Abstract
An experimental system was developed to generate infectious human respiratory syncytial virus (HRSV) lacking matrix (M) protein expression (M-null virus) from cDNA. The role of the M protein in virus assembly was then examined by infecting HEp-2 and Vero cells with the M-null virus and assessing the impact on infectious virus production and viral protein trafficking. In the absence of M, the production of infectious progeny was strongly impaired. Immunofluorescence (IF) microscopy analysis using antibodies against the nucleoprotein (N), attachment protein (G), and fusion protein (F) failed to detect the characteristic virus-induced cell surface filaments, which are believed to represent infectious virions. In addition, a large proportion of the N protein was detected in viral replication factories termed inclusion bodies (IBs). High-resolution analysis of the surface of M-null virus-infected cells by field emission scanning electron microscopy (SEM) revealed the presence of large areas with densely packed, uniformly short filaments. Although unusually short, these filaments were otherwise similar to those induced by an M-containing control virus, including the presence of the viral G and F proteins. The abundance of the short, stunted filaments in the absence of M indicates that M is not required for the initial stages of filament formation but plays an important role in the maturation or elongation of these structures. In addition, the absence of mature viral filaments and the simultaneous increase in the level of the N protein within IBs suggest that the M protein is involved in the transport of viral ribonucleoprotein (RNP) complexes from cytoplasmic IBs to sites of budding.
INTRODUCTION
Human respiratory syncytial virus (HRSV) is an important viral agent of respiratory tract disease in infants, children, immunosuppressed individuals, and the elderly (15, 24, 48). In the absence of a vaccine, the prevention and treatment of HRSV disease remain a significant challenge. HRSV is a single-stranded negative-sense RNA virus of the family Paramyxoviridae. Its genome expresses 11 known proteins, among which are three transmembrane glycoproteins (the small hydrophobic protein [SH], G, and F) and the viral M protein (14, 28). G is a highly glycosylated protein that is expressed as a secreted form and a membrane-anchored form, with the latter serving as a viral attachment protein (34). F resembles the prototypic paramyxovirus fusion protein but can induce membrane fusion in the absence of G (26, 39). F also appears to play a role in viral attachment, and nucleolin was recently identified as a cellular receptor for the F protein (57). M is a nonglycosylated phosphorylated protein of 256 amino acids and a structural component of the HRSV virion (19, 27, 52). M is thought, in part by analogy to the role of matrix proteins in other virus systems, to play a key role in virion assembly by inhibiting viral transcription and by forming a bridge between the viral ribonucleoprotein (RNP) and envelope (13, 21, 25, 27, 54, 56). However, the roles of HRSV M in virion assembly have not been well characterized. A better understanding of the mechanisms underlying assembly has the potential to improve the yield and purity of HRSV stocks for the large-scale production and worldwide distribution of future vaccines, to help predict the potential for cell-cell transmission and the safety of live-vaccine candidates, and to lay the groundwork for the use of viruslike particles (VLPs) as an alternative vaccine strategy.
HRSV infectivity is associated predominantly with a filamentous form (1, 22, 41, 51, 60). These viral filaments are produced at the cell surface late in the infection cycle and remain largely attached to the infected cells, where they can be readily visualized by immunofluorescence (IF) or electron microscopy (EM). Viral filaments range in length from 2 to 8 μm; contain high concentrations of N, G, and F proteins; and often appear as aggregates (1, 16, 31, 43, 51, 53, 55). In a previous study where anti-RhoA drugs were used to block the formation of viral filaments, the virion morphology was reported to shift from predominantly filamentous to predominantly round, without affecting the total yield of infectivity (22). This finding suggests that while the filamentous phenotype is perhaps the “preferred” morphology, the virus has significant potential to generate infectious virions of a nonfilamentous morphology.
Recent studies using viral genomic RNA (vRNA) visualization, transmission EM (TEM) tomography, and proteomic analysis of purified viral filaments suggested that the filaments contain multiple viral RNPs (49, 53). How the RNPs are recruited to budding sites and incorporated into viral filaments is not known. HRSV RNPs are believed to be synthesized in virus-induced inclusion bodies (IBs). IBs form in the cytoplasm relatively early in the infection cycle and contain all the components of the viral polymerase complex (N, the phosphoprotein P, the catalytic polymerase subunit L, and the processivity factor M2-1), M, and cellular proteins, many of which are also found in purified viral filaments (17, 49). Previous TEM studies reported that IBs and sites where viral filaments form are sometimes in close proximity (30, 49), suggesting that IBs may be the scaffold from which viral filaments are generated. However, the composition and functions of IBs, including a potential role in viral filament formation, are poorly understood. Several reports have implicated cytoskeletal elements in the processes that lead to virus egress, in particular microtubules, myosin V, actin, and actin-regulatory proteins such as profilin and RhoA (30, 32, 59). In addition, a role for an actin/myosin-based motility system in HRSV exit has been proposed (6, 53, 59), and there is strong evidence for the involvement of lipid rafts (8, 9, 22, 27, 31, 36, 49, 50, 53).
Studies of other paramyxoviruses have shown that their M proteins play a role in the assembly of virus particles (12, 29, 40, 46). A requirement for HRSV M for cell culture propagation was previously reported, based on an HRSV minireplicon system (58). Studies of host cell infection by HRSV in the absence of the SH, G, and F proteins pointed to M as an important determinant of virion release (2, 3). The roles of M in the viral assembly process likely include a function in bringing together the RNP and the viral envelope, since an M-containing sheath was revealed when the lipid membrane was removed from HRSV-induced surface filaments (1, 27). In HRSV-infected cells, the M protein is first detected in the cytoplasm and nucleus (19). The purpose of nuclear targeting is not well understood but may be to inhibit host cell transcription or to temporarily divert M away from sites of viral transcription, which it was shown to inhibit (18, 21). At later times, M is increasingly detected in virus-induced IBs (18, 21, 35). M was shown previously to associate with viral RNPs through interactions with the M2-1 protein and also has an RNA-binding capacity (35, 52). M binds both plasma and internal cellular membranes and was reported to interact with the G protein cytoplasmic tail (CT) (20, 27). Several of these observations agree with a predicted role for M in the late stages of virion production. In carrying out this role, HRSV M appears unique in that known viral late domains have not been identified, and budding was found previously to be independent of ubiquitination and vacuolar protein sorting-associated protein 4 (13, 60). Another unique characteristic of the HRSV M protein is its structural relatedness to the Ebola virus matrix protein VP40 (33, 38).
To characterize the roles of HRSV M in the late stages of the infection cycle, we generated an infectious M-null virus and analyzed progeny virus production and viral protein distribution in the absence of M. The “null”-virus approach was pursued chiefly because of the possible downstream advantages for studying the role of M, such as the generation of engineered viruses with debilitating M mutations. The results of this study present novel insights into the process of viral filament formation and provide a platform from which to further dissect the role of M in the viral life cycle.
MATERIALS AND METHODS
Cells and primary antibodies.
Vero and HEp-2 cells were acquired from the American Type Culture Collection and grown in standard growth medium containing 5% fetal bovine serum (FBS). Monoclonal antibodies L9 (anti-G antibody) and A5 (anti-F antibody) were provided by Edward Walsh (University of Rochester School of Medicine, Rochester, NY). Synagis (anti-F) and anti-N antibodies were acquired from MedImmune, Inc., and AbD Serotec, respectively. A rabbit polyclonal anti-M peptide serum (M residues 18 to 31) was produced and affinity purified by Genscript.
Construction of a codon-optimized M ORF.
The M open reading frame (ORF) of the HRSV A2 strain was codon optimized according to methods described previously by Haas et al. (23). Overlapping oligonucleotides (70 nucleotides [nt] with a 22-nt overlap; Operon Biotechnologies), representing the entire codon-optimized ORF, were assembled and PCR amplified with flanking BsrGI and XhoI restriction sites. The product was cloned by BsrGI and XhoI restriction sites into a pcDNA3-derived plasmid (modified to lack the T7 promoter and the neomycin gene cassette and to contain an additional BsrGI restriction site). The codon-optimized M ORF was sequence verified. Errors due to the use of long primers were corrected by using site-directed mutagenesis, and the final plasmid was named pc-Mopt.
Generation of an inducible M-expressing cell line.
The codon-optimized M ORF was PCR amplified with flanking EcoRI and XbaI sites and cloned into pTRE-Tight (Clontech). The resulting plasmid (pTRE-Mopt) was used to generate an inducible M-expressing HEp-2-derived cell line in two steps. First, HEp-2 cells were transfected with the plasmid pTet-on-Advanced (Clontech), and G418-resistant colonies were identified and amplified in G418-containing medium. Resistant colonies were screened by transfection with a plasmid expressing enhanced green fluorescent protein (EGFP) from a Tet-inducible promoter (pTRE-EGFP) and incubating the transfected cells in the presence of doxycycline (DOX). A cell line with strong induction as well as low EGFP expression levels in the absence of DOX was subcloned twice by limiting dilution and named H2-tet. Second, plasmid pTRE-Mopt, along with the linearized hygromycin gene (Clontech), was transfected into H2-tet cells, and G418/hygromycin-resistant colonies were selected. Resistant colonies were amplified and screened for their levels of M expression by adding DOX to the medium, fixing cells 24 h after addition, and staining with anti-M serum followed by goat anti-rabbit antibodies carrying Alexa-488. A colony with a high level of inducible M expression was identified with a fluorescence microscope, subjected to another round of subcloning by limiting dilution, and named H2-M.
Western blot analysis.
Equivalent cell numbers (∼6,000 cells per lane) of each sample were electrophoresed on reducing 12% SDS-PAGE gels, and a Western blot was generated. The blot was incubated with anti-M peptide serum followed by goat anti-mouse antibodies conjugated to horseradish peroxidase and developed by using ECL (Pierce).
Construction of M-null and recWT cDNAs.
In previous work, we generated a cDNA (A2 strain) in which the SH ORF was replaced with that of EGFP (pRSΔSH) (43). The virus recovered from cDNA pRSΔSH (which contained artificial restriction sites to facilitate glycoprotein gene exchange) replicated to levels indistinguishable from those of an unaltered A2 virus, and EGFP expression from this location was shown previously to be an accurate indicator of infectivity correlating with the number of PFU (10, 43, 44). For this project, a second-generation cDNA was constructed which also had an exact replacement of the SH ORF with that of EGFP but which did not contain artificial restriction sites. This cDNA was designated pRSV-ΔSH/GFP. A cDNA that lacked the M ORF and contained instead a linker region with BsmBI restriction sites was constructed from pRSV-ΔSH/GFP (pRSV-ΔSH/GFP-ΔM/Bsm). Next, seven of the eight methionine codons in the authentic M ORF were mutated (methionine 1 to AAC and methionines 2 to 7 to UAA). Methionine 8 is located near the COOH-terminal end of the ORF and was left intact. The mutated M ORF was designated “MΔAUG.” The MΔAUG ORF was PCR amplified with flanking BsmBI sites, such that after the ligation of the product into pRSV-ΔSH/GFP-ΔM/Bsm following BsmBI digestion, no artificial sequences were retained other than the intended AUG mutations of the M ORF and the exact replacement of the SH ORF with that of EGFP (see Fig. 2A). The M-mutated cDNA was designated “pRSV-M-null.”
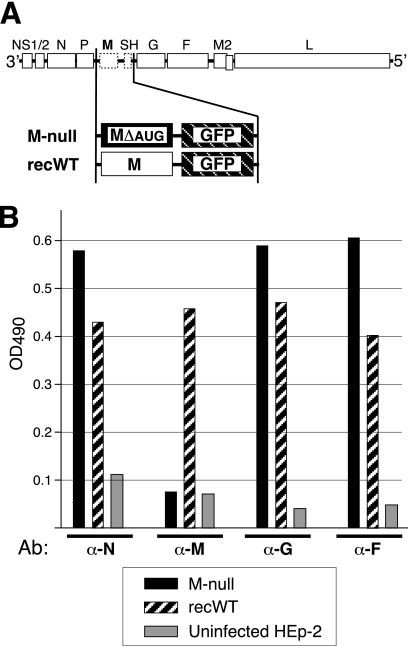
Fig 2.
Composition of engineered virus genomes and viral protein expression. (A) Genome content of engineered viruses. In the “M-null” virus, the M ORF was replaced with a modified M ORF in which AUGs were mutated to ablate expression, and the SH ORF was replaced with the EGFP ORF for tracking purposes. (A virus with the SH ORF being replaced with that of EGFP was previously shown to replicate in a manner indistinguishable from that of the wt virus [10, 43, 44].) The properties of the M-null virus were compared to those of the “recWT” control virus. The recWT virus also has a replacement of the SH ORF with that of EGFP but contains an unaltered (wt) M ORF. The recWT virus resembles the previously reported RSΔSH virus (43) but is a second-generation virus in which all artificial restriction sites were removed. (B) Viral protein expression determined by cell ELISA. HEp-2 cells were infected with the M-null or recWT virus or left uninfected as a control and were fixed and permeabilized at 26 hpi. Relative protein levels were measured with anti-N, -M, -G, and -F antibodies (Ab) by a cell ELISA as previously described (42, 44). OD490, optical density at 490 nm. A representative result of several independent experiments is shown.
Recovery of infectious virus from cDNA.
Infectious virus was recovered from the pRSV-M-null and pRSV-ΔSH/GFP cDNAs as previously described (45), with the following modifications (for cDNA pRSV-ΔSH/GFP, only the first and second modifications apply): (i) the initial transfection of the cDNA and support plasmids was done with BHK-21 cells expressing T7 polymerase from a nonpathogenic alphavirus replicon (47); (ii) the N, P, M2-1, and L support plasmids contained an internal ribosome entry site (IRES) preceding the ORF; (iii) plasmid pc-Mopt was included in the initial transfection to ensure virion production; and (iv) infectious virus was amplified in H2-M cells. Virus derived from pRSV-Δ was designated recWT, and virus derived from pRSV-M-null was designated M-null. Viral RNA was harvested from cells infected with the recovered viruses (passage 3 for the recWT virus and passage 5 for M-null virus) and amplified by reverse transcription-PCR. Modified areas (the M and EGFP genes and the surrounding intergenic regions) plus the N, G, and F genes were verified by bulk nucleotide sequence analysis. No unintended nucleotide changes were found. Passage 3 stocks of recWT virus and passage 5 and 6 stocks of M-null virus were used for the experiments described above.
Cell ELISA.
Relative protein expression levels were determined by a cell enzyme-linked immunosorbent assay (ELISA) as previously described (42, 44), with minor modifications. Briefly, infected cells were fixed at 26 h postinfection (hpi) and permeabilized with 0.2% Triton (for N, G, and F) or 0.1% SDS (for M). Fixed, permeabilized cells were blocked and labeled with anti-N, -M, -G, or -F (A5) antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-mouse antibody for the N, G, and F antibodies and anti-rabbit antibody for the M antibody [Pierce]). After washing, cells were incubated in an O-phenylenediamine-based substrate solution. At short time intervals after the addition of the substrate, small aliquots were collected and immediately added to 3 M sulfuric acid to stop the reaction. The optical density at 400 nm (OD490) was determined with an ELISA plate reader. The experiment was carried out in triplicate, yielding similar relative protein expression levels. Due to a significant overall variation in ELISA signal strengths between replicate experiments, a single result from three independent experiments is shown.
Growth analysis (flow cytometry).
HEp-2 cells plated into six-well plates were infected by the addition of an inoculum (∼0.2 PFU/cell) and centrifugation at 3,000 × g for 10 min (Allegra X-15R; Beckman Coulter) to boost the infection rate. Total (cell-associated and released) progeny virus was harvested immediately after infection and at 1-day intervals thereafter by scraping cells into the medium and storing them at −80°C. Samples were assayed simultaneously by flow cytometry as previously described (43). Briefly, samples (20% of the total volume harvested) were thawed, mixed by gentle pipetting, cleared by low-speed centrifugation (5 min at 750 × g), and used to infect freshly plated (receiver) HEp-2 cells. At 24 hpi, receiver cells were trypsinized and fixed with 4% paraformaldehyde in suspension, and the percentage of EGFP-expressing cells was determined by flow cytometry, counting 50,000 cells per sample.
IF microscopy.
Cells were fixed with freshly dissolved 4% paraformaldehyde, permeabilized with 0.1% SDS, and incubated with anti-M peptide serum. Following incubation with Alexa-488-conjugated anti-rabbit antibodies, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI), washed, and photographed at a ×200 magnification on a Nikon inverted fluorescence microscope (Fig. 1C) or washed, mounted onto slides, and photographed at a ×600 magnification (Fig. 1B). To examine EGFP expression within infected cells (see Fig. 3B), cells were photographed at a ×100 magnification without fixation or processing, using UV light to visualize EGFP or halogen light for phase-contrast images.
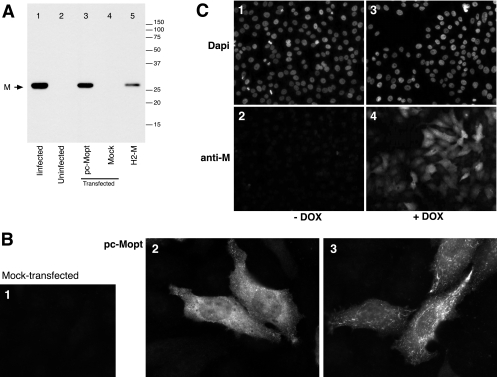
Fig 1.
Transient and stable expression of the M protein from a codon-optimized ORF. (A) Western blot analysis. Lysates of transfected HEp-2 cells were prepared at 30 hpx, and a Western blot was generated after SDS-PAGE under reducing conditions. Lane 3 represents cells transfected with a plasmid expressing M from a codon-optimized ORF (pc-Mopt). As controls, lysates with equivalent cell numbers of mock-transfected cells (lane 4), wt virus-infected cells (lane 1), and uninfected cells (lane 2) were included. (For lane 5, see panel C.) The positions of molecular weight markers (in thousands) and the M protein are indicated. (B) Transient expression of M determined by IF microscopy. pc-Mopt-transfected cells (panels 2 and 3) or mock-transfected cells (panel 1) on glass coverslips were fixed and permeabilized at 30 hpx and incubated with anti-M peptide serum and Alexa-488-conjugated anti-rabbit antibodies. Samples were examined with a fluorescence microscope and photographed at a ×600 magnification. Panels 2 and 3 represent differential expression patterns observed within the same sample. (C) Analysis of the M-expressing cell line H2-M. Tet-responsive H2-M cells were induced for M expression by the addition of doxycycline (DOX) to the medium (+DOX) (panels 3 and 4) or were left uninduced (−DOX) (panels 1 and 2), and cells were fixed and permeabilized 30 h after induction. M was visualized as described above for panel B (panels 2 and 4), and cells were counterstained with DAPI to visualize nuclei (panels 1 and 3). The samples were examined on a fluorescence microscope and photographed at a ×200 magnification. A lysate of induced H2-M cells at 30 h postinduction was also examined by Western blotting (panel A, lane 5).
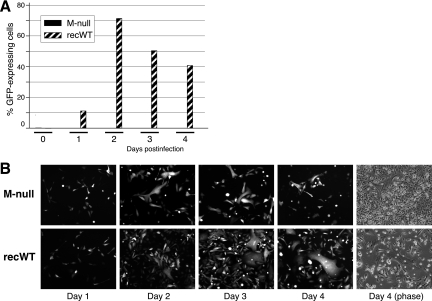
Fig 3.
Viral replication in cell culture in the absence of M. (A) Flow cytometry. HEp-2 cells were infected with the M-null or recWT virus by the addition of an inoculum (∼ 0.2 PFU/cell) and centrifugation for 10 min at 3,000 × g. Immediately after infection and at 1-day intervals, progeny virus was harvested by scraping cells into the medium and transferring the samples to −80°C. Frozen samples were thawed, mixed by gentle pipetting, cleared by low-speed centrifugation, and used to infect freshly plated (receiver) HEp-2 cells. At 24 hpi, receiver cells were trypsinized and fixed with 4% paraformaldehyde in suspension, and the percentage of GFP-expressing cells was determined by flow cytometry. The data represent the average values of duplicate samples. (B) HEp-2 cells were similarly infected with the M-null (top) or recWT (bottom) virus. At days 1, 2, 3, and 4 postinfection, GFP expression within the infected cultures was examined with a fluorescence microscope and photographed at a magnification of ×100. A phase-contrast image at day 4 postinfection was also included [Day 4 (phase)].
Confocal microscopy.
Vero cells on glass coverslips were infected for 1.5 h at 37°C (∼0.2 PFU/cell). At 26 hpi, cells were fixed and processed for confocal microscopy as previously described (43), using L9 (G) and A5 (F) as primary antibodies and anti-mouse secondary antibodies conjugated to Alexa-594. Cells were photographed with a Leica TCS SP2 inverted confocal microscope system using a 63× objective and a ∼3.5× zoom.
Field emission SEM.
HEp-2 cells on plastic coverslips were infected as described above in “Growth analysis (flow cytometry).” At 26 hpi, the medium was replaced with minimal essential medium (MEM) reduced-serum medium (MEM-RS; HyClone) containing anti-G (L9) and anti-F (Synagis) antibodies, 50 mM HEPES, and 0.1% bovine serum albumin (BSA), and cells were incubated for 1 h at room temperature. A total of 1 mM sodium azide was added to the medium 30 min prior to antibody incubation, and during primary antibody incubation, as a precaution to avoid the internalization of antibodies. The inclusion of sodium azide had no measurable impact on the appearance of surface filaments on the surfaces of recWT-infected cells (not shown). Cells were washed twice and incubated with goat anti-mouse or goat anti-human antibodies (conjugated to 15-nm and 25-nm colloidal gold, respectively; Aurion) in MEM-RS containing 0.1% BSA for 1 h on ice. Cells were washed three times and fixed in 2.5% glutaraldehyde for 30 min at room temperature. After fixation, cells were washed once, incubated in 1% osmium tetroxide for 1 h, and washed three additional times. Cells were then dehydrated in ethanol in a stepwise fashion and incubated in hexamethyldisilazane for 1 min. Following hexamethyldisilazane treatment, samples were air dried, carbon coated, and examined with an FEI Quanta 600 field emission gun scanning electron microscope. Samples were examined by using the secondary electron (SE) and backscattered electron (BSE) modes at various magnifications and photographed at magnifications of ×20,000 and ×100,000. For each sample, approximately 25 fields containing infected cells (identified by the presence of anti-G antibody- and anti-F antibody-conjugated gold particles) were examined. No gold particles were found in the uninfected cell sample. Images at a magnification of ×100,000 were overlaid to determine the location of gold particles relative to identified surface filaments.
RESULTS
Transient and stable expression of the M protein from a codon-optimized ORF.
Due to the generally low levels of nuclear promoter-driven expression of HRSV proteins, a synthetic, codon-optimized M ORF was assembled from oligonucleotides and cloned into a pcDNA3-derived vector (pc-Mopt). To examine transient M expression from plasmid pc-Mopt, HEp-2 cells were transfected and processed for IF microscopy and Western blot analysis (Fig. 1). For the latter, transfected cells were harvested 30 h after the start of transfection (hpx) and subjected to SDS-PAGE under reducing conditions. As negative and positive controls for M expression, lysates from an equal number of mock-transfected HEp-2 cells and cells infected with wild-type (wt) HRSV were included. The M protein was detected by using an anti-M peptide serum (see Materials and Methods). In lysates of pc-Mopt-transfected cells (Fig. 1, lane 3) and wt HRSV-infected cells (Fig. 1, lane 1), anti-M antibodies identified a protein of ∼27 to 28 kDa, consistent with data from previous reports (19, 52). No obvious difference in molecular mass was observed between M expressed alone and in the context of a viral infection. For IF microscopy, pc-Mopt-transfected cells were fixed at 30 hpx, permeabilized with 0.1% SDS, and incubated with anti-M serum followed by Alexa-488-conjugated secondary antibodies. Consistent with the Western blot results, the M protein was abundantly expressed in pc-Mopt-transfected (Fig. 1B2 and B3) but not in mock-transfected (Fig. 1B1) cells. In most cells examined, M was present throughout the cytoplasm (Fig. 1B2). Sometimes, M appeared to be concentrated in cell structures near the plasma membrane (Fig. 1B3). M was also routinely detected in the nucleus (Fig. 1B2), in agreement with previous observations of virus-infected cells (17). At late times posttransfection, M expression resulted in a high rate of cell rounding and detachment.
To avoid cytotoxic effects of constitutive expression, the pc-Mopt ORF was cloned behind a Tet-inducible promoter (pTRE-Mopt), and an inducible M-expressing cell line was generated as described in Materials and Methods and named H2-M. Figure 1C documents M expression by the cell line H2-M in the presence (Fig. 1C3 and C4) or absence (Fig. 1C1 and C2) of the DOX inducer at 30 h postinduction. DOX-induced H2-M cells were also examined by Western blotting (Fig. 1A, lane 5), with equivalent cell numbers loaded per lane. H2-M cells expressed a peptide of the correct molecular mass albeit at a level lower than that in virus-infected or transiently transfected cells.
Generation of an M-null virus using the trans-complementing cell line H2-M.
The HRSV A2 strain M protein contains eight methionines. Seven of these occur within the approximate amino-terminal half of the ORF and were mutated to ablate expression (MΔAUG). A cDNA in which the M ORF was replaced with a spacer containing remote-cutting BsmBI restriction sites was also generated (see Materials and Methods). In this cDNA, the SH ORF was replaced with that of EGFP to allow the monitoring of infectious-virus recovery and cell-to-cell spread. SH was previously shown to be dispensable for virus replication in cell cultures, and EGFP expression from the SH location was shown to be an accurate and stable indicator of infectivity correlating with the number of PFU (10, 43, 44). While previous studies did not suggest a major role for the SH protein in viral assembly (2, 3, 42–44, 58), the reader should keep in mind that these studies were carried out in its absence. The resulting cDNA was named pRSV-ΔM/Bsm-ΔSH/GFP. Next, the MΔAUG ORF was PCR amplified by using primers to incorporate flanking BsmBI sites and ligated into BsmBI-digested pRSV-ΔM/Bsm-ΔSH/GFP cDNA, such that no artificial sequences were remaining anywhere in the cDNA other than the intended M mutations and an exact replacement of the SH ORF with that of EGFP. The gene content of the resulting cDNA (“M null”) is shown in Fig. 2A. A cDNA also having EGFP in place of SH but containing the unaltered M ORF was constructed to serve as a control (“recWT”) (Fig. 2A). Note that the recWT cDNA is a second-generation cDNA with SH being replaced with EGFP, engineered such that artificial restriction sites (to facilitate glycoprotein exchange) contained within the 2003 version (43) are no longer present.
Infectious virus was recovered from the above-mentioned cDNAs as previously described (44) (see Materials and Methods). The recovery of the M-null virus required the inclusion of plasmid pc-Mopt in the initial transfection mixture and the use of H2-M cells. While M-containing virus (designated recWT) was amplified to a relatively high titer in two passages in HEp-2 cells, the M-lacking virus (designated M null) required four passages in H2-M cells to obtain a titer of 1 × 105 PFU/ml. Virus stocks used for the experiments described above were verified by reverse transcription-PCR on RNA harvested from infected cells at passage 5 (M null) or passage 3 (recWT), followed by sequence analysis as previously described (44). No unintended changes were found (data not shown).
Characterization of protein expression by the M-null virus.
To examine protein expression by the recovered viruses, HEp-2 cells were infected with the M-null virus or the M-expressing recWT control virus and examined at 26 hpi by a cell ELISA as previously described (45) (Fig. 2B). The results consistently demonstrated both the lack of M expression in M-null virus-infected cells and moderately higher levels of N, G, and F proteins in the absence of M. A representative result of several independent experiments is shown in Fig. 2B. Viral protein expression was also verified by IF microscopy (data not shown). The results of cell ELISA and IF microscopy analyses were in agreement with the gene content indicated in Fig. 2A.
The M protein is required for cell-cell transmission of HRSV.
Using a minireplicon system, it was previously shown that the M protein is required for virus transmission (58). However, that study was done in the context of vaccinia virus and wt HRSV helper virus and examined only virus released into the supernatant, which typically constitutes a minority of the total HRSV infectivity. To determine whether HRSV cell-cell transmission, be it through released virions or by alternate mechanisms, can occur in the absence of M, HEp-2 cells were infected with the M-null or recWT virus (0.2 PFU/cell), and progeny virus was harvested at day 0 and at 1-day intervals thereafter. Total (cell-associated and released) virus was assayed by flow cytometry as previously described (43). Briefly, clarified lysates of infected cells were used to infect receiver HEp-2 cells. At 24 hpi, receiver cells were trypsinized and fixed, and the percentage of EGFP-expressing cells was determined to determine the relative amount of progeny virus present at each time point (Fig. 3A). As expected, the recWT virus produced a significant amount of progeny virus by day 1 postinfection. By day 2, sufficient recWT progeny virus was produced to infect the majority (>70%) of receiver cells. In contrast, the level of infectious progeny virus production in the absence of M was very low, with fewer than <0.01% of receiver HEp-2 cells expressing EGFP at days 2, 3, and 4. The propagation of the viruses was also examined by monitoring EGFP-expressing cells within the infected culture at daily intervals (Fig. 3B). In agreement with the flow cytometry results, cells infected with the control recWT virus spread to neighboring cells at a high rate, and by day 3, the majority of cells were expressing EGFP (Fig. 3B, bottom panels). Cells infected with the M-null virus also displayed high levels of EGFP expression, but the virus failed to spread and invade the culture (Fig. 3B, top panels). In many cases, small, strongly fluorescent, multinucleated syncytia formed at sites where individual cells were initially infected. However, the M-null virus did not spread beyond the observed syncytia, even at several days postinfection, and uninfected cells continued to divide rapidly for the duration of the experiment (Fig. 3B). Taken together, these results demonstrate that the M protein was critical for virus propagation in cell culture. However, the F protein remained fusion competent in the absence of M and induced a moderate amount of virus spreading via syncytium formation.
Distribution of viral proteins is altered in the absence of M.
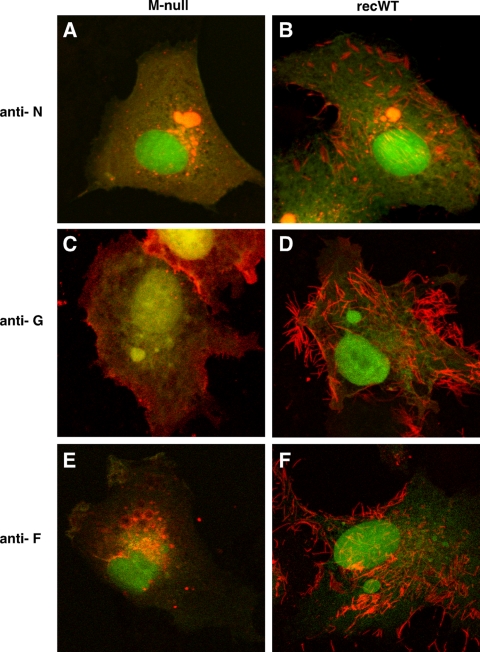
M is thought to facilitate virion assembly by bringing together the RNP and envelope glycoproteins. In this experiment, we examined the impact of the absence of M on the subcellular targeting of the N, G, and F proteins using IF microscopy (Fig. 4). This was done with Vero cells, since they attach more readily to glass coverslips. Cells were infected with the M-null or recWT virus, fixed and permeabilized at 26 hpi, and incubated with anti-N, anti-G, or anti-F antibodies. Following incubation with Alexa-594-conjugated secondary antibodies, cells were examined by confocal microscopy. In both M-null- and recWT-infected cells, EGFP expression was abundant, indicating successful infection and viral replication (Fig. 4). In recWT-infected cells, the typical distribution pattern for the N protein was observed: N was concentrated in cytoplasmic IBs and in viral filaments located at or near the cell surface and was also present diffusely in the cytoplasm (Fig. 4B). In addition, N was abundantly present in long filaments extending far from the cell surface. However, these long filaments were thin relative to filaments labeled with G or F antibodies and difficult to capture with photography. The latter may be due to the location of N inside the virion structure. In M-null virus-infected cells, the expression of the N protein was substantially different: while high levels of N were also observed in the cytoplasm, viral filaments were not detected, and the amount of N protein present within IBs appeared to have increased (Fig. 4A). To quantitate the observed increase, 10 random fields in M-null- and recWT-infected cells were chosen and photographed, and the number and area of N-labeled IBs were measured by using Nikon NIS Elements software. We found that in the absence of M, the number of IBs was unchanged, but the average area occupied per IB had increased by approximately 2-fold (data not shown). Thus, the lack of M expression led to an increase in the amount of N protein in IBs and a notable absence of N-containing viral filaments.
Fig 4.
Subcellular distribution of the N, G, and F proteins in infected cells in the absence of M. Vero cells infected with the recWT (B, D, and F) or M-null (A, C, and E) virus were fixed at 26 hpi and detergent permeabilized. Samples were incubated with anti-N (A and B), anti-G (C and D), or anti-F (E and F) antibodies. Following incubation with Alexa-594-conjugated secondary antibodies, samples were examined with a confocal microscope by scanning sequentially for EGFP expression (green) and N, G, or F expression (red). Images were overlaid. Magnification, approximately ×2,000.
In Vero cells infected with the recWT virus, the G and F proteins were also concentrated in viral surface filaments (Fig. 4D and F). In contrast, in the absence of M, viral filaments were not detected by using anti-G and anti-F antibodies. Rather, G and F were found at the plasma membrane in an evenly distributed although punctate manner (Fig. 4C and E). In addition, round structures resembling IBs were often observed near the plasma membrane of M-null virus-infected cells labeled with the anti-F antibody (Fig. 4E). The surface expression of F and G in infected cells was verified in duplicate, nonpermeabilized samples (data not shown). Combined with the N protein data described above, it appeared that the absence of M precluded the formation of cell surface filaments. Whereas F protein subcellular targeting appeared to be altered, significant amounts could still be detected at the cell surface. The altered distribution of the N, G, and F proteins in the absence of M was also observed in HEp-2 cells (not shown), indicating that the results were not a cell type-dependent phenomenon.
Virus-induced cell surface filaments are stunted in the absence of M.
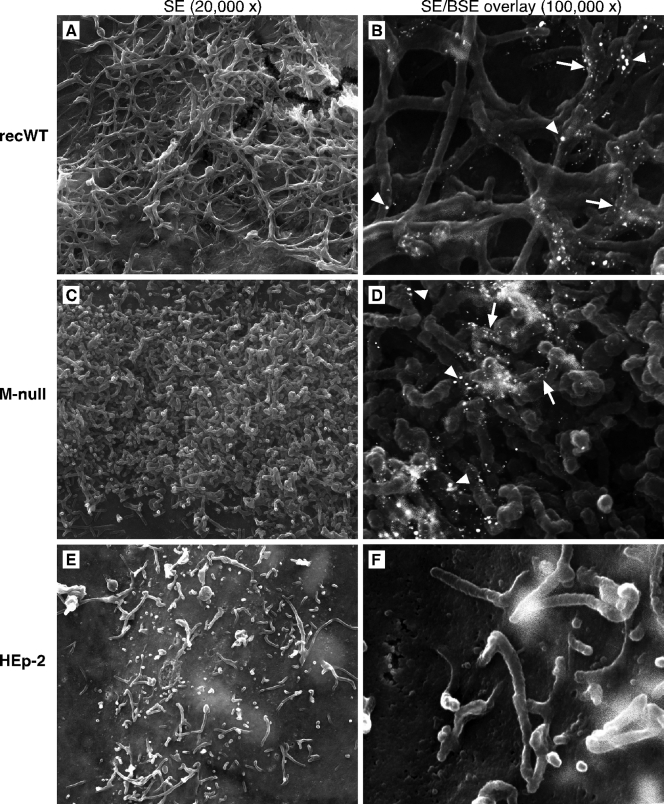
To determine whether the inability to detect viral filaments in M-null virus-infected cells by IF microscopy (Fig. 4) represented a failure of filament formation or of viral protein targeting to existing filaments, we examined the surfaces of M-null- and recWT-infected cells at a higher resolution. A procedure described previously by Jeffree et al. (31) was applied, in which field emission SEM was combined with immunogold labeling (Fig. 5). Infected cells were fixed at 26 hpi, labeled with anti-G and anti-F antibodies and secondary antibodies conjugated to 15- or 25-nm gold, respectively, and processed for SEM as described in Materials and Methods. Fields in the samples were analyzed at various magnifications and photographed at magnifications of ×20,000 using the secondary electron (SE) detection mode to visualize surface filaments (Fig. 5A, C, and E) and ×100,000 using both SE and backscattered electron (BSE) detection modes to also identify gold particles bound to the G and F proteins (Fig. 5B, D, and F). At a ×20,000 magnification, the cell surface of recWT virus-infected cells displayed an abundance of filaments (Fig. 5A). Such filaments have been reported previously and were not detected at the surfaces of uninfected cells (Fig. 5E). Immunogold labeling and overlays of SE and BSE images provided further evidence that the identified cells were indeed infected (Fig. 5B). Fewer 25-nm particles (F protein) than 15-nm particles (G protein) were observed, which may be a consequence of the variation in the antibody-binding kinetics and the size of gold particles or may reflect a high G-to-F protein ratio in viral filaments. Despite the failure to detect filament structures by IF microscopy in Fig. 4, SEM analysis of M-null virus-infected cells revealed an abundance of densely packed short filaments, often covering the majority of the available cell surface (Fig. 5C and D). Both the diameter of the short filaments and the extent of G and F labeling by gold particles were similar to those of filaments induced by the recWT virus. The uniformity of length and appearance of the short filaments on M-null virus-infected cells (Fig. 5C) suggests that these filaments did not develop normally after initiation. The data thus indicate that while the M protein is not required for the initial stage of virus-induced surface filament formation, in its absence, filaments fail to elongate or mature properly.
Fig 5.
High-resolution analysis of the surface of M-null virus-infected cells. HEp-2 cells were infected, as described in the legend of Fig. 3A, with the recWT (A and B) or M-null (C and D) virus or left uninfected (E and F). At 26 hpi, cells were incubated stepwise with anti-G (L9) and anti-F (Synagis) antibodies and goat anti-mouse or goat anti-human antibodies conjugated to 15-nm and 25-nm colloidal gold, respectively, and processed for field emission SEM analysis as described in Materials and Methods. Samples (approximately 25 fields containing infected cells) were examined by SEM and scanned at magnifications of ×20,000 using the secondary electron (SE) detection mode (A, C, and E) and ×100,000 using both SE and backscattered electron (BSE) detection modes to also visualize gold particles. Photographs of SE and BSE scans were overlaid (B, D, and F). White arrowheads indicate 25-nm gold particles (F protein), and white arrows indicate 15-nm gold particles (G protein).
DISCUSSION
A better understanding of HRSV assembly will help overcome challenges in current vaccine strategies and may lead to new opportunities for HRSV therapeutics. This study addresses the role of the viral M protein and shows that M is a critical factor in the organization of infectious progeny production. Filamentous virions have been described as the predominant infectious HRSV virion phenotype in cell culture, even after extensive passaging, and do not appear to be a tissue culture adaptation. However, their relative abundance in vivo, or the role that the cell surface-attached filaments might play in virus dissemination within an infected host, is not understood. Irrespective of the in vivo significance, detailed knowledge of the assembly process of viral filaments in cell culture is important, as vaccine manufacture, be it live-attenuated or killed or in the form of viruslike particles, will most likely depend on a cell culture platform. In addition, the M protein of HRSV has unique characteristics within the paramyxoviruses, including the absence of a known viral late domain and structural similarity with the VP40 matrix protein of Ebola virus (33, 38). Hence, characterizing the role of the M protein in viral assembly may also provide novel insights into viral replication mechanisms.
This study describes the generation and characterization of an M-null virus and its use in dissecting the role of the M protein in late-stage viral assembly. We used a null-virus approach because of potential downstream advantages such as the generation of viruses with debilitating M mutations for in vivo studies. Through the complementation of the M protein by an M-expressing cell line, we were able to generate infectious virus stocks lacking an intact M protein gene. The resulting infectious M-null virus allowed for the first time an investigation of the HRSV infection cycle in the complete absence of M. It is important to keep in mind that this study was done in the absence of the viral SH protein. Prior studies did not suggest a major role for the SH protein in viral assembly or filament formation, and our results are in agreement with those previous findings. However, a minor direct or indirect impact of SH on filament production in vitro, or a possible significant impact in vivo, cannot be excluded by the presented data.
Characterization of the M-null virus.
Titers of M-null virus stocks were generally low (approximately 105 PFU/ml), which may reflect cytotoxic effects by M during amplification in H2-M cells or a suboptimal processing or spatiotemporal regulation of M when expressed from the host cell nucleus. Analysis of protein expression by ELISA showed that the levels of expression of other viral proteins were moderately higher in the absence of M. The previously reported inhibition of host and viral transcription by M (18, 21) may explain the overall increase in HRSV protein expression levels in the absence of M.
The M protein is critical for production of infectious virus.
An important role for M in virion production was anticipated based on a previously reported HRSV minireplicon system (58) and analogy to the roles of M proteins from other paramyxoviruses (12, 29, 40, 46). The growth analysis shown in Fig. 3 directly demonstrates that HRSV M is critical for the production of both released and cell-associated infectious progeny. In the absence of M, the level of production of infectious virus was reduced by ∼1,000- to 10,000-fold. Whether the remaining infectivity represents true infectious progeny production in the absence of M or is a result of the experimental conditions of the sensitive flow cytometry-based assay is not known. Syncytium formation was observed in both recWT virus- and M-null virus-infected cell cultures. This was not surprising, since the expression of the F protein alone is capable of inducing extensive cell-cell membrane fusion (5, 26). In the absence of M, however, syncytia were limited in size, and healthy uninfected cells in the culture kept dividing throughout the analysis. In contrast, an M-lacking measles virus, which also showed strongly reduced titers, was reported previously to propagate through a Vero cell culture as efficiently as wt measles virus due to enhanced cell-cell fusion (12). These differences may be the result of the unique properties of closely related viruses. In the case of Sendai virus, another paramyxovirus, it was shown that VLPs were not or were poorly generated when M expression was suppressed (40). While our data show that the production of infectious HRSV progeny was nearly abrogated in the absence of M, we cannot exclude the possibility that noninfectious VLPs were released.
Role of the M protein in viral filament formation.
HRSV-induced surface filaments have been suggested to be the equivalent of virions (27, 49, 53, 60), and the loss of viral filaments typically results in the loss of the majority of viral infectivity (7, 42, 49, 53). An exception to the above-mentioned data was a study in which wt HRSV-infected HEp-2 cells were treated with RhoA inhibitors (22). In those instances, the amount of surface filaments was strongly reduced, but the yield of infectious virus was unchanged relative to the yield from untreated cells. Moreover, gradient fractionation and EM analyses showed that upon treatment with RhoA inhibitors, the virion morphology shifted from predominantly filamentous to predominantly spherical. Whether more than one infectious HRSV morphology exists in vivo and whether distinct morphologies might have distinct roles are not known. Similarly, the machinery and mechanisms that underlie the abundant filament formation observed in cell cultures are not understood. Our studies provide new insights into the process of viral filament formation. By IF microscopy (Fig. 4), the typical N-, G-, and F-containing filaments were notably absent in M-null virus-infected cells. Instead, the N protein accumulated in IBs, while G and, to a lesser degree, F were present at the plasma membrane in an evenly distributed but punctate manner. High-resolution analysis of the surface of M-null virus-infected cells (Fig. 5) revealed the presence of abundant, uniformly short, G- and F-containing filaments with a diameter similar to those seen in wt virus-infected cells. Although both IF and SEM analyses thus demonstrated clear differences in filament formation in the absence versus the presence of M, the contrast in G and F targeting appeared to be greater when analyzed by IF microscopy. This may be related to the ease with which G and F proteins on the cell surface are labeled during the IF procedure and the significantly more challenging and nonquantitative labeling of G and F proteins associated with the SEM protocol.
The uniformity of the short filaments on M-null virus-infected cells is highly suggestive of an assembly process that was initiated but arrested. It thus appears that M is not required for the initiation of virus-induced surface filaments, but the filaments are unable to elongate in the absence of M. These observations appear to be in agreement with the recent findings that M has an intrinsic property to self-assemble into helical arrays when incubated in the presence of select lipid mixtures (37). While the latter finding points to a capacity for M to drive both filament initiation and elongation, the abundance of stunted filaments on M-null virus-infected cells suggests that other viral factors, or processes induced after viral infection, are capable of initiating filament formation in the absence of M. A role for the F protein in driving filament formation was suggested previously by other studies in which IBs and growing filaments were occasionally found in close proximity, with F and P proteins colocalizing in projections originating from the IBs (30, 49). In addition, in the absence of the F protein cytoplasmic tail (CT), the production of infectious virus was nearly abrogated, and viral filaments could not be detected by anti-F and anti-G antibodies (42). The presence of G and F proteins in stunted filaments (Fig. 5) may support an M-independent role for F, or F and G proteins combined, in filament initiation. However, the nonquantitative labeling in the presented SEM data does not exclude the possibility that G and F proteins are associated with stunted filaments by their mere presence at the plasma membrane, especially in the case of the surface-abundant G protein. Furthermore, VLPs were found previously to be released from polarized cells with a high efficiency in the absence of the glycoproteins (2, 3). Although the morphology of the released VLPs was not examined, it indicates that the remaining viral proteins (which include M) can efficiently drive the budding of particles in the complete absence of the glycoproteins. In short, further studies are needed to establish the relative contributions of the M, G, and F proteins to the initiation of viral filament formation.
RNP transport: IBs, M, and the cytoskeleton.
Cytoplasmic IBs are believed to be sites of RNP production. M was previously shown both to target to cytoplasmic IBs at a stage of the infection cycle prior to virion release and to associate with RNPs via interactions with the M2-1 protein (21, 35), presumably to shut down RNA synthesis and initiate the assembly process. The M2-1-dependent association of M with viral RNPs differs from the previously reported findings for other paramyxovirus M proteins, in which biochemical and IF microscopy-based evidence suggested a direct interaction between M and N proteins (25). The above-mentioned reports as well as the observed presence of growth-arrested viral filaments in the absence of M (Fig. 5) and the simultaneous increase in the presence of N in IBs (Fig. 4) support a model in which the M proteins are involved in targeting RNPs to budding sites. How HRSV M-RNP complexes would be transported from IBs to sites of budding is not known but likely involves the cytoskeleton. Several cytoskeletal proteins, including actin isoforms, cofilin, and filamin-1, were detected by mass spectrometry analyses of purified viral filaments (although purity was difficult to assess due to the cell-associated nature of filaments). There is ample evidence of a role for polymerized actin and actin-regulatory proteins in assembly (4, 6, 11, 22, 30, 32, 49, 53, 59). However, the role of polymerized actin seems to be limited to the actual budding/release event (11, 30, 32), which was shown previously to occur at sites of lipid rafts (8, 9, 22, 27, 31, 36, 42, 49, 50). In contrast to polymerized actin, microtubules were reported previously to be involved in virus production at a step prior to budding (32). Although the role of cytoskeletal components in RNP transport are far from understood, our data implicate the M protein in this process. Alternatively, RNP transport to sites of budding continues in the absence of M, but M-lacking RNPs fail to reach their destination or to support filament elongation once they have arrived. The latter finding seems inconsistent with the observed accumulation of N in IBs of M-null virus-infected cells.
In conclusion, an improved understanding of the origin and formation of viral filaments in cell culture is important, because these filaments represent the majority of HRSV infectivity, and cell culture is the manufacturing platform for current candidate vaccine stocks. The presented work allowed an examination of infected cells in the complete absence of the M protein for the first time and showed that M plays a role in the maturation and/or maintenance of viral filaments. The increased presence of the N protein in IBs in the absence of M suggests that this role may be played through an interaction of M with RNPs and the subsequent transport of the resulting complexes to sites of filament production. Currently, the M-null system is being developed to serve as a platform to further probe the mechanisms underlying assembly and the role of the M protein therein.
ACKNOWLEDGMENTS
We thank the members of the Oomens laboratory and Rich Eberle for helpful discussions during the preparation of the manuscript, Edward Walsh for providing antibodies, and Terry Colberg and Charlotte Ownby of the Oklahoma State University Microscopy Laboratory for technical expertise.
This work was supported by an OHRS award, project number HR08-139S, from the Oklahoma Center for the Advancement of Science and Technology.
Footnotes
Published ahead of print 8 February 2012
REFERENCES
- 1. Bachi T, Howe C. 1973. Morphogenesis and ultrastructure of respiratory syncytial virus. J. Virol. 12:1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batonick M, Oomens AG, Wertz GW. 2008. Human respiratory syncytial virus glycoproteins are not required for apical targeting and release from polarized epithelial cells. J. Virol. 82:8664–8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batonick M, Wertz GW. 2011. Requirements for human respiratory syncytial virus glycoproteins in assembly and egress from infected cells. Adv. Virol. 2011:pii=34308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bitko V, Oldenburg A, Garmon NE, Barik S. 2003. Profilin is required for viral morphogenesis, syncytium formation, and cell-specific stress fiber induction by respiratory syncytial virus. BMC Microbiol. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Branigan PJ, et al. 2005. Use of a novel cell-based fusion reporter assay to explore the host range of human respiratory syncytial virus F protein. Virol. J. 2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brock SC, Goldenring, Crowe JE., Jr 2003. Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 100:15143–15148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brock SC, Heck JM, McGraw PA, Crowe JE., Jr 2005. The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence. J. Virol. 79:12528–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown G, Aitken J, Rixon HW, Sugrue RJ. 2002. Caveolin-1 is incorporated into mature respiratory syncytial virus particles during virus assembly on the surface of virus-infected cells. J. Gen. Virol. 83:611–621 [DOI] [PubMed] [Google Scholar]
- 9. Brown G, et al. 2004. Analysis of the interaction between respiratory syncytial virus and lipid-rafts in Hep2 cells during infection. Virology 327:175–185 [DOI] [PubMed] [Google Scholar]
- 10. Bukreyev A, Whitehead SS, Murphy BR, Collins PL. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J. Virol. 71:8973–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burke E, Dupuy L, Wall C, Barik S. 1998. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 252:137–148 [DOI] [PubMed] [Google Scholar]
- 12. Cathomen T, Naim HY, Cattaneo R. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen BJ, Lamb RA. 2008. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology 372:221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collins PL, Huang YT, Wertz GW. 1984. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc. Natl. Acad. Sci. U. S. A. 81:7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couch RB, Englund JA, Whimbey E. 1997. Respiratory viral infections in immunocompetent and immunocompromised persons. Am. J. Med. 102:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuchs H, Bachi T. 1975. Scanning electron microscopical demonstration of respiratory syncytial virus antigens by immunological markers. J. Ultrastruct. Res. 52:114–119 [DOI] [PubMed] [Google Scholar]
- 17. Garcia J, Garcia-Barreno B, Vivo A, Melero JA. 1993. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology 195:243–247 [DOI] [PubMed] [Google Scholar]
- 18. Ghildyal R, Baulch-Brown C, Mills J, Meanger J. 2003. The matrix protein of human respiratory syncytial virus localises to the nucleus of infected cells and inhibits transcription. Arch. Virol. 148:1419–1429 [DOI] [PubMed] [Google Scholar]
- 19. Ghildyal R, Ho A, Jans DA. 2006. Central role of the respiratory syncytial virus matrix protein in infection. FEMS Microbiol. Rev. 30:692–705 [DOI] [PubMed] [Google Scholar]
- 20. Ghildyal R, et al. 2005. Interaction between the respiratory syncytial virus G glycoprotein cytoplasmic domain and the matrix protein. J. Gen. Virol. 86:1879–1884 [DOI] [PubMed] [Google Scholar]
- 21. Ghildyal R, Mills J, Murray M, Vardaxis N, Meanger J. 2002. Respiratory syncytial virus matrix protein associates with nucleocapsids in infected cells. J. Gen. Virol. 83:753–757 [DOI] [PubMed] [Google Scholar]
- 22. Gower TL, et al. 2005. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J. Virol. 79:5326–5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haas J, Park EC, Seed B. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315–324 [DOI] [PubMed] [Google Scholar]
- 24. Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25–30 [DOI] [PubMed] [Google Scholar]
- 25. Harrison MS, Sakaguchi T, Schmitt AP. 2010. Paramyxovirus assembly and budding: building particles that transmit infections. Int. J. Biochem. Cell Biol. 42:1416–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heminway BR, et al. 1994. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology 200:801–805 [DOI] [PubMed] [Google Scholar]
- 27. Henderson G, Murray J, Yeo R. 2002. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology 300:244. [DOI] [PubMed] [Google Scholar]
- 28. Huang YT, Collins PL, Wertz GW. 1985. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 2:157–173 [DOI] [PubMed] [Google Scholar]
- 29. Inoue M, et al. 2003. A new Sendai virus vector deficient in the matrix gene does not form virus particles and shows extensive cell-to-cell spreading. J. Virol. 77:6419–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeffree CE, et al. 2007. Ultrastructural analysis of the interaction between F-actin and respiratory syncytial virus during virus assembly. Virology 369:309–323 [DOI] [PubMed] [Google Scholar]
- 31. Jeffree CE, Rixon HW, Brown G, Aitken J, Sugrue RJ. 2003. Distribution of the attachment (G) glycoprotein and GM1 within the envelope of mature respiratory syncytial virus filaments revealed using field emission scanning electron microscopy. Virology 306:254–267 [DOI] [PubMed] [Google Scholar]
- 32. Kallewaard NL, Bowen AL, Crowe JE., Jr 2005. Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 331:73–81 [DOI] [PubMed] [Google Scholar]
- 33. Latiff K, Meanger J, Mills J, Ghildyal R. 2004. Sequence and structure relatedness of matrix protein of human respiratory syncytial virus with matrix proteins of other negative-sense RNA viruses. Clin. Microbiol. Infect. 10:945–948 [DOI] [PubMed] [Google Scholar]
- 34. Levine S, Klaiber-Franco R, Paradiso PR. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521–2524 [DOI] [PubMed] [Google Scholar]
- 35. Li D, et al. 2008. Association of respiratory syncytial virus M protein with viral nucleocapsids is mediated by the M2-1 protein. J. Virol. 82:8863–8870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCurdy LH, Graham BS. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77:1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McPhee HK, et al. 2011. Influence of lipids on the interfacial disposition of respiratory syncytical [sic] virus matrix protein. Langmuir 27:304–311 [DOI] [PubMed] [Google Scholar]
- 38. Money VA, McPhee HK, Mosely JA, Sanderson JM, Yeo RP. 2009. Surface features of a Mononegavirales matrix protein indicate sites of membrane interaction. Proc. Natl. Acad. Sci. U. S. A. 106:4441–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morton CJ, et al. 2003. Structural characterization of respiratory syncytial virus fusion inhibitor escape mutants: homology model of the F protein and a syncytium formation assay. Virology 311:275–288 [DOI] [PubMed] [Google Scholar]
- 40. Mottet-Osman G, et al. 2007. Suppression of the Sendai virus M protein through a novel short interfering RNA approach inhibits viral particle production but does not affect viral RNA synthesis. J. Virol. 81:2861–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norrby E, Marusyk H, Orvell C. 1970. Morphogenesis of respiratory syncytial virus in a green monkey kidney cell line (Vero). J. Virol. 6:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oomens AG, Bevis KP, Wertz GW. 2006. The cytoplasmic tail of the human respiratory syncytial virus F protein plays critical roles in cellular localization of the F protein and infectious progeny production. J. Virol. 80:10465–10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oomens AG, Megaw AG, Wertz GW. 2003. Infectivity of a human respiratory syncytial virus lacking the SH, G, and F proteins is efficiently mediated by the vesicular stomatitis virus G protein. J. Virol. 77:3785–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oomens AG, Wertz GW. 2004. The baculovirus GP64 protein mediates highly stable infectivity of a human respiratory syncytial virus lacking its homologous transmembrane glycoproteins. J. Virol. 78:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oomens AG, Wertz GW. 2004. trans-Complementation allows recovery of human respiratory syncytial viruses that are infectious but deficient in cell-to-cell transmission. J. Virol. 78:9064–9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peeples ME. 1991. Paramyxovirus M proteins: pulling it all together and taking it on the road. Plenum Press, New York, NY [Google Scholar]
- 47. Petrakova O, et al. 2005. Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in mammalian cells. J. Virol. 79:7597–7608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pringle CR. 1987. Progress towards control of the acute respiratory viral diseases of childhood. Bull. World Health Organ. 65:133–137 [PMC free article] [PubMed] [Google Scholar]
- 49. Radhakrishnan A, et al. 2010. Protein analysis of purified respiratory syncytial virus particles reveals an important role for heat shock protein 90 in virus particle assembly. Mol. Cell. Proteomics 9:1829–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rixon HW, et al. 2004. The small hydrophobic (SH) protein accumulates within lipid-raft structures of the Golgi complex during respiratory syncytial virus infection. J. Gen. Virol. 85:1153–1165 [DOI] [PubMed] [Google Scholar]
- 51. Roberts SR, Compans RW, Wertz GW. 1995. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J. Virol. 69:2667–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodriguez L, Cuesta I, Asenjo A, Villanueva N. 2004. Human respiratory syncytial virus matrix protein is an RNA-binding protein: binding properties, location and identity of the RNA contact residues. J. Gen. Virol. 85:709–719 [DOI] [PubMed] [Google Scholar]
- 53. Santangelo PJ, Bao G. 2007. Dynamics of filamentous viral RNPs prior to egress. Nucleic Acids Res. 35:3602–3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmitt AP, Lamb RA. 2004. Escaping from the cell: assembly and budding of negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:145–196 [DOI] [PubMed] [Google Scholar]
- 55. Stope MB, Karger A, Schmidt U, Buchholz UJ. 2001. Chimeric bovine respiratory syncytial virus with attachment and fusion glycoproteins replaced by bovine parainfluenza virus type 3 hemagglutinin-neuraminidase and fusion proteins. J. Virol. 75:9367–9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takimoto T, Portner A. 2004. Molecular mechanism of paramyxovirus budding. Virus Res. 106:133–145 [DOI] [PubMed] [Google Scholar]
- 57. Tayyari F, et al. 2011. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 17:1132–1135 [DOI] [PubMed] [Google Scholar]
- 58. Teng MN, Collins PL. 1998. Identification of the respiratory syncytial virus proteins required for formation and passage of helper-dependent infectious particles. J. Virol. 72:5707–5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ulloa L, Serra R, Asenjo A, Villanueva N. 1998. Interactions between cellular actin and human respiratory syncytial virus (HRSV). Virus Res. 53:13–25 [DOI] [PubMed] [Google Scholar]
- 60. Utley TJ, et al. 2008. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc. Natl. Acad. Sci. U. S. A. 105:10209–10214 [DOI] [PMC free article] [PubMed] [Google Scholar]