Abstract
Chronic and juvenile myelomonocytic leukemias (CMML and JMML) are aggressive myeloproliferative neoplasms that are incurable with conventional chemotherapy. Mutations that deregulate Ras signaling play a central pathogenic role in both disorders, and Mx1-Cre, KrasLSL-G12D mice that express the Kras oncogene develop a fatal disease that closely mimics these two leukemias in humans. Activated Ras controls multiple downstream effectors, but the specific pathways that mediate the leukemogenic effects of hyperactive Ras are unknown. We used PD0325901, a highly selective pharmacological inhibitor of mitogen-activated protein kinase kinase (MEK), a downstream component of the Ras signaling network, to address how deregulated Raf/MEK/ERK signaling drives neoplasm formation in Mx1-Cre, KrasLSL-G12D mice. PD0325901 treatment induced a rapid and sustained reduction in leukocyte counts, enhanced erythropoiesis, prolonged mouse survival, and corrected the aberrant proliferation and differentiation of bone marrow progenitor cells. These responses were due to direct effects of PD0325901 on Kras mutant cells rather than to stimulation of normal hematopoietic cell proliferation. Consistent with the in vivo response, inhibition of MEK reversed the cytokine hypersensitivity characteristic of KrasG12D hematopoietic progenitor cells in vitro. Our data demonstrate that deregulated Raf/MEK/ERK signaling is integral to the growth of Kras-mediated myeloproliferative neoplasias, and further suggest that MEK inhibition could be a useful way to ameliorate functional hematologic abnormalities in patients with CMML and JMML.
Introduction
Somatic mutations in the NRAS or KRAS oncogenes are identified in 20–40% of patients with chronic or juvenile myelomonocytic leukemias (CMML or JMML). Mutations in related molecules collectively implicate hyperactive Ras signaling in ~50% of CMML and 90% of JMML (1). The lack of compounds that directly inhibit oncogenic Ras has led to widespread efforts to find alternative therapeutic targets. We previously developed a genetically engineered mouse model that recapitulates many features of human myeloproliferative neoplasms. In this animal model, KrasLSL-G12D mice carry a conditional Kras allele that expresses oncogenic K-RasG12D after an upstream “stop” cassette is removed by Cre recombinase (2). Mx1-Cre transgenic mice express Cre in response to polyinosinic-polycytidylic acid (pIpC). Therefore, Mx1-Cre, KrasLSL-G12D mice express KrasG12D from the endogenous locus after treatment with pIpC. These mice (hereafter designated Mx1-Cre, KrasG12D) rapidly develop a progressive myeloproliferative neoplasm that is characterized by leukocytosis, splenomegaly, and severe anemia (3, 4). The similarities of this model to human myeloproliferative neoplasms suggest that it might be useful for studying disease mechanisms and for testing potential therapeutic strategies.
We previously showed that the Raf/MEK/ERK signaling pathway is modestly hyperactive in primary hematopoietic progenitor cells from the bone marrow of Mx1-Cre, KrasG12D mice (5). Because numerous pathways are potentially deregulated by oncogenic Ras, the importance of deregulated Raf/MEK/ERK signaling in KrasG12D-driven myeloproliferative neoplasia remains unclear (6). To address this question and to evaluate alternative therapeutic strategies for CMML and JMML, we treated Mx1-Cre, KrasG12D mice with PD0325901, a potent and highly specific inhibitor that binds to an allosteric site on mitogen-activated protein kinase kinase (MEK) that is not conserved in other protein kinases (7–9). We show that PD0325901 treatment improves multiple hematologic abnormalities in Mx1-Cre, KrasG12D mice by direct effects on bone marrow progenitor cells that express oncogenic Kras. This demonstrates that deregulated Raf/MEK/ERK signaling is integral to Kras-mediated myeloproliferative neoplasia, and suggests that MEK inhibition may be a useful approach for treating patients with CMML and JMML.
RESULTS
PD0325901 inhibits MEK in vivo
The ability of PD0325901 to inhibit MEK in vivo was validated by measuring ERK phosphorylation induced by GM-CSF stimulation of primary hematopoietic progenitor cells (Fig. 1A). In a flow cytometry based assay, Lin−/lo c-kit+ CD34+ CD105− bone marrow cells were enriched for myeloid progenitors that responded to GM-CSF. We treated Mx1-Cre, KrasG12D mice with PD0325901 and measured the ability of GM-CSF to evoke protein phosphorylation in bone marrow harvested at various times after administration. An oral dose of 5 mg/kg suppressed the ability of GM-CSF to phosphorylate ERK in mouse bone marrow cells for 18–24 h (Fig. 1B), which is consistent with previous data in this mouse strain (10). Phosphorylation of STAT5, which is independent of Raf/MEK/ERK activity, was unimpaired (Fig. 1C), consistent with the expected specificity of PD0325901.
Fig. 1. PD0325901 inhibits MEK in vivo.
(A) Flow cytometry was used to measure ERK and STAT5 phosphorylation in primary myeloid progenitor cells from the bone marrow of Mx1-Cre, KrasG12D and wild-type mice in the presence or absence of 10 ng/mL GM-CSF. After gating for surface-marker expression, phosphoprotein staining was analyzed as a histogram (open) in comparison with an unstimulated control samples (gray). (B) Mx1-Cre, KrasG12D mice were treated with a single dose of the MEK inhibitor PD0325901 (PD) or vehicle, and bone marrow and spleens were harvested 12 h, 18 h, and 24 h later. Bone marrow and spleen cells were stimulated with GM-CSF and analyzed for phosphorylated ERK (pERK). (C) Bone marrow and spleen cells were analyzed for phosphorylation of STAT5 (pSTAT5), 12 h after treatment with PD0325901. The phosphorylation of STAT5 in cells that do not phosphorylate ERK indicates that some signals induced by GM-CSF are intact after exposure to PD0325901, demonstrating specific inhibition of the Raf/MEK/ERK pathway.
PD0325901 controls disease in Mx1-Cre, KrasG12D mice
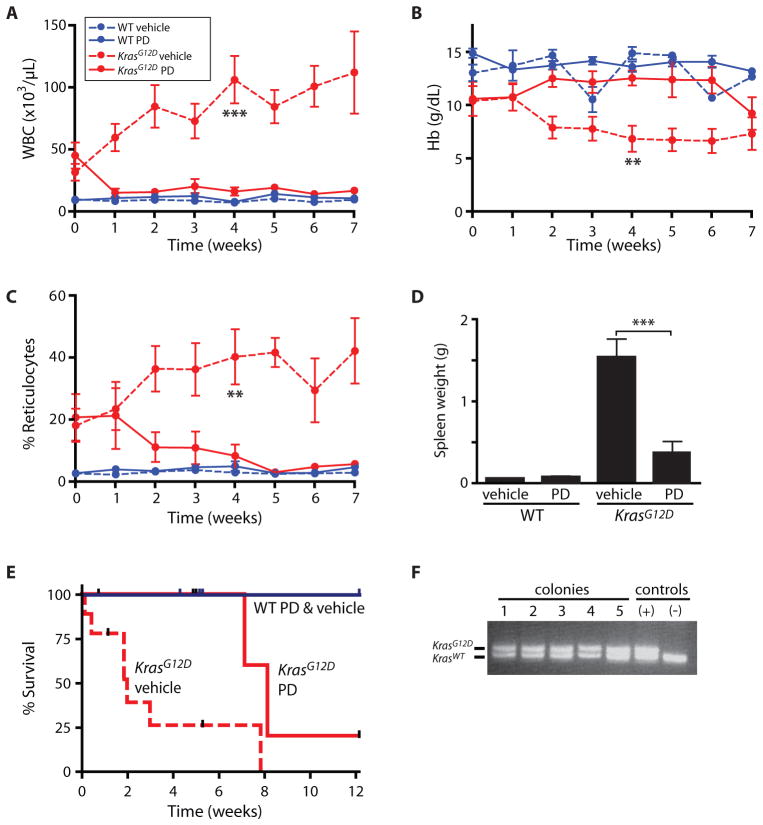
To investigate whether PD0325901 reduces the severity of disease in Mx1-Cre, KrasG12D mice, we induced KrasG12D expression in 3–4 week old pups and allowed the myeloproliferative neoplasia to progress until the age of 8 weeks. The disease was well-established by this time, as indicated by high blood leukocyte counts (41,000/μL ± 25,000 s.d.) (Fig. 2A) and low hemoglobin concentrations (10.6 g/dL ± 3.8 s.d.) (Fig. 2B), compared with wild-type control mice. Mx1-Cre, KrasG12D mice and wild-type littermates were then randomized to receive PD0325901 at a dose of 5 mg/kg/day or vehicle treatment. Mx1-Cre, KrasG12D mice that received the PD0325901 MEK inhibitor demonstrated rapid improvements in composition of the peripheral blood, with reduced leukocyte counts (Fig. 2A), disappearance of anemia (Fig. 2B) and reticulocytosis (Fig. 2C), and reduced splenomegaly (Fig. 2D). Daily treatment with PD0325901 also prolonged dramatically the survival of Mx1-Cre, KrasG12D mice compared with vehicle-treated mice (8.1 vs. 2.0 weeks on trial; p<0.0001 by log rank test) (Fig. 2E). Two of three Mx1-Cre, KrasG12D mice treated for 12 weeks died with KrasG12D T-lineage leukemia/lymphoma (T-ALL), suggesting that some hematopoietic malignancies are not susceptible to MEK inhibition (10). There were no adverse effects of PD0325901 administration observed in wild-type mice.
Fig. 2. PD0325901 controls myeloproliferative neoplasia in Mx1-Cre, KrasG12D mice.
(A) Total leukocyte (WBC) count, (B) hemoglobin (Hb) concentration, and (C) reticulocyte frequency in peripheral blood of Mx1-Cre, KrasG12D (red) or wild-type control (blue) mice treated with PD0325901 (PD; solid line) or vehicle (dashed line). Error bars show s.e.m. (n=5–7 per group). Significance by Student t test comparing PD0325901 treated vs. vehicle treated Mx1-Cre, KrasG12D mice at the 4 week time point: *p<0.05; **p< 0.01; ***p< 0.001. (D) Mouse spleen weight after 5 weeks of therapy. (E) Kaplan-Meier survival estimates indicating a survival benefit to PD0325901 treatment in Mx1-Cre, KrasG12D mice with significance of p<0.0001 by the log rank test (n=6 and 7, respectively, for wild-type mice treated with PD0325901 and vehicle; n=8 and 9 for Mx1-Cre, KrasG12D mice treated with PD0325901 and vehicle).
(F) Genotypes of individual granulocyte/macrophage colonies (lanes 1–5) derived from the bone marrow of a representative Mx1-Cre, KrasG12D mouse treated daily with PD0325901 for five weeks. Each colony was derived from a single myeloid progenitor, indicating a predominance of cells with a heterozygous KrasG12D/+ genotype in the progenitor population. Lanes (+) and (−) show controls for KrasG12D/+ and wild-type genotypes, respectively. A total of 44 colonies grown from three independent KrasG12D mice that received PD0325901 were analyzed, and all demonstrated Cre-mediated activation of the KrasG12D allele.
PD0325901 improves myeloid and erythroid differentiation in vivo
To investigate the mechanism of this response to MEK inhibition, we first explored whether PD0325901 treatment eliminated Kras mutant cells from the bone marrow. We used PCR genotyping to determine the configuration of the conditional Kras allele in myeloid colonies obtained from Mx1-Cre, KrasG12D mice treated with PD0325901. Because each colony is derived from a single progenitor cell, termed a granulocyte/macrophage colony forming unit (CFU-GM), this analysis estimates the frequency of progenitor cells that express oncogenic KrasG12D. This assay demonstrated that the majority of CFU-GM contained the mutant Kras allele in the expressed configuration that lacks the “stop” cassette (Fig. 2F), even after 5 weeks of PD0325901 treatment. The improvement in blood counts without a change in the genotype of progenitor cells suggests that MEK inhibition alters the behavior of hematopoietic cells expressing oncogenic K-RasG12D.
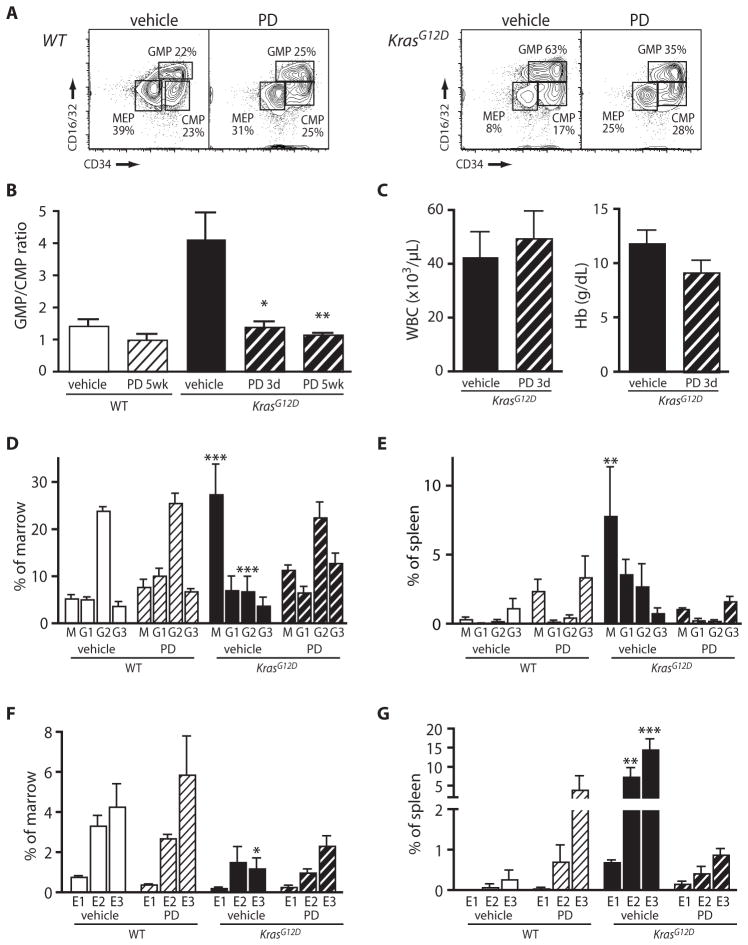
Bone marrow contains discrete populations of multi-potent progenitors with the potential to generate granulocytes and macrophages, megakaryocytes and erythrocytes, or all four myeloid lineages. We used flow cytometry to ascertain whether improved blood composition reflected changes in the abundance of these populations(Fig. 3A) (11). Vehicle-treated Mx1-Cre, KrasG12D mice demonstrated a bias toward granulocyte and macrophage progenitors at the expense of megakaryocyte and erythroid progenitors and progenitors of the four myeloid lineages; conversely, PD0325901 treatment restored the distribution seen in wild-type control mice, in which these populations are approximately equal in size (Fig. 3A,B). Analysis after only three daily doses of PD0325901 showed that changes in progenitor cell distributions precede any improvement in the peripheral blood (Fig. 3B,C). We did not find a significant difference in the numbers of cells expressing hematopoietic stem cell markers in the bone marrow of treated versus control mice (Fig. S1). Together, these data suggest that MEK inhibition reduces myeloproliferative neoplasia severity by modulating the behavior of hematopoietic progenitor cells, but not stem cells, in the bone marrow.
Fig. 3. PD0325901 improves myeloid and erythroid differentiation in vivo.
Frequencies of precursor populations were determined by flow cytometry(A) Typical distributions of myeloid progenitors in bone marrow of wild-type and Mx1-Cre, KrasG12D mice treated with vehicle or PD0325901 (PD) for five weeks. Cells gated for lineage negative/low (Lin−/lo), c-kit+, Sca1− are shown. GMP: granulocyte/macrophage progenitors, MEP: megakaryocyte/erythroid progenitors, CMP: common myeloid progenitors that populate all four myeloid lineages (B) Ratios of GMP to CMP in bone marrow of wild-type control (white bars) or Mx1-Cre, KrasG12D (black bars) mice treated with vehicle (solid bar) or PD0325901 (striped bar) for 3 days or 5 weeks, as indicated. All error bars show s.e.m. (n=3–9 per group). In Mx1-Cre, KrasG12D mice, differences in treatment with vehicle vs. PD are significant according to Welch’s corrected t test at 3 days (*p<0.05) and 5 weeks (**p<0.01). (C) Peripheral blood concentrations of leukocytes (WBC) and hemoglobin (Hb) in Mx1-Cre, KrasG12D mice after 3 days of PD0325901 treatment. Differences are not statistically significant (n=4–6 per group). (D) Bone marrow and (E) spleen populations of monocytes (M; Mac1+ Ly6C+), immature granulocytes (G1; Mac1+ Ly6Cmed Gr1med), intermediate granulocytes (G2; Mac1med Ly6Cmed Gr1high), and mature granulocytes (G3; Mac1high Gr1high). Differences from the vehicle-treated wild-type cohort are indicated (*p<0.05, **p< 0.01, ***p< 0.001), as determined by Bonferroni testing after 2-way ANOVA (n=3–4 mice per group). (F) Bone marrow and (G) spleen distributions of erythroid populations, with erythroid precursors at sequential stages of maturation labeled as E1 (c-kit+ CD105+), E2 (CD71high TER119+), or E3 (CD71med TER119+). Statistical significance is indicated as in Fig. 3D.
Treatment with PD0325901 also normalized the distributions of differentiating myeloid cells. Mx1-Cre, KrasG12D mice demonstrated a relative increase in the percentage of monocytes and a decrease in the percentage of intermediate granulocytes in the bone marrow and the spleen, but treatment with PD0325901 restored percentages of these populations to near normal levels (Fig. 3D, 3E) (4–10% monocytes and 20–30% intermediate granulocytes in bone marrow, and <1% of each in spleen). Similarly, untreated Mx1-Cre, KrasG12D mice progressively lose the TER119+ erythroid population in the marrow, and accumulate immature erythroid cells in the spleen (12). Treatment with PD0325901 partially corrected the distribution of differentiating cells in the bone marrow (Fig. 3F), and eliminated the massive accumulation of splenic erythroblasts (Fig. 3G). Collectively, these studies demonstrate that MEK inhibition alleviates aberrant myelopoiesis and ineffective erythropoiesis in Mx1-Cre, KrasG12D mice. PD0325901 treatment had no adverse hematological effects, such as anemia or leukopenia, in wild-type mice, although we observed an increase in splenic erythroid cells, suggesting that MEK inhibition may impose some stress on normal erythropoiesis. (Fig. 3G).
PD0325901 corrects the hypersensitive growth pattern of Mx1-Cre, KrasG12D myeloid progenitors in vitro
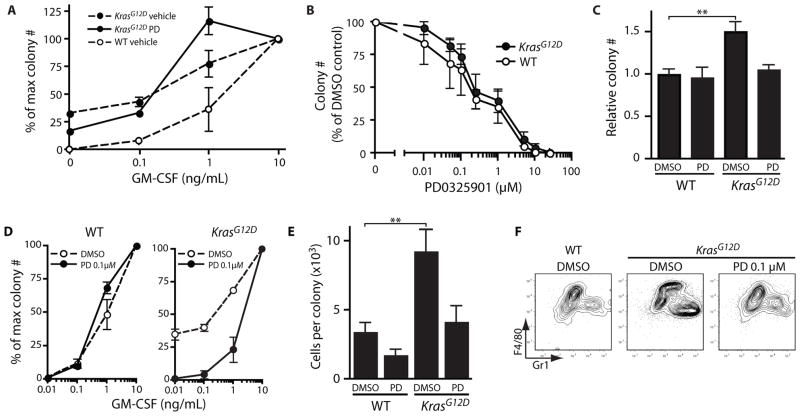
Bone marrow cells from patients with JMML and CMML and from Mx1-Cre, KrasG12D mice display a characteristic hypersensitive growth pattern, resulting in greater production of granulocyte-macrophage colonies in response to GM-CSF (3, 13). CFU-GM progenitors from Mx1-Cre, KrasG12D mice treated with PD0325901 remained hypersensitive to GM-CSF in vitro (Fig. 4A), which is consistent with retention of the activated KrasG12D allele (Fig. 2F). Similarly, we did not observe selective inhibition of Kras mutant CFU-GM progenitor colony growth over a range of PD0325901 concentrations in the presence of a saturating concentration of GM-CSF (Fig 4B). Nevertheless, some specificity was observed in cultures containing a low concentration of PD0325901 (0.1 μM). Whereas this had only modest effects on total number of colonies when GM-CSF was present at saturating levels (Fig. 4C), PD0325901 0.1 μM both abrogated growth of cytokine-independent myeloid colonies and restored a normal response to increasing concentrations of GM-CSF (Fig. 4D). At saturating doses of GM-CSF, a low concentration of PD0325901 (0.1 μM) was sufficient to normalize the numbers and types of cells within the colonies (Fig. 4E,F). Together, these data show that a low concentration of PD0325901 is sufficient to overcome the dominant effects of oncogenic KrasG12D expression and impart a normal program of proliferation and differentiation in primary myeloid progenitors. A structurally distinct MEK inhibitor, PD98059, similarly inhibited the growth of cytokine-independent colonies, confirming that MEK is the physiological target of PD0325901 in this assay (Fig. S2) (14).
Fig. 4. PD0325901 improves function of Mx1-Cre, KrasG12D myeloid progenitors in vitro.
(A) Bone marrow was harvested from wild-type and Mx1-Cre, KrasG12D mice after 5 weeks of treatment with PD0325901 (PD) or vehicle and plated in methylcellulose with a range of GM-CSF concentrations. CFU-GM from Mx1-Cre, KrasG12D mice treated with PD0325901 remain hypersensitive to GM-CSF. Error bars for all graphs show s.e.m., which is smaller than the plotting symbol for some data points. (B) Bone marrow cells from pIpC-treated wild-type or Mx1-Cre, KrasG12D mice were plated in methylcellulose medium containing a high concentration of GM-CSF (10 ng/mL) and varying doses of PD0325901. The DMSO concentration was constant. Colonies were enumerated after 8 days, and counts were normalized to the number arising without PD0325901. CFU-GM from wild-type and Mx1-Cre, KrasG12D mice have similar sensitivities to PD0325901 at saturating concentrations of GM-CSF. (C) A low concentration of PD0325901 (0.1 μM) causes a modest reduction in the number of colonies derived in the presence of 10 ng/mL GM-CSF. A DMSO control is shown for comparison. To account for variation, the number of colonies for each sample was normalized by dividing colony number by the average number of colonies arising from a control sample in (wild-type cells in DMSO) in the same experiment. **p<0.01 vs. wild-type DMSO control by Bonferroni testing after 2-way ANOVA. (D) Bone marrow cells from pIpC-treated wild-type or Mx1-Cre, KrasG12D mice were plated with a range of GM-CSF concentrations and a fixed concentration of PD0325901 (0.1 μM) or DMSO control. (E) CFU-GM from pIpC-treated wild-type or Mx1-Cre, KrasG12D mice were harvested from methylcellulose culture after 7 days with 10 ng/mL GM-CSF and either 0.1 μM PD0325901 or DMSO. Average colony size was calculated as total cell number per plate divided by colony number (n = 3–5). Statistical significance is denoted as in Fig. 4C. (F) Harvested cells were stained for myeloid differentiation antigens F4/80 and Gr1. All colony assays were performed in triplicate from independent mice.
DISCUSSION
Patients with well-differentiated forms of cancer may derive substantial clinical benefit from therapies that modify the aberrant behavior of the malignant clone, without damaging normal tissues, even if the tumor is not eradicated. In this case, the value of therapy lies in reducing the symptoms caused by the neoplasm for a meaningful time period. A classic example of this paradigm is the use of anti-proliferative agents, such as hydroxyurea, in the treatment of chronic myelogenous leukemia (CML), a myeloproliferative neoplasm driven by the aberrant fusion kinase Bcr-Abl (15). Even as highly effective small-molecule-targeted inhibitors of the Bcr-Abl kinase have markedly prolonged the survival of patients with CML, the causative mutation often remains detectable at low levels, and clinical experience supports the idea that a highly beneficial treatment does not always eliminate all mutant stem cells (16). In our studies, Mx1-Cre, KrasG12D mice treated with PD0325901 demonstrated a rapid and sustained improvement in hematopoietic abnormalities, even though the KrasG12D oncogene persisted at high levels in the bone marrow. This is similar to patients with myeloproliferative neoplasms that undergo clinical, but not molecular, remissions. An implication of this idea is that a reduction in oncogene ‘allele burden,’ which is frequently used as a surrogate of disease response in clinical trials, may not always be required for a therapeutic agent to demonstrate clinical utility.
Our data indicate that the dramatic hematological responses observed in Mx1-Cre, KrasG12D mice treated with the MEK inhibitor PD0325901 are not due to a purely anti-proliferative effect, as observed with conventional chemotherapy. The effects of MEK inhibition in Mx1-Cre, KrasG12D mice also contrast with those of Abl kinase inhibitors in patients with CML, where treatment provides a selective advantage to normal myeloid progenitor populations (17). Instead, the resolution of both leukocytosis and anemia indicates that PD0325901 rebalances the output of the hematopoietic system, despite continued KrasG12D expression. This “rebalancing” might indicate that MEK activity regulates the expression of genes controlling lineage choice during hematopoietic differentiation. Such a model would be consistent with studies showing that cytokine stimulation instructs lineage choice in multipotent progenitors (18), and that Ras activation promotes monocytic over granulocytic differentiation (19). Further experiments to identify genes that respond to MEK activity in multipotent cells, and to test their influence on cell fate, are required to address this hypothesis.
Curative therapy for myeloproliferative neoplasia will need to eliminate neoplastic stem cells. Whether or not Kras mutant alleles could be eliminated by MEK inhibition was not fully addressed in these experiments. Because recombination in the bone marrow of Mx1-Cre mice is usually incomplete, Mx1-Cre, KrasG12D mice can be expected to harbor a small pool of stem cells in which the conditional KrasLSL-G12D allele remains in the germline, non-expressed configuration. We have demonstrated such genetic chimerism in the bone marrow of young Mx1-Cre, KrasG12D mice (20). However, we did not determine if this persisted until the initiation of PD0325901 treatment. Interestingly, a similar question applies to patients with myeloproliferative neoplasia; some patients might lose their normal hematopoietic elements over the course of their disease. Although we did not observe emergence of cells lacking oncogenic Kras in these studies, it is possible that longer or more intense MEK inhibition might be more effective. Alternatively, inhibition of other signals evoked by mutant Kras may be required for elimination of mutant stem cells. The retention of KrasG12D stem cells in the bone marrow likely contributed to the development of T-ALL in some mice after prolonged drug treatment, because KrasG12D stem cells can create a reservoir of lymphoid progenitors that are susceptible to undergoing leukemic transformation (20). The observation that these acute leukemias arise during ongoing treatment suggests they are less dependent than myeloproliferative neoplasia on hyperactive Raf/MEK/ERK signaling.
In this study, we have provided direct evidence in vivo that aberrant hematopoiesis caused by hyperactive Ras signaling is mediated by the Raf/MEK/ERK pathway. These results are consistent with a previous investigation, which showed that constitutive activation of MEK could block erythroid differentiation in vitro, (21). However, the requirement for MEK in myeloproliferative neoplasia has been called into question by our previous biochemical studies, which revealed that endogenous levels of KrasG12D do not strongly activate Raf/MEK/ERK signaling (3, 5). This implied that aberrant hematopoiesis might be mediated by other effector pathways regulated by Ras (22). Therefore, the importance of Raf/MEK/ERK signaling in mediating the myeloproliferative phenotype provides insight into the mechanism by which excessive Ras activity causes hematologic malignancies.
Our preclinical data imply that MEK inhibitors might be useful for treating patients with JMML and CMML, by alleviating the burdens of anemia, splenomegaly, and complications from myeloid cell overproduction such as tissue infiltration. In particular, treating children with JMML prior to hematopoietic stem cell transplantation might improve their clinical status by reducing the morbidity caused by infiltration of organs with leukemic cells (1). Many adults with CMML suffer substantial morbidity owing to chronic anemia (23), and a treatment that both reduces myeloproliferation and enhances erythropoiesis, as seen with MEK inhibitors, could prove clinically beneficial.
Materials and Methods
In Vivo MEK Inhibition
All procedures were approved by the institutional animal research committee. F1 (129Sv/Jae x C57BL/6) Mx1-Cre, KrasLSL-G12D mice and littermates were injected intraperitoneally with 250 μg of pIpC (Sigma-Aldrich) at 4 weeks of age. Mice were randomly assigned to cohorts that received either 5 mg/kg PD0325901 (Pfizer) or hydroxypropylmethylcellulose (Sigma-Aldrich) vehicle by gavage once daily. Blood cell and reticulocyte counts were measured weekly using a Hemavet blood analyzer (Drew Scientific) and Retic-Count reagent (BD Biosciences). Mice were removed from study after 5 weeks of PD0325901 treatment, or when Hb <6 g/dL.
Flow Cytometry
Nucleated bone marrow and spleen cells were stained with the following antibodies (eBiosciences, Biolegend, AbCam and BD Biosciences): lineage markers (PE-Cy7–conjugated CD3, CD4, CD5, CD8, B220, TER119, CD11b, or Gr1), FITC-CD34, PE-CD16/32, Pacific Blue-Sca1, APC-750–C-kit for stem cells and progenitors, PE-Cy7-CD11b, FITC-Gr1, PE-Ly6C for myeloid and monocytic precursor maturation (24) and FITC-CD71, PE-Ter119, PE-Cy7-C-kit, Pacific Blue-CD105 for erythroid precursor maturation (25, 26). Intracellular phosphoproteins were analyzed as described (5), with the addition of staining for CD34 and CD105. Antibodies were titrated individually. Data were collected on a LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo (TreeStar).
Colony Assays
Nucleated bone marrow and spleen cells (5 × 104 cells per culture) were suspended in M3231 methylcellulose medium (StemCell) supplemented with recombinant murine GM-CSF (PeproTech) 0.01–10 ng/ml, and/or PD0325901 0.01–25 μM, PD98059 (Cell Signaling Technology) or DMSO 0.2%. CFU-GM colonies were counted on day 7. To evaluate recombination of the KrasLSL-G12D allele, colonies were individually harvested into 10 μL distilled water, and 2 μL was analyzed by PCR (2).
Polymerase chain reaction (PCR)
Genotyping was performed using primers SD5′ (AGCTAGCCACCATGGCTTGAGTAAGTCTGCA), DT5′ IO (GTCGACAAGCTCATGCGGGTG), and LJ3′ (CCTTTACAAGCGCACGCAGACTGTAGA), with 35 cycles of 94°C (30 s), 65°C (90 s), and 72°C (60 s). This reaction yields amplicons of 500 bp for the wild-type Kras allele and 550 bp for the KrasLSL-G12D allele. Recombination of the KrasLSL-G12D allele was determined by PCR with the Advantage-GC kit (Clontech) using primers 5′-1 (GGGTAGGTGTTGGGATAGCTG) and 3′-3 (TCCGAATTCAGTGACTACAGATGTACAGAG), with 35 cycles of 95°C (30 s), 58°C (30 s), and 72°C (30 s); the wild-type Kras allele yields a 285-bp amplicon, and the recombined KrasLSL-G12D allele yields a 315-bp amplicon. The non-recombined KrasLSL-G12D allele does not amplify under these conditions.
Statistics
Data were analyzed using Prism 4.0 software (GraphPad). Student’s t tests, 2-tailed, unpaired, were used to compare blood cell counts and spleen weights after treatment. WBC data were log transformed to correct heteroscedasticity; otherwise, Welsh’s correction was applied when variances were unequal. For bone marrow and spleen population frequencies, and for colony counts, effects of genotype/treatment cohort and populations were analyzed by 2-way ANOVA, and Bonferroni post-test comparisons were performed within each population against the wild-type/vehicle cohort. Kaplan Meier survival analysis was analyzed by the log rank test.
Supplementary Material
Summary.
Inhibiting the Raf/MEK/ERK pathway reverses the harmful effects of oncogenic Kras on hematopoietic differentiation, suggesting a strategy for treating myeloproliferative neoplasms.
Acknowledgments
We are indebted to D. Tuveson and T. Jacks for KrasLSL-G12D mice, and to J. Sebolt-Leopold and Pfizer, Inc. for PD0325901.
Funding: This work was supported by grants from the National Institutes of Health (K08CA103868, K08CA119105, R37CA72614, U01CA84221, and T32CA128583); the Leukemia and Lymphoma Society (LLS7019-04); the V Foundation for Cancer Research; the Aplastic Anemia and Myelodysplastic Syndrome Foundation; the MPD Foundation; the Ronald McDonald House Charities of Southern California/Couples Against Leukemia; the Jeffrey and Karen Peterson Family Foundation; and the Frank A. Campini Foundation. N.L. was supported by the UCSF Helene and Charles Linker Fellowship.
Footnotes
Author contributions: N.L. participated in the design and execution of all experiments. M.F.G. designed and performed initial experiments to evaluate the phenotypic effects of PD325901 in Mx1-Cre, KrasG12D mice. J.O.L. helped define pharmacokinetic and pharmacodynamic properties of PD0325901 and its toxicity in this mouse strain. W.X.H. designed and performed colony assay experiments. J.K.A. maintained mouse strains, and designed and performed pharmacodynamic studies of PD0325901 using flow cytometry. K.S. was instrumental in obtaining PD0325901, aided in the design and interpretation of experiments, and provided ongoing suggestions and discussions throughout the study. B.S.B. conceived and supervised this study, was involved in the design and evaluation of all experiments, and wrote the manuscript with assistance from N.L. and K.S.
Competing interests: None of the authors have competing interests. J.O.L. was not affiliated with Genentech while contributing to this work.
References
- 1.Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22:1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- 2.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J, Le Beau MM, Jacks TE, Shannon KM. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci USA. 2004;101:597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, Johnson L, Akashi K, Tuveson DA, Jacks T, Gilliland DG. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Meter ME, Díaz-Flores E, Archard JA, Passegué E, Irish JM, Kotecha N, Nolan GP, Shannon K, Braun BS. K-RasG12D expression induces hyperproliferation and aberrant signaling in primary hematopoietic stem/progenitor cells. Blood. 2007;109:3945–3952. doi: 10.1182/blood-2006-09-047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AP, Carlson TCG, Loi C, Graziano MJ. Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemother Pharmacol. 2007;59:671–679. doi: 10.1007/s00280-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 9.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 10.Lauchle JO, Kim D, Le DT, Akagi K, Crone M, Krisman K, Warner K, Bonifas JM, Li Q, Coakley KM, Diaz-Flores E, Gorman M, Przybranowski S, Tran M, Kogan SC, Roose JP, Copeland NG, Jenkins NA, Parada L, Wolff L, Sebolt-Leopold J, Shannon K. Response and resistance to MEK inhibition in leukaemias initiated by hyperactive Ras. Nature. 2009;461:411–414. doi: 10.1038/nature08279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 12.Braun BS, Archard JA, Van Ziffle JAG, Tuveson DA, Jacks TE, Shannon K. Somatic activation of a conditional KrasG12D allele causes ineffective erythropoiesis in vivo. Blood. 2006;108:2041–2044. doi: 10.1182/blood-2006-01-013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emanuel PD, Bates LJ, Castleberry RP, Gualtieri RJ, Zuckerman KS. Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood. 1991;77:925–929. [PubMed] [Google Scholar]
- 14.Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, Delaney AM, Kaufman M, LePage S, Leopold WR, Przybranowski SA, Sebolt-Leopold J, Van Becelaere K, Doherty AM, Kennedy RM, Marston D, Howard WA, Smith Y, Warmus JS, Tecle H. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg Med Chem Lett. 2008;18:6501–6504. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Bolin RW, Robinson WA, Sutherland J, Hamman RF. Busulfan versus hydroxyurea in long-term therapy of chronic myelogenous leukemia. Cancer. 1982;50:1683–1686. doi: 10.1002/1097-0142(19821101)50:9<1683::aid-cncr2820500904>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen HG, Holyoake TL. Characterization of cancer stem cells in chronic myeloid leukaemia. Biochem Soc Trans. 2007;35:1347–1351. doi: 10.1042/BST0351347. [DOI] [PubMed] [Google Scholar]
- 17.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 18.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 19.Dorrell C, Takenaka K, Minden MD, Hawley RG, Dick JE. Hematopoietic cell fate and the initiation of leukemic properties in primitive primary human cells are influenced by Ras activity and farnesyltransferase inhibition. Mol Cell Biol. 2004;24:6993–7002. doi: 10.1128/MCB.24.16.6993-7002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabnis AJ, Cheung LS, Dail M, Kang HC, Santaguida M, Hermiston ML, Passegué E, Shannon K, Braun BS. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol. 2009;7:e59. doi: 10.1371/journal.pbio.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Lodish HF. Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood. 2004;104:1679–1687. doi: 10.1182/blood-2004-04-1362. [DOI] [PubMed] [Google Scholar]
- 22.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellström-Lindberg E, Gulbrandsen N, Lindberg G, Ahlgren T, Dahl IMS, Dybedal I, Grimfors G, Hesse-Sundin E, Hjorth M, Kanter-Lewensohn L, Linder O, Luthman M, Löfvenberg E, Oberg G, Porwit-MacDonald A, Rådlund A, Samuelsson J, Tangen JM, Winquist I, Wisloff F. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120:1037–1046. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 24.Trottier MD, Newsted MM, King LE, Fraker PJ. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci USA. 2008;105:2028–2033. doi: 10.1073/pnas.0712003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 26.Pronk CJH, Rossi DJ, Månsson R, Attema JL, Norddahl GL, Chan CKF, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.