Abstract
RIG-I is a cytosolic pathogen recognition receptor that engages viral RNA in infected cells to trigger innate immune defenses through its adaptor protein MAVS. MAVS resides on mitochondria and peroxisomes, but how its signaling is coordinated among these organelles has not been defined. Here we show that a major site of MAVS signaling is the mitochondrial-associated membrane (MAM), a distinct membrane compartment that links the endoplasmic reticulum to mitochondria. During RNA virus infection, RIG-I is recruited to the MAM to bind MAVS. Dynamic MAM tethering to mitochondria and peroxisomes then coordinates MAVS localization to form a signaling synapse between membranes. Importantly, the hepatitis C virus NS3/4A protease, which cleaves MAVS to support persistent infection, targets this synapse for MAVS proteolysis from the MAM, but not from mitochondria, to ablate RIG-I signaling of immune defenses. Thus, the MAM mediates an intracellular immune synapse that directs antiviral innate immunity.
Keywords: interferon, IPS-1
MAVS, formerly also called VISA/CARDIF/IPS-1, is essential for innate immunity to RNA viruses (1, 2). In accordance with the HUGO Gene Nomenclature Committee–approved nomenclature, we now refer to this molecule as MAVS. During acute infection by hepatitis C virus (HCV) and other RNA viruses, viral RNA is recognized in the host cell as nonself by the cytosolic pathogen recognition receptor RIG-I, resulting in a signaling cascade that induces innate antiviral immunity by triggering the production of type I IFN and proinflammatory cytokines from infected tissue (3, 4). The signals that drive this response are relayed through MAVS, which is localized to both peroxisomes and mitochondria, for assembly of a macromolecular signaling complex (5, 6). The importance of MAVS in innate immunity is underscored by the fact that HCV, which is sensed by RIG-I in hepatocytes (7), uses its multifunctional NS3/4A protease to cleave MAVS from membranes to evade RIG-I signaling (8–11). This innate immune evasion strategy contributes to HCV's ability to establish chronic infection in nearly 200 million people worldwide and provides a foundation for liver disease. The NS3/4A protease has been reported by us and others to cleave MAVS on the outer mitochondrial membrane (OMM); however, its actions in HCV replication occur on the endoplasmic reticulum (ER) (10, 12). Interestingly, the ER contains a specialized subdomain, the mitochondrial-associated membrane (MAM), that physically connects the ER to mitochondria and provides a mitochondrial–ER axis that compartmentalizes both stress and metabolic signaling (13, 14). The MAM plays a central role in calcium signaling and phospholipid transport to mitochondria (15). In addition, the MAM has recently been implicated as a platform for inflammasome signaling, suggesting that it might play a role in infection and immunity (16). We hypothesized that the MAM may coordinate MAVS signaling of innate immunity from peroxisomes and mitochondria, and thus that MAM-localized MAVS could represent the actual substrate target of the HCV NS3/4A protease to facilitate viral immune evasion.
Results
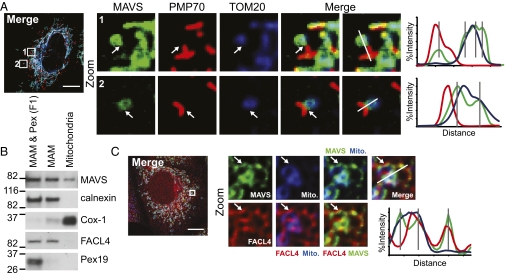
We assessed MAVS cellular distribution by confocal microscopy of human hepatoma (Huh7) cells expressing MAVS-mEGFP. In addition to the previously described mitochondrial and peroxisomal localization of MAVS (5, 6), our higher-resolution analyses also revealed MAVS staining directly adjacent to and around mitochondria (Fig. 1A). The MAM is known to link the ER to mitochondria (14), and we assessed the possibility that it also could make contacts with peroxisomes, which are derived from ER membranes (17). Indeed, confocal microscopy of Huh7 cells expressing mEGFP-PSS-1, a MAM-enriched marker protein (18, 19), revealed an interconnected MAM network, distinct from the ER, between mitochondria and peroxisomes (Fig. S1 A and B). Thus, we biochemically assessed the cellular distribution of MAVS and various intracellular organelle marker proteins by fractionating Huh7 cells to isolate the MAM from mitochondria and microsomes, the latter of which contains light membranes of the ER and plasma membrane (Fig. S2A) (18). MAVS was present in Huh7 cells within both MAM and mitochondria, as well as in “F1”, a membrane fraction of lighter density than MAM alone that contains both MAM and peroxisomes (Fig. 1B), but not in microsomal fractions (Fig. S2B). The MAM-localized MAVS cofractionated with a previously described MAM-enriched marker, FACL4 (Fig. 1B and Fig. S2 B–D) (15, 18). The MAM fractions were largely devoid of both peroxisomal (Pex19) and mitochondrial markers (Cox-1 and VDAC1) (Fig. 1B and Fig. S2B), whereas standard mitochondrial fractions contained all of these markers (Fig. S2B). In addition, MFN2, a MAM- and mitochondrial-localized protein that tethers the ER to mitochondria and interacts with MAVS (20, 21), cofractionated with MAVS and was present in both MAM and mitochondria (Fig. S2C).
Fig. 1.
MAVS is localized to MAM, mitochondria, and peroxisomes. (A) Confocal microscopy analysis of immunostained Huh7 cells expressing MAVS-mEGFP for PMP70 (peroxisomes) and TOM20 (OMM). (B) Immunoblot analysis of fractionated Huh7 cells. F1, containing MAM and peroxisomes, is labeled as MAM & Pex. Fractionation markers: calnexin, ER; Cox-1, mitochondria; FACL4, MAM; Pex19, peroxisomes. (C) Confocal microscopy analysis of endogenous MAVS and FACL4 Huh7 cells preloaded with Mitotracker. In A and C, arrows indicate MAVS on MAM or between peroxisomes and mitochondria. Single-cell images are representative of at least 10 cells analyzed. Histograms display measured fluorescence intensity along the line in the merge panels, with gray lines indicating local peaks of MAVS intensity. (Scale bar: 10 μm.)
To verify biochemical identification of the MAM localization of MAVS, we performed confocal microscopy of endogenous MAVS in Huh7 cells. This revealed MAVS staining directly adjacent to mitochondria and partially overlapping with three different MAM-enriched proteins: FACL4, sigma-1 receptor (Sig-1R), and mEGFP-PSS-1 (Fig. 1C and Figs. S2D and S3) (15, 18). This MAVS distribution pattern at the MAM–mitochondrial axis was apparent in cells that were either mock-infected or infected with Sendai virus (SenV), which activates RIG-I signaling and accurately models HCV activation of the RIG-I pathway (Fig. S3) (7, 10). Thus, MAVS is localized to the MAM, peroxisomes, and mitochondria, likely residing at the junctions between these organelles.
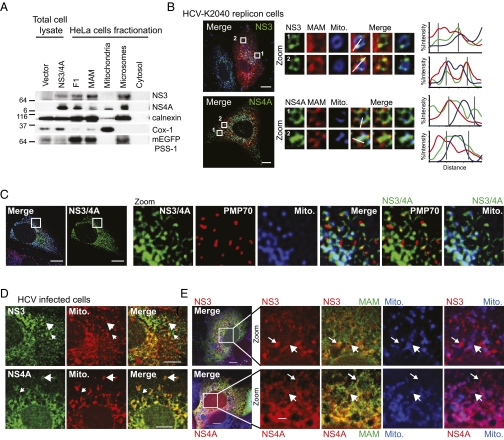
The HCV NS3/4A protease complex is localized within infected cells to intracellular membranes and regions near mitochondria by membrane-anchor domains within NS3 and the NS4A cofactor (22), suggesting that MAM localization of NS3/4A could govern its dual distribution between the ER and mitochondria. Therefore, we assessed the distribution of NS3/4A transiently expressed in HeLa cells that coexpress mEGFP-PSS-1. We found that NS3 and NS4A fractionated with MAM, F1 (representing peroxisomes with MAM), and microsomes (representing the ER and HCV replication complex; described in ref. 12), with a small amount of NS4A also detected in the mitochondria (Fig. 2A). Confocal microscopy confirmed NS3/4A localization adjacent to peroxisomes and mitochondria, both of which are sites of MAVS localization (Fig. 2C) (5, 6). In addition, confocal microscopy revealed that in cells propagating a self-replicating HCV RNA genome (a model of persistent HCV infection; Huh7-HCV K2040 cells) (3), both NS3 and NS4A were present at MAM–mitochondrial junctions (Fig. 2B). In HCV-infected Huh7.5 cells (3), both NS3 and NS4A were associated with the MAM either (i) at junctions with mitochondria (Fig. 2 D and E, large arrows) or (ii) independent of mitochondria (Fig. 2 D and E, small arrows), similar to what was observed in Huh7-HCV K2040 cells (compare Fig. 2 B and E). Thus, biochemical fractionation and direct imaging of cells establish that NS3/NS4A localizes to the junctions among MAM, mitochondria, and peroxisomes during HCV infection and persistent viral RNA replication.
Fig. 2.
NS3/4A localizes to junctions between MAM and mitochondria. (A) Immunoblot analysis of subcellular fractions from HeLa cells stably expressing mEGFP-PSS-1 (MAM) and transiently expressing NS3/4A. (B–E) Confocal micrographs of cells transfected with mEGFP-PSS-1 or left untransfected, preloaded with Mitotracker, and immunostained. (B) Huh7-HCV K2040 cells. White arrows indicate NS3 and NS4A adjacent to mitochondria. Histograms display the measured fluorescence intensity along the line in the merge panels, with gray lines marking local peaks of HCV protein intensity. (C) Huh7 cells transiently expressing HCV NS3/4A. (D and E) Huh7.5 cells infected with HCV for 48 h. Large arrows indicate NS3 and NS4A near mitochondria, and small arrows indicate membranous areas devoid of mitochondria. Single-cell images are representative of at least 10 cells analyzed. (Scale bars: 10 μm in B–D; 2 μm in E.)
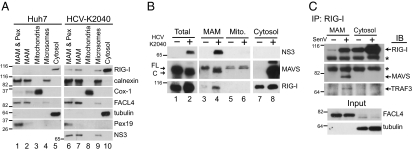
In cells replicating HCV RNA, NS3 was found in both MAM and microsomal fractions (Fig. 3 A and B and Fig. S2C). MAVS was cleaved during HCV replication by NS3/4A and accumulated within the cytosolic fraction (Fig. 3B, lane 8 and Fig. S2C), as described previously (10). Importantly, we detected cleaved MAVS in the MAM, but not in the mitochondrial fraction, during HCV RNA replication (Fig. 3B, compare lanes 4, 6, and 8; Fig. S2C). The residual cleaved MAVS present in the MAM likely reflects the homotypic interaction of MAVS with uncleaved protein and/or binding to other MAM-associated signaling cofactors, with the cleaved MAVS in the cytosol likely representing both MAM and peroxisomal-MAVS cleavage by NS3/4A. Surprisingly, neither the mass nor the relative amount of mitochondrial MAVS was affected by the presence of HCV proteins (Fig. 3B, lanes 5 and 6, with markers of these samples shown in Fig. 3A; see also Fig. S2C). We note that the lack of MAVS cleavage from mitochondria by NS3/4A differs from findings in previous studies by us and others in which analysis of crude mitochondrial fractions led to the conclusion that MAVS was cleaved from the OMM (10, 23). We have now found that such mitochondrial fractions indeed contain MAM, explaining these differences (Fig. S2B). Moreover, confocal microscopy analysis of cells expressing NS3/4A identified residual mitochondrial MAVS staining, thus defining a pool of uncleaved mitochondrial-associated MAVS (Fig. S4). Therefore, MAVS is cleaved by NS3/4A on the MAM, but not on mitochondria, during HCV infection.
Fig. 3.
The MAM is an innate immune signaling platform. (A and B) Immunoblot analysis of fractionated Huh7 (−) and Huh7-HCV K2040 (+) cells. Arrows indicate full-length (FL) and cleaved (C) MAVS. (C) Immunoblot analysis of fractions immunoprecipitated with anti–RIG-I from mock- or SenV-infected (for 8 h) PH5CH8 cells and input. *Nonspecific band. Fractionation markers: calnexin, ER; Cox-1, mitochondria; FACL4, MAM; tubulin, cytosol; Pex19, peroxisomes.
Because MAM, but not mitochondrial MAVS, is the proteolytic target of NS3/4A, we evaluated the possibility that the MAM-localized MAVS is capable of transducing RIG-I signaling. We assessed MAM fractions from Huh7 and Huh7-HCV K2040 cells for the presence of RIG-I and found that a portion of RIG-I was redistributed from cytosol into the MAM, but not into the mitochondria, in cells chronically replicating HCV RNA (Fig. 3 A and B and Fig. S2C). This redistribution was not due to cytosol contamination of these fractions or to changes in MAM levels during HCV RNA replication, because the cytosol marker tubulin was absent from the MAM and microsomes, and MAM marker levels were unchanged by HCV RNA replication (Fig. 3 A and C and Fig. S2C). RIG-I levels are known to increase after RIG-I pathway activation (9) and were accordingly increased during the course of SenV infection (Fig. 3C). RIG-I levels also were moderately increased within Huh7-K2040 cells, reflecting the cellular compensation due to MAVS cleavage and RIG-I signaling suppression (9, 10). We also conducted confocal microscopy analysis and confirmed that a portion of RIG-I was redistributed into the MAM near sites of MAVS localization during SenV infection (Fig. S5). Furthermore, immunoprecipitation analyses of the MAM fraction from PH5CH8 hepatocytes revealed that the portion of RIG-I that redistributed to the MAM during acute RNA virus infection interacted with both MAVS and TRAF3 (Fig. 3C). This indicates that RIG-I and its signaling cofactor TRAF3 are distributed to the MAM during RNA virus infection for interaction with MAVS.
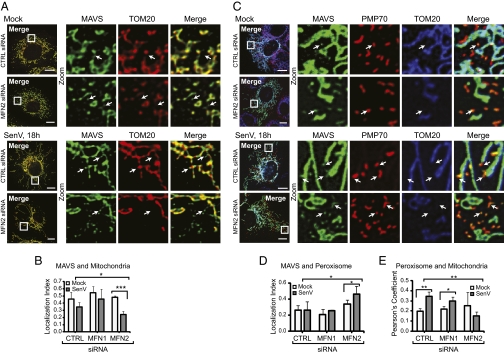
Contacts between the MAM and mitochondria are maintained by the mitochondrial fusion protein MFN2 (20). To assess the role of MAM contacts in coordinating MAVS localization and signaling during virus infection, we used siRNA technology to knock down MFN2 expression in Huh7 cells. Although MFN2 is the MAM tethering protein, it shares homology with the related MFN1 protein, and both are involved in mitochondrial fusion and bind to MAVS (21, 24). Therefore, we also used siRNA to knock down MFN1 to control for the shared functions of these proteins and to specifically isolate the MAM–mitochondrial tethering role of MFN2. Manders’ colocalization coefficient analysis (25) of confocal micrographs confirmed that loss of MFN2 expression, but not of MFN1 expression, disrupted the ER–mitochondria interaction (Fig. S6) (20). Colocalization analysis of MAVS and TOM20 in confocal micrographs through an object-based overlap approach (26) revealed that knockdown of MFN2, but not of MFN1, resulted in a slight but significant increase in nonmitochondrial MAVS during SenV infection (Fig. 4 A and B and Fig. S7B). Knockdown data are provided in Fig. 5A. Colocalization analysis with the peroxisomal marker PMP70 established that this increase in nonmitochondrial MAVS corresponded with its enhanced association with peroxisomes (Fig. 4 C and D). In addition, it appeared that detection of peroxisomes by immunofluorescence was easier because of an increased number or a reduced association with mitochondria when MFN2 was knocked down (Fig. 4E), perhaps explaining the increased MAVS association with peroxisomes on MFN2 knockdown.
Fig. 4.
Mitochondrial–ER contacts regulate MAVS localization and signaling. (A) Immunostain of endogenous MAVS and TOM20 (OMM). Arrows indicate MAVS localized independent of TOM20. (C) Immunostain of cells expressing MAVS-mEGFP for PMP70 (peroxisomes) and TOM20. (B, D, and E) Values (mean ± SD; n = 3–4 cells) of MAVS localization to mitochondria (B) or peroxisomes (D) (object-based overlap approach) (26), or overlap between peroxisomes and mitochondria (E) (Pearson's coefficient) (26). *P ≤ 0.05; **P ≤0.005; ***P ≤0.0005, by unpaired Student t test. Single-cell images are representative of two experiments and >10 cells analyzed per experiment. (Scale bar: 10 μm.)
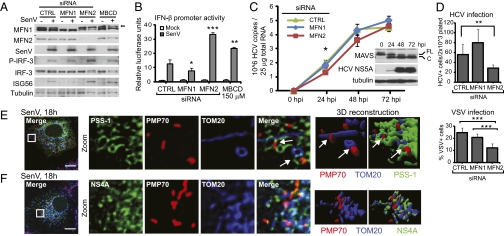
Fig. 5.
Mitochondrial–ER contacts regulate RIG-I pathway antiviral signaling. (A and B) Immunoblot analysis and IFN-β promoter luciferase expression of Huh7 cells treated with nontargeting control (CTRL), MFN1, or MFN2 siRNA pools or methyl-β cyclodextrin and then mock- or SenV-infected. Values are mean ± SD (n = 3) of one of three representative experiments. *P ≤ 0.05; **P ≤0.005; ***P ≤0.0005, by unpaired Student t test. (C) HCV copy number (mean ± SD; n = 3). (Inset) Immunoblot analysis of MAVS; full-length (FL) and cleaved (C). (D) (Upper) HCV-positive cells after 72 h of infection (mean ± SD; n = 5–6). (Lower) Percent VSV-positive cells after 16 h of VSV infection (mean ± SD; n = 3–5, >1000+ cells counted). (E and F) Confocal micrographs of immunostained SenV-infected Huh7 cells expressing mEGFP-PSS-1 (MAM) (E) or HCV NS4A-mEGFP (F) with PMP70 (peroxisomes) and TOM20 (OMM) and 3D reconstruction of Z stacks. Arrows mark synapses. Single-cell images are representative of two experiments and >10 cells analyzed per experiment. (Scale bar: 10 μm.)
In addition to increasing MAVS localization with peroxisomes, treatment of cells with siRNA specific to MFN2 or with methyl-β-cyclodextrin, which disrupts MAM–mitochondria contacts through distinct mechanisms (27), enhanced RIG-I pathway signaling as defined by an increase in phosphorylated active IRF-3, enhanced IFN-β promoter activity, and ISG56 expression after SenV infection (Fig. 5 A and B). We note that knockdown of MFN2 enhanced RIG-I signaling despite an attenuation of the previously described virus-induced mitochondrial elongation (Fig. S7A), demonstrating that its role in the antiviral response is actually independent of a role in mitochondrial elongation (28). MFN1 knockdown attenuated SenV-induced RIG-I signaling, as described previously (24, 28), while maintaining ER–mitochondria contacts (Fig. S6). When infected with HCV, MFN2 knockdown of cells resulted in a moderate but significant reduction in HCV RNA early after infection up to the point of MAVS cleavage by NS3/4A, consistent with acute enhancement of innate immune defenses induced by RIG-I–HCV RNA interaction due to deregulated RIG-I pathway signaling (Fig. 5C) (7, 29). Indeed, MFN2 knockdown reduced the permissiveness of cells for infection by HCV and vesicular stomatitis virus (VSV), consistent with enhanced innate immune defenses (Fig. 5D). Therefore, MAVS localization and antiviral signaling are supported by the MAM–mitochondrial tethering function of MFN2, which may regulate MAVS distribution among mitochondria, MAM, and peroxisomes.
We also found that during SenV infection, the interaction between peroxisomes and mitochondria increased in an MFN2-dependent manner (Fig. 4 C and E). This observation suggests that MAM–mitochondrial tethering mediates an increased interaction between these organelles during RIG-I pathway activation. Indeed, confocal microscopy of SenV-infected Huh7 cells revealed that the MAM forms a synapse between peroxisomes and mitochondria (Fig. 5E). An apparent synapse between peroxisomes and mitochondria anchored by MAVS also can be detected on MAVS overexpression (Fig. 1A). Moreover, we found that this synapse is targeted by the NS4A subunit of the HCV protease complex (Fig. 5F). These observations raise the possibility that NS3/4A targeting of this MAM-anchored synapse may regulate innate immune signaling by MAVS from MAM, peroxisomes, and mitochondria.
Discussion
Our results identify an innate immune signaling synapse in which the MAM serves as the central scaffold that coordinates MAVS-dependent signaling of the RIG-I pathway between mitochondria and peroxisomes. This model implicates the MAM in immune signaling to a variety of viruses in addition to HCV that are engaged by RIG-I–like receptors to trigger the immune response (2). The MAM-anchored synapse contains both MAVS and RIG-I pathway factors that physically interact in RNA virus-infected cells to drive the immune actions of an MAVS signalosome. STING, a putative RIG-I signaling cofactor and IPS-1 binding protein, also may have MAM localization, but this localization is likely specific to cell type and context (30). Because the viral NS3/4A protease targets the MAM-anchored synapse and cleaves MAVS from the MAM, but not from mitochondria, to ablate innate immune signaling during HCV infection, the MAM-resident MAVS likely mediates the RIG-I pathway function against HCV. This notion is further supported by previous studies showing that cells supporting chronic HCV RNA replication (which, as we show here, contain intact mitochondrial-localized MAVS but not intact MAM-localized MAVS) are unable to signal IRF-3 activation and the expression of IRF-3 target genes despite the presence of MAVS on the mitochondria (9). Although MAM-localized MAVS directs innate immunity against HCV, our observations do not exclude a role for mitochondrial MAVS in innate immune signaling. In this respect, mitochondrial MAVS supports apoptotic signaling of innate immune actions against virus infection (31, 32), and also could serve as a local source of MAVS for distribution among peroxisomes and MAM through dynamic membrane trafficking regulated by MFN2. This latter idea is supported by previous work showing that peroxisomes are not required for populating mitochondria with MAVS (6), suggesting that peroxisomes may receive MAVS by membrane trafficking through MAM–mitochondrial interactions.
We found that when MAM–mitochondrial contacts were disrupted, there was increased localization of endogenous native MAVS to peroxisomes, with a concomitant increase in signaling to IFN-β compared with cells maintaining MAM–mitochondria contacts and harboring less peroxisomal MAVS. In contrast, previous work has shown that ectopic MAVS targeted solely to peroxisomes through replacement of the native MAVS transmembrane domain with a peroxisome-targeting domain does not signal to IFN-β (6). In that case, the presence of the nonnative MAVS transmembrane domain likely precluded essential membrane interactions with signaling factors that drive IFN-β production, thus explaining the divergent findings. In the present study, peroxisomal MAVS enhanced signaling to IFN-β, possibly because when localized to peroxisomes, MAVS no longer interacts with mitochondrial negative regulatory proteins of innate immune signaling, including NLRX1 (33). In support of this, NLRX1 has been shown to be unable to suppress signaling by peroxisomal MAVS (6). Placement of NLRX1 or of other negative regulators of RIG-I signaling on mitochondria also might explain why MAVS must be redistributed to MAM to initiate innate immune signaling. In this case, the mitochondria-to-MAM-to-peroxisome redistribution of MAVS would release it from signaling inhibition by a negative regulator, and the MAM would provide the platform for recruiting positive signaling effector proteins to interact with MAVS for innate immune signaling.
Our study and others indicate that dynamic immune signaling is coordinated by the MAM through membrane–protein interactions governed by MFN2, with membrane tethering sites acting as signaling microdomains that contain localized sources of MAVS and other signaling factors (15). This organization is analogous to the immunologic synapse that forms between a T cell and an antigen-presenting cell in which adhesion molecules tether opposing plasma membranes to establish T-cell receptor signaling microdomains supplied with key signaling factors through membrane interactions (34). Taken together with the role of the MAM in NLRP3 inflammasome signaling (16), our findings indicate that the MAM plays a central role in initiating both the innate immune and inflammatory responses to infection. Therapeutic strategies directed toward regulating MAM processes could serve to preserve and enhance antiviral immunity.
Materials and Methods
Additional information is provided in SI Materials and Methods.
Cell Culture and Viruses.
Huh7, Huh7.5, and Huh7-HCV K2040 replicon cells that propagate culture-adapted variants of the Con1 HCV (genotype 1B) subgenomic replicon RNA (3), along with immortalized human hepatocyte PH5CH8 cells, were cultured according to standard techniques. SenV strain Cantell was obtained from Charles River Laboratory. Cell culture adapted HCV JFH1 genotype 2A strain was propagated and infectivity titrated, as described previously (35). VSV-GFP infections were done at MOI 1 for 16h. Methyl-β-cyclodextrin treatment used a concentration of 150 μm in DMSO.
Subcellular Fractionation.
MAM, mitochondria, and microsomes were isolated from cells using Percoll gradient fractionation (18). Equivalent amounts of protein from each fraction were analyzed by SDS/PAGE and immunoblot.
Supplementary Material
Acknowledgments
We thank our colleagues for reagents, including A. Colberg-Poley for pmEGFP-huPSS-1; the W. M. Keck Center for Advanced Studies in Neural Signaling for microscopy assistance; K. Chang for technical assistance; and D. Stetson, M. Diamond, and members of the M.G. laboratory for discussion and a critical reading of the manuscript. This work was supported by the National Institutes of Health and the Burroughs Wellcome Fund. S.M.H. is a fellow of the Irvington Institute Fellowship Program of the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110133108/-/DCSupplemental.
References
- 1.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loo YM, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumpter R, Jr, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 5.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Dixit E, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foy E, et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 9.Foy E, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid–inducible gene-I signaling. Proc Natl Acad Sci USA. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loo YM, et al. Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc Natl Acad Sci USA. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 12.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 13.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 14.Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: More than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 17.Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Bozidis P, Williamson CD, Colberg-Poley AM. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts. Curr Protoc Cell Biol. 2007;chap 3:unit 3.27. doi: 10.1002/0471143030.cb0327s37. [DOI] [PubMed] [Google Scholar]
- 19.Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 20.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 21.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 22.Brass V, et al. Structural determinants for membrane association and dynamic organization of the hepatitis C virus NS3-4A complex. Proc Natl Acad Sci USA. 2008;105:14545–14550. doi: 10.1073/pnas.0807298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin R, et al. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKε molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol. 2006;80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onoguchi K, et al. Virus-infection or 5′ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6:e1001012. doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manders EM, et al. Four-dimensional imaging of chromatin dynamics during the assembly of the interphase nucleus. Chromosome Res. 2003;11:537–547. doi: 10.1023/a:1024995215340. [DOI] [PubMed] [Google Scholar]
- 26.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 27.Sano R, et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I–like receptor antiviral pathway. EMBO Rep. 2010;11:133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzri D, Gehrke L. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J Virol. 2009;83:4174–4184. doi: 10.1128/JVI.02449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holm GH, et al. Retinoic acid–inducible gene-I and interferon-β promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J Biol Chem. 2007;282:21953–21961. doi: 10.1074/jbc.M702112200. [DOI] [PubMed] [Google Scholar]
- 32.Yu CY, Chiang RL, Chang TH, Liao CL, Lin YL. The interferon stimulator MAVS facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases. J Virol. 2010;84:2421–2431. doi: 10.1128/JVI.02174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 34.Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 35.Kato T, et al. Cell culture and infection system for hepatitis C virus. Nat Protoc. 2006;1:2334–2339. doi: 10.1038/nprot.2006.395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.