Abstract
Selenium is an essential micronutrient required for cellular antioxidant systems, yet at higher doses it induces oxidative stress. Additionally, in vertebrates environmental exposures to toxic levels of selenium can cause paralysis and death. Here we show that selenium-induced oxidative stress leads to decreased cholinergic signaling and degeneration of cholinergic neurons required for movement and egg-laying in Caenorhabditis elegans. Exposure to high levels of selenium leads to proteolysis of a soluble muscle protein through mechanisms suppressible by two pharmacological agents, levamisole and aldicarb which enhance cholinergic signaling in muscle. In addition, animals with reduction-of-function mutations in genes encoding post-synaptic levamisole-sensitive acetylcholine receptor subunits or the vesicular acetylcholine transporter developed impaired forward movement faster during selenium-exposure than normal animals, again confirming that selenium reduces cholinergic signaling. Finally, the antioxidant reduced glutathione, inhibits selenium-induced reductions in egg-laying through a cellular protective mechanism dependent on the C. elegans glutaredoxin, GLRX-21. These studies provide evidence that the environmental toxicant selenium induces neurodegeneration of cholinergic neurons through depletion of glutathione, a mechanism linked to the neuropathology of Alzheimer’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease.
Keywords: Cholinergic, Glutathione, Neurodegeneration, Oxidative stress, Selenium
1. Introduction
Selenium (Se) is an essential micronutrient that has been shown to improve the outcome of several models of neurodegenerative disorders (van Eersel et al., 2010; Ishrat et al., 2009; Wood-Allum et al., 2006; Zafar et al., 2003) and has been examined in treatment paradigms for human disease (Fairweather-Tait et al., 2011). These studies and others (Bellinger et al., 2009) aimed at elucidating the functions of the many selenoproteins that incorporate Se in the form of the amino acid selenocysteine, have demonstrated that Se is both absorbed by the central nervous system (Schweizer et al., 2004) and required for muscle function (Rederstorff et al., 2006). Not surprisingly, it is these same two systems which have been observed to be affected in both animals and humans after toxic Se exposures (Koller and Exon, 1986). Chicks exposed in vivo to toxic levels of sodium selenite, an inorganic form of Se, experienced paralysis and died from respiratory failure which in vitro studies suggested resulted in part, from both sustained muscle contracture and blockade of presynaptic neuromuscular transmission (Lin-Shiau et al., 1990). Humans poisoned with high doses of Se exhibited muscle weakness prior to cardiovascular collapse and death (Spiller and Pfiefer, 2007) and showed evidence of neurological decline (Koller and Exon, 1986). Isolated populations chronically exposed to Se in their environment were reported to have an increased risk of developing amyotrophic lateral sclerosis (ALS), a neuromuscular disease typified by progressive muscle weakness, paralysis, and death (Vinceti et al., 2010). Similarly, in the soil nematode Caenorhabditis elegans, high dose Se exposure induced a progressive movement impairment culminating in an irreversible paralysis phenotype and death (Morgan et al., 2010) that parallels the progressive decline observed with ALS. This suggests that Se may similarly be targeting the worm neuromuscular system.
The coordinate regulation of the 95 body wall muscles in C. elegans is controlled by 70 motor neurons whose cell bodies are positioned along the ventral nerve cord and that function to affect a normal sinusoidal wave pattern of movement (Duerr et al., 2008; de Bono and Maricq, 2005). Two of the major neurotransmitters conserved in C. elegans, acetylcholine and gamma-aminobutyric acid respectively provide the excitatory and inhibitory inputs for forward and backward movement (Duerr et al., 2008; McIntire et al., 1993). Reduction-or-loss-of-function mutations in genes affecting these neurotransmitter systems can result in uncoordinated movement behaviors (Brenner, 1974) which phenocopy many aspects observed in animals after Se-exposure including the inability to complete a normal backwards or forwards wave pattern, and paralysis (Morgan et al., 2010). One of the major advantages of using C. elegans as a model organism is the ability to directly correlate these behavioral changes to pathological changes in muscles and/or neurons that can be easily observed in vivo or in situ due to the clear body plan of the animal (Dimitriadi and Hart, 2010; Silverman et al., 2009).
In order to determine how high dose Se exposure leads to motility impairment in worms, we began a systematic examination of both the nervous and muscular systems after high dose Se-exposure to determine the pathological changes induced by this toxicant metalloid. Previously we defined a model for selenium-induced toxicity in C. elegans showing that selenium similarly induces motor impairment and increases mortality. Here we demonstrate that selenium exerts these effects by damaging cholinergic motor neurons and reducing their secretion of acetylcholine. This alters the ability of muscles to respond to environmental stimuli and affects two motor behaviors essential for survival, movement, and egg-laying. This model is of particular significance because it addresses both how environmental toxins such as selenium induce changes in motor behaviors and provides a possible explanation for the link between exposures and neurodegenerative disease processes.
2. Materials and methods
2.1. Strains, maintenance, and growth conditions
The C. elegans N2 Bristol strain was used in all experiments requiring WT animals. In addition, the following strains were used: BL5717 inIs179[Pida-1::gfp]II;him-8(e1489)IV, CB193 unc-29(e193)I, CB211 lev-1(e211)IV, CB382 unc-49(e382)III, CB904 unc-38(e264)I, CB993 unc-17(e245)IV, CB1331 unc-17(e245)IV;snb-1(e1563)V, NC571 wdIs20[Punc-4::snb-1::gfp] (Hung et al., 2007), OM316 inIs179[Pida-1::gfp]II;glrx-21(tm2921)III, PD55 tra-3(e1107)IV;ccIs55 [Punc-54::unc-54::lac Z;sup-7(st5)]V, PJ727 ccIs55;jIs01[Pmyo-3::gfp::myo-3;rol-6(su1006)] (Fostel, 2003), RB918 acr-16(ok789)V, RB756 gar-2(ok520)III, VC731 unc-63(ok1075)I, VZ54 glrx-21(tm2921)III.
Animals were maintained on modified NGM agar plates (Estevez et al., 2004), and assayed as described previously (Morgan et al., 2010), except where noted. Mock-exposure plates had a volume of water added which was equivalent to that added to the experimental plates. Eserine salicylate salt (Sigma, St. Louis, MO) was diluted in distilled water to the final concentrations indicated and was added to plates along with Se. Se concentration was 5 mM Na2SeO3.
2.2. Behavioral assays
Assays for movement were as described (Morgan et al., 2010) unless indicated below. Many of the following assays included analysis of movement prior to and/or following additional treatments or studies.
2.2.1. Levamisole-induced hyper-contraction
After 24 h of Se exposure, 15 paralyzed animals were picked and placed onto fresh agar plates containing a final concentration of 100 mM levamisole. Fifteen mock-exposed normal animals were similarly treated. Animals were treated with levamisole for 30 min then examined for shortening of body length and mass egg release, indications of hyper-contraction of the body wall and vulval muscles, respectively (Trent et al., 1983; Lewis et al., 1980).
2.2.2. Aldicarb-induced paralysis
To test for resistance to aldicarb, 20 animals that failed to back after 24 h exposure to Se, and 20 age-synchronized mock-exposed animals were placed on plates containing a final concentration of 0.5 mM aldicarb (Nonet et al., 1998). These animals were assayed for forward movement every 30 min for a total of 2 h and the number of paralyzed animals was scored.
2.2.3. Forward movement
Animals containing mutations in genes affecting pre- and post-synaptic inputs along the NMJ, and WT-N2 animals, were scored for forward movement only since mutations in some of the genes result in backing defects (Hosono and Kamiya, 1991). Thus, the “% forward” category includes backing deficient animals.
2.2.4. Egg-laying assay
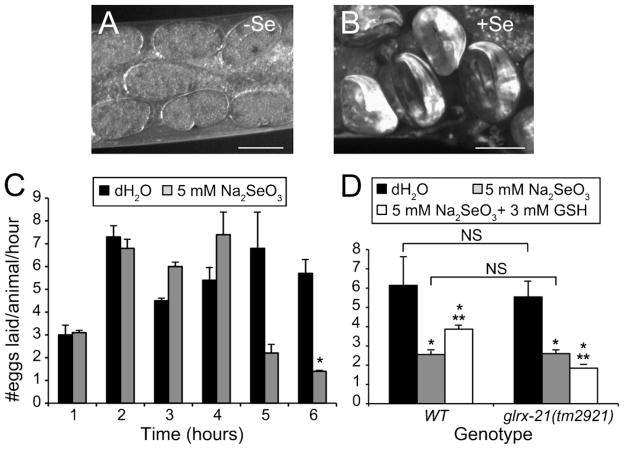
Populations of animals were exposed to Se or mock-exposed for one to 6 h at 25 °C. At 1 h intervals, ten animals were removed from each plate and placed on a “recovery” plate which contained no added Se. After 24 h, the adult animals were removed and the total number of eggs and hatched progeny counted. The number of eggs laid/animal/hour was calculated by dividing the number of eggs and progeny counted on each plate by the number of adult animals originally plated (n = 10), and the number of hours (1–6 h) they were incubated before moving to the recovery plate. Since the 6 h time point showed the greatest difference between Se exposed and unexposed animals, subsequent egg-laying experiments were performed at this time point only.
Serotonin (5-hydroxytrptamine-hydrochloride, Spectrum Chemical, Gardena, CA) was freshly diluted in distilled water prior to assay, and added to agar plates to a final concentration of 3 mg/ml (serotonin plates). Adult animals were exposed to Se or mock-exposed to water for 6 h then transferred to plates with serotonin or an equal volume of carrier (water) (three animals/plate). After 1 h the adult animals were removed and the eggs/plate were counted. The rate of egg-laying was calculated from three plates/condition.
The comparisons between the WT and the glrx-21(tm2921) mutant strains were as above, except as follows. To pretreat with GSH (L-glutathione reduced, Sigma, St. Louis, MO), synchronized late larval stage (L4) animals were placed on agar plates with 3 mM GSH overnight. The next day these pretreated young adults and age matched controls, were placed on agar plates with or without added Se for 6 h, and then moved to recovery plates for 24 h. Five animals from each recovery plate were placed onto four “egg-lay” plates/condition (e.g. control, GSH pretreatment, etc.) and allowed to lay eggs for a total of 3 h before being removed. This assay was repeated four times totaling 16 plates/condition.
2.3. Staining for β-galactosidase activity
Animals expressing the E. coli lacZ reporter gene fused in-frame to 5′ and 3′ sequences of the C. elegans major muscle myosin heavy chain gene, unc-54 were histochemically stained for β-galactosidase activity as described (Szewczyk et al., 2000).
2.4. Staining for filamentous (F)-actin
Animals exposed to Se or mock-exposed for either 24 or 48 h were treated to two to three freeze/thaw cycles to crack their cuticles then fixed overnight at 4 °C in a solution of 4% formaldehyde (http://dx.doi.org/10.1895/wormbook.1.105.1). The fixed animals were washed three times in 1× phosphate buffered saline (PBS; http://dx.doi.org/10.1895/wormbook.1.105.1) then placed in a solution of Alex Fluor-594-phalloidin (Invitrogen, Carlsbad, CA) diluted to 0.2 mU/μl in 1 × PBS for 24–48 h at 4 °C followed by three to four washes with 1 × PBS/1% triton X-100. Animals were stored in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) until visualized.
2.5. Cell counting
In order to count the number of VC neurons expressing Pida-1::gfp, adult animals expressing the construct were Se-exposed or mock-exposed for a total of 48 h. After 24 h, an average 50 each of: mock-exposed animals, Se-exposed normal forward moving, and Se-exposed paralyzed animals were examined on a Leica MZ16FA dissecting stereomicroscope equipped with epifluorescence (Leica Microsystems Inc., Buffalo Grove, IL) and utilizing a GFP filter. The number of VC neuron cells per animal was scored with each animal removed from the plate after counting. Since mortality is directly proportional to time of Se exposure (Morgan et al., 2010), in some instances <50 each of the paralyzed or normal moving animals were left alive on the Se-exposed plates; in those cases all living animals were counted. Additional sets of animals were counted at 48 h. The experiment was repeated three times for strain OM316 and six times for strain BL5717 at both 24 and 48 h. Because VC neuron loss was observed in a small percentage of the mock-exposed animals at both 24 and 48 h, the percentage of Se-exposed animals with VC neuron loss was normalized to this data. This was achieved by subtracting the percentage of mock-exposed animals with VC neuron loss from the percentage of Se-exposed animals with VC neuron loss at the same time points (i.e. 51.8% of Se-exposed animals had VC neurons missing after 24 h. The VC neuron loss for the mock-exposed animals at 24 h was 1.6%, thus the normalized Se-exposed 24 h data presented in Table 1 = 51.8–1.6 = 50.2%).
Table 1.
Effects of Se-exposure on the cholinergic VC motor neurons.
| Strainb | Exposure time | %Population per strain with <6 VC cell bodies ±SEM (n)
|
p valuea
|
||
|---|---|---|---|---|---|
| Se(−) | Se(+)c | [(−) to (+)] | [WT to glrx] | ||
| WT | 24 h | 1.6 ± 0.1 (306) | 50.2 ± 15.4 (581) | 1.1 × 10−3(WT) | 3.5 × 10−2 (−) |
| glrx | 6.0 ± 0.3 (150) | 41.5 ± 9.4 (316) | 2.8 × 10−2(glrx) | 0.40 (+) | |
| WT | 48 h | 3.3 ± 0.3 (305) | 38.1 ± 11.4 (536) | 2.1 × 10−3(WT) | 0.33 (−) |
| glrx | 3.8 ± 1.4 (105) | 58.3 ± 3.7 (177) | 4.2 × 10−2(glrx) | 3.2 × 10−2 (+) | |
p value is determined by comparing the conditions or strains at the top of the column within the condition or strain in parenthesis following the p value.
WT (wild-type) = N2; glrx = glrx-21(tm2921).
Se(+): includes approximately equal ratios of normal forward moving and paralyzed animals; averages are standardized by subtracting the average of baseline control animals [Se(−)] for each experimental set from the corresponding Se-exposed set.
2.6. Fluorescence imaging
Individual animals were visualized on an Olympus BX51 microscope equipped with epifluorescence (Olympus America Inc., Center Valley, PA) and utilizing a FITC (GFP) or Texas Red filter (phalloidin). Images were documented with a QImaging Retiga 1300 camera and imaging system (QImaging, Surrey, British Columbia, Canada) and processed for publication using Adobe CS5 Software (Adobe Systems Inc., San Jose, CA).
2.7. Statistical analysis
Statistical analysis was performed using Excel 2010 with Analysis Toolpak (Microsoft, Seattle, WA). The averages and standard error of means (SEM) reported were calculated from data obtained from all the plates of each strain or population type (e.g. treated or untreated) counted. p values were determined by applying one-way or two-way ANOVA analysis across populations followed by post hoc analysis using two tailed Student’s t-test (to avoid an error of the first kind) or by Student’s t-test only. All Student’s t-tests were two-tailed with unequal variance. Graphs were initially drawn with Excel and were prepared for publication using Adobe Illustrator CS5.
3. Results
3.1. Selenium does not disrupt muscle structure in C. elegans
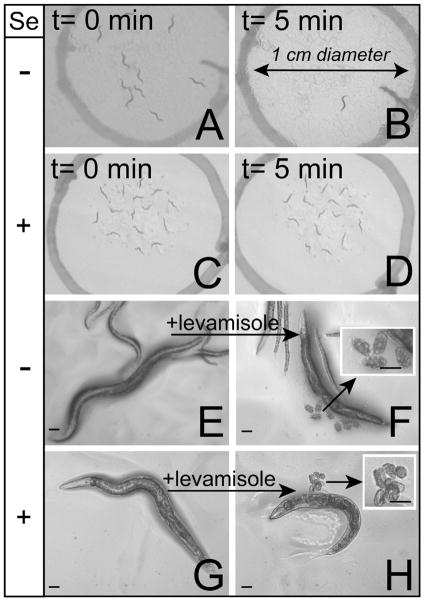
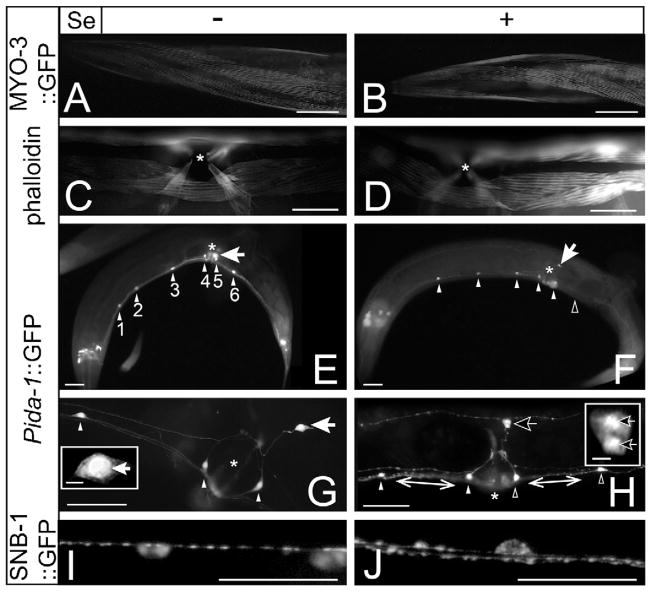
The C. elegans musculature consists of two major types, the body wall and pharyngeal muscles which respectively mediate movement and feeding behaviors, as well as minor muscle groups involved in egg-laying (vulval and uterine), intestinal contraction, defecation and mating (Moerman et al., 1997). Exposure to the anthelmintic levamisole has been reported in C. elegans to cause rapid paralysis due to hyper-contraction of body wall muscles (Lewis et al., 1980) and to induce egg-laying due to contraction of vulval muscles in wild-type (WT) animals (Trent et al., 1983). If the Se-induced progressive movement deficits previously reported (Morgan et al., 2010) resulted from significant damage to muscles, then we hypothesized that animals paralyzed by Se-exposure would not hyper-contract or lay eggs in response to levamisole treatment. This did not prove to be the case. Although all mock-exposed animals were able to leave a 1 cm circle 5 min after being placed within it (Fig. 1A and B), paralyzed animals exposed for 24 h to Se did not (Fig. 1C and D). Yet, despite this difference, both mock-exposed and Se-exposed animals showed no difference in their responses to levamisole, i.e. both exhibited a shortening of body length (i.e. hyper-contraction of body wall muscles; compare Fig. 1E and G to Fig. 1F and H, respectively), and induction of egg-laying (Fig. 1F and H, insets) suggesting that the muscles were not damaged by Se exposure. Further support of this assumption comes from microscopic examination of the myosin and F-actin filaments of body wall and vulval muscles of mock-exposed (Fig. 2A and C, respectively) and Se-exposed animals (Fig. 2B and D, respectively) where no detectable difference in their structural integrity was observed after 48 h of exposure, a time point when less than 20% of the animals were previously shown to exhibit normal movement behavior (Morgan et al., 2010). These data together suggest that in animals exposed to Se, both the body wall and egg-laying muscles were still functionally and structurally intact.
Fig. 1.
Selenium causes paralysis independent of muscle function. Animals paralyzed by high dose Se-exposure responded normally to treatment with levamisole, an anthelmintic which functions as a cholinergic agonist and induces muscle contraction in C. elegans. “+” indicates exposure to 5 mM Na2SeO3 while “−” indicates mock-exposure to water only. Scale bars (E–H, including insets), 50 μm. (A–D) Nearly all mock-exposed adult WT animals leave a 1 cm diameter circle after 5 min (compare A to B) while paralyzed Se-exposed adults do not (compare C to D). (E–H) When treated with the levamisole, both mock- and Se-exposed adults were observed to hyper-contract (compare (E) and (F), and (G) to (H), respectively) and lay eggs (F and H, insets). Late stage embryos were observed in the eggs released by the Se-exposed animals only (H, inset).
Fig. 2.
Neurons, but not muscles appear structurally damaged by selenium. In adult hermaphrodites, exposure to high dose Se damages cholinergic motor neurons in the ventral cord and those required for egg-laying, but does not affect muscles. “*” = vulva (C–H). “Arrowheads” = VC neurons, “arrows” = HSNs, white color indicates normal and black indicates damaged or missing (E–H). “+” and “−”, and scale bars are as in Fig. 1, except inset scale bars = 5 μm. (A and B) Animals expressing an integrated myosin-GFP translational fusion protein (MYO-3::GFP) were grown for 48 h “+” or “−” Se. The regular linear pattern of the MYO-3::GFP in the head (A) was not grossly altered by Se exposure (B). (C and D) Similarly, in phalloidin staining of F-actin no apparent structural differences were observed between mock- (C) or Se-exposed animals (D), as shown in the vulval region. (E–H) Nearly all mock-exposed WT animals expressed the Pida-1::gfp transgene in all six VC neurons and the two HSNs (E) while Se-exposed animals were significantly more likely (Table 1) to have lost GFP expression in one or more VC neurons (F). 400×, mock-exposed animals were observed to exhibit normal cellular morphology in their VC neurons (G) and HSNs (G, inset) while neurons from Se-exposed animals showed evidence of neurodegeneration, including nuclear swelling (H; VC neuron), nuclear fragmentation (H, inset; HSN), and axonal beading (H, double arrowhead lines). (I and J) Presynaptic densities were visualized using an unc-4::snb-1::GFP reporter construct that expresses a fusion of GFP and the C. elegans synaptobrevin protein, SNB-1 in a subset of cholinergic motor neurons in the ventral cord. A regular pattern of expression was observed after 24 h in mock-exposed adult animals (I). This pattern was disrupted in a significant percentage of the Se-exposed population (J).
3.2. Selenium induces neuronal damage and degeneration in C. elegans
Since our examination of both body wall and vulval muscles after Se-exposure could not detect any damage, we next focused our investigation on whether injury to neurons resulted in the observed Se-induced deficits. In order to explore this possibility, we compared expression of the green fluorescent protein (GFP) under the control of the promoter for the ida-1 gene (Zahn et al., 2001) in mock- and Se-exposed animals. The neuroendocrine protein IDA-1 is expressed in about 30 neurons in adult hermaphrodites including eight motor neurons (Zahn et al., 2001): the six VC neurons which lie along the ventral cord and innervate both body wall and vulval muscles (White et al., 1986), and the two hermaphrodite specific neurons (HSNs) which are required for egg-laying (Desai et al., 1988). In mock-exposed WT animals, all six VC neurons were observed under lower power magnification (200×; Fig. 2E, white arrowheads) to be present in over 98% of animals (Table 1) while with Se-exposure greater than 50% of the animals were missing one or more of these neurons after 24 h (Table 1, 24 h and Fig. 2F, black arrowhead). As reported previously (Morgan et al., 2010), the percentage of animals with motility impairment behavior proportionally increased with exposure time. Yet this was not the case for VC neuron cell loss, as the percentage of WT animals with missing cells did not increase significantly after an additional 24 h of exposure time (p = 0.24; Student’s t-test comparing 24–48 h Se-exposed WT animals). This was also true when comparing only animals with the most severe phenotype of paralysis, i.e. no significant difference was observed between the two time points (p = 0.28, Student’s t-test comparing paralyzed 24–48 h Se-exposed WT animals).
Previous studies showed that the GLRX-21 glutaredoxin is required to prevent Se-induced oxidative stress and that animals lacking the protein are more sensitive to Se’s effects on motility than are WT animals (Morgan et al., 2010). Given the role of the VC neurons in movement behavior, we predicted that animals lacking the GLRX-21 protein would show a significantly increased percentage of animals with cell loss in response to Se-exposure. Consistent with this hypothesis, we observed that glrx-21(tm2921) animals expressing the Pida-1::gfp construct were one and a half times more likely than WT animals to be missing a cell after 48 h of Se-exposure although no significant difference was observed between the two strains at the 24 h exposure time (Table 1). The additional observation that mock-exposed glrx-21(tm2921) animals were nearly four times more likely than WT animals to be missing one or more VC neurons after 24 h (Table 1) may partially explain why glrx-21(tm2921) animals are more sensitive to the effects of Se on movement (Morgan et al., 2010). No difference was observed between the two mock-exposed strains after 48 h (Table 1).
Since paralysis occurs prior to death and is the most severe movement behavior observed with high-dose Se-exposure (Morgan et al., 2010), we examined paralyzed animals for direct anatomical evidence of neuronal injury after 24 h Se-exposure under higher magnification (400×; Fig. 2H and J) and compared them to mock-exposed age matched animals (Fig. 2G and I). Nuclear boundary loss (Fig. 2H, VC and HSN) and cellular fragmentation (HSN-Fig. 2H inset) were apparent in the Se-exposed paralyzed animals expressing the Pida-1::gfp transgene, but not in the mock-exposed animals (Fig. 2G and G inset). Additional evidence of neuronal degeneration in Se-exposed animals was observed along the ventral cord and included axonal beading identified in animals expressing the Pida-1::gfp transgene (Fig. 2H, white lines), as well as an irregular pattern of presynaptic densities detected in animals expressing a synaptobrevin (SNB-1) GFP fusion protein (Fig. 2J). This disruption of the SNB-1::GFP pattern was significantly increased (p = 4.0 × 10−3) when comparing populations of Se-exposed [51.3(+4.6)%] to mock-exposed animals [19.2(+1.4)%]. Together, these studies suggest that high dose Se-exposure induces damage to and loss of motor neurons in C. elegans that is consistent with the observed movement deficits reported previously (Morgan et al., 2010).
3.3. Muscles in animals exposed to selenium receive less cholinergic signal
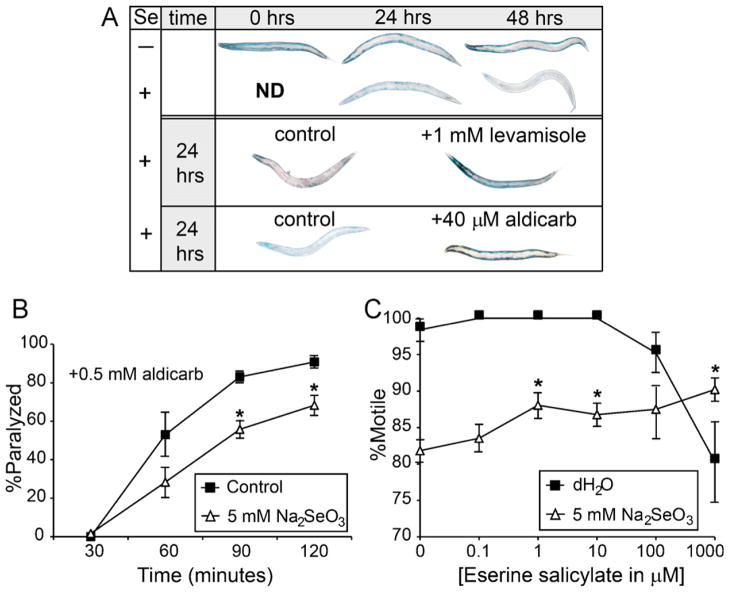
We used an in vivo assay that detects changes in cholinergic signaling through the activity of a soluble myosin-β-galactosidase fusion protein that is stably expressed in both anterior and posterior body wall, and vulval muscles in fed normal animals, but lost in all the posterior body wall muscles after starvation (Szewczyk et al., 2000). This reduction in protein stability upon starvation was shown to be due to reduced cholinergic signaling across the neuromuscular junction (NMJ) (Szewczyk et al., 2000). After just 24 h of Se-exposure, the β-galactosidase activity was reduced in all muscles and was lost completely after 48 h when compared to the age matched mock-exposed controls (Fig. 3A, upper panel). This pattern of activity loss is similar to that described for starved acetylcholine (ACh) deficient animals (Szewczyk et al., 2000) suggesting that cholinergic signaling is similarly reduced by Se-exposure. Animals exposed for 24 h to both Se and either the cholinergic agonist levamisole (Fig. 3A, middle panel), or the acetylcholinesterase (AChE) inhibitor aldicarb (Fig. 3A, lower panel), retained activity in all their muscles similar to controls (Fig. 3A, upper panel, mock-exposed animals at 24 h). The levamisole result was comparable to that previously reported for starved animals, reporter protein stability was maintained throughout the body, and suggests that stimulation of muscle ACh receptors prevented the loss of the β-galactosidase activity (Szewczyk et al., 2000). Aldicarb similarly stabilized the reporter protein expression throughout the animals after Se-exposure, but this was different from the drug’s effect in starved animals where protein stability was only maintained in the anterior region (Szewczyk et al., 2000).
Fig. 3.
Selenium alters cholinergic signaling. Pharmacological agents that increase cholinergic signaling at the NMJ, either by preventing breakdown of ACh (aldicarb and eserine salicylate) or through activating AChRs on muscles (levamisole), reduced movement deficits caused by high dose Se-exposure and prevented Se-induced loss of a soluble muscle protein, but could not induce a time dependent increase in paralysis suggesting that ACh levels at the NMJ are decreased after Se-exposure. (A) Animals expressing a myosin::lac-z reporter were histochemically stained for β-galactosidase activity. Reporter activity was reduced in muscles of Se-exposed, but not mock-exposed animals at 24 and 48 h (upper panel). After 24 h exposure to Se, levamisole (mid panel) and aldicarb (lower panel) treatment both suppressed protein degradation in muscle cells. Controls for the Se-exposed worms at 0 h were not determined (ND) because they were no different from mock-exposed. “+” and “−” are as in Fig. 1. (B) The percentage of adult WT (N2) animals paralyzed by aldicarb gradually increases over time. A significant reduction was observed for the Se-exposed populations (△) that were backing deficient prior to aldicarb treatment as compared to mock-exposed populations (■) at ≤90 min. Data was reported for each time point as the average percentages of 20 paralyzed animals with six repetitions (n = 120). Error bars = ±SEM. *p = 3.2 × 10−17, by two-way ANOVA, comparing Se-exposed to mock-exposed animals at the same time points after aldicarb treatment; post hoc analysis by Student’s t-test. (C) Populations of animals exposed to a concentration range (△, 0.1–1000 μm) of eserine salicylate and Se show a dose dependent increase in the percentage of animals that are motile (move both forwards and backwards) which is significantly different when compared to the Se-exposed controls (△, 0 μm). Although animals not exposed to Se, but treated with the two highest concentrations of eserine salicylate (■, 100 and 1000 μM) were observed to move slower than the water only controls (■, 0 μM), they were not significantly different (p > 0.05 comparing 100 and 1000 μM eserine salicylate with water). *p = 1.4 × 10−2 (one-way ANOVA, comparing Se-exposed and drug treated animals to Se-exposed only animals, post hoc analysis by Student’s t-test). Error bars = ±SEM.
Since AChE inhibitors prevent breakdown of ACh at the synaptic cleft, the observation that aldicarb prevents protein breakdown in the muscles of Se-exposed animals suggests that reduced levels of ACh at the NMJ may contribute to the Se-induced movement deficits. To test this theory, Se-exposed animals were tested for resistance to aldicarb-induced paralysis. If ACh levels are reduced at the neuromuscular synapse, then Se-exposed animals treated with aldicarb would be expected to paralyze slower than mock-exposed animals, similar to what has been classically observed with aldicarb treatment of mutant animals shown to have reduced levels of ACh (Mahoney et al., 2006). Because Se-exposure induces a range of assayable movement deficits (normal, backing, and paralyzed) (Morgan et al., 2010), the population used for the aldicarb assay was normalized by selecting animals that were backing deficient after 24 h of exposure. These animals would be expected to already have sufficient Se-induced damage, but would not already be paralyzed prior to aldicarb treatment. Not surprisingly, the percentage of Se-exposed animals that exhibited aldicarb-induced paralysis was significantly less than that observed for the mock-exposed animals 90 and 120 min after being placed on aldicarb containing agar plates (Fig. 3B). Since nearly all WT animals are paralyzed within 5 h in the presence of 0.5 mM aldicarb (Mahoney et al., 2006), we tested an additional AChE inhibitor, eserine salicylate. Animals treated with eserine salicylate did not paralyze or die after 24 h of exposure although at the highest concentrations tested (100 and 1000 μM) they were observed to be marginally hyper-contracted and slower in movement (Fig. 3C, (■)-H2O). Thus, animals could be exposed to both Se and an AChE inhibitor for 24 h. When populations of animals were coincidentally exposed to Se and eserine salicylate, they showed significantly improved motility across most of the concentration range tested (Fig. 3C, (Δ)-5 mM Na2SeO3). These data together coupled with the levamisole data presented above (Fig. 1E–H) suggest that the damage due to Se-exposure mimics behaviors that would be expected if muscles are experiencing reduced levels of ACh at the NMJ (Miller et al., 1996).
3.4. Reductions in cholinergic muscle reception enhance selenium’s effect on movement
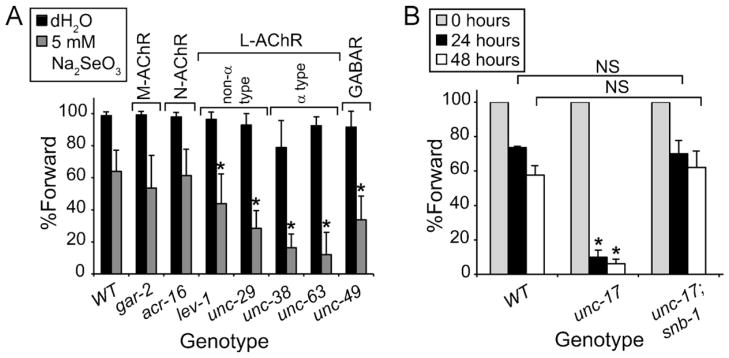
Postsynaptic cells at the NMJ in C. elegans contain three types of receptors, one ionotropic GABAA receptor (GABAR) composed of subunits encoded by the unc-49 gene, and two ACh receptors (AChRs) (Richmond and Jorgensen, 1999). One of the two AChRs is sensitive to nicotine (N-AChR) and is composed of subunits encoded by the acr-16 gene (Touroutine et al., 2005). The second is levamisole sensitive (L-AChR) and composed of a multimer of three α-(LEV-8, UNC-38, UNC-63) and two non-α subunits (LEV-1, UNC-29) (Boulin et al., 2008). GAR-2, a muscarinic AChR (M-AChR) is expressed in motor neurons located along the ventral cord and is not expressed in muscles (Lee et al., 2000). Previous studies (Miller et al., 1996) suggested that animals responding normally to levamisole and resistant to aldicarb-induced paralysis do not have impaired ACh reception. Based on these studies and those presented thus far in this paper, we examined strains containing mutations in genes affecting ACh reception at the NMJ both pre-(gar-2) and post-synaptically (lev-1, unc-29, unc-38, unc-63), and the post-synaptic GABAR (unc-49) for their resistance or sensitivity to Se-induced damage in order to estimate the site of the Se-induced effects. Because some of these mutant strains displayed defects in backwards movement (Hosono and Kamiya, 1991), animals were measured only for their ability to move forward as previously described (Morgan et al., 2010). Animals containing mutations in any of the levamisole-sensitive AChR subunits tested, lev-1, unc-29, unc-38, and unc-69, showed significantly greater sensitivity to Se-induced damage as indicated by a decrease in the percentage of Se-exposed animals moving forward normally while both the M- and N-AChR mutant animals were no different from WT (Fig. 4A). Animals with a mutation in the unc-49 GABAR were also significantly more sensitive to Se-exposure (Fig. 4A). Thus, Se seems to exert its greatest effect on movement behavior in those strains containing mutations affecting post-synaptic reception while the presynaptic GAR-2 M-AChR was not affected. Coupled with the anatomical observations that Se induced damage to neurons (Fig. 2E–J), but not muscles (Fig. 2A–D), these data support the hypothesis that Se induces damage to the presynaptic motor neurons affecting the levels of ACh available to muscles.
Fig. 4.
Presynaptic cholinergic release is altered by selenium. Mutations in some genes which reduce either post-synaptic muscle reception (lev-1, unc-29, unc-38, unc-63) or affect ACh release from motor neurons (unc-17) adversely alter forward movement behavior in response to high dose Se exposure. (A) Reduction- or loss-of-function mutations in some of the C. elegans AChRs (lev-1, unc-29, unc-38, unc-63) and one GABAR (unc-49), but not others (acr-16, gar-2), conferred additional sensitivity to Se (black bars) when compared to WT animals as indicated by a decrease in the percentage of animals within each population that were able to move forward; black bars = mock-exposed controls. Each graph bar represents an average of four plates with twenty animals each repeated three to four times (average n/graph bar = 300); error bars = ±SEM. “non-α” and “α” refer to the subtypes of the L-AChR. *p = 2.8 × 10−20, by one-way ANOVA comparing the Se-exposed mutant strains to the Se-exposed WT strain, post hoc analysis was by Student’s t-test. (B) Populations of animals with a reduction-of-function mutation (e245) in the C. elegans VAChT, unc-17 had significantly less normal forward movement in comparison to WT populations of animal after 24 h (black bars) and 48 h (white bars) of Se exposure. A dominant mutation (e1563) in the gene for the vesicle-associated membrane protein, synaptobrevin (snb-1) was able to suppress this unc-17(e245) Se-induced phenotype such that there was no significant difference (NS) between populations of the unc-17;snb-1 double mutant strain when compared to WT populations exposed for the same amount of time (Student’s t-test). Since animals with the unc-17(e245) mutation do not normally move backwards, forward movement only was scored such that direct comparisons of the three strains could be made. Each graph bar represents at least five plates of twenty animals (n ≥ 100) of each genotype (WT, unc-17, unc-17;snb-1) which were scored at 24 and 48 h to determine the percentage of animals within each population expressing each phenotype; error bars = ±SEM. *p = 6.8 × 10−7 (two-way ANOVA comparing unc-17 to both WT and unc-17;snb-1 across phenotypes and time, post hoc analysis was by Student’s t-test).
3.5. Pre-synaptic defect in acetylcholine release induces hypersensitive to selenium
Mutations in the unc-17 gene which encodes the vesicular ACh transporter (VAChT) (Alfonso et al., 1993) confer an uncoordinated-coiler phenotype with difficulty backing (Rand and Russell, 1984) and resistance to aldicarb (Nguyen et al., 1995). Although unc-17 mutants have normal ACh levels (Nguyen et al., 1995), their resistance to aldicarb suggests a reduction in ACh secretion. Synaptobrevin proteins are part of the SNARE (soluble N-ethylmaleimide-sensitive-factor attachment protein receptor) complex that is involved in fusion at the plasma membrane of neurotransmitter filled vesicles. In C. elegans, the synaptobrevin protein is encoded by snb-1 (Nonet et al., 1998). The uncoordinated and aldicarb resistance phenotypes of the unc-17(e245) mutant are suppressed by the dominant snb-1(e1563) allele by presumably restoring secretion of ACh to near normal levels (Sandoval et al., 2006). Because our cumulative data suggested that ACh levels at the NMJ were reduced after Se-exposure (Figs. 3 and 4A), we tested both the unc-17(e245) and unc-17(e245);snb-1(e1563) strains for their sensitivity to the Se-induced motility deficits (Fig. 4B). Animals with a mutation in the unc-17 gene only were significantly more sensitive to the Se-induced effects on movement (Fig. 4B) while the unc-17(e245);snb-1(e1563) was no different in its response than was the WT strain, N2 (Fig. 4B). Hence, these data suggest that a reduction in ACh reception at the NMJ is sufficient to increase sensitivity to Se-exposure most likely due to decreases in presynaptic release of ACh.
3.6. Glutathione requires GLRX-21 glutaredoxin to reduce Se-induced egg-laying defect
Egg-laying in C. elegans is a well-defined circuit involving eight neurons and 16 vulval and uterine muscles (White et al., 1986). Animals that are abnormal in egg-laying are termed egg-laying defective (e.g. do not lay eggs either completely or at a slower rate than normal) (Trent et al., 1983) or egg-laying constitutive (e.g. lay eggs continuously without regard to normal inhibitory cues) (Bany et al., 2003). In studies of the progressive impairment of motility seen in animals exposed to high dose Se, we noted that Se-exposed animals retained late stage embryos, thus displaying an egg-laying defective (Egl-d) phenotype (Fig. 1H inset and 5B) that was not observed in mock-exposed animals (Figs. 1F inset and 5A). In order to definitively determine if high dose Se exposure caused a statistically significant effect on egg-laying, we measured the egg-laying rate (eggs laid/animal/hour) of age-synchronized WT adult animals growing on agar plates with or without Se for 1–6 h (Fig. 5C). Under these conditions, Se-exposure reduced their overall egg-laying rate by 75% after 6 h when compared to mock-exposed controls (Fig. 5C). Intriguingly, at the same 6 h time point we had observed severe motility impairment (paralyzed or dead) in only 30% of the Se-exposed animals (Morgan et al., 2010). This implies that substantial and detectable amounts of damage to the egg-laying circuit must occur at a much earlier time point than damage to the motor circuit.
Fig. 5.
Decreased egg-laying rates after selenium-exposure. Animals exposed to high dose Se were not only observed to retain late stage embryos, but to reduce their egg-laying rate overtime, a phenomena that appeared to be partially mediated through oxidative stress-induction. (A and B) Adult animals expressing GFP under the control of the promoter for the pan-neuronal expression gene, unc-119 and maintained continuously in the presence of an adequate food supply do not normally retain embryos past the multicellular stages (A) although late stage embryos (two-fold and beyond) were observed after only 24 h exposure to Se under the same conditions (B). “+” and “−”, and scale bars are as in Fig. 1. (C) The egg-laying rate (#eggs laid/animal/hour) of WT animals was reduced after 6 h of exposure to Se as compared to mock-exposed animals (H2O). Each bar graph represents the average progeny from 30 animals (three plates of ten animals each); error bars = ±SEM. *p = 1.9 × 10−2, comparing Se-exposed to mock-exposed animals at the same time point, Student’s t-test (two-tailed, unequal variance). (D) The Se-induced reduction in the egg-laying rate was mediated by oxidative stress since treatment with the cellular antioxidant glutathione (GSH) was able to partially rescue this phenotype. In addition, the glrx-21(tm2921) null mutant could not be rescued by the GSH pretreatment. NS, not significant (Student’s t-test). *p = 4.1 × 10−13-WT and p = 3.8 × 10−14-glrx-21 (one-way ANOVA comparing across conditions within strains). **p = 1.6 × 10−2-WT and p = 5.2 × 10−4-glrx-21, comparing “Se-exposed only” to “Se-exposed pretreated with GSH” animals within the same strain (Student’s t-test).
The application of exogenous serotonin has been demonstrated to stimulate egg-laying behavior through its direct action on serotonin receptors located on the egg-laying muscles (Shyn et al., 2003; Carre-Pierrat et al., 2006; Hobson et al., 2006). If exogenous application of serotonin could rescue the reduction in the egg-laying rate observed after Se-exposure (Fig. 5C), then it could be presumed that the primary site of the Se-induced damage to egg-laying lies primarily in the neurons controlling egg-laying. Conversely, if it did not rescue the egg-laying rate then muscles or both may be affected (Shyn et al., 2003). Therefore, in order to clarify the site of Se damage, exogenous serotonin was applied to age-synchronized adult WT populations exposed with or without Se for 6 h (Table 2). Here it was found that serotonin was able to significantly increase the egg-laying rates of both populations of animals (Table 2). This data coupled with those of the levamisole studies presented above (Fig. 1E–H) suggest that Se-exposure primarily induces damage to the neurons controlling the egg-laying process while sparing muscle function.
Table 2.
Effects of serotonin on egg-laying rates after Se-exposure.
| Se | #Eggs laid/animal/hour ± SEMa
|
p valuesb | ||
|---|---|---|---|---|
| Water (H2O) | Serotonin (5HT)c | [H2O to 5HT] | [(−) to (+)] | |
| − | 3.9 ± 0.3 | 15.5 ± 0.7 | 8.0 × 10−4 (−) | 6.4 × 10−3 (H2O) |
| + | 1.8 ± 0.2 | 11.1 ± 0.5 | 1.1 × 10−3 (+) | 7.6 × 10−3 (5HT) |
Eggs from three plates containing three adult animals each (progeny from nine
animals) per condition [H2O or 5HT, and Se(−) or Se(+)].
p value is determined by comparing the conditions at the top of the column within the condition in parenthesis following the p value.
Final serotonin concentration on agar plates = 3 mg/ml.
Previous studies have shown that the progressive Se-induced movement phenotype was affected by oxidative stress damage (Morgan et al., 2010). Based on this, we predicted that the Se-induced Egl-d phenotype was mediated similarly. To test this hypothesis WT animals were pretreated with the cellular antioxidant reduced glutathione (GSH) or mock-pretreated with water prior to being placed on culture plates with high dose Se, then scored for their egg-laying ability after 6 h of Se-exposure (Fig. 5D). These studies found that Se-exposed animals pretreated with GSH had a significantly increased rate of egg-laying when compared to animals not pretreated with GSH prior to Se exposure (Fig. 5D) suggesting that Se induces oxidative stress damage to neurons involved in movement and the egg-laying behavior.
Glutaredoxins are well known GSH-dependent oxidoreductases that have been shown to utilize Se as a substrate (Wallenberg et al., 2010). We have previously shown that GLRX-21, one of the five worm glutaredoxins, mediates the protective effects of GSH on Se-induced lethality (Morgan et al., 2010). Similarly, animals with a null mutation in glrx-21(tm2921) did not increase their egg-laying rate after Se exposure with GSH pretreatment (Fig. 5D). This observation is consistent with a role for the antioxidant GLRX-21 glutaredoxin in mediating the protective effects of GSH against Se-induced oxidative damage that impairs the egg-laying behavior.
4. Discussion
4.1. Overview
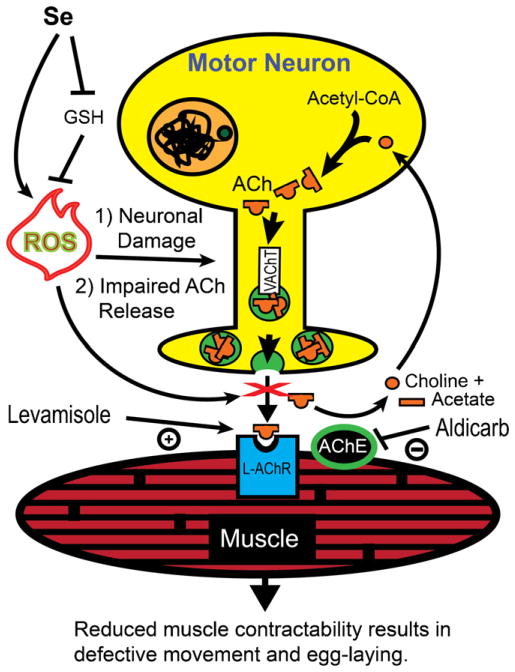
The range of tissue damage observed in various vertebrate species exposed to toxic levels of Se in their environment has shown that Se can induce necrotic changes in multiple organs and tissues including muscles and the nervous system (Koller and Exon, 1986). The mechanisms mediating this observed tissue damage have begun to be defined in various model systems including C. elegans where it has recently been shown that Se induces oxidative stress capable of causing lethality and paralysis in a dose dependent manner (Morgan et al., 2010). Here we provide evidence that high dose Se exposure exerts this effect by inducing damage to and degeneration of cholinergic motor neurons affecting two essential behavioral phenotypes in C. elegans, locomotion and egg-laying (summarized in Fig. 6). In addition, we present evidence that the GLRX-21 glutaredoxin is required in egg-laying neurons to mediate glutathione-induced protection against oxidative stress as was observed previously for lethality and movement (Morgan et al., 2010), and for protection from age-related cell loss of motor neurons.
Fig. 6.
Summary. Motor neuron signaling to muscles is altered by selenium-induced oxidative stress. Reduced glutathione (GSH) normally prevents reactive oxygen species (ROS) from damaging cells. Excess selenium (Se) from the environment can both increase the formation of ROS and decrease the pool of GSH, thus inducing motor neuron (yellow) degeneration and impairing release of acetylcholine (ACh) filled synaptic vesicles (green circles) loaded by the vesicular acetylcholine transporter (VAChT). The reduction in ACh available to muscles reduces contractility, but does not affect the function of muscles since exogenous application of the pharmaceutical aldicarb induces muscle paralysis by artificially increasing ACh in the neuromuscular junction (space between muscle and neuron). Aldicarb inhibits the action of the enzyme acetylcholinesterase (AChE) which normally functions to inactivate ACh by breaking it down into its constituent parts choline (orange circle) and acetate (orange rectangle). In addition, the levamisole-sensitive ACh receptors (L-AChR) still respond by causing muscle hyper-contraction when the AChR agonist levamisole is exogenously applied. ChAT = choline acetyl transferase. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4.2. Altered acetylcholine signaling a mechanism for selenium-induced toxicity in C. elegans
The observed reduction in egg-laying rates after Se-exposure described here, like the decreased locomotion rates previously described (Morgan et al., 2010), point to the neuromuscular system as a target for Se damage. The ability of levamisole which acts on the post-synaptic side of the NMJ (Martin et al., 2005), to induce muscle contraction in Se-exposed animals (Fig. 6), and the observation that myosin and actin filaments in the body wall and egg-laying muscles appeared to be unaffected by Se-exposure suggested that Se does not directly damage muscles, but instead alters the levels of cholinergic signaling. This was supported by our observations that animals with mutations that altered cholinergic reception on muscles were more sensitive to Se’s effects on movement. Although both the L-AChR and N-AChR are localized to muscles (Touroutine et al., 2005; Richmond and Jorgensen, 1999), this increased sensitivity was only significant in strains containing mutations in the L-AChR subunits (LEV-1, UNC-29, UNC-38, UNC-63; Fig. 4A) and parallels previous studies in C. elegans that have demonstrated the function of the L-AChR has a greater impact on locomotion then does the N-AChR (Touroutine et al., 2005). Interestingly, a reduction-of function mutation in the muscle GABAR encoding gene unc-49 (Richmond and Jorgensen, 1999) also demonstrated enhanced sensitivity to Se-induced paralysis suggesting GABAergic signaling is potentially protective against Se-induced toxicity, and that disinhibition of signaling through some other pathway normally inhibited by UNC-49 function may potentiate Se-induced neurotransmission problems. Although these studies could not rule out the possibility that Se induces damage to sub-cellular muscle compartments (e.g. mitochondria), a recent study examining mutations in genes affecting movement behavior suggested that these types of behavioral effects were least likely to result from sub-cellular muscle defects (Shephard et al., 2011).
High dose Se levels induced catabolism of a soluble cytosolic reporter protein expressed in muscle that has been shown previously to occur with impaired cholinergic signaling (Szewczyk et al., 2000; Corbett et al., 1982). This catabolism could be efficiently suppressed by either increasing cholinergic signaling through levamisole-induced AChR activation or by suppressing ACh breakdown at the NMJ with the AChE inhibitor aldicarb. That the pattern of suppression observed with levamisole and aldicarb treatment of Se-exposed animals did not directly mimic that observed in starved animals where levamisole, but not aldicarb, was observed to completely suppress catabolism (Szewczyk et al., 2000) suggests that Se unlike starvation does not induce anterior–posterior distinctions, but rather causes a global decrease in the levels of ACh signaling measured within this assay. Gene mutations which decrease cholinergic synaptic transmission are resistant to aldicarb-induced paralysis (Miller et al., 1996). Similarly, animals which could not move backwards after suffering Se-induced damage were significantly more resistant to the paralyzing effects of aldicarb relative to control animals. That Se-damaged animals were slower to paralyze than their mock-exposed counterparts in the presence of the AChE-inhibitor aldicarb supports a hypothesis that exposures to high dose Se reduces the amount of ACh available at the NMJ (Fig. 6). In addition, mutant animals with impaired synaptic release of ACh were more sensitive to the Se-induced loss of motility as demonstrated by the susceptibility of animals with a reduction-of-function mutation (e245) in the unc-17 gene which impairs vesicular loading of ACh (Alfonso et al., 1993). Loss of GAR-2/M-AChR which has been shown to initiate a negative feedback loop on cholinergic motor neurons in response to increased levels of ACh (Dittman and Kaplan, 2008) did not increase the Se-induced movement defects. This result is consistent with our hypothesis because GAR-2 function responds to increased levels of ACh at the NMJ and should therefore not affect movement if high dose Se-exposure reduces ACh levels. Together this evidence supports a neurotoxicity model in which high levels of Se-exposure in the environment directly cause loss of cholinergic signaling that results in the effects on motility observed and is consistent with previous studies hinting at Se-induced cholinergic signaling defects (Li et al., 2011).
4.3. Direct evidence of motor neuron degeneration with toxic selenium exposure models neurodegenerative disease
In our examination of Se-exposed C. elegans adults, the cholinergic motor neurons in their ventral cords exhibited signs of neurodegeneration that included axonal beading, cellular swelling, and nuclear cytoplasmic boundary loss and fragmentation. These changes bore striking similarities to the Se-induced neuronal pyknosis and necrosis observed in the ventral horns of pigs (Nathues et al., 2010; Panter et al., 1996), as well as the cellular blebbing of hepatocytes from rainbow trout exposed to SeMet (Misra et al., 2012). Se-exposure resulted in a significant increase in the percentage of WT adult animals with motor neuron cell loss suggesting that the neurodegenerative processes observed in the Se-exposed worms lead to cell death. This accumulation of degeneration and cell loss in the cholinergic motor neurons of the ventral cord most likely contributes to the increasing motility impairment observed over time with Se exposure (Morgan et al., 2010), a phenomenon that has been documented to occur in neurodegenerative disease models of ALS, Huntington’s and Parkinson’s Diseases from Drosophila, mice, and C. elegans (Estes et al., 2011; Tamura et al., 2011; Bonilla-Ramirez et al., 2011; Chesselet and Richter, 2011; Kanning et al., 2010; Cao et al., 2010; Wang et al., 2009; Morley et al., 2002; Mangiarini et al., 1996). Additionally, the disruption of the orderly array of the presynaptic densities observed along the ventral cord after Se-exposure paralleled changes observed at the NMJ within the SOD1 mouse model of ALS (Fischer et al., 2004) further suggesting that the neurodegenerative process targeting cholinergic neurons initiated by Se toxicity is similar to changes observed during neurodegen-erative disease processes that target motor neurons in other organisms.
4.4. GLRX-21 glutaredoxin is required for preventing age-related loss of motor neurons
Although nerve cell integrity is generally well maintained during normal aging in comparison to other tissues, enhanced cellular stress is known to increase age-related neuronal cell death in C. elegans and other organisms (Takács-Vellai et al., 2006). Intriguingly, we observed that mock-exposed animals lacking the GLRX-21 protein were more likely than WT animals to be missing at least one cholinergic VC neuron at the 24 h time point, suggesting that GLRX-21 protects against age-related neuron loss. This difference was most likely not developmental, or due to programmed cell death, since VC neuron loss was random rather than cell-specific. The base-line difference between WT and the glrx-21(tm2921) animals when not exposed to Se goes away by the 48 h time point suggesting that compensatory antioxidant protective mechanisms might be activated in the absence of normal GLRX-21 function to prevent further neuron loss. If cellular protection against ROS is impaired in animals lacking GLRX-21, the additional oxidative stress provided by Se-exposure should again increase cell loss in the glrx-21(tm2921) animals versus WT. This was observed in the older glrx-21(tm2921) animals which showed an increased prevalence of cell loss over WT animals when examined at the 48 h time point after Se-exposure. Thus, GLRX-21 appears to be required both to mediate GSH oxidative stress relief, and to prevent age related ROS-induced cell loss in the cholinergic VC neurons.
5. Conclusion
We have demonstrated that exposure to high dose Se in the environment is sufficient to induce neuronal damage and cell loss and to alter cholinergic signaling in C. elegans. Although we have shown previously that the GLRX-21 protein was required to mediate the antioxidant effects of GSH, here we present evidence for a role in cell maintenance of cholinergic motor neurons under oxidative stress. Intriguingly, a GLRX-21 ortholog, glutaredoxin 2 has been shown to prevent aggregation of mutant SOD1 in a mouse model of ALS, a neurodegenerative disease affecting cholinergic motor neurons (Ferri et al., 2010). The association between high dose environmental Se and the increased risk of ALS within exposed populations (Vinceti et al., 2010), coupled with the data presented suggests that an ALS model based on Se-induced oxidative stress may be an alternative and complimentary model to those based on SOD1 for examining the connections between oxidative stress and ALS pathology.
Acknowledgments
We would like to thank L.A. Jacobson and D. Miller for providing strains PJ727 and NC571, respectively. This research was supported by grants from the Jim Himelic Foundation (AE and ME), Southern Arizona Foundation (ME), Keatings Institute (ME), National Institutes of Environmental Health Sciences [R21-ES012305 to AE and ME], Arthritis and Musculoskeletal and Skin Diseases [R01-AR054342 to NJS], Instituto de Salud Carlos III [Projects PI050065 and PI080557, co-financed by Fondo Social Europeo, FEDER to AMV] and Junta de Andalucía [Projects CVI-3629 and CVI-2697to AMV]. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
DISCLOSURE
The study sponsor had no involvement in study design, collection, analysis, and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Contributor Information
Annette O. Estevez, Email: aestevez@email.arizona.edu.
Catherine L. Mueller, Email: clm58@email.arizona.edu.
Kathleen L. Morgan, Email: kathleen@immunetrics.com.
Nathaniel J. Szewczyk, Email: Nathaniel.Szewczyk@nottingham.ac.uk.
Luke Teece, Email: lteece89@brandeis.edu.
Antonio Miranda-Vizuete, Email: amiranda-ibis@us.es.
References
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261(5121):617–9. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Bany IA, Dong M-Q, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23(22):8060–9. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422(1):11–22. doi: 10.1042/BJ20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study Parkinsonism. Biometals. 2011;24(6):1045–57. doi: 10.1007/s10534-011-9463-0. [DOI] [PubMed] [Google Scholar]
- Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105(47):18590–5. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Yuan Y, Pehek EA, Moise AR, Huang Y, Palczewski K, et al. Alpha-synuclein disrupted dopamine homeostasis leads to dopaminergic neuron degeneration in Caenorhabditis elegans. PLoS One. 2010;5(2):e9312. doi: 10.1371/journal.pone.0009312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre-Pierrat M, Baillie D, Johnsen R, Hyde R, Hart A, Granger L, et al. Characterization of the Caenorhabditis elegans G protein-coupled serotonin receptors. Invert Neurosci. 2006;6(4):189–205. doi: 10.1007/s10158-006-0033-z. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10(12):1108–18. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- Corbett AJ, Griggs RC, Moxley RT., 3rd Skeletal muscle catabolism in amyotrophic lateral sclerosis and chronic spinal muscular atrophy. Neurology. 1982;32(5):550–2. doi: 10.1212/wnl.32.5.550. [DOI] [PubMed] [Google Scholar]
- de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336(6200):638–46. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- Dimitriadi M, Hart AC. Neurodegenerative disorders: insights from the nematode Caenorhabditis elegans. Neurobiol Dis. 2010;40(1):4–11. doi: 10.1016/j.nbd.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J Neurosci. 2008;28(28):7104–12. doi: 10.1523/JNEUROSCI.0378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Han HP, Fields SD, Rand JB. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 2008;506(3):398–408. doi: 10.1002/cne.21551. [DOI] [PubMed] [Google Scholar]
- Estevez M, Estevez AO, Cowie RH, Gardner KL. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J Neurochem. 2004;88(1):102–13. doi: 10.1046/j.1471-4159.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- Estes PS, Boehringer A, Zwick R, Tang JE, Grigsby B, Zarnescu DC. Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Hum Mol Genet. 2011;20(12):2308–21. doi: 10.1093/hmg/ddr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–83. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Ferri A, Fiorenzo P, Nencini M, Cozzolino M, Pesaresi MG, Valle C, et al. Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity. Hum Mol Genet. 2010;19(22):4529–42. doi: 10.1093/hmg/ddq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185(2):232–40. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fostel JL, Benner Coste L, Jacobson LA. Degradation of transgene-coded and endogenous proteins in the muscles of Caenorhabditis elegans. Biochem Biophys Res Commun. 2003;312(1):173–7. doi: 10.1016/j.bbrc.2003.09.248. [DOI] [PubMed] [Google Scholar]
- Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki PR, Komuniecki RW. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics. 2006;172(1):159–69. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R, Kamiya Y. Additional genes which result in an elevation of acetylcholine levels by mutations in Caenorhabditis elegans. Neurosci Lett. 1991;128(2):243–4. doi: 10.1016/0304-3940(91)90270-4. [DOI] [PubMed] [Google Scholar]
- Hung W, Hwang C, Po MD, Zhen M. Neuronal polarity is regulated by a direct interaction between a scaffolding protein, Neurabin, and a presynaptic SAD-1 kinase in Caenorhabditis elegans. Development. 2007;134(2):237–49. doi: 10.1242/dev.02725. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, et al. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009;1281:117–27. doi: 10.1016/j.brainres.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–40. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Koller LD, Exon JH. The two faces of selenium deficiency and toxicity are similar in animals and man. Can J Vet Res. 1986;50:297–306. [PMC free article] [PubMed] [Google Scholar]
- Lee Y-S, Park YS, Nam S, Suh SJ, Lee J, Kaang BK, et al. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 2000;75:1800–9. doi: 10.1046/j.1471-4159.2000.0751800.x. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5(6):967–89. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Li WH, Hsu FL, Liu JT, Liao VH. The ameliorative and toxic effects of selenite on Caenorhabditis elegans. Food Chem Toxicol. 2011;49(4):812–9. doi: 10.1016/j.fct.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Lin-Shiau SY, Liu SH, Fu WM. Neuromuscular actions of sodium selenite on chick biventercervicis nerve-muscle preparation. Neuropharmacology. 1990;29(5):493–501. doi: 10.1016/0028-3908(90)90172-n. [DOI] [PubMed] [Google Scholar]
- Mahoney TR, Luo S, Nonet ML. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat Protoc. 2006;1(4):1772–7. doi: 10.1038/nprot.2006.281. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Bates GP Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Verma S, Levandoski M, Clark CL, Qian H, Stewart M, et al. Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology. 2005;131:S71–84. doi: 10.1017/S0031182005008668. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364(6435):337–41. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci U S A. 1996;93:12593–8. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Hamilton C, Niyogi S. Induction of oxidative stress by selenomethionine in isolated hepatocytes of rainbow trout (Oncorhynchus mykiss) Toxicol ceitalicIn Vitro/ceitalic. 2012 doi: 10.1016/j.tiv.2012.02.001. http://dx.doi.org/10.1016/j.tiv.2012.02.001. [DOI] [PubMed]
- Moerman GD, Fire A. Muscle: structure, function, and development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. New York: Cold Spring Harbor; p. 1997418418. [PubMed] [Google Scholar]
- Morgan KL, Estevez AO, Mueller CL, Cacho-Valadez B, Miranda-Vizuete A, Szewczyk NJ, et al. The glutaredoxin GLRX-21 functions to prevent selenium-induced oxidative stress in Caenorhabditis elegans. Toxicol Sci. 2010;118(2):530–43. doi: 10.1093/toxsci/kfq273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99(16):10417–22. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathues H, Boehne I, grosse Beilage T, Gerhauser I, Hewicker-Trautwein M, Wolf P, et al. Peracute selenium toxicosis followed by sudden death in growing and finishing pigs. Can Vet J. 2010;51(5):515–8. [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Alfonso A, Johnson CD, Rand JB. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics. 1995;140(2):527–35. doi: 10.1093/genetics/140.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18(1):70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO. Comparative toxicity of selenium from seleno-DL-methionine, sodium selenate, and Astragalus bisculcatus in pigs. Fundam Appl Toxicol. 1996;32:217–23. [PubMed] [Google Scholar]
- Rand JB, Russell RL. Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics. 1984;106(2):227–48. doi: 10.1093/genetics/106.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rederstorff M, Krol A, Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci. 2006;63(1):52–9. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One G.A.B.A two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–7. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval GM, Duerr JS, Hodgkin J, Rand JB, Ruvkun G. A genetic interaction between the vesicular acetylcholine transporter VAChT/UNC-17 and synaptobrevin/SNB-1 in C. elegans. Nat Neurosci. 2006;9(5):599–601. doi: 10.1038/nn1685. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev. 2004;45(3):164–78. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shephard F, Adenle AA, Jacobson LA, Szewczyk NJ. Identification and functional clustering of genes regulating muscle protein degradation from amongst the known C. elegans muscle mutants. PLoS One. 2011;6(9):e24686. doi: 10.1371/journal.pone.0024686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Kerr R, Schafer WR. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr Biol. 2003;13:1910–5. doi: 10.1016/j.cub.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Luke CJ, Bhatia SR, Long OS, Vetica AC, Perlmutter DH, et al. Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatr Res. 2009;65(1):10–8. doi: 10.1203/PDR.0b013e31819009b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Pfiefer E. Two fatal cases of selenium toxicity. Forensic Sci Int. 2007;171(1):67–72. doi: 10.1016/j.forsciint.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, Hartman JJ, Barmada SJ, Jacobson LA. Genetic defects in acetylcholine signaling promote protein degradation in muscle cells of Caenorhabditis elegans. J Cell Sci. 2000;113(Pt 11):2003–10. doi: 10.1242/jcs.113.11.2003. [DOI] [PubMed] [Google Scholar]
- Takács-Vellai K, Bayci A, Vellai T. Autophagy in neuronal cell loss: a road to death. Bioessays. 2006;28(11):1126–31. doi: 10.1002/bies.20489. [DOI] [PubMed] [Google Scholar]
- Tamura T, Sone M, Iwatsubo T, Tagawa K, Wanker EE, Okazawa H. Ku70 alleviates neurodegeneration in Drosophila models of Huntington’s disease. PLoS One. 2011;6(11):e27408. doi: 10.1371/journal.pone.0027408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, 3rd, Richmond JE. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem. 2005;280:27013–21. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104(4):619–47. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Götz J, et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci U S A. 2010;107(31):13888–93. doi: 10.1073/pnas.1009038107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Bonvicini F, Bergomi M, Malagoli C. Possible involvement of overexposure to environmental selenium in the etiology of amyotrophic lateral sclerosis: a short review. Ann Ist Super Sanita. 2010;46(3):279–83. doi: 10.4415/ANN_10_03_09. [DOI] [PubMed] [Google Scholar]
- Wallenberg M, Olm E, Hebert C, Björnstedt M, Fernandes AP. Selenium compounds are substrates for glutaredoxins: a novel pathway for selenium metabolism and a potential mechanism for selenium-mediated cytotoxicity. Biochem J. 2010;429(1):85–93. doi: 10.1042/BJ20100368. [DOI] [PubMed] [Google Scholar]
- Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, et al. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009;5(1):e1000350. doi: 10.1371/journal.pgen.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wood-Allum CA, Barber SC, Kirby J, Heath P, Holden H, Mead R, et al. Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen. Brain. 2006;129(Pt 7):1693–709. doi: 10.1093/brain/awl118. [DOI] [PubMed] [Google Scholar]
- Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F. Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem. 2003;84(3):438–46. doi: 10.1046/j.1471-4159.2003.01531.x. [DOI] [PubMed] [Google Scholar]
- Zahn TR, Macmorris MA, Dong W, Day R, Hutton JC. IDA-1, a Caenorhabditis elegans homolog of the diabetic autoantigensIA-2 and phogrin, is expressed in peptidergic neurons in the worm. J Comp Neurol. 2001;429(1):127–43. doi: 10.1002/1096-9861(20000101)429:1<127::aid-cne10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]