Abstract
PYY is a gut-derived putative satiety signal released in response to nutrient ingestion and is implicated in the regulation of energy homeostasis. Pyy-expressing neurons have been identified in the hindbrain of river lamprey, rodents, and primates. Despite this high evolutionary conservation, little is known about central PYY neurons. Using in situ hybridization, PYY-Cre;ROSA-EYFP mice, and immunohistochemistry, we identified PYY cell bodies in the gigantocellular reticular nucleus region of the hindbrain. PYY projections were present in the dorsal vagal complex and hypoglossal nucleus. In the hindbrain, Pyy mRNA was present at E9.5, and expression peaked at P2 and then decreased significantly by 70% at adulthood. We found that, in contrast to the circulation, PYY-(1–36) is the predominant isoform in mouse brainstem extracts in the ad libitum-fed state. However, following a 24-h fast, the relative amounts of PYY-(1–36) and PYY-(3–36) isoforms were similar. Interestingly, central Pyy expression showed nutritional regulation and decreased significantly by acute starvation, prolonged caloric restriction, and bariatric surgery (enterogastroanastomosis). Central Pyy expression correlated with body weight loss and circulating leptin and PYY concentrations. Central regulation of energy metabolism is not limited to the hypothalamus but also includes the midbrain and the brainstem. Our findings suggest a role for hindbrain PYY in the regulation of energy homeostasis and provide a starting point for further research on gigantocellular reticular nucleus PYY neurons, which will increase our understanding of the brain stem pathways in the integrated control of appetite and energy metabolism.
Keywords: peptide YY, brain stem, fasting, energy homeostasis, satiety
peptide tyrosine-tyrosine (PYY) is a member of the pancreatic polypeptide (PP)-fold family that also includes neuropeptide Y (NPY) and pancreatic polypeptide (PPY). Five Y-receptor subtypes (Y1, Y2, Y4, Y5, and y6) mediate the effects of the PP-fold peptides (15, 33). PYY is a gut-derived putative satiety hormone released from gastrointestinal (GI) tract enteroendocrine L-cells in response to nutrient ingestion (2). PYY-(1–36) and PYY-(3–36) are the two main isoforms, with the latter being a truncated 34-amino acid form produced by cleavage of the two NH2-terminal residues by the enzyme dipeptidyl-peptidase IV (DPPIV) (42). PYY-(1–36) binds to all Y receptor (YR) subtypes with similar affinity, whereas PYY-(3–36) exhibits Y2R selectivity. Consequently, PYY-(1–36) and PYY-(3–36) exert different physiological effects (19, 37).
Endogenous circulatory PYY levels are affected in rodents by different dietary and physiological conditions as well as in obese subjects and patients with eating disorders and following weight loss surgery (5, 6, 16, 23, 39, 40, 44, 47, 50, 62). PYY levels in the cerebrospinal fluid were also reported to be significantly increased in bulimia nervosa patients (10, 36). These suggest a role for endogenous PYY in the regulation of appetitive behavior and energy metabolism both in physiological and in pathological conditions.
Peripheral PYY-(3–36) administration reduces feeding and adiposity in rodents, acting through the Y2R (1, 6). Moreover, peripheral PYY-(3–36) infusion reduces food intake in both lean volunteers and obese subjects (5, 6). We (7) previously showed in humans that peripheral PYY-(3–36) modulates neuronal activity within homeostatic and hedonic brain regions. In view of these findings, therapies aimed at increasing circulating PYY-(3–36) concentrations or Y2R agonists are being developed as potential treatments for obesity (48).
While high-dose peripheral PYY-(1–36) also inhibits feeding in rodents, this effect is absent in DPPIV-deficient rats, suggesting that the conversion of PYY-(1–36) to PYY-(3–36) is critical for the regulation of appetite by peripheral PYY-(1–36) (17, 63). In contrast, intracerebroventricular injection of PYY-(1–36) produces a strong orexigenic response (29). The effects of centrally administered PYY-(3–36) on feeding vary depending on the dose and site of administration. We found that low-dose intra-arcuate PYY-(3–36) administration reduces feeding (6), whereas previous studies showed that lateral ventricle PYY-(3–36) administration increased feeding and that this effect was reduced in y1r and y5r knockout mice (31, 51).
The majority of research to date has focused on peripheral PYY. However, Pyy is also expressed within the central nervous system (CNS) (21, 27, 49). The characterization of the CNS PYY system has been hampered by antibody cross-reactivity between NPY and PYY and the lack of a specific assay that can differentiate between PYY-(1–36) and PYY-(3–36) in rodents. Pieribone et al. (49) reported Pyy expression in the gigantocellular reticular nucleus (Gi) of the rostral medulla and in a neuronal population near the nucleus ambiguus. More recently, Glavas et al. (27) also reported PYY neurons in the Gi region in rodents and monkeys, but they were unable to detect PYY cell bodies near the nucleus ambiguus. Interestingly, similar PYY immunoreactive neurons were also reported in the rostral medulla of the river lamprey (Lampetra fluviatilis), a vertebrate group that diverged from the main vertebrates about 450 million years ago (14). Furthermore, PYY neuronal projections in the rostral medulla were very similar in the rat and the lamprey (49, 56). The unique location of PYY neurons in the Gi region of lampreys, rodents, and nonhuman primates indicates an anatomic conservation of the central PYY system across species throughout evolution and suggests a functional importance. Despite this high degree of evolutionary conservation, our knowledge of central PYY neurons is limited. In the present study, we examined the regulation of brainstem Pyy expression under different feeding conditions and following enterogastroanastomosis (EGA, a surrogate for human gastric bypass surgery) surgery in mice. In addition, we evaluated PYY-(1–36) and PYY-(3–36) peptide levels in mouse brain stem extracts under ad libitum feeding and following 24-h fasting. In agreement with the findings reported by Glavas et al. and Pieribone et al., we identified PYY cell bodies in the Gi region and projections in the dorsal vagal complex (DVC) and hypoglossal nucleus (12N). We found that PYY-(1–36) is the predominant isoform in brain stem extracts under ad libitum feeding. Finally, central Pyy expression was decreased significantly by acute starvation, prolonged caloric restriction, and EGA surgery.
MATERIALS AND METHODS
Mouse studies.
Mice were maintained on a 12:12-h light-dark cycle (lights on at 0700). All in vivo studies were performed in accordance with the United Kingdom Home Office Animal Procedures Act (1986) and the UCL Animal Ethics Committee. C57Bl/6 mice were purchased from Charles River (Margate, UK). Timed matings were set up between adult C57BL6/J mice. Females were inspected daily for plugs with the day of plug detection considered as E0.5. Embryos from three pregnant females were dissected for each stage (E9.5 and E13.5) and eight tissue blocks covering the mesencephalon to myelencephalon (E9.5) or brain stem (E13.5) were pooled for gene expression analysis. In addition, brain stems were dissected from P2 and P13 pups and P70 adults.
The generation of Pyy-Cre transgenic mice was described previously (52). Cre-positive offspring were back-crossed to C57BL6/J for 10 generations. In the resulting Pyy-Cre transgenic mice, the expression of Cre recombinase is under the control of the regulatory regions located in the first untranslated exon of the murine Pyy gene. ROSA26R-YFP reporter mice, in which Yfp expression is blocked by a loxP flanked STOP cassette, were obtained from Jackson Laboratories (Bar Harbor, ME). Crossing of YFP reporter mice to Pyy-Cre transgenic mice resulted in the generation of PYY-Cre;ROSA-EYFP mice, in which Cre-mediated excision of the STOP cassette limits Yfp expression only to Pyy-expressing cells. Homozygotic mice with a deletion of the gene encoding Npy and their wild-type (WT) littermates were derived by heterozygous breeding (22). The generation and breeding of PyyKO mice was described previously (8).
Dietary studies.
Six-month-old male C57BL6/J mice (n = 20) were singly housed for 1 wk and then randomized in two groups: mice in the first group were maintained on a normal chow diet (n = 10); those in the second group were calorie restricted for 4 wk (CR; n = 10). CR was carried out by a step-down regimen (16, 55), and mice received 80% of their control group's (Control diet) food. Food pellets were given at the onset of the dark phase (1800). At the end of the study, mice were culled during the first 3 h of the light phase after an overnight fast (14 h, between 1800 and 0800). After decapitation, brain stems were dissected, snap-frozen, and stored at −80°C until further use.
Surgical procedures.
Four-week-old male C57BL6/J mice were fed a high-fat diet (HFD) for 16 wk. Macronutrient composition and fat content of the control diet were 70% carbohydrate; 10% fat, and 20% protein; macronutrient composition and fat content of HFD were 35% carbohydrate, 45% fat, and 20% protein. They were then transported to facilities where they underwent surgery. Following 1 wk of acclimatization, mice were divided into two weight-matched groups to undergo either the EGA procedure (n = 8) or sham operation (n = 8) (60). In the EGA procedure, a laparotomy was performed, and the pyloric sphincter was ligatured, followed by an anastomosis between midjejunum and stomach, resulting in the exclusion of the duodenum and the proximal jejunum from the alimentary tract (60). Sham-operated mice underwent the same duration of anesthesia and a laparotomy was performed. Postoperatively, mice were fed the HFD and culled 10 days after the surgery, following an overnight fast. Tissue harvesting was performed as described above. Hormonal assays in mice exposed to different dietary regimens and EGA procedure were performed as described previously (16).
Transcardiac perfusion, immunohistochemistry, and in situ hybridization.
Transcardiac perfusion was accomplished using a peristaltic pump with 4% paraformaldehyde (PFA). After the perfusion, brains were removed from the skulls and stored in the same PFA solution at 4°C overnight. Fixed brains were then cryoprotected in 30% sucrose at 4°C overnight and stored at −80°C until sectioning. Immunohistochemistry (IHC) and in situ hybridization (ISH) were performed on 30-μm coronal sections as previously described (18). Rabbit anti-peptide YY (Peninsula Laboratories, San Carlos, CA) and rabbit anti-5HT (Immunostar, Wisconsin) antibodies were used to detect PYY and serotonin [5-hydroxytryptamine (5-HT)] neurons. DIG-labeled ISH riboprobe was generated using Pyy mouse sequence. Imaging was performed with an Olympus BX51 microscope combined with SimplePCI software. Confocal microscopy was performed on a Bio-Rad MRC1000 microscope. For phospho-signal transducer and activator of transcription 3 (pSTAT3) immunostaining, 24-h-fasted 3-mo-old PYY-Cre;ROSA-EYFP mice were injected with 5 μg/kg ip recombinant mouse leptin (R&D Systems, Abingdon, UK) or vehicle and perfused 45 min later. Brain stem and hypothalamus sections were stained for pSTAT3 as previously described (54, 54) using rabbit pSTAT3 (Tyr705) antibody (Cell Signaling Technology, UK).
Gene expression analysis.
The effect of peripheral PYY-(3–36) administration on central Pyy expression was assessed in C57BL6/J mice. Twelve-week-old male mice (n = 20) were divided into two weight-matched groups to receive an injection of either saline or PYY-(3–36) (50 ng/g ip; Bachem, UK). All injections were started at 0800 following an overnight fast, and mice were culled 6 h post-injection. Tissue harvesting was performed as described above.
For gene expression analysis, total RNA was extracted from frozen tissues using TRIzol reagent, and 2 μg of RNA was reverse transcribed to cDNA. Real-time quantitative PCR was performed on an ABI Prism 7900HT system (Applied Biosystems, Foster City, CA) using predesigned assays. Various housekeeping genes were tested for their expression stability across different samples; glyceraldehyde-3-phosphate dehydrogenase and ubiquitin C (Applied Biosystems) were found to be most stable and thus used as endogenous controls. Relative expression of the gene was determined by comparing its expression to that of the endogenous control. Data evaluation was carried out using SDS v. 2.1 software.
Peptide extraction and chromatographic and RIA procedures.
Fed and 24-h-fasted mice were used for the assessment of PYY isoforms in the brain stem. For peptide extraction, harvested tissues were boiled (95°C for 10 min) in 10× their volume of 1 M acetic acid. The extraction solutions were homogenized and centrifuged. The supernatant was loaded onto a 360-mg C18 SepPak cartridge (Waters, Milford, MA), which had been activated with acetonitrile (ACN) and equilibrated with 0.12% trifluoroacetic acid (TFA). After loading of the supernatant, the SepPak was washed with 0.12% TFA and eluted with 80% ACN-water solution containing 0.1% TFA, and dried SepPak fractions were then dissolved in 100 μl of water prior to loading of the column. Reverse-phase HPLC was carried out on a Shimadzu Class VP System using an Alltech Alltime C18 column (15 × 4.6 mm). The solvent systems used were: (A) 0.1% (vol/vol) TFA in water and (B) 0.1% (vol/vol) TFA in ACN. The solvent flow rate was 1 ml/min, and the elution was carried out with an initial gradient of 29% solvent B over the first minute followed by a gradient of 29–31% solvent B over the next 50 min. Brain stem extracts from fed and 24-h-fasted mice (n = 3 per group) were injected onto the column, and based on the retention times of the standards, 250-μl fractions were collected between 5 and 25 min and tested for the presence of PYY-like immunoreactivity (PYY-LI) using a rat/mouse total PYY RIA (Millipore,Watford, UK).
Statistical analysis.
All group data are expressed as means ± SE unless otherwise indicated. Variations in Pyy and receptor mRNA levels were assessed using Student's t-test or one-way ANOVA. Post hoc tests were performed in case a significant effect was detected (the Bonferroni correction was used when the equality of variances assumption held, and the Dunnett t3 correction was used otherwise). For all statistical analyses, P < 0.05 was considered significant. Data were analyzed using SPSS v. 14.0.
RESULTS
Distribution of PYY neurons in mouse brain stem and validation of PYY-Cre;ROSA-EYFP mouse.
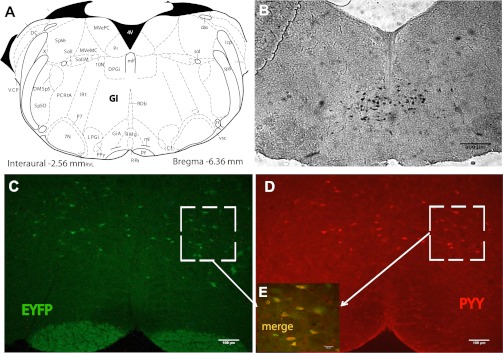
In agreement with the studies undertaken by Glavas et al. (27), ISH on mouse brain stem sections indicated only one population of Pyy-expressing neurons scattered throughout the Gi region (Fig. 1, A and B). In addition, Yfp-expressing neurons were present in the Gi region of PYY-Cre;ROSA-EYFP mice (Fig. 1C). Nearly all PYY immunoreactive cells expressed EYFP, confirming that the expression of the PYY-Cre transgene occurred in all PYY cells as expected. Approximately 70% of EYFP-expressing cells stained for PYY, indicating that a fraction of the EYFP-positive cells that arose from PYY cells no longer expressed the peptide (Fig. 1, D and E). PYY IHC on Gi sections from Pyy-null mice (PyyKO) did not reveal any PYY-ir.
Fig. 1.
Peptide YY (PYY) neurons are localized in the gigantocellular reticular nucleus (Gi) region of the hindbrain. A: coronal section of mouse hindbrain containing Gi region (24). B: representative in situ hybridization (ISH) image in brain slices from control mice showing Pyy expression in neurons located in the Gi region. C: representative immunofluorescence image in hindbrain slices from PYY-Cre;ROSA-EYFP mice. D: representative immunohistochemistry (IHC) image using PYY antibody on EYFP-positive cells in C. E: high magnification confocal image showing merge of C and D. Red, PYY immunostaining; green, EYFP; yellow: colocalization.
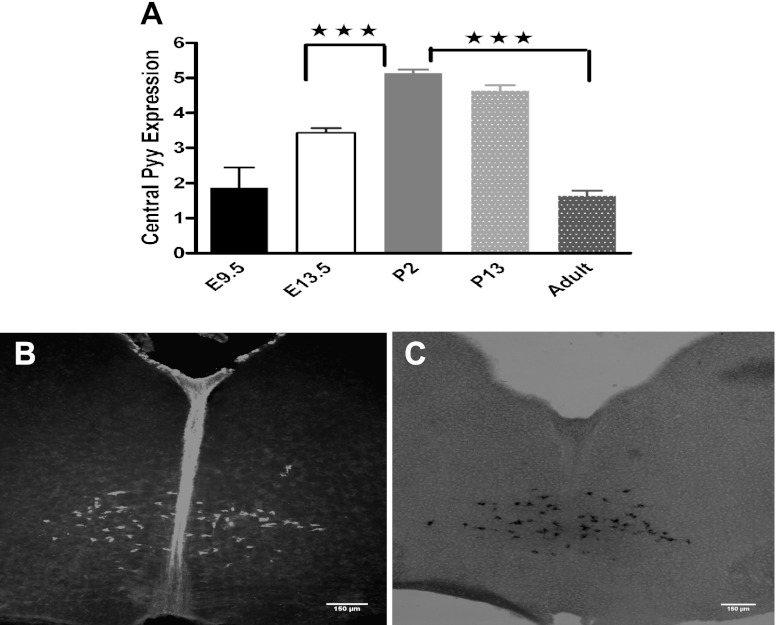
Because lineage tracing experiments showed that some descendants of PYY cells discontinued producing the peptide, we examined the developing hindbrain for Pyy mRNA expression. More specifically, we investigated brain stem Pyy expression at E9.5 and E13.5 (n = 4 per age) and at P2, P13, and P70 (n = 6 per age). Pyy mRNA was already present at E9.5 in the part of the brain covering mesencephalon to myelencephalon, and its level changed significantly at various developmental stages [F (5,28) = 34.10, P = 0.0001; Fig. 2A]. Pyy expression increased from day E9.5 and reached a peak at P2 with a significant increase between E13.5 and P2 (Dunnett's post hoc P = 0.0001). From P2 onward, Pyy expression started to decrease, and by P70 its expression had decreased by 69% compared with P2 (P = 0.0001; Fig. 2A). The presence of Pyy mRNA and protein at the P2 stage was further confirmed by ISH and IHC undertaken on brain stem sections from NpyKO mice (Fig. 2, B and C).
Fig. 2.
Brain stem Pyy expression reaches a peak at postnatal day 2 (P2) and decreases by 70% at 10 wk of age. A: real-time PCR assay using a predesigned assay for Pyy showed that Pyy mRNA is already present in the hindbrain at embryonic day (E)9.5, expression reaches peak at P2 and decreases to its E9.5 level by 10 wk of age. B: presence of PYY in the brain stem was confirmed with IHC on brain stem sections prepared from neuropeptide Y knockout (NpyKO) P2 pups. C: representative ISH image on brain stem sections from P2 pups, confirming the presence of Pyy mRNA. Bregma, 6.36 mm.
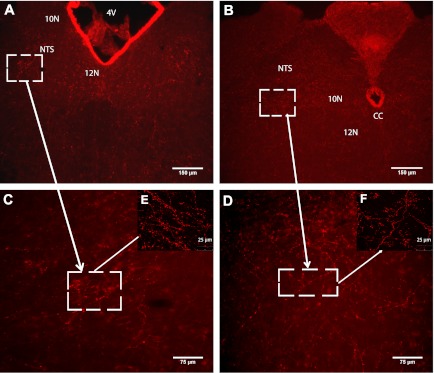
Due to the concerns of cross-reactivity of the PYY antibody with NPY, we investigated the distribution of PYY-ir fibers in mice lacking NPY (NpyKO). We observed PYY-ir fibers scattered throughout the DVC, including the nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus (10N), and the hypoglossal nucleus (12N) (Fig. 3, A–F). Taken together, our data indicate the presence of PYY-expressing cell bodies in the Gi region of the hindbrain and positive fibers throughout the DVC and 12N.
Fig. 3.
PYY-immunoreactive (ir) fibers are present in NTS, 10N, and 12N regions of the hindbrain. DVC, dorsal vagal complex; NTS, nucleus of the solitary tract; 10N, dorsal motor nucleus of the vagus; 12N, hypoglossal nucleus; Bregma, 7.08–7.64 mm. A and B: representative IHC images using PYY antibody showing the presence of PYY-ir projections in DVC and 12N regions. C and D: high magnification images of squared areas in A and B, respectively. E and F: high magnification confocal images of squared areas in C and D, respectively.
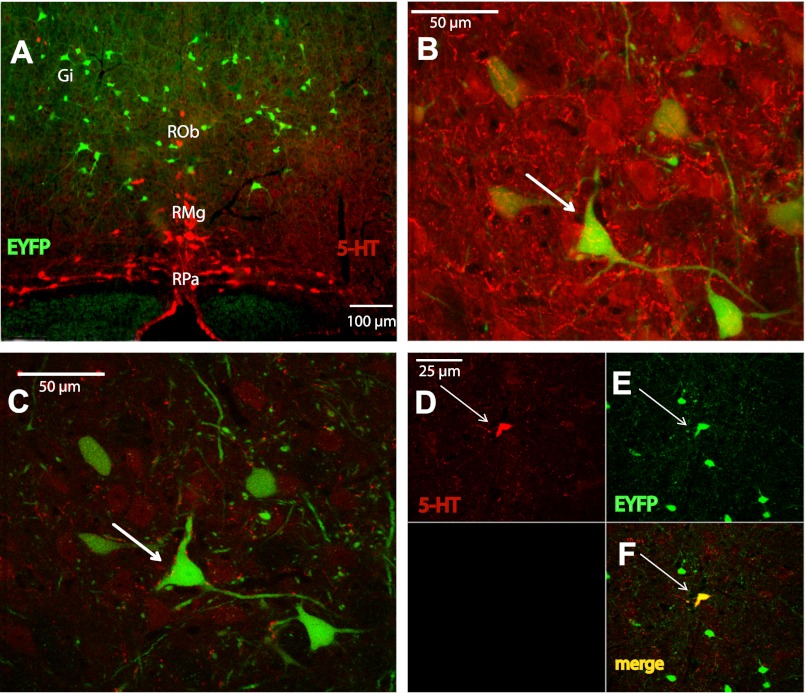
Raphe magnus (RMg), raphe obscurus (Rob), and raphe pallidus (RPa) of the brain stem raphe nuclei are located in close proximity to the Gi region (59). 5-HT IHC on hindbrain sections from PYY-Cre;ROSA-EYFP mice showed that PYY neurons lie just above the RMg and RPa and lateral to Rob cell bodies (Fig. 4A). Single scan and serial z-stack images obtained at 2-μm intervals demonstrated close appositions between 5-HT and PYY cell bodies (Fig. 4B). 5-HT fibers were also in close apposition with PYY neurons, suggestive of possible synaptic contacts (Fig. 4C). Furthermore, ∼8% of the PYY cell bodies in the RMg and Rob nuclei costained with 5-HT antibody (Fig. 4, D–F).
Fig. 4.
Serotonergic neurons are in close proximity to PYY cell bodies. RMg, Raphe magnus; Rob, Raphe obscures; RPa, raphe pallidus. A: representative IHC image using serotonin (5-HT) antibody on brain stem sections prepared from PYY-Cre;ROSA-EYFP mice, showing close proximity of two neuron groups. Bregma, 6.36 mm. B: high magnification confocal image of the section in A showing close apposition between 5-HT and PYY cell bodies. C: high magnification confocal image of the section in A showing close apposition between 5-HT-ir projections and PYY cell bodies. D: representative confocal image of an EYFP-positive neuron in the Rob nuclei costained with 5-HT antibody. Red, 5-HT immunostaining; green, EYFP; yellow, colocalization.
Changes in brain stem Pyy and Y-receptor mRNA levels in response to food deprivation and prolonged CR.
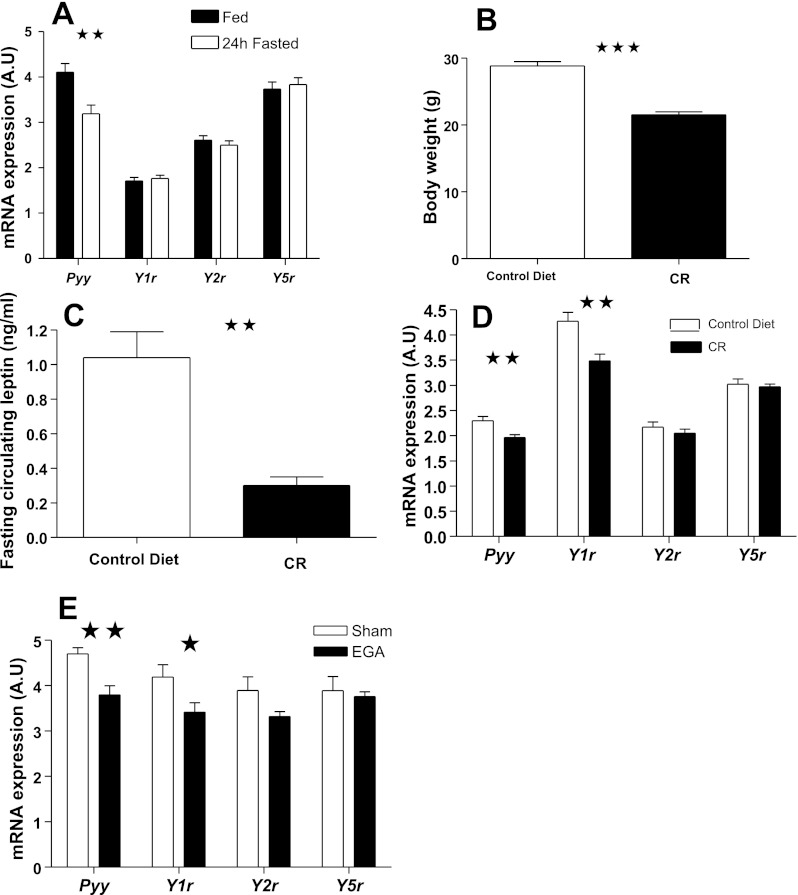
Because of the peripheral expression of the Y4r (15) and studies indicating a lack of involvement of y6r in feeding regulation and absence of a functional y6r in humans and rats (12, 15, 46), we investigated the expression of brain stem Pyy, Y1r, Y2r, and Y5r under different feeding conditions and following weight loss surgery. The expression of Pyy and the three receptors in male C57BL/6 mice was unaltered by overnight fasting (data not shown). However, 24-h fasting led to a significant reduction in Pyy expression [t(28) = 3.38, P = 0.002] but did not alter Yr expression (Fig. 5A). Four weeks of CR caused a significant decrease in body weight [Fig. 5B; t(17) = −8.18, P = 0.0001]. Whereas fasting peripheral acyl-ghrelin and PYY levels were unaltered (data not shown), circulating leptin levels were significantly decreased in response to CR [Fig. 5C; t(4.44) = 10.24, P = 0.001]. Brain stem Pyy and Y1r expressions were reduced in the CR mice [t(15.64) = 3.29, P = 0.005; t(17) = 3.49, P = 0.003, respectively; Fig. 5D] and were positively correlated (r = 0.498, P = 0.03). Neither Y2r nor Y5r expression was altered by 4 wk of CR. Body weight loss correlated negatively with both Pyy (r = −0.561, P = 0.015) and Y1r expression (r = −0.576, P = 0.012). Furthermore, Y1r expression correlated positively with fasting leptin levels (r = 0.686, P = 0.002).
Fig. 5.
Brain stem Pyy mRNA expression is reduced significantly in response to 24-h fasting, prolonged calorie restriction (CR), and following enterogastroanastomosis (EGA) procedure. A: change in brain stem Pyy mRNA expression in mice in response to 24-h fasting. B and C: changes in body weight and circulating leptin levels, respectively, in mice subjected to prolonged CR. D and E: changes in Pyy and Y1, Y2, and Y5 receptor mRNA expression levels in mice subjected to prolonged CR and in mice that underwent EGA procedure, respectively.
Changes in brain stem Pyy and Y-receptor expression following EGA surgery.
We (16) recently reported that EGA procedure resulted in a significant reduction in body weight and fasting circulating leptin levels, whereas acyl-ghrelin and total PYY levels were markedly elevated. Brain stem Pyy and Y1r expression decreased significantly in response to EGA procedure [t(11.86) = 3.76, P = 0.003; t(13) = 2.20, P = 0.046, respectively; Fig. 5E] and were positively correlated (r = 0.753, P = 0.001). Both Pyy and Y1r expression correlated with body weight loss (r = −0.526 P = 0.044; r = −0.533 P = 0.041, respectively), circulating leptin (r = 0.623, P = 0.022; r = 0.546, P = 0.044), and PYY levels (r = −0.627, P = 0.012; r = −0.574, P = 0.025). Y2r and Y5r expression was not affected by EGA surgery.
In view of the relationship between circulating leptin and PYY levels and central Pyy expression, we investigated whether intraperitoneal leptin and PYY-(3–36) administration acted on brain stem PYY neurons. Peripheral leptin administration following 24-h fasting induced pSTAT3 staining in the arcuate nucleus, ventromedial hypothalamic nucleus, and dorsomedial hypothalamic nucleus of the hypothalamus (data not shown). However, no pSTAT3 staining was observed in brain stem PYY neurons, suggesting that leptin does not act directly on PYY neurons in 24-h-fasted mice (data not shown). TaqMan real-time PCR assay showed no effect of peripheral PYY-(3–36) injection on brain stem Pyy mRNA levels in mice following an overnight fast (mRNA expression in AU: Saline, 2.19 ± 0.08; PYY, 2.10 ± 0.07).
Assessment of brain stem PYY-(1–36) and PYY-(3–36) levels using HPLC/RIA.
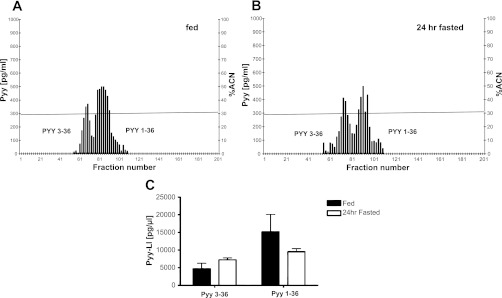
All chromatographic procedures were performed in NpyKO mice to prevent false positive results due to cross-reactivity of NPY with the PYY antibody. Therefore, prior to HPLC, we determined Pyy expression in the brain stems of NpyKO and WT mice to ensure that the expression was unaffected by Npy deletion; Pyy mRNA levels were similar in both groups (data not shown). Following the injection of a mixture containing both peptides, the first peak, corresponding to PYY-(3–36), was detected at 16 min and the second peak, corresponding to PYY-(1–36), at 18 min. The ratio of PYY-(1–36) to PYY-(3–36) in brain stem extracts from fed and 24-h-fasted mice was significantly different [3.26 ± 0.29 fed vs. 1.33 ± 0.33 fasted group; t(2.05) = 6.48, P = 0.002]. Figure 6, A and B, shows data from one representative chromatogram from fed and 24-h-fasted mice. Under fed conditions, PYY-(1–36) was the dominant form in the brain stem, whereas under fasted conditions PYY-LI in fractions corresponding to both isoforms was similar. This was due both to an increase in PYY-(3–36)-LI and to a decrease in PYY-(1–36)-LI in the fasted state (Fig. 6C). The high SEM in the fed group is due to one mouse having lower PYY-(3–36) and PYY-(1–36)-LI. However, the ratio of the two isoforms was similar to that of the other two mice.
Fig. 6.
PYY-(1–36) is the predominant form under ad libitum feeding, and the level of both isoforms is similar following 24-h fasting. A and B: representative chromatograms from one fed and one 24-h-fasted mouse, respectively. C: changes in PYY-like immunoreactivity corresponding to PYY-(1–36) and PYY-(3–36) isoforms in response to 24-h fasting.
DISCUSSION
Following the initial report by Batterham et al. indicating an implication of gut-derived peripheral PYY in energy metabolism (6), several studies reported changes in endogenous PYY levels in rodents under various dietary conditions and humans with eating disorders and obesity (5, 6, 16, 23, 39, 40, 44, 47, 50, 62). Peripheral PYY is an essential key for the effect of EGA in mice, since body weight regulation after EGA is lost in PyyKO mice (16). However, to our knowledge, there has been no study assessing the changes in central Pyy expression in mice in response to food restriction and weight loss surgery. Our study shows that central Pyy expression changes in response to starvation, long-term caloric restriction, and the EGA weight loss surgery. With the development of a human RIA specific for PYY-(3–36), this isoform was shown to be the predominant circulatory form in both fed and fasted states (8). A RIA specific for the truncated form of the polypeptide is not available in mice, and to our knowledge, there is no study that has investigated tissue or plasma levels of both isoforms in mice under fed and fasted conditions. In this study, we established a method using HPLC and RIA to measure brain levels of both isoforms in mice and showed that, contrary to the circulation, the untruncated PYY isoform was predominant in the fed state but levels of both isoforms became similar following 24 h fasting. Our findings provide a starting point for further studies into the functional significance of central PYY, which in turn will contribute to an increased understanding of the complex central mechanisms involved in the regulation of food intake and energy metabolism.
Our studies with PYY-Cre;ROSA-EYFP transgenic mice showed that Pyy-expressing cells are located in the Gi region. Both IHC and ISH experiments confirmed this distribution, and this is consistent with previous reports (27, 49). All cells that are stained with the PYY antibody are also EYFP positive, and this validates the PYY-Cre;ROSA-EYFP mice, which enables us to subsequently use these mice as a tool to further investigate PYY neurons. The advantage of using PYY-Cre;ROSA-EYFP mice to localize PYY neurons is that the localization does not require an NpyKO background, as the absence of NPY could affect PYY localization. Approximately 70% of EYFP-expressing cells were PYY immunoreactive, indicating that some of the EYFP-expressing cells were descendants of cells where Pyy was transiently expressed during hindbrain development and these cells no longer express PYY peptide. The peak Pyy expression at P2 and 70% decrease in the expression by 10 wk of age suggests that Pyy is transiently expressed in a percentage of brain stem neurons during embryonic and postnatal development.
The Gi region is implicated in cardiovascular and respiratory inhibition (58), motor aspects of feeding, such as chewing and swallowing (3, 30) and antinociception (61, 64). IHC experiments using a PYY-specific antibody in NpyKO mice showed the presence of PYY-ir fibers in the caudal brain stem, including the DVC and 12N. This pattern of PYY-ir fibers is similar to that previously described by Glavas et al. (27). Caudal brain stem nuclei respond to feeding modulatory effects of the gastrointestinal satiety signals and are involved in the regulation of motor aspects of feeding behavior, such as chewing, licking, and swallowing and the parasympathetic control of the gastric motility (11, 26, 28, 53, 66). The unique localization of PYY neurons in the Gi region and their projections to the DVC and the 12N suggest that central PYY neurons are involved in the regulation of the motor and visceral aspects of feeding behavior.
We observed intense PYY-ir within the ependymal cells lining the fourth ventricle. One potential explanation for this finding is that circulating PYY enters the CNS via this route, enabling area postrema (AP) neurons to “sense” peripheral PYY concentrations. Evidence for direct uptake of peripheral PYY-(3–36) into hindbrain regions comes from the studies of Dumont et al. (20), who examined the distribution of peripherally administered radiolabeled PYY-(3–36). They detected radioactive PYY-(3–36) in the AP within 30 min of administration. Additional studies are now warranted to investigate this further.
To examine a possible role for central PYY in energy homeostasis, we assessed whether central Pyy expression is regulated under different dietary conditions and following EGA surgery. Fasting for 24 h led to a downregulation of Pyy mRNA without altering Yr mRNA levels. Long-term CR and EGA surgery caused a significant decrease in both Pyy and Y1r expression, and both were correlated with body weight loss. These findings suggest that brain stem Pyy and Y1r expression are regulated both acutely by the animal's nutritional state and by long-term alterations in body weight. Moreover, these findings suggest that central PYY may modulate appetite via the Y1R.
We observed a close apposition between 5-HT fibers and PYY neurons. Furthermore, PYY-ir was colocalized with 5-HT-ir in few neurons. Brain stem raphe serotonin neurons influence pain processing via projections to the spinal cord (9, 65). By using wheat germ agglutinin transneuronal tracer, Braz et al. have shown the postsynaptic targets of raphe serotonin neurons in the Gi region, which together constitute a part of the serotoninergic descending pathway modulating spinal nociceptive processing (13). The close apposition between 5-HT fibers and PYY cell bodies that we observed in our study suggests that Gi PYY neurons may be involved in the processing of pain perception via its connections with raphe serotonin neurons. Further studies with rodent models of nociception will help to delineate the implication of Gi PYY and raphe neurons in the modulation of pain perception.
There was a correlation between central Pyy expression and fasting circulating leptin following weight loss as a result of EGA surgery but not as a result of CR. Glucocorticoids released in response to stress are implicated in the impairment of leptin sensitivity (57, 67). Mice in the CR group were exposed to stress as a result of 4-wk restricted food intake. In contrast, mice that underwent EGA surgery were culled 10 days post-surgery, during which time they had ad libitum access to a high-fat diet. Thus, the stress in the CR mice and the resulting impairment in leptin sensitivity may underlie the lack of correlation between central Pyy expression and circulating leptin levels. Furthermore, fasting decreases the entry of leptin to the brain (34, 35). It is possible that in CR mice, the transport of circulating leptin across the blood brain barrier is decreased as a way to reduce its satiety effect and this may underlie the observed lack of regulation of central Pyy by leptin (4).
In view of the correlation between central Pyy expression and fasting circulating leptin in mice that underwent EGA surgery, we further investigated a possible regulation of PYY neurons by peripheral leptin by performing pSTAT3 immunostaining. Peripheral leptin administration to 24-h-fasted PYY-Cre;ROSA-EYFP mice did not induce STAT3 phosphorylation in Gi PYY neurons, suggesting that these neurons are not directly regulated by peripheral leptin after a 24-h fast. We did not examine p-STAT3 immunostaining in response to leptin injection in mice that had undergone weight loss surgery; hence, we do not know whether leptin directly acts upon Gi PYY neurons following EGA.
The negative correlation between fasting circulating total PYY and central Pyy expression in sham- and EGA-operated mice suggests a possible regulation of central Pyy expression by peripheral PYY. To further investigate this, we measured central Pyy expression in overnight-fasted mice injected with PYY-(3–36). Peripheral injection of PYY-(3–36) did not alter hindbrain Pyy mRNA levels. This could be due to nonresponsiveness of PYY neurons to single peptide administration following an overnight fast. Additional studies following longer fasting periods, EGA surgery, and with repeated peptide injections will help to determine a possible regulation of central Pyy expression by circulating PYY.
Two main PYY isoforms, PYY-(1–36) and PYY-(3–36), exert opposing effects on feeding due to their different YR specificity, with PYY-(1–36) stimulating appetite and promoting weight gain by acting through Y1R and Y5R and PYY-(3–36) enhancing satiety and promoting weight loss through its interactions with Y2R (1, 6, 25, 31, 32, 41). With the development of a human RIA specific for PYY-(3–36), we previously showed that, in humans, PYY-(3–36) is the predominant circulatory form in both fed and fasted states (8). Currently, there are no RIAs or ELISAs that can specifically detect the two main murine isoforms in the brain or periphery. Furthermore, there are no data regarding whether PYY-(1–36), PYY-(3–36), or both are present in the CNS and whether these are regulated by feeding. To investigate this, we used HPLC and a total murine RIA kit in combination. By separating the two isoforms differing in only two amino acids using HPLC, we were then able to quantify the relative amounts of both isoforms in brain stem extracts using the RIA. Under fed conditions, PYY-(1–36) was the predominant form in brain stem extracts. Following a 24-h fast, the amounts of the two isoforms were similar due to both an increase in PYY-(3–36) and a decrease in PYY-(1–36) levels, suggesting an increased conversion of PYY-(1–36) to PYY-(3–36) in the fasted state. The increase in PYY-(3–36)-LI in fasted animals seems counterintuitive if central PYY-(3–36) similarly inhibits food intake to circulating PYY-(3–36). On the other hand, Pyy mRNA decreases as expected during fasting. Given the available assays, it may be difficult to determine the form of PYY that is present at receptor-bearing cells, as the enzyme DPPIV is widely distributed throughout the CNS (38, 43, 45). The predominance of orexigenic PYY-(1–36) in the fed state and the similar levels of both isoforms following a 24-h fast suggest a potential role for central PYY in body weight regulation. Further studies, with larger numbers of mice and with extracts from more selected regions of the brain stem, will help to further clarify the functional significance of the relative amounts of each isoform under different feeding states.
In conclusion, our data provide a starting point for future studies into the functional significance of central PYY neurons upon whole body energy homeostasis regulation. To date, much effort has been placed on understanding the hypothalamic pathways in the regulation of feeding. It is accepted that the neural networks responsible for the overall control of energy metabolism are not limited to the hypothalamus but also include other areas of the CNS such as the midbrain and the brainstem. Drugs based on targeting the PYY system are being developed for the treatment of obesity, but still, little is known about the function and regulation of central PYY system. A greater understanding of the central PYY system is required to harness the full therapeutic potential of PYY-based obesity drugs.
GRANTS
This work was supported by grants from the Medical Research Council (R.L. Batterham, D.J. Withers, K. Chandarana, and M. Claret), Wellcome Trust (D. J. Withers and E. E. Irvine), and the Benjamin Delessert Institute (F. Andreeli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.G., F.A., and R.L.B. conception and design of research; C.G., K.C., A.I.C., H.A.-Q., I.M.E., E.E.I., C.B.H., and S.E.S. performed experiments; C.G. and R.L.B. analyzed data; C.G. and R.L.B. interpreted results of experiments; C.G. prepared figures; C.G. and R.L.B. drafted manuscript; C.G., M.C., F.A., A.B.L., D.J.W., and R.L.B. edited and revised manuscript; R.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Benoit Viollet and Veronique Fauveau (Cochin Institute, Paris, France) for their contribution to the generation of this paper. Breeding pairs for NpyKO mice were provided by Dr. Richard Palmiter (University of Washington, Seattle, WA).
REFERENCES
- 1.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 1043: 139–144, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Athanassiadis T, Olsson KA, Kolta A, Westberg KG. Identification of c-Fos immunoreactive brainstem neurons activated during fictive mastication in the rabbit. Exp Brain Res 165: 478–489, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349: 941–948, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical, hypothalamic brain areas predicts feeding behaviour in humans. Nature 450: 106–109, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation, body-weight regulation. Cell Metab 4: 223–233, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 60: 355–366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrettini WH, Kaye WH, Gwirtsman H, Allbright A. Cerebrospinal-fluid peptide YY immunoreactivity in eating disorders. Neuropsychobiology 19: 121–124, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Berthoud HR, Sutton GA, Townsend RL, Patterson LM, Zheng HY. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav 89: 517–524, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Blomqvist AG, Herzog H. Y-receptor subtypes-how many more? Trends Neurosci 20: 294–298, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J Comp Neurol 507: 1990–2003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodin L, Rawitch A, Taylor T, Ohta Y, Ring H, Hokfelt T, Grillner S, Terenius L. Multiple forms of pancreatic polypeptide-related compounds in the lamprey CNS: partial characterization and immunohistochemical localization in the brain stem and spinal cord. J Neurosci 9: 3428–3442, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrele C, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J Pept Sci 6: 97–122, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, reelli F, Withers DJ, Batterham RL. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60: 810–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY(3–36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287: R1064–R1070, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest 115: 940–950, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corp ES, McQuade J, Krasnicki S, Conze DB. Feeding after fourth ventricular administration of neuropeptide Y receptor agonists in rats. Peptides 22: 493–499, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Dumont Y, Moyse E, Fournier A, Quirion R. Distribution of peripherally injected peptide YY ([125I] PYY (3–36)) and pancreatic polypeptide ([125I] hPP) in the CNS: enrichment in the area postrema. J Mol Neurosci 33: 294–304, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ekman R, Wahlestedt C, Bottcher G, Sundler F, Hakanson R, Panula P. Peptide YY-like immunoreactivity in the central nervous system of the rat. Regul Pept 16: 157–168, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Evans S, Pamuklar Z, Rosko J, Mahaney P, Jiang N, Park C, Torquati A. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin KBJ, Paxinos GM. The Mouse Brain in Stereotaxic Coordinates. New York: Academic, 1997 [Google Scholar]
- 25.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 382: 168–171, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol 147: 159–176, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Glavas MM, Grayson BE, Allen SE, Copp DR, Smith MS, Cowley MA, Grove KL. Characterization of brainstem peptide YY (PYY) neurons. J Comp Neurol 506: 194–210, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 33, Suppl 1: S11–S15, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Hagan MM. Peptide YY: a key mediator of orexigenic behavior. Peptides 23: 377–382, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto N, Katayama T, Ishiwata Y, Nakamura Y. Induction of rhythmic jaw movements by stimulation of the mesencephalic reticular formation in the guinea pig. J Neurosci 9: 2887–2901, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, Macneil DJ, Van der Ploeg LH, Saga Y, Nishimura S, Ihara M. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology 141: 1011–1016, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Karra E, Batterham RL. The role of gut hormones in the regulation of body wt and energy homeostasis. Mol Cell Endocrinol 316: 120–128, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol 587: 19–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides 21: 679–682, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Kastin AJ, Pan W. Dynamic regulation of leptin entry into brain by the blood-brain barrier. Regul Pept 92: 37–43, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Kaye WH, Berrettini W, Gwirtsman H, George DT. Altered cerebrospinal-fluid neuropeptide-y, peptide-Yy immunoreactivity in anorexia and bulimia-nervosa. Arch Gen Psychiatry 47: 548–556, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Keire DA, Mannon P, Kobayashi M, Walsh JH, Solomon TE, Reeve JR., Jr Primary structures of PYY, [Pro(34)]PYY, and PYY-(3–36) confer different conformations and receptor selectivity. Am J Physiol Gastrointest Liver Physiol 279: G126–G131, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyi-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Labor Sci 40: 209–294, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, Lydecker J, Herzog D, Klibanski A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol 164: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Mashiko S, Moriya R, Ishihara A, Gomori A, Matsushita H, Egashira S, Iwaasa H, Takahashi T, Haga Y, Fukami T, Kanatani A. Synergistic interaction between neuropeptide Y-1 and Y-5 receptor pathways in regulation of energy homeostasis. Eur J Pharmacol 615: 113–117, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Mentlein R, Dahms P, Grandt D, Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept 49: 133–144, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Mentzel S, Dijkman HBPM, Vanson JPHF, Koene RAP, Assmann KJM. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 44: 445–461, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 91: 1027–1033, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Mitro A, Lojda Z. Histochemistry of proteases in ependyma, choroid-plexus and leptomeninges. Histochemistry 88: 645–646, 1988 [DOI] [PubMed] [Google Scholar]
- 46.Mullins DE, Guzzi M, Xia L, Parker EM. Pharmacological characterization of the cloned neuropeptide Y y(6) receptor. Eur J Pharmacol 395: 87–93, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Munsch S, Biedert E, Meyer AH, Herpertz S, Beglinger C. CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT). Physiol Behav 97: 14–20, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther 124: 44–56, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Pieribone VA, Brodin L, Friberg K, Dahlstrand J, Soderberg C, Larhammar D, Hokfelt T. Differential expression of mRNAs for neuropeptide Y-related peptides in rat nervous tissues: possible evolutionary conservation. J Neurosci 12: 3361–3371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahardjo GL, Huang XF, Tan YY, Deng C. Decreased plasma peptide YY accompanied by elevated peptide YY and Y2 receptor binding densities in the medulla oblongata of diet-induced obese mice. Endocrinology 148: 4704–4710, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Raposinho PD, Pierroz DD, Broqua P, White RB, Pedrazzini T, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol Cell Endocrinol 185: 195–204, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Schonhoff S, Baggio L, Ratineau C, Ray SK, Lindner J, Magnuson MA, Drucker DJ, Leiter AB. Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol Cell Biol 25: 4189–4199, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz GJ. Brainstem integrative function in the central nervous system control of food intake. Front Eating Weight Regul 63: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selman C, Kerrison ND, Cooray A, Piper MD, Lingard SJ, Barton RH, Schuster EF, Blanc E, Gems D, Nicholson JK, Thornton JM, Partridge L, Withers DJ. Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol Genomics 27: 187–200, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Soderberg C, Pieribone VA, Dahlstrand J, Brodin L, Larhammar D. Neuropeptide role of both peptide YY and neuropeptide-Y in vertebrates suggested by abundant expression of their messenger-RNAs in a cyclostome brain. J Neurosci Res 37: 633–640, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Solano JM, Jacobson L. Glucocorticoids reverse leptin effects on food intake, body fat in mice without increasing NPY mRNA. Am J Physiol Endocrinol Metab 277: E708–E716, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Stremel RW, Waldrop TG, Richard CA, Iwamoto GA. Cardiorespiratory responses to stimulation of the nucleus reticularis gigantocellularis. Brain Res Bull 24: 1–6, 1990 [DOI] [PubMed] [Google Scholar]
- 59.Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci 600: 9–34, 1990 [DOI] [PubMed] [Google Scholar]
- 60.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, reelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8: 201–211, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Tseng LLF, Tang R, Stackman R, Camara A, Fujimoto JM. Brain-stem sites differentially sensitive to beta-endorphin and morphine for analgesia and release of met-enkephalin in anesthetized rats. J Pharmacol Exper Therapeut 253: 930–937, 1990 [PubMed] [Google Scholar]
- 62.Ukkola OH, Puurunen VP, Piira OP, Niva JT, Lepojarvi ES, Tulppo MP, Huikuri HV. High serum fasting peptide YY (3–36) is associated with obesity-associated insulin resistance and type 2 diabetes. Regul Pept 170: 38–42, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Unniappan S, McIntosh CH, Demuth HU, Heiser U, Wolf R, Kieffer TJ. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia 49: 1915–1923, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Urban MO, Smith DJ. Nuclei within the rostral ventromedial medulla mediating morphine antinociception from the periaqueductal gray. Brain Res 652: 9–16, 1994 [DOI] [PubMed] [Google Scholar]
- 65.VanderHorst VGJM, Ulfhake B. The organization of the brainstem, spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat 31: 2–36, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148: 1868–1881, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes 46: 717–719, 1997 [DOI] [PubMed] [Google Scholar]