Abstract
Myeloid cells are the most abundant nucleated hematopoietic cells in the human body and are a collection of distinct cell populations with many diverse functions. The three groups of terminally differentiated myeloid cells — macrophages, dendritic cells and granulocytes — are essential for the normal function of both the innate and adaptive immune systems. Mounting evidence indicates that the tumour microenvironment alters myeloid cells and can convert them into potent immune suppressive cells. Here, we consider myeloid cells as an intricately connected, complex, single system and we focus on how tumours manipulate the myeloid system to evade the host immune response.
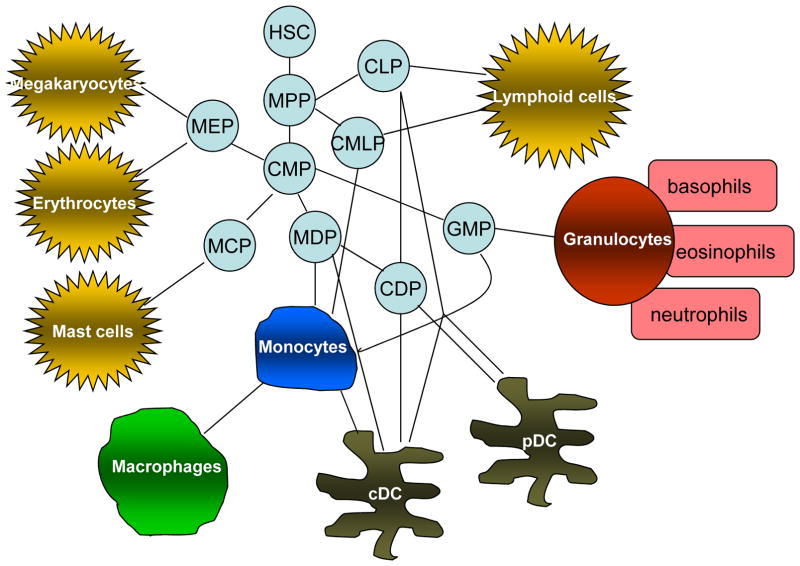
Myeloid cells are the most abundant hematopoietic cells in the human body with diverse functions. All myeloid cells arise from multipotent hematopoietic stem cells (HSCs) that develop into mature myeloid cells through sequential steps of differentiation. However, myeloid progenitors do not form a hierarchical system but can instead be considered as a network of cells that can differentiate into various more specialized myeloid cell subsets (Fig. 1).
Figure 1. Myeloid cell differentiation under normal physiological conditions.
Myeloid cells are a subpopulation of hematopoietic cells and originate from a network of hematopoietic stem cells (HSC) and multi-potent progenitor cells (MPP). The network of progenitor cells that gives rise to various hematopoietic cells includes common myeloid progenitor cells (CMP); common lymphoid progenitor cells (CLP); MΦ and DC progenitors (MDP); common DC progenitors (CDP), granulocyte/macrophage progenitors (GMP), megakaryocyte/erythroid progenitors (MEP); - mast cell progenitors (MCP). cDC – conventional DCs, pDC – plasmacytoid DCs
The three groups of terminally differentiated myeloid cells — macrophages (MΦ), dendritic cells (DCs) and granulocytes (G) — are essential for the normal functions of the innate and adaptive immune systems. Classically, they protect organisms from pathogens, eliminate dying cells, and mediate tissue remodeling. Although the contribution of myeloid cells to tumour pathogenesis has been recognized for over 100 years, only during the past two decades has their crucial role in promoting tumour angiogenesis, cell invasion, and metastasis been appreciated (reviewed in1–3). Mast cells were also implicated in regulation of tumor progression (reviewed in4). Mounting evidence indicates that the tumour microenvironment alters myeloid cells by converting them into potent immunosuppressive cells. In recent years the concept of myeloid-derived suppressor cells (MDSCs) (described below) has emerged. However, the wealth of new information concerning myeloid cells in cancer has also produced confusion. In most studies, individual myeloid cell populations were examined independently, generating fragmented information that contributed to a convoluted view of their role in immune responses in cancer. In addition, their expression of overlapping cell surface markers has made it difficult to distinguish between different myeloid cell populations, further obscuring the nature of specific myeloid cell subsets in cancer. These complications limit our understanding of myeloid cell biology and hamper attempts to develop and optimize therapeutic interventions.
In this Review, we present a cohesive view of the effects of the tumour on myeloid cells. Our goal is not to provide a comprehensive overview of changes in individual populations of myeloid cells as this has been accomplished in other recent reviews. Instead, we will briefly summarize the effects that tumours have on terminally differentiated myeloid cell subsets and will then focus on discussing myeloid cell interactions and responses during tumour development as an intricately connected single, albeit complex, system.
Dendritic cells
DCs are terminally differentiated myeloid cells that specialize in antigen processing and presentation. DCs differentiate in the bone marrow from various progenitors5, 6,7, 8. They can also differentiate from monocytes under certain conditions, although most DCs in mouse lymphoid organs are not monocyte-derived5, 9. In contrast, monocytes are the major precursors of DCs in humans10.
Two major subsets of DCs are currently recognized: conventional (cDCs) and plasmacytoid (pDCs). Although these cells share some common progenitors, they differentiate along distinct genetic programs and have different morphologies, markers, and functions11 (Box 1). The centerpiece of DC biology is the concept of functional activation and maturation in response to ‘dangerous’ stimuli. Differentiated DCs reside in tissues as ‘immature’ cells that actively take up tissue antigens, but are poor antigen presenters and do not promote effector T cell differentiation. Only functionally activated DCs can effectively stimulate immune responses. DCs are activated in response to stimuli associated with bacteria, viruses or damaged tissues; such stimuli are commonly referred to as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Activation of DCs leads to profound changes in their gene expression, resulting in increased expression of co-stimulatory molecules and cytokines that promote T cell activation, and also in upregulation of chemokine receptors that drive DC migration to lymphoid tissues. pDCs constitute a minor population of DCs that have a morphology reminiscent of plasma cells, express TLR7 and TLR9 (this receptor is not expressed on human cDCs) and produce large amounts of IFNα in response to activation of TLRs by viruses and self-DNA 11. A more detailed discussion of DC biology can be found in recent reviews12, 13.
Box 1. Phenotypic definition useful for separation of different myeloid populations.
Phenotypic definition useful for separation of myeloid populations in lymphoid organs of mice
-
Dendritic cells: CD11c+F4/80−Gr-1− MHC class II+
-
cDCs: CD11c+CD11b+MHC class II+CD205+F4/80−Gr-1−CD115low
Expression of 33D or DEC205/CD205 is specific for DCs but these markers are not expressed on all cells
pDCs: CD11c+CD11b−B220+Siglec H+Gr-1+F4/80
-
-
Monocytes: CD11b+ Ly6C+ Ly6G− CD11c− CD115+
Resident monocytes: CD11b+ Ly6ClowLy6G− CD115+ MHC class II− F4/80high CD11c−
Inflammatory monocytes: CD11b+ Ly6highLy6G− CD115+ MHC class II− F4/80+ CD11c−
-
Macrophages: F4/80+CD11b+Gr-1−
M1 macrophages iNOS+IL-12+CD86+MHC class IIhigh
M2 macrophages CD206+ CD163+ CD36+ ARG1+MHC class IIlow IL-10+IL- 4R α+FIZZ1+YM1+
Granulocytes: CD11b+Ly6G+Ly6Clow F4/80−CD11c−
Phenotypic definition useful for separation of myeloid populations in human blood
-
DCs (mononuclear fraction separated on standard ficoll gradient) Lin− (CD3− CD14−CD19− CD56−) HLA-DR+BDCA-1+ CD209+
-
DCs: Lineage (Lin) cocktail (CD3,CD14,CD19, CD56) − CD11c+ CD11b+ CD33+ BDCA-1/CD1c+ BDCA-3/CD141, CD209/DC-SIGN+
Expression DEC205/CD205 is specific for DCs but this marker is not expressed on all cells
pDCs: Lineage cocktail (CD3,CD14,CD19, CD56) − CD123+ BDCA-2/CD303+ BDCA-4/CD304
-
Monocytes (mononuclear fraction separated on standard ficoll gradient) CD14+HLA-DR+CD15−
-
Macrophages CD14+CD68+
M1 macrophages iNOS+IL-12+CD86+HLA-DR+
M2 macrophages CD206+ CD163+ CD36+HLA-DRlow IL-10+CD124+
Granulocytes (usually are not present in mononuclear fraction and require sedimentation after removal of mononuclear cells): CD15+CD14− CD66b+ CD16+
Typical phenotype of mouse MDSCs
-
CD11b+Gr-1+CD11c−F4/80+/−CD124+
PMN-MDSCs: CD11b+Gr-1hiLy6ClowLy6G+ CD49d−
M-MDSCs: CD11b+Gr-1midLy6ChiLy6G−CD49d+
Typical phenotype of human MDSCs
These cells are purified on standard ficoll gradient for isolation of mononuclear cells
CD11b+CD14−CD33+ (PMN-MDSCs in addition express CD15 and/or CD66b)
Lin− (CD3, CD14, CD16, CD19) HLA-DR−CD33+
CD14+HLA-DRlow/− − M-MDSC
Effects of cancer on DCs
That cancer can have profound effects on the function of DCs has been known for more than 20 years. It is well established that DCs in tumour-bearing hosts do not adequately stimulate an immune response, which potentially contributes to tumour evasion of immune recognition. Significant evidence from numerous studies strongly indicates that abnormal myelopoiesis is the dominant mechanism responsible for DC defects in cancer14. This abnormal differentiation produces at least three main results: decreased production of mature functionally competent DCs; increased accumulation of immature DCs at the tumour site; and increased production of immature myeloid cells 14. In recent years multiple clinical studies have confirmed the findings of earlier studies and have indicated there is a decreased presence and defective functionality of mature DCs in patients with breast15, non-small cell lung16, pancreatic17, cervical18, hepatocellular19, prostate cancers and glioma20.
In addition to the many tumour-derived soluble factors previously implicated in abnormal DC differentiation, such as vascular endothelial growth factor (VEGF), macrophage colony-forming factor (M-CSF) and IL-6, recent studies have shown that other factors present in the tumour microenvironment impair normal DC functions. The tumour microenvironment is predominantly characterized by hypoxia, accumulation of extracellular adenosine, increased lactate, and a decreased pH. DC migration and function are severely impaired by hypoxia and adenosine21, 22. The transcription factor hypoxia-inducible factor (HIF) is upregulated by DCs in the hypoxic tumour environment and was shown to induce expression of adenosine receptor A2b by human DCs, causing these DCs to drive the development of T helper 2 (TH2) cells rather than more potent anti-tumour TH1 cells23. DCs differentiated in the presence of adenosine showed impaired allostimulatory activity in a mixed leukocyte reaction, and they expressed higher levels of the pro-angiogenic cytokine VEGF, the pro-inflammatory cytokines IL-6 and IL-8, and the immunosuppressive mediators IL-10, cyclooxygenase (COX2), transforming growth factor β (TGFβ) and indoleamine 2,3 dioxygenase (IDO)24. Addition of lactic acid during DC differentiation in vitro also induced a phenotype comparable with that of tumour-associated DCs. Blockade of lactic acid production reverted the tumour-induced DC phenotype to normal25. DCs found in the peripheral blood and lymphoid organs of tumor-bearing mice and cancer patients, and especially those closely associated with the tumor, show increased accumulation of lipids. This is mediated primarily via up-regulation of macrophage scavenger receptor types I and II and impairs the ability of DCs to process soluble proteins and stimulate tumor-specific T cell responses26
Some DCs in tumour-bearing hosts actively suppress T cell function and both phenotypically immature and mature DCs may be conditioned by the environment to support immune tolerance or immunosuppression27, 28. MHCII+CD11b+CD11c+ tumour-infiltrating mouse DCs have been shown to suppress CD8+ T cells and antitumour immune responses by producing arginase 1 (ARG1) 29, an immunosuppressive mechanism previously attributed only to murine tumour-associated macrophages (TAMs) and MDSCs (see below). Interestingly, pDCs infiltrating prostate cancer also use ARG1 and indoleamine 2,3-dioxygenase (IDO) to alter the functions of intratumoral CD8+ T cells, suggesting that immunosuppressive programmes might be shared across different myeloid cells in cancer30. Human lung tumour cells can convert mature DCs into TGFβ-producing cells31 and mouse lung cancer can drive DCs to express high levels of IL-10, nitric oxide (NO), VEGF, and ARG132. Accumulation of IDO-expressing DCs (most of which are pDCs) in tumour-bearing mice and in some cancer patients33, 34 provides another possible mechanism of immune suppression by limiting T cell growth via depletion of L-tryptophan and promotion of T cell apoptosis by generating L-tryptophan metabolites and by altering redox potentials through consumption of superoxide radicals. Evidence supports the hypothesis that IDO-expressing DCs enhance the suppressive abilities of forkhead box protein P3 (FOXP3+) regulatory T (TReg) cells in certain settings of chronic inflammation35. As mentioned above, such immunosuppressive activities are primarily associated with DCs localized in tumour sites. However, abnormal DC differentiation and defective DC function is a systemic phenomenon that affects the myeloid cell lineage during cancer, as will be described further below.
Macrophages
MΦ are a group of terminally differentiated myeloid cells closely related to DCs. MΦ are tissue-resident cells derived from monocytes circulating in peripheral blood. They include a broad spectrum of cells whose markers and functions reflect their tissue microenvironment (Box 1). Their function in healthy individuals is to eliminate infectious agents, promote wound healing, and regulate adaptive immunity (reviewed in36). The terminology ‘M1’ and ‘M2’ was coined to describe the different functional states of MΦ and was originally based on studies of murine macrophages 37. M1 or ‘classically activated’ MΦ are activated by IFNγ and bacterial products, express high levels of IL-12, low levels of IL-10, and are tumouricidal. In contrast, M2 or ‘alternatively activated’ MΦ are activated by IL-4, IL-13, IL-10 and glucocorticoid hormones, express high levels of IL-10, low levels of IL-12, and facilitate tumour progression. As discussed by Mantovani38, the M1/M2 nomenclature is useful, but oversimplified because MΦ form a continuum of phenotypes. Although there are some differences between M2-like mouse and human MΦ, phenotypically and functionally the MΦ in these two species are quite similar (Box 1).
Role of macrophages in promoting tumorigenesis
There is an extensive literature demonstrating that in both mouse and man MΦ are co-opted during malignancy to facilitate tumour growth (reviewed in 1, 2,38, 39) (Fig. 2). Their presence is associated with poor clinical outcome1, 40, and their pivotal role in cancer was recently highlighted by the demonstration that TAMs with a specific gene signature are associated with primary treatment failure in patients with Hodgkin’s lymphoma 41. TAMs are M2-like MΦ and mediate their effects via both non-immune and immune mechanisms. Non-immune mechanisms include the promotion of angiogenesis42, facilitation of tumour cell invasion and metastasis43, and protection of tumour cells from chemotherapy-induced apoptosis44 (see also reviews by 1, 2, 38, 39, 45).
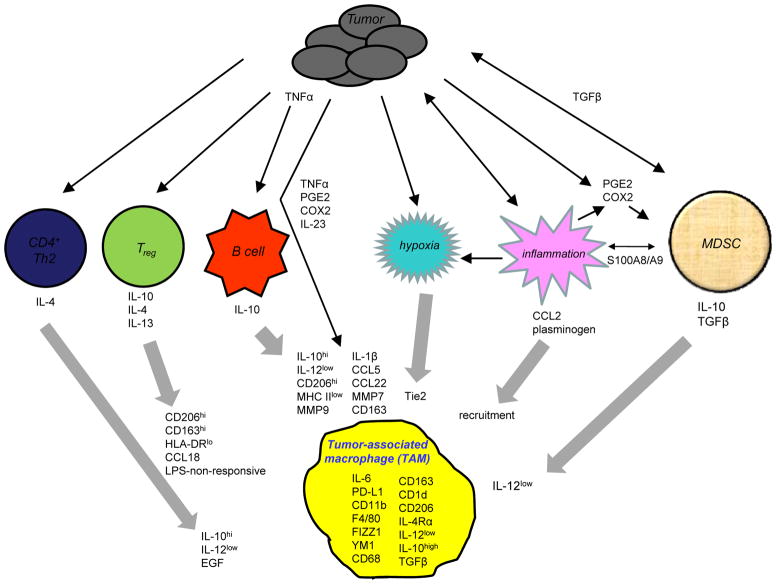
Figure 2. The tumor microenvironment polarizes macrophages towards a tumor-promoting phenotype.
Tumor cells produce factors that drive the generation of multiple regulatory cells, including CD4+ Th2, Tregs, B cells, and MDSC. Tumor cells also modify their microenvironment to produce hypoxia and inflammation (thin black arrows). The regulatory cells and the modified tumor microenvironment subsequently produce cytokines, chemokines, and other molecules that polarize MΦ by regulating MΦ gene expression (e.g. Tie2, HLA-DR, CD163, etc.), by modifying MΦ cytokine expression (e.g. IL-10, IL-12, etc.) and by enhancing MΦ recruitment to the tumor site (thick gray arrows). Tumors also produce factors (ie. TNFα, etc.) that directly polarize MΦ. The resulting MΦ share some characteristics with alternatively activated MΦ and other characteristics unique to TAMs. Cross-talk between tumor cells and MDSC and between tumor cells and inflammation within the tumor microenvironment amplify the effects (thin black double-headed arrows). See the text for references and for which molecules and cellular interactions are known for murine macrophages vs. human macrophages.
TAMs sabotage anti-tumour immunity by eliminating M1 macrophage-mediated innate immune responses and by impairing T cell activation. Studies with transgenic mice showed that IL-12 produced by M1 MΦ promotes the activation of natural killer (NK) cells and TH1 cells, which facilitate the activation of cytotoxic T lymphocytes (CTLs). However, because TAMs do not produce IL-12, they do not contribute to activation of NK cells and TH1 cells. Instead, they produce IL-10 and drive the development of TH2 cells. TH2 cells do not support the development of CTL responses, and their production of IL-4 drives the development of TAMs46. IL-10 produced by MΦ in inflamed lamina propria is required to maintain TReg cell activity and prevent autoimmune colitis47, raising the possibility that TAM-produced IL-10 may also promote tumor progression by enhancing TReg cell activity.
TAMs are ineffective APCs and produce CCL22, which chemoattracts TReg cells that inhibit T cell activation48. Secretion of prostaglandin E2 (PGE2) and TGFβ49 by TAMs further contribute to immune suppression. TAMs also can cause T cell apoptosis through their expression of programmed death ligand-1 (PD-L1), which binds to its receptor PD-1 on activated T cells 50, and murine MΦ produce ARG1, which deprives T cells of L-arginine51. A similar mechanism is also utilized by MDSC (see below). Inflammation is important for the recruitment of macrophages to tumour sites, with the pro-inflammatory mediators CCL252 and plasminogen53, 54 playing essential roles.
TAM polarization
Because areas within solid tumours contain distinct microenvironments, TAMs within an individual tumour will vary. Seven subsets of TAMs have been identified in mouse mammary carcinoma and lung adenocarcinoma based on expression of Ly6C, MHC class II, CX3CR1, CCR2, and CD62L. These subsets have different half-lives and their relative quantities change as the tumour microenvironment evolves with disease progression55. Pro-angiogenic TAMs may express the angiopoietin receptor Tie256 and/or be MHC IIlow and localize to hypoxic regions55. TAMs that promote early tumour cell invasion are enriched for Wnt7b57. Tumour-derived TGFβ and PGE2 promote the differentiation of MΦ that show high levels of Gr1 expression and express low levels of markers associated with M1-type MΦ49.
T cells play an important role in MΦ regulation during tumorigenesis. In a mouse model of breast cancer driven by transgenic expression of the polyoma middle T (PyMT) antigen, mice with mammary adenocarcinomas developed CD4+ TH2 cells that produced IL-4 and polarized TAMs to an M2 phenotype. These TAMs produced epidermal growth factor (EGF), which initiates tumour cell invasion, migration, and metastasis by signaling through the corresponding receptor on the malignant mammary epithelial cells46. TReg cells also regulate macrophages by orchestrating monocyte differentiation. A population of human CD4+CD25+CD127lowFOXP3+ TReg cells was shown to induce monocytes to differentiate into M2 MΦ by inhibiting their responsiveness to lipopolysaccharide (LPS)-induced M1 polarization, and by increasing their expression of mannose (CD206) and scavenger (CD163) receptors. TReg cell production of IL-10, IL-4, and IL-13 promotes the non-responsiveness of MΦ to LPS58. In contrast to TReg cells, Vα24-invariant NKT cells show cytotoxic activity towards TAMs and facilitate tumour rejection59.
B lymphocytes also polarize macrophages towards a tumor-promoting phenotype60. Recent study in a mouse B16 melanoma model has shown that B cells decreased macrophage production of TNFα, IL-1β, and CCL3 while increasing MΦ production of IL-10 which in turn facilitated pro-tumor macrophage activity as well as synthesis of the M2-like markers Ym1 and Fizz161. In another report, autoantibodies polarized CD45+ leukocytes, including MΦ, towards a tumor-promoting phenotype by interacting with activating Fc receptors on the leukocytes 62.
Polarization of MΦ towards an M2 phenotype is also mediated directly by tumour cells. Human ovarian cancer cells cause increased MΦ production of IL-10, IL-1β, CCL5, CCL22, MMP7, MMP9, CD206 and CD163, with tumour cell-produced TNF being partially responsible for this polarization through its induction of MSR163.
Granulocytes
Granulocytes are myeloid cells that are characterized by the presence of cytoplasmic granules and specific nuclear morphology. The most abundant type of granulocytes in the body are polymorphonuclear neutrophils (PMNs) named after their poly-lobed nuclei. PMN possess a complex machinery to engulf and destroy bacteria. PMN are not released from bone marrow until they reach full maturity, but during inflammation, neutrophil precursors (myelocytes and promyelocytes) can be released 64.
Human tumours can be infiltrated with mature granulocytes whose numbers can also constitute independent prognostic factors for recurrence 65–68. Recent evidence has linked granulocytes, and particularly PMNs, with tumour angiogenesis and metastasis, and has provided initial clues about the immunoregulatory role of these cells in cancer. Tumor and tumor-associated stromal cells produce PMN-attracting CXC-chemokines and the orthologue of the secreted protein Bv8, prokineticin 269, 70. Tumour-released G-CSF also mobilized granulocytes to pre-metastatic niches in the lung and supported subsequent metastasis formation, whereas prokineticin 2 aided tumour cell migration through activation of the Bv8 receptor, prokineticin receptor 171. It is likely that granulocytes facilitate the angiogenic switch by expressing matrix metalloproteinase 9 (MMP9), which promotes tumour angiogenesis by inducing VEGF expression within neoplastic tissue72. MMP9 also causes the release of elastase, which enters endosomal compartments of neoplastic cells and degrades insulin receptor substrate 1 (IRS1). Degradation of IRS1 facilitates interaction between phosphatidylinositol 3-kinase (PI3K) and the mitogen platelet-derived growth factor receptor, thus promoting tumour cell proliferation73. In contrast to these observations, a recent study demonstrated that in 4T1 breast tumour-bearing mice, PMN inhibited tumor metastases via direct antitumor effects mediated by reactive oxygen species74. These new data revisited the old concept of tumour cytotoxic PMN and suggest a possible dichotomic polarization of PMNs. Similar to M1 and M2 polarization in MΦ, PMNs have been shown to shift from an anti-tumoral ‘N1 phenotype’ to a pro-tumoral ‘N2 phenotype’ in the cancer environment75 TGFβ drives the N2 phenotype, whereas TGFβ blockade promotes an N1 phenotype with antitumor activity. In lung adenocarcinoma and mesothelioma models, TGFβ favours a pro-tumour phenotype among tumour-infiltrating PMNs, which are characterized by ARG1 expression and low levels of TNF, CCL3, and ICAM1. In tumour-bearing animals, depletion of N2 PMN led to an increase in CD8+ T cell activity75. In line with these findings, serum amyloid A1 protein induced the expansion of IL-10-secreting PMN that were able to suppress antigen-specific proliferation of CD8+ T cells in human melanomas76. However, IL-10 production by activated human PMNs was not confirmed in a subsequent study 77
Myeloid cells as a single integrated system
Neoplastic cells condition distant sites, such as the bone marrow and spleen, by releasing soluble factors that drive the accumulation of myeloid cells; these myeloid cells subsequently promote neovascularization and metastasis. This creates de facto a tumour-driven ‘macroenvironment’. As discussed above this macroenvironment conditions DCs, MΦ and granulocytes to become immunosuppressive. However, the most prominent effect is accumulation of highly immunosuppressive, immature myeloid cells. These cells were named MDSCs to highlight their common myeloid origin and immunoregulatory properties78 (Box 1). Immature myeloid cells with the same phenotype as MDSCs are continually generated in the bone marrow of healthy individuals and differentiate into mature myeloid cells without causing detectable immunosuppression. However, in cancer, normal myeloid cell differentiation is diverted from its intrinsic pathway of terminal differentiation of mature MΦ, DCs, or granulocytes and instead favours differentiation of pathological MDSCs (Fig. 3).
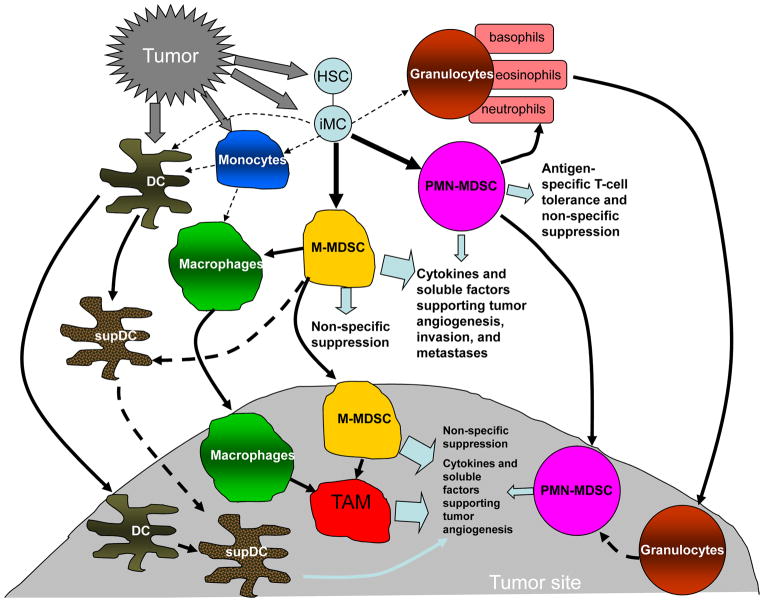
Figure 3. Changes that occur in myeloid cells in cancer.
Factors in the tumor microenvironment produced by tumor cells and stromal cells (including myeloid cells) modulate myeloid cell phenotype and function. iMC – denote immature myeloid cells, a combination of myeloid progenitors described in Figure 1. Thin dotted line - regular pathways of myeloid cell differentiation from iMC to DCs, MΦ and granulocytes. Solid thick lines – pathways of myeloid cell differentiation in cancer. Dotted thick line – suggested, not yet confirmed direction of myeloid cell differentiation. supDCs – DC with immune suppressive activity. TAM- tumor associated MΦ.
Characteristics of MDSCs
MDSCs were originally identified in tumor-bearing mice as cells that co-express CD11b and Gr1, however their phenotype in cancer is rather diverse 79, 80. Currently, two main MDSC populations have been characterized: monocytic MDSCs (M-MDSC) and polymorphonuclear (also called granulocytic) MDSCs (PMN-MDSC) (Box 1). In tumour-bearing mice, PMN-MDSCs is the prevalent population of MDSC. They suppress antigen-specific CD8+ T cells predominantly by production of reactive oxygen species (ROS). PMN-MDSCs represent the major subset of circulating MDSCs; however, they are less immunosuppressive than M-MDSCs when assessed on a per cell basis81–83. In human studies, the number of monocytic but not PMN-MDSCs correlated directly with suppression of in vitro T lymphocyte activation 84
M-MDSCs in addition to their specific markers (Box 1) co-express varying levels of classic monocyte markers, such as F4/80, CD115, 7/4 and CCR281–83, 85 They suppress CD8+ T cells predominantly via expression of iNOS and ARG1 enzymes and through the production of reactive nitrogen species81–83. This subset of MDSCs may also include progenitors that give rise to a subset of CD11bhiGr-1lowLy6G−F4/80hiMHC class II+ MΦ with potent immunosuppressive properties 83, 86–88.
MDSCs with the phenotype LIN−HLA-DR−CD33+CD11b+ have been isolated from the blood of patients with glioblastoma, breast cancer, colon cancer, lung cancer and kidney cancer.80, 89–92. These cells share features and properties with progranulocytes91. The frequency of this immature cell population may reflect the tumour burden and correlates with a poor prognosis and radiographic progression in breast and colorectal cancer patients90, 91, 93. In addition, the frequency of each MDSC subset appears to be influenced by the type of cancer. Patients with renal cancer have immunosuppressive CD11b+CD14−CD15+CD66b+VEGFR1+ PMN-MDSCs94, while CD14+CD11b+HLA-DRlow/neg M-MDSCs circulate in the blood of patients with melanoma, multiple myeloma, prostate cancer, hepatocellular carcinoma, and head and neck cancer84, 95–98.
Relationship of MDSCs to other myeloid cells
Despite their morphologic similarity, PMN-MDSCs and PMNs are functionally and phenotypically different. PMN-MDSCs, but not PMNs, are immunosuppressive 99. Expression of CD115 (also known as M-CSFR) and CD244 is up-regulated in PMN-MDSCs, whereas CXCR1 and CXCR2 are down-regulated99, 100 Compared with PMNs, PMN-MDSCs are less phagocytic, express higher levels of ARG1 and myeloperoxidase, show increased ROS production and reduced chemotaxis toward supernatants from human carcinomas99, 100.
Similarly, although M-MDSCs and inflammatory monocytes share a common phenotype and morphology, these cell populations are functionally distinct. M-MDSCs are highly immunosuppressive, expressing, among other factors, high levels of both iNOS and ARG1. In contrast, these two proteins are not coordinately up-regulated in monocytes. Furthermore, although in M1 MΦ iNOS expression is a hallmark of a tumoricidal phenotype, in M-MDSCs iNOS expression promotes suppressive activities37. This shift in iNOS activity likely reflects the interplay of iNOS with other enzymes expressed by MDSCs, such as ARG1 and NADPH oxidase, as the coordinated activity of these enzymes was shown to promote the production of peroxinitrite that inhibits the proliferation, effector functions and migration of T cells101–104. Although differences exist in the expression of ARG1 and NOS2 among mouse and human myeloid cells (ARG1 is constitutively expressed in human granulocytes105 but not monocytes), evidence indicates that human MDSCs can also co-express these enzymes 98, 106.
MDSCs include direct progenitors of DCs, MΦ and granulocytes. Within 24 hours of culture, PMN-MDSCs phenotypically and functionally resemble PMNs99. Culture of tumour-derived MDSCs in the absence of tumour-derived factors or the transfer of MDSCs to tumour-free recipients results in the generation of mature MΦ and DCs107–109. In contrast, the presence of tumour-derived soluble factors or adoptive transfer into tumour-bearing hosts promotes the differentiation of MDSCs into immunosuppressive MΦ109, 110. MDSCs can also differentiate into DCs following transfer into tumour-bearing recipients 111, but whether these DCs are immunosuppressive is not currently known. Furthermore, hypoxia in the tumor microenvironment drives the differentiation of MDSCs into TAM111, 112 (Fig. 3).
Immunomodulatory functions of MDSCs
MDSCs exploit a plethora of redundant mechanisms to influence both innate and adaptive immune responses. Broadly speaking, these mechanisms can be grouped into four classes.
The first is lymphocyte nutrient depletion: L-arginine depletion by ARG1-dependent consumption51 and L-cysteine deprivation via its consumption and sequestration113. These depletions cause down-regulation of the ζ-chain in the T cell receptor (TCR) complex and proliferative arrest of antigen-activated T cells.
The second is the generation of oxidative stress, which is caused by their production of ROS and reactive nitrogen species (RNS). Peroxynitrite and hydrogen peroxide are produced by the combined and cooperative activity of phagocytic oxidase, ARG1 and iNOS in different MDSC subsets and they drive a number of molecular blocks in T cells, ranging from the loss of ζ-chain expression114 and interference with IL-2 receptor signalling115, to nitration and subsequent desensitization of the TCR 103.
The third set of mechanisms interferes with lymphocyte trafficking and viability. Plasma membrane expression of ADAM17 (a disintegrin and metalloproteinase domain 17) by MDSCs decreases L-selectin expression on the surface of naïve CD4+ and CD8+ T cells, thereby limiting T cell recirculation to lymph nodes116. Another example is the modification of CCL2 by MDSC-derived peroxynitrite, a process which impairs migration of effector CD8+ T cells to the tumour core117. MDSCs express galectin 9, which binds to TIM3 on lymphocytes and induces T cell apoptosis118. MDSCs mostly through membrane contact-dependent mechanisms, i.e. membrane bound TGF-β (mouse MDSCs) and interaction with the NK receptor NKp30 decrease the number and inhibit function of mouse and human NK cells 119–121.
The fourth is the activation and expansion of TReg cells. MDSCs expand antigen-specific natural Treg (nTReg) cells and also promote conversion of naive CD4+ T cells into induced TReg (iTReg) cells. The mechanisms are not completely understood, but may involve cell-to-cell contact, including CD40–CD40L interactions122, production of soluble factors by MDSCs, such as IFNγ, IL-10, and TGFβ123, and possibly also MDSC expression of ARG124 (Fig. 4). Human CD14+HLA-DR−/low MDSCs promote the transdifferentiation of Foxp3+ iTReg from Th17 lymphocytes by producing TGF-β and retinoic acid 125.
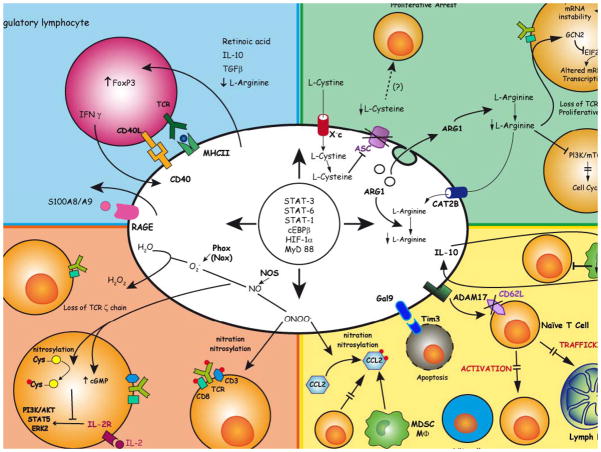
Figure 4. Mechanisms of myeloid cell-dependent inhibition of T cell activation and proliferation.
Myeloid cells conditioned by tumors can induce paralysis of T lymphocytes by expanding/converting Tregs (top left), depriving the environment of amino acids (top right), releasing oxidizing molecules (bottom left), and/or altering T cell migratory properties and viability (bottom right). Since induction of these pathways is regulated by common transcription factors, they can operate in more than one myeloid cell type, as reported in Figure 5. By binding to RAGE, S100A8/A9 also provides autocrine stimulation (middle left). TGFβ, transforming growth factor-β; Xc-, cystine/glutamate transporter; CAT2B, cationic amino acid transporter (L-arginine transporter); ASC, sodium-dependent neutral amino acid transporter (L-cysteine transporter); IFNγ, interferon-γ; IL, interleukin; MYD88, myeloid differentiation primary response protein 88; HIF-1α, hypoxia inducible factor 1α; CEBPβ, CCAAT/enhancer-binding protein β; FOXP3, forkhead box protein P3; Phox, phagocyte oxidase; STAT, signal transducer and activator of transcription; NO, nitric oxide; ARG, arginase; NOS, nitric oxide synthase; Gal9, galectin 9; TIM3, T-cell immunoglobulin and mucin domain-containing protein 3; ADAM17, a disintegrin and metalloproteinase domain 17; CD62L, L-selectin. S100A8/A9, S100 calcium binding protein A8/A9; RAGE, receptor for advanced glycation end products.
In peripheral lymphoid organs MDSC-mediated suppression of CD8+ T cells is usually antigen-specific and requires the presentation of antigens by MDSCs and direct MDSC–T cell contact 103, 126. The activity of MDSCs is also enhanced by activated T cells in the periphery129 and at the tumour site111, 112, 127, 128. As a result, MDSCs are able to suppress nearby T cells in an antigen-nonspecific manner. However, if T cells are activated and become FASL+, they may induce apoptosis of FAS+ MDSCs 129.
The co-dependence of cells in the myeloid lineage is further demonstrated by the regulation of mature DCs and MΦ by MDSCs. Through an IL-10- and cell contact-dependent mechanism, MDSCs skew MΦ towards an M2 phenotype by decreasing MΦ production of IL-12130. IL-12 down-regulation is exacerbated by the MΦ themselves, since MΦ increase MDSC production of IL-10 (Fig. 4). As MDSC potency is enhanced by inflammation131, it is not unexpected that inflammation enhances the cross-talk between MDSCs and MΦ. Inflammation mediates these effects by increasing MDSC expression of CD14 and signaling through the TLR4 pathway132. MDSCs similarly impair DC function by producing IL-10, which inhibits TLR-induced IL-12 production by DCs and reduces DC-mediated activation of T cells133.
Common mechanisms of tumour impact on myeloid cell recruitment and function
Neoplastic and tumour-associated stromal cells release multiple tumour-derived soluble factors that perturb the myeloid compartment. Cytokines such as GM-CSF, G-CSF, M-CSF, SCF, VEGF, and IL-3 promote myelopoiesis and contribute, in part, to a blockade of myeloid cell maturation88, 107 (Fig. 5). Tumour-derived soluble factors that are pro-inflammatory, such as IL-1β, IL-6, and S100A8-9134–136, as well as cytokines released by activated T cells, such as IFN-γ, IL-4, IL-13, IL-10127, initiate the immunosuppressive pathways that commit immature myeloid cells to become MDSCs and then further promote MDSC differentiation towards immune suppressive MΦ and DCs (Fig. 5). The tumour-derived factors CCL2, prokineticin 2, CXCL5, S100A8-9, and CCL12 recruit immature myeloid cells to tumor stroma70, 137, 138. Immature myeloid cells are also chemoattracted by CCL2 that is nitrated/nitrosylated within the tumour environment. In contrast, effector CD8+ T cells are not recruited by modified CCL2, which may explain the selective enrichment of myelomonocytic cells within mouse and human tumours117. LPS, in combination with IFNγ, promotes MDSC population expansion, probably by inhibiting DC differentiation139. Tumor-derived TGFβ also regulates MDSC accumulation140 and neutrophil polarization75 (Fig. 5). Neoplastic cells and their associated stromal cells also release into the bloodstream subcellular components known as exosomes, which contain signal peptides, mRNAs, microRNAs, and lipids and promote MDSC expansion (reviewed in141).
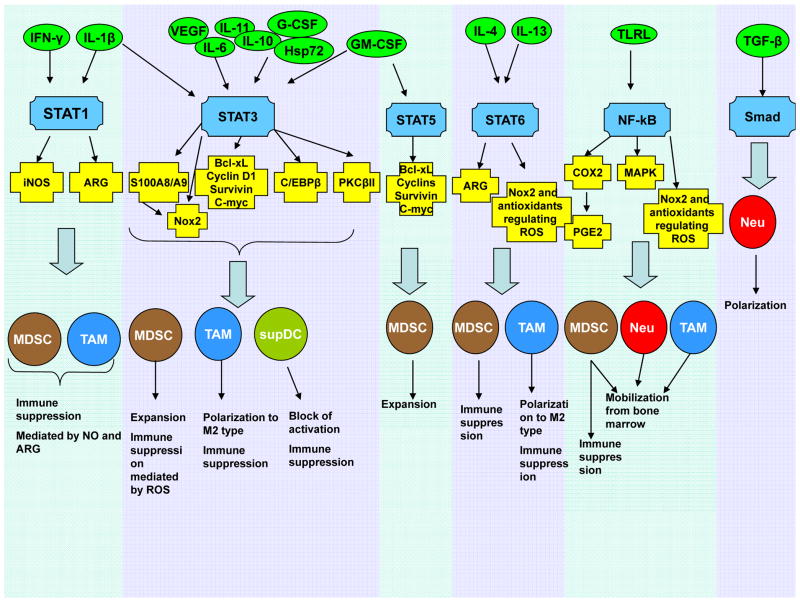
Figure 5. Molecular mechanisms affecting myeloid lineage in cancer.
The tumour microenvironment secretes many different cytokines (green circles) that affect myeloid progenitors as well as mature myeloid cells by regulating the activity of multiple transcription factors (blue). These transcription factors, in turn, regulate synthesis of their protein targets (yellow) affecting myeloid cell functions (black).
Tumour-derived soluble factors regulate myeloid lineage cells on multiple levels involving a variety of transcription factors88, 107, 131 (Fig. 5), with STAT3 playing a major role. Early studies identified STAT3 as a critical regulator of DC and MΦ defects142, 143 and MDSC expansion144,145,146. STAT3 not only prevents apoptosis and promotes cell proliferation via up-regulation of BCL-XL, MYC, cyclin D1, or survivin107, 147, but also regulates expression of multiple proteins critical for differentiation of myeloid cells. One such pathway involves the calcium-binding pro-inflammatory proteins S100A8 and S100A9148. STAT3-mediated up-regulation of these proteins in myeloid progenitors inhibits DC differentiation and promotes MDSC accumulation149. S100A8 and S100A9 also enhance MDSC suppressive activity and recruit MDSCs to the tumour site135. Myeloid cell NADPH-oxidase (NOX2) is another important target of STAT3. STAT3 up-regulation of the NOX2 components p47phox and gp91phox increases ROS levels, thereby making MDSCs more suppressive92. STAT3 also down-regulates PKCβII, which is required for DC differentiation and thus prevents the development of HPCs into mature cells 150. In addition, STAT3 regulates the transcription factor CCAAT-enhancer-binding protein beta (C/EBPβ). C/EBPβ regulates myelopoiesis in healthy individuals151 and plays a crucial role in controlling differentiation of myeloid progenitors to functional MDSCs134. STAT3, at least partially, induces MDSC expansion via up-regulation of C/EBPβ and plays an indirect role in myeloid cell mobilization, accumulation, and survival152.
The transcription factor STAT1 regulates subsets of myeloid cells via its effects on NOS2 and is crucial for MΦ and MDSC-mediated immune suppression127, 153,154. Other characteristics of MDSCs and MΦ, including the up-regulation of ARG1155–157, increased TGFβ production124, 158, and possibly expansion of MDSCs159, are controlled by STAT6. IL-4-induced polarization of TAMs activates STAT6, which binds to the promoter of the gene-encoding the demethylase Jumonji domain-containing 3 (JMJD3). Activated JMJD3 demethylates histone H3 lysine-27, which then increases expression of ARG1, YM1, and FIZZ1, resulting in M2 polarization160 However, the genetic ablation of Jmjd3 gene in mice caused a defective and irf4-dependent M2 polarization in response to M-CSF, helminth infection or chitin administration, but not following IL-4 stimulation, suggesting a more complex regulatory network 161.
The TLR family also plays a prominent role in myeloid cell development, primarily via the activation of MYD88 and the downstream induction of NF-κB. NF-κB signaling is important for mobilization of myeloid cells to sites of infection, injury, or tumour growth162, 163. TLR4 regulates inflammation-driven MDSC suppressive potency through an NF-κB-dependent mechanism164. The pro-inflammatory mediators COX2 and PGE2, which enhance MDSC accumulation and suppressive activity140, 165–167, are also potential targets for NF-κB168.
Two-stage model of MDSC involvement in tumour progression
Recent studies of autochthonous tumor formation in transgenic mice indicate that cells with an MDSC phenotype probably intervene in the very early stages of cancer progression. Mice with autochthonous pancreatic cancer undergo progressive waves of myeloid cell recruitment after initiation of the transforming programme driven by the Kras oncogene169. Recruited myeloid cells contribute to the local production of IL-6 and IL-11 that activate STAT3. STAT3, in turn, induces anti-apoptotic and pro-proliferative genes, fuelling tumour initiation, promotion, and progression170, 171. During early events of colitis-associated cancer, myeloid cells act as tumour promoters by enhancing proliferation of tumour-initiating cells and by protecting premalignant intestinal epithelial cells from apoptosis172. The oncogenic fusion protein RET/PTC3 (RP3) in thyroid carcinomas directly regulates CCL2 and GM-CSF production, which recruits CD11b+Gr-1+ cells173, 174.
An important question is whether these early recruited cells are immunosuppressive MDSCs. Unfortunately, only a few studies in autochthonous tumor models have determined the immunosuppressive activity of the tumour-associated CD11b+Gr-1+ cells. In models of spontaneous breast, pancreatic, or lung cancer, accumulated myeloid cells had both the phenotype and immunosuppressive features of MDSCs111, 169, 175. In a recent study, conditional deletion of p120ctn in mice caused formation of invasive squamous cell cancer and desmoplasia associated with production of GM-CSF, CCL2, M-CSF, and TNF. These events resulted in accumulation of immunosuppressive CD11b+Gr-1+CD124+ MDSCs that promoted tumor progression by activating stromal fibroblasts176.
In another model of multistep squamous carcinogenesis driven by the HPV16 early-region genes (including the E6/E7 oncogenes) under the control of the human keratin-14 promoter/enhancer, CD11b+Gr1+F4/80−CD11c− cells constituted the most abundant leukocyte subtype in premalignant skin, and accumulated progressively in the spleen. However, these cells failed to inhibit polyclonal activation of either CD4+ or CD8+ T cells and did not produce ROS62.
These results, together with the data discussed above from transplantable tumour models, support a two-stage model of MDSC involvement in cancer. The almost universal feature of tumour progression is activation of abnormal myelopoiesis and recruitment of immature myeloid cells into tissues. This process is governed by diverse soluble factors and is dependent upon up-regulation of STAT3 and other key transcription factors (Fig. 5). Myelopoiesis during acute infections, stress, or trauma results in rapid terminal differentiation of myeloid cells. In contrast, cancer myelopoiesis is associated with defective myeloid cell differentiation, which results in accumulation and persistence of immature myeloid cells. Although necessary, these events are not sufficient to generate immunosuppressive MDSCs: activation of cells via a network of regulatory mechanisms is also required (Fig. 5). Activation of these mechanisms in mice with most transplantable tumours and many, but not all, spontaneous tumours, results in the accumulation of immunosuppressive MDSCs. In tumor sites these cells further differentiate into TAMs and possibly into suppressive DCs. In patients with cancer, cells with an MDSC phenotype are almost universally immune suppressive, which may reflect their isolation from patients with advanced disease. If immunosuppressive activity is not a property of the first wave of immature myeloid cells recruited to tumors, continuous stimulation of myelopoiesis and activation of immature myeloid cells by tumour-derived soluble factors may drive the subsequent accumulation of immunosuppressive MDSCs that support tumor promotion and form the metastatic niche. Accordingly, the oncogenic programme may influence the functional immunosuppressive activity more than the accumulation of CD11b+Gr-1+ cells. Thus, the transition from immature myeloid cells to MDSCs might be defective in some experimental tumour models, and different oncogenic programmes may differentially affect the kinetics of immature myeloid cell to MDSC conversion. Combinations of GM-CSF, G-CSF, IL-6 and IL-13 induce the rapid differentiation of cells similar to MDSCs from human and mouse bone marrow precursors in vitro 91, 134, 177,140. These studies may provide the framework for identifying key molecules governing the stages of MDSC maturation.
Therapeutic targeting of myeloid cells
Knowledge of the molecular mechanisms responsible for accumulation of MDSCs, immune suppressive macrophages and DCs in cancer has allowed for therapeutic targeting of these cells as it is increasingly clear that successful cancer immunotherapy will require limiting the immunosuppressive effects of myeloid cells. This targeting is focused on six main goals: first, inhibiting the molecular mechanisms used by myeloid cells to block lymphocyte reactivity and proliferation; second, inhibiting the expansion of MDSCs from bone marrow progenitors or inducing apoptosis of circulating MDSC; third, forcing MDSCs to mature into proficient APCs; fourth, preventing trafficking of myeloid cells from bone marrow to peripheral lymphoid organs and to tumors; fifth, repolarizing or eliminating TAMs and replacing them with M1 macrophages; sixth, restoring the antigen-presenting capabilities of DCs and macrophages (Table 1).
Table 1.
Pharmacological regulation of myeloid cells in cancer
| Treatment | Type of cancer tested In bold – cancer patients | Molecular events | Effect on myeloid cells | Ref. |
|---|---|---|---|---|
| Nitroaspirin | Colon carcinoma | Downregulation of ARG1, NOS2, PNT | Inhibition of the MDSC suppressive effects | 178 |
| Phosphodiesterase-5 inhibitors (sildenafil and tadalafil) | Mammary, colon carcinomas and fibrosarcoma (mice) | Downregulation of ARG1, NOS2, and CD124 in MDSCs | Inhibition of the MDSC suppressive effects | 98 |
| AT38 ( NO-donor based on furoxan molecule) | Fibrosarcoma, thymoma | Downregulation of ARG1, NOS2, PNT; nitrated/nitrosylated CCL2 | Inhibition of the MDSC suppressive effects; MDSCs/CD8+ T cells ratio in tumors | 104 |
| Triterpenoids | Colon, lung carcinomas, thymoma | Inhibition of ROS | Inhibition of the MDSC suppressive effects | 179 |
| Tyrosin kinase inhibitor (Sunitinib) | Fibrosarcoma, colon, breast, lung and kidney cancer. Renal cell carcinoma | Possible c-Kit blockade; STAT3 inhibition; GM-CSF confers resistance by activating STAT5 in intratumoral MDSCs | Inhibition of MDSC expansion in lymphoid organs but not in tumor stroma; modest inhibition of MDSC expansion in patients | 180–184 |
| Cyclooxygenase 2 inhibitor(SC58236, SC58125, celecoxib) | Mammary carcinoma, mesothelioma, lung carcinoma, glioma | Downregulation of PGE2, ARG1, ROS, CCL2. Increase in CXCL10 | Inhibition of the MDSC suppressive effects | 140, 168, 185 |
| Anti-cKit antibody | Colon carcinoma | cKit-SCF interaction blockade | Inhibition of MDSC expansion | 186 |
| CSF1 and cKit receptor tyrosine kinases inhibitor (PLX3397) | Mammary carcinoma | CSF1R and cKit blockade | Inhibition of TAM recruitment | 187 |
| Anti-CCL2 monoclonal antibody | Mammary carcinoma | Interference with CCL2/CCR2 binding and VEGFA upregulation | Inhibition of metastatic spread by targeting inflammatory monocytes and macrophages | 52 |
| Amino-bisphosphonate (zoledronate) | Mammary tumors, mesothelioma | Reduction in VEGF and pro-MMP9 serum levels | Inhibition of MDSC expansion | 188, 189 |
| Very small size proteoliposomes | Lymphomas and sarcoma | NOS2 downregulation | Changes in MDSC subset distribution | 190 |
| Antagonist of CXCR2- (S-265610) and CXCR4 (AMD3100) | Breast cancer | Interference with SDF-1 and CXCL5 chemokines | altered recruitment of iMCs to tumor | 137 |
| Anti-BV8 antibody | Various human and mouse tumors in nude mice | Interference with the BV8 pleiotropic activity | Inhibition of PMN-MDSC expansion and recruitment to tumor and pre-metastatic niches | 70, 71 |
| CSF-1 receptor antagonist (GW2580) | Lung carcinoma Prostate cancer |
CSF-1R interference, ARG1 decrease in MDSCs, VEGF and MMP9 reduction in tumor | Inhibition of expansion and recruitment of MDSC and MΦ to tumor | 191 |
| VEGF-trap, anti-VEGF antibody (avastin) | Various solid tumors Metastatic renal cell cancer | VEGF interference | Improvement of DC differntiation | 192, 193 |
| Gemcitabine | Lung cancer, breast cancer | MDSC apoptosis | Inhibition of MDSC expansion | 130, 194 |
| 5-fluorouracil | Thymoma | MDSC apoptosis | Inhibition of MDSC expansion | 195 |
| Doxorubicin- | Breast cancer | MDSC apoptosis (?) | Weak inhibition of MDSC expansion | 196 |
| Docetaxel | Mammary carcinoma | MDSC apoptosis with differentiation to M1 MΦ of surviving cells | Inhibition of MDSC expansion, MΦ polarization | 197 |
| All trans retinoic acid | Sarcoma, colon carcinioma Metastatic renal cell carcinoma | Differentiation of iMCs to mature leukocytes | Inhibition of MDSC acumulation | 198, 199 |
| Vitamin D3 | Head and neck cancer | Forced differentiation of CD34+ iMCs | Moderate effect on inhibition of MDSC expansion | 200 |
| IL-12, CCL16 + CpG + anti-IL-10 receptor monoclonal antibody | Lung cancer Breast cancer |
Decrease in IL-10, MCP-1, and TGF-β and increase in TNFα, IL-15, and IL-18; | TAM reprogramming | 201, 202 |
| Tumor specific CTLs engineered to release IL-12; Chimeric antigen receptor (CAR)-redirected T-cells engineered to release IL-12 | Melanoma Colon carcinoma |
Increased antigen cross-presentation and costimulation; acute inflammation signature (including IFN-γ); increased TNFα production | DC, MDSC, and TAM reprogramming | 203, 204 |
| IL-2 plus anti-CD40 monoclonal antibodies | Renal cell carcinoma | Increased NOS2 and tissue inhibitor of MMP 1 | TAM reprogramming in lung metastasis but not in primary tumor | 205 |
| Agonist anti-CD40 monoclonal antibodies and gemcitabine | Pancreatic carcinoma Pancreatic ductal adenocarcinoma | Targeting and activation of blood circulating macrophages | TAM reprogramming | 206 |
| Histidine-rich glycoprotein (HRG) | Fibrosarcoma, pancreatic and breast cancer | Downregulation of placental growth factor | TAM reprogramming | 207 |
| Inhibition of NF-κB signaling by targeting IκB kinase | Ovarian cancer | IL-12 production by NK cells; TAMs become IL-12high, IL-10low, MHC class IIhigh, arginase-1low | TAM reprogramming to M1 phenotype | 208 |
The proposed two-stage model for MDSC involvement might have implications for the further development of therapies. Some immunosuppressive mechanisms are common to all myeloid cells but others are unique to individual populations. Therefore, targeting common effector molecules is likely to be more effective than targeting individual suppressive pathways.
Conclusions and perspective
It is not clear whether abnormal myelopoiesis and pathological activation of myeloid cells are temporarily regulated. Do cancer cells first condition mature leukocytes, MDSCs, DCs, and macrophages that are then recruited in response to suppressor factors produced by the progressing tumor? Or, do the two processes occur concurrently and MDSCs are recruited as a pre-requisite to tumor progression? More sophisticated tumor models and techniques will be required to address this key question. It is clear that the myeloid lineage is globally altered in cancer as a single, closely integrated system involving all terminally differentiated myeloid cells and their pathologically activated immature progenitors. Although there are a multitude of phenotypic and functional changes in different myeloid cell subpopulations, these changes are governed by common tumour-derived suppressor factors and transcriptional programmes. These commonalities provide an opportunity for therapeutic interventions that may concomitantly normalize multiple myeloid cell abnormalities.
Online summary.
Tumours directly affect mature myeloid cells by converting some of them into immunosuppressive populations that facilitate tumour growth.
In cancer, normal myeloid cell differentiation is also diverted from its intrinsic pathway of terminal differentiation to mature myeloid cells (dendritic cells, macrophages, and granulocytes) towards pathologically activated immature cells, which are known as myeloid-derived suppressor cells(MDSCs).
MDSCs are immune suppressive, immature, and pathologically activated myeloid cells. However, in the absence of tumor-derived factors they are still able to differentiate into mature myeloid cells. MDSCs consist of two major populations: polymorphonuclear MDSCs and monocytic MDSCs. MDSCs suppress antigen-specific and non-specific immune responses via a variety of different mechanisms.
Myeloid cell responses in cancer are regulated by common tumour-derived factors that activate a diverse set of transcription factors shared by myeloid cells. These transcription factors promote myelopoiesis and initiate the immunosuppressive pathways that commit immature myeloid cells to become MDSCs .
A two-stage model of MDSC involvement in tumour development and progression is proposed. The universal feature of tumour progression is activation of abnormal myelopoiesis and recruitment of immature myeloid cells into tissues. These cells may or may not possess immunosuppressive features, depending on the activation signals provided by the tumor microenvironment. If immunosuppression is not a property of the first wave of immature myeloid cells that are recruited to tumors, continuous stimulation of myelopoiesis and activation of immature myeloid cells by tumor-derived factors drives the subsequent accumulation of immunosuppressive MDSCs, which support tumor growth and formation of the metastatic niche.
Glossary terms
- Myeloid-derived suppressor cells (MDSCs)
A group of immature CD11b+GR1+ cells (which include precursors of macrophages, granulocytes, DCs and myeloid cells) that are produced in response to various tumour-derived cytokines. These cells have been shown to inhibit tumour-specific immune responses
- Pathogen-associated molecular patterns (PAMPs)
These are molecular motifs that are found in pathogens but not mammalian cells. Examples include terminally mannosylated and polymannosylated compounds, which bind the mannose receptor, and various microbial products that activate host Toll-like receptors, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA
- Danger-associated molecular patterns (DAMPs)
As a result of cellular stress, cellular damage and non-physiological cell death, DAMPs are released from the degraded stroma (for example, hyaluronate), from the nucleus (for example, high-mobility group box 1 protein (HMGB1)) and from the cytoplasm (for example, adenosine triphosphate, uric acid, S100 calcium-binding proteins and heat-shock proteins). Such host-derived DAMPs are thought to promote local inflammatory reactions
- Mixed leukocyte reaction
A tissue-culture technique for testing T cell reactivity and APC activity. A population of T cells is cultured with MHC-mismatched APCs, and proliferation of the T cells is determined by measuring the incorporation of 3H-thymidine into the DNA of dividing cells
- Indoleamine 2,3 dioxygenase (IDO)
An intracellular haem-containing enzyme that catalyses the oxidative catabolism of tryptophan. IDO suppresses T cell responses and promotes immune tolerance in mammalian pregnancy, tumour resistance, chronic infection, autoimmunity and allergic inflammation
- Regulatory T (TReg) cells
A specialized subset of CD4+ T cells that can suppress both innate and adaptive immune responses. These cells provide a crucial mechanism for the maintenance of peripheral self tolerance, but may also limit the effectiveness of anti-tumour immune responses
- Natural Treg (nTReg) cells
A subset of TReg cells that undergoes maturation in the thymus where these cells acquire the ability to recognize with intermediate avidity self antigens presented by host MHC class II molecules before being released to the periphery
- Induced (iTreg) cells
A subset of TReg cells that derives from the direct conversion of CD4+ effector T cells in peripheral lymphoid organs under several situations, including the interaction with tumor-conditioned myelo-monocytic cells in tumor-bearing hosts
- TH17 cell
A subset of CD4+ T helper cells that produce IL-17 and that are thought to be important in mediating host defence against certain infections, particularly at mucosal tissues. They are also though to drive pathology in certain inflammatory and autoimmune diseases, such as Crohn’s disease
- Plasminogen
Plasminogen is the inactive precursor of plasmin, a serine protease involved in the dissolution of fibrin blood clots. A causal role has been advanced for plasmin generation in cancer cell invasion through the extracellular matrix remodeling
- Invariant NKT cells
A lymphocyte thought to be particularly important in bridging innate and adaptive immunity. They express a particular variable gene segment, V 14 (in mice) and V 24 (in humans), precisely rearranged to a particular J (joining) gene segment. Typically, NKT cells co-express cell-surface markers encoded by the natural killer (NK) locus, and are activated by recognition of CD1d
- Autochthonous tumor
Differently from transplanted tumors, which arise from the experimental transfer of neoplastic cells or tissues, autochthonous tumors develop spontaneously in the host. Autochthonous tumors can derive from either chemical carcinogenesis or targeted tissue expression of oncogenes by genetic manipulation of the mouse
References
- 1.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khazaie K, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 6.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 7.Onai N, et al. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–16. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 8.Naik SH, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–26. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 9.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Idoyaga J, Steinman RM. SnapShot: Dendritic Cells. Cell. 2011;146:660–660. e2. doi: 10.1016/j.cell.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 15.Pinzon-Charry A, et al. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer. 2007;97:1251–9. doi: 10.1038/sj.bjc.6604018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrot I, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–9. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 17.Bellone G, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177:3448–60. doi: 10.4049/jimmunol.177.5.3448. [DOI] [PubMed] [Google Scholar]
- 18.Lee BN, et al. Deficiencies in myeloid antigen-presenting cells in women with cervical squamous intraepithelial lesions. Cancer. 2006;107:999–1007. doi: 10.1002/cncr.22092. [DOI] [PubMed] [Google Scholar]
- 19.Ormandy LA, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol. 2006;12:3275–82. doi: 10.3748/wjg.v12.i20.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 21.Mancino A, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–34. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 22.Elia AR, et al. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84:1472–82. doi: 10.1189/jlb.0208082. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88:165–71. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 24.Novitskiy SV, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–31. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottfried E, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–21. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 26.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiringhelli F, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Norian LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–94. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins SK, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–72. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, et al. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182:6207–16. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 33.Lee JR, et al. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest. 2003;83:1457–66. doi: 10.1097/01.lab.0000090158.68852.d1. [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baban B, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–83. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Current opinion in immunology. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. This study identified a gene signature of TAMs that was associated with primary treatment failure of Hodgkin's lymphoma patients, and demonstrated that the level of macrophages in Hodgkin's lymphoma patients is prognostic of clinical outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 43.Qian B, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. This study demonstrated that macrophages play a critical role in metastatic cell seeding and in progression of metastatic disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–8. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 46.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. This study demonstrated that in transgenic mice with mammary carcinoma TAMS achieve a pro-tumor and pro-metastatic phenotype in response to CD4+ T cell-produced IL-4. These findings demonstrate that the adaptive immune system significantly drives the pro-tumor activity of the innate immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 49.Torroella-Kouri M, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–9. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 50.Kuang DM, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. This study demonstrated that CD68+ monocytes in peritumoral stroma of hepatocellular carcinoma patients express PD-L1 (B7-H1/CD274), induce T cell anergy, promote tumor progression, and are associated with poor survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez PC, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 52.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. Using a transgenic mouse mammary carcinoma system, this study demonstrated that tumor- and stromal cell-produced CCL2 recruits inflammatory monocytes and metastasis-associated macrophages that promote metastasis, and that blocking CCL2-CCR2 signaling blocks the recruitment of inflammatory monocytes, prolongs survival, and inhibits metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136–46. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- 54.Phipps K, Surette A, O'Connell P, Weaisman D. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Cancer Research. 2011 doi: 10.1158/0008-5472.CAN-11-1748. in press. [DOI] [PubMed] [Google Scholar]
- 55.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. This study demonstrated that distinct subpopulations of TAMs can be idnetified based on their constellation of M1 and M2-like markers and that the different subpopulations localize to different regions of solid tumors where they have distinct functions. [DOI] [PubMed] [Google Scholar]
- 56.Pucci F, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 57.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–12. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiemessen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song L, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Wong SC, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 62.Andreu P, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagemann T, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–32. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 64.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 66.Jensen HK, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–17. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 67.Ilie M, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil- to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 68.Li YW, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 69.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 71.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–55. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houghton AM, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–14. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. Neutrophil granulocytes are demonstrated to undergo a shift among different transitional activation stages, which is regulated by TGFβ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Santo C, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davey MS, et al. Failure to detect production of IL-10 by activated human neutrophils. Nat Immunol. 2011;12:1017–8. doi: 10.1038/ni.2111. [DOI] [PubMed] [Google Scholar]
- 78.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peranzoni E, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 80.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dolcetti L, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 82.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 84.Mandruzzato S, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–8. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 85.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 86.Van Ginderachter JA, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–35. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 87.Bronte V, et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 88.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kusmartsev S, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 90.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solito S, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011 doi: 10.1182/blood-2010-12-325753. A relationship between human MDSCs and immature promyelocyte is shown in this manuscript; more importantly, blood levels of these cells are inversely correlated with the response to chemotherapy in breast and colon cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raychaudhuri B, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez PC, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vuk-Pavlovic S, et al. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–55. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoechst B, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 97.Filipazzi P, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 98.Serafini P, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Youn J-I, Collazo M, Shalova I, Biswas S, Gabrilovich D. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012 doi: 10.1189/jlb.0311177. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandau S, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–7. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 101.Lu T, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–29. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 103.Nagaraj S, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molon B, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. References 101,103,104 illustrate the complex negative influence of peroxinitrite produced within tumor environment or tumor-draining lymph nodes by either myeloid or transformed cells on T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raes G, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. author reply 6561–2. [DOI] [PubMed] [Google Scholar]
- 106.Lechner MG, Liebertz DJ, Epstein AL. Characterization of Cytokine-Induced Myeloid-Derived Suppressor Cells from Normal Human Peripheral Blood Mononuclear Cells. J Immunol. 2010 doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–9. doi: 10.1158/0008-5472.can-03-1715. [DOI] [PubMed] [Google Scholar]
- 109.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- 110.Kusmartsev S, Gabrilovich D. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–91. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 111.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. This study demonstrated that MDSC in tumor microenvironment rapidly differentiate into TAMs and that this effect is mediated by hypoxia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 115.Mazzoni A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 116.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molon B, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011 doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–9. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 120.Hoechst B, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elkabets M, et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–57. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan PY, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 124.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 126.Watanabe S, et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 127.Gallina G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagaraj S, et al. Antigen-specific CD4+ T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sinha P, et al. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–90. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 131.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. JLleukocBiol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–64. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 134.Marigo I, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. This manuscript shows that the transcription factor C/EBPβ, essential for the emergency granulopoiesis during inflammatory conditions, regulates the immunosuppressive activity of myeloid cells conditioned by growing tumors and provide the basis for molecular targeting of common tolerogenic pathways in myeloid cells. [DOI] [PubMed] [Google Scholar]
- 135.Sinha P, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 137.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang B, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–76. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 140.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 141.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–64. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 143.Cheng F, et al. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–436. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 144.Nefedova Y, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 145.Nefedova Y, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 147.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 149.Cheng P, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:3325–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Farren MR, Carlson LM, Lee KP. Tumor-mediated inhibition of dendritic cell differentiation is mediated by down regulation of protein kinase C beta II expression. Immunol Res. 2010;46:165–76. doi: 10.1007/s12026-009-8118-5. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H, et al. STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood. 2010 doi: 10.1182/blood-2009-12-259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sander LE, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–91. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 154.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 155.Sinha P, Clements V, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 156.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–51. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 157.Bronte V, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 158.Terabe M, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Munera V, et al. Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg. 2010;251:120–6. doi: 10.1097/SLA.0b013e3181bfda1c. [DOI] [PubMed] [Google Scholar]
- 160.Ishii M, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–54. doi: 10.1182/blood-2009-04-217620. This study demonstrates that M2 macrphage polarization is partially regulated epigenetically by chromatin/histone remodeling through an IL-4/STAT6-dependent mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Satoh T, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–44. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 162.Martino A, et al. Mycobacterium bovis bacillus Calmette-Guerin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R-dependent nitric oxide production. J Immunol. 2010;184:2038–47. doi: 10.4049/jimmunol.0903348. [DOI] [PubMed] [Google Scholar]
- 163.Liu Y, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176:2490–9. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y, et al. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol. 2009;182:3801–8. doi: 10.4049/jimmunol.0801548. [DOI] [PubMed] [Google Scholar]
- 166.Donkor MK, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9:937–48. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 167.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE2 catabolism in myeloid cells. J Leukoc Biol. 2010 doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rodriguez PC, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clark CE, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 170.Lesina M, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 171.Fukuda A, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pufnock JS, Rothstein JL. Oncoprotein signaling mediates tumor-specific inflammation and enhances tumor progression. J Immunol. 2009;182:5498–506. doi: 10.4049/jimmunol.0801284. [DOI] [PubMed] [Google Scholar]
- 174.Borrello MG, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–45. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 176.Stairs DB, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. In the model of autochthonous cancer, release of soluble factors by tumor cells expanded and recruited immature myeloid cells, which possess immunosuppressive properties and promote desmoplasia by activating fibroblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Highfill SL, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–47. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.De Santo C, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–90. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nagaraj S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of myeloid-derived suppressor cells and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–36. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 182.Ozao-Choy J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van Cruijsen H, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14:5884–92. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 184.Xin H, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Veltman JD, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.DeNardo D, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–46. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Veltman JD, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010;103:629–41. doi: 10.1038/sj.bjc.6605814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fernandez A, et al. Inhibition of tumor-induced myeloid-derived suppressor cell function by a nanoparticulated adjuvant. J Immunol. 2011;186:264–74. doi: 10.4049/jimmunol.1001465. [DOI] [PubMed] [Google Scholar]
- 191.Priceman SJ, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fricke I, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–8. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 193.Kusmartsev S, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181:346–53. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 194.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 195.Vincent J, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 196.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kodumudi KN, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mirza N, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kusmartsev S, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 200.Lathers D, Clark J, Achille N, Young M. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 202.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 203.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011 doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chmielewski M, Kopecky C, Hombach A, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that shut down tumor antigen expression. Cancer research. 2011 doi: 10.1158/0008-5472.CAN-11-0103. References 203 and 204 show that it is possible to reprogram tumor environment by adoptive transfer of T lymphocytes recognizing a tumor antigen and engineered to release IL-12. [DOI] [PubMed] [Google Scholar]
- 205.Weiss JM, et al. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 2010;207:2455–67. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 208.Hagemann T, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–8. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]