1. Discovery of KSHV miRNAs and introduction
Kaposi's sarcoma-associated herpesvirus (KSHV or human herpesvirus 8) is the agent associated with multiple human diseases including Kaposi's sarcoma (one of the most common AIDS-associated malignancies), primary effusion lymphoma and Multicentric Castleman's disease. Like other members of the herpesvirus family, KSHV exhibits two main transcriptional programs, the latent or the lytic program. Upon infection, the default latency program is established, during which the virus only expresses a handful of viral protein-encoding genes. An exciting discovery by Pfeffer et al [1] and built upon by others [2],[3], [4], [5] was first the discovery of multiple microRNAs (miRNAs) encoded by EBV and later KSHV, which are expressed during latency. This finding presented the possibility that KSHV could modulate host gene expression using viral miRNAs and by using miRNAs instead of viral proteins, the virus could avoid expressing additional proteins that could potentially be detected by the host immune system.
As viruses have borrowed protein-encoding genes from their hosts, we now have multiple examples of viruses utilizing the RNA interference pathway by expressing their own miRNAs. Recent reviews have wonderfully covered mechanisms of miRNA-mediated repression [6] and miRNAs of various viruses [7] [8] [9]. Here we will specifically focus on the miRNAs of KSHV, their expression patterns, target genes and functions. Viral miRNAs offer an attractive system to study the roles of miRNAs since one can utilize the ideal negative control, which is the uninfected cell that lacks any viral miRNAs. In addition, tools exist to express viral miRNAs in uninfected cells and inhibit viral miRNAs in infected cells. The combination of gain and loss of miRNA functions in relevant cell types is an asset for dissecting miRNA functions.
2. Sequence and expression analysis
Most miRNAs begin as an RNA-polymerase II transcript (this is true for the majority of both cellular and viral miRNAs). This transcript can encode for both protein and miRNAs or miRNAs alone. Along the miRNA biogenesis pathway, the primary miRNA is further processed into a hairpin structure called a pre-miRNA (around 65 nucleotides long). KSHV encodes 12 pre-miRNAs and like other organisms, these pre-miRNAs are processed into short double-stranded duplexes. Usually only one strand is incorporated into the RNA-induced silencing complex (RISC), where it can associate with a target mRNA and typically repress gene expression by stimulating mRNA degradation and/or inhibiting protein translation[10]. In some cases, both strands of the short double-stranded duplexes can be incorporated into the RISC complex. With sensitive sequencing methods, all 24 strands from the 12 pre-miRNAs can be detected. In addition, mature miRNAs from pre-miR-K12-10 can undergo an RNA editing event along with variations at the 5' end of the mature miRNA, leading to at least five different mature miRNAs arising from the pre-miR [1, 11] [12]. In total, at least 25 KSHV mature miRNAs have been detected by multiple groups.
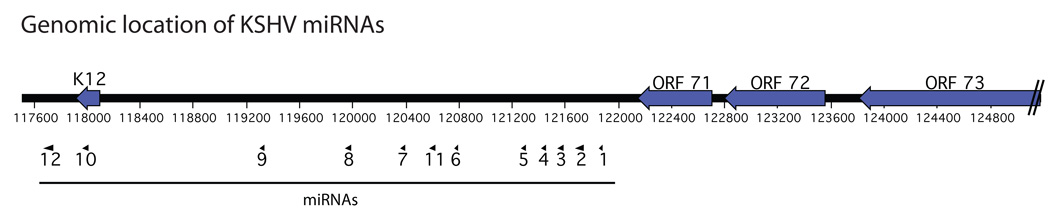
Similar to other herpesvirus miRNAs, KSHV miRNAs are clustered into a region known as the latency locus (Fig. 1). Within this locus, 10 of the 12 pre-miRNA hairpins are located in the intron of an mRNA encoding the latent gene K12/Kaposin. The remaining two pre-miRNAs are located in the protein-coding region and the 3' untranslated region (UTR) of K12/Kaposin gene. There is no evidence currently to suggest KSHV miRNAs have a dedicated promoter or are processed differently than human miRNAs. Others have previously described the limited sequence conservation between KSHV miRNAs and the related primate virus RRV [13]. There is however, a perfect seed homology (but limited overall sequence homology) between KSHV-K12-11 and human miR-155, and miR-K12-6-5p and human miR-15a and –16 [14] [15]. While, sequence conservation can be an effective tool to study cellular miRNAs, the limited conservation between viral miRNAs and the specific tropism of a given virus likely diminishes the utility of this approach in studying viral miRNAs.
Figure 1.
The genomic location of the KSHV major latency locus. Open reading frames are displayed as solid arrows. Pre-miRNAs are symbolized by triangles. The pre-miRNA labeled “1” corresponds to miR-K12-1 and so forth.
While most of the KSHV miRNAs originate from the same transcript, the levels of mature miRNAs are dramatically different. Deep sequencing data from a primary effusion lymphoma (PEL) cell line latently infected with KSHV showed over a five log difference in the frequency of sequence counts between different KSHV miRNAs expressed at the highest and lowest levels [16]. When sequencing small RNAs from a PEL cell line, a remarkable 92% of the combined viral and human miRNAs were KSHV miRNAs. While expression levels may be suggestive of functional relevance, levels of incorporation of miRNAs into RISC may likely be a better indicator of functional relevance. Other studies compared miRNA expression during latent and lytic infections in a B cell line and showed (along with others) that only pre-miRNAs miR-K12-10 and miR-K12-12 (with corresponding mature miRNAs) increased by significant amounts during lytic infection compared to latency [4, 17]. These recent deep sequencing studies also presented the addition complexities of mature miRNA sequences. Both studies revealed conserved 5' ends of the mature miRNAs, but variability of additional bases at the 3' end. Currently, it is unknown if this variability can affect the function of these miRNAs.
While all KSHV miRNAs are expressed during latency, they also detected during lytic replication. However, most cell culture models of lytic infection contain a mixture of latent and lytically infected cells. Therefore, detection of miRNAs could result from a number of sources. During the lytic phase, ORF37/SOX expression induces host-shutoff which dramatically reduces host gene expression [18]. Host-shutoff may have a more dramatic effect on host gene expression during lytic replication than miRNAs, but miRNAs may also prove to have specific functions during lytic replication.
Most of the miRNA expression studies have been focused on a limited number of PEL cell lines. However, one recent study examined the expression of pre-miRNAs using qPCR in multiple PEL cell lines and KS skin biopsies [19]. This study revealed some variability in pre-miRNA expression levels across different PEL cell lines and KS skin biopsies, but the greater differences were observed when comparing different models of infection, with KS biopsies and PEL cells showing higher levels of pre-miRNA expression compared to in vitro infection models. This pattern suggests a correlation of increased KSHV pre-miRNA expression with tumor formation, but it is unknown if the altered expression patterns are functionally significant. In addition, another group demonstrated overall in vivo sequence conservation among five KSHV PEL cell lines and seventeen clinical specimens [20]. This conservation was observed across disease states and geographical locations. Sequence conservation in this region could be a reflection of the biological importance of KSHV miRNAs and/or additional messages expressed in this region [21].
Recent sensitive sequencing approaches have discovered small RNAs encoded proximally to some viral miRNAs [11, 12]. The sequence reads were relatively low and displayed anti-sense orientation to a subset of the viral miRNAs. Expression of these small RNAs was primarily detected during the lytic cycle and their function is currently unknown. These small RNAs will likely need to associate with proteins (like siRNAs, miRNAs and lncRNAs) for functional roles. It should also be noted these studies utilized infected cell lines in culture. The expression of these small RNAs may be elevated by external stimuli only confronted in an organism.
3. Finding targets and functions
3.1 Target discovery methods
While the discovery of miRNAs has been extremely productive, progress to identify and validate targets of miRNAs has been challenging. A common method of identifying miRNA targets uses bioinformatics to search for mRNA sequences with complementary bases to the 5' end or seed region of the mature miRNA [22] [23–25]. This method can be employed quickly to search thousands of genes, but has multiple limitations. First, most of these programs search only the 3’UTR regions of potential targets. While genome-wide searches for miRNA targets show an enrichment for sites in the 3’UTR, multiple reports have demonstrated miRNA target sites within the coding region of a message. Second, the sequences in the 3’UTR database may not perfectly match the mRNA sequences found in cell types being tested in the laboratory. Third, multiple target genes are targeted through an imperfect seed-matching sequences. Given the small number of nucleotides in seed-matching sequences, seed-matching alone can predict many false positive targets. These methods have been innovated to utilize sequence conservation to improve target prediction. Given the very limited sequence conservation across viral miRNAs, this additional filter may have little benefit for viral miRNA target prediction. Consequently, other methods are being used to identify miRNA targets, including, microarrays to measure changes at the mRNA level [26] [15, 27] [28], measuring changes at the protein level [29], and purifying miRNAs and associated mRNAs, followed by sequence analysis [30] [31]. Each assay has inherent advantages and disadvantages depending on the mechanism of miRNA-induced gene repression. Those targets repressed by deadenylation will be reflected at the mRNA level while targets repressed by decreased translation efficiency will only be reflected at the protein level. Additionally, measuring steady-state mRNA or protein levels assumes miRNA-repressed genes will display altered levels at the specific time point tested. The ideal time point to assess repression may vary based on the expression and decay kinetics of a specific gene product. Biochemical purification of miRNA/mRNA complexes followed by deep sequencing can be difficult to interpret given the results reflect interactions from hundreds of miRNAs (from both the virus and the host cell). This often requires filtering the data for transcripts containing seed matching sequences, which leads to the limitations of bioinformatic approaches discussed above.
3.2 Viral targets of KSHV miRNAs
Given the fact that KSHV miRNAs are expressed during latency, it was hypothesized that the miRNAs could function to suppress lytic gene expression in order to maintain latency. One possible mechanism would be to repress expression of the viral latent-lytic switch protein RTA. Currently, three published reports describe roles of KSHV miRNAs in regulating RTA expression. Bellare et al. [32] systematically checked all of the KSHV miRNAs for their ability to modulate RTA expression. It was discovered miR-K12-9* was able to suppress expression of a luciferase reporter containing the RTA 3’UTR. Furthermore, inhibition of miR-K12-9* in latently infected cells increased lytic reactivation. The authors propose this as an additional mechanism for maintaining latency. While miRNAs from other viruses have been shown to target viral genes at a site with perfect complementarity [1, 33], targeting of RTA by a KSHV miRNA is a relatively rare example of a viral miRNA targeting a viral mRNA through imperfect complementary base pairing.
Following this study, another group sought to identify KSHV miRNAs with the ability to repress RTA. Using expression constructs to ectopically express each of the KSHV pre-miRNAs, the authors observed repression of a luciferase construct containing the RTA 3’UTR with both miR-K12-7 and miR-K12-9 [34]. In addition, endogenous RTA proteins levels were repressed by overexpressing miR-K12-7 and derepressed when cells were treated with an inhibitor to miR-K12-7-5p. Overexpression of miR-K12-7 also correlated with decreased production of virions. MiR-K12-7-5p is not abundantly expressed in KSHV-infected cells relative to other KSHV miRNAs and was not tested in the Bellare et al. report; however, overlapping results between the two reports suggest a role of miR-K12-9/9* in repression of RTA.
A third report [35], described in detail below, also investigated the effects of KSHV miRNAs on RTA expression. Using luciferase reporter assays and RT-PCR, miR-K12-5 (but not miR-K12-7) demonstrated a two-fold repression on endogenous RTA mRNA levels. At the moment, no consensus exists for the roles of specific KSHV miRNAs in regulating RTA and lytic reactivation. Evidence from deep sequencing assays revealed a PEL cell line, BC-3, does not express miR-K12-9 [11]. However, this cell line is able to maintain latency, correlating with the conclusion of Bellare et al. [32] that miR-K12-9 is not the major mechanism of maintaining latency. It should be stressed the effects on lytic replication by modulating KSHV miRNA activity are modest in all cases. In some cases, different methods of inactivation or ectopic expression of miRNAs were employed which could partially explain discrepancies. Antisense inhibitors to target endogenous miRNAs also do not extinguish all targeted miRNA activity. One possible solution to the controversy would be to measure lytic replication using a series of mutant forms of KSHV which have a single pre-miRNA deleted. Reversing the mutant phenotypes through ectopic expression of individual miRNAs will be essential to clarify the roles of miRNA in lytic replication.
3.3 Host targets of viral miRNAs
During the course of a chronic latent infection, one could imagine a role for KHSV miRNAs in protecting the infected cells from destruction and increasing proliferation in order to maintain and spread the viral infection. Determining if KSHV miRNAs alter these phenotypes would aid our understanding of pathogenesis mechanisms. The first major step down this path was the discovery that KSHV miRNAs target the human gene thrombospondin (THBS1), an angiogenesis inhibitor and regulator of cell-cell and cell-ECM interactions. Thrombospondin and other host genes were repressed in a microarray analysis to assess miRNA-induced changes in mRNA levels. Further evidence showed KSHV miRNAs could repress thrombospondin at the mRNA and protein level [27]. In agreement, others have previously shown by IHC staining that expression of thrombospondin was reduced in KS lesions [36]. These results suggest KSHV miRNAs could influence the angiogenesis hallmark of KS by repressing expression of a repressor of angiogenesis.
Another study using the microarray approach to identify human targets of KSHV miRNAs revealed BCL-2 associated factor (BCLAF1) as a target of at least four miRNAs (miR-K12-5, -9, -10a, and -10b) [28]. BCLAF1 was originally identified in a yeast two-hybrid screen for binding to adenovirus E1B and can also bind human bcl-2 [37]. While these four KSHV miRNAs can suppress caspase activity induced by a DNA damage drug, the miRNAs unexpectedly cannot protect primary endothelial cells from apoptosis, suggesting caspase activity is suppressed by viral miRNAs for a different reason. Inhibition of these four miRNAs correlated with decreased lytic reactivation and replication (also observed by Bellare et al. [32]). Knocking down BCLAF1 in latently infected cells increased lytic replication. Taken together, these miRNAs appear to have functions beneficial to viral replication.
As mentioned earlier, KSHV miR-K12-11 shares a seed sequence with human miR-155. These two miRNAs were shown to function as orthologs, as both can repress similar gene sets [14, 15] including BACH-1, which can act as a transcriptional activator or repressor. While the significance of BACH-1 in KSHV replication and pathogenesis was explored, previous studies have shown a correlation of miR-155 overexpression and certain lymphomas. Interestingly, multiple KSHV-infected B cell lines display decreased levels of miR-155, suggesting that expression of miR-K12-11 may be substituting for miR-155. During EBV infection human, miR-155 expression is induced and inhibition of miR-155 inhibits growth of EBV-transformed cells [38]. An exciting role for a miR-155 viral ortholog, miR-M4 (expressed by Marek's disease virus), in lymphomagenesis in chickens was recently described [39]. Taken together, miR-155 and viral orthologs have important roles in pathogenesis.
BACH-1 repression by miR-K12-11 also correlates with increased expression of an amino acid transport protein, xCT, which enables KSHV dissemination by cell fusion [40]. A search for miRNAs that repress xCT using a sequence-based bioinformatics method predicted xCT could be targeted by multiple KSHV miRNAs. Validation assays show miR-K12-11 can repress BACH-1 and, as a result, increase xCT expression. In murine macrophages, upregulation of xCT by miRNAs increased the number of infected cells after de novo infection. This increase in infection rate was not observed in endothelial cells, but an increase in KSHV episomes per cell was observed when the miRNA cluster was expressed prior to infection. Additional data suggests KSHV miRNAs can protect cells from reactive nitrogen species-induced apoptosis.
Earlier discoveries established a link between a HCMV miRNA and MHC class I polypeptide-related sequence B (MICB) expression. A bioinformatics search was initiated to look for KSHV and EBV miRNAs that also target MICB [41]. Flow analysis of 293 cells transfected with KSHV miRNAs revealed miR-K12-7 inhibited MICB protein expression, but mRNA levels were not significantly changed. MICB is a surface marker that can be upregulated by viral infection and recognized by NK cells. The authors demonstrate cells expressing miR-K12-7 were partially protected from NK killing. Importantly, these combined results show HCMV, EBV and KSHV use different miRNA sequences to independently repress MICB. It is expected that more examples of convergent evolution will be discovered in future studies.
Another example of miRNAs functioning in immune defense comes from the identification of tumor necrosis factor receptor superfamily, member 12A (TNFRSF12A/TWEAKR/Fn14) as a target of miR-K12-10a [42]. This host gene was identified by mRNA expression profiling of B cells and endothelial cells following gain or loss of miRNA function. Two mature KSHV miRNAs (miR-K12-10a and miR-K12-10b) are expressed by the miR-K12-10 pre-miRNA due to an RNA editing event, which causes a single nucleotide difference in the seed region of the mature miRNA. Luciferase and Western blot assays demonstrated miR-K12-10a, but not miR-K12-10b, can repress TNFRSF12A expression, further emphasizing the target specificity of miRNAs. Previous literature suggested TNFRSF12A plays a role in apoptosis and the proinflammatory response. Multiple assays revealed miR-K12-10a repression of TNFRSF12A protects cells from cytokine-induced apoptosis and suppresses a proinflammatory cytokine response induced by TNFSF12/TWEAK.
KSHV has previously been shown to modulate expression of multiple cytokines as well as encoding a viral IL-6 homolog. Thus, modulation of external signals might be expected as a function of viral miRNAs during latency. The first example of KSHV miRNAs affecting human cytokine expression came from studies showing miRNAs could induce IL-6 and IL-10 expression in murine macrophages [43]. A bioinformatics sequence analysis revealed this was likely due to viral miRNA-mediated repression of C/EBPβ p20, a known repressor of these cytokines. Together, these miRNAs could be partially responsible for the elevated levels of IL-6 and IL-10 observed during KSHV infections.
In addition to cytokine modulation, miRNAs were hypothesized to play a role in increasing proliferation and survival of latently infected cells. One group discovered multiple sequences that could be targeted by KSHV miR-K12-1 within the 3’UTR of the cyclin-dependent kinase inhibitor p21 (CDKN1A) [44]. Inhibition of miR-K12-1 in PEL cells resulted in increased cell cycle arrest following activation of p53. This represents a strategy of the virus to avoid p53-induced cell cycle arrest.
As with other viral mechanisms of host interactions, viral miRNAs display redundancy by utilizing multiple miRNAs to target an individual host gene for repression. This redundancy creates an experimental challenge when attempting to detect significant phenotypic changes upon inhibition of a single miRNA. Recently, two groups have overcome this challenge by creating mutant versions of KSHV in which 10 of the 12 pre-miRNAs are deleted. One study investigated the role of the miRNAs in viral replication. Using a mutant form of KSHV that lacks the region encoding all pre-miRNAs except miR-K12-10 and miR-K12-12, they observed an increase in lytic protein expression in 293T cells compared to the wildtype virus. This phenotype was suppressed when the deleted miRNAs were expressed ectopically. A previous study suggested vFLIP limits lytic replication by increasing NF-κB activity [45] and this finding motivated others to determine the effects of miRNA expression on NF-κB activity. In fact, NF-κB activity was diminished in cells transfected with a mutant KSHV genome lacking miRNAs compared to wildtype [46]. An analysis of IKK members revealed that miR-K12-1 directly inhibits NFKBIA/IKBalpha, thus increasing NF-κB activity and inhibiting lytic replication.
Another study utilizing a similar but independently developed mutant virus lacking miRNAs assessed changes in viral replication as well. This report also showed an increase in viral lytic gene expression, including RTA, during infection with the mutant virus, however the intracellular amounts of viral DNA were not changed [35]. Measurement of RTA mRNA and luciferase assays suggested miR-K12-5 can suppress RTA expression. Bioinformatics approaches did not predict a direct interaction of miR-K12-5 with the 3’UTR of RTA. Therefore, the authors hypothesized that viral miRNAs may be controlling RTA expression by modifying chromatin modifications. A genome-wide analysis revealed less histone H3 K9 trimethylation in certain regions of the viral genome and decreased levels of DNA methylation across the viral and human genome in cells infected with the KSHV mutant. Previous studies proposed a miRNA could affect global DNA methylation by inhibiting a transcriptional repressor retinoblastoma-like 2 (Rbl2), which can control expression of DNA methylase transferase 3a and 3b. Using bioinformatics, a link between KSHV miR-K12-4-5p and Rbl2 was established. Expressing miR-K12-4-5p in the context of the miRNA deletion mutant virus increased DNA methylation. Overall, this report provided evidence for KSHV miRNAs influencing RTA expression, histone methylation and genome-wide DNA methylation.
While a genetic miRNA deletion mutant is a more effective way to inhibit miRNA functions than antisense inhibitors or sponges, studies involving mutant viruses are currently restricted to 293T cells. The fact that 293T cells infected with recombinant KSHV r.219 show dramatically more lytic gene expression than during infection in other cell types may complicate results and interpretations [47]. In the future, it will be important to assess changes the latent/lytic balance in relevant cell types for KSHV infection.
A recent report also investigated the potential roles of KSHV miRNAs to affect the switch between latent and lytic infection. In this study, a KSHV-infected PEL cell line was transduced to overexpress individual KSHV miRNAs and levels of RTA expression were analyzed [48]. It was observed that miR-K12-3 could repress RTA expression and decrease the amount of viral DNA in both unstimulated and lytically reactivated cells. The authors used a bioinformatics program to predict potential host targets of miR-K12-3 and identified nuclear factor I/B (NFIB, a transcription factor), which was previously found to promote KSHV reactivation in a genome-wide screen. Luciferase, mRNA and protein assays supported a role of miR-K12-3 in repressing NFIB expression, while studies using short-hairpin RNA-mediated knock-down of NFIB confirmed the resulting reduction in RTA expression and viral DNA replication. Several potential NFIB binding sites were observed in the RTA promoter and NFIB can modestly activate a RTA promoter luciferase reporter. Taken together, a model where miR-K12-3 represses NFIB and indirectly repress RTA expression to maintain latency is proposed.
An additional KSHV miRNA target influencing NF-κB signaling was discovered using a bioinformatics approach [49]. This analysis identified I-kappa-B kinase epsilon (IKBKE) as a predicted target of miR-K12-11. Using an immunofluorescent assay with KSHV-infected PEL cells, it was shown that cells transduced with a sponge miRNA inhibitor to miR-K12-11 had higher expression of I-kappa-B kinase epsilon (IKBKE) compared to control cells. Similar results were obtained from miRNA inhibitor studies after de novo KSHV infection of a lung cancer cell line. Since IKBKE influences the interferon pathway, it was determined lung cancer cells transfected with miR-K12-11 expressed less IFNA1, IFNA2, IFNB and IFNA14 after infection with Sendai virus. Other anti-viral interferon-stimulated genes were also repressed in the presence of miR-K12-11. Finally, the combination of VSV infection and a sponge miRNA inhibitor to miR-K12-11 resulted in higher levels of RTA, suggesting a role of miR-K12-11 in maintaining KSHV latency.
Previous studies have shown KSHV infection of lymphatic endothelial cells (LEC) leads to expression of blood endothelial cell (BEC) markers. The converse is also true, and this results in KSHV-infected cells expressing a mix of LEC and BEC markers. One study [50] tested whether miRNAs could play a role in this process. Using mRNA expression profiling of LECs expressing a cluster of KSHV miRNAs, it was discovered musculoaponeurotic fibrosarcoma oncogene homology (MAF) was repressed by KSHV miRNAs and also during infection. Gene expression analysis also revealed increased expression of BEC markers when MAF was repressed by KSHV miRNAs, suggesting that miRNAs play a role in altering cell-specific expression profiles.
A relatively new method to identify miRNA target genes involves immunoprecipitation of RNA-induced silencing complexes (RISC) to biochemically purify miRNAs and associated mRNAs. This method was used in a KSHV-infected PEL cell line or uninfected cells transduced to stably express 10 of the 12 pre-miRNAs [31]. Potential host targets were identified (n=114) and these displayed an enrichment for genes involved in regulation of gene expression, spliceosomes, and nuclear import. Six targets were randomly selected for further validation (NHP2L1, LRRC8D, EXOC6, GEMIN8, ZNF684, and CDK5RAP1) and all six were validated with luciferase assays. It is important to note this method was able to identify two targets that did not have responsive elements in their 3'UTR, but rather the elements targeted by the miRNAs were in the coding sequence of the transcript, which has been observed with other miRNAs. Therefore, these targets would not have been identified using target prediction programs which largely query only the 3'UTR.
4. Relationships among host targets
Currently, the validated targets of KSHV miRNAs (Table 1) represent a collection of genes identified by unbiased methods or chosen by previously identified functions. While this list contains targets that were subjectively studied because of roles in certain processes, it may be helpful to inspect the list for emerging themes among the targets. One method analyzes gene ontology terms enriched in the list of KSHV miRNA targets (Table 2), which reveals an enrichment of gene products involved in apoptosis (25% of the list) and gene regulation (55% of the list). The significance of gene ontology term enrichment is very limited since the target genes were chosen subjectively in many cases and the list of validated target genes is quite small.
Table 1. Validated targets of KSHV miRNAs.
KSHV miRNA validated targets. All targets have been validated using ectopic expression of miRNAs to demonstrate that a luciferase reporter or ectopic reporter are repressed. Additional validations include (1) exogenous reporters have been mutated to disrupt targeting by a ectopic miRNA, (2) ectopic miRNA suppresses endogenous target mRNA and/or protein levels, (3) de novo infection with KSHV represses endogenous target expression, (4) miRNA inhibitors and/or mutant virus display derepression of endogenous target mRNA and/or protein levels, and (5) repression of miRNA target is observed in KSHV clinical samples.
| Target | miRNA(s) | Prediction | Validations | Proposed Consequence | Reference |
|---|---|---|---|---|---|
| MICB | miR-K12-7 | bioinformatics | 1,2,4 | Immune evasion | Nachmani et al., 2009 |
| CEBPB | miR-K12-3,-7 | bioinformatics | 2 | Cytokine secretion | Qin et al., 2010 |
| CDKN1A/p21 | miR-K12-1 | bioinformatics | 1,2,4 | Suppress growth arrest | Gottwein and Cullen, 2010 |
| NFKBIA | miR-K12-1 | bioinformatics | 1,2,3,4 | NF-κB activation, maintain latency | Lei et al., 2010 |
| RBL2 | miR-K12-4-5p | bioinformatics | 1,2 | DNA methylation alterations | Lu et al., 2010b |
| IKBKE | miR-K12-11 | bioinformatics | 1,2,3,4 | Suppress interferon response | Liang et al., 2011 |
| NFIB | miR-K12-3 | bioinformatics | 1 | Maintain latency | Lu et al., 2010a |
| THBS1 | miR-K12-1,-3-3p,-6-3p,-11 | mRNA profiling | 1,2,5 | Angiogensis | Samols et al., 2007 |
| BCLAF1 | miR-K12-5,-9,-10a,-10b | mRNA profiling | 1,2,3,4 | Viral replication, Caspase inhibition | Ziegelbauer et al., 2009 |
| BACH1 | miR-K12-11 | mRNA profiling | 1,2 | Skalsky et al., 2007; Gottwein et al., 2007 | |
| TNFRSF12A/TWEAKR | miR-K12-10a | mRNA profiling | 1,2,3,4,5 | Anti-apoptosis, anti-inflammation | Abend et al., 2010 |
| MAF1 | miR-K12-1,-6-5p,-11 | mRNA profiling | 1,2,3,4 | Cell type differentiation | Hansen et al., 2010 |
| NHP2L1 | miR-K12-3 | RISC precipitation | 1 | Dolken et al., 2010 | |
| LRRC8D | miR-K12-3, intronic cluster | RISC precipitation | 1 | Dolken et al., 2010 | |
| EXOC6/SEC15L | miR-K12 intronic cluster | RISC precipitation | Dolken et al., 2010 | ||
| GEMIN8 | miR-K12-4-3p | RISC precipitation | 1,2 | Dolken et al., 2010 | |
| ZNF684 | miR-K12 intronic cluster | RISC precipitation | Dolken et al., 2010 | ||
| CDK5RAP1 | miR-K12 intronic cluster | RISC precipitation | Dolken et al., 2010 | ||
| KSHV ORF50/RTA | miR-K12-9* | Speculation/systematic testing | 1,4 | Maintain latency | Bellare and Ganem, 2009 |
| KSHV ORF50/RTA | miR-K12-7-5p | Speculation/systematic testing | 1,2,4 | Maintain latency | Lin et al., 2011 |
| KSHV ORF50/RTA | miR-K12-5 | Speculation/systematic testing | 2 | Maintain latency | Lu et al., 2010b |
Table 2. Enriched biological processes of targets of KSHV miRNAs.
The gene ontology biological processes enriched among KSHV miRNA human targets in Table 1. The percent refers to the percentage of targets with the corresponding gene ontology term. The p-values reflect the probability of randomly selecting the same GO term given the specific number of gene products in the input list using a hypergeometric distribution[57]. This is influenced by all of the GO terms and gene products in the MetaCore database. Analysis was performed using MetaCore from GeneGo.
| Process | Percent of targets | p-Value |
|---|---|---|
| induction of apoptosis | 25 | 4.57E-05 |
| induction of programmed cell death | 25 | 4.76E-05 |
| gene expression | 55 | 9.15E-05 |
| cellular macromolecule metabolic process | 70 | 1.05E-04 |
| negative regulation of cyclin-dependent protein | 10 | 1.10E-04 |
| kinase activity | ||
| nucleic acid metabolic process | 55 | 1.13E-04 |
| positive regulation of apoptosis | 25 | 3.24E-04 |
| macromolecule metabolic process | 70 | 3.33E-04 |
| positive regulation of programmed cell death | 25 | 3.38E-04 |
| positive regulation of cell death | 25 | 3.63E-04 |
| cellular process | 95 | 4.65E-04 |
| nucleobase, nucleoside, nucleotide and nucleic | 55 | 5.27E-04 |
| acid metabolic process |
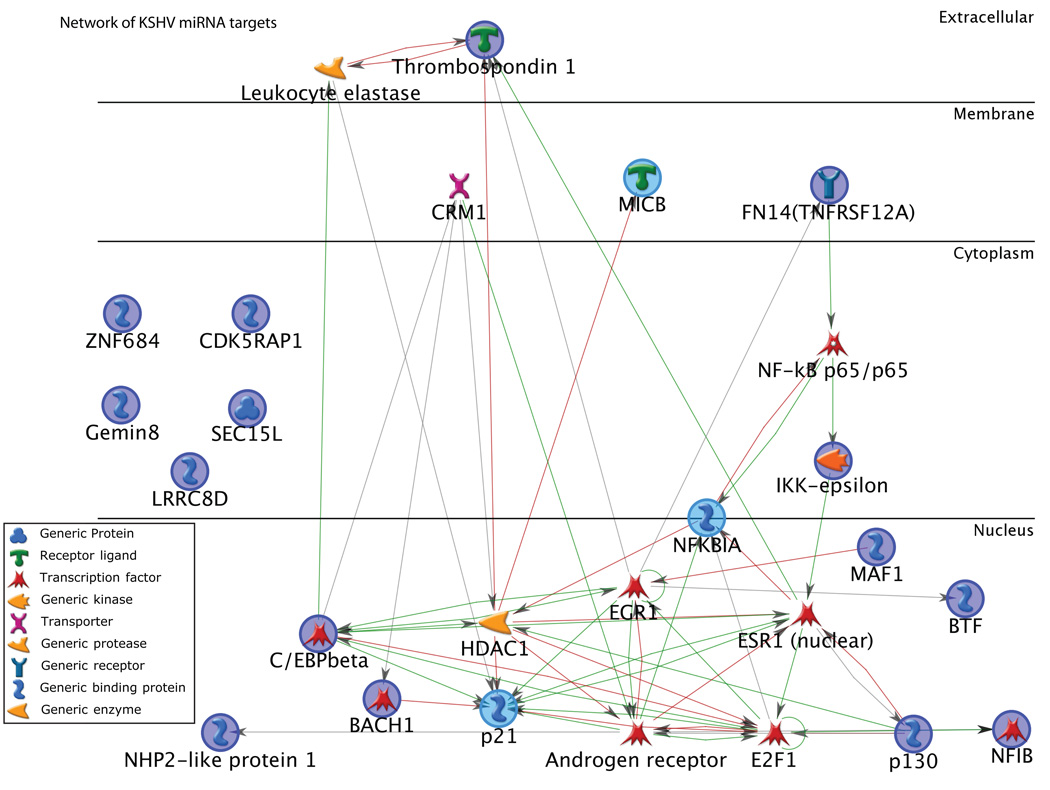
Many reports have focused on the functional significance of a single target gene, but understanding connections between validated targets may foster a deeper understanding of the network disturbances by KSHV miRNAs. Figure 2 shows known direct and indirect interactions between validated targets. The network highlights previous studies demonstrating both C/EBPbeta and BACH1 can transcriptionally regulate p21. In addition, six miRNA targets interact with histone deacetylase 1 (HDAC1), which has roles in cellular proliferation and differentiation. A tumor suppressor factor, early growth response (EGR1) transcription factor, also interacts with six miRNA targets and EGR1 has been shown to control RTA expression in infected cells [51]. This analysis highlights proteins that are connected directly and closely indirectly to miRNA target gene products. The network in Figure 2 also displays an enrichment of interactions within the network compared to random proteins (the clustering coefficient is four times higher than random networks of the same size [52]). As the number of validated targets increases, these types of analyses will become more informative to the global cellular changes that occur during KSHV infection.
Figure 2.
Network of KSHV miRNA human targets. The network [58] was started using the list of validated miRNA human targets and Dijkstra's shortest path algorithm [59] with a maximum of two steps in between gene products[60]. Interactions between gene products come from manually curated interactions described in the literature. Validated miRNA targets are highlighted by colored circles. Activation interactions are shown by green arrows, repression by red arrows, and unspecified are shown in gray. Analysis was performed using MetaCore from GeneGo.
5. Conclusions and future issues
While many studies have found interesting functions mediated by a single miRNA repressing a single host gene, others show multiple miRNAs can target the same gene. Complex gene regulatory networks, similar to transcription regulatory networks, are emerging. In order to study these networks, it is likely we will need to express or repress multiple miRNAs simultaneously and to validate these phenotypes by expressing or inhibiting multiple host or viral genes. Since miRNAs may target hundreds of genes, the resulting phenotypes may not be phenocopied by repressing a single target. In many mammalian studies, deletion of a single miRNA frequently displays little or no phenotypic difference from the wild type animal [53]. Furthermore, deletion of RNA machinery in embryonic stem cells which suppresses the expression of hundreds of miRNAs, results in fully viable cells [54]. This suggests miRNAs are not essential to maintain cell viability. Similarly, deleting 10 of the 12 KSHV pre-miRNAs did not render the virus incapable of replication. Rather, miRNAs appear to influence a response to external stimuli or act in combination with canonical pathway members. Thus, these masked functions of miRNAs may only be revealed by studies performed in the appropriate background or conditions.
Many reports are focused on a single mRNA target of a miRNA after using bioinformatics methods, but it should be noted most target prediction programs predict dozens of miRNA targets while experimental limitations force the focus on a single gene. As other predicted targets are validated, it will be interesting to understand the functional contribution of additional targets to specific phenotypes. Deep sequencing data from RISC immunoprecipitations studies will be useful in determining whether viral miRNAs target mRNA sequences that are conserved among mammals. Since KSHV only naturally infects humans, one might assume this sequence conservation is not a high priority for the virus. However, one could also imagine viral miRNAs targeting conserved regions of mRNAs since these might reflect desirable traits, such as target site accessibility, when compared to other regions of a specific transcript. As more targets are validated, we may be able to use examples of convergent evolution among viral miRNAs to aid in deciphering the functions of KSHV miRNAs.
A number of studies have independently demonstrated a wide range of expression levels across the KSHV miRNAs. At this point, we do not understand the functional importance of this differential expression. Since the miRNA-mediated repression models suggest at least one RISC interaction per mRNA, abundant miRNAs may be better suited to target abundant mRNAs or a greater number of different mRNAs. It is also curious that some mature miRNAs display enormous differences in expression despite originating from the same pri-miRNA. Would this suggest consequential mechanisms of differential miRNA biogenesis regulation? How is this maturation modified by host signals in the microenvironment of infection?
While it is convenient to imagine the mature miRNAs as stable identical molecules integrated in RISC, new complexities are emerging. Some miRNAs display chemical modifications [55], KSHV miRNA mature sequences show variability primarily at the 3’ end of the miRNA [16], and EBV miRNAs can be transported intercellularly via exosomes [56]. Are KSHV miRNAs chemically modified and how does this alter stability and targeting? Is repression of target genes modified by nucleotide variability at the 3’ end? Are KSHV miRNAs also transported via exosomes?
While most KSHV miRNA studies have been conducted in various cell culture systems, it will important to assess the functions of miRNAs in animal models and at sites of infection in humans. Since KSHV is associated with different diseases, it is assumed KSHV miRNAs will have different targets and functions depending on the context of infection. Given the reports showing the role of KSHV miRNAs in maintaining latency, how is this barrier to the lytic cycle overcome during infection? Since the seed regions of mature miRNAs appear to be well-conserved, could we target viral miRNAs for inhibition and effectively deliver these inhibitors? Many questions remain as we understand this newly discovered mechanism of gene regulation by viral miRNAs and apply the lessons learned.
Highlights.
> KSHV expresses 12 pre-microRNAs during latent and lytic infection. > Methods to identify microRNAs targets include: microarrays, sequence comparison of microRNAs and mRNAs, and immunoprecipitation of miRNA:mRNA complexes. > Themes of cellular targets targeted by KSHV miRNAs include: cell cycle regulation, innate immunity, and apoptosis.
Acknowledgements
Thank you to J. Abend and P. Kieffer-Kwon for comments on the manuscript. Research is supported by intramural funds from NCI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;vol. 304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grässer FA, van Dyk LF, Ho CK, Shuman S, Chien M, Russo JJ, Ju J, Randall G, Lindenbach BD, Rice CM, Simon V, Ho DD, Zavolan M, Tuschl T. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;vol. 2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci USA. 2005;vol. 102:5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J Virol. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;vol. 12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipowicz, Bhattacharyya, Sonenberg Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008 doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 7.Umbach JL, Cullen BR. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 2009;vol. 23:1151–1164. doi: 10.1101/gad.1793309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boss I, Plaisance K, Renne R. Role of virus-encoded microRNAs in herpesvirus biology. Trends Microbiol. 2009 doi: 10.1016/j.tim.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundhoff A, Sullivan CS. Virus-encoded microRNAs. Virology. 2011 doi: 10.1016/j.virol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Umbach JL, Cullen BR. In-depth analysis of Kaposi's sarcoma-associated herpesvirus microRNA expression provides insights into the mammalian microRNA-processing machinery. J Virol. 2010;84:695–703. doi: 10.1128/JVI.02013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YT, Kincaid RP, Arasappan D, Dowd SE, Hunicke-Smith SP, Sullivan CS. Small RNA profiling reveals antisense transcription throughout the KSHV genome and novel small RNAs. RNA. 2010;16:1540–1558. doi: 10.1261/rna.1967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walz N, Christalla T, Tessmer U, Grundhoff A. A global analysis of evolutionary conservation among known and predicted gammaherpesvirus microRNAs. J Virol. 2010;84:716–728. doi: 10.1128/JVI.01302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skalsky, Samols, Plaisance, Boss, Riva, Lopez, Baker, Renne Kaposi's Sarcoma-associated Herpesvirus Encodes an Ortholog of miR-155. J Virol. 2007 doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umbach J, Cullen B. In-depth analysis of KSHV microRNA expression provides insights into the mammalian microRNA processing machinery. J Virol. 2009 doi: 10.1128/JVI.02013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y-T, Kincaid RP, Arasappan D, Dowd SE, Hunicke-Smith SP, Sullivan CS. Small RNA profiling reveals antisense transcription throughout the KSHV genome and novel small RNAs. RNA. 2010 doi: 10.1261/rna.1967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell. 2004;13:713–723. doi: 10.1016/s1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 19.O'Hara AJ, Chugh P, Wang L, Netto EM, Luz E, Harrington WJ, Dezube BJ, Damania B, Dittmer DP. Pre-micro RNA signatures delineate stages of endothelial cell transformation in Kaposi sarcoma. PLoS Pathog. 2009;vol. 5:e1000389. doi: 10.1371/journal.ppat.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, Wyvil KM, Aleman K, Little RF, Yarchoan R, Renne R, Whitby D. Conservation of virally encoded microRNAs in Kaposi sarcoma--associated herpesvirus in primary effusion lymphoma cell lines and in patients with Kaposi sarcoma or multicentric Castleman disease. J Infect Dis. 2007;vol. 195:645–659. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- 21.Chandriani S, Xu Y, Ganem D. The lytic transcriptome of Kaposi's sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes. J Virol. 2010;84:7934–7942. doi: 10.1128/JVI.00645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimson, Farh, Johnston, Garrett-Engele, Lim, Bartel MicroRNA Targeting Specificity in Mammals: Determinants beyond Seed Pairing. Mol Cell. 2007;vol. 27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue D, Liu H, Huang Y. Survey of Computational Algorithms for MicroRNA Target Prediction. Curr Genomics. 2009;10:478–492. doi: 10.2174/138920209789208219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;vol. 37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 25.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;vol. 5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;vol. 433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 27.Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, Renne R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007;vol. 3:e65. doi: 10.1371/journal.ppat.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat Genet. 2009;vol. 41:130–134. doi: 10.1038/ng.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grosshans H, Filipowicz W. Proteomics joins the search for microRNA targets. Cell. 2008;vol. 134:560–562. doi: 10.1016/j.cell.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Orom UA, Lund AH. Isolation of microRNA targets using biotinylated synthetic microRNAs. Methods. 2007;43:162–165. doi: 10.1016/j.ymeth.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Dolken L, Malterer G, Erhard F, Kothe S, Friedel CC, Suffert G, Marcinowski L, Motsch N, Barth S, Beitzinger M, Lieber D, Bailer SM, Hoffmann R, Ruzsics Z, Kremmer E, Pfeffer S, Zimmer R, Koszinowski UH, Grasser F, Meister G, Haas J. Systematic analysis of viral and cellular microRNA targets in cells latently infected with human gamma-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe. 2010;7:324–334. doi: 10.1016/j.chom.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Bellare P, Ganem D. Regulation of KSHV lytic switch protein expression by a virus-encoded microRNA: an evolutionary adaptation that fine-tunes lytic reactivation. Cell Host Microbe. 2009;vol. 6:570–575. doi: 10.1016/j.chom.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo GJ, Fink LH, O'Hara B, Atwood WJ, Sullivan CS. Evolutionarily conserved function of a viral microRNA. J Virol. 2008;82:9823–9828. doi: 10.1128/JVI.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin X, Liang D, He Z, Deng Q, Robertson ES, Lan K. miR-K12-7-5p Encoded by Kaposi's Sarcoma-Associated Herpesvirus Stabilizes the Latent State by Targeting Viral ORF50/RTA. PLoS One. 2011;6:e16224. doi: 10.1371/journal.pone.0016224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic Regulation of KSHV Latency by Viral-Encoded MicroRNAs that Target Rta and the Cellular Rbl2-DNMT Pathway. J Virol. 2010 doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraboletti G, Benelli R, Borsotti P, Rusnati M, Presta M, Giavazzi R, Ruco L, Albini A. Thrombospondin-1 inhibits Kaposi's sarcoma (KS) cell and HIV-1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J Pathol. 1999;188:76–81. doi: 10.1002/(SICI)1096-9896(199905)188:1<76::AID-PATH312>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;vol. 19:4390–4404. doi: 10.1128/mcb.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J Virol. 2010;84:11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek's disease lymphomas. PLoS Pathog. 2011;7:e1001305. doi: 10.1371/journal.ppat.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress. PLoS Pathog. 2010;vol. 6:e1000742. doi: 10.1371/journal.ppat.1000742. EP - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse Herpesvirus MicroRNAs Target the Stress-Induced Immune Ligand MICB to Escape Recognition by Natural Killer Cells. Cell Host Microbe. 2009;vol. 5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Abend JR, Uldrick T, Ziegelbauer JM. Regulation of TWEAKR expression by KSHV microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression. J Virol. 2010 doi: 10.1128/JVI.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin Z, Kearney P, Plaisance K, Parsons C. Pivotal Advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. Journal of Leukocyte Biology. 2009 doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottwein E, Cullen BR. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J Virol. 2010;84:5229–5237. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye FC, Zhou FC, Xie JP, Kang T, Greene W, Kuhne K, Lei XF, Li QH, Gao SJ. Kaposi's sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-kappaB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J Virol. 2008;82:4235–4249. doi: 10.1128/JVI.02370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei X, Bai Z, Ye F, Xie J, Kim C-G, Huang Y, Gao S-J. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol. 2010 doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira J, O'Hearn PM. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology. 2004;vol. 325:225–240. doi: 10.1016/j.virol.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 48.Lu C-C, Li Z, Chu C-Y, Feng J, Feng J, Sun R, Rana TM. MicroRNAs encoded by Kaposi's sarcoma-associated herpesvirus regulate viral life cycle. EMBO Rep. 2010 doi: 10.1038/embor.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang D, Gao Y, Lin X, He Z, Zhao Q, Deng Q, Lan K. A human herpesvirus miRNA attenuates interferon signaling and contributes to maintenance of viral latency by targeting IKKvarepsilon. Cell Res. 2011 doi: 10.1038/cr.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen A, Henderson S, Lagos D, Nikitenko L, Coulter E, Roberts S, Gratrix F, Plaisance K, Renne R, Bower M, Kellam P, Boshoff C. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010;vol. 24:195–205. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyson OF, Traylen CM, Akula SM. Cell membrane-bound Kaposi's sarcoma-associated herpesvirus-encoded glycoprotein B promotes virus latency by regulating expression of cellular Egr-1. J Biol Chem. 2010;285:37491–37502. doi: 10.1074/jbc.M110.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park CY, Choi YS, McManus MT. Analysis of microRNA knockouts in mice. Hum Mol Genet. 2010;19:R169–R175. doi: 10.1093/hmg/ddq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese JM, Seila AC, Yeo GW, Sharp PA. RNA sequence analysis defines Dicer's role in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:18097–18102. doi: 10.1073/pnas.0709193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu B, Chen X. Analysis of miRNA Modifications. Methods Mol Biol. 2010;592:137–148. doi: 10.1007/978-1-60327-005-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GeneGo p-value calculations. https://portal.genego.com/help/P-value_calculations.pdf.
- 58.Network building algorithms. https://portal.genego.com/help2/MetaCore/baggage/Network_building_algorithms.pdf.
- 59.Dijkstra EW. A note on two problems in connexion with graphs. Numerische Mathematik. 1959;1:269–271. [Google Scholar]
- 60.Ekins S, Bugrim A, Brovold L, Kirillov E, Nikolsky Y, Rakhmatulin E, Sorokina S, Ryabov A, Serebryiskaya T, Melnikov A, Metz J, Nikolskaya T. Algorithms for network analysis in systems-ADME/Tox using the MetaCore and MetaDrug platforms. Xenobiotica. 2006;36:877–901. doi: 10.1080/00498250600861660. [DOI] [PubMed] [Google Scholar]