Abstract
Cross-presentation of IgG-containing immune complexes (ICs) is an important means by which dendritic cells (DCs) activate CD8+ T cells, yet it proceeds by an incompletely understood mechanism. We show that monocyte-derived CD8−CD11b+ DCs require the neonatal Fc receptor for IgG (FcRn) to conduct cross-presentation of IgG ICs. Consequently, in the absence of FcRn, Fcγ receptor (FcγR)-mediated antigen uptake fails to initiate cross-presentation. FcRn is shown to regulate the intracellular sorting of IgG ICs to the proper destination for such cross-presentation to occur. We demonstrate that FcRn traps antigen and protects it from degradation within an acidic loading compartment in association with the rapid recruitment of key components of the phagosome-to-cytosol cross-presentation machinery. This unique mechanism thus enables cross-presentation to evolve from an atypically acidic loading compartment. FcRn-driven cross-presentation is further shown to control cross-priming of CD8+ T-cell responses in vivo such that during chronic inflammation, FcRn deficiency results in inadequate induction of CD8+ T cells. These studies thus demonstrate that cross-presentation in CD8−CD11b+ DCs requires a two-step mechanism that involves FcγR-mediated internalization and FcRn-directed intracellular sorting of IgG ICs. Given the centrality of FcRn in controlling cross-presentation, these studies lay the foundation for a unique means to therapeutically manipulate CD8+ T-cell responses.
Dendritic cells (DCs) function as central orchestrators of immunity by providing necessary innate signals and adaptive functions through the processing and presentation of antigens to which T cells respond. DCs are highly specialized for the processing of extracellular antigens in the context of major histocompatibility complex class II (MHC-II) molecules for presentation to CD4+ T cells and MHC class I (MHC-I) molecules for presentation to CD8+ T cells (1). This latter process, known as “cross-presentation,” is important for the induction of immune responses against a variety of pathogens, including viruses and bacteria, that do not primarily infect DCs (2, 3). Not all DCs are equal in their ability to cross-present antigens because a very specific intracellular environment is required for this process. In particular, CD8+CD11b− DCs in mice and their recently identified human counterparts, CD141+(BCDA-3)+ DCs, are major mediators of cross-presentation (4, 5). These DC subsets uniquely process antigen in a minimally degradative manner that relies upon maintaining a near-neutral compartmental pH and preserving antigenicity to generate peptides compatible with MHC-I loading (6, 7). CD8+CD11b− DCs have been shown to use two predominant mechanisms to achieve this result. In the vacuolar pathway, antigens are degraded by proteases, such as cathepsins, within phagosomes where loading of peptides directly onto MHC-I occurs. In the phagosome-to-cytosol pathway, antigens are protected from local degradation and exported to the cytosol for proteasomal degradation. Antigenic peptides are either transported back to the endoplasmic reticulum (ER) for loading onto MHC-I or reimported into the phagosomal compartment containing components of the ER retrotranslocation and protein loading machinery (8, 9). All pathways of cross-presentation are characterized by carefully controlled acidic degradation, which is achieved by balancing acidification and oxidation within the relevant intracellular compartments (6).
To date, the majority of studies examining cross-presentation mechanisms have focused on the processing of soluble antigens. However, extracellular antigens can also be directed to intracellular cross-presentation pathways by specific receptor-mediated internalization. An important example of the latter is uptake of IgG-containing immune complexes (IgG ICs) by activating Fcγ receptors (FcγR; refs. 10–14). It was therefore interesting to consider the role played by the neonatal Fc receptor for IgG (FcRn) in cross-presentation and its relationship to FcγR-mediated internalization. FcRn is a nonclassical Fc receptor encoded by the Fcgrt gene that binds its ligand, IgG, exclusively at acidic pH ≤ 6.5 and resides predominantly intracellularly (15). Despite the insinuation of its name, FcRn is expressed throughout life in a variety of cell types. These include mouse and human DCs (15, 16), where it has been shown to play a role in MHC-II-restricted presentation of IgG IC-derived antigens (17, 18).
Given these characteristics, we sought to determine whether FcRn is necessary for directing antigen as an IgG IC toward a cross-presented fate subsequent to FcγR-mediated entry of antigen. Our results demonstrate that FcRn indeed plays an indispensible role downstream of FcγR in the intracellular sorting of IgG IC-delivered antigens to the appropriate destination for cross-presentation to occur. This pathway is especially important in monocyte-derived CD8−CD11b+ DCs wherein FcRn expression is required for the in vivo induction of CD8+ T cells. Collectively, our data identify a previously undescribed role for FcRn and as such delineate a unique intracellular mechanism by which FcγR-initiated cross-presentation proceeds.
Results
FcRn Enables Cross-Presentation of IgG-Complexed Antigens in CD8−CD11b+ DCs.
To investigate the role of FcRn in cross-presentation of IgG ICs, we used a series of previously described chimeric anti-4-hydroxy-3-iodo-5-nitrophenylacetic acid (anti-NIP) antibodies, one of which, IHH-IgG, harbors three mutations that disable binding to FcRn [I253A, H435A, and H436A (IHH); refs. 18 and 19; Fig. S1A].
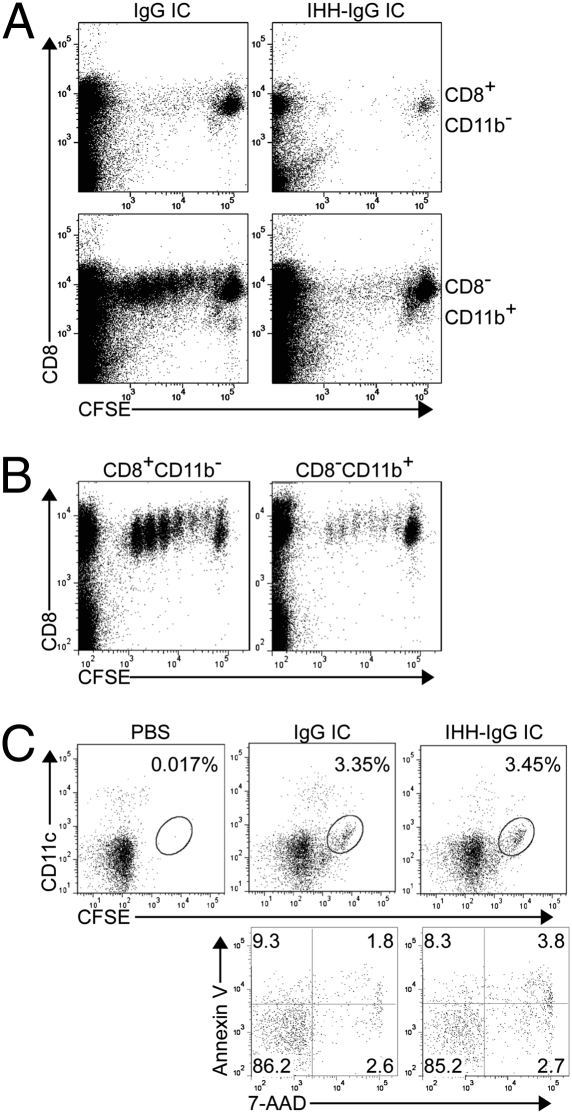
We compared CD8−CD11b+ DCs and CD8+CD11b− DCs for their ability to elicit cross-priming of a CD8+ T-cell response in vivo. In the first series of experiments, the desired subsets of DCs were obtained from the spleens of mice s.c. inoculated with either GM-CSF or Flt-3L secreting melanoma cells to selectively enrich for CD8−CD11b+ or CD8+CD11b− DCs, respectively, as described in SI Methods (18, 20, 21). The different DC subsets were isolated to purity by flow cytometric sorting and characterized for expression of cell surface markers (Fig. S1B). The sorted DCs were pulsed with IgG- or IHH-IgG–complexed NIP-ovalbumin (OVA) and adoptively transferred into the opposite hind footpads of recipient mice that had received carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-I T cells (Fig. S1C). Ipsilateral popliteal lymph nodes (PLN) were harvested after 72 h, and antigen-specific T-cell proliferation was assessed. Strikingly, CD8−CD11b+ DCs loaded with IgG-complexed OVA (0.5 μg/mL) induced robust proliferation of adoptively transferred OT-I T cells and did so to a much greater extent than CD8+CD11b− DCs and in an entirely FcRn-dependent manner (Fig. 1A and Fig. S1D). In contrast, CD8+CD11b− DCs were more potent than CD8−CD11b+ DCs in inducing proliferation to 5 μg/mL soluble antigen (Fig. 1B), consistent with previous observations (4, 22). These differences in the ability of CD8+CD11b− and CD8−CD11b+ DCs to cross-present antigen as an IC with IgG versus an FcRn-disabled (IHH-IgG) ICs were not due to unequal FcRn expression (Fig. S1E), the binding of the IgG containing IC to FcγR on the cell surface (Fig. S1 F–H), the quantities of internalized antigen (Fig. S1I), the ability of adoptively transferred DCs to reach the draining LN, or the viability of homed DCs (Fig. 1C and Fig. S1 J and K). Therefore, whereas CD8+CD11b− DCs are superior in the cross-presentation of soluble antigen, CD8−CD11b+ DCs are more effective in cross-presenting antigen as an IgG IC in a pathway that is dependent upon FcRn.
Fig. 1.
CD8−CD11b+ DCs use FcRn-dependent cross-presentation in vivo to induce primary CD8+ T-cell responses to low-dose antigen in IgG ICs. (A) Only CD8−CD11b+ DCs efficiently cross-prime when loaded with IgG ICs containing low doses of antigen (0.5 μg/mL) and do so in an FcRn-dependent manner. IC-loaded DCs were injected into the opposite hind footpads of recipient mice (see Fig. S1C). (B) CD8+CD11b− DCs efficiently cross-prime CD8+ T cells when loaded with a standard dose (5 μg/mL) of soluble antigen. (C) Viable CD8−CD11b+ DCs loaded with IgG ICs or IHH-IgG ICs migrate equally to the draining PLN. DCs were labeled with CFSE before IC loading and footpad injection and assessed 12 h later. Percentages indicate the fraction of MHC-II+CD11c+CFSE+ cells. Annexin V and 7-AAD staining confirmed DCs viability.
FcRn-Mediated Cross-Presentation by CD8−CD11b+ DCs Is Highly Efficient at Inducing T-Cell Responses to Low Doses of Complexed and Particulate Antigen.
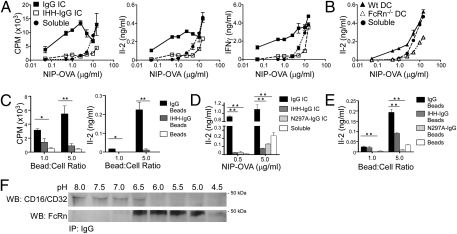
To investigate the efficiency of FcRn-mediated cross-presentation, we studied the ability of these cells to activate OT-I cells over a broad dose range of IgG ICs and particulate antigens in vitro using CD11c-microbead isolated DCs from mice inoculated with GM-CSF or Flt3L secreting melanoma cells (SI Methods and Fig. S2A). We observed that CD8−CD11b+ DCs were able to cross-present OVA at doses as low as 0.05 μg/mL in a dose-dependent and entirely FcRn-restricted fashion when provided as an IgG IC (Fig. 2 A and B). FcRn dependence for cross-presentation extended to opsonized particulate antigens because only OVA-coated beads opsonized with IgG, but not IHH-IgG, efficiently activated CD8+ T cells (Fig. 2 C). Furthermore, the ability of FcRn to promote cross-presentation was not limited to CD8−CD11b+ DCs generated under inflammatory conditions because spleen CD8−CD11b+ DCs from untreated mice loaded with IgG ICs were also capable of activating CD8+ T cells in an FcRn-dependent manner (Fig. S2 B and C). Thus, CD8−CD11b+ DCs are extremely potent in the cross-presentation of low doses of antigen in various forms when delivered in an IgG-complexed form.
Fig. 2.
FcRn-mediated cross-presentation by CD8−CD11b+ DCs is particularly efficient at inducing T-cell responses over a low-dose range of complexed and particulate antigen. (A) FcRn enables cross-presentation of very low antigen concentrations contained within IgG ICs. DCs were loaded with ICs or soluble NIP-OVA and cocultured with primary OT-I cells. (B) CD8−CD11b+ DCs from FcRn−/− mice fail to cross-present IgG ICs. (C) FcRn enables cross-presentation of antigens derived from large opsonized particles. DCs were loaded with opsonized 3-μm beads at the indicated ratios and cocultured with OT-I cells. (D) FcRn-mediated cross-presentation of ICs is abrogated in the absence of FcγR binding. (E) Internalization via FcγR is required for FcRn-mediated cross-presentation of antigen from IgG-opsonized particulate antigen. (F) FcγR binds IgG at neutral pH, whereas FcRn binds IgG at acidic pH. CD8−CD11b+ DCs were lysed in buffers of the indicated pH and immunoprecipitated with IgG. All results are representative of at least three independent experiments with n = 3 per group. All data are mean ± SEM of triplicates. *P < 0.05; **P < 0.01.
We next examined the relationship between FcγR and FcRn in the cross-presentation of antigen as IC with IgG. As shown in Fig. 2D, cross-presentation by CD8−CD11b+ DCs of small ICs was highly dependent upon the ability of ICs to bind to both FcRn and FcγR since CD8+ T-cell activation was greatly reduced when ICs were formed with either IHH-IgG or N297A-IgG, the latter of which cannot bind FcγRs but retains the ability to bind FcRn (23). The requirement for FcγR was similarly observed for opsonized particulate antigen (Fig. 2E). Given that FcRn characteristically binds IgG at acidic pH not typical of the extracellular milieu where FcγRs function, we considered whether pH might be a primary factor in determining at which stage each of the IgG binding receptors is engaged while shuttling antigen into a cross-presentation pathway. As shown in Fig. 2F, IgG was observed to immunoprecipitate CD16/CD32 between pH levels of 8.0 and 6.5, consistent with what would be observed in an extracellular or early endosomal environment. In contrast, FcRn was only precipitated by IgG between pH levels 6.5 and 5.0, a pH more likely to be found within deeper endocytic compartments. Our data are consistent with a model in which IgG ICs interact sequentially with FcγRs and FcRn, binding first to FcγRs at the neutral pH of the cell surface and subsequently, following internalization, to FcRn within acidic intracellular endolysosomal compartments.
FcRn Mediates Entry of IgG ICs into a Rab27a, Vacuolar ATPase, and gp91phox Containing Acidic Phagosome That Allows for Cross-Presentation.
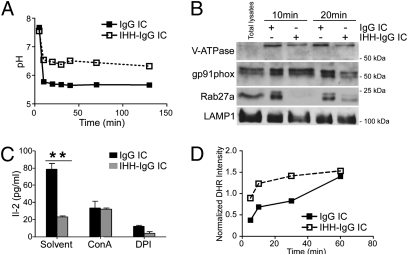
Given our proposed pH-dependent mechanism, we next sought to elucidate the characteristics of the intracellular loading compartments involved in FcRn-mediated cross-presentation in CD8−CD11b+ DCs. We first examined the pH reached by phagosomal compartments after IgG IC internalization within CD8−CD11b+ DCs using FITC- and Alexa647-conjugated 3-μm beads. As shown in Fig. 3A, phagosomes that had internalized FcRn-binding IgG-opsonized beads maintained a pH of ∼5.5, which was consistent with 1-log lower acidification of the phagosomes than that observed within DCs that had internalized IHH-IgG-opsonized beads (pH ∼ 6.5). To understand the mechanism for this increased phagosomal acidification in the presence of IC binding to FcRn, we performed biochemical studies on phagosomes purified from CD8−CD11b+ DCs having internalized magnetic particles opsonized with IgG or IHH-IgG (Fig. 3B). Phagosomes from DCs that received IgG-opsonized particles rapidly acquired greater quantities of vacuolar ATPase (V-ATPase), gp91phox, and Rab27a than those from DCs treated with IHH-IgG-opsonized particles. Consistent with the functional importance of the recruited phagosomal proteins, blockade of V-ATPase activity with concanamycin A or gp91phox-mediated oxidation with DPI completely abrogated the contribution of FcRn to cross-presentation (Fig. 3C). We therefore next examined the oxidation state of internalized IgG-opsonized beads conjugated to the oxidation-sensitive probe dihydrorhodamine (DHR). Interestingly, despite the presence of increased gp91phox within the phagosomes of IgG-containing beads, beads opsonized by IgG underwent less oxidation than beads opsonized by IHH-IgG (Fig. 3D).
Fig. 3.
FcRn-dependent cross-presentation of IgG ICs relies on acidification and oxidation of the loading compartment. (A) Phagosomes containing IgG-opsonized beads acidify more efficiently than do those containing IHH-IgG-opsonized beads. DCs were pulsed with opsonized 3-μm beads labeled with FITC and Alexa647 dyes and chased as indicated. (B) Phagosomes containing IgG-opsonized beads were enriched in Rab27a, V-ATPase, and gp91phox. Phagosomes were purified from ICs pulsed DCs by using magnetic separation. (C) FcRn-mediated cross-presentation of IgG-complexed antigens is blocked by inhibitors of acidification (concanamycin A, 50 nM) and oxidation (DPI, 5 μM). (D) IgG-complexed antigens that bind FcRn are protected from oxidation. DCs were pulsed with opsonized beads conjugated to DHR and Alexa647 and chased as indicated. Results are representative of at least three independent experiments. Data are mean ± SEM of triplicates. **P < 0.01.
FcRn Retains IgG ICs in an Intracellular Compartment Equipped with Components of the Cross-Presentation Machinery.
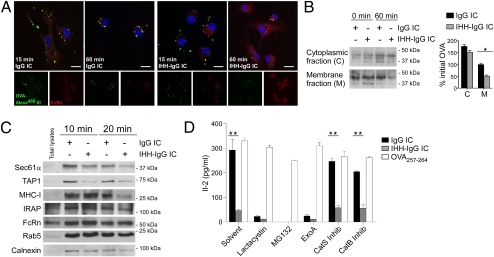
In light of these findings, we examined the stability and distribution of ICs using confocal microscopy on CD8−CD11b+ DCs obtained from bone marrow (BMDCs), as described in SI Methods (Fig. S3A). In cells pulsed with IgG ICs, the antigenic component of the ICs remained visible within intracellular compartments 60 min after pulsing and remained localized with FcRn (Fig. 4A). In comparison, despite containing comparable quantities of internalized antigen at 15 min after the pulse, BMDCs that received IHH-IgG containing ICs exhibited little evidence of the internalized antigen after 60 min and loss of FcRn coalescence into punctate structures (Fig. 4A). Consistent with the morphologic evidence for persistence of antigen within phagosomes when IgG ICs bind to FcRn, quantitatively more OVA was detected after 60 min within the membrane fractions of CD8−CD11b+ DCs pulsed with IgG ICs than in DCs pulsed with IHH-IgG ICs (Fig. 4B and Fig. S3B). We therefore conclude that FcRn protects IgG ICs from rapid degradation in the acidic phagosomes of CD8−CD11b+ DCs.
Fig. 4.
FcRn ligation by IgG ICs traps the complexed antigen in a membrane-bound compartment optimized for cross-presentation. (A) IgG ICs are retained for prolonged periods within endosomal compartments where they colocalize with FcRn. BMDCs were pulsed with ICs containing OVA-Alexa488, chased as indicated, and stained for FcRn. (Scale bar: 10 μm.) (B) IgG-complexed antigen persists within a membrane-bound compartment when bound to FcRn. DCs were pulsed with ICs and chased as indicated before isolation of cytoplasmic and membrane fractions. The density of each major band at 60 min was quantified for each of three independent experiments. *P < 0.05. (C) Key proteins of the cross-presentation machinery, including TAP1, Sec61α, and MHC-I, are enriched in phagosomes containing FcRn-binding IgG-opsonized beads. (D) Generation of peptides for FcRn-mediated cross-presentation is dependent on proteasomal processing rather than vacuolar protease digestion and requires Sec61α endosomal export. DCs were pretreated with solvent, Lactacystin (10 μM), MG132 (4 μM), Cathepsin S inhibitor (0.1 μM), Cathepsin B inhibitor (10 μM), or Exotoxin A (5 μg/mL) before ICs or OVA257–264 (100 pg/mL) loading. All results are representative of at least two independent experiments. All data are mean ± SEM of triplicates. **P < 0.01.
Because of the prolonged sequestration of FcRn-bound IgG ICs within phagosomal compartments, we sought to determine whether these compartments contained known components of cross-presentation machinery. Indeed, only the phagosomes containing beads capable of FcRn ligation were enriched in TAP1, Sec61α, and MHC-I molecules (Fig. 4C). These data suggest that FcRn facilitates IC trafficking into a compartment equipped for cross-presentation. Furthermore, inhibition of FcRn-mediated cross-presentation by proteasome (lactacystin and MG132) and Sec61α (Exotoxin A; Fig. 4D and Fig. S3 C and D) inhibitors indicated that this process proceeds via a phagosome-to-cytosol cross-presentation pathway. A contribution for the alternative vacuolar pathway was ruled out by the lack of insulin-regulated amino peptidase (IRAP) enrichment within phagosomes from IgG-opsonized beads (Fig. 4C) and the inability of cathepsin inhibitors to abrogate IgG IC-mediated cross-presentation (Fig. 4D). FcRn is thus necessary for both the movement of IgG ICs to an acidified endosome equipped for phagosome-to-cytosol mediated cross-presentation as well as protection of the complexed antigen from rapid degradation in this harsh environment.
FcRn-Mediated Cross-Presentation Induces Cross-Priming of CD8+ T-Cell Responses in Vivo During Intestinal Inflammation.
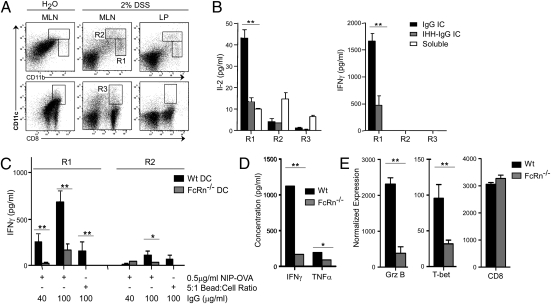
To demonstrate the physiological relevance of our findings, we investigated whether CD8−CD11b+ DCs were capable of inducing FcRn-mediated cross-presentation during the course of an active inflammatory response in vivo. To do so, we chose a chronic model of intestinal inflammation induced by sequential rounds of dextran sodium sulfate (DSS) administration that is associated with the generation of high levels of IgG anti-bacterial antibodies (Fig. S4A; refs. 17 and 24). Both the mesenteric lymph nodes (MLN) and the colonic lamina propria (LP) of DSS-treated mice contained a marked increase in CD8−CD11b++CD11c+ cells, consistent with infiltrating monocyte-derived inflammatory DCs, and CD8−CD11b+CD11c++ cells, consistent with expanded resident DCs, but not CD8+CD11b−CD11c+ DCs (Fig. 5A). These infiltrating DC subsets were present in equivalent proportions in both wild-type and FcRn−/− mice, did not display differences in the level of activation, and exhibited similar levels of MHC-I, MHC-II, and FcγR (Fig. S4B) as well as FcRn expression (Fig. S4C). When these three DC subsets were sorted by flow cytometry and loaded ex vivo with IgG- or IHH-IgG–complexed OVA, only the CD8−CD11b++CD11c+ monocyte-derived DC subset efficiently cross-presented the IC-derived antigen and did so in an FcRn-dependent fashion (Fig. 5B). Furthermore, only FcRn-mediated cross-presentation by the CD8−CD11b++CD11c+ DCs was associated with strong induction of IFNγ secretion by primary OT-I cells (Fig. 5B). These findings were confirmed by DSS treatment of FcRn−/− mice whose CD8−CD11b++CD11c+ DCs failed to elicit strong cross-priming under a variety of different conditions (Fig. 5C). Moreover, when total CD8+ T cells were isolated from the MLNs, but not spleens, of the colitic mice, it was observed that the T cells from wild-type mice exhibited significantly higher levels of IFNγ secretion upon ex vivo restimulation than did the CD8+ T cells from FcRn−/− mice (Fig. 5D). These CD8+ T cells also expressed significantly greater quantities of transcripts for Granzyme-B and T-bet relative to the amounts observed in those obtained from FcRn−/− mice (Fig. 5E and Fig. S4D). Given the monocyte-derived origin of CD8−CD11b++CD11c+ DCs, we ruled out a possible contribution of monocytic cells by demonstrating that peritoneal macrophages entirely lack the ability to induce FcRn-dependent cross-presentation (Fig. S4 E and F). Thus, FcRn-dependent cross-presentation of IgG ICs is carried out by monocyte-derived CD8−CD11b++CD11c+ DCs and leads to greater levels of cytotoxic CD8+ T-cell activation in vivo during the course of colitis.
Fig. 5.
FcRn-dependent cross-presentation of IgG ICs by CD8−CD11b++CD11c+ inflammatory DCs induces a proinflammatory T-cell response during chronic DSS colitis. (A) The MLN and LP of mice chronically treated with DSS contain a population of CD8−CD11b++CD11c+ inflammatory DCs. R1, CD8−CD11b++CD11c+; R2, CD8−CD11b+CD11c++; R3, CD8+CD11b−CD11c++. (B) Only FcRn-mediated cross-presentation by CD8−CD11b++CD11c+ DCs induces the production of proinflammatory IFNγ by CD8+ T cells. Sorted DCs were loaded with ICs and cocultured with OT-I T cells. (C) CD8−CD11b++CD11c+ DCs from DSS-treated FcRn−/− mice are deficient in their ability to induce proinflammatory cytokine production by CD8+ T cells in response to IgG ICs. (D and E) CD8+ T cells from the MLNs of chronically DSS-treated WT mice, but not those from FcRn−/− mice, secrete large quantities of IFNγ (D) and produce large quantities of Granzyme B and T-bet (E) upon anti-CD3/CD28 restimulation. Data are representative of at least two independent experiments with n = 5 per group. All data are mean ± SEM of triplicates. *P < 0.05; **P < 0.01.
Discussion
Despite existing literature documenting that antigens within IgG ICs are efficiently cross-presented after FcγR-mediated internalization (10–14), the intracellular mechanisms responsible for directing IgG-complexed antigen into cross-presentation-enabling compartments is not known. In the present study, we show that intracellular routing by FcRn after FcγR-mediated uptake is necessary for the cross-presentation of extremely low concentrations of IgG-complexed antigens by CD8−CD11b+ DCs.
FcRn is perfectly suited to facilitate cross-presentation because of several unique characteristics. These characteristics include the fact that the pH range in which FcRn binds its ligand, pH 5.0–6.5, is complementary to the relatively neutral pH binding range of FcγR on the cell surface. Together with the predominantly intracellular distribution of FcRn in DCs, this bias toward binding IgG at acidic pH means that FcRn is perfectly poised to bind IgG ICs as soon as they are released by the internalizing FcγR within acidic endosomes. Consistent with this hypothesis, our data demonstrate that the intracellular compartment within which FcRn functions in CD8−CD11b+ DCs is atypically acidic for a cross-presenting compartment but is one that would be predicted to permit IC interactions with FcRn. Our results therefore favor a pH-dependent relay model in which FcγR and FcRn act consecutively to shuttle IgG ICs from the cell surface into a cross-presentation compartment.
We have also shown that the acidic microenvironment of the phagosome not only stabilizes the IC–FcRn interaction but also slows the rate of antigen degradation and enables retention in a compartment equipped for cross-presentation. These latter points are a key feature of cross-presentation because antigenic epitopes require conservation for efficient loading onto MHC-I (7, 25). The intracellular compartment to which FcRn directs IgG ICs is enriched in Sec61α, TAP1, and MHC-I (9). The presence of these membrane-associated proteins within FcRn-expressing phagosomes containing multimeric IgG ICs, together with our data demonstrating their functional importance, indicate that FcRn-enabled cross-presentation occurs by reuptake of proteasomally generated peptides into a cross-presentation equipped vesicle for direct loading onto MHC-I molecules. Together, these studies show that FcRn not only functions to relay FcγR internalized IgG ICs to the appropriate intracellular locale but also, potentially, directly controls the recruitment of components necessary for optimized acidic degradation as well as phagosome-to-cytosol associated cross-presentation. The net effect of FcRn ligation in CD8−CD11b+ DCs may thus be to emulate for the immune-complexed antigen the mildly degradative conditions experienced by soluble antigens within CD8+CD11b− DCs.
A notable aspect of our study is that a role for FcRn in IgG IC-mediated cross-presentation is mainly demonstrable, especially at low antigen concentrations, within a subset of monocyte-derived CD8−CD11b+ DCs. Prior studies that have identified a role for these DCs in cross-presentation have examined instances of in vivo inflammation in the context of parasite- or adjuvant-induced disease (26–28). Under such circumstances, although not directly shown, it is highly likely that the observed effects were at least partially mediated by IgG-complexed antigen rather than strictly by soluble antigen, thereby implicating a role for FcRn. Most studies on cross-presentation to date have focused on the presentation of soluble antigen by CD8+CD11b− DCs. However, several of the very elements that enable this subset of DCs to efficiently cross-present such antigens may also limit FcRn from optimally participating in the cross-presentation of IgG IC-derived antigens in these DCs. Specifically, whereas the phagosomes of CD8−CD11b+ DCs are buffered at a highly acidic pH (≤5.5), which is optimal for FcRn function, the phagosomes within CD8+CD11b− DCs are buffered at near-neutral pH (6.5–7.0), a range at which FcRn exhibits minimal IgG binding (7). This finding may explain the relatively limited ability of CD8+CD11b− DCs to exhibit cross-presentation of antigen as an IC relative to that observed with soluble antigen. This result is in stark contrast to the very potent ability of CD8−CD11b+ DCs, which can achieve a high degree of acidification amenable to IgG–FcRn binding, to exhibit cross-presentation of antigen as a complex with IgG. We believe these data complement a previous study demonstrating cross-presentation of IgG ICs by CD8−CD11b+ DCs (11). The ICs used in the study by den Haan & Bevan (11) contained very high concentrations of antigen (250 μg/mL), whereas the antigen concentrations in our ICs were >2 orders of magnitude lower (0.5 μg/mL). Thus, it is likely that our findings reflect the specific behaviors of CD8+CD11b− and CD8−CD11b+ DCs at different points along a spectrum of IC antigen concentrations. Although this concept undoubtedly requires further study, the present data indicate that CD8−CD11b+ DCs are primarily involved in initiating immune responses as a consequence of cross-presentation under conditions of low-dose antigen, whereas CD8+CD11b− DCs are more potent in controlling such immune responses once they are underway and abundant antigen is present. Even so, the demonstrable level of proliferation detected with IgG ICs, but not IHH-IgG ICs, by the CD8+CD11b− DCs in our footpad assay supports a role for FcRn-dependent T-cell activation via cross-presentation at low antigen concentrations in this DC subset. Therefore, it is likely that cross-presentation of IgG ICs by both subsets of DCs is nearly entirely dependent upon the expression of FcRn, only with varying degrees of efficiency.
Importantly, we show that FcRn-mediated cross-presentation by CD8−CD11b+ DCs is critical for cross-priming of CD8+ T cells in vivo when antigen is provided as an IC with IgG and that during inflammation, the generation of CD8+ cytotoxic T cells is dependent upon FcRn expression by these DCs. Recently, FcRn in hematopoietic cells was shown to play a pathogenic role in colitis in the presence of flagellin containing IgG ICs (17, 18). Furthermore, FcRn has been implicated in the pathogenesis of several CD8+ T cell-dependent inflammatory diseases, including glomerular disease, arthritis, and myasthenia gravis (29–34). Given that FcRn-mediated cross-presentation by infiltrating monocyte-derived CD8−CD11b+ DCs likely operates at extremely low-dose ranges of antigen and that pathogen-, autoimmune-, and tumor-associated immune responses are typically associated with a humoral IgG response (35), our results indicate that these DCs have evolved the ability to use FcRn to be highly sensitive in the detection of antigen and to convert this stimulus into a functional CD8+ T-cell response. Thus, the knowledge that FcRn plays a critical role in cross-presentation significantly extends its importance to immunity beyond its previously identified roles in MHC-II-restricted antigen presentation, antibody protection, and transcellular transport of IgG (18, 19, 30).
In summary, our data reveal an important role for FcRn in the proper intracellular trafficking of IgG-derived ICs during cross-presentation. Our studies show that, in the absence of FcRn function, FcγR-dependent internalization of ICs is not able to elicit cross-presentation, indicating that these two molecules function in tandem and are cooperative. This role for FcRn is especially notable in CD8−CD11b+ DCs that are recruited to sites of inflammation, such that in the absence of FcRn function, the induction of CD8+ T-cell responses are disabled. Thus, although it is well-established that cross-presentation of antigen in the context of IgG ICs can be initiated by FcγR-mediated internalization, our data not only elucidate the intracellular mechanism by which this process proceeds but also demonstrate the necessity of FcRn for the induction of CD8+ T-cell responses during the course of inflammation.
Methods
Mice.
OT-I mice were obtained from The Jackson Laboratory. FcRn−/− mice on a C57BL/6 background, which are deficient in the Fcgrt gene, have been described (18). In all experiments, wild-type (Wt) mice and FcRn−/− mice were matched littermates. All procedures were approved by the Harvard Medical Area Standing Committee on Animals.
Antigen Presentation Assays.
ICs were preformed as indicated in SI Methods. DCs isolated as described in SI Methods were loaded with ICs or opsonized beads for 2–3 h and cocultured with primary CD8+ T cells isolated from naïve OT-I mice.
Isolation of Intracellular Compartments.
Phagosomes were prepared as described (SI Methods).
In Vivo T-Cell Proliferation.
Splenic CD8−CD11b+ and CD8+CD11b− DCs were sorted from spleens of donor mice, loaded with IgG or IHH-IgG ICs for 3 h, and injected into opposite hind footpads of Wt mice adoptively transferred with CFSE-labeled OT-I T cells, as outlined in Fig. S1C. For assessing DC migration and viability, DCs were stained with CFSE before transfer and for Annexin V and 7-AAD after isolation of the draining LN, as described in SI Methods.
Chronic DSS Colitis.
Chronic DSS colitis was induced by using a 2% DSS solution (36). On day 52, cells were isolated from the colonic LP and MLN as described (37). CD8−CD11b++CD11c+, CD8−CD11b+CD11c++, and CD8+CD11b−CD11c++ DC subsets were sorted, loaded with ICs, and cocultured with primary OT-I T cells. In separate experiments, CD8+ T cells were isolated and restimulated for 24 h with anti-CD3/anti-CD28. Supernatants were analyzed by using a Cytokine Bead Array (BD Pharmingen). RNA was analyzed by using a Nanostring assay (NanoString Technologies).
Statistical Analysis.
Statistical significance was assessed by two-tailed unpaired Student's t test.
Supplementary Material
Acknowledgments
We thank Dr. Sherie Morrison (University of California Los Angeles) for the J558L cells, Dr. Siew Yeen Chai (University of Melbourne, Australia) for the anti-IRAP antibody, and Dr. Glenn Dranoff (Dana–Farber Cancer Institute) for the Flt3L- and GM-CSF-secreting melanoma cell lines. This work was supported by the Canadian Institutes of Health Research (K.B.); National Institutes of Health Grants DK071798 (to T.T.K.), AI075037 (to E.F.), and DK53056 (to W.I.L. and R.S.B.); and the Harvard Digestive Diseases Center (National Institutes of Health Grant DK34854 to W.I.L. and R.S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019037108/-/DCSupplemental.
References
- 1.McCurley N, Mellman I. Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PLoS ONE. 2010;5:e11949. doi: 10.1371/journal.pone.0011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurts C, Robinson BWS, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 3.Bozzacco L, et al. HIV gag protein is efficiently cross-presented when targeted with an antibody towards the DEC-205 receptor in Flt3 ligand-mobilized murine DC. Eur J Immunol. 2010;40:36–46. doi: 10.1002/eji.200939748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 9.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 10.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin Immunol. 1999;11:385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 11.den Haan JMM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci USA. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnault A, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 15.Baker K, et al. Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Semin Immunopathol. 2009;31:223–236. doi: 10.1007/s00281-009-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi K, et al. An FcRn-dependent role for anti-flagellin immunoglobulin G in pathogenesis of colitis in mice. Gastroenterology. 2009;137:1746–1756. doi: 10.1053/j.gastro.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao SW, et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA. 2008;105:9337–9342. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claypool SM, et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mach N, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 21.Naik SH, et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 22.Savina A, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989;143:2595–2601. [PubMed] [Google Scholar]
- 24.Vijay-Kumar M, et al. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock KL, Farfán-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Borgne M, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci USA. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheong C, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akilesh S, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akilesh S, et al. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 2004;113:1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent A, et al. Determinant spreading and immune responses to acetylcholine receptors in myasthenia gravis. Immunol Rev. 1998;164:157–168. doi: 10.1111/j.1600-065x.1998.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 32.Kang YM, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178:5390–5398. doi: 10.4049/jimmunol.178.8.5390. [DOI] [PubMed] [Google Scholar]
- 34.Tackenberg B, et al. Expanded TCR Vbeta subsets of CD8(+) T-cells in late-onset myasthenia gravis: Novel parallels with thymoma patients. J Neuroimmunol. 2009;216:85–91. doi: 10.1016/j.jneuroim.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 36.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 37.Chang S-Y, et al. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180:4361–4365. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.