Abstract
Summary
We report the primary analysis of the safety and efficacy of the MRKad5 gag/pol/nef HIV-1 sub-type B vaccine in South Africa (SA), where the major circulating clade is sub-type C.
Methods
This phase IIb double-blind, randomized test-of-concept study was conducted in sexually active HIV-1 sero-negative participants in SA. The co-primary endpoints were a vaccine-induced reduction in HIV-1 acquisition or viral-load setpoint. These were assessed independently in the modified intent-to-treat (MITT) cohort with two-tailed significance tests stratified by gender. Immunogenicity was assessed by interferon-gamma (IFNγ) ELISPOT in peripheral blood mononuclear cells. Following the lack of efficacy of the MRKAd5 HIV-1 vaccine in the Step study, enrollment and vaccination in this study was halted, treatment unblinding occurred and follow-up continued. This study is registered with the SA National Health Research Database (DOH-27-0207-1539) and ClinicalTrials.gov (NCT00413725).
Results
801 of a scheduled 3000 participants were enrolled, of whom 360 (44.9%) were women, more than half (55.6%) had Ad5 titres > 200, and almost a third (29.3%) of men were circumcised. 62 MITT participants were diagnosed with HIV-1, 34 in the vaccine arm and 28 in the placebo arm, with infection rates of 4.54 and 3.70 per 100 person-years, respectively. There was no evidence of vaccine efficacy (VE); the hazard ratio adjusted for gender was 1.25 (95% CI: 0.76, 2.05). VE did not differ by Ad5 titre, gender, age, HSV-2 status, or circumcision. The geometric mean viral load setpoint was 20,483 copies/ml (N=33) in vaccinees and 34,032 copies/ml (N=28) in placebo recipients (p=0.39). The vaccine elicited IFNγ-secreting T cells recognizing both clade B (89.2%) and C (77.4%) antigens.
Conclusion
The MRKAd5 HIV-1 vaccine did not prevent HIV-1 infection or lower viral-load setpoint however early stopping likely compromised our ability to draw conclusions. The high incidence rates seen in SA highlight the critical need for intensified efforts to develop an efficacious vaccine.
Keywords: HIV, HIV vaccine efficacy studies, South Africa
Introduction
South Africa (SA), the country with the largest number of people infected with HIV-1 (5.7 million) 1 and an HIV-1 prevalence of 11% in the general population 2 is in dire need of a biomedical intervention to prevent HIV-1 infection. The HVTN 503 or Phambili (Zulu for “forward!”) study was the second phase IIb HIV vaccine test-of-concept study to evaluate the efficacy of the MRKAd5 HIV-1 gag/pol/nef sub-type B vaccine. This vaccine was designed to elicit T-cell mediated immune responses capable of providing complete or partial protection from HIV-1 infection or a decrease in viral load post-acquisition3–9. The Ad5 vaccine was shown to elicit immune responses in participants regardless of Ad5 serostatus10. With promising safety and immunogenicity data from earlier clinical studies 10, 11, the Step test-of-concept of study12 was designed to assess the efficacy of this vaccine in regions of the world where the predominant circulating sub-type is clade B. The Phambili study was designed soon after to test the vaccine in a clade C region of the world13, 14, in populations with high levels of pre-existing immunity to Ad5 15, 16. Cross-clade T cell immunity to HIV-1 gag, pol and nef genes had been demonstrated in both HIV-1 infected and Clade B vaccinated HIV seronegatives, providing an immunization rationale for the trial in SA 17, 18. Enrollment and vaccinations in the Phambili study, were stopped in September 2007 following the Step study’s interim analysis, which found that the vaccine did not protect against HIV-1 infection or reduce early viral load in those who acquired infection. In an exploratory analysis of Step data, the risk of HIV-1 infection also appeared to be higher in a sub-group of male vaccine recipients, those who were Ad5 sero-positive or uncircumcised 12. We report our findings from the Phambili study on HIV-1 acquisition and disease progression as well as describe and investigate factors associated with HIV-1 acquisition as a means of guiding future biomedical interventions directed at reducing HIV-1 acquisition.
Methods
Study Design and Population
The study, a two-arm, double-blind, placebo controlled randomized clinical trial, was initiated in January 2007 and designed to enroll 3000 healthy HIV uninfected, predominantly heterosexual adults between the ages of 18–35 years at 5 sites within SA (Soweto, Cape Town, Klerksdorp-Orkney-Stilfontein-Hartbeesfontein [KOSH], eThekwini, and MEDUNSA). Because of the generalized nature of the HIV-1 epidemic in SA, the only behavioral risk eligibility criterion was being sexually active within the six months prior to enrollment. Pregnant or breastfeeding women were ineligible, and women had to agree to use two methods of contraception (barrier and other effective method e.g. hormonal contraception) to avoid pregnancy from 21 days prior to their first vaccination until one month after their last vaccination.
The study was registered with the Food and Drug Administration (FDA) in the USA; and approved by the SA Medicines Control Council; the Genetically Modified Organism Review Committee of the SA Department of Agriculture; and the ethical review committees and institutional biosafety committees of the University of the Witwatersrand, University of Cape Town, University of Limpopo and the University of KwazuluNatal. Participants provided written informed consent in English or their local language. The trial was registered in clinicaltrials.gov (NCT00413725) and the SA National Health Research Database (DOH-27-0207-1539).
On 19 September 2007, enrollment and vaccinations were halted based on the interim analyses of the Step study12, and in October 2007, unblinding of participants began, with concomitant HIV-1 testing, risk evaluation and counselling. After unblinding, follow-up visits were changed from every six to every three months for more frequent HIV-1 testing and risk reduction counseling, and are ongoing.
Randomization
Randomization was 1:1 between vaccine and placebo and stratified by site and gender. The randomization sequence was based on computer-generated random numbers and provided to site pharmacists by a central statistical and data monitoring center (SDMC).
Masking
The participants, study team and laboratory were blinded to treatment allocation.
Products and Procedures
The study product, MRKAd5 HIV-1 gag/pol/nef vaccine, manufactured by Merck and Co., Inc, has been described elsewhere12, but in brief, the vaccine was given as a dose of 1.5×1010 Ad viral genomes/1mL; the placebo was a 1ml solution of the vaccine diluent with no Ad5 vector. Study products were administered by intramuscular injection on a 0, 1, 6 month schedule. Serum samples were obtained at enrolment for Ad5 neutralizing antibody titres 19 and herpes simplex virus, type 2 (HSV-2) serology 20. Clinical assessments and risk reduction counseling occurred at every visit. Before each vaccination, pregnancy was excluded. Full Blood Count (FBC) with differential, platelet counts and alanine aminotransferase values (ALT) were obtained 14 days following first vaccination.
An assessment of HIV-1 risk behaviors in the previous six months was administered at screening and every six months prior to termination of enrolment and vaccinations; after participant unblinding, these assessments were performed every three months using a three-month time frame for behaviors. At screening, men were also assessed for circumcision status by physical exam. HIV-1 prevention interventions were provided to all participants throughout the trial including: risk reduction counseling, provision of male condoms; partner and couple HIV testing and counseling; male circumcision 21; STI management; and post-sexual exposure antiretroviral prophylaxis.
Before enrollment and vaccination were suspended, HIV-1 testing was done on blood drawn on the day of first vaccination, week 12, week 30, and every six months thereafter using an algorithm that distinguishes true infection from vaccine-induced seropositivity: an initial positive test result is confirmed by a second blood draw, which includes HIV RNA detection. HIV-1 testing occurred at unblinding and subsequently every three months. HIV-1 infected participants underwent physical examination, post-test counseling and regular blood draws for monitoring of viral load and CD4 count and were referred for medical care including ART.
Vaccine immunogenicity was measured on a subset of participants in the HVTN laboratory using a validated IFN-γ ELISPOT assay using two panels of peptide pools, Clade B vaccine-matched and Clade C potential T-cell epitopes (PTE-C), in previously cryopreserved PBMCs22. The primary immunogenicity assessment was conducted on samples obtained by venipuncture at Week 8, 4 weeks following the second vaccination from the first 186 participants enrolled (93 vaccinees/93 placebo recipients) who were HIV-1 antibody negative at the Week 12 visit; had received the second study injection, and whose thawed PBMC had ≥ 66% cell viability23.
Study Objectives and Endpoints
The objectives of the study were to assess the safety, tolerability and efficacy of the MRKAd5 vaccine. The study had two co-primary efficacy endpoints: acquisition of HIV-1 infection and HIV-1 viral-load setpoint in participants who become HIV-1 infected.
Statistical Analysis
This was an event-driven trial, designed to accrue at least 120 per-protocol events among 3000 participants to provide 80% power to detect a vaccine efficacy (VE) against infection of at least 45% and/or at least a 0.75 log10 copies per mL reduction in the mean viral-load setpoints of vaccinees vs. placebos. VE was defined as 100 × [1 − (vaccine infection rate/placebo infection rate)]. Viral-load setpoint was defined as the geometric mean of HIV plasma viral load (Roche COBAS Amplicor Monitor HIV-1 Standard, Roche Diagnostics, Location) measurements at ~2 months through ~3 months post-diagnosis. Results from earlier time points were used when both month 2 and 3 values were missing.
The two endpoints were assessed independently with two-tailed significance tests stratified by gender. A Hochberg multiplicity adjustment was applied to the p-values to adjust for the two endpoints 24. Follow-up for HIV infection is through 31 August 2009, with viral load and CD4 data through 15 January 2010 to allow sufficient time to calculate viral-load setpoint.
The efficacy endpoint analyses were modified intent-to-treat (MITT). The original study design called for a per-protocol (PP) efficacy analysis, limiting to participants who received at least two vaccinations and were sero-negative at the week 12 visit. However, due to the early stopping and consequent reduction in the PP and overall sample size, the analysis plan was changed to MITT prior to unblinding. The MITT population includes all vaccinated participants apart from those diagnosed as HIV-1 infected on the day of first vaccination.
Safety analyses included all randomized participants who received at least one study injection. Differences between treatment arms for reactogenicity and adverse events were assessed with Fisher’s exact tests. Differences in STI rates were assessed with tests of homogeneity of the Poisson rates.
For HIV-1 infection, a Cox proportional hazards model was used to estimate the infection hazard ratio for vaccine:placebo (HR) adjusted for gender with Wald-based 95% confidence interval (CI). Time to HIV-1 infection for infected participants was defined as the time from first study injection to the midpoint between the last plasma HIV-1 RNA negative and first RNA positive test; for uninfected participants it was the time from first study injection to last day of study follow-up. Cumulative incidence plots of time to HIV-1 infection by treatment and gender are provided for illustration. To assess changes in the HR over time, the point estimate and 95% confidence bands for the instantaneous hazard ratio were plotted 25. Cox proportional hazards models were used to assess other predictors of HIV-1 infection: age (in quartile groups), baseline Ad5 titre (both 18 and 200 cut-points assessed), number of vaccinations received (placebo arm = 0), baseline HSV-2 seropositivity, baseline behavioral risk factors (except for having an HIV positive partner and exchange of sex, which could not be tested due to small numbers), baseline circumcision status (men), and use of hormonal contraceptives at baseline (women). Cox models were also used to evaluate time to CD4 decline <350, a typical ART-initiation guideline.
For viral load setpoint, values below the limit of detection (<400) were set to 400 copies/mm3. The difference in the distributions of viral load setpoint between vaccine and placebo groups were assessed with a Wilcoxon rank sum test stratified by gender.
Funding
The study was funded by grants from the National Institute of Allergy and Infectious Diseases to the HIV Vaccine Trials Network – Core, #5U01 AI068614, Lab, 5U01 AI068618, and SDMC 5U01 AI068635; and to the clinical study sites: the PHRU Soweto: 5U01 AI069453; Cape Town: 5U01 AI069519; and KOSH/Klerksdorp: 5U01 AI069469; as well as Merck & Co., Inc. The South African AIDS Vaccine initiative (SAAVI) provided support to the clinical trial sites.
Results
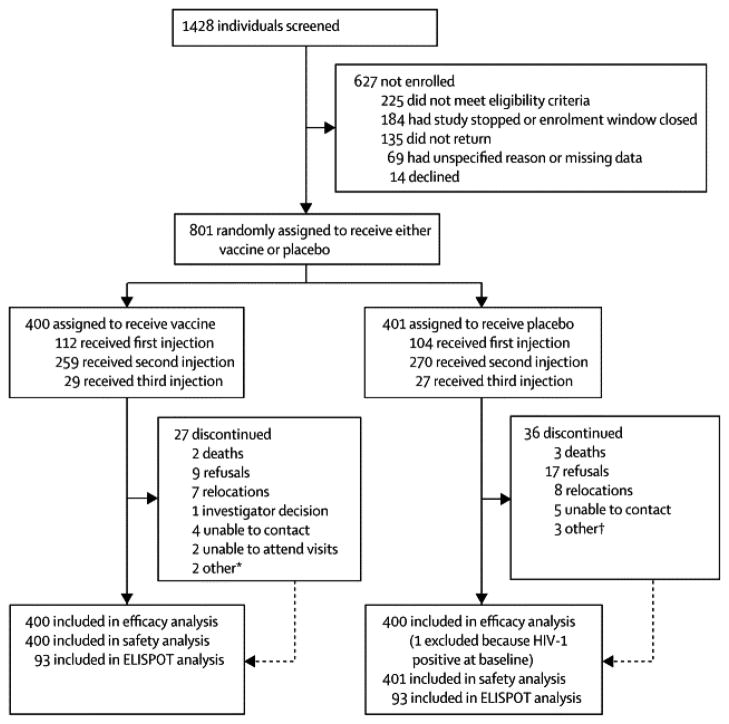
We enrolled 801 participants between 24 January 2007 and 19 September 2007, before enrolment and vaccinations were discontinued, in five sites in SA (Figure 1). Of those enrolled, 360 (44.9%) were women (Table 1), and two thirds (522) were under the age of 25. Nearly 20% (154) of participants had baseline Ad5 antibody titres ≤18 and 55.6% (445) had Ad5 antibody titres of >200. At enrollment, 129 men (29.3%) were circumcised (61 on the vaccine arm and 68 on the placebo arm). An additional 109 men were circumcised post-enrollment (52 vaccine, 57 placebo); half of these occurred pre-unblinding (26 vaccine, 29 placebo). HSV-2 prevalence at baseline was significantly higher in women than in men, (177/360=49.2% vs 72/441=16.3%, respectively, p<0.001). Baseline demographic and HIV risk behaviors were similar between study arms except for women reporting drinking or taking drugs during sex in the 6 months prior to screening (26/178=14.6% of vaccinees and 14/182=7.7% of placebo recipients, p = 0.04). Our cohort was predominantly heterosexual with 457/801=57.1% of participants reporting unprotected vaginal or anal sex as a risk factor. Men were more likely than women to report more than one sexual partner (247/441=56.0% vs 56/360=15.6%, respectively, p<0.001) and to report having a casual or anonymous partner (209/441=47.4% vs 40/360=11.1%, p<0.001). Most participants (304/441=68.9% of men and 302/360=83.9% of women) had a main partner, with 349/606=57.6% of those with a main partner regularly living apart. Less than 10% of both men and women reported having an HIV positive partner, exchanging sex for money or gifts, being forced to have sex, or being diagnosed with an STI and few women reported heavy drinking (Table 1). In total, 216 (27.0%) received one study injection, 529 (66.0%) two injections, and 56 (7.0%) three injections. There were no differences between the treatment arms in the number of injections received or in reasons for discontinuation of vaccination. Overall, 63 (7.9%) participants withdrew from the study by 31 August 2009 (Figure 1), the proportion being similar between treatment arms. Post unblinding, more participants in the placebo vs vaccine arm (n=13 vs 6) refused further participation.
Figure 1.
Table 1.
Participant baseline characteristics stratified by gender
| Men (N=441) | Women (N=360) | ||||
|---|---|---|---|---|---|
|
| |||||
| Vaccine (N=222) | Placebo (N=219) | Vaccine (N=178) | Placebo (N=182) | Total (N=801) | |
| Demographics | |||||
| Adenovirus 5 titer | |||||
| <= 18 | 50 (22.5%) | 48 (21.9%) | 25 (14.0%) | 31 (17.0%) | 154 (19.2%) |
| 19–200 | 46 (20.7%) | 55 (25.1%) | 43 (24.2%) | 58 (31.9%) | 202 (25.2%) |
| 201–1000 | 94 (42.3%) | 95 (43.4%) | 83 (46.6%) | 62 (34.1%) | 334 (41.7%) |
| > 1000 | 32 (14.4%) | 21 (9.6%) | 27 (15.2%) | 31 (17.0%) | 111 (13.9%) |
| Age (years) | |||||
| Median (range) | 22 (18,35) | 22 (18,35) | 23 (18, 35) | 23 (18,34) | 22 (18,35) |
| Race | |||||
| Black | 222 (100.0%) | 215 (98.2%) | 176 (98.9%) | 180 (98.9%) | 793 (99.0%) |
| Other | 0 (0.0%) | 4 (1.8%) | 2 (1.1%) | 2 (1.1%) | 8 (1.0%) |
| HSV-2 status | |||||
| Positive | 40 (18.0%) | 32 (14.7%) | 90 (50.8%) | 87 (47.8%) | 249 (31.2%) |
| Negative | 177 (79.7%) | 181 (83.0%) | 84 (47.5%) | 93 (51.1%) | 535 (67.0%) |
| Atypical | 5 (2.3%) | 5 (2.3%) | 3 (1.7%) | 2 (1.1%) | 15 (1.9%) |
| Unknown | 0 (0.0%) | 1 (0.5%) | 1 (0.6%) | 0 (0.0%) | 2 (0.2%) |
| Circumcised (men only) | 61 (27.5%) | 68 (31.1%) | -- | -- | 129 (29.3%) |
| Study site | |||||
| Soweto-PHRU | 82 (36.9%) | 81 (37.0%) | 72 (40.4%) | 73 (40.1%) | 308 (38.5%) |
| Cape Town | 34 (15.3%) | 31 (14.2%) | 50 (28.1%) | 51 (28.0%) | 166 (20.7%) |
| KOSH | 72 (32.4%) | 72 (32.9%) | 38 (21.3%) | 39 (21.4%) | 221 (27.6%) |
| CAPRISA | 14 (6.3%) | 15 (6.8%) | 12 (6.7%) | 12 (6.6%) | 53 (6.6%) |
| Medunsa | 20 (9.0%) | 20 (9.1%) | 6 (3.4%) | 7 (3.8%) | 53 (6.6%) |
| Risk behaviors (previous 6 months) | |||||
| Number of sexual partners | |||||
| 0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1 | 89 (40.1%) | 105 (47.9%) | 146 (82.0%) | 158 (86.8%) | 498 (62.2%) |
| 2 | 60 (27.0%) | 46 (21.0%) | 29 (16.3%) | 19 (10.4%) | 154 (19.2%) |
| 3–4 | 55 (24.8%) | 52 (23.7%) | 3 (1.7%) | 5 (2.7%) | 115 (14.4%) |
| >=5 | 18 (8.1%) | 16 (7.3%) | 0 (0.0%) | 0 (0.0%) | 34 (4.2%) |
| Median (range) | 2 (1, 20) | 2 (1, 14) | 1 (1,3) | 1 (1,3) | 1 (1,20) |
| Serostatus of sexual partners | |||||
| Any HIV positive | 4 (1.8%) | 3 (1.4%) | 2 (1.1%) | 1 (0.5%) | 10 (1.2%) |
| Any HIV unknown | 163 (73.4%) | 163 (74.4%) | 87 (48.9%) | 88 (48.4%) | 501 (62.5%) |
| Any HIV negative | 92 (41.4%) | 90 (41.1%) | 103 (57.9%) | 99 (54.4%) | 384 (47.9%) |
| Unprotected vaginal sex | 129 (58.1%) | 125 (57.1%) | 103 (57.9%) | 96 (52.7%) | 453 (56.6%) |
| Unprotected receptive anal sex | 0 (0.0%) | 1 (0.5%) | 4 (2.2%) | 5 (2.7%) | 10 (1.2%) |
| Unprotected insertive anal sex (men only) | 4 (1.8%) | 8 (3.7%) | -- | -- | 12 (2.7%) |
| Drinking/drugs with sex | 93 (41.9%) | 81 (37.0%) | 26 (14.6%) | 14 (7.7%) | 214 (26.7%) |
| Had a main partner | 155 (69.8%) | 149 (68.0%) | 151 (84.8%) | 151 (83.0%) | 606 (75.7%) |
| ≥ 10 years younger (men only) | 7 (3.2%) | 4 (1.8%) | -- | -- | 11 (2.5%) |
| ≥ 10 years older (women only) | -- | -- | 14 (7.9%) | 13 (7.1%) | 27 (7.5%) |
| Apart regularly | 97 (43.7%) | 95 (43.4%) | 83 (46.6%) | 74 (40.7%) | 349 (43.6%) |
| Had a casual or anonymous partner | 109 (49.1%) | 100 (45.7%) | 22 (12.4%) | 18 (9.9%) | 249 (31.1%) |
| Exchanged sex for money/gifts | 12 (5.4%) | 12 (5.5%) | 2 (1.1%) | 2 (1.1%) | 28 (3.5%) |
| Forced to have sex | 6 (2.7%) | 3 (1.4%) | 5 (2.8%) | 2 (1.1%) | 16 (2.0%) |
| Away from home regularly (men only) | 46 (20.7%) | 48 (21.9%) | -- | -- | 94 (21.3%) |
| Sexually transmitted infection | 17 (7.7%) | 10 (4.6%) | 10 (5.6%) | 9 (4.9%) | 46 (5.7%) |
| Heavy drinking | 49 (22.1%) | 59 (26.9%) | 7 (3.9%) | 9 (4.9%) | 124 (15.5%) |
Behavioral risk data are based on self-reported behaviour within 6 months prior to screening. Apart regularly from main partner is defined as living in a different location or partner regularly away from home for 3 or more days per week or for men, being away from home for 3 or more days per week. Sexually transmitted infection (STI) is based on participant self-report of a STI diagnosis. Heavy drinking is defined as having >5 drinks per day on at least 10 days within the six month reporting period.
Vaccines were well tolerated. Adverse events, local and systemic reactogenicity and in Table 2 and pregnancies are reported in web appendix 3. Self reported STI rates were similar between vaccine and placebo arms over the entire study period (7.5 vs 9.8 per 100 person-years) as well as over the pre- and post-unblinding periods (9.9 vs 9.6 and 7.7 vs 9.9 per 100 person-years). Women were more likely than men to report an STI (11.1 vs 6.7 per 100 person-years, p=0.007).
Table 2.
| Vaccine (N=400) | Placebo (N=401) | ||
|---|---|---|---|
| n (%) | n (%) | p-valuec | |
| Reactogenicity symptoms of any severity | |||
| Local pain/tenderness | 247 (61.8%) | 134 (33.4%) | <0.0001 |
| Any systemic symptom | 262 (65.5%) | 220 (54.9%) | 0.002 |
| Headache | 184 (46.0%) | 149 (37.2%) | 0.01 |
| Malaise/fatigue | 155 (38.8%) | 118 (29.4%) | 0.006 |
| Myalgia | 107 (26.8%) | 61 (15.2%) | <0.0001 |
| Arthralgia | 82 (20.5%) | 59 (14.7%) | 0.03 |
| Nausea | 44 (11.0%) | 38 (9.5%) | 0.49 |
| Chills | 44 (11.0%) | 28 (7.0%) | 0.049 |
| Diarrhoea | 16 (4.0%) | 19 (4.7%) | 0.73 |
| Vomiting | 13 (3.3%) | 18 (4.5%) | 0.46 |
|
| |||
| Adverse events (AEs) | |||
|
| |||
| No. of participants with 1 or more AEs | 270 (67.5%) | 270 (67.3%) | 1.00 |
| AEs with ≥ 1% frequency in either the vaccine or placebo group and occurring with ≥ frequency in the vaccine group | |||
| n (%) | n (%) | p-value | |
| Upper respiratory tract infection | 55 (13.8%) | 54 (13.5%) | 0.92 |
| Headache | 26 (6.5%) | 15 (3.7%) | 0.08 |
| Influenza | 19 (4.8%) | 14 (3.5%) | 0.38 |
| Neutropenia | 20 (5.0%) | 10 (2.5%) | 0.07 |
| Hypertension | 15 (3.8%) | 14 (3.5%) | 0.85 |
| Genital discharge | 15 (3.8%) | 14 (3.5%) | 0.85 |
| Alanine aminotransferase increased | 7 (1.8%) | 4 (1.0%) | 0.38 |
| Haemoglobin decreased | 8 (2.0%) | 3 (0.7%) | 0.14 |
| Urinary tract infection | 6 (1.5%) | 5 (1.2%) | 0.77 |
| Pharyngitis | 5 (1.3%) | 4 (1.0%) | 0.75 |
| Skin laceration | 5 (1.3%) | 4 (1.0%) | 0.75 |
| Dizziness | 6 (1.5%) | 2 (0.5%) | 0.18 |
| Fungal skin infection | 4 (1.0%) | 3 (0.7%) | 0.73 |
| Oropharyngeal pain | 4 (1.0%) | 3 (0.7%) | 0.73 |
| Rash | 4 (1.0%) | 3 (0.7%) | 0.73 |
| Anaemia | 5 (1.3%) | 1 (0.2%) | 0.12 |
| Genital herpes | 4 (1.0%) | 2 (0.5%) | 0.45 |
| Leukopenia | 4 (1.0%) | 2 (0.5%) | 0.45 |
| Cough | 4 (1.0%) | 1 (0.2%) | 0.22 |
Reactogenicity symptoms were a set of specific symptoms commonly associated with vaccination that had an onset within the first 3 days following a study injection.
Adverse events (AEs) were non-reactogenicity events that had an onset within 28 days following a study injection or were new chronic conditions requiring medical intervention of more than 30 days or newly diagnosed or treated sexually transmitted infections occurring at any time on study.
At four weeks following the 2nd vaccination, 83/93=89.2% of vaccinees had developed an IFN-γ secreting T cell response to Clade B peptides and 72/93=77.4% to Clade C peptides (Table 3). For Clade B, Gag-specific responses were highest (74/93=79.6%); for Clade C, Pol-specific responses were highest (65/93=69.9%). All Clade C responders also had a Clade B response. Among responders to both Clades B and C, the overall magnitude of response to the Clade B vaccine-matched panel was significantly higher than to Clade C PTE panel; the same pattern held for responses to individual antigens (Table 3). Although not statistically different, vaccinees seronegative for Ad5 (titre ≤18) at baseline tended to demonstrate consistently higher response rates for both clades, overall and by gene, than those Ad5 seropositive (Table 3). Among responders, the Ad5 seronegative vaccinees also had a greater magnitude of response than those Ad5 seropositive for both clades overall (p = 0.004 for B and 0.007 for C), for Clade B Nef and Pol (p = 0.049 and = 0.002), and Clade C Pol (p = 0.01).
Table 3.
Interferon-γ secreting T cell responses by ELISPOT in vaccine recipients at Week 8
| Peptide Panel
|
||||||
|---|---|---|---|---|---|---|
| Clade B vaccine matched | Clade C PTE | |||||
| Ad5 antibody titre ≤18 (n=18) | Ad5 antibody titre > 18 (n=75) | Overall (n=93) | Ad5 antibody titre ≤18 (n=18) | Ad5 antibody titre > 18 (n=75) | Overall (n=93) | |
|
N (%) GM |
||||||
|
| ||||||
| Gag | 16 (88.9%) 293 |
58 (77.3%) 192 |
74 (79.6%) 210 |
11 (61.1%) 220 |
37 (49.3%) 193 |
48 (51.6%) 199 |
|
| ||||||
| Pol | 15 (83.3%) 837 |
50 (66.7%) 349 |
65 (69.9%) 427 |
15 (83.3%) 481 |
50 (66.7%) 216 |
65 (69.9%) 260 |
|
| ||||||
| Nef | 13 (72.2%) 318 |
52 (69.3%) 184 |
65 (69.9%) 205 |
5(27.8%) 168 |
15 (20.0%) 148 |
20 (21.5%) 153 |
|
| ||||||
| ≥1 antigen (overall) | 17 (94.4%) 1242 |
66 (88.0%) 587 |
83 (89.2%) 684 |
15 (83.3%) 787 |
57 (76.0%) 381 |
72 (77.4%) 443 |
|
| ||||||
| ≥2 antigen | 15 (83.3%) | 57 (76.0%) | 72 (77.4%) | 12 (66.7%) | 35 (46.7%) | 47 (50.5%) |
|
| ||||||
| ≥3 antigen | 12 (66.7%) | 37 (49.3%) | 49 (52.7%) | 4 (22.2%) | 10 (13.3%) | 14 (15.1%) |
IFN-γ ELISPOT assay results are presented for the first 93 vaccinees enrolled in the trial who were HIV-1 plasma RNA negative at the week 12 visit, received the 2nd vaccination within the visit window, had a week 8 blood draw within the visit window, and whose PBMC specimen had ≥ 66% cell viability. Data are number (%) of responders. GM=geometric mean of spot forming cells per 1 million PBMC for responders only. For clade B, the Pol magnitude is the sum of the SFCs for the 2 Pol peptide pools. For clade C, the magnitudes for Gag and Pol are the maximum SFCs among the multiple peptide pools for the protein. The GM for ≥ 1 antigen is based on the sum of the gene-specific magnitudes. Responders were defined as those with antigen-stimulated responses significantly greater than twice their background responses as assessed by a bootstrap test (1-sided α=0.05) after adjusting for the multiple antigens within each clade33; in addition, background-subtracted responses had to exceed 10 SFC/200,000 PBMC. Among 93 placebo recipients assayed, 4 (4.3%) had a positive response for Clade B and 2 of the 4 had a Clade C response. The Clade B peptide pools were synthetic peptide pools spanning the Clade B proteins encoded by the vaccine constructs consisting of 1 Gag, 1 Nef, and 2 Pol pools. Clade C peptide pools were: 2 Gag PTE-C (potential T cell epitope-Clade C), 1 Nef PTE-C, and 3 Pol PTE-C pools.
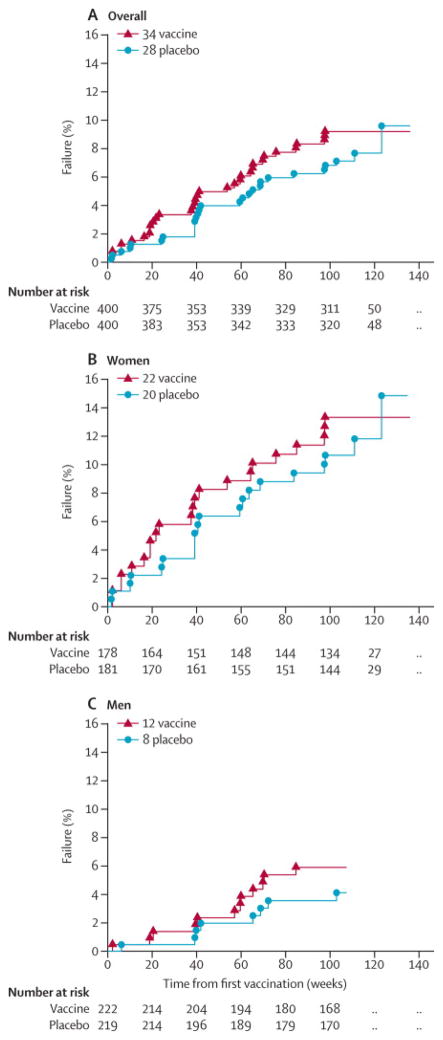
There was no evidence of VE (Figure 2): the treatment HR adjusted for gender was 1.25 (95% CI: 0.76, 2.05). Accounting for the time at which participants were notified of their treatment assignment did not alter this finding: the HR adjusted for unblinding time was 1.15 (95% CI: 0.69, 1.90). The treatment HR was higher in the 6 months (26 weeks) after first vaccination, but this finding was not significant as can be seen in Web appendix Figure 1 which illustrates the log instantaneous HR for treatment over time since first vaccination. HIV incidence was similar between vaccine and placebo arms (4.54 vs 3.70/100 person-years, n=34 vs 28, Web appendix 1). The majority of these infections were among women (n=42, Figure 2) with high HIV-1 incidence rates both in the vaccine arm (6.79) and placebo arm (5.86).
Figure 2.
In multivariate Cox proportional hazard models, gender, age, HSV-2 and the interaction of gender and HSV-2 were significant predictors of HIV-1 infection (Table 4). Baseline Ad5 titre, categorized using either 18 or 200 as a cut-point, was not a significant predictor of HIV-1 infection and adjusting for Ad5 had little effect on the treatment HR; (HR= 1.24 – 1.31, depending on model). The number of vaccinations and behavioural risk factors were also not significant predictors of infection. Ad5 titre, gender, age or HSV-2 did not modify the treatment HR, nor did the effect of age and Ad5 titre differ by gender.
Table 4.
Risk factors for HIV-1 Infection
| Group | Variable | Hazard Ratio Contrast | Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| MITT Cohort | Treatment arm | Vaccine:placebo | 1.31 | 0.79, 2.16 |
| Age quartiles | 18–20 yrs (ref) | 1 | ||
| 21–22 yrs | 3.28 | 1.47, 7.32 | ||
| 23–26 yrs | 1.82 | 0.79, 4.20 | ||
| 27–35 yrs | 1.95 | 0.85, 4.46 | ||
| MITT Men | HSV-2 | positive:negative | 5.23 | 2.09, 13.10 |
| MITT Women | HSV-2 | positive:negative | 1.00 | 0.53, 1.89 |
| MITT HSV-2 seronegative | Gender | women:men | 4.60 | 2.16, 9.79 |
| MITT HSV-2 seropositive | Gender | women:men | 0.88 | 0.41, 1.88 |
The modified-intention-to-treat population includes all vaccinated participants apart from those diagnosed as HIV-1 infected on the day of first vaccination. Time to HIV-1 infection was calculated as the time from first study injection to last day of study follow-up for uninfected participants; for infected participants, from first study injection to the midpoint of the time between the last negative and first positive HIV-1 RNA PCR test. Estimates are from the final multivariate Cox proportional hazards model of time to HIV-1 infection which included treatment arm (Wald p=0.30), age quartiles (likelihood ratio p=0.03), gender (Wald p = <0.0001), baseline HSV-2 status (Wald p < 0.0004), and the interaction term of gender x HSV-2 (Wald p = 0.0025). Ad5 titre was not a significant predictor or effect modifier.
HSV-2 increased the risk of HIV-1 in men but not in women (Table 4). For men, HSV-2 increased the risk of infection more than 5-fold (5.23, 95% CI: 2.09, 13.10). For those HSV-2 negative at baseline, women had an increased risk of HIV-1 compared to men (HR 4.60; 2.16, 9.79). In an analysis restricted to women, there were no significant predictors of HIV-1 infection. In multivariable analysis for men (Web appendix Table 2), HSV-2 (HR 4.90, 95% CI 2.03, 11.80) and having a main partner but living apart regularly (HR 3.61, 95% CI 1.31, 9.99) were associated with HIV-1 infection; neither Ad5 titre nor circumcision were significant. HSV-2 status post-enrollment was not assessed; among 31 participants who acquired HIV-1 who were HSV-2 seronegative at enrollment, 22 either did not change HSV-2 status (19) or acquired HSV-2 prior to HIV-1 infection (3), and all nine remaining participants were identified as HSV-2 and HIV-1 positive at the same visit.
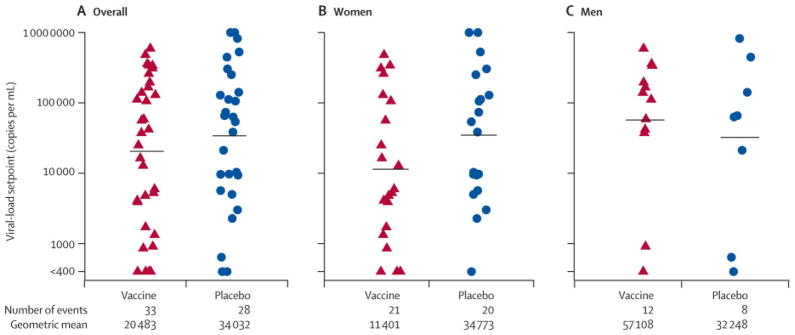
Viral-load setpoint was calculated for 61 of the HIV-1 infected participants. One woman on the vaccine arm was excluded from the viral-load setpoint analysis because of a six month delay in confirming infection. There was no significant difference in the distribution of viral load setpoint between the vaccine arm (geometric mean titre 20,483 copies/mL, N=33) and the placebo arm (34,032 copies/mL, N=28) (Figure 3, p=0.39, stratified by gender). Women tended to have lower viral-load setpoints than men (geometric mean 19,642 vs 45,438 copies/mL), which was not statistically significant (p = 0.15). Women who received vaccine (n=21) tended to have lower viral-load setpoints than women on the placebo arm (n=20) although this was not statistically significant (11,401 vs 34,773; p=0.16). Participants with baseline Ad5 titre > 18 (n=50) tended to have higher viral-load setpoint than those ≤ 18 (n=11), (31,924 vs 9,924), but this too, was not statistically significant (p =0.12).
Figure 3.
Neither of the p-values for the co-primary endpoints of HIV-1 infection and viral-load setpoint were statistically significant, adjusted p’s=0.39. Seven infected participants (3 vaccine, 4 placebo) started ART, all after the 3rd month visit post HIV-1 diagnosis. Two women with CD4 counts above 350 initiated ART to prevent mother-to-infant transmission and data from these women were censored at 3 months and 1 year, respectively. Treatment was not a significant predictor of CD4 decline to less than 350 overall or among men, but among women, vaccinees were at significantly lower risk of this event than placebos: HR=0.33 (95% CI: 0.12, 0.91) (Web appendix Figure 2). No other significant predictors of time to CD4 count less than 350 were found among baseline covariates considered: site, Ad5 titre, age quartiles, HSV-2 and their interactions with treatment.
Discussion
This study agrees with the Step findings that the MRKAd5 HIV-1 vaccine did not prevent HIV-1 infection or lower early viral load in either Ad5 sero-positive or -negative vaccinees. However, Phambili had limited ability to assess the vaccine due to the discontinuation of enrollment and vaccination, possible changes in risk behavior after unblinding, and the limited number of infections particularly those occurring close to vaccination. This lack of vaccine effect is despite the relatively high frequency and magnitude of HIV-1 specific T cell responses measured by INF-γ ELISPOT to both clade B and C antigens. Most vaccinees developed an immune response to both Clades B and C, although the responses were greater to the vaccine-matched Clade B peptides. Although the vaccine was immunogenic in this and previous studies 26, the responses elicited did not translate to vaccine efficacy.
The high incidence of HIV-1 in women permitted the first evaluation of VE among heterosexual women and highlights the pressing need to find effective preventive interventions for women. There was no evidence of increased risk of HIV-1 infection among vaccinated participants compared to placebo. There was also no such evidence among Ad5 seropositive women or men or among uncircumcised men.
Although our results differ from Step, the premature interruption of the study, discontinuation of vaccinations, and unblinding may have affected risk behaviour, impacted on our statistical power to show these associations; or may have altered susceptibility to infection. In addition, the men in our study were predominantly heterosexual, therefore risk factors would have differed from the predominantly homosexual population studied in Step.
Prior HSV-2 infection has been associated with increased HIV-1 infection 27–30 and was associated with increased HIV-1 acquisition among men who have sex with men in the Step trial. In Phambili, prior HSV-2 infection was associated with HIV-1 infection among heterosexual men but not women. The finding in women that HIV-1 risk was not associated with prevalent HSV-2 has been documented previously31. The lack of association between oral or injectable hormonal contraception use at baseline and HIV-1 acquisition is consistent with other studies. 32 In men, HSV-2 status and living apart from the main partner were associated with HIV infection, and interventions addressing these factors need to be studied.
The lower viral-load setpoint and slower CD4 decline observed in vaccinated women may indicate a vaccine effect on disease progression, although, because enrolment in the study was halted prematurely, this study was not powered to detect this effect. Pre-existing immunity to the vaccine vector may alter VE. Further studies are required to understand the interplay between vaccination and these factors, including gender. A study of sexual partners of Phambili participants to provide new insights on the natural history of HIV infection and transmission dynamics in this population has been initiated.
Stratification by sex should be considered in future vaccine efficacy trials, to examine the effects of HIV-1 vaccines on gender. Longer follow-up of incident infections is warranted to assess the effects of vaccination on disease progression.
Supplementary Material
Acknowledgments
We thank the Phambili Study volunteers and the staff and community members at each of the Phambili Study sites.
Members of the HVTN 503 study team include:
HVTN, Seattle: Sarah Alexander, Larry Corey, Constance Ducar, Ann Duerr, Niles Eaton, Julie McElrath, Renée Holt. John Hural, Jim Kublin, Margaret Wecker. SCHARP, Seattle: Gina Escamilla, Drienna Holman, Barbara Metch, Zoe Moodie, Steve Self. DAIDS, Washington DC: Mary Allen, Alan Fix, Dean Follman, Peggy Johnston, Mary Anne Luzar, Ana Martinez. Merck Research Lab: Danny Casimiro, Robin Isaacs, Lisa Kierstead, Randi Leavitt, Devan Mehrotra, Mike Robertson. Perinatal Health Research Unit, Soweto: Guy DeBruyn, Glenda Gray, Busi Nkala, Tebogo Magopane, Baningi Mkhise. Medunsa, Pretoria: Innocentia Lehobye, Matsontso (Peter) Mathebula, Maphoshane Nchabeleng. Desmond Tutu HIV Centre, Cape Town: Linda-Gail Bekker, Agnes Ronan, Surita Roux. The Aurum Institute, Klerksdorp: Gavin Churchyard, Mary Latka, Carien Lion-Cachet Kathy Mngadi, Tanya Nielson, Pearl Selepe. CAPRISA, Durban: Thola Bennie, Koleka Mlisana, Nivashnee Naicker. NICD, Sandringham: Adrian Puren. Community Representative: David Galetta. SAAVI, Cape Town: Elise Levendal
Footnotes
The opinions expressed in this article are those of the authors and do not represent the official views of the National Institute of Allergy and Infectious Diseases.
Authors Contributions:
Conducted the analyses: ZM, BM
Analyzed the data: ZM, BM
Designed study and wrote the manuscript: GEG, MA, ZM, BM, GC, LGB, MN, KM, GdB, MHL, MM, MR, JGK, LC
Oversaw laboratory, conducted and interpreted lab data: DC, DKC, AP, MJMc
Conducted the Phambili study, oversaw study, managed participants: GEG, GC, LGB, NM, KM, GdB, SR, NN
Final editing: GEG, MA, ZM, BM, LC, JGK
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. Report on the global AIDS epidemic. Geneva, CH: Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- 2.Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Pillay-van-Wyk V, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 3.Klein MR. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–72. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, Feinberg MB, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12(7):585–92. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 5.Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS, Jones NG, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73(8):6715–20. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 7.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–72. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 8.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–40. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 10.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46(11):1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 11.Tobery TW, Dubey SA, Anderson K, Freed DC, Cox KS, Lin J, et al. A comparison of standard immunogenicity assays for monitoring HIV type 1 gag-specific T cell responses in Ad5 HIV Type 1 gag vaccinated human subjects. AIDS Res Hum Retroviruses. 2006;22(11):1081–90. doi: 10.1089/aid.2006.22.1081. [DOI] [PubMed] [Google Scholar]
- 12.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredell H, Martin DP, Van Harmelen J, Varsanii A, Sheppard HW, Donovan R, et al. HIV Type 1 Subtype C gag And nef Diversity in Southern Africa. AIDS Res Hum Retroviruses. 2007;23(3):477–81. doi: 10.1089/aid.2006.0232. [DOI] [PubMed] [Google Scholar]
- 14.Woodman Z, Williamson C. HIV molecular epidemiology: transmission and adaptation to human populations. Curr Opin HIV AIDS. 2009;4(4):247–52. doi: 10.1097/COH.0b013e32832c0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11(2):351–7. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28(4):950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari G, Humphrey W, McElrath MJ, Excler JL, Duliege AM, Clements ML, et al. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci U S A. 1997;94(4):1396–401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coplan PM, Gupta SB, Dubey SA, Pitisuttithum P, Nikas A, Mbewe B, et al. Cross-reactivity of anti-HIV-1 T cell immune responses among the major HIV-1 clades in HIV-1-positive individuals from 4 continents. J Infect Dis. 2005;191(9):1427–34. doi: 10.1086/428450. [DOI] [PubMed] [Google Scholar]
- 19.Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther. 2004;15(3):293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 20.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26(4):662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bruyn G, Martinson NA, Nkala BD, Tshabangu N, Shilaluka G, Kublin J, et al. Uptake of male circumcision in an HIV vaccine efficacy trial. J Aquir Immune Defic Syndr. 2009;51(1):108–10. doi: 10.1097/QAI.0b013e3181a03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra U, Nolin J, Horton H, Li F, Corey L, Mullins JI, et al. Functional properties and epitope characteristics of T-cells recognizing natural HIV-1 variants. Vaccine. 2009;27(48):6678–87. doi: 10.1016/j.vaccine.2009.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Aquir Immune Defic Syndr. 2007;45(1):20–7. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–2. [Google Scholar]
- 25.Gilbert PB, Wei LJ, Kosorok MR, Clemens JD. Simultaneous inferences on the contrast of two hazard functions with censored observations. Biometrics. 2002;58(4):773–80. doi: 10.1111/j.0006-341x.2002.00773.x. [DOI] [PubMed] [Google Scholar]
- 26.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 28.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Aquir Immune Defic Syndr. 2004;35(5):435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 29.Corey L. Synergistic copathogens--HIV-1 and HSV-2. N Engl J Med. 2007;356(8):854–6. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 30.Weiss HA, Buve A, Robinson NJ, Van Dyck E, Kahindo M, Anagonou S, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. Aids. 2001;15 (Suppl 4):S97–108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 31.Ramjee G, Williams B, Gouws E, Van Dyck E, De Deken B, Karim SA. The impact of incident and prevalent herpes simplex virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. J Aquir Immune Defic Syndr. 2005;39(3):333–9. doi: 10.1097/01.qai.0000144445.44518.ea. [DOI] [PubMed] [Google Scholar]
- 32.Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, et al. Hormonal contraception and the risk of HIV acquisition. Aids. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 33.Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Lwer M, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother. 2010;59(10):1489–501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010 Sep;5(5):357–61. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fellay J, Frahm N, Shianna KV, Cirulli ET, Casimiro DR, Robertson MN, Haynes BF, Geraghty DE, McElrath MJ, Goldstein DB for the National Institute of Allergy and Infectious Diseases (NIAID) Center for HIV/AIDS Vaccine Immunology (CHAVI) and the NIAID HIV Vaccine Trials Network (HVTN) Host Genetic Determinants of T Cell Responses to the MRKAd5 HIV-1 gag/pol/nef Vaccine in the Step Trial. J Infect Dis. 2011 Mar;203(6):773–779. doi: 10.1093/infdis/jiq125. Epub 2011 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.