Abstract
Magnesium lithospermate B (MLB) is one of the major active components of Salvia miltiorrhizae. The anti-oxidative effects of Salvia miltiorrhizae have been previously reported. The aim of this study was to investigate the effect of purified MLB on hepatic fibrosis in rats and on the fibrogenic responses in hepatic stellate cells (HSCs). Hepatic fibrosis was induced in rats by intraperitoneal thioacetamide (TAA) injections over a period of 8 or 12 weeks. MLB was orally administered daily by gavage tube. Serum AST and ALT levels in TAA + MLB group were significantly lower than those in TAA only group at week 8. Hepatic fibrosis was significantly attenuated in TAA + MLB group than in TAA only group at week 8 or 12. Activation of HSCs was also decreased in TAA + MLB group as compared to TAA only group. Hepatic mRNA expression of α-smooth muscle actin (α-SMA), TGF-β1, and collagen α1(I) was significantly decreased in TAA + MLB group as compared to TAA only group. Incubation with HSCs and MLB (≥100 µM) for up to 48 h showed no cytotoxicity. MLB suppressed PDGF-induced HSC proliferation. MLB inhibited NF-κB transcriptional activation and monocyte chemotactic protein 1 (MCP-1) production in HSCs. MLB strongly suppressed H2O2-induced reactive oxygen species (ROS) generation in HSCs, and MLB inhibited type I collagen secretion in HSCs. We concluded that MLB has potent antifibrotic effect in TAA-treated cirrhotic rats, and inhibits fibrogenic responses in HSCs. These data suggest that MLB has potential as a novel therapy for hepatic fibrosis.
Keywords: liver cirrhosis, Salvia miltiorrhiza, hepatic stellate cells, lithospermate B, reactive oxygen species
Introduction
Hepatic fibrosis refers to a wound-healing response to various kinds of liver injuries caused by alcohol, hepatitis virus, and toxins (Bataller and Brenner, 2005). The accumulation of the extracellular matrix (ECM) including type I collagen is the main pathological alteration in hepatic fibrosis. The terminal outcome of liver fibrosis is liver cirrhosis accompanied by portal hypertension, hepatic failure and hepatocellular carcinoma.
Hepatic stellate cells (HSCs) are the main fibrosing cell type in the liver. Quiescent HSCs are desmin-positive, perisinusoidal cells that are the primary sites of vitamin A storage in our bodies. Upon fibrogenic stimuli, quiescent HSCs transform into activated myofibroblasts, which produce ECMs such as type I collagen (Bataller and Brenner, 2005). Activated HSCs also produce proinflammatory cytokines and chemokines, which recruit HSCs and inflammatory cells to the site of liver injury. Until now there has been no effective antifibrotic therapy available, and many investigators have been trying to develop an antifibrotic agent that targets fibrogenic responses in HSCs.
It has been demonstrated that reactive oxygen species (ROS) play an important role in hepatic fibrogenesis from various liver injuries including alcohol abuse, hepatitis C virus infection, iron overload and chronic cholestasis (Poli and Parola, 1997; Pietrangelo, 1998; Parola and Robino, 2001). ROS is directly fibrogenic because it induces the activation of the collagen type I gene in HSCs (Nieto et al., 2000). ROS also stimulates proliferation and invasiveness of HSCs (Galli et al., 2005). Activation of MAPK pathways and NF-κB signaling cascades has been implicated in liver injury from oxidative stress (Czaja, 2007; Eng and Friedman, 2001). Free radicals or lipid peroxidation products stimulate the activation of HSCs through induction of c-myb and NF-κB, which is blocked by antioxidants such as α-tocopherol (Lee et al., 1995).
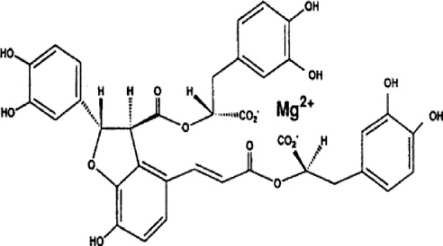
Salvia miltiorrhizae, also known as 'Danshen,' is a traditional herbal medicine that has been widely used for the treatment of vascular diseases in China (Zhou et al., 2005). It has been reported that Danshen contains multiple chemical constituents including lipophilic and hydrophilic compounds. Polyphenolic acids are the major type of hydrophilic components of Danshen. Magnesium lithospermate B (MLB) is one of the major active polyphenolic acids from Salvia miltiorrhizae (Zhou et al., 2005) (Figure 1). It was reported that administration of water-soluble extract of Salvia miltiorrhizae reduces hepatic fibrosis caused by carbon tetrachloride (CCl4) administration or bile duct ligation (BDL) (Wasser et al., 1998; Nan et al., 2001; Lee et al., 2003). However, it is still unknown which hydrophilic component of Salvia miltiorrhizae exerts an antifibrotic effect in the liver. In this study we investigated the effects of purified magnesium lithospermate B (MLB) on thioacetamide (TAA)-induced hepatic fibrosis in rats and on the fibrogenic responses in HSCs.
Figure 1.
The chemical structure of magnesium lithospermate B.
Results
MLB attenuates TAA-induced liver injury and hepatic fibrosis in rats
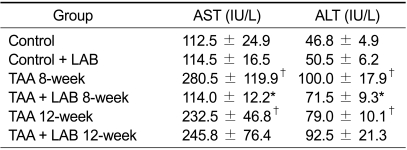
The extent of liver injury was assessed by measuring serum aminotransferase levels (Table 1). Eight or 12 weeks of TAA treatment induced significant elevation of AST and ALT levels as compared to the control group (P < 0.05). The levels of AST and ALT were significantly lower in the TAA + LAB 8-week group than in the TAA 8-week group (P < 0.05). However, MLB failed to decrease AST or ALT levels in 12 weeks of TAA treatment.
Table 1.
Serum aminotransferase levels
†P < 0.05 compared with Control. *P < 0.05 compared with TAA 8-week.
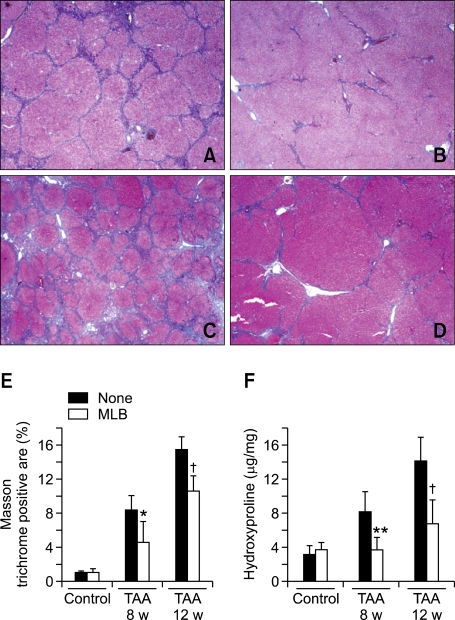
The extent of hepatic fibrosis was analyzed by morphometric quantitation of Masson's trichrome stained area in liver sections. The representative photomicrographs are shown in Figure 1. Fibrous septa developed in rats treated with TAA for 8 weeks. These septa incompletely surrounded the regenerative parenchyma and a partial nodular formation was present without development of obvious cirrhosis (Figure 2A). The septal fibrosis presented in the TAA 8-week group was attenuated by MLB (Figure 2B). After 12 weeks of TAA treatment, multiple cirrhotic nodules of varying size with fibrous septa were noted (Figure 2C), and these overt cirrhotic changes were also attenuated by MLB (Figures 2D). The morphometric measurement of Masson Trichrome stained area using a computerized imaging analysis system demonstrated that hepatic fibrosis was significantly attenuated by MLB in both the 8-week and 12-week TAA treatment groups (P < 0.05, P < 0.01, respectively) (Figure 2E). Treatment with MLB significantly decreased the amount of hepatic hydroxyproline content in both the 8-week and 12-week TAA treatment groups (P < 0.01, P < 0.01, respectively) (Figure 2F).
Figure 2.
MLB suppresses hepatic fibrosis in TAA-treated rats. The extent of hepatic fibrosis was assessed by Masson's trichrome staining (A, B, C, and D, original magnification × 40); (A) TAA 8-week, (B) TAA 8-week + MLB, (C) TAA 12-week, (D) TAA 12-week + MLB. (E) Masson's trichrome-stained area was quantified using a computerized imaging analysis system. (F) Hepatic hydroxyproline content was colorimetrically quantified from liver samples. *P < 0.05 compared with TAA 8-week, **P < 0.01 compared with TAA 8-week, †P < 0.01 compared with TAA 12-week.
MLB decreases activation of HSCs in TAA-treated rats
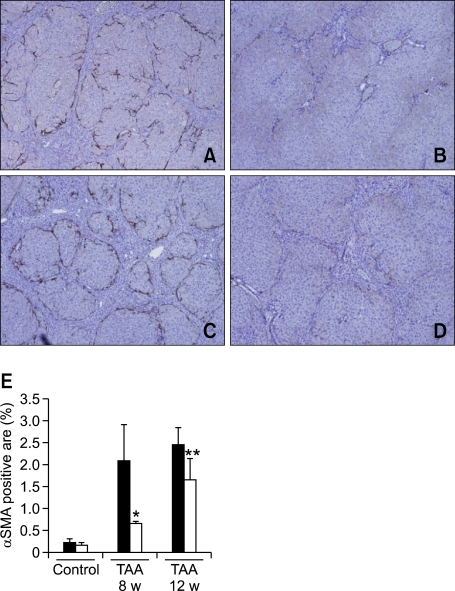
Activation of HSCs was assessed by measuring the α-SMA stained area in the liver. Immunohistochemistry showed that α-SMA positive cells were evident on the periphery of regenerating nodules in rats treated with TAA for both 8 and 12 weeks (Figures 3A and 3C). Expression of α-SMA was decreased in rats treated with TAA + MLB (Figures 3B and 3D). The morphometric measurement of the α-SMA stained area demonstrated that activation of HSCs was significantly decreased by MLB in both the 8-week and 12-week TAA treatment groups (P < 0.05, P < 0.05, respectively) (Figure 3E).
Figure 3.
MLB inhibits HSC activation in TAA-treated rats. Activation of HSCs was assessed by α-SMA staining (A, B, C, and D, original magnification × 40); (A) TAA 8-week, (B) TAA 8-week + MLB, (C) TAA 12-week, (D) TAA 12-week + MLB. (E) The α-SMA-stained area was quantitated using a computerized imaging analysis system. *P < 0.05 compared with TAA 8-week, **P < 0.05 compared with TAA-12 week.
MLB decreases the hepatic fibrogenic responses in TAA-treated rats
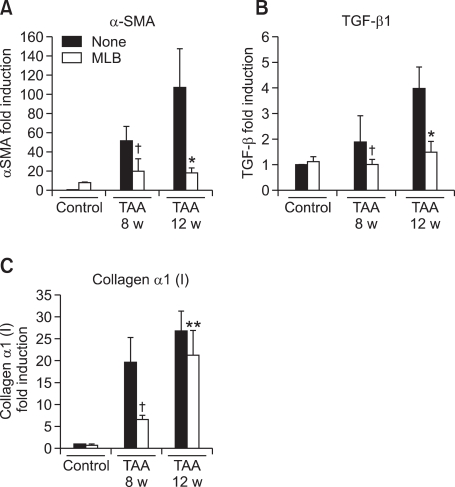
We next evaluated the hepatic expression of fibrosis-related genes in TAA-treated rats. Treatment with TAA increased hepatic expression of fibrosis-related genes including α-SMA, TGF-β1, and collagen α1(I). The expression of these fibrosis-related genes was significantly decreased by MLB in both the 8-week and 12-week TAA treatment groups (Figures 4A-C).
Figure 4.
Hepatic mRNA expression of fibrosis-related genes in TAA-treated rats. (A) α-SMA, (B) TGF-β1, (C) collagen α1(I) mRNA levels in rat livers were quantified by quantitative RT-PCR using Taqman probe after reverse transcription. The mRNA expression of these molecules was normalized with rat PBGD expression. Data represent the mean ± SD of two independent experiments. †P < 0.01 compared with TAA-8 week, *P < 0.01 compared with TAA-12 week, **P < 0.05 compared with TAA 12-week.
MLB suppresses cell proliferation of HSCs
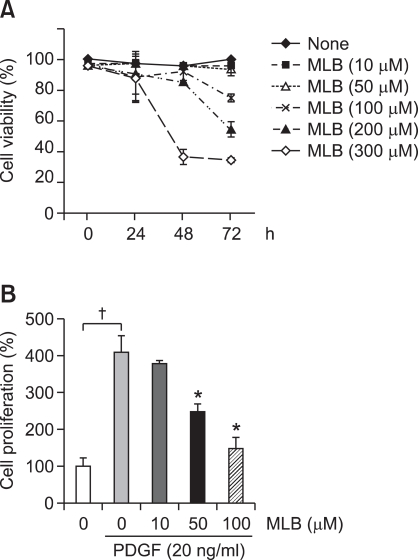
To investigate the underlying mechanism of the antifibrotic effects of MLB, we performed in vitro studies using immortalized human HSCs, the main fibrogenic cell type in the liver. First, we examined the cytotoxicity of MLB in cultured HSCs. Incubation of HSCs with MLB (≤100 µM) for up to 48 h did not show any discernable cytotoxicity. The estimated cell viability as assessed by MTT assay after 72 h of treatment with 50 µM MLB was 93.4% ± 4.1% (Figure 5A). We then assessed the effect of MLB on HSC proliferation. Cell proliferation measured by MTT assay after 24 hours is shown in Figure 5B. The cell number of HSCs increased significantly after PDGF stimulation (P < 0.01). PDGF-induced HSC proliferation was significantly suppressed by pretreatment with MLB in a dose-dependent manner (P < 0.05).
Figure 5.
Effects of MLB on HSCs viability and proliferation. (A) Cytotoxicity by MLB was tested in HSCs. HSCs were treated with various concentrations (0-300 µM) of MLB for up to 72 h in a serum-free condition. Cytotoxicity was assessed by MTT assay. Data represent the mean ± SD of two independent experiments. (B) Effect of MLB on cell proliferation was tested in HSCs. HSCs were stimulated with PDGF (20 ng/ml) for 24 h with various concentrations (0-100 µM). Data represent the mean ± SD of two independent experiments. †P < 0.01 between no treatment and PDGF only, *P < 0.05 compared with PDGF only.
MLB suppresses NF-κB activation and MCP-1 production in HSCs
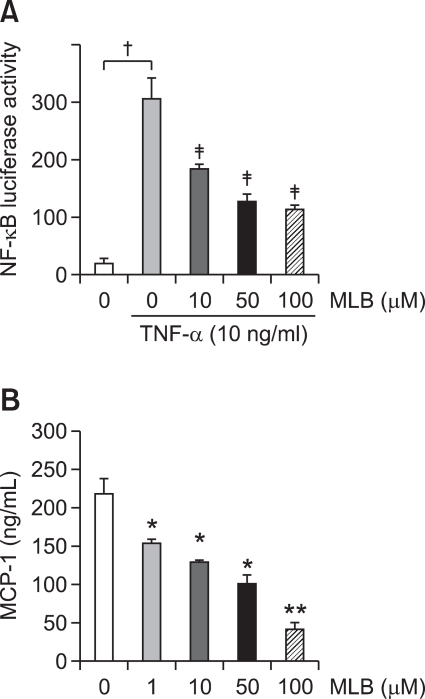
We next examined whether MLB has an anti-inflammatory effect on HSCs. NF-κB transcriptional activation was assessed using an NF-κB-dependent luciferase assay and monocyte chemotactic protein-1 (MCP-1) from the culture supernatant was measured using an ELISA assay. As shown in Figures 6A and 6B, MLB inhibited NF-κB activation and MCP-1 production in HSCs in a dose-dependent manner, indicating that MLB has an anti-inflammatory effect on HSCs.
Figure 6.
MLB attenuates NF-κB transcriptional activation and inflammatory chemokine MCP-1 production in HSCs. (A) HSCs were infected with the Ad5NF-κBLuc (MOI 500) for 12 h in DMEM containing 0.5% FBS. At 20 h post-infection, HSCs were treated with TNF-α (10 ng/ml) for 8 h with pretreatment of various concentrations of MLB (0-100 µM) for 1 h. Cells were lysed and NF-κB-mediated luciferase activity was quantified. Data represent the mean ± SD of 2 independent experiments and are expressed as fold-increase over unstimulated cells. All measurements of luciferase activity were normalized to the protein concentration. †P < 0.01 between no treatment and TNF-α only, ‡P < 0.01 compared with TNF-α alone. (B) Secreted MCP-1 in culture medium was quantified by ELISA. After 48 h serum starvation, HSCs were cultured in medium with 1% FBS for 24 h with various concentrations of MLB (0-100 µM). Data represent the mean ± SD of two independent experiments. *P < 0.05 compared with no MLB treatment, **P < 0.01 compared with no MLB treatment.
MLB inhibits ROS generation and type I collagen secretion in HSCs
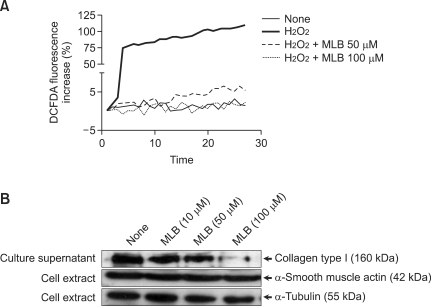
It has been reported that ROS generation stimulates type I collagen production in HSCs. We continuously measured intracellular ROS formation in HSCs using a fluorometer after loading cells with the redox-sensitive dye CM-H2DCFDA. As shown in Figure 7A, 100 µM of H2O2 markedly induced ROS production in HSCs. However, HSCs pre-incubated with MLB (≥50 µM) showed markedly reduced ROS production after stimulation with H2O2. Finally, we measured the secreted type I collagen from HSCs in the culture supernatant. Secretion of type I collagen was inhibited by treatment with MLB (≥50 µM) (Figure 7B). However, the expression of α-SMA was not affected by treatment with MLB.
Figure 7.
MLB causes decreases in ROS production and type I collagen secretion in HSCs. (A) Serum starved HSCs were pre-incubated with various concentrations of MLB (0-100 µM). After loading redox-sensitive dye CM-H2DCFDA (10 µM) into HSCs, cells were stimulated with 100 µM H2O2 and fluorescence was measured with a spectrometer. (B) HSCs were treated with various concentrations of MLB (0-100 µM) for 48 h. Western blot of the precipitated proteins from the culture supernatant probed for type I collagen; Western blot of cellular proteins (20 µg/ml) probed for α-SMA or α-tubulin.
Discussion
Several investigators reported that water-soluble crude extracts of Salvia miltiorrhizae reduced hepatic fibrosis caused by CCl4 administration and BDL in rats (Wasser et al., 1998; Nan et al., 2001; Lee et al., 2003). Water-soluble components extracted from Salvia miltiorrhizae contain at least six phenolic constituents including MLB, rosmarinic acid (RA), lithospermic acid (LA), caffeic acid (CAA), protocatechuic aldehyde (PAL), and 3,4-dihydroxyphenyllactic acid (danshensu) (Li et al., 2005). However, it is still unknown which hydrophilic component of Salvia miltiorrhizae exerts antifibrotic effects in the liver. In this study, we demonstrated that purified MLB has an anti-fibrotic effect on TAA-induced hepatic fibrosis in rats. We also demonstrated that purified MLB inhibits the proliferation, chemokine production, ROS production and type I collagen secretion of HSCs, a main fibrogenic cell type in the liver. Our data indicates that MLB is an active anti-fibrogenic component of Salvia miltiorrhizae and exerts an antifibrotic effect through the suppression of proinflammatory and fibrogenic responses in HSCs.
In this study we investigated the effects of purified MLB on TAA-induced hepatic fibrosis in rats. TAA is a potent hepatotoxin. It is transformed into TAA sulfoxide or TAA sulfone by cytochrome P450 and induces oxidative damage to the liver (Wasser and Tan, 1999). When compared to CCl4-induced hepatic fibrosis, TAA-induced hepatic fibrosis in rodents shows more prominent regenerating of cirrhotic nodules and is histologically more similar to human liver cirrhosis. In our in vivo study, the rats were sacrificed after 8 or 12 weeks of TAA treatment. The condition of the rats treated with TAA for 8 weeks corresponded to the early stages of liver cirrhosis, and that of the rats treated for 12 weeks corresponded to overt liver cirrhosis. We used 40 mg/kg/d of MLB for the in vivo experiment, because 25 mg/kg/d crude extract of Salvia miltiorrhizae had a modest hepatoprotective effect and 50 mg/kg/d of Salvia miltiorrhizae showed a significant hepatoprotective effect in a CCl4-treated liver (Lee et al., 2003). Oral administration of MLB significantly attenuated liver fibrosis and activation of HSCs in both the early and late stages of liver cirrhosis in rats. Interestingly, MLB attenuated the extent of TAA-induced liver injury only in 8-week treated rats, but not in 12-week treated rats. Taken together, these results suggest that MLB has a direct anti-fibrogenic effect and has a modest hepato-protective effect as well.
Based on the in vivo results, we investigated the underlying mechanism of the anti-fibrotic effect of MLB in HSCs, a main fibrogenic cell type in the liver. It was reported that Salvia miltiorrhizae attenuates TGF-β1-induced mRNA upregulation of fibrosis related genes, including α-SMA, connective tissue growth factor (CTGF), and TIMP-1 in rat HSCs (Hsu et al., 2005). HSC activation and proliferation is of crucial importance in hepatic fibrogenesis. PDGF appears to be the most potent mitogen of HSCs during hepatic fibrogenesis (Marra et al., 1994). Our in vitro experiments demonstrated that MLB inhibits PDGF-induced HSC proliferation in a dose-dependent manner. Activated HSCs produce various kinds of cytokines and chemokines in response to liver injuries, resulting in the recruitment of other inflammatory cells and HSCs to the injured site (Bataller and Brenner, 2005). NF-κB is an important signaling molecule involved in inflammatory signaling in HSCs (Paik et al., 2003). Our results show that MLB inhibits NF-κB transcriptional activation and suppresses downstream pro-inflammatory chemokine MCP-1 production in HSCs. These anti-proliferative and anti-inflammatory effects of MLB on HSCs may contribute at least partially to the in vivo anti-fibrotic effects of MLB.
It has been demonstrated that ROS plays an important role in hepatic fibrogenesis from various liver injuries including alcohol abuse, hepatitis C virus infection, iron overload and chronic cholestasis (Pietrangelo, 1998; Poli and Parola, 1997; Parola and Robino, 2001). Type I collagen is a main extracellular matrix that is accumulated in cirrhotic liver. ROS is directly fibrogenic because it induces activation of the collagen type I gene in HSCs (Nieto et al., 2000). ROS also stimulates the proliferation and invasiveness of HSCs (Galli et al., 2005). We demonstrated that MLB suppresses ROS production and type I collagen production in HSCs. These results indicate that MLB has a potent anti-oxidative effect and a direct anti-fibrotic effect on HSCs.
In this study, we demonstrated the anti-fibrotic effect of MLB in TAA-induced hepatic fibrosis in rats. Our in vitro data indicate that MLB has anti-proliferative, anti-inflammatory, anti-oxidative, and anti-fibrotic effects on activated HSCs. Taken together, these results suggest that MLB may be used as an effective anti-fibrotic agent for the hepatic fibrosis. Further clinical trials examining the anti-fibrotic effects of MLB in patients with liver cirrhosis are warranted.
Methods
Magnesium lithospermate B preparation
MLB was isolated and purified from Salvia miltiorrhizae as described previously (Jung et al., 2002). Slices of the dried roots of Salviae miltiorrhizae (1.0 kg) were placed in water at 100℃ for two hours. The heating water extract was evaporated to dryness under reduced pressure at 70℃. The heating water extract was suspended in water (500 mL), and the water extract was adjusted to pH 3.5 with hydrogen chloride (HCl) and extracted with saturated butyl alcohol (300 ml × 5 each). The butyl alcohol extract was evaporated to dryness under reduced pressure at 70℃. Then, the butyl alcohol extracts were suspended in water (300 ml) and the water extracts were washed with hexane (300 ml × 3 each). The water extract was evaporated to dryness under reduced pressure at 70℃. The water extract was extracted with ethyl acetate (300 ml × 3 each). The ethyl acetate extract and water extract were evaporated to dryness under reduced pressure at 70℃. The ethyl acetate extract and the water extract were purified by recrystallization and fractional crystallization. The yield of the MLB as amorphous powder was 10 g from Salviae miltiorrhizae (1.0 kg). All spectral data (NMR, 13C-NMR, IR, HR-MS) of MLB were identical to those of literature (Yokozawa et al., 1989). The chemical structure of MLB is illustrated in Figure 1.
Animal experiments of hepatic fibrosis
Six-week-old male Sprague-Dawley rats were prepared. Hepatic fibrosis was induced by an intraperitoneal injection of TAA (Sigma, St. Louis, MO) at a dose of 200 mg/kg twice a week for up to 12 weeks. Rats were fed MLB once daily for up to 12 weeks at a dose of 40 mg/kg using a gavage tube. The rats were randomly assigned to six experimental groups: the control group (n = 5), the control + MLB group (n = 5), the TAA 8-week group (n = 5), the TAA + MLB 8-week group (n = 5), the TAA 12-week group (n = 5), and the TAA + MLB 12-week group (n =5). The TAA 8-week group and the TAA + LAB 8-week groups were sacrificed 8 weeks after the first TAA injection. All of the other rats were sacrificed at week 12. At the time of sacrifice, blood and liver samples were harvested. The livers were fixed in 10% formalin for histological analysis. Portions of liver tissue were immediately frozen in liquid nitrogen for further RNA and protein analysis. All experimental procedures were performed according to the guidelines for the care and use of animals established by Yonsei University College of Medicine.
Assessment of hepatic fibrosis
Four-micrometer-thick sections of formalin-fixed and paraffin-embedded livers from all the experimental groups were processed for hematoxylin and eosin staining (H&E) and Masson's trichrome staining. Tissue sections were immunostained for α-SMA (Dako North America, Inc., Carpinteria, CA), diluted at 1:1000. Stained liver sections were analyzed with the computerized imaging analysis system (MetaMorph version 4.65, Universal Imaging Corp., Downingtown, PA). Hydroxyproline content was quantified colorimetrically from 0.2 g liver samples as previously described (Bataller et al., 2003). The results were expressed as micrograms of hydroxyproline per grams of liver.
Serum biochemistry
Rat whole blood was obtained at the time of sacrifice via vena cava puncture. After centrifugation of each blood sample, the serum was obtained and examined for both aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
RNA isolation and quantitative RT-PCR
Total RNA was extracted from frozen rat liver tissues, using Takara RNA PCR (AMV) Ver 3.0 (Takara Bio Inc., Shiga, Japan) as described in the product protocol, and cDNA was generated from 1 µg of total RNA, using oligo dT-Adaptor primers and Avian Myeloblastosis Virus reverse transcriptase (Life Science, Boston, MA). α-SMA, TGF-β1, collagen α1(I) mRNA in rat liver samples were quantified by quantitative RT-PCR using a Taqman probe (Roche, Mannheim, Germany) and LightCycler® (Roche Diagnostics GmbH, Mannheim, Germany). The PCR primers for α-SMA were 5'-CGATAGAACACGGCATCATCAC-3' sense and 5'-GCATAGCCCTCATAGATAGGCA-3' antisense. The PCR primers for TGF-β1 were 5'-CCTGGAAAGGGCTCAACAC-3' sense and 5'-CAGTTCTTCTCTGTGGAGCTGA-3' antisense. The PCR primers for collagen α1(I) were 5'-CATGTTCAGCTTTGTGGACCT-3' sense and 5'-GCAGCTGACTTCAGGGATGT-3' antisense. The relative expression of target gene mRNA was normalized to the amount of rat porphobilinogen deaminase (PBGD) mRNA in an identical cDNA sample (de Kok et al., 2005). The PCR primers for PBGD were 5'-CACCTGGAATTCAAGAGTATTCG-3' sense and 5'-CCAGGATAATGGCACTGAACT-3' antisense. The relative abundance of the target genes was obtained by calculation using the comparative threshold cycle (Ct) method.
HSCs culture
We used immortalized human HSCs that were established by retroviral expression of human telomerase reverse transcriptase (hTERT) in this study (Schnabl et al., 2002). Telomerase-positive HSCs exibit morphological and functional characteristics of activated HSCs. It was proven that HSCs with hTERT are very useful in researching the biology of activated HSCs (Paik et al., 2006, 2009). HSCs were cultured in a 5% CO2 humidified incubator at 37℃. The cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY) containing 10% fetal bovine serum (FBS) (Invitrogen) and a 1% streptomycin-penicillin mixture.
Cell viability and proliferation assay
Cytotoxicity of MLB in HSCs was assessed using MTT assay. HSCs were seeded at a density of 3 × 104 cells per well in 24-well plates. After 24 h of serum starvation, cells were treated with various concentrations of MLB (0-300 µM). After incubation for up to 72 h, 125 µl of MTT solution (2 mg/ml in PBS) was added to each well and incubated for 4 h. The supernatant was removed and the cells solubilized in DMSO (250 µl) for 30 min. The optical density at 540 nm was measured using an enzyme-linked immunosorbent VERSAmax™ reader (Molecular Devices, Sunnyvale, CA). For the cell proliferation assay, serum-starved HSCs were incubated with PDGF-BB (R&D systems, Minneapolis, MN) with MLB (0-100 µM) for 24 h. The number of viable cells was measured by MTT assay at a wavelength of 540 nm on an ELISA reader.
NF-κB responsive luciferase assay
Recombinant adenoviral vectors expressing a luciferase reporter gene driven by NF-κB transcriptional activation (Ad5NF-κBLuc) were used for the assessment of NF-κB transcriptional activation as described previously (Paik et al., 2003). Briefly, HSCs were infected with Ad5NF-κBLuc (multiplicity of infection 500) for 12 h in DMEM containing 0.5% FBS. After infection, the culture medium was changed to a fresh medium without FBS, and the culture was continued for an additional 8 h. At 20 h post-infection, HSCs were stimulated with TNF-α for 8 h in serum-free conditions with or without pretreatment with MLB. A luciferase assay kit with luciferase cell culture lysis buffer (Promega, Madison, WI) was used to measure NF-κB mediated transcriptional induction according to the manufacturer's protocol. The relative light intensity was measured by a spectrometer and all measurements of luciferase activity were normalized to the protein concentration.
MCP-1 enzyme-linked immunosorbent assay
After 48 h of serum strarvation, HSCs were cultured in medium with 1% FBS for 24 h after pretreatment with MLB for 1 h. Measurements of secreted MCP-1 in the culture supernatant were done by ELISA (R & D Systems, Minneapolis, MN).
Measurement of reactive oxygen species
HSCs were plated at a density of 2 × 104 cells/well in a 96-well plate. After serum starvation for 24 h, cells were preincubated with MLB (0, 50, 100 µM) for 1h. After washing, cells were loaded with a redox-sensitive dye, 10 µM of CM-H2DCFDA (Molecular Probes, Eugene, OR) at 37℃ for 30 minutes, and then cells were rinsed twice with PBS and stimulated with 100 µM hydrogen peroxide (H2O2). CM-H2DCFDA fluorescence was detected at excitation and emission wavelengths of 488 nm and 520 nm, respectively. ROS formation was measured over a time course of 30 minutes using a multi-well fluorescence scanner (Fluostar Optima, BMG Labtech, Cary, NC).
Western blot
For detection of collagen I secretion, cells were cultured in 100 mm culture dishes (106 cells per dish). Cells were treated with MLB for 48 h, and 4 ml of cell media were precipitated with 0.76 g of sodium sulfite at 4℃ for 3 h and centrifuged at 10,000 g for 30 min. Pellets were resuspended in 0.5 M acetic acid, and 40 µl aliquots were subjected to electrophoresis on a 7.5% acrylamide gel. After blotting, membranes were probed with anti-type I collagen antibody (1:1,000; Biodesign International, Saco, MA), which recognized the heterotrimer of type I collagen. For the detection of cellular α-SMA and α-tubulin, whole-cell extracts were prepared using Triton lysis buffer containing protease and phosphatase inhibitors. Forty micrograms of protein was electrophoresed on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. The gels were blotted onto a nitrocellulose membrane. Antibodies against α-SMA (Dako) and α-tubulin (Santa Cruz biotechnology), all diluted to 1:1000, were used.
Statistics
The data are shown as mean ± standard deviation. Data were analyzed using the Mann-Whitney U test. Data were considered to be statistically significant when P < 0.05.
Acknowledgements
This study was supported by a grant from the 2006 Korean Association for the Study of the Liver (KASL) Research Fund (to P.Y.H) and from the Good Health R & D Project from the Ministry of Health and Welfare, Republic of Korea (A050021) (to H.K.H).
Abbreviations
- HSC
hepatic stellate cell
- MLB
magnesium lithospermate B
- TAA
thioacetamide
- α-SMA
α-smooth muscle actin
- MCP-1
monocyte chemotactic factor-1
- ROS
reactive oxygen species
- CCl4
carbon tetrachloride
- BDL
bile duct ligation
- ECM
extracellular matrix
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- 4.de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–159. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- 5.Eng FJ, Friedman SL. Transcriptional regulation in hepatic stellate cells. Semin Liver Dis. 2001;21:385–395. doi: 10.1055/s-2001-17553. [DOI] [PubMed] [Google Scholar]
- 6.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, Surrenti C, Casini A. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology. 2005;41:1074–1084. doi: 10.1002/hep.20683. [DOI] [PubMed] [Google Scholar]
- 7.Hsu YC, Lin YL, Chiu YT, Shiao MS, Lee CY, Huang YT. Antifibrotic effects of Salvia miltiorrhiza on dimethylnitrosamine-intoxicated rats. J Biomed Sci. 2005;12:185–195. doi: 10.1007/s11373-004-8167-7. [DOI] [PubMed] [Google Scholar]
- 8.Jung M, Lee HC, Ahn CW, Park W, Choi S, Kim H, Cho D, Lee GT, Li HR. Effective isolation of magnesium lithospermate B and its inhibition of aldose reductase and fibronectin on mesangial cell line. Chem Pharm Bull (Tokyo) 2002;50:1135–1136. doi: 10.1248/cpb.50.1135. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TY, Wang GJ, Chiu JH, Lin HC. Long-term administration of Salvia miltiorrhiza ameliorates carbon tetrachloride-induced hepatic fibrosis in rats. J Pharm Pharmacol. 2003;55:1561–1568. doi: 10.1211/0022357022098. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Yu C, Cai Y, Liu G, Jia J, Wang Y. Simultaneous determination of six phenolic constituents of danshen in human serum using liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820:41–47. doi: 10.1016/j.jchromb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Marra F, Choudhury GG, Pinzani M, Abboud HE. Regulation of platelet-derived growth factor secretion and gene expression in human liver fat-storing cells. Gastroenterology. 1994;107:1110–1117. doi: 10.1016/0016-5085(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 13.Nan JX, Park EJ, Kang HC, Park PH, Kim JY, Sohn DH. Anti-fibrotic effects of a hot-water extract from Salvia miltiorrhiza roots on liver fibrosis induced by biliary obstruction in rats. J Pharm Pharmacol. 2001;53:197–204. doi: 10.1211/0022357011775406. [DOI] [PubMed] [Google Scholar]
- 14.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 15.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 16.Paik YH, Lee KS, Lee HJ, Yang KM, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Moon YM, Brenner DA. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006;86:676–686. doi: 10.1038/labinvest.3700422. [DOI] [PubMed] [Google Scholar]
- 17.Paik YH, Kim JK, Lee JI, Kang SH, Kim DY, An SH, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Lee KS, Brenner DA. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58:1517–1527. doi: 10.1136/gut.2008.157420. [DOI] [PubMed] [Google Scholar]
- 18.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 19.Pietrangelo A. Iron, oxidative stress and liver fibrogenesis. J Hepatol. 1998;28(Suppl 1):8–13. doi: 10.1016/s0168-8278(98)80368-1. [DOI] [PubMed] [Google Scholar]
- 20.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 21.Schnabl B, Choi YH, Olsen JC, Hagedorn CH, Brenner DA. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82:323–333. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- 22.Wasser S, Ho JM, Ang HK, Tan CE. Salvia miltiorrhiza reduces experimentally-induced hepatic fibrosis in rats. J Hepatol. 1998;29:760–771. doi: 10.1016/s0168-8278(98)80257-2. [DOI] [PubMed] [Google Scholar]
- 23.Wasser S, Tan CE. Experimental models of hepatic fibrosis in the rat. Ann Acad Med Singapore. 1999;28:109–111. [PubMed] [Google Scholar]
- 24.Yokozawa T, Chung HY, Oura H, Nonaka G, Nishioka I. Isolation of a renal function-facilitating constituent from the Oriental drug, salviae miltiorrhizae radix. Nippon Jinzo Gakkai Shi. 1989;31:1091–1098. [PubMed] [Google Scholar]
- 25.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]