 (4,51–9,49) | 7,58 (4,71–10,34) |
| 7 days | 5,82 (4,23–7,42) | 6,41 (4,28–8,53) |
| 45 days* | 6,23 (4,45–8,01) | 8,56 (5,88–11,23) |
| Myeloperoxidase (μg)*# | 3,67 (1,56–5,77) | 5,33 (2,82–8,11) |
| 7 days | 2,08 (1,08–3,09) | 2,35 (1,34–3,36) |
| 45 days | 3,50 (1,61–5,40) | 5,89 (1,90–9,88) |
| Calprotectin (μg/μl)* | 0,11 (0,075–0,14) | 0,12 (0,086–0,15) |
| 7 days | 0,24 (0,14–0,33) | 0,21 (0,14–0,28) |
| 45 days* | 0,20 (0,12–0,27) | 0,23 (0,16–0,31) |
| Myeloperoxidase (μg/μl)* | 0,047 (0,029–0,064) | 0,075 (0,047–0,102) |
| 7 days | 0,082 (0,039–0,125) | 0,078 (0,04–0,11) |
| 45 days* | 0,099 (0,051–0,150) | 0,12 (0,06–0,18) |
N=21 #p<0.001;
p-values represent differences between test group and control group.
At baseline, statistically significant differences were detected for the μg/sample of Myeloperoxidase between the two groups (p<0.02). No other differences between groups were found.
Gingival crevicular fluid volume (GCF)
At 1-week, a tendency towards a decrease in GCF was observed in both groups (test group Tbaseline=74,09 (CI: 56,93–91,26) T7 days=32,82 (CI: 23,93–40,71) T45 days =33,38 (CI: 27,58–39,18); control group Tbaseline=70,95 (CI: 52,57–89,34) T7 days=34,45 (CI: 27,68–41,23) T45 days =38,36 (CI: 30,56–46,17)). Statistical significance was achieved at re-evaluation at 1-week and at 45 days post-instrumentation (p<0.001). However, no statistical significance was found for any re-evaluation for both groups.
Calprotectin
Significant reductions in μg/sample of calprotectin were found after 1-week (p<0.01) and an increase at 45 days was found in both groups (p<0.01). The concentration of calprotectin elevated after 7 days (p<0.001). There was no evidence for difference for concentrations and in μg/sample between test and control group.
Myeloperoxidase
Significant reductions in μg/sample of MPO were found after 1-week (p<0.01) and an increase at 45 days was found in both groups (p<0.01). The concentration of MPO elevated after 7 and 45 days (p<0.001). There was no evidence for difference for concentrations and in μg/sample between test and control group.
Discussion
The aim of this study was to evaluate whether the application of amino acids and sodium hyaluronate gel could enhance the results obtained by subgingival ultrasonic instrumentation only in patients with chronic periodontitis.
In this study, ultrasonic periodontal debridement with and without amino acids and sodium hyaluronate gel resulted in an improvement in all clinical parameters. These results are consistent with those of Badersten et colleagues (33) who stated that nonsurgical periodontal therapy is effective in improving periodontal clinical parameters.
In the present investigation, the use of amino acids and sodium hyaluronate gel in adjunction to subgingival debridement in test sites has shown statistically significant reduction of bleeding and probing depth, while no statistically significant difference between the two treatment groups was observed regarding plaque index score (PI), clinical attachment level (CAL) and crevicular fluid volume.
From a clinical point of view, probing pocket depth and bleeding reduction exerts a positive influence in the prognosis of a dental element by decreasing specific risk parameters. Nevertheless, amino acids and sodium hyaluronate gel addition could not outcome in a complete elimination of periodontal pockets, leading the clinician to perform corrective surgical therapy.
The use of HA has shown statistically significant improvement of clinical parameters in the treatment of gingivitis (15) and accelerates periodontal wound healing (18). Besides, the topical application of hyaluronic acid in combination with non-surgical periodontal treatment might be useful in reducing bleeding and probing pocket depth, owing to its properties of increasing the extent of fibrin polymerization, stabilizing the clot and exerting antiexudative activity (34,35).
Conversely, from a biochemical perspective there was no evidence for any Aminogam-related difference in calprotectin levels and peroxidase activity in crevicular fluid. Previous data reported by Engström and colleagues also failed to demonstrate the anti-inflammatory effect of HA in relation to immunological (PGE2, IgG, C3) responses in gingival crevicular fluid (9). Gingival crevicular fluid MPO and calprotectin are directly related with both the extent of polymorphonuclear neutrophils (PMNs) infiltration and the severity of periodontal disease (8). HA modulation of PMNs function in vitro and in vivo is still controversial, since both stimulating (36,37,38) and inhibiting activity (39,40) have been reported. Our findings reported that concentrations of myeloperoxidase and calprotectin did not change during the time of treatment, indicating that the hyaluronic acid does not significantly affect the activity and recruitment of crevicular neutrophils in the inflammatory response as recently reported for human isolated PMNs exposed to a hyaluronate-carboxymethylcellulose membrane (Seprafilm).
Recently it has been reported that hyaluronan plays an important role as moderator of inflammation and it also functions in the negative feedback loop of inflammatory activation, through interacting with fibroblasts’s TSG6 (14). On this basis further investigations should be performed to analyze the effect of hyaluronic acid and amino acids on the activity of cells involved in the healing process, through exploring inflammatory markers, such as metalloproteinases.
Acknowledgments
The authors declare that they have no conflicts of interests. The study was self-supported by Department of Clinical Medical Sciences, Surgical and Health of University of Trieste. Company Errekappa Euroterapici Spa (Milan, Italy) provided free materials to be used in the study.
References
- 1.Tunkel J, Heinecke A, Flemmig TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):72–81. doi: 10.1034/j.1600-051x.29.s3.4.x. discussion 90–1. Review. [DOI] [PubMed] [Google Scholar]
- 2.Van der Weijden GA, Timmerman MF. A systematic review on the clinical efficacy of subgingival debridment in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:55–71. doi: 10.1034/j.1600-051x.29.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Wennström JL, Tomasi C, Bertelle A, Dellasega E. Full-mouth ultrasonic debridement versus quadrant scaling and root planing as an initial approach in the treatment of chronic periodontitis. J Clin Periodontol. 2005 Aug;32(8):851–9. doi: 10.1111/j.1600-051X.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- 4.Sherman PR, Hutchens LH, Jewson LG, Moriarty JM, Greco GW, McFall WT. The effectiveness of subgingival scaling and root planning. I. Clinical detection of residual calculus. J Periodontol. 1990;61:3–8. doi: 10.1902/jop.1990.61.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Kaner D, Bernimoulin JP, Hopfenmüller W, Kleber BM, Friedmann A. Controlled-delivery chlorhexidine chip versus amoxicillin/metronidazole as adjunctive antimicrobial therapy for generalized aggressive periodontitis: a randomized controlled clinical trial. J Clin Periodontol. 2007 Oct;34(10):880–91. doi: 10.1111/j.1600-051X.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Cruz J, Hujoel PP, Maupome G, Saver B. Systemic antibiotics and tooth loss in periodontal disease. J Dent Res. 2008 Sep;87(9):871–6. doi: 10.1177/154405910808700916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennström JL, Newman HN, MacNeil SR, Killoy WJ, Griffith GS, Gillam DG, Krok L, Needleman IG, Weiss G, Garrett S. Utilisation of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multicentre trial of 2 treatment approaches. J Clin Periodontol. 2001;28:753–761. doi: 10.1034/j.1600-051x.2001.280806.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaner D, Bernimoulin JP, Kleber BM, Heizmann WR, Friedmann A. Gingival crevicular fluid levels of calprotectin and myeloperoxidase during therapy for generalized aggressive periodontitis. J Periodontal Res. 2006 Apr;41(2):132–9. doi: 10.1111/j.1600-0765.2005.00849.x. [DOI] [PubMed] [Google Scholar]
- 9.Engström PE, Shi XQ, Tronje G, Larsson A, Welander U, Frithiof, Engstrom GN. The effect of hyaluronan on bone and soft tissue and immune response in wound healing. J Periodontol. 2001 Sep;72(9):1192–200. doi: 10.1902/jop.2000.72.9.1192. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Höfling K, Fimmers R, Frentzen M, Jervøe-Storm PM. Clinical and microbiological effects of topical subgingival application of hyaluronic acid gel adjunctive to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2004 Aug;75(8):1114–8. doi: 10.1902/jop.2004.75.8.1114. [DOI] [PubMed] [Google Scholar]
- 11.Mesa FL, Aneiros J, Cabrera A, Bravo M, Caballero T, Revelles F, del Moral RG, O’Valle F. Antiproliferative effect of topic hyaluronic acid gel. Study in gingival biopsies of patients with periodontal disease. Histol Histopathol. 2002;17(3):747–53. doi: 10.14670/HH-17.747. [DOI] [PubMed] [Google Scholar]
- 12.Giannobile WV, Riviere GR, Gorski JP, Tira DE, Cobb CM. Glycosaminoglycans and periodontal disease: analysis of GCF by safranin O. J Periodontol. 1993 Mar;64(3):186–90. doi: 10.1902/jop.1993.64.3.186. [DOI] [PubMed] [Google Scholar]
- 13.Laurent TC, Laurent UBG, Fraser JR. Functions of hyaluronan. Ann Rheum Dis. 1995 May;54(5):429–32. doi: 10.1136/ard.54.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999 Mar-Apr;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 15.Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003 Feb;30(2):159–64. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 16.Sapna N, Vandana KL. Evaluation of hyaluronan gel (Gengigel ®) as a topical applicant in the treatment of gingivitis. Journal of Investigative and Clinical Dentistry. 2011;2(3):162–170. doi: 10.1111/j.2041-1626.2011.00064.x. [DOI] [PubMed] [Google Scholar]
- 17.Pistorius A, Martin M, Willershausen B, Rockmann P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005 Jul-Aug;36(7–8):531–8. [PubMed] [Google Scholar]
- 18.Sukumar S, Drízhal I. Hyaluronic acid and periodontitis. Acta Medica (Hradec Kralove) 2007;50:225–8. [PubMed] [Google Scholar]
- 19.Johannsen A, Tellefsen M, Wikesjö U, Johannsen G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2009 Sep;80(9):1493–7. doi: 10.1902/jop.2009.090128. [DOI] [PubMed] [Google Scholar]
- 20.Favia G, Mariggio MA, Maiorano F, Cassano A, Capodiferro S, Ribatti D. Accelerated wound healing of oral soft tissues and angiogenic effect induced by a pool of aminoacids combined to sodium hyaluronate (AMINOGAM) J Biol Regul Homeost Agents. 2008 Apr-Jun;22(2):109–16. [PubMed] [Google Scholar]
- 21.Vanden Bogaerde L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: clinical report of 19 consecutive lesions. Int J Periodontics Restorative Dent. 2009 Jun;29(3):315–23. [PubMed] [Google Scholar]
- 22.Fawzy El-Sayed KM, Dahaba MA, Aboul-Ela S, Darhous MS. Local application of hyaluronan gel in conjunction with periodontal surgery: a randomized controlled trial. Clin Oral Investig. 2011 Oct 20; doi: 10.1007/s00784-011-0630-z. [DOI] [PubMed] [Google Scholar]
- 23.Kido J, Nakamura T, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease. J Clin Periodontol. 1999;26:653–7. doi: 10.1034/j.1600-051x.1999.261004.x. [DOI] [PubMed] [Google Scholar]
- 24.Dupont WD, Plummer WD. PS power and sample size program available for free on the Internet. Controlled Clin Trials. 1997;18:274. [Google Scholar]
- 25.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J. Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 26.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975 Dec;25(4):229–35. [PubMed] [Google Scholar]
- 27.Goodson JM. Gingival crevice fluid flow. Periodontol 2000. 2003;31:43–54. doi: 10.1034/j.1600-0757.2003.03104.x. Review. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Menegazzi R, Zabucchi G, Knowles A, Cramer R, Patriarca P. A new, one-step assay on whole cell suspensions for peroxidase secretion by human neutrophils and eosinophils. J Leukoc Biol. 1992 Dec;52(6):619–24. doi: 10.1002/jlb.52.6.619. [DOI] [PubMed] [Google Scholar]
- 30.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008 Aug;23:328–35. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabucchi G, Soranzo MR, Menegazzi R, Bertoncin P, Nardon E, Patriarca P. Uptake of human eosinophil peroxidase and myeloperoxidase by cells involved in the inflammatory process. J Histochem Cytochem. 1988;37:499–508. doi: 10.1177/37.4.2538504. [DOI] [PubMed] [Google Scholar]
- 32.Karhuvaara L, Tenovuo J, Sievers G. Crevicular fluid myeloperoxidase--an indicator of acute gingival inflammation. Proc Finn Dent Soc. 1990;86:3–8. [PubMed] [Google Scholar]
- 33.Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy.II Severely advanced periodontitis. J Clin Periodontol. 1984 Jan;11(1):63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 34.Weigel PH, Frost SJ, LeBoeuf RD, McGary CT. The specific interaction between fibrin(ogen) and hyaluronan: possible consequences in haemostasis, inflammation and wound healing. Ciba Found Symp. 1989;143:248–61. doi: 10.1002/9780470513774.ch15. discussion 261–4, 281–5. [DOI] [PubMed] [Google Scholar]
- 35.Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986 Mar 21;119:219–34. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- 36.Håkansson L, Hällgren R, Venge P, Artursson G, Vedung S. Hyaluronic acid stimulates neutrophil function in vitro and in vivo. A review of experimental results and a presentation of a preliminary clinical trial. Scand J Infect Dis Suppl. 1980;(Suppl 24):54–7. [PubMed] [Google Scholar]
- 37.Håkansson L, Hällgren R, Venge P. Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion, and metabolism. J Clin Invest. 1980 Aug;66(2):298–305. doi: 10.1172/JCI109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Håkansson L, Venge P. The molecular basis of the hyaluronic acid-mediated stimulation of granulocyte function. J Immunol. 1987 Jun 15;138(12):4347–52. [PubMed] [Google Scholar]
- 39.Tamoto K, Nochi H, Tada M, Shimada S, Mori Y, Kataoka S, Suzuki Y, Nakamura T. High-molecular-weight hyaluronic acids inhibit chemotaxis and phagocytosis but not lysosomal enzyme release induced by receptor-mediated stimulations in guinea pig phagocytes. Microbiol Immunol. 1994;38:73–80. doi: 10.1111/j.1348-0421.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 40.Tamoto K, Tada M, Shimada S, Nochi H, Mori Y. Effects of high-molecular-weight hyaluronates on the functions of guinea pig polymorphonuclear leukocytes. Semin Arthritis Rheum. 1993 Jun;22:4–8. doi: 10.1016/s0049-0172(10)80014-9. [DOI] [PubMed] [Google Scholar]