Abstract
OBJECTIVE:
To evaluate the association of late-preterm birth with asthma severity among young children.
METHODS:
A retrospective cohort study was performed with electronic health record data from 31 practices affiliated with an academic medical center. Participants included children born in 2007 at 34 to 42 weeks of gestation and monitored from birth to 18 months. We used multivariate logistic or Poisson models to assess the impact of late-preterm (34–36 weeks) and low-normal (37–38 weeks) compared with term (39–42 weeks) gestation on diagnoses of asthma and persistent asthma, inhaled corticosteroid use, and numbers of acute respiratory visits.
RESULTS:
Our population included 7925 infants (7% late-preterm and 21% low-normal gestation). Overall, 8.3% had been diagnosed with asthma by 18 months. Compared with term gestation, late-preterm gestation was associated with significant increases in persistent asthma diagnoses (adjusted odds ratio [aOR]: 1.68), inhaled corticosteroid use (aOR: 1.66), and numbers of acute respiratory visits (incidence rate ratio: 1.44). Low-normal gestation was associated with increases in asthma diagnoses (aOR: 1.34) and inhaled corticosteroid use (aOR: 1.39).
CONCLUSION:
Birth at late-preterm and low-normal gestational ages might be an important risk factor for the development of asthma and for increased health service use in early childhood.
Keywords: late preterm, gestational age, asthma, persistent asthma, health care use
WHAT'S KNOWN ON THIS SUBJECT:
Infants born late preterm (34–36 completed gestational weeks) are at increased risk for respiratory morbidity in the neonatal period. Few studies have focused on the possible associations between late-preterm birth and long-term respiratory outcomes such as asthma.
WHAT THIS STUDY ADDS:
Asthma severity in a primary care cohort was measured. Results revealed that late-preterm birth was associated with diagnoses of persistent asthma, inhaled corticosteroid use, and more acute respiratory visits.
In the past 2 decades, the birth rate for late-preterm infants (delivered between 34 and 36 completed gestational weeks) has increased by >20%, to yield ∼9% of all births in the United States.1 Compared with term infants, late-preterm infants have increased risks of morbidity and death in the neonatal period.2–11 Furthermore, there is evidence that, in the first year of life, late-preterm infants have a propensity for more severe illnesses and might require more medical services when hospitalized.12–14 To date, however, the full magnitude of potential long-term morbidities for this population and their public health impact have not been well studied. The Eunice Kennedy Shriver National Institute of Child Health and Human Development has identified the economic impact and long-term health impact of late prematurity as priority areas of research.15
One important long-term outcome is asthma. An association between extreme preterm birth and chronic respiratory morbidity, including asthma, has been established.16–22 However, most studies of late prematurity and respiratory morbidity have focused on short-term outcomes, such as respiratory distress syndrome, transient tachypnea of the newborn, and transfer to the NICU for mechanical ventilation.3,4,10,23 Few studies have focused on the impact of late prematurity on long-term respiratory outcomes, such as the development of asthma.24,25
In this study, we analyzed the association of late-preterm birth with the development of asthma within the first 18 months of life, by using electronic health record (EHR) data from a large primary care network. We measured asthma severity on the basis of physician assessments (information not available in administrative data). Specifically, we examined whether children born late preterm were more likely to have persistent asthma diagnosed and whether their use of controller medication and primary care acute asthma visits increased. In addition, we examined the association of asthma with low-normal gestational age (37–38 weeks), which has not been well studied to date.26 Given the significant health and societal costs related to childhood asthma, a better understanding of the association between asthma and gestational age is critical for understanding the true impact of late prematurity on health and health care costs.
METHODS
Study Population and Setting
We conducted a retrospective cohort analysis of infants born at 34 to 42 weeks of gestation between January 1, 2007, and December 31, 2007. The study was conducted at 31 practices within the Children's Hospital of Philadelphia Pediatric Research Consortium, a multistate, hospital-owned, primary care practice-based research network caring for >235 000 children and adolescents at 31 practice locations. Study sites included 4 urban teaching practices, where approximately one-third of children have private insurance, and 26 urban or suburban practices not involved in resident teaching, where the majority of children are privately insured. All practices used the ambulatory EHR EpicCare (Epic, Verona, WI) for physician documentation and order entry. The Children's Hospital of Philadelphia institutional review board approved the study.
Eligible subjects included children who were seen at a primary care site for well-child care within the first 30 days of life and subsequently were monitored for 18 months. Children who left the primary care network before 18 months of age were excluded. We also excluded children with major congenital anomalies and hereditary disorders, because their patterns of health care utilization might vary significantly from those of unaffected children. These diagnoses were identified in the chronic problem list for each patient, on the basis of International Classification of Diseases, Ninth Revision (ICD-9), codes, and were clustered into homogeneous categories by using a taxonomy developed previously for acute health problems (Appendix).27
Primary Predictor Variable
Gestational age was recorded as part of routine care, on the basis of available birth hospital discharge records. We categorized infants as late preterm (34–36 completed weeks of gestation), low-normal gestation (37–38 weeks of gestation), or term (39–42 weeks of gestation). Because physicians might have been less likely to document gestational-age information for term infants, compared with preterm infants, missing gestational-age data (7.6% of the sample) were likely nonrandom. We imputed missing gestational-age data on the basis of birth weight, gender, race, insurance status, income, and delivery method, by using single imputation. For univariate and multivariate regression analyses, we then used multiple imputation, a technique that pools analyses from multiple imputed data sets and yields a more-conservative estimate by adjusting the SEs for missing data uncertainty.28
Outcome Variables
We measured several outcomes to evaluate the relationship between late prematurity and the development of asthma. By using ICD-9 codes from the chronic problem lists and visit histories, we measured any diagnosis of asthma (code 493.X) within the 18-month period as a binary outcome.25,29 We also measured the diagnosis of persistent asthma as a binary outcome, including mild, moderate, and severe persistent asthma. These classifications, which were developed by the Children's Hospital of Philadelphia in 2001 on the basis of ICD-9 codes to help physicians designate the severity of asthma, were determined through physician judgment on the basis of National Asthma Education and Prevention Program guidelines.30,31 Asthma management tools available to all practices in the EHR included a pediatric asthma control tool to capture asthma symptom frequency, as well as standardized documentation templates and order sets to facilitate severity classification and prescription of controller medications. The internal consistency and construct validity of the pediatric asthma control tool were demonstrated in previous work.32
We also queried the EHR for prescription information to create an indicator variable for inhaled corticosteroid use, including the following medications: beclomethasone dipropionate, budesonide, fluticasone propionate, and fluticasone-salmeterol.25 For the subgroup of children with diagnosed asthma, we measured the total number of acute office visits within the 18-month period with a visit diagnosis of wheezing or asthma (Current Procedural Terminology codes 99201–99205 and 99211–99215; ICD-9 codes 786.07, 466.X, and 493.X), which were all captured in the EHR database.
Confounding Variables
Clinical and demographic covariates to control for potential confounding were chosen on the basis of previous studies of asthma risk factors and included delivery method,33 breastfeeding,34 gender,35,36 birth weight,16,36,37 atopy,38,39 race,36,40 and socioeconomic status.37,41 We obtained information on the method of delivery (cesarean versus vaginal) and breastfeeding status (any breastfeeding versus formula feeding only) by searching EHR text for the documented birth history and visit notes. Small-for-gestational-age infants were identified by using growth curves for birth weight and gestational age.42 Although additional clinical factors, such as respiratory distress syndrome, surfactant use, and neonatal intensive care, might have been useful to evaluate, this type of information was documented inconsistently and data were not reliable for analyses. These factors also might be on the causal pathway between gestational age and childhood respiratory outcomes and therefore would be inappropriate for inclusion in the final regression model.43 Atopy was ascertained through physician diagnoses on the problem list, including diagnoses of seasonal and food allergies and eczema (ICD-9 codes V15.01–V15.05, V15.09, 995.3, 995.6–995.7, 708.0, 693.1, 477.X, 372.14, 691.8, and 692.9). Patient race (black, white, or other) and insurance status (private insurance at any time during the study versus none) also were included. To approximate family income, we linked 5-digit zip codes to US Census tract data and grouped the zip codes on the basis of the proportions of residents living below the federal poverty level.44,45
Statistical Analyses
First, to assess correlations between variables, we conducted bivariate analyses for all covariates. If 2 variables were colinear, then we chose the most clinically meaningful variable to include for analysis. Then, we used univariate regression analyses to analyze the independent association of each covariate with the outcome variables. We built our base multivariate model by including primary care site as a fixed effect, to adjust for clustering. Then, we added terms for interactions between gestational age and other covariates, such as gender and race, to the base model. Because no interaction term remained statistically significant after adjustment of the significance level for multiple testing, the final regression model did not include any interaction terms.
We used multivariate logistic regression analyses to analyze the association between gestational age and each binary outcome (diagnosis of asthma, diagnosis of persistent asthma, and inhaled corticosteroid use), with adjustment for all other clinical and demographic covariates. For the subgroup of patients with asthma, we used multivariate Poisson regression analysis to analyze the association between gestational age and the number of outpatient visits with a diagnosis of wheeze. Comparisons of asthma, persistent asthma, and inhaled corticosteroid use from logistic regression analyses were then transformed into standardized proportions by using marginal standardization.46 Standardized proportions are interpreted as the outcomes expected if the entire sample alternatively was subjected to the intervention or was monitored as a control group. We repeated all analyses after exclusion of children with missing gestational-age data, and results were similar (data not shown). Analyses were performed by using Stata 11.0 (Stata Corp, College Station, TX).
RESULTS
Study Population
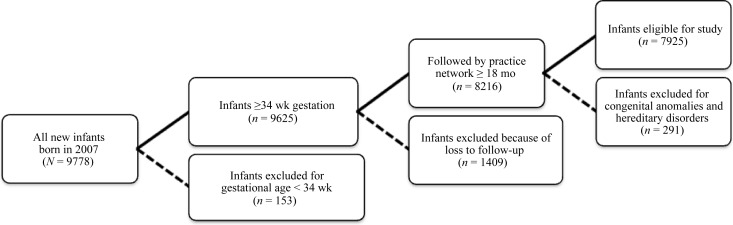
In the original data set containing data for all infants born in 2007 and seen within the practice network by 30 days of life, 9625 infants were born at gestational ages of ≥34 weeks (Fig 1). We excluded 1409 children who dropped out of the practice network before 18 months of life. In addition, we excluded 291 patients with major congenital or hereditary disorders. The remaining sample consisted of 7925 children, including 581 (7.3%) born at 34 to 36 weeks of gestation, 1716 (21.7%) born at 37 to 38 weeks, 5027 (63.4%) born at 39 to 42 weeks, and 601 (7.6%) with imputed gestational-age data (Table 1).
FIGURE 1.
Study population. Dashed lines indicate when infants were excluded from the study; solid lines indicate when infants were included.
TABLE 1.
Patient Characteristics and Outcomes Associated With Gestational Age (Unadjusted)
| 34–36 wk (N = 582) | 37–38 wk (N = 1803) | 39–42 wk (N = 5540) | P | |
|---|---|---|---|---|
| Demographic variables | ||||
| Female, n (%) | 293 (50.3) | 844 (46.8) | 2745 (49.6) | .10 |
| Race, n (%) | ||||
| White | 274 (47.1) | 931 (51.6) | 2959 (53.4) | |
| Black | 218 (37.5) | 521 (28.9) | 1605 (29.0) | .001 |
| Other | 89 (15.3) | 346 (19.2) | 966 (17.4) | |
| Any private insurance, n (%) | 419 (72.0) | 1371 (76.0) | 4252 (76.8) | .04 |
| Income, n (%)a | ||||
| <5% below FPL | 216 (37.1) | 732 (40.6) | 2242 (40.5) | |
| 5%–10% below FPL | 130 (22.3) | 454 (25.2) | 1429 (25.8) | .09 |
| 10%–20% below FPL | 87 (15.0) | 222 (12.3) | 717 (12.9) | |
| >20% below FPL | 148 (25.4) | 394 (21.9) | 1143 (20.6) | |
| Clinical variables | ||||
| Vaginal delivery, n (%) | 317 (54.5) | 1090 (60.5) | 3724 (67.2) | <.001 |
| Any breastfeeding, n (%) | 300 (51.6) | 1100 (61.0) | 3687 (66.6) | <.001 |
| Atopy, n (%) | 93 (16.0) | 307 (17.0) | 904 (16.3) | .74 |
| Outcome variables | ||||
| Asthma, n (%)b | 54 (9.3) | 180 (10.2) | 422 (7.6) | .002 |
| Persistent asthma, n (%)c | 19 (3.3) | 46 (2.6) | 108 (2.0) | .04 |
| Inhaled corticosteroid use, n (%) | 52 (8.9) | 139 (7.7) | 310 (5.6) | <.001 |
| Visits with wheeze, mean | 4.6 | 3.5 | 3.6 | .06d |
Proportion of zip code residents with incomes below the federal poverty level (FPL).
Any diagnosis of asthma, including intermittent or unspecified asthma and mild, moderate, or severe persistent asthma.
Diagnosis of mild, moderate, or severe persistent asthma.
F-statistic P value.
We conducted χ2 analyses to compare our final study sample with the patients who were excluded because of dropout from the primary care network before 18 months of age. There was no statistically significant difference in gestational age or gender between our final study sample and the excluded patients. However, patients in our study sample were more likely to be white (53% vs 43%; P < .001), to have any private insurance (76% vs 62%; P < .001), and to live in zip codes with <5% of residents below the federal poverty limit (40% vs 32%; P < .001).
Unadjusted Analyses
The overall proportion of children in the sample with diagnosed asthma was 8.3%. Unadjusted analyses of gestational age and outcomes demonstrated statistically significant differences in diagnoses of asthma (P = .002), diagnoses of persistent asthma (P = .04), and inhaled corticosteroid use (P < .001) (Tables 1 and 2). Unadjusted differences in covariates across gestational-age groups are listed in Table 1. In univariate analyses, several covariates, including black race (odds ratio [OR]: 1.46 [95% confidence interval [CI]: 1.23–1.74]) and lower income (OR: 1.58 [95% CI: 1.27–1.96]), also were associated with diagnoses of asthma (Table 2).
TABLE 2.
Univariate Associations of Predictors With Outcomes
| OR (95% CI) |
Incident Rate Ratio (95% CI) for Acute Outpatient Visits With Wheeze | |||
|---|---|---|---|---|
| Asthmaa | Persistent Asthmab | Inhaled Corticosteroids | ||
| Gestational age | ||||
| 34–36 wk | 1.24 (0.92–1.67) | 1.70 (1.03–2.79)c | 1.66 (1.22–2.25)c | 1.30 (1.14–1.49)c |
| 37–38 wk | 1.35 (1.12–1.62)c | 1.32 (0.93–1.87) | 1.41 (1.15–1.73)c | 0.99 (0.91–1.09) |
| 39–42 wk | Reference | Reference | Reference | Reference |
| Gender | ||||
| Male | 1.84 (1.56–2.18)c | 2.48 (1.77–3.46)c | 1.81 (1.50–2.18)c | 1.18 (1.08–1.29)c |
| Female | Reference | Reference | Reference | Reference |
| Race | ||||
| Black | 1.46 (1.23–1.74)c | 2.86 (2.06–3.97)c | 1.17 (0.96–1.44) | 0.63 (0.57–0.69)c |
| Other | 0.84 (0.66–1.07) | 0.94 (0.56–1.58) | 0.87 (0.67–1.13) | 0.97 (0.87–1.09) |
| White | Reference | Reference | Reference | Reference |
| Insurance | ||||
| Private | 0.68 (0.57–0.81)c | 0.50 (0.36–0.68)c | 0.90 (0.73–1.11) | 1.45 (1.32–1.60)c |
| Public | Reference | Reference | Reference | Reference |
| Incomed | ||||
| >20% below FPL | 1.58 (1.27–1.96)c | 2.58 (1.77–3.78)c | 1.06 (0.83–1.37) | 0.55 (0.49–0.62)c |
| 10%–20% below FPL | 1.37 (1.06–1.78)c | 1.70 (1.05–2.76)c | 1.15 (0.86–1.54) | 0.75 (0.65–0.86)c |
| 5%–10% below FPL | 1.61 (1.32–1.98)c | 1.16 (0.75–1.80) | 1.33 (1.06–1.66)c | 1.00 (0.91–1.10) |
| <5% below FPL | Reference | Reference | Reference | Reference |
| Delivery method | ||||
| Vaginal | Reference | Reference | Reference | Reference |
| Cesarean | 1.15 (0.97–1.36) | 1.10 (0.80–1.51) | 1.22 (1.01–1.48)c | 1.09 (1.00–1.19)c |
| Breastfeeding | ||||
| Any breastfeeding | Reference | Reference | Reference | Reference |
| Formula feeding only | 1.50 (1.28–1.77)c | 1.57 (1.16–2.13)c | 1.28 (1.06–1.54)c | 0.79 (0.73–0.86)c |
| Atopy | 1.93 (1.60–2.32)c | 2.05 (1.46–2.86)c | 1.84 (1.50–2.27)c | 1.03 (0.94–1.13) |
Any diagnosis of asthma, including intermittent or unspecified asthma and mild, moderate, or severe persistent asthma.
Diagnosis of mild, moderate, or severe persistent asthma.
P < 0.05.
Proportion of zip code residents with incomes below the federal poverty level (FPL).
Multivariate Analyses
Compared with gestational ages of 39 to 42 weeks, late-preterm gestation was associated with a trend toward increased diagnoses of asthma, although this difference was not statistically significant (Table 3). However, late-preterm gestation was significantly associated with increased diagnoses of persistent asthma (adjusted OR [aOR]: 1.68 [95% CI: 1.01–2.80]) and receipt of ≥1 inhaled corticosteroid prescription (aOR: 1.66 [95% CI: 1.20–2.29]). In addition, late-preterm infants with asthma had more acute respiratory visits (incident rate ratio: 1.45 [95% CI: 1.25–1.68]), compared with children born at term. Children with gestational ages of 37 to 38 weeks also had increased odds of asthma outcomes, compared with term children, including diagnoses of asthma (aOR: 1.34 [95% CI: 1.11–1.63]) and inhaled corticosteroid use (aOR: 1.41 [95% CI: 1.15–1.73]) (Table 3).
TABLE 3.
Multivariate Associations of Predictors With Outcomes
| aOR (95% CI) |
Incident Rate Ratio (95% CI) for Acute Outpatient Visits With Wheeze | |||
|---|---|---|---|---|
| Asthmaa | Persistent Asthmab | Inhaled Corticosteroids | ||
| Gestational age | ||||
| 34–36 wk | 1.26 (0.92–1.73) | 1.68 (1.01–2.80)c | 1.66 (1.20–2.29)c | 1.44 (1.24–1.67)c |
| 37–38 wk | 1.34 (1.11–1.63)c | 1.28 (0.89–1.84) | 1.39 (1.12–1.72)c | 0.98 (0.89–1.07) |
| 39–42 wk | Reference | Reference | Reference | Reference |
| Gender | ||||
| Male | 1.88 (1.58–2.24)c | 2.41 (1.72–3.40)c | 1.82 (1.50–2.22)c | 1.14 (1.04–1.25)c |
| Female | Reference | Reference | Reference | Reference |
| Race | ||||
| Black | 1.81 (1.35–2.42)c | 2.04 (1.21–3.44)c | 1.56 (1.13–2.17)c | 0.87 (0.75–1.02) |
| Other | 0.75 (0.57–0.97)c | 0.92 (0.53–1.58) | 0.79 (0.60–1.05)c | 1.03 (0.90–1.16) |
| White | Reference | Reference | Reference | Reference |
| Insurance | ||||
| Private | 0.69 (0.54–0.88)c | 0.83 (0.55–1.26) | 0.80 (0.60–1.05) | 1.06 (0.93–1.19) |
| Public | Reference | Reference | Reference | Reference |
| Incomed | ||||
| >20% below FPL | 0.71 (0.48–1.06) | 0.69 (0.35–1.34) | 0.78 (0.50–1.23) | 0.72 (0.58–0.90)c |
| 10%–20% below FPL | 0.90 (0.64–1.27) | 0.73 (0.39–1.38) | 0.96 (0.66–1.39) | 0.78 (0.66–0.93)c |
| 5%–10% below FPL | 1.02 (0.79–1.32) | 0.90 (0.53–1.53) | 1.00 (0.76–1.33) | 0.88 (0.78–0.99)c |
| <5% below FPL | Reference | Reference | Reference | Reference |
| Delivery method | ||||
| Vaginal | Reference | Reference | Reference | Reference |
| Cesarean | 1.13 (0.94–1.35) | 1.11 (0.80–1.55) | 1.15 (0.94–1.40) | 1.06 (0.97–1.15) |
| Breastfeeding | ||||
| Any breastfeeding | Reference | Reference | Reference | Reference |
| Formula feeding only | 1.39 (1.16–1.66)c | 1.27 (0.91–1.76) | 1.24 (1.01–1.51)c | 0.87 (0.80–0.95)c |
| Atopy | 1.80 (1.47–2.19)c | 1.63 (1.15–2.31)c | 1.70 (1.36–2.12)c | 1.01 (0.91–1.11) |
The analysis included adjustment for primary care site.
Any diagnosis of asthma, including intermittent or unspecified asthma and mild, moderate, or severe persistent asthma.
Diagnosis of mild, moderate, or severe persistent asthma.
P < 0.05
Proportion of zip code residents with incomes below the federal poverty level (FPL).
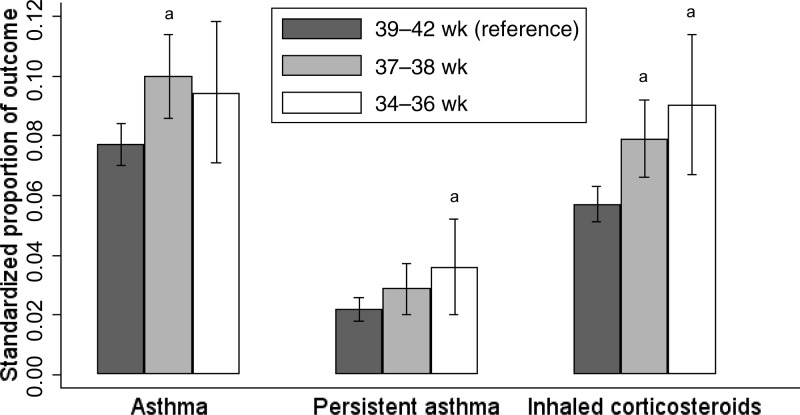
The standardized proportion of asthma in the reference group was 7.7%, compared with 9.4% for children born late preterm (P = .15) and 9.9% for children born at 37 to 38 weeks (P = .003) (Fig 2). The proportion of persistent asthma in the reference group was 2.2%, compared with 3.6% for children born late preterm (P = .04) and 2.8% for children born at 37 to 38 weeks (P = .18). The proportion of inhaled corticosteroid use in the reference group was 5.7%, compared with 9.0% for children born late preterm (P = .002) and 7.7% for children born at 37 to 38 weeks (P = .003).
FIGURE 2.
Standardized proportions of asthma outcomes. Standardized proportions of asthma-related outcomes according to gestational-age category are shown. Estimates were standardized for all covariates. Brackets represent 95% CIs. a Risk differences are statistically significant compared with the reference group at 39 to 42 weeks of gestation (P < .05).
DISCUSSION
Our study examined the association of late-preterm birth with asthma in the first 18 months of life. We demonstrated that children born late preterm were at increased risk for persistent asthma and use of an inhaled corticosteroid. Children born late preterm with asthma also had increased utilization, as measured on the basis of acute respiratory outpatient visits. In addition, we found that diagnoses of asthma and inhaled corticosteroid were more common among children with low-normal gestational age, compared with term children. Extending previous work that focused on infants <1 year of age, this study documents an association of asthma severity with late prematurity.
Previous studies on asthma and preterm birth focused on the impact of gestational age for very preterm infants (≤32 weeks) or all preterm infants (<37 weeks), and the magnitude of the association varied substantially between studies.16–22 Mechanistically, it has been proposed that chorioamnionitis, because of the associated proinflammatory state and heightened immune response, might play a role in asthma development for infants born preterm.20,29 The few studies of late prematurity and the development of asthma showed mixed results. Using survey data from a nationally representative sample, Abe et al24 did not find an association with late-preterm birth when asthma was assessed on the basis of caregiver reports. Escobar et al,25 using a large cohort from Kaiser Permanente, did find a statistically significant association between late prematurity and recurrent wheeze in children up to 3 years of age; recurrent wheeze was defined as a composite measure on the basis of ICD-9 codes, prescription utilization, and visit history.
Our study specifically evaluated the association of birth at 34 to 36 weeks of gestation with asthma in a practice-based setting, using physician classifications of severity as well as utilization measures. Our findings that late-preterm children were more likely to have persistent asthma diagnosed and to be given inhaled corticosteroids indicate that late-preterm birth is associated with differences in asthma disease severity and health resource utilization for asthma. The implication that late-preterm children have a propensity for more-severe illness is consistent with the results of previous studies that demonstrated higher rehospitalization rates and higher hospital costs for late-preterm infants, compared with term infants, in the first year of life.12,13
Our findings also suggest that low-normal gestational ages of 37 to 38 weeks might confer increased risk of early childhood asthma, compared with gestational ages of ≥39 weeks, as suggested in other studies.25,26 This association is noteworthy, given recent epidemiological trends in gestational length for US births. Since the 1990s, the distribution of gestational ages has shifted downward; 39, rather than 40, weeks is now the most common length of gestation.47 The proportion of births at severely preterm gestational ages has remained stable in the past 2 decades, whereas there have been increases primarily in the proportions of births at 37 to 39 weeks and 34 to 36 weeks of gestation.1,47 The reasons for this trend are likely multifactorial, including increases in labor induction and cesarean delivery rates, patient and provider preferences, and obesity and other demographic changes.47–50 Given the large contributions of late-preterm and low-normal gestation deliveries to the total volume of US births, associations of asthma with these gestational-age groups could have a significant public health impact, which warrants further investigation.
This study had several limitations, primarily related to regression adjustment for nonrandomization. We lacked data on certain prenatal and postnatal risk factors, including chorioamnionitis, maternal smoking, and family history. Although more-detailed information on obstetric and neonatal histories, including the indications for delivery, was available for some infants, such data were documented inconsistently and therefore were not reliable for analysis. Although such unobserved factors might have influenced selection into gestational-age groups and biased results, the large ORs make these unmeasured variables less likely to account for observed differences between groups. We also acknowledge that there might have been errors in ICD-9 coding that led to misclassification bias. However, this would be minimized by the fact that each ICD-9 code in the chronic problem list was paired with a physician-documented diagnosis in the medical records, which we used as a cross-reference. An additional limitation is that we were unable to assess outcomes beyond 18 months of age; therefore, we missed asthma diagnoses made after that time. Although diagnosis of asthma in younger age groups can be difficult, our study used an EHR that provides a unique, standardized approach for physicians to measure and to document asthma symptoms among infants and young children; the validity of this approach was demonstrated previously.30,32 Strengths of this study include racial and socioeconomic diversity of the sample, which contributes to the generalizability of results. This birth cohort, in which all patients were born in 2007, reflects recent trends in late-preterm birth rates and asthma.
CONCLUSIONS
We demonstrated an association between late prematurity and asthma severity by 18 months of age in a cohort of 7925 children who sought primary care in a practice network. Although children born late preterm were not statistically more likely than term children to have asthma diagnosed, they were more likely to have persistent asthma diagnosed, to be given an inhaled corticosteroid, and to be seen for acute outpatient respiratory visits. Our findings suggest that the impact of late prematurity on health and health care utilization extends beyond the neonatal period and into the second year of life. Moreover, we demonstrated that birth at low-normal gestations also was associated with physician diagnoses of asthma and increased health care utilization. Additional study is needed to confirm our observations. Because asthma rates have continued to increase, contributing substantially to morbidity and health care spending for children,51 pediatric and obstetric providers, as well as families, should recognize the role that early gestation might play in the development of asthma.
ACKNOWLEDGMENTS
This work was performed while Dr Goyal was a Robert Wood Johnson Foundation Clinical Scholar at the University of Pennsylvania, with funding support from the Robert Wood Johnson Foundation. Drs Fiks and Lorch were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants K23 HD059919 and R01 HD057168).
We thank the network of primary care physicians and their patients and families for their contributions to clinical research through the Pediatric Research Consortium at the Children's Hospital of Philadelphia. In addition, we thank Nicholas Boscamp for research assistance, Diane Richardson for critical review of the manuscript, and Mark Ramos and Robert Grundmeier for assistance with study implementation.
APPENDIX.
ICD-9 Codes for Exclusion Diagnoses
| Diagnosis | ICD-9 Code |
| Congenital anomalies of palate and lip | 524.04, 524.06, 749, 749.1, 749.12, 749.2, 749.22, 750.26 |
| Endocrine, genetic, and inherited metabolic disorders | 237.7, 237.71, 243, 244.9, 245.2, 250.01, 250.1, 252, 252.1, 253, 253.2, 253.3, 255.2, 270.1, 270.2, 270.6, 273.4, 277.5, 277.85, 277.87, 277.9, 756.16, 756.51, 756.59, 758, 758.39, 758.5, 758.7, 758.89, 758.9, 759.1, 759.2, 759.6, 759.82, 759.89, 795.2 |
| Congenital heart anomalies | 416, 425.1, 425.4, 426.5, 426.82, 428, 429.3, 441.02, 745.1, 745.12, 745.19, 745.2, 745.69, 746.01, 746.02, 746.09, 746.1, 746.4, 746.81, 746.83, 746.85, 746.87, 746.89, 746.9, 747.1, 747.21, 747.6, 747.61, 747.81, 747.9 |
| Inherited hematologic disorders | 282, 282.3, 282.49, 282.6, 282.8, 286, 286.3, 286.4 |
| Congenital disorders of musculoskeletal system | 213.9, 359.21, 359.9, 737.39, 745.2, 756.1, 756.19 |
| Congenital respiratory anomalies | 277, 748.3, 748.4, 748.5 |
| Congenital gastrointestinal anomalies | 537, 550.03, 553.2, 553.3, 553.9, 750.5, 751.2, 751.3, 751.4, 751.5, 751.61, 751.69, V44.1, V44.3 |
| Congenital neurologic anomalies | 331.3, 331.4, 348.4, 433.31, 434.01, 434.91, 655, 741.9, 742.2, 742.3, 742.4, 742.59, 756.17, V45.2 |
| Congenital urologic and renal anomalies | 753, 753.19, 753.21, 753.29, 753.9 |
| Neoplastic disorders | 183, 187.1, 189, 190.5, 191, 191.6, 191.9, 192, 194, 204, 208.01, 208.9, 211.3, 225, 238.5, 273.3 |
| Immunologic disorders | 279, 279.01, 279.11, 279.3, 279.9, 288.09, 710.9, 714.3, 714.32, 714.89, 759 |
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Robert Wood Johnson Foundation or the Eunice Kennedy Shriver National Institute of Child Health and Human Development. E-mail: goyaln@email.chop.edu
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- EHR
- electronic health record
- OR
- odds ratio
- aOR
- adjusted odds ratio
- ICD-9
- International Classification of Diseases, Ninth Revision
- CI
- confidence interval
REFERENCES
- 1. Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a Bit Too Early: Recent Trends in Late Preterm Births. Hyattsville, MD: National Center for Health Statistics; 2009. NCHS Data Brief No. 24 [PubMed] [Google Scholar]
- 2. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90(2):125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gestational age and birth weight. Obstet Gynecol. 2003;102(3):488–492 [DOI] [PubMed] [Google Scholar]
- 4. Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304(4):419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284(7):843–849 [DOI] [PubMed] [Google Scholar]
- 6. Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young PC. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123(6). Available at: www.pediatrics.org/cgi/content/full/123/6/e1072 [DOI] [PubMed] [Google Scholar]
- 7. Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114(2):372–376 [DOI] [PubMed] [Google Scholar]
- 8. Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G. Mortality of late-preterm (near-term) newborns in Utah. Pediatrics. 2007;119(3). Available at: www.pediatrics.org/cgi/content/full/119/3/e659 [DOI] [PubMed] [Google Scholar]
- 9. Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121(1). Available at: www.pediatrics.org/cgi/content/full/121/1/e170 [DOI] [PubMed] [Google Scholar]
- 10. Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks' gestation: a population-based cohort study. Pediatrics. 2009;123(1):109–113 [DOI] [PubMed] [Google Scholar]
- 11. Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151(5):450–456, 456.e1 [DOI] [PubMed] [Google Scholar]
- 12. Bird TM, Bronstein JM, Hall RW, Lowery CL, Nugent R, Mays GP. Late preterm infants: birth outcomes and health care utilization in the first year. Pediatrics. 2010;126(2). Available at: www.pediatrics.org/cgi/content/full/126/2/e311 [DOI] [PubMed] [Google Scholar]
- 13. McLaurin KK, Hall CB, Jackson EA, Owens OV, Mahadevia PJ. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. 2009;123(2):653–659 [DOI] [PubMed] [Google Scholar]
- 14. Palmer L, Hall CB, Katkin JP, et al. Healthcare costs within a year of respiratory syncytial virus among Medicaid infants. Pediatr Pulmonol. 2010;45(8):772–781 [DOI] [PubMed] [Google Scholar]
- 15. Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–1214 [DOI] [PubMed] [Google Scholar]
- 16. Dombkowski KJ, Leung SW, Gurney JG. Prematurity as a predictor of childhood asthma among low-income children. Ann Epidemiol. 2008;18(4):290–297 [DOI] [PubMed] [Google Scholar]
- 17. Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118(4):823–830 [DOI] [PubMed] [Google Scholar]
- 18. Kase JS, Pici M, Visintainer P. Risks for common medical conditions experienced by former preterm infants during toddler years. J Perinat Med. 2009;37(2):103–108 [DOI] [PubMed] [Google Scholar]
- 19. Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20(6):682–687 [DOI] [PubMed] [Google Scholar]
- 20. Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121(4):878–884.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steffensen FH, Sorensen HT, Gillman MW, et al. Low birth weight and preterm delivery as risk factors for asthma and atopic dermatitis in young adult males. Epidemiology. 2000;11(2):185–188 [DOI] [PubMed] [Google Scholar]
- 22. von Mutius E, Nicolai T, Martinez FD. Prematurity as a risk factor for asthma in preadolescent children. J Pediatr. 1993;123(2):223–229 [DOI] [PubMed] [Google Scholar]
- 23. Pulver LS, Denney JM, Silver RM, Young PC. Morbidity and discharge timing of late preterm newborns. Clin Pediatr (Phila). 2010;49(11):1061–1067 [DOI] [PubMed] [Google Scholar]
- 24. Abe K, Shapiro-Mendoza CK, Hall LR, Satten GA. Late preterm birth and risk of developing asthma. J Pediatr. 2010;157(1):74–78 [DOI] [PubMed] [Google Scholar]
- 25. Escobar GJ, Ragins A, Li SX, Prager L, Masaquel AS, Kipnis P. Recurrent wheezing in the third year of life among children born at 32 weeks' gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med. 2010;164(10):915–922 [DOI] [PubMed] [Google Scholar]
- 26. Raby BA, Celedón JC, Litonjua AA, et al. Low-normal gestational age as a predictor of asthma at 6 years of age. Pediatrics. 2004;114(3). Available at: www.pediatrics.org/cgi/content/full/114/3/e327 [DOI] [PubMed] [Google Scholar]
- 27. Alessandrini EA, Alpern ER, Chamberlain JM, Shea JA, Gorelick MH. A new diagnosis grouping system for child emergency department visits. Acad Emerg Med. 2010;17(2):204–213 [DOI] [PubMed] [Google Scholar]
- 28. Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598 [DOI] [PubMed] [Google Scholar]
- 29. Getahun D, Strickland D, Zeiger RS, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164(2):187–192 [DOI] [PubMed] [Google Scholar]
- 30. Bell LM, Grundmeier R, Localio R, et al. Electronic health record–based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e770 [DOI] [PubMed] [Google Scholar]
- 31. Fiks AG, Hunter KF, Localio AR, et al. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics. 2009;124(1):159–169 [DOI] [PubMed] [Google Scholar]
- 32. Zorc JJ, Pawlowski NA, Allen JL, et al. Development and validation of an instrument to measure asthma symptom control in children. J Asthma. 2006;43(10):753–758 [DOI] [PubMed] [Google Scholar]
- 33. Debley JS, Smith JM, Redding GJ, Critchlow CW. Childhood asthma hospitalization risk after cesarean delivery in former term and premature infants. Ann Allergy Asthma Immunol. 2005;94(2):228–233 [DOI] [PubMed] [Google Scholar]
- 34. Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139(2):261–266 [DOI] [PubMed] [Google Scholar]
- 35. Wijga A, Tabak C, Postma DS, et al. Sex differences in asthma during the first 8 years of life: the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study. J Allergy Clin Immunol. 2011;127(1):275–277 [DOI] [PubMed] [Google Scholar]
- 36. Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States: association with social class, perinatal events, and race. Am Rev Respir Dis. 1990;142(3):555–562 [DOI] [PubMed] [Google Scholar]
- 37. Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford Record Linkage Study. BMC Pulm Med. 2010;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan-Yeung M, Dimich-Ward H, Becker A. Atopy in early life and effect of a primary prevention program for asthma in a high-risk cohort. J Allergy Clin Immunol. 2007;120(5):1221–1223 [DOI] [PubMed] [Google Scholar]
- 39. Sly PD, Boner AL, Bjorksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117(5). Available at: www.pediatrics.org/cgi/content/full/117/5/e868 [DOI] [PubMed] [Google Scholar]
- 41. Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90(3):428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168 [DOI] [PubMed] [Google Scholar]
- 43. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482 [DOI] [PubMed] [Google Scholar]
- 45. US Census Bureau Statistical brief: poverty areas, 1995. Available at: www.census.gov/population/socdemo/statbriefs/povarea.html Accessed August 1, 2010
- 46. Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659 [DOI] [PubMed] [Google Scholar]
- 47. Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30(1):8–15 [DOI] [PubMed] [Google Scholar]
- 48. MacDorman MF, Mathews TJ, Martin JA, Malloy MH. Trends and characteristics of induced labour in the United States, 1989–98. Paediatr Perinat Epidemiol. 2002;16(3):263–273 [DOI] [PubMed] [Google Scholar]
- 49. Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56(6):1–103 [PubMed] [Google Scholar]
- 50. Reddy UM, Ko CW, Raju TN, Willinger M. Delivery indications at late-preterm gestations and infant mortality rates in the United States. Pediatrics. 2009;124(1):234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bloom B, Dey AN, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2005. Vital Health Stat 10. 2006;(231):1–84 [PubMed] [Google Scholar]