Abstract
Segmental identity along the anteroposterior axis of bilateral animals is specified by Hox genes. These genes encode transcription factors, harboring the conserved homeodomain and, generally, a YPWM motif, which binds Hox cofactors and increases Hox transcriptional specificity in vivo. Here we derive synthetic Drosophila Antennapedia genes, consisting only of the YPWM motif and homeodomain, and investigate their functional role throughout development. Synthetic peptides and full-length Antennapedia proteins cause head-to-thorax transformations in the embryo, as well as antenna-to-tarsus and eye-to-wing transformations in the adult, thus converting the entire head to a mesothorax. This conversion is achieved by repression of genes required for head and antennal development and ectopic activation of genes promoting thoracic and tarsal fates, respectively. Synthetic Antennapedia peptides bind DNA specifically and interact with Extradenticle and Bric-à-brac interacting protein 2 cofactors in vitro and ex vivo. Substitution of the YPWM motif by alanines abolishes Antennapedia homeotic function, whereas substitution of YPWM by the WRPW repressor motif, which binds the transcriptional corepressor Groucho, allows all proteins to act as repressors only. Finally, naturally occurring variations in the size of the linker between the homeodomain and YPWM motif enhance Antennapedia repressive or activating efficiency, emphasizing the importance of linker size, rather than sequence, for specificity. Our results clearly show that synthetic Antennapedia genes are functional in vivo and therefore provide powerful tools for synthetic biology. Moreover, the YPWM motif is necessary—whereas the entire N terminus of the protein is dispensable—for Antennapedia homeotic function, indicating its dual role in transcriptional activation and repression by recruiting either coactivators or corepressors.
Keywords: Hox specificity, synthetic genes, homeotic transformations, antenna-to-tarsus transformation
Homeotic (Hox) genes encode transcription factors that specify body segments along the anterior–posterior axis of bilaterians (1–3). Hox transcription factors harbor the homeodomain (HD), a helix-turn-helix DNA-binding domain that has been highly conserved throughout animal evolution and shares remarkable sequence similarity among different Hox paralogs (4–7). For Hox proteins to confer segmental identity in the developing embryo, they need to regulate differentially—notwithstanding their sequence similarity—a large number of target genes, most of the time in a paralog-specific manner. Despite our current knowledge of their DNA-binding properties (8), the identification of Hox cofactors and “collaborators” that increase their target-selection precision (9), the possibility of performing genomewide analyses (10, 11), and our understanding of transcriptional regulation in vivo, we fall short of providing a complete explanation about the specificity of Hox transcription factors that would allow us to predict or identify novel Hox functions.
We have demonstrated previously that DNA-binding and the functional specificity of Antennapedia (Antp) reside in the HD and its flexible N-terminal arm, respectively (8, 12–14), and that the YPWM motif directly links Antp to the transcriptional machinery (15). In the present study, we find that the YPWM motif, but not its flanking regions, is required for Antp homeotic function in vivo. We derive synthetic Antp genes, which encode a small portion of the full-length protein (spanning the region between the YPWM motif and the beginning of the C terminus), and examined their function in vivo, compared with their full-length counterparts. We find that synthetic peptides and full-length Antp proteins featuring a YPWM motif are able to generate head-to-thorax transformations in the embryo, to repress endogenous Sex combs reduced (Scr), and to activate Teashirt (Tsh) ectopically. They also cause antenna-to-mesothoracic tarsus transformations through repression of Spalt major (Salm) and distal antenna (dan) and ectopic activation of grain (grn) in the antennal disc. In a twin of eyeless (toy) mutant background, which fails to launch the antennal morphogenetic program (5), they additionally transform eyes to wings. Moreover, they activate transcription and interact with the Antp cofactors Extradenticle (Exd) and Bric-à-brac interacting protein 2 (Bip2) in vitro and in Drosophila tissue culture. When the YPWM motif is replaced by WRPW, a domain interacting with the generic transcriptional corepressor Groucho (Gro) (16), Antp repressive activity is retained in vivo. Finally, we evaluate the effect of either a long or a short linker between the YPWM motif and the HD, comprising eight or four amino acids, respectively, which occur naturally in different Antp isoforms (17), and find that they play an important role in transcriptional potency and/or specificity in vivo.
Results
The Antp HD and YPWM Motifs Are Required for Homeotic Function in the Embryo.
We initially evaluated the requirement of the HD, the conserved YPWM motif, and its flanking regions for Antp function in vivo. We constitutively expressed constructs that either lacked the entire HD and YPWM motif or contained alanine substitutions in either the YPWM motif or in the four amino acids situated N- or C-terminally to it (SI Appendix, Fig. S1), using the hsp70 promoter, as previously described (18). Abnormalities in the development of the anterior (head) structures of the embryo, caused by transformation toward second thoracic segment identity (T2), are caused upon ectopic expression of Antp in the segments anterior to its normal expression domain (12, 19, 20). The transformation includes head-involution defects, repression of prothoracic (T1) “beards,” and formation of ectopic denticle bands in the head segments (12, 20). Substitution of the YPWM motif or deletion of both the HD and YPWM motif failed to generate head-to-thorax transformations, whereas mutations in either of the sequences flanking the YPWM motif generated a phenotype similar to the one caused by ectopic expression of the WT Antp protein (SI Appendix, Fig. S1). This result indicates that the HD and an intact YPWM motif are required for Antp homeotic function in vivo, whereas the amino acids flanking the YPWM motif are dispensable.
Synthetic Antp Peptides, Consisting of Only the HD and the YPWM Motif, Cause Homeotic Transformations in the Embryo.
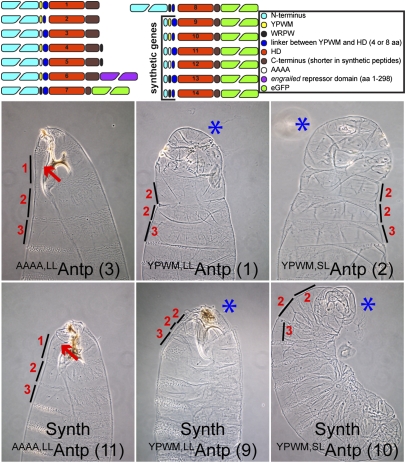
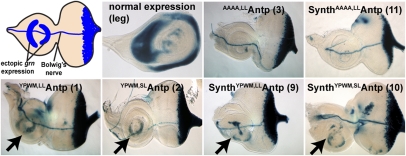
The functional requirement of the YPWM motif prompted us to construct various versions of full-length and synthetic Antp genes to study the functional specificity of each module and to derive the minimal requirements for Antp homeotic function in vivo. We used constructs with long and short linkers between the HD and the YPWM motif, replaced the YPWM motif by quadruple alanines (AAAA), fused additional repressor domains to the C terminus of the protein, replaced the YPWM motif with WRPW, and also constructed synthetic Antp genes, comprising only the HD and YPWM motif but lacking the entire N terminus, to test whether they retain their homeotic function in vivo. For this purpose we used Upstream Activating Sequence (UAS) constructs and tagged the synthetic genes with eGFP (Fig. 1) to allow the visualization and immunohistochemical detection of each peptide. Using the nullo-Gal4 driver (5) for strong expression of the Antp genes at the preblastoderm and blastoderm stages of the embryo, we observed that all synthetic peptides and full-length Antp proteins featuring a YPWM motif cause head-involution defects of variable strength and repression of the prothoracic beards (Fig. 1 and SI Appendix, Fig. S2). In contrast, the full-length and synthetic AAAAAntp exhibited WT-like phenotypes with no repression of T1 beards (Fig. 1, lines 3 and 11 and SI Appendix, Fig. S2, line 12 and close-up). All synthetic and full-length constructs featuring a WRPW repressor motif (Fig. 1 and SI Appendix, Fig. S2) also caused head-to-thorax transformation and repression of the prothoracic beards. The most dramatic phenotype was displayed by the full-length Antp with C-terminal fusions of WRPW (SI Appendix, Fig. S2, line 4 and 5), whereas the addition of the engrailed (en) repressor domain to the 3′ end of the full-length Antp (SI Appendix, Fig. S2, line 6) did not result in any phenotype considerably different from the one caused by the WT Antp gene. In addition, we analyzed variations in the size of the linker region between the YPWM motif and the HD, consisting of four or eight amino acids that normally occur in different Antp isoforms (Fig. 1, lines 1, 2, 9 and 10). Both full-length and synthetic YPWMAntp short-linker peptides gave rise to a more pronounced head-involution defect with no detectable mouth parts in the cuticle. Therefore, the YPWM motif, but not the N terminus of Antp, is required for homeotic function in the embryo, with transformations being favored by short-linker isoforms. Replacing the tetrapeptide with WRPW retains part of its specificity in vivo.
Fig. 1.
Full-length and synthetic Antp genes cause homeotic transformations and head-involution defects in the embryo. (Upper) UAS Antp constructs (full-length and synthetic) generated for the functional analysis of the YPWM motif and the N terminus. The short linker (SL) contains the sequence RSQF and the long linker (LL) contains the sequence RSQFGKCQ. The C terminus of synthetic peptides lacks the terminal 14 residues and contains only the sequence TKGEPGS. All construct numbers are used for reference in the subsequent figures. (Lower) Comparative analysis of head-involution defects generated by constitutive expression of full-length and synthetic Antp genes, using nullo-Gal4. Only proteins bearing a YPWM motif (Center and Right column) could induce transformation of head to mesothorax (T2). The size of thoracic segments is indicated by lines. The numbers 1–3 indicate segment identity: (1) prothoracic, (2) mesothoracic, or (3) metathoracic. Red arrows show the prothoracic beards, and blue asterisks indicate pronounced head-involution defects, where present. Note the mild head defects in the synthetic AAAAAntp line, shown by the abnormal development of the mouth hook.
The YPWM Motif of Antp Is Essential for Repression of Endogenous Scr and Ectopic Activation of Tsh in the Embryo.
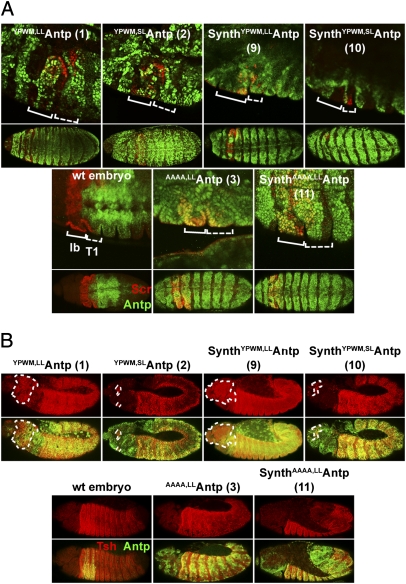
Target genes repressed or activated by Antp in the embryo were then used as a system to determine the ability of synthetic peptides and full-length Antp proteins to act, directly or indirectly, as transcriptional repressors and activators. Such genes include Scr (21), normally expressed in the labial and T1 segments of the embryo, and Tsh, expressed in the trunk in parasegments 3–13 (22). Using the patched (ptc) enhancer (23) for ectopic Antp expression in the anterior compartment of every parasegment, we observed partial to complete repression of Scr by the full-length and synthetic Antp lines in the labial and T1 segments (Fig. 2A). A comparison of the Scr protein levels among the different Antp lines is shown in SI Appendix, Fig. S3. Interestingly, Antp full-length proteins and synthetic peptides bearing a YPWM-to-AAAA substitution did not repress Scr (Fig. 2A, lines 3 and 11 and SI Appendix, Fig. S4A, line 12). A clear difference in repression potency was observed between the synthetic YPWMAntp long- and short-linker genes, with the short-linker peptide exhibiting greater repressor activity than its long-linker counterpart (Fig. 2A, lines 9 and 10). However, the synthetic WRPWAntp peptides conferred only partial repression (SI Appendix, Fig. S4A, lines 13 and 14). The presence of a repressor motif (WRPW) seems to suffice for functionality of the transcription factor in the repression of Scr. To ensure comparable expression levels among constructs and to compare the relative protein concentration of each line with the WT protein levels, we quantified the relative fluorescence intensity of each protein after staining with antibodies, as in ref. 24. Surprisingly, all constructs tested were expressed in approximately equal or lower amounts than the endogenous Antp protein, indicating that the observed phenotypes are not a result of overexpression (SI Appendix, Fig. S5).
Fig. 2.
YPWM-dependent repression and activation of Antp target genes in the embryo mediated by full-length and synthetic Antp genes. (A) Repression of Scr in the labial (lb, solid brackets) and prothoracic (T1, dashed brackets) segments by full-length and synthetic Antp constructs. Ectopic Antp is depicted in green and Scr in red. For each line a close-up of the region of repression is shown, and below it an overview of an entire embryo is shown. Note the overlay of both gene products in the AAAA-substituted lines, indicated by the yellow color. Staining in the WT embryo is for the endogenous proteins. (B) Ectopic activation of Tsh in the embryonic head segments by full-length and synthetic Antp genes. Tsh is shown in red and ectopic Antp in green. The upper image for each construct depicts Tsh, and the lower image shows its overlay with ectopic Antp. The domain of ectopic Tsh expression is outlined by dashed lines. Staining in the WT embryo is for the endogenous genes. All UAS lines were induced using a ptc-Gal4 driver.
However, activation of Tsh by full-length proteins and synthetic Antp peptides requires a functional YPWM motif (Fig. 2B and SI Appendix, Fig. S4B). Differences in the potency of ectopic Tsh activation were also observed between the full-length (Fig. 2B, lines 1 and 2) and synthetic YPWMAntp genes (Fig. 2B, lines 9 and 10), but the trend was the opposite of that displayed in homeotic transformations in the embryo or in the repression of Scr. In this case, the long-linker construct activated Tsh in a domain as extensive as the full-length WT Antp, whereas the effect of the short-linker construct was weaker. Therefore, the YPWM motif is indispensable for transcriptional activation, but repression can be achieved at least partially by its substitution by WRPW. Also, the long linker between the HD and the YPWM motif seems to favor Antp function in activation, whereas the short linker favors repression of downstream genes.
Synthetic Antp Genes Featuring a Functional YPWM Motif Cause Antenna-to-Tarsus and Eye-to-Wing Transformations.
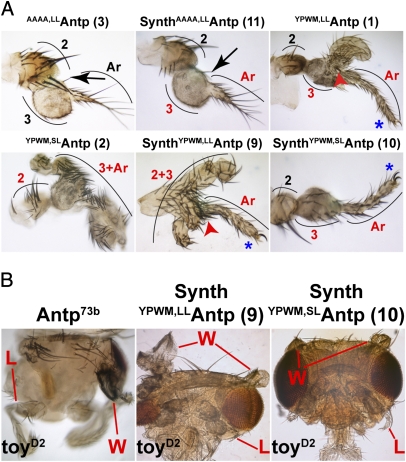
We used the Distal-less (Dll) enhancer to generate tarsal transformations in the antenna. Ectopic T2-legs bearing apical and/or preapical bristles on the distal tibia were generated only by full-length proteins and synthetic Antp peptides featuring a functional YPWM motif (Fig. 3A and SI Appendix, Fig. S6). As anticipated, no transformation or a mild thickening of the arista with a few ectopic leg bristles on the third antennal segment (A3) were observed with the full-length AAAA,LLAntp (Fig. 3A, line 3) and the corresponding synthetic peptide (Fig. 3A, line 11), suggesting mild transformation by the HD alone. The same rule applied to AAAA,LLAntpWRPW, where numerous tarsal bristles and absence of the arista were identified on the antenna (SI Appendix, Fig. S6, line 5). Synthetic peptides and full-length Antp proteins with YPWM-to-WRPW substitutions caused overgrowth of the A3 segment (SI Appendix, Fig. S6, lines 7, 8, 13 and 14), which occasionally displayed leg bristles. Finally, the antennal tarsi generated by both the full-length and synthetic YPWMAntp lines also showed a strong dependence on the size of the linker between the HD and YPWM motif, with the long-linker constructs giving rise to more dramatic phenotypes than the short-linker ones (compare line 1 with 2 and line 9 with 10 in Fig. 3A). The strength of the transformation was assessed by (i) whether only the arista or both the arista and the third antennal segment were transformed, (ii) the presence of claws on the distal tarsi, and (iii) whether tarsal and tibial or only tarsal structures could be detected. Thus, a functional YPWM motif, but not the N terminus, is indispensable for Antp gain of function in the antenna. The WRPW cannot substitute for the tetrapeptide in this case; however, the linker size seems to play a considerable role, with the long-linker lines favoring stronger transformations (SI Appendix, Fig. S6, lines 7 and 8).
Fig. 3.
Tarsal transformations caused by full-length proteins and synthetic Antp peptides. (A) The presence of the YPWM motif in Antp full-length and synthetic peptides greatly enhances the transformation, but its absence in synthetic peptides does not completely abolish it. Note the leg bristles (black arrows) that indicate weak transformation of the third antennal segment (A3) to a tarsus and the presence of apical/preapical bristles (red arrowheads), indicating mesothoracic leg (T2) identity. Curved lines show antennal segments. 2, second antennal segment; 3, third antennal segment; Ar, arista; transformed segments are identified in red type; tarsal claws are indicated by blue asterisks. (B) Eye-to-wing transformations induced in a toyD2 mutant background. The synthetic genes behave comparably to the full-length Antp73b allele. W, wings; L, legs.
However, Antp is responsible for specifying the entire mesothorax with a pair of legs (ventral appendages) and a pair of wings (dorsal appendages). We have shown previously that Antp can induce ectopic wings in the dorsal head when misexpressed in a toy mutant background (5). Because the toy gene is required for antennal morphogenesis, we expressed Antp ectopically in a hypomorphic toy mutant background (toyD2) using decapendaplegic (dppblink)-Gal4. We observed eye-to-wing transformations with the synthetic YPWM Antp constructs, comparable to the Antp73b gain-of-function allele (Fig. 3B), whereas the synthetic AAAAAntp lines did not show this transformation. Again, as in the tarsal transformations described above, the long-linker construct conferred a more dramatic phenotype than the short-linker line (Fig. 3B, lines 9 and 10).
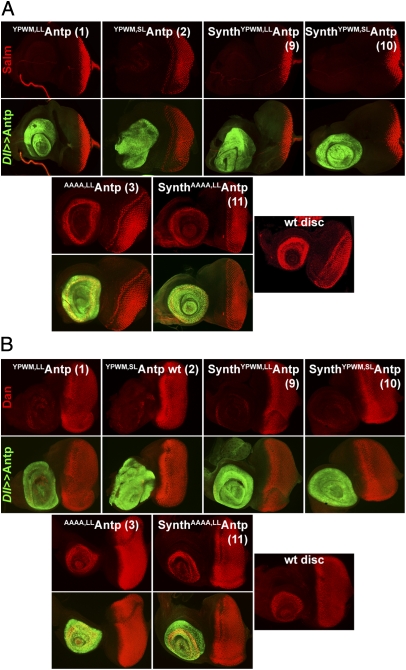
Synthetic Antp Genes Repress Antennal-Specific and Activate Leg-Specific Genes in Antennal Discs Undergoing Tarsal Transformations.
Tarsal transformations in the antennal disc require the repression of genes needed for antennal morphogenesis and ectopic activation of leg-specific genes; this repression allows antennal disc cells to commit themselves to the leg-determination program (5, 25). We and others have shown previously that antennal-specific genes such as Salm (26) and dan (27) are repressed in the antennal disc, whereas leg-specific markers such as grn are ectopically activated upon expression of Antp (28). That full-length or synthetic Scr genes (29) also confer the aforementioned changes in the antennal determination program suggests a general mechanism by which the regulation of Salm, dan, and grn is altered in discs undergoing tarsal transformations. As expected, Salm (Fig. 4A and SI Appendix, Fig. S7) and dan (Fig. 4B and SI Appendix, Fig. S8) were repressed completely in all Antp lines tested, with the exception of lines bearing the AAAA substitution for the YPWM motif (Fig. 4 A and B, lines 3 and 11, and SI Appendix, Figs. S7 and S8, line 12) and proteins with the WRPW substitution (SI Appendix, Figs. S7 and S8). The synthetic WRPWAntp discs bearing the long linker were largely overgrown compared with the eye portion of the disc (SI Appendix, Figs. S7 and S8, line 13) and gave rise to an enlarged A3 segment in adult flies (SI Appendix, Fig. S6, line 13), but no pronounced tarsal phenotypes were observed. Staining for Salm has been performed using both an anti-Salm antibody and a previously described Salm-lacZ enhancer trap chromosome (26). In both cases, all lines behaved in the same fashion, an indication that Antp acts at the transcriptional level to repress Salm.
Fig. 4.
Repression of genes required for antennal development by ectopic expression of full-length and synthetic Antp genes. (A) Repression of Salm in the antennal portion of the eye-antennal disc mediated by full-length and synthetic Antp genes. All proteins featuring a functional YPWM motif repress Salm. The constructs with the AAAA substitution do not repress Salm. (B) Repression of dan in the antennal disc by full-length and synthetic Antp genes. All lines behave as in A.
On the other hand, the ectopic activation of grn in the antennal disc (Fig. 5 and SI Appendix, Fig. S9) followed the same principle as the ectopic activation of Tsh in the embryo: An absolute requirement for a functional YPWM motif (Fig. 5, Lower) was observed, but the constructs with the AAAA substitution were indistinguishable from the control disc bearing a single enhancer trap chromosome (SI Appendix, Fig. S9). Moreover, constructs in which WRPW replaced the YPWM motif (SI Appendix, Fig. S9, lines 7, 8, 13 and 14) failed to activate transcription of the reporter, thus confirming our previous findings regarding the strict requirement for the YPWM motif in transcriptional activation. All observed phenotypes are summarized quantitatively in SI Appendix, Table S1.
Fig. 5.
Ectopic activation of leg-specific genes by full-length and synthetic Antp genes in the antennal disc. Ectopic activation of grn in the antennal disc by synthetic and full-length Antp constructs. Arrows show the ectopic expression of β-galactosidase upon Antp gain of function in the antennal portion of the disc, as represented schematically. A Dll-Gal4 driver has been used throughout.
Full-Length Proteins and Synthetic Antp Peptides Interact with Exd and Bip2 in a YPWM-Dependent Fashion.
To analyze the ability of full-length proteins and synthetic Antp peptides to bind DNA and interact with cofactors via the YPWM motif, we performed gel-shift assays using the consensus HD binding site BS2 (SI Appendix, Fig. S10) (30). As expected, all constructs bind to the labeled oligonucleotide, but those bearing a YPWM motif form additional complexes in the presence of Exd (31–33) or Bip2 (15, 34), which migrate higher than the Antp proteins alone. Each construct is represented by three lanes, the first containing Antp and the second and third also containing Exd or Bip2, respectively. The synthetic YPWMAntp peptides also interact with Exd and Bip2, albeit more weakly. We also used an ex vivo assay to evaluate the aforementioned interaction quantitatively. For this purpose, we performed transactivation experiments in cell culture using a luciferase reporter gene under the control of BS2 sites (SI Appendix, Figs. S11 and S12).
Discussion
Earlier studies have shown that the HD plays an essential role in Hox gene function. However, ectopic expression of the HD alone results in little, if any, homeotic transformation (14, 18). By combining the HD with the YPWM motif in synthetic gene constructs, we successfully reconstructed the homeotic functions of two Hox genes, Scr (29, 35) and Antp (this study). Substitutions of the YPWM motif by WRPW retain the repressive activity of Antp, and C-terminal fusions of the WRPW motif to full-length proteins convert them into stronger repressors. On the other hand, the en repression domain—also able to interact with Gro in vivo (36)—does not confer significant changes to Antp functioning as a repressor or activator. We also evaluated the role of the size of the linker between the YPWM motif and the HD, using naturally occurring splicing variants of Antp with an eight- or a four-amino acid linker. We found that the long-linker variant renders the protein a stronger activator, and the short-linker makes the protein a stronger repressor of transcription in vivo. This result, combined with the finding that mutation of the linker sequence did not alter its specificity, suggests that Antp specificity depends partially on linker size but not on sequence. Finally, we observed that synthetic peptides and full-length proteins can interact with Exd and Bip2, but all require an intact YPWM motif. The WRPW motif cannot substitute for YPWM in this case.
The Antp YPWM Motif Has a Dual Role in Transcriptional Activation and Repression.
The YPWM motif is required for Antp homeotic function in the embryo, larva, and adult fly, but the flanking regions of YPWM have no effect on Antp function. The YPWM motif has a dual role in Antp function, because it is necessary and indispensable for activation and repression of all Antp target genes tested: repression of endogenous Scr, ectopic activation of Tsh, repression of Salm and dan, and ectopic activation of grn. These properties indicate the essential role of the YPWM motif in recruiting both corepressors and coactivators. The YPWM motif is also required for ectopic induction of mesothoracic leg structures and for interaction with Exd and Bip2 cofactors in gel shift and transactivation assays. However, substitution by WRPW retains to a large extent its repressor activity in both synthetic peptides and full-length proteins and results in partial to complete repression of reporter genes.
We have shown previously that the YPWM motif acts as an activation domain in transactivation experiments (15). To examine whether the YPWM motif also can act as a repressor domain, we fused it to the C terminus of the Gal4 protein and tested its binding to UAS and its transactivation efficiency. We observed that the Gal4YPWM protein decreased transcription of the reporter by about 60%, whereas a quadruple-alanine fusion (Gal4AAAA) did not (SI Appendix, Fig. S13). Therefore, the YPWM motif, in addition to its role as an activation module, can act as a potent repressor module in vivo.
The differential requirements for a functional YPWM motif in Antp support the notion that different rules may apply in Hox-mediated transcriptional repression versus activation. Sequence comparisons among Hox binding preferences to direct targets (9) show that Pbc–Hox sites usually are found in enhancers of genes activated by a Pbc–Hox complex, whereas regulatory elements bound by Hox factors alone (without any Pbc input) are distributed equally among the genes activated and repressed by Hox factors. This observation supports the idea that Hox-mediated activation depends largely on cofactors, whereas repression does not always require them.
Our phenotypic observations in the embryo, in which both the YPWM,LLAntpWRPW and the AAAA,LLAntpWRPW proteins activated Tsh ectopically in the region of the embryonic head (Fig. 2B, lines 4 and 5), but the synthetic or full-length AAAAAntp proteins did not, suggest that this target gene is activated indirectly in the embryo (possibly through repression of a repressor). However, the finding that the YPWM,LLAntpWRPW construct can ectopically activate grn (Fig. 5, line 4), whereas the AAAA,LLAntpWRPW line cannot (Fig. 5, line 5), indicates that Antp can act as a bona fide activator and that the activation of grn may be direct.
The substitution of the YPWM motif by alanines strongly decreases but does not entirely abolish Antp homeotic function in full-length and synthetic AAAAAntp constructs, because they still exhibit aristal transformations and display ectopic leg bristles on the A3 segment, suggesting weak transformations toward leg identity. Similar behavior of AAAA-substituted Antp has been reported in flies for the full-length AAAA,LLAntp, coinduced with a constitutively active Notch (15), or the beetle fushi tarazu gene encoding a protein with a YPWM-to-AAAA substitution (37). Also, the phenotypes of AAAAAntp in the antenna are in line with the head-involution defects in the larval cuticle early in development. Although the AAAA-substituted synthetic peptides fail overall to exhibit significant phenotypes, they still infer mild malformations in the larval head, including defects in the formation of the mouth hooks and, in these cases, lethality. However, unlike their homeotic effect in the antenna, these lines retain T1 identity in the cuticle, as demonstrated by the presence of prothoracic beards.
The Long and Short Linkers Between the YPWM Motif and the HD Enhance the Activation or Repression Capacity of Antp, Respectively.
In the embryo, the antennal disc, and in Drosophila S2 cells the long-linker Antp proteins behaved as potent activators, whereas the short-linker constructs acted as strong repressors. The long-linker variant is expressed predominantly in embryonic stages, and equal amounts of both variants are detected in larval, pupal, and adult stages (17). Thus, different Antp transcripts might play distinct, albeit similar, roles in development. Isoforms with different linker sizes also are found in Ultrabithorax (Ubx) (38), and in Abdominal A mutations in the linker decrease its activation capacity on the wingless enhancer (39). Recently, the size of the linker between the YPWM and the HD of Ubx was found to play a regulatory role in Ubx function, with the long- and short-linker variants not being interchangeable in vivo (40). In the same study, the short-linker variant was found to bind DNA more strongly than its long-linker relative, in the presence of Exd. In line with these arguments, the Antp short-linker variant also interacted more strongly with Exd and more weakly with Bip2 than did its long-linker counterpart (SI Appendix, Fig. S12).
A C-Terminal Portion of Antp Containing the HD and YPWM Motif Suffices for Homeotic Function in Drosophila.
One possible N-terminal domain to which potency could be attributed is the poly-glutamine (poly-Q) stretch (present in the N terminus of Antp and absent in synthetic Antp peptides), which has been proposed to function as an activator domain in Bicoid (41) and Abdominal B (39). Another conserved motif present in the N terminus of many homeoproteins (24) and reported to participate in transcriptional potency in the fly Scr (24) and the mouse Hoxa5 (42) is the TSYF motif (SSYF in Scr). An alignment of various Antp orthologs showed that the N and C termini of all proteins are largely variable between insects and mammals, with the latter displaying shorter N termini devoid of poly-Q stretches (SI Appendix, Fig. S14). The residues flanking the YPWM motif are slightly conserved in arthropods but not between arthropods and mammals, an observation that is in line with our finding that their substitution by alanines does not affect the homeotic function of the protein (SI Appendix, Fig. S1). However, the linker between the HD and the YPWM motif, differentially spliced in the transcripts of at least some Hox transcripts (Antp, Ubx), could account for increased specificity of target selection in vivo. Recent evidence shows that the regions flanking the Ubx YPWM motif (alternatively spliced in Ubx isoforms) are responsible for Ubx specificity in vivo (40, 43). Moreover, the YPWM motif and the linker region (termed “the specificity module”) of Deformed and Scr result in their unique regulatory behavior in vivo, and nonconserved domains N-terminal to this region allow subtle differences in Hox-DNA recognition properties. These differences can have a considerable impact on the transcriptional output—activation versus repression—of target genes (44). Finally, decisions on whether certain Hox factors function as activators or repressors in a specific context can result from the abundance of their isoforms rather than from the regulatory sequences to which they bind. The finding that the same enhancer element upstream of the murine Six2 gene is activated by Hox11 and repressed by Hoxa2 (45) substantiates this hypothesis.
The Power of Synthetic Genes.
Our study has increased our understanding of synthetic genes. Hypothesizing that protein function is a sum of the functions of its protein domains, the latter being oriented in a precise 3D conformation that allows normal interactions to take place, we may not be not far from attempting to engineer and possibly predict the functions of novel synthetic peptides on the basis of the structural architecture of their protein domains. Inversely, functions of proteins might be scaled down to fractions of their sequences, with each domain retaining only a subset of these functions. So far, we have reconstructed successfully, to a great extent, the function of two Hox proteins, Antp and Scr, using their synthetic-peptide versions, and have demonstrated that they act predictably in vivo. If biomedically relevant proteins also fell in this category, the development and use of their corresponding synthetic peptides might present advantages over their full-length counterparts for application in vivo, because of their smaller size and more easily understood molecular properties. Novel proteins with predictable functions have been constructed successfully, e.g., with the neuronal Wiskott–Aldrich protein, N-WASP (46), and guanine nucleotide exchange factors (47), in which integrating artificial domains that respond to nonnative inputs led to the precise control of physiological responses, such as the formation of filopodia or lamellipodia.
Materials and Methods
Plasmids were generated using standard procedures, and fly transgenesis was performed as described (48). Preparation of embryos was performed as in ref. 9, and imaginal discs were prepared as described (29). Embryonic cuticles were prepared according to ref. 19. Gel-shift assays were performed as described (49). Detailed methods are outlined in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the kantons of Basel-Stadt and Basel-Landschaft, a grant from the Swiss National Science Foundation, the European Network of Excellence “Cells into Organs,” and the Consejo Nacional de Ciencia y Tecnología of Mexico, Project 39479-Q and Scholarship 46659. D.K.P. also was supported by a European Molecular Biology Organization short-term fellowship (ASTF 499-2010).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108686108/-/DCSupplemental.
References
- 1.Gehring WJ. Homeo boxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 3.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis W, Levine MS, Hafen E, Kuroiwa A, Gehring WJ. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature. 1984;308:428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- 5.Gehring WJ, Kloter U, Suga H. Evolution of the Hox gene complex from an evolutionary ground state. Curr Top Dev Biol. 2009;88:35–61. doi: 10.1016/S0070-2153(09)88002-2. [DOI] [PubMed] [Google Scholar]
- 6.Kmita-Cunisse M, Loosli F, Bièrne J, Gehring WJ. Homeobox genes in the ribbonworm Lineus sanguineus: Evolutionary implications. Proc Natl Acad Sci USA. 1998;95:3030–3035. doi: 10.1073/pnas.95.6.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott MP, Weiner AJ. Structural relationships among genes that control development: Sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci USA. 1984;81:4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehring WJ, et al. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 9.Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hueber SD, Lohmann I. Shaping segments: Hox gene function in the genomic age. Bioessays. 2008;30:965–979. doi: 10.1002/bies.20823. [DOI] [PubMed] [Google Scholar]
- 11.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 12.Furukubo-Tokunaga K, Flister S, Gehring WJ. Functional specificity of the Antennapedia homeodomain. Proc Natl Acad Sci USA. 1993;90:6360–6364. doi: 10.1073/pnas.90.13.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian YQ, Resendez-Perez D, Gehring WJ, Wüthrich K. The des(1-6)antennapedia homeodomain: Comparison of the NMR solution structure and the DNA-binding affinity with the intact Antennapedia homeodomain. Proc Natl Acad Sci USA. 1994;91:4091–4095. doi: 10.1073/pnas.91.9.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson G, Schier A, LeMotte P, Gehring WJ. The specificities of Sex combs reduced and Antennapedia are defined by a distinct portion of each protein that includes the homeodomain. Cell. 1990;62:1087–1103. doi: 10.1016/0092-8674(90)90386-s. [DOI] [PubMed] [Google Scholar]
- 15.Prince F, et al. The YPWM motif links Antennapedia to the basal transcriptional machinery. Development. 2008;135:1669–1679. doi: 10.1242/dev.018028. [DOI] [PubMed] [Google Scholar]
- 16.Paroush Z, et al. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 17.Stroeher VL, Gaiser JC, Garber RL. Alternative RNA splicing that is spatially regulatedGgeneration of transcripts from the Antennapedia gene of Drosophila melanogaster with different protein-coding regions. Mol Cell Biol. 1988;8:4143–4154. doi: 10.1128/mcb.8.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bello B, Resendez-Perez D, Gehring WJ. Spatial and temporal targeting of gene expression in Drosophila by means of a tetracycline-dependent transactivator system. Development. 1998;125:2193–2202. doi: 10.1242/dev.125.12.2193. [DOI] [PubMed] [Google Scholar]
- 19.Gibson G, Gehring WJ. Head and thoracic transformations caused by ectopic expression of Antennapedia during Drosophila development. Development. 1988;102:657–675. [Google Scholar]
- 20.Zeng W, Andrew DJ, Mathies LD, Horner MA, Scott MP. Ectopic expression and function of the Antp and Scr homeotic genes: The N terminus of the homeodomain is critical to functional specificity. Development. 1993;118:339–352. doi: 10.1242/dev.118.2.339. [DOI] [PubMed] [Google Scholar]
- 21.Andrew DJ, Horner MA, Petitt MG, Smolik SM, Scott MP. Setting limits on homeotic gene function: Restraint of Sex combs reduced activity by teashirt and other homeotic genes. EMBO J. 1994;13:1132–1144. doi: 10.1002/j.1460-2075.1994.tb06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick A, Coré N, Kerridge S, Scott MP. Homeotic response elements are tightly linked to tissue-specific elements in a transcriptional enhancer of the teashirt gene. Development. 1995;121:2799–2812. doi: 10.1242/dev.121.9.2799. [DOI] [PubMed] [Google Scholar]
- 23.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 24.Tour E, Hittinger CT, McGinnis W. Evolutionarily conserved domains required for activation and repression functions of the Drosophila Hox protein Ultrabithorax. Development. 2005;132:5271–5281. doi: 10.1242/dev.02138. [DOI] [PubMed] [Google Scholar]
- 25.Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. doi: 10.1038/292635a0. [DOI] [PubMed] [Google Scholar]
- 26.Wagner-Bernholz JT, Wilson C, Gibson G, Schuh R, Gehring WJ. Identification of target genes of the homeotic gene Antennapedia by enhancer detection. Genes Dev. 1991;5(12B):2467–2480. doi: 10.1101/gad.5.12b.2467. [DOI] [PubMed] [Google Scholar]
- 27.Emerald BS, Curtiss J, Mlodzik M, Cohen SM. Distal antenna and distal antenna related encode nuclear proteins containing pipsqueak motifs involved in antenna development in Drosophila. Development. 2003;130:1171–1180. doi: 10.1242/dev.00323. [DOI] [PubMed] [Google Scholar]
- 28.Grieder NC, Morata G, Affolter M, Gehring WJ. Spalt major controls the development of the notum and of wing hinge primordia of the Drosophila melanogaster wing imaginal disc. Dev Biol. 2009;329:315–326. doi: 10.1016/j.ydbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos DK, et al. Function and specificity of synthetic Hox transcription factors in vivo. Proc Natl Acad Sci USA. 2010;107:4087–4092. doi: 10.1073/pnas.0914595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukubo-Tokunaga K, et al. In vivo analysis of the helix-turn-helix motif of the fushi tarazu homeo domain of Drosophila melanogaster. Genes Dev. 1992;6:1082–1096. doi: 10.1101/gad.6.6.1082. [DOI] [PubMed] [Google Scholar]
- 31.Passner JM, Ryoo HD, Shen L, Mann RS, Aggarwal AK. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 32.Piper DE, Batchelor AH, Chang CP, Cleary ML, Wolberger C. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: Role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 33.Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangloff YG, et al. The TFIID components human TAF(II)140 and Drosophila BIP2 (TAF(II)155) are novel metazoan homologues of yeast TAF(II)47 containing a histone fold and a PHD finger. Mol Cell Biol. 2001;21:5109–5121. doi: 10.1128/MCB.21.15.5109-5121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vukojevic V, Papadopoulos DK, Terenius L, Gehring WJ, Rigler R. Quantitative study of synthetic Hox transcription factor-DNA interactions in live cells. Proc Natl Acad Sci USA. 2010;107:4093–4098. doi: 10.1073/pnas.0914612107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolkunova EN, Fujioka M, Kobayashi M, Deka D, Jaynes JB. Two distinct types of repression domain in engrailed: One interacts with the groucho corepressor and is preferentially active on integrated target genes. Mol Cell Biol. 1998;18:2804–2814. doi: 10.1128/mcb.18.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Löhr U, Pick L. Cofactor-interaction motifs and the cooption of a homeotic Hox protein into the segmentation pathway of Drosophila melanogaster. Curr Biol. 2005;15:643–649. doi: 10.1016/j.cub.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor MB, Binari R, Perkins LA, Bender W. Alternative RNA products from the Ultrabithorax domain of the bithorax complex. EMBO J. 1988;7:435–445. doi: 10.1002/j.1460-2075.1988.tb02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merabet S, et al. The hexapeptide and linker regions of the AbdA Hox protein regulate its activating and repressive functions. Dev Cell. 2003;4:761–768. doi: 10.1016/s1534-5807(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 40.Reed HC, et al. Alternative splicing modulates Ubx protein function in Drosophila melanogaster. Genetics. 2010;184:745–758. doi: 10.1534/genetics.109.112086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janody F, Sturny R, Schaeffer V, Azou Y, Dostatni N. Two distinct domains of Bicoid mediate its transcriptional downregulation by the Torso pathway. Development. 2001;128:2281–2290. doi: 10.1242/dev.128.12.2281. [DOI] [PubMed] [Google Scholar]
- 42.Zhao JJ, Lazzarini RA, Pick L. Functional dissection of the mouse Hox-a5 gene. EMBO J. 1996;15:1313–1322. [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Matthews KS, Bondos SE. Internal regulatory interactions determine DNA binding specificity by a Hox transcription factor. J Mol Biol. 2009;390:760–774. doi: 10.1016/j.jmb.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi R, Sun L, Mann R. Dissecting the functional specificities of two Hox proteins. Genes Dev. 2010;24:1533–1545. doi: 10.1101/gad.1936910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yallowitz AR, Gong KQ, Swinehart IT, Nelson LT, Wellik DM. Non-homeodomain regions of Hox proteins mediate activation versus repression of Six2 via a single enhancer site in vivo. Dev Biol. 2009;335:156–165. doi: 10.1016/j.ydbio.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dueber JE, Yeh BJ, Chak K, Lim WA. Reprogramming control of an allosteric signaling switch through modular recombination. Science. 2003;301:1904–1908. doi: 10.1126/science.1085945. [DOI] [PubMed] [Google Scholar]
- 47.Yeh BJ, Rutigliano RJ, Deb A, Bar-Sagi D, Lim WA. Rewiring cellular morphology pathways with synthetic guanine nucleotide exchange factors. Nature. 2007;447:596–600. doi: 10.1038/nature05851. [DOI] [PubMed] [Google Scholar]
- 48.Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 49.Plaza S, et al. Molecular basis for the inhibition of Drosophila eye development by Antennapedia. EMBO J. 2001;20:802–811. doi: 10.1093/emboj/20.4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.