Abstract
Background
Translating Ribosome Affinity Purification (TRAP), a method recently developed to generate cell type-specific translational profiles, relies on creating transgenic lines of animals in which a tagged ribosomal protein is placed under regulatory control of a cell type-specific promoter. An antibody is then used to affinity purify the tagged ribosomes so that cell type-specific mRNAs can be isolated from whole tissue lysates.
Results
Here, cell type-specific transgenic lines were generated to enable TRAP studies for retinal ganglion cells and rod photoreceptors in the Xenopus laevis retina. Using real time quantitative PCR for assessing expression levels of cell type-specific mRNAs, the TRAP method was shown to selectively isolate mRNAs expressed in the targeted cell and was efficient at purifying mRNAs expressed at both high and low levels. Statistical measures used to distinguish cell type-specific RNAs from low level background and non-specific RNAs showed TRAP to be highly effective in Xenopus.
Conclusions
TRAP can be used to purify mRNAs expressed in rod photoreceptors and retinal ganglion cells in Xenopus laevis. The generated transgenic lines will enable numerous studies into the development, disease and injury of the Xenopus laevis retina.
Keywords: Xenopus laevis, Translating ribosome affinity purification, TRAP, retina, rod photoreceptor cells, retinal ganglion cells, cell type-specific transgenic lines
Introduction
The vertebrate retina is a highly ordered laminar structure with seven major cell types each having unique structure, function, development, and susceptibility to injury or disease. The structure and function of the retina is highly conserved across vertebrates, and the Xenopus laevis (X. laevis) retina has been a favored experimental system, particularly for studies of retinal development. A significant challenge for molecular studies of the retina, including those in X. laevis, is the ability to characterize the response of one cell type separate from the rest. Methods to isolate the response of individual cell classes typically rely on cell purification procedures such as fluorescence activated cell sorting or immunopanning. The major limitation of these approaches is that they introduce changes to the expression profile during the purification procedures (Emery and Barres, 2008). These methods are particularly problematic when trying to compare transcriptomes of animals under different conditions or treatments because cell purifications increase the variability between samples that can, in turn, mask emerging patterns. Recently, several methods have been developed to genetically target and isolate mRNAs undergoing protein translation by expressing a tagged ribosomal protein to isolate fully assembled ribosomes and their associated mRNAs (Doyle et al., 2008; Heiman et al., 2008; Halbeisen et al., 2009). Specifically, the Translating Ribosome Affinity Purification (TRAP) method uses a transgene encoding the enhanced green fluorescent protein (EGFP) fused to the ribosomal protein L10a (Rpl10a), a large ribosomal subunit component strategically chosen for its location at the ribosome surface (Heiman et al., 2008). The EGFP-Rpl10a fusion constructs can then be put under the control of cell type-specific regulatory sequences(Gong et al., 2010). To isolate mRNAs from a single cell type, the tissue of interest is dissected from the transgenic animals, and cycloheximide is added to arrest nascent mRNAs in the process of translational elongation. These ribosomes with their associated mRNAs are then affinity purified using an antibody to EGFP conjugated to magnetic beads. After removal of any unbound ribosomes, polysomal mRNAs are isolated and the mRNAs are further purified for analysis by microarray, quantitative real time PCR (qPCR), or high throughput sequencing. In principle, translating mRNAs for any specific cell type can be purified by the TRAP method provided an animal expressing a cell type-specific transgene can be engineered.
Since the 2008 description of TRAP, relatively few studies have used this method in mice (Doyle et al., 2008; Dougherty et al., 2010; Fomchenko et al., 2011; Okaty et al., 2011; Schmidt et al., 2012; Warner-Schmidt et al., 2012). The TRAP method also has been shown to be effective in Drosophila to purify cell type-specific mRNAs (Thomas et al, 2012). Recently, a modification of the TRAP method has been used in X. laevis to analyze mRNAs locally translated within retinal ganglion cell (RGC) axons (Yoon et al., 2012). Yoon et al. injected in vitro transcribed mRNA encoding the EGFP-Rpl10a fusion protein used in mice (Heiman et al., 2008) into individual blastomeres of developing X. laevis embryos, and then transplanted eye primordia from these animals into unlabeled hosts, before dissecting the brain hemispheres for analyses of locally translated mRNAs. While this study demonstrates that the TRAP methology works in X. laevis, the experimental protocol is technically demanding and can be used only in the rare settings where tissue transplantation can help to isolate specific mRNAs.
Here, restriction enzyme mediated integration was used to create X. laevis lines that stably express TRAP transgenes in RGCs and rod phototoreceptors (rods). By measuring transcripts expressed specifically in these cells, the TRAP method was shown to be highly efficient in isolating cell type-specific mRNAs expressed at both high and low levels. The high throughput and low cost of transgenesis, the large number of F1 progeny generated from a single mating, and the wealth of information about the X. laevis retina, make this an ideal system to exploit the capabilities of the TRAP method. These and future TRAP transgenic lines will enable molecular profiling studies of X. laevis retina structure and function, development, and disease.
Results
Generation of X. laevis lines with cell type-specific expression of TRAP transgenes
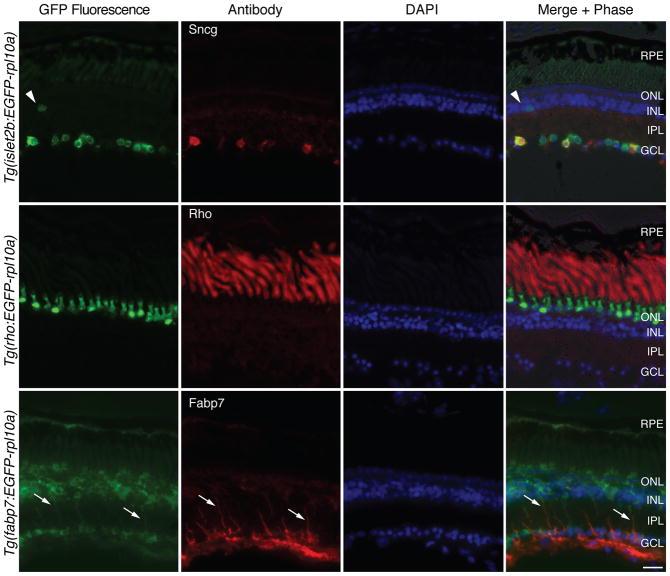
An optimal construct for carrying out TRAP studies in the X.laevis retina was determined to be a direct fusion of enhanced green fluorescent protein (EGFP) coding sequence, a linker sequence [2x SGGGG], and the full coding sequence of X. laevis L10a gene (see methods). This EGFP-rpl10a cDNA was placed behind three upstream regulatory control sequences: 17.5 kb from the zebrafish islet2b gene (isl2b; (Pittman et al., 2008)), 1.3 kb from the X. laevis rhodopsin gene (xop (Zhang et al., 2008), here referred to as rho), or 1.7 kb from the X. tropicalis fattyacid binding protein 7 gene (fabp7, also named basic lipid binding protein or blbp), to drive expression in X. laevis RGCs, rods, and Müller cells, respectively. The resulting transgenic F0 tadpoles were screened for EGFP expression using an epifluorescence stereomicroscope and grown to sexual maturity. These transgenic frogs were then mated to wildtype frogs. The resulting transgenic F1 progeny, as selected by EGFP fluorescence, were grown to the tadpole stage 57 (Nieuwkoop and Faber, 1994), and their retinas were examined for EGFP fluorescence together with antibody markers for RGCs, rods and Müller cells. In retina sections from Tg(isl2b:EGFP-rpl10a) embryos, EGFP fluorescence was confined to the ganglion cell layer (GCL) with an occasional cell in the innernuclear layer (INL), presumed to be a displaced RGC (arrowhead, Fig. 1, Tg(isl2b:EGFP-rpl10a)). Labeling with an antibody to γ-synuclein (sncg), a gene expressed in most or all RGCs of multiple mammals (Surgucheva et al., 2002; Mu et al., 2004; Soto et al., 2010)confirmed EGFP-Rpl10a to be expressed within RGC somata. Two of three Tg(isl2b:EGFP-rpl10a) lines showed nearly identical expression. A third line with very high expression of the transgene in RGCs also had low-level expression in the outer nuclear layer (ONL) and was discarded. In retinas from tadpoles expressing the EGFP-rpl10a transgene under the control of the rho upstream sequences, the EGFP-Rpl10a fusion protein localized in the outer nuclear layer (ONL), the location of photoreceptor cells (Fig. 1, Tg(rho:EGFP-rpl10a)). Labeling with a rhodopsin antibody showed that the transgene was expressed only in ONL cells that are rods and was not expressed in cone photoreceptors. Three different lines with the Tg(rho:EGFP-rpl10a) transgene showed rod-specific expression, though their expression differed in intensity. The expression of the EGFP-rpl10a transgene under regulatory control of the fabp7 upstream sequences was expected to occur exclusively in Müller cells. However, by comparison to immunostaining using a Müller cell-specific anti-Fabp7 antibody, the Tg(fabp7:EGFP-rpl10a) transgene expression was highest in Müller cells but also occurred in other retinal cells in all of six different Tg(fabp7:EGFP-rpl10a) frog lines tested. Thus, the Tg(fabp7:EGFP-rpl10a) frog lines were used only as a reference for the two other lines, Tg(isl2b:EGFP-rpl10a) and Tg(rho:EGFP-rpl10a), which did have cell type-specific expression.
Figure 1. X. laevis lines have specific expression of EGFP-Rpl10a in rods or RGCs.
Representative images show GFP fluorescence of the EGFP-rpl10a transgene in retinal sections of stage 57 F1 progeny (green; left column) from lines expressing the EGFP-rpl10a cDNA under the control of zebrafish islet2b (isl2b), X. laevis rhodopsin (rho), or X. tropicalis fatty acid binding protein 7(fabp7) upstream regulatory sequences. Immunostaining using cell type-specific antibodies (red; second column) specific for RGCs (Sncg), rods (Rho), and Müller cells (Fabp7) confirms cell type-specificity of the Tg(rho:EGFP-rpl10a) and Tg(isl2b:EGFP-rpl10a) lines but not Tg(fabp7:EGFP-rpl10a) line. DAPI shows the nuclear cell layers (blue; 3rd column). Arrowhead points to a presumed displaced RGC. Arrows point to Müller cell processes. RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 20 μm.
To determine whether the transgenic animals expressed the expected protein products, Western blotting of whole eye lysates from F1 embryos expressing the EGFP-Rpl10a fusion protein and from F1 embryos expressing a cytoplasmic GFP transgene (GFP-cyto), both under regulatory control of the RGC-specific isl2b promoter, were analyzed. Antibodies against the GFP variants confirmed that the Tg(isl2b:GFP) and Tg(isl2b:EGFP-rpl10a) transgenes each produced only one major protein product of the expected size, 27 kD and 56 kD, respectively (Fig. 2A). To confirm the ribosomal localization of the transgenic EGFP-Rpl10a protein, confocal microscopy was used to determine the distribution of the EGFP-Rpl10a protein within rods and RGCs (Fig. 2B–C). In rods, the EGFP-Rpl10a protein localized primarily to the photoreceptor inner segment, the main site of protein translation, but also to nucleoli, site of ribosome assembly (arrowhead, Fig. 2B). Similarly, in RGCs the EGFP-Rpl10a protein localized to the cytoplasm, where most translation occurs, as well as to the nucleoli (Fig. 2C).
Figure 2. EGFP-Rpl10a protein localizes properly and can be used to selectively enrich for RNAs.
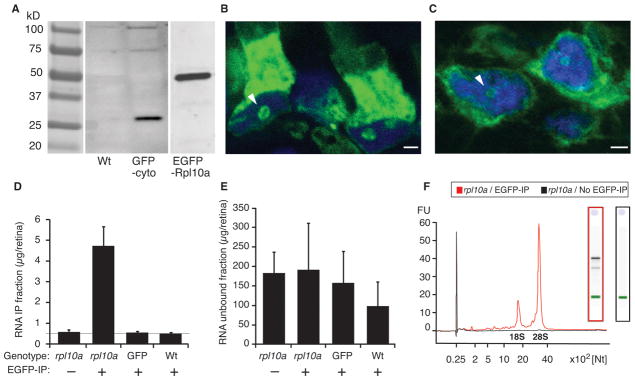
(A) Western analysis of whole eye lysates from transgenic tadpoles expressing cytoplasmic GFP (GFP-cyto) and EGFP-Rpl10a, both under control of the same islet2b regulatory sequence, and from wildtype control tadpoles (Wt) show transgene protein products of the expected size. (B) Representative image shows a 2D projection of a series of optical sections (Z-stack) showing nucleolar and inner segment localization of the EGFP-Rpl10a protein in rods. (C) Representative image shows a 2D projection of a Z-stack showing nucleolar and cytoplasmic localization of the transgenic EGFP-Rpl10a protein in RGCs. (D) RNA recovery from unconjugated beads (-) or EGFP-coated beads (+) using lysates of Tg(isl2b:EGFP-Rpl10a) (L10a), Tg(isl2b:GFP) (GFP-cyto), and from non-transgenic wildtype (Wt) retinas, show significant RNA recovery only in Tg(isl2b:EGFP-Rpl10a) lysates in the presence of the EGFP antibody. Grey horizontal line shows the 0.5 ng limit of detection of the bioanalyzer. (E) The residual unbound RNA fraction from the samples shown in D was comparable in all samples. Data in D and E represent the mean RNA levels from 12 retinas averaged for three replicates, and statistical significance between individual samples was established by pair-wise ANOVA comparisons (*** P < 0.001). (F) Representative superimposed electropherogram traces from TRAP EGFP-rpl10a transgenic samples affinity purified with EGFP conjugated beads (red trace) and unconjugated beads (black trace) show high abundance 18S and 28S ribosomal RNAs and lower abundance mRNAs only in EGFP-rpl10a samples extracted using EGFP-conjugated beads. Right insets show corresponding RNA gels for each of the traces. Arrowheads in B and C point to nucleoli. Scale bars = 2 μm.
To determine whether the tagged ribosomes could be used to selectively enrich for translating mRNAs, retina extracts from tadpoles expressing the EGFP-rpl10a or GFP cDNAs under the control of the isl2b upstream sequences and from wildtype control embryos were subjected to affinity purification using magnetic beads coated with anti-EGFP antibodies. Following the affinity purification, RNA was purified from both the bead-bound IP fraction and the unbound total fraction (Fig. 2D). Control unconjugated beads failed to pull down any significant amount of RNA. Amongst the EGFP-conjugated bead-bound fractions, only retinas from EGFP-rpl10a embryos yielded significant amounts of mRNA (one way ANOVA; p < 0.001). As expected, the RNA extracted from the residual unbound total fractions were variable and exceeded the immunoprecipitated amounts, but showed no significant difference between the groups (one way-ANOVA; p > 0.5) (Fig. 2E). Confirming a selective pull-down of mRNAs associated with transgenic ribosomes, sharp ribosomal 28S and 18S RNA peaks and lower molecular weight mRNAs were only detected in the case where EGFP-conjugated beads were used on lysates from Tg(isl2b:EGFP-rpl10a) retinas (Fig. 2F). No TRAPed RNAs were detectable in the control samples (Fig. 2F).
TRAP mRNAs accurately represent expression profiles of individual cell types
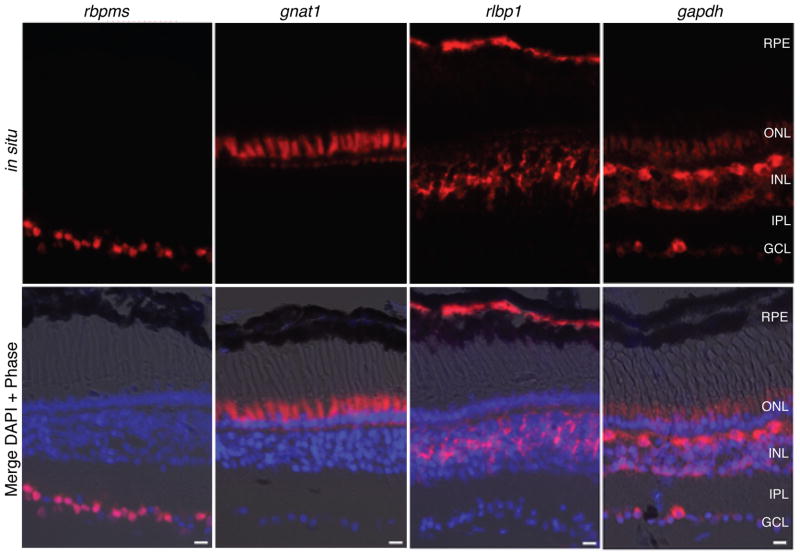
Four genes known or presumed to be expressed at low, middle or high levels were selected to represent each of three cell types (RGCs, rods and Müller cells): sncg, rbpms (a.k.a. hermes), brn3d (a.k.a. pou4f1.2), and barhl2 (a.k.a. xbh1) for RGCs (Gerber et al., 1999; Patterson et al., 2000; Hutcheson and Vetter, 2001; Agathocleous et al., 2009), rho, gnat1, pde6a and crx for rods (Batni et al., 1996; Knox et al., 1998; Calvert et al., 2000; Viczian et al., 2003; Muradov et al., 2010), and rlbp1, vim, fabp7 and notch1 for Müller cells (Dent et al., 1989; Coffman et al., 1990; Dorsky et al., 1995). Most of these genes are expected to be expressed in only one cell type, whereas others such as barhl2 and crx are highest in one cell type but also expressed in other cell types (Patterson et al., 2000; Viczian et al., 2003; data not shown Fig. 3S). In addition, a set of three genes (gapdh, rpl6, rpl10a) presumed to have broad expression in all retinal cell types and typically used as reference genes (Kusner et al., 2004; Chen et al., 2008; Roesch et al., 2008)were also selected. To confirm the expression levels and the cell type-specificity of the genes, all 15 genes were analyzed by in situ hybridization (ISH) using retina sections from wildtype stage 57 tadpoles. Representative images of the mRNA expression pattern detected by ISH for three genes (rbpms, gnat1 and rlpb1) exemplify the cell type-specificity in RGCs, rods and Müller cells, respectively (Fig. 3). All twelve genes chosen for their cell type-specific expression were confirmed as specific or highly enriched in the expected cell type by ISH or immunostaining (Figs. 1 and 3, and data not shown Fig. 3S). Surprisingly, the genes presumed to be ubiquitous were broadly expressed but revealed differences in expression levels between different cell types. For instance, gapdh was expressed more highly in a subset of cells in the INL and GCL, presumed to be horizontal cells and a subset of RGCs, respectively, based on cell size and morphology, and was not observed in pigment epithelial cells (Fig. 3). The two other genes presumed to be ubiquitous (rpl6 and rpl10a) were expressed at comparable levels in differentiated retinal cells, but were expressed at higher levels in the undifferentiated cells of the ciliary marginal zone (data not shown Fig. 4S).
Figure 3. In situ hybridization shows expression patterns of a subset of the genes used to assess cell-type enrichment by TRAP.
Representative images of retina sections from wildtype stage 57 tadpoles show mRNA expression patterns for genes selectively expressed in RGCs, rods and Müller cells (rbpms, gnat1, rlpb1, respectively) and a gene expressed widely in multiple cell types (gapdh). RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar= 20 μm.
To determine whether the TRAP method provided effective enrichment of mRNAs from the targeted cell types, qPCR was used to quantify the expression of the described 15 genes in the mRNA eluted from the beads (TRAPed mRNA) obtained from the retinas of tadpoles expressing EGFP-rpl10a under control of the three regulatory sequences. Additionally, EGFP primers were used to assess transgene expression levels in the various transgenic lines. Changes in gene expression are typically assessed by measuring the genes of interest relative to a ubiquitously expressed control gene such as gapdh (relative qRT-PCR methods) (Pfaffl, 2001). However, since ISH revealed differences in expression between different cell types of all tested control genes, absolute qPCR methods were used to measure mRNA copy number based on standard curves generated for each of the 16 genes of interest (data not shown Fig. 2S).
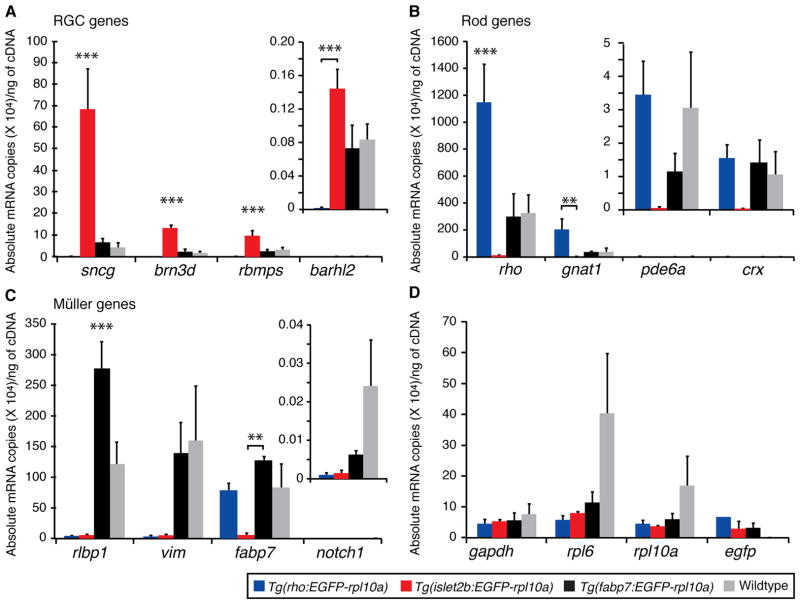
To determine whether the TRAP method could enrich for mRNAs from different cell types, the TRAPed mRNAs in each of the three transgenic genotypes (Tg(isl2b:EGFP-rpl10a), Tg(rho:EGFP-rpl10a) and Tg(fabp7:EGFP-rpl10a)) were compared to mRNA harvested from unbound fraction obtained from whole eye lysates (no transgene), and, are here presented in four graphs according to whether the genes were expected to be enriched in RGCs (Fig. 4A), rods (Fig. 4B), Müller cells (Fig. 4C), or were expected to be ubiquitous (Fig. 4D). These data represent averages from a minimum of three independent biological replicate experiments. For the chosen RGC-specific genes (Fig. 4A), there were highly significant differences among genotypes (p < 0.001). In pair–wise comparisons between individual genotypes, Tg(isl2b:EGFP-rpl10a) and Tg(rho:EGFP-rpl10a) were significantly different for all four RGC genes (Tukey test, p < 0.005). The degree of enrichment of genes between the Tg(isl2b:EGFP-rpl10a) and Tg(rho:EGFP-rpl10a) lines varied, with levels of RGC-specific genes expressed at high (sncg), moderate (brn3d; rbpms) and low (barhl2) levels showing enrichments of 350, 500, 87 and 87 fold, respectively (Fig. 4A). There was also a significant enrichment of these genes when comparing TRAPed mRNA from the Tg(isl2b:EGFP-rpl10a) lines to the two control samples expected to contain mRNA from all retina cell types, the TRAPed mRNAs from the Tg(fabp7:EGFP-rpl10a) lines and the unbound mRNA from whole eyes (Figure 4A; Tukey test, p < 0.05). The average enrichment in the Tg(isl2b:EGFP-rpl10a) samples for brn3d, sncg, and rbpms was 73, 15 and 3 fold relative to the Tg(fabp7:EGFP-rpl10a) samples, and 58, 10, and 4 fold relative to whole eye mRNA, respectively (Tukey test, p < 0.05). Barhl2, a gene with inherently low expression, showed the least enrichment between the Tg(isl2b:EGFP-rpl10a) and both control genes, 3 fold and 2 fold over Tg(fabp7:EGFP-rpl10a) TRAPed and whole eye mRNAs, respectively (Fig. 4A).
Figure 4. Lines expressing EGFP-rpl10a in rods and RGCs enable efficient enrichment of cell type-specific mRNAs.
Graphs show absolute expression levels of mRNA for affinity purified TRAPed mRNAs from transgenic lines (blue=Tg(rho:EGFP-Rpl10a); red=Tg(isl2b:EGFP-Rpl10a); black=Tg(fabp7:EGFP-Rpl10a)) and from the unbound fraction of wildtype control eyes (grey=Wildtype). To determine the gene expression copy number, a standard curve was generated for each of the genes tested by qPCR (data not shown Fig. 2S). Due to large differences in absolute gene expression values, genes expressed at low levels require a different y-axis scale (see inset bar graphs for barhl2 (A), pde6a and crx (B), and notch1 (C)). Asterisks reflect the level of significance for individual pair-wise comparisons between the marked genotype and either one other genotype (bracket) or all other genotypes (Tukey test; *p<0.05; ** p<0.005; ***p<0.001).
For genes selected for their expression in rods (Fig. 4B), overall, there were significant differences among genotypes (p < 0.005). As expected, enrichment was largest in comparing the Tg(rho:EGFP-rpl10a) lines relative to the Tg(isl2b:EGFP-rpl10a) lines. In pair-wise comparisons among genotypes of genes expressed at high (rho) or moderate (gnat1) levels, there was 100 and 95 fold enrichment, respectively (Tukey test, p < 0.05). For genes expressed at low levels (pde6a and crx), the magnitude of the enrichment was less, 48 and 41 fold (Figure 4B). While enrichment was also observed in rod-specific genes when comparing Tg(rho:EGFP-rpl10a) TRAPed mRNA to Tg(fabp7:EGFP-rpl10a) TRAPed mRNA and unbound whole retina mRNA, the magnitude of this enrichment was considerably less and did not reach statistical significance.
Overall, significant differences among genotypes were also seen in Müller cell-specific genes (p < 0.005) (Fig. 4C). Pair-wise comparison of the Müller cell-specific gene expressed at highest levels (rlbp1) was enriched in the Tg(fabp7:EGFP-rpl10a) TRAPed mRNAs relative to that of unbound whole eye mRNA, confirming that the Tg(fabp7:EGFP-rpl10a) transgene is expressed at higher levels in Müller cells relative to other retinal cells (Tukey test, p < 0.05). With the additional exception of fabp7 expression in RGCs (pair-wise comparison of Tg(fabp7:EGFP-rpl10a) and Tg(isl2b:EGFP-rpl10a); Tukey test, p < 0.05), Müller cell genes were selectively under represented in the TRAPed mRNAs from rho-and isl2b-driven EGFP-rpl10a lines (Fig. 4C). As expected, control genes were found in both the Tg(rho:EGFP-rpl10a) and Tg(isl2b:EGFP-rpl10a) TRAPed mRNAs at comparable levels (Fig. 4D; p > 0.78). Since among the mRNAs translated in the transgenic animals were the EGFP-rpl10a transgenes themselves, their level of expression was also assessed. While a trend indicating higher EGFP-rpl10a mRNA expression levels in rod-specific lines was observed, the level of expression was generally comparable in the different EGFP-rpl10a lines (Fig. 4D).
As a means of assessing the efficiency of TRAP in the X. laevis retina, two independent metrics previously used to asses TRAP efficiency in mice (Dougherty et al., 2010) were calculated. First, the ratio of affinity purified TRAPed RNAs (IP) relative to the unbound fraction of RNA present in the whole eyes from wild type samples (TotalWt) were determined for each genotype (Fig. 5A–B). Plots showing the IP/TotalWt ratio confirmed that both the Tg(isl2b:EGFP-rpl10a) (Fig. 5A) and Tg(rho:EGFP-rpl10a) (Fig. 5B) lines show cell type-specificity in RGC genes and rod genes, respectively. Second, a specificity index (SI), which rank-orders IP ratios between genotypes, was also calculated. In this analysis, 50 sets of permuted non-rank ordered SI data were compared to the normalized and rank-ordered data (according to http://www.bactrap.org/downloads/Specificity.r). The distributions of these data were then compared to determine the probability that a particular gene was present in the population above chance. As evidenced by the clustering of SIs in RGC and rod genes (Fig. 5C), this second statistical measure also identified these genes as being cell type-specific. In addition, the Müller cell gene rlbp1 was also shown to be enriched in the Tg(fabp7:EGFP-rpl10a) TRAPed mRNA (Fig. 5C).
Figure 5. The IP/Total ratio and Specificity Index (SI) provide independent measures to identify genes enriched above background levels in different cell types.
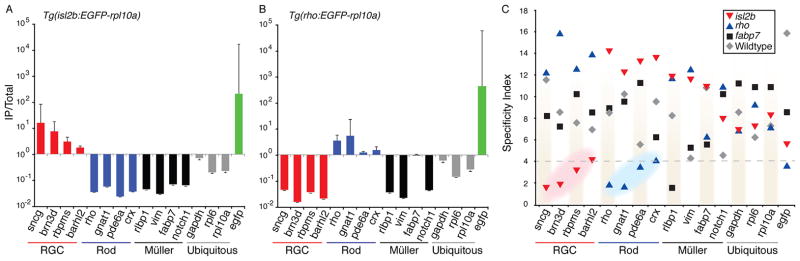
(A, B) Data for each of three biological replicates are plotted as the ratio of the absolute expression level for the IP for Tg(isl2b:EGFP-Rpl10a) (A) and Tg(rho:EGFP-Rpl10a) (B) TRAPed mRNAs over the absolute expression level for RNAs purified from total unbound fraction from a reference wildtype sample (Totalwt) for each gene. Standard error of the mean (error bars) was calculated based on the standard deviation for a ratio as described in the methods. (C) Scatter plot of the SI for all cell types plotted against individual genes shows a clustering of cell type-specific enrichment for RGCs (red highlighted cluster) and rod phototoreceptor cells (blue highlighted cluster). Genotypes on graph are as indicated isl2b = Tg(isl2b:EGFP-rpl10a); rho = Tg(rho:EGFP-rpl10a); fabp7 = Tg(fabp7:EGFP-rpl10a). P-values are significant at an SI less than 4 (dashed line, P < 0.1).
To determine the line-to-line reproducibility of the TRAP method in the X. laevis retina, two additional lines expressing each of Tg(rho:EGFP-rpl10a) and Tg(fabp7:EGFP-rpl10a) transgenes and one additional line expressing the Tg(isl2b:EGFP-rpl10a) transgene were also examined by qPCR. These data, comprising multiple replicates of individual lines and multiple lines of the same genotype, were examined using a principal components analysis (PCA). In plotting the compound data (Fig. 6A), the first principal component plotted along the X-axis (PC1) showed that the highest variation through the data was largely attributable to genotype. On one end of PC1, all Tg(isl2b:EGFP-rpl10a) samples, including data from two different transgenic lines, formed a distinct cluster (Fig. 6A; red inverted triangles), while all the Tg(rho:EGFP-rpl10a) samples, generated from three different transgenic lines, formed a second cluster at the opposite end of the axis (Fig. 6A; blue triangles). Both the whole eye mRNAs (Fig. 6A; grey diamonds) and the Tg(fabp7:EGFP-rpl10a) TRAPed mRNAs (Fig. 6A; black squares) were virtually contiguous and showed little variation along the PC1 axis. The second principal component plotted along the Y-axis (PC2) showed significant variation among different biological replicates, including within the Tg(isl2b:EGFP-rpl10a) and Tg(rho:EGFP-rpl10a) lines. A scree plot showed that the third component contributed significantly to the variance(data not shown Fig. 5S). Analysis of the PCA plot along the second and third dimensions (data not shown Fig. 5S) also showed separation of the two cell type-specific groups (RGCs and rods) and an overlap between the third group (Tg(fabp7;EGFP-rpl10a)) and the wild type samples.
Figure 6. TRAP provides cell-type enrichment for genes of different absolute expression levels and reproducibly across different transgenic lines in the X. laevis retina.
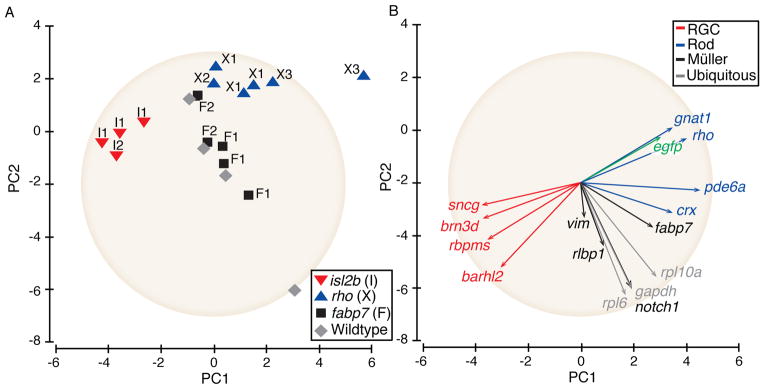
(A) PCA of individual samples showing qPCR-based expression levels from TRAPed replicates from the same GFP-rpl10a frog lines as well as from different lines. (B) PCA of the contribution of each of the 16 analyzed genes on the overall variance. The placement and length of the vectors show the contribution to the variance for each gene. The gene expression data have been standardized to a value of 1, demarked by the tan circle(see methods).
To assess the relative contribution of each gene to the variability in the data, individual gene data for all samples were analyzed in a second PCA plot, where vector length approximates the standard deviation and angles between the vectors approximate the degree of correlation between the genes (Greenacre, 2010). This analysis showed cell type-specific genes to be tightly clustered along PC1 with more variability along PC2, and that genes expressed at both high and low levels contributed nearly equally to the separation of different genotypes along PC1. Overall, PCA analysis demonstrated that TRAP in the X. laevis retina shows low variability both within individual replicates for a given line and between different lines.
Discussion
Xenopus laevis lines have been created which enable efficient translational profiling for RGCs and rods. The Tg(isl2b:EGFP-rpl10a) and the Tg(rho:EGFP-rpl10a) transgenes used in this study showed expression patterns exclusive to RGCs and rods, respectively (Fig. 1). Lines intended to be specific to Müller cells based on the fabp7 promoter showed highest expression in Müller cells but also lower expression in other cell types. In one discarded line for the Tg(isl2b:EGFP-rpl10a) transgene, tadpoles showed the expected expression in RGCs but also additional expression in cells located in the ONL. Occasional transgene misexpression in other than the intended cells has also been observed in bacTRAP mouse lines (Dougherty et al., 2010). Because of these examples, future use of the TRAP method in X. laevis or any other model organism should confirm the specificity of the EGFP-rpl10a expression before translational profiling, especially when using promoters for genes that exhibit dynamic expression patterns. There were also differences in the expression level of the EGFP-rpl10a transgene between the three Tg(rho:EGFP-rpl10a) frog lines studied, as observed by the relative brightness of the transgene in the F1 progeny. Such variability in expression was expected, and likely depends on the integration site and copy number. Nonetheless, the level of expression of the EGFP-rpl10a transgene per cell must be considered as a variable in the translational profiling of different cell types, as this expression level can contribute to the experimental variability as measured by PCA (Fig. 6B). One important consideration in the choice of promoters for TRAP studies is that they should express at sufficiently high levels to ensure that a significant proportion of translating ribosomes incorporate a tagged Rpl10a protein. The fraction of Rpl10a that was of transgenic origin within ribosomes could not here be measured directly because of the lack of suitable antibodies for the detection on non-transgenic Rpl10a in X. laevis. However, comparison of egfp and rpl10a mRNAs in both transgenic and control animals demonstrates both that the EGFP-rpl10a transgene was expressed at levels comparable to the endogenous rpl10a and that the EGFP-rpl10a transgene did not alter significantly the overall rpl10a amount, at least at the level of mRNA (Figure 4D).
As a means of assessing the ability of the TRAP method to select for cell type-specific translating mRNAs in the X. laevis retina, mRNAs purified from lines expressing EGFP-rpl10a under the control of three regulatory sequences were analyzed by qPCR for cell type-specific genes. Comparison between the two cell type-specific (RGC and rod) lines expressing the EGFP-rpl10a transgene showed robust enrichment for cell type-specific genes, demonstrating TRAP to be highly efficient in the X. laevis retina. While the magnitude of the enrichment varied according to the individual genes examined, and whether the comparison was made between two cell types or between one cell type and whole retina mRNA, the enrichment between genotypes was maintained regardless of whether the genes were expressed at high, middle or low levels. As might be expected, genes expressed at high levels in specific cells, such as sncg in RGCs and rho in rods, provided the biggest differences between cell types. However, because of their high expression, they also might be expected to contribute more to variability both within and between lines expressing the same transgene. A rough estimate of TRAP sensitivity in the X. laevis retina can be calculated. Stage 58 retinas have approximately 25,000 RGCs (Wilson, 1970) and comparable numbers of rods (Wilhelm and Gabriel, 1999). Based on these estimates and the measured expression levels (Figure 4A–B), the TRAP method was here shown capable of quantifying messages expressed at less than 10 copies per cell for genes such as rbpms and brn3d in RGCs, and pde6a and crx in rods. Since these estimates are approximate and assume full mRNA recovery, they certainly represent the upper limit of method sensitivity. Regardless, a combination of genes expressed at different levels is likely optimal to distinguish between cell types, and analysis of even a small number of such genes expressed at different levels will be sufficient to ensure the detection of cell type-specific signatures (Fig. 6A). The low inter-sample variability demonstrated here within and even between lines is essential to the success of future studies that use these lines to assess the effect of controlled modifications of experimental conditions on the translational profiles of individual cell types.
Any method designed to acquire a complete set of mRNA transcripts from a given cell type starting from heterogeneous tissues is likely to isolate some non-specific mRNAs as well. While the total amount of RNA purified from all control samples was negligible in terms of yield (Fig. 2), measurements by qPCR clearly demonstrate that mRNAs originating outside the cell of interest are recovered when using TRAP. Such contaminating mRNAs can be attributed to two main sources. The major source is likely to be mRNAs originating from cells other than those expressing the EGFP-rpl10a transgene that are nonetheless purified because of their overall high abundance in the tissue mRNA pool. For instance, rho, a gene expressed in rods at high levels, was also present in the mRNA purified from the Tg(isl2b:EGFP-rpl10a) lines, albeit at very low levels. The second source of non-specific mRNAs likely results from non-specific binding of free mRNAs or ribosomes to the magnetic beads. Both these non-specific signals were expected and have been reported by other authors (Heiman et al., 2008; Dougherty et al., 2010; Okaty et al., 2011), and statistical methods have been developed to differentiate signal from noise. Using two previously developed independent measures (Dougherty et al., 2010), TRAP in the X. laevis retina was shown to be highly efficient at providing cell type-specific signals above background levels. The two such statistical measures, IP/totalWt ratio and the SI, correctly identified the RGC and rod genes as being enriched in the Tg(isl2b:EGFP-rpl10a) and Tg(rho:EGFP-rpl10a) transgenic lines, respectively (Fig. 5). Some genes inherently expressed at low levels, such as barhl2 in RGCs and crx and pde6a in rods, were identified as being enriched using a mixed model comparison for each group (p < 0.05) but fell just outside the 10% confidence interval using the SI index. However, determining whether one statistical test is preferable over the other is complicated by the fact that both barhl2 and crx are expressed also in cell types in the inner nuclear layer (Patterson et al., 2000; Viczian et al., 2003; data not shown Fig. 3S).
In the current study, all genes examined by qPCR were also examined by ISH or immunostaining, and there was good correspondence between methods in terms of cell type-specificity. One surprise in the ISH analyses was that all three genes presumed to be ubiquitous showed variation in gene expression among the different retinal cell types, with some genes such as gapdh being expressed at higher levels in some cells and absent in others. In mammals as well, there has been reported variability in gapdh between different cell types and even differences between individuals (Barber et al., 2005). These results underscore the importance of choosing the proper method of qPCR data normalization. For comparative studies using the same cell type, relative qPCR methods can be used to compare expression levels using a reference gene such as gapdh only when there is solid evidence that the reference gene itself is not regulated (Thellin et al., 1999; Larionov et al., 2005). However, for comparative studies between different cell types, such as the one presented here, absolute qPCR methods in which the expression of each gene is determined relative to its own standard curve may be preferred.
There are a number of reasons that make the X. laevis retina a particularly attractive system for the use of the TRAP method. The generation of transgenic X. laevis is efficient and inexpensive. Individual frogs often produce hundreds to thousands of progeny in a single breeding. Most importantly, X. laevis is a well-established animal model for studies of the retina. One of the studies enabled by the TRAP method that may be of most general interest is a comprehensive molecular characterization of cell types in the retina. Such a characterization has been carried out in mammals using single cell isolation (Roesch et al., 2008), but this method is technically challenging, subject to high noise, and involves a cell purification step that can introduce changes in the transcriptomes (Okaty et al., 2011; Emery and Barres 2010). Another use of the TRAP method that may be of general interest is the study of how cell types change during development. Numerous other more specialized questions can also now be addressed through the TRAP method. For example, RNAs TRAPed from RGCs whose axon shave been crushed are being used to uncover novel transcripts important in the process of nerve regeneration, neural protection and survival. Second, mRNAs that are regulated by thyroid hormone in RGCs are being TRAPed to uncover cell-autonomous processes that underlie the dramatic optic nerve shortening that occurs at metamorphosis. Given the specificity of the islet2b promoter in the X. laevis central nervous system, isolating RNAs from not only the eyes, but also the optic nerves and optic tecta of Tg(isl2b:EGFP-rpl10a) frogs may be used in the future to selectively enrich for mRNAs expressed within RGC axons and nerve terminals, rather than their cell bodies. The ability to TRAP these locally expressed mRNAs may be particularly useful in uncovering novel mechanisms involved in axon growth, axon guidance, regeneration, resistance to axonal insults, and perhaps even synaptic plasticity (Deglincerti and Jaffrey, 2012; Jung et al 2012). The Tg(rho:EGFP-rpl10a) lines may be particularly useful in molecular studies of rod differentiation, and of the response of rods to light or transgene induced degeneration (Tam and Moritz, 2006; Zhang et al., 2008). The ability to measure transcriptomes for specific cell types will become powerful tools to address a wide variety of biological questions of relevance to the retina. Further, the TRAP method can be easily adapted for other tissues and other experimental animal models.
Experimental Procedures
TRAP transgenes
The X. laevis rpl10a coding sequence (Open Biosystems, IMAGE: 4684157) was amplified with primers acgcgaattcaggaggaggaagtagcaaagtgtctcgagatacc and aaggagtctagattagtacaggcgttgtggcttgcc and cloned into EcoRI and XbaI sites of pCS2:GFP* (Huang et al., 1999). Egfp was amplified with primers (aaggagggatccggcaccatggtgagcaagggcgagcagc and acgcgaattcttgtacagctcgtccatgccga) and replaced gfp between the BamHI and EcoRI sites, creating a construct pCS2:EGFP-rpl10a. A linker (a serine followed by four glycines, or SGGGG) was inserted between the gfp and rpl10a ORFs by ligating annealed oligonucleotides aattctggtggaggtggttctggtggtggg and aattcccaccaccagaaccacctccaccag into the EcoRI sites of pCS2:GFP*-rpl10a or pCS2:EGFP-rpl10a. Clones containing two SGGGG linkers were identified by sequencing. After evaluating the above clones for fluorescence intensity in transgenic X. laevis and the ability to immuno-precipitate mRNA (data not shown Fig. 1S), the pCS2:EGFP-rpl10a with two tandem SGGGG linkers was chosen as the one with which to make cell type-specific transgenes. The construct used to drive the TRAP transgene in rods, Tg(rho:EGFP-rpl10a), was made by replacing the CMV promoter with 1.3 kb of the X.laevis rhodopsin promoter(Zhang et al., 2008). To generate a construct to drive EGFP-rpl10a in Müller cells, the 1.7 kb fabp7 promoter was amplified from Xenopus tropicalis (X. tropicalis) liver DNA using primers (aaggaggtcgaccctatgaaagagtgtgttttactg and aaggagaagcttcacaaggcagtggaacagatc) and cloned into pCS2:GFP3 with SalI and HindIII, and then from there blunt-end subcloned to generate Tg(fabp7:EGFP-rpl10a). To generate a construct to drive EGFP-rpl10a in RGCs, first a construct to carry out recombineering (Chan et al., 2007)had to be generated, as the RGC-specific isl2b promoter (Pittman et al., 2008)is too large to manipulate by restriction enzyme based cloning. The generic targeting construct pCS2:GFP3-Frt-Kan-Frt was generated by subcloning into pCS2+GFP3 a cassette from pIGCN21 (gift from N. Jenkins, NCI-Frederick) containing the Kanamycin selection gene flanked by FRT sites. A 1 kb homology arm for the isl2b promoter was amplified and cloned into SalI and HindIII sites of pCS2:GFP3-Frt-Kan-Frt to facilitate future generation of Isl2b driven constructs. The GFP3 cassette was replaced by EGFP-rpl10a. The final construct, Tg(isl2b:EGFP-rpl10a), was made by transforming EL250 cells carrying the isl2b:EGFP construct using linear pCS2:1kbIsl2b:GFP3-Frt-Kan-Frt cut with AloI and SalI to prevent re-circularization. Recombineering was performed by heat induction of the RecBCD recombinase followed by arabinose-induced Flip recombinase-mediated removal of the Kanamycin cassette, essentially as described (Chan et al., 2007). For convention, all gene names and symbols conform to those adopted by Xenbase (www.xenbase.org) and transgenic construct names Tg(isl2b:EGFP-rpl10a), Tg(rho:EGFP-rpl10a), and Tg(fabp7:EGFP-rpl10a) conform to ZFIN zebrafish nomenclature guidelines(www.zfin.org). Frog lines were generated by REMI transgenesis (Kroll and Amaya, 1996)as previously described (Marsh-Armstrong et al., 1999a). All animal experiments were carried out using procedures approved by the Johns Hopkins University and Washington and Lee University IACUCs.
Immunostaining and in situ hybridization
Retinas from stage 55–62 tadpoles were fixed in 4% MEMPFA (0.1 MOPS (pH 7.4), 2 mM EGTA, 1 mM MgSO4, 4 % paraformadehyde)overnight at 4°C, infiltrated with 30% sucrose in PBS for 4 to 6 hours, cryo-embedded using Shandon M-1 embedding matrix (Thermo Scientific Inc.), stored at −80°C, and cryosectioned at 14 μm thickness. Antibody labeling and in situ hybridization were carried out as previously described (Zhang et al., 2008). Primary antibodies used were a monoclonal antibody to rhodopsin (1D4, gift of R.S. Molday, U. British Columbia, Vancouver; at 1:10 dilution), a polyclonal antibody to Fabp7(also known as Blbp; Abcam ab32423, at 1:1000 dilution), and an affinity purified rabbit polyclonal antibody generated against the 14 terminal amino acids (EATESTEQVGDGEN) of X. tropicalis γ-synuclein (Covance; at 1:20,000 dilution). Secondary antibodies conjugated to Alexa-488 and Cy3 (Life Technologies) were used at 1:1,000 dilutions. In situ hybridization was carried out with hydrolyzed digoxigenin labeled riboprobes transcribed from cDNAs (clones listed in Table 1), most of which were commercially available (Open Biosystems). Sub-cellular localization of the transgene protein product was determined using a laser scanning confocal microscope (FV1000; Olympus Microscopes).
Table 1. List of primers used for qPCR and IMAGE number of clones used to generate both riboprobes for ISH and standard curves for qPCR.
Table lists the full name of the genes, their symbol and the synonym used in referenced literature, the Genbank accession number, primers used for qPCR, and IMAGE clone number.
| Gene Identification | Gene symbol | Genbank | Forward Primer | Reverse Primer | IMAGE |
|---|---|---|---|---|---|
| glyceraldehyde 3-phosphate dehydrogenase | gapdh | U41753.1 | ctttgatgctgatgctggaa | gaagaggggttgacaggtga | 5571163 |
| ribosomal protein L6 | rpl6 | BC075222.1 | gagggcgagatctttgacac | ggcaagagctgagagtccac | 6958815 |
| ribosomal protein L10a | rpl10a | BC041308.1 | tcccttccttgctcactcat | ccaactgctacagccagaca | 4684157 |
| enhanced green fluorescent protein | Egfp | acgacggcaactacaagacc | gtcctccttgaagtcgatgc | * | |
| rhodopsin | Rho | BC054145.1 | caatgctcatgcggagtaga | tctgggtggtagcagattcc | 4740890 |
| cone-rod homeobox; orthodenticle homolog 5 | crx; otx5; xotx5b | BC084194.1 | ctatgcacccacagctctca | gtgaagccaagactggaagc | 5156432 |
| guanine nucleotide binding protein; alpha transducing activity polypeptide 1 | gnat1 | BC111509.1 | acatgcgacgagatgtcaag | cattgggtgatggggtaaag | 7019014 |
| phosphodiesterase 6A; cGMP-specific, rod, alpha | pde6a | CD302881.1 | gtgacctctcagccatagcc | tcctttccaaatcaccttgc | 6955150 |
| gamma synuclein; breast cancer- specific protein 1 | sncg; bcsg1 | BC054269.1 | aaccaaagaacaggccaatg | ggttcttctggctgatctgg | 6876189) |
| RNA binding protein with multiple splicing | rbpms; hermes | BC060391.1 | tccgttttgacccagagaac | aagtgctgggtggaagtttg | 4030673) |
| POU class 4 homeobox 1 | brn3d; pou4f1.2 | CF290338.1 | ttggccaatttgaagattcc | tcaggtttggccattttctc | 4970250 |
| BarH-like homeobox 2 | barhl2; xbh1 | BC087470.1 | ttagggatgtcagggctacg | atggacgctgtccactaacc | 3300989 |
| vimentin | vim | BC080051.1 | tcgccagcagtatgagaatg | gattagcagcttccgacagg | 7008042 |
| fatty acid binding protein 7, brain | fabp7; blbp | BC087470.1 | aggcaggttggaaatgtggac | tcatctgcagtggcttcatc | 7201992 |
| retinaldehyde binding protein 1 | rlbp1; cralbp | BC054209.1 | tgctggaaaacgaggagact | caccctctccagtagcttgc | 6878468 * |
| notch1 | notch1; xnotch1 | M33874.1 | acctgtccgcaaggatacac | aaacaccagtccagccactc | Ŧ |
Was first subcloned into pBluescript before probe making.
Clone, gift of W. Harris (U. Cambridge, U.K.) was used as in Marsh-Armstrong et al. (1999b).
Protein analysis
Whole eyes from stage 40–62 tadpoles were pooled and lysed in RIPA buffer (Sigma) with protease inhibitors (Roche Mini Complete, EDTA-Free) using a 0.5 ml Teflon-glass Potter-Elv tissue grinder, followed by cell disruption using a 30 gauge needle and syringe. Cellular debris was removed by collecting the supernatant following a 2,000 X g centrifugation for 10 minutes at 4°C, and protein concentration was determined by a colorometric assay (Pierce 660). Proteins were resolved on 12% polyacrylamide gels(BioRad), transferred to PVDF membranes (Millipore), incubated with primary antibodies that detect GFP variants (from Torrey Pines and Cell Signaling; each used at 1:1,000), followed by a HRP-conjugated secondary antibody (Pierce; 1:10,000 dilution), and visualized by a Immun-Star™ HRP chemiluminescent reagent (BioRad).
Translating Ribosome Purification
The original TRAP method, as described in Heiman et al. (Heiman et al., 2008)and http://www.bactrap.org/downloads.html, was modified for use in X. laevis retinas, as described. Between 10 and 15 stage 47–60 tadpoles (28 eyes) or 3–4 adult frogs (6 retinas) were used for each TRAP experiment. Freshly dissected eyes or retinas were placed in ice-cold lysis buffer (20 mM HEPES KOH (pH 7.4), 5 mM MgCl2, 150 mM KCl) to which was added freshly prepared 100 μg/ml cycloheximide, 0.5 mM DTT, protease inhibitors (Roche Mini Complete, EDTA-Free), and 40 U/ml recombinant RNasin (Promega). Samples were gently homogenized in a 1 ml glass-on-glass homogenizer (Wheaton) or a 0.5 ml Teflon-glass Potter-Elv tissue grinder (Corning; Thomas Scientific) containing a final volume of 350 μl lysis buffer, and then transferred into pre-chilled centrifuge tubes. To a post-nuclear supernatant, obtained by centrifugation at 4°C for 10 minutes at 2,000 × g., was added NP-40 for a final concentration of 1%, and 1,2-diheptanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) for a final concentration of 30 mM. After 5 minute incubation at 4°C, a post-mitochondrial supernatant was obtained by centrifugation at 4°C for 10 minutes at 20,000 × g. To this post-mitochondrial supernatant were added 50 μl freshly-prepared EGFP conjugated Dynal Protein G magnetic beads (Life Technologies) and 0.5 mM DTT and 100 μg/ml cycloheximide. These beads were freshly prepared by conjugating anti-EGFP antibodies (a total of 94 μl of Dynal Protein G beads per immunoprecipitation (IP) for a final concentration of 25 μg anti-EGFP antibodies using equal amounts of 19C8 and 19F7 anti-EGFP antibodies per IP (Memorial Sloan-Kettering Monoclonal Antibody Facility))to Dynal Protein G magnetic beads as previously described (Heiman et al., 2008). After a 30 minute incubation of tissue extracts with the EGFP conjugated beads, beads were precipitated with a DynaMag-2 magnet (Life Technologies) for 1 minute. The supernatant was saved as the unbound fraction. After four washes (20 mM HEPES-KOH (pH 7.4), 5 mM MgCl2, 350 mM KCl, 1% NP-40), beads were resuspended, the RNA was eluted by incubation in 350 μl RLT buffer with beta-mercaptoethanol from Qiagen’s Micro RNeasy kit (Qiagen), and the RNA was purified using the manufacturer instructions. Quantity and quality of the RNA was assessed by microfluidic analysis (Agilent Technologies’ Bioanalyzer 1200 picochip).
Quantitative PCR
RNA samples eluted and purified from the magnetic beads (IP fraction) and samples purified from the supernatants (unbound fractions) were reverse transcribed using oligo poly d(T)20 and the SuperScript III kit (Life Technologies) according to the manufacturer instructions, using no more than 50 μg of polysomal mRNA per sample. Real time quantitative PCR (qPCR) was carried out using SYBR-green compatible primers to amplify 90–200 bp amplicons using IQ SYBR green Supermix (Bio-Rad) on a CFX96 touch Real-Time PCR Detection System (Bio-Rad). Primers were designed using Primer 3 (http://frodo.wi.mit.edu/) and were selected based on their amplification efficiency and their ability to amplify a single dominant peak from a template-specific plasmid cDNA. To obtain absolute transcript levels, three-to eight-point standard curves ranging from 100 to 107 plasmid copies were generated for each of the 16 amplicons (data not shown Fig. 2S), using the same cDNA templates used to generate the riboprobes for in situ hybridization (Table 1). Technical replicates were pooled and averaged; few outliers were discarded. Then, to determine expression levels, three biological replicates were averaged.
Statistics
One-way ANOVA was used to compare TRAP RNA samples for each of three biological replicate experiments. A mixed model analysis was used to compare the qPCR-generated expression levels between the four genotypes (Tg(isl2b:EGFP-rpl10a), Tg(rho:EGFP-rpl10a), Tg(fabp7:EGFP-rpl10a) IP fractions, and the whole retina unbound fraction) within each set of genes expressed within a cell type. Individual frog lines were treated as a random effect in this analysis. Further comparison between individual genotypes for each gene was carried out using a two-way ANOVA (Tukey test). To assess enrichment of gene expression in specific cells within the context of non-specific background, ratios of affinity purified RNAs for a given genotype (IPx) over RNAs purified from total unbound fraction from a reference wildtype sample (Totalwt) were calculated. Standard deviation of the IPx/Totalwt ratio was calculated based on the variance for the ratio of two means using the Taylor expansion (Rice, 2007). Specificity index (SI) was calculated and a scatter plot was generated as previously described (Dougherty et al., 2010). To determine the major sources of variance when comparing replicates for the same lines as well as across multiple lines for the same genotypes, principal components analysis (PCA) was carried out. In order to generate the PCA and vector plots, data was first normalized to mean 0 and variance 1 using PRIMER (Clarke and Gorley, 2006). To facilitate interpretation of the PCA-vector biplot, figure 6 was split into figures 6a and 6b, wherein the tan circle provides a scale of normalized data with the outer perimeter equal to one.
Supplementary Material
Data show absolute number of gapdh transcript copies based on a standard curve.
Representative standard curves shown are based on three to eight-point standard curves ranging from 100 to 107 plasmid copies generated for each of the 16 amplicons.
Representative images from retina sections from wildtype stage 57 tadpoles show mRNA expression patterns for genes selectively expressed in RGCs (brn3d, barhl2), photoreceptors (rho; pde6a; crx) and Müller cells (vim; fabp7; notch1) and genes expressed widely in multiple cell types (rpl6; rpl10a). Note that barhl2 and crx are also expressed in INL cells. RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar= 20 μm.
Representative images show mRNA expression patterns for rpl6 and rpl10a in retina from stage 57 wildtype tadpoles. CMZ, ciliary marginal zone; RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar= 20 μm.
(A) A scree plot shows the loading values for each of the components superimposed against a broken stick diagram. Analysis of the first three components contribute significantly to the variance while the 4th component is equal to that of the broken stick value and does not contribute significantly towards the variance within the data. (B) A PCA-vector biplot of the 2nd and 3rd components show variability among the qPCR-based expression levels from TRAPed replicates from the same GFP-rpl10a frog lines as well as from different lines. The second (PCA2; X-axis) and third (PCA3; Y-axis) dimensions show separation of the two cell type-specific groups (RGCs and rods) and an overlap between the third group (Tg(fabp7;EGFP-rpl10a)) and the wildtype samples. Two outliers (one each from X3 (Tg(rho;EGFP-rpl10a)) and Wt) represent extreme variance that are maintained within the same groupings. These results recapitulate those shown in the PCA plot comparing the first two dimensions. PCA of the contribution of each of the 16 analyzed genes on the overall variance. The placement and length of the biplot vectors show the contribution to the variance for each gene. The gene expression data have been standardized to a value of 1, demarked by the tan circle (see methods).
Bullet points.
Xenopus laevis transgenic lines drive TRAP transgenes specifically in retinal ganglion cells and rod photoreceptor cells.
TRAP selectively purifies mRNAs from targeted cells.
Statistical tests demonstrate high efficiency of TRAP in the Xenopus laevis retina.
Acknowledgments
Authors thank R. Humston, D. Marsh, J. Pevsner and J. Stough for statistical consultation. Authors also thank M. Vetter for helpful comments on the manuscript. Work was supported by grants from the Foundation Fighting Blindness and NIH grant 1R01EY019960(NMA) and the Thomas F. and Kate Miller Jeffress Memorial Trust J-1026, NSF DBI-1126118, and the H. F. Lenfest Endowment for Faculty Support (FLW).
Grant Sponsor (grant number):
F.L. Watson: Thomas F. and Kate Miller Jeffress Memorial Trust (J-1026); NSF DBI (1126118); and the H. F. Lenfest Endowment for Faculty Support (425380/425382).
N. Marsh-Armstrong: Foundation Fighting Blindness (C-NP-0707-0418-JHU04) and 1R01EY019960.
References
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Batni S, Scalzetti L, Moody SA, Knox BE. Characterization of the Xenopus rhodopsin gene. J Biol Chem. 1996;271:3179–3186. doi: 10.1074/jbc.271.6.3179. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN, Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci US A. 2000;97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res. 2007;35:e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu M, Sezate SA, Matsumoto H, Ramsey M, McGinnis JF. Interaction of glyceraldehyde-3-phosphate dehydrogenase in the light-induced rod alpha-transducin translocation. J Neurochem. 2008;104:1280–1292. doi: 10.1111/j.1471-4159.2007.05081.x. [DOI] [PubMed] [Google Scholar]
- Clarke K, Gorley R. PRIMER v6: User Manual/Tutorial. PRIMER-E; Plymouth: 2006. [Google Scholar]
- Coffman C, Harris W, Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990;249:1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- Deglincerti A, Jaffrey S. Insights into the roles of local translation from the axonal transcriptome. Open Biology. 2012;2:e120079. doi: 10.1098/rsob.120079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989;105:61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Rapaport DH, Harris WA. Xotch inhibits cell differentiation in the Xenopus retina. Neuron. 1995;14:487–496. doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- Dougherty JD, Schmidt EF, Nakajima M, Heintz N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 2010;38:4218–4230. doi: 10.1093/nar/gkq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Barres BA. Unlocking CNS cell type heterogeneity. Cell. 2008;135:596–598. doi: 10.1016/j.cell.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Fomchenko EI, J Dougherty JD, Helmy KY, Katz AM, Pietras A Brennan C, Huse JT, Milosevic A, Holland EC. Recruited Cells Can Become Transformed and Overtake PDGF-Induced Murine Gliomas In Vivo during Tumor Progression. PLOS One. 2011;6:e20605. doi: 10.1371/journal.pone.0020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber WV, Yatskievych TA, Antin PB, Correia KM, Conlon RA, Krieg PA. The RNA-binding protein gene, hermes, is expressed at high levels in the developing heart. Mech Dev. 1999;80:77–86. doi: 10.1016/s0925-4773(98)00195-6. [DOI] [PubMed] [Google Scholar]
- Gong S, Kus L, Heintz N. Rapid bacterial artificial chromosome modification for large-scale mouse transgenesis. Nat Protoc. 2010;5:1678–1696. doi: 10.1038/nprot.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre M. Rubes Editorial, editor. Biplots in Practice Bilbao. Fundación BBVA; 2010. Multidimensional Scaling Biplots; Principal Component Analysis Biplots; p. 237. [Google Scholar]
- Halbeisen RE, Scherrer T, Gerber AP. Affinity purification of ribosomes to access the translatome. Methods. 2009;48:306–310. doi: 10.1016/j.ymeth.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Marsh-Armstrong N, Brown DD. Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proc Natl Acad Sci U S A. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Dev Biol. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox BE, Schlueter C, Sanger BM, Green CB, Besharse JC. Transgene expression in Xenopus rods. FEBS Lett. 1998;423:117–121. doi: 10.1016/s0014-5793(98)00018-0. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Kusner LL, Sarthy VP, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Muller cells. Invest Ophthalmol Vis Sci. 2004;45:1553–1561. [PubMed] [Google Scholar]
- Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Berry DL, Brown DD. Germ-line transmission of transgenes in Xenopus laevis. Proc Natl Acad Sci U S A. 1999a;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Lui TT, Brown DD. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type-III deiodinase. Neuron. 1999b;24:871–878. doi: 10.1016/s0896-6273(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Muradov H, Boyd KK, Artemyev NO. Rod phosphodiesterase-6 PDE6A and PDE6B subunits are enzymatically equivalent. J Biol Chem. 2010;285:39828–39834. doi: 10.1074/jbc.M110.170068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland Publishing Inc; 1994. [Google Scholar]
- Okaty BW, Sugino K, Nelson SB. Cell Type-Specific Transcriptomics in the Brain. J Neurosci. 2011;31(19):6939–6943. doi: 10.1523/JNEUROSCI.0626-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KD, Cleaver O, Gerber WV, White FG, Krieg PA. Distinct expression patterns for two Xenopus Bar homeobox genes. Dev Genes Evol. 2000;210:140–144. doi: 10.1007/s004270050020. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AJ, Law MY, Chien CB. Path finding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–2871. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JA. Mathematical Statistics and Data Analysis. University of California; Berkeley: 2007. Expected Values; p. 688. [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Identification of the Cortical Neurons that Mediate Antidepressant Responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci. 2010;52:434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgucheva I, McMahan B, Ahmed F, Tomarev S, Wax MB, Surguchov A. Synucleins in glaucoma: implication of gamma-synuclein in glaucomatous alterations in the optic nerve. J Neurosci Res. 2002;68:97–106. doi: 10.1002/jnr.10198. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:3234–3241. doi: 10.1167/iovs.06-0213. [DOI] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291–295. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Thomas A, Lee P-J, Dalton JE, Nomie KJ, Stoica L, Costa-Mattioli M, Chang P, Nuzhdin S, Arbeitman MN, Dierick HA. A Versatile Method for Cell-Specific Profiling of Translated mRNAs in Drosophila. PLOS One. 2012;7:e40276. doi: 10.1371/journal.pone.0040276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczian AS, Vignali R, Zuber ME, Barsacchi G, Harris WA. XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina. Development. 2003;130:1281–1294. doi: 10.1242/dev.00343. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Gabriel R. Functional anatomy of the photoreceptor and second-order cell mosaics in the retina of Xenopus laevis. Cell Tissue Res. 1999;297:35–46. doi: 10.1007/s004410051331. [DOI] [PubMed] [Google Scholar]
- Wilson MA. Optic nerve fibre counts and retinal ganglion cell counts during development of Xenopus laevis (Daudin) Q J Exp Physiol Cogn Med Sci. 1971;56:83–91. doi: 10.1113/expphysiol.1971.sp002110. [DOI] [PubMed] [Google Scholar]
- Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Oglesby E, Marsh-Armstrong N. Xenopus laevis P23H rhodopsin transgene causes rod photoreceptor degeneration that is more severe in the ventral retina and is modulated by light. Exp Eye Res. 2008;86:612–621. doi: 10.1016/j.exer.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data show absolute number of gapdh transcript copies based on a standard curve.
Representative standard curves shown are based on three to eight-point standard curves ranging from 100 to 107 plasmid copies generated for each of the 16 amplicons.
Representative images from retina sections from wildtype stage 57 tadpoles show mRNA expression patterns for genes selectively expressed in RGCs (brn3d, barhl2), photoreceptors (rho; pde6a; crx) and Müller cells (vim; fabp7; notch1) and genes expressed widely in multiple cell types (rpl6; rpl10a). Note that barhl2 and crx are also expressed in INL cells. RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar= 20 μm.
Representative images show mRNA expression patterns for rpl6 and rpl10a in retina from stage 57 wildtype tadpoles. CMZ, ciliary marginal zone; RPE, retinal pigment epithelium; ONL/INL, outer and inner nuclear layers; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar= 20 μm.
(A) A scree plot shows the loading values for each of the components superimposed against a broken stick diagram. Analysis of the first three components contribute significantly to the variance while the 4th component is equal to that of the broken stick value and does not contribute significantly towards the variance within the data. (B) A PCA-vector biplot of the 2nd and 3rd components show variability among the qPCR-based expression levels from TRAPed replicates from the same GFP-rpl10a frog lines as well as from different lines. The second (PCA2; X-axis) and third (PCA3; Y-axis) dimensions show separation of the two cell type-specific groups (RGCs and rods) and an overlap between the third group (Tg(fabp7;EGFP-rpl10a)) and the wildtype samples. Two outliers (one each from X3 (Tg(rho;EGFP-rpl10a)) and Wt) represent extreme variance that are maintained within the same groupings. These results recapitulate those shown in the PCA plot comparing the first two dimensions. PCA of the contribution of each of the 16 analyzed genes on the overall variance. The placement and length of the biplot vectors show the contribution to the variance for each gene. The gene expression data have been standardized to a value of 1, demarked by the tan circle (see methods).