Abstract
The transcription factor nuclear factor kappa B (NF-κB) is activated in human breast cancer tissues and cell lines. However, it is unclear whether NF-κB activation is a consequence of tumor formation or a contributor to tumor development.
We developed a doxycycline-inducible mouse model, termed DNMP, to inhibit NF-κB activity specifically within the mammary epithelium during tumor development in the polyoma middle T oncogene (PyVT) mouse mammary tumor model. DNMP females and PyVT littermate controls were treated with doxycycline from 4 to 12 weeks of age. We observed an increase in tumor latency and a decrease in final tumor burden in DNMP mice compared to PyVT controls. A similar effect with treatment from 8 to 12 weeks indicates that outcome is independent of effects on postnatal virgin ductal development. In both cases, DNMP mice were less likely to develop lung metastases than controls. Treatment from 8 to 9 weeks was sufficient to impact primary tumor formation. Inhibition of NF-κB increases apoptosis in hyperplastic stages of tumor development and decreases proliferation at least in part by reducing CyclinD1 expression. To test the therapeutic potential of NF-κB inhibition, we generated palpable tumors by orthotopic injection of PyVT cells and then treated systemically with the NF-κB inhibitor thymoquinone (TQ). TQ treatment resulted in a reduction in tumor volume and weight as compared to vehicle-treated control. This data indicates that epithelial NF-κB is an active contributor to tumor progression and demonstrates that inhibition of NF-κB could have a significant therapeutic impact even at later stages of mammary tumor progression.
Keywords: NF-κB, mammary, tumorigenesis, apoptosis, proliferation
Introduction
To aid discovery of new therapeutics, additional investigation into molecular factors responsible for breast cancer development and progression is required. Nuclear factor kappa B (NF-κB) is a transcriptional regulator of proteins that modulate survival, proliferation, angiogenesis and metastasis (Baud and Karin, 2009). NF-κB is activated in human breast cancer tissues and cell lines (Nakshatri et al., 1997; Sovak et al., 1997; Biswas et al., 2004). However, the precise roles played by NF-κB during breast tumor development have not been extensively investigated in vivo. In particular, the role of NF-κB activity specifically within epithelial cells has not been studied. The transcription factor NF-κB is a dimer formed from a multi-subunit family consisting of p65 (Rel A), Rel B, c-Rel, p105/p50 (NF-κB1), and p100/p52 (NF-κB2) (Ghosh et al., 1998; Vallabhapurapu and Karin, 2009). Activation of NF-κB is regulated by the binding of inhibitory proteins termed IκBs (Baeuerle and Baltimore, 1988; Hayden and Ghosh, 2008). In the canonical NF-κB pathway, a dimer composed of the p65 and p50 subunits is held in the cytosol through binding of the inhibitor IκBα. The phosphorylation of IκBα by IκB Kinase 2 (IKK2) acts as a signal for the ubiquitination and degradation of the inhibitor. p50/p65 then translocates to the nucleus and binds to the promoter of target genes (Baldwin, 1996; Ghosh et al., 1998; Hayden and Ghosh, 2008).
Many investigations concerning activation of NF-κB in human breast cancer cells in vitro have focused on a role of NF-κB in providing resistance to chemotherapeutics. Expression of a super repressor of IκBα in MDA-MB-231 cells increases sensitivity to paclitaxel-induced apoptosis (Patel et al., 2000). Doxorubicin treatment increases NF-κB activity in BT-474 human breast cancer cells and treatment of cells with siRNA against NF-κB subunits p65, p52 and c-Rel leads to a 30–40% increase in doxorubicin toxicity (Tapia et al., 2007). While these studies illustrate the potential for inhibition of NF-κB in chemoresistant tumors they do not determine the role of NF-κB in tumor development.
Few studies have investigated the contribution of NF-κB activity to tumor progression in vivo. MDA-MB-231 human breast cancer cells treated with a dominant negative IKK2 construct prevented xenograft tumor growth in nude mice (Singh et al., 2007). A similar reduction in tumor formation was observed in a study where NAFA murine mammary tumor cells transfected with a dominant negative IκBα construct were injected subcutaneously into FVB mice (Liu et al., 2009). While these studies suggest that NF-κB may contribute to mammary tumor progression, in both cases cells were injected into areas distinct from the mammary gland such that, the models may not accurately represent the appropriate microenvironment for mammary tumor progression. More importantly, both studies used established tumor cell lines so do not address the role of NF-κB activity during the early process of cellular transformation. More relevant to this question was a recent study in which a dominant inhibitor of NF-κB, similar to that used here, was targeted directly to the mammary epithelium under the control of the MMTV promoter and mice subjected to systemic chemical carcinogenesis with MPA/DMBA (Pratt et al., 2009). While NF-κB inhibition had no effect on squamous lesions, induction of mammary luminal lesions was reduced.
In the current study we have developed an inducible mouse model that allows us to inhibit NF-κB activity specifically within mammary epithelial cells in vivo as they are transformed and in discrete stages during tumor progression which we define as the continuum from benign lesion initiated by oncogene expression to malignant tumor. We used the polyoma middle T oncogene (PyVT) transgenic model that effectively represents human mammary tumor development (Lin et al., 2003). We recently generated a transgenic model in which doxycycline (dox) treatment allows the MMTV-reverse tetracycline transactivator (rtTA) to drive inducible mammary epithelial specific expression of a mutated form of IκBα that acts a dominant inhibitor of NF-κB activity (Connelly et al., 2010). In the current study, we have combined these models to generate triple transgenics, termed DNMP, in which NF-κB activity can be inhibited by dox treatment in defined windows during PyVT induced tumorigenesis. Using this new model we demonstrate that inhibition of NF-κB signaling leads to increased tumor latency and decreased tumor burden. In addition, we demonstrate that significant inhibition of tumor progression can be achieved even after initiation of primary tumor formation. This is mediated by an increase in apoptosis in early stage tumors and an inhibition of proliferation throughout tumor development. In an additional model, we demonstrate that systemic inhibition of NF-κB by Thymoquinone (TQ) also inhibits mammary tumor progression. This data shows that NF-κB is an active contributor to tumor progression, not merely up-regulated as a result of tumor formation, and provides in vivo evidence that inhibition of NF-κB may be an effective therapeutic strategy.
Results
Increased NF-κB activity is associated with mammary tumor development in the PyVT model
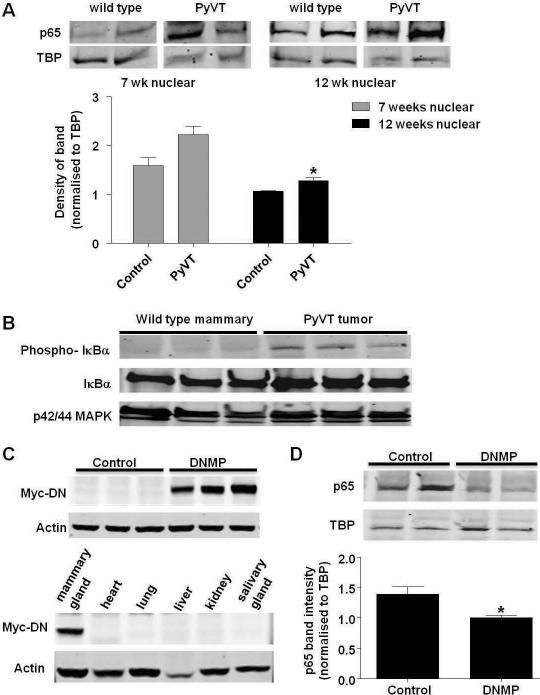
The PyVT mouse mammary tumor model recapitulates the stages of human disease (Lin et al., 2003), however it not known whether this includes activation of NF-κB. Mammary gland nuclear extracts were prepared and western blot performed for the NF-κB p65 subunit as nuclear translocation of p65 indicates NF-κB activation. An increase in nuclear p65 levels was observed in extracts from 7 week old PyVT mice as compared to control. This increase reached significance in extracts from 12 week old PyVT (Figure 1A). We observed phosphorylation of IκBα in orthotopic tumors grown with polyoma mouse mammary tumor cells but not in normal mammary tissue (Figure 1B). These studies confirmed that NF-κB is activated in PyVT tumors which represent an appropriate model to investigate effects of inhibiting NF-κB during mammary tumorigenesis.
Figure 1.
Inhibition of NF-κB activity in the PyVT model. A) Expression of NF-κB p65 in nuclear extracts from 7 week and 12 week old wild type and PyVT mammary glands. Protein expression was analyzed by western blot of nuclear extracts. Western blot for TATA binding protein (TBP) was performed as a loading control. Densitometry was performed on Western blots and levels normalized to TBP loading control. Data are represented as mean ± S.E. band density. * indicates p<0.05, significantly different from control; n=3. B) Expression of phosphorylated IκBα in cytoplasmic extracts from wild type mammary glands and PyVT (L129) orthotopic tumors. Western blot of IκB and p42/44 MAPK was performed as loading controls. Expression of myc-tagged DN-IκBα transgene in C) mammary glands (top) or tissues (bottom) from PyVT control and DNMP mice treated with dox (2g/L from 4 to 12 weeks old). Protein expression was analyzed by western blot of whole cell extracts. Western blot for actin was performed as a loading control (n=5). D) Levels of NF-κB p65 in nuclear extracts from PyVT control and DNMP mammary glands treated with dox (2g/L from 8 to 9 weeks old). Protein expression was analyzed by western blot of nuclear extracts. Western blot for TATA binding protein (TBP) was performed as a loading control. Densitometry was performed on Western blots and levels normalized to TBP loading control. Data are represented as mean ± S.E. band density. * indicates p<0.05, significantly different from control; n=4.
We developed a modular transgenic mouse model to investigate the effects of inhibiting NF-κB in the mammary epithelium during tumorigenesis. The model combines three transgenes, MMTV-rtTA, (tet-O)7-IκB-αDN-Myc-His and PyVT such that treatment of DNMP females with dox induces mammary epithelial expression of the NF-κB inhibitor IκB-αDN on the PyVT background. We have previously used this transgene to inhibit NF-κB activity (Connelly et al., 2010). Littermates lacking the MMTV-rtTA transgene were used as controls (PyVT controls).
Characterization of DNMP mice
DNMP mice and PyVT control littermates were treated with dox from 4 to 12 weeks. Mammary glands were harvested and western blots performed on whole cell extracts to detect expression of the myc-tagged DN-IκBα transgene. Expression of the transgene was observed in DNMP mammary glands and was undetectable in PyVT controls (Figure 1C). Transgene expression was observed in mammary gland but not in the heart, lung, liver, kidney or salivary glands of dox-treated DNMP mice (Figure 1C). To confirm inhibition, western blot for NF-κB p65 was performed on nuclear extracts from mammary glands of DNMP and PyVT control mice treated with dox from 8 to 9 weeks of age. A significant decrease in nuclear levels of p65 was apparent in DNMP mice compared with PyVT controls (Figure 1D). This demonstrates that the DN-IκBα transgene is expressed specifically within mammary and functions to inhibit NF-κB activity.
An increase in tumor latency and a reduction in tumor burden is observed in DNMP mice treated with dox from 4 to 12 weeks old
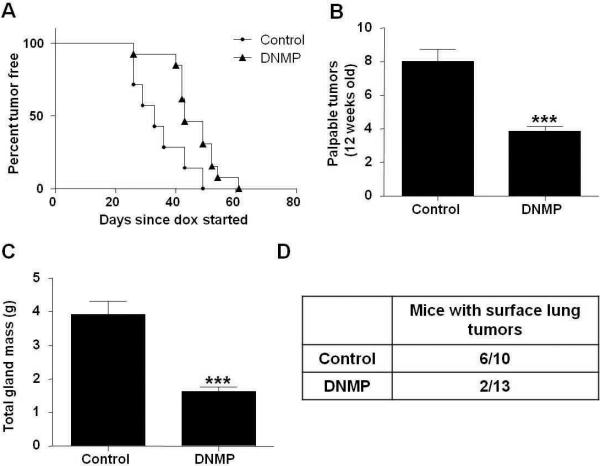
DNMP mice and PyVT controls were treated with dox from 4 to 12 weeks of age, i.e. encompassing primary tumor development through to metastasis to the lungs. There was a significant increase in tumor latency in DNMP mice as compared to PyVT controls (Figure 2A). Control mice and DNMP mice developed palpable tumors with a median time of 33 days or 43 days from dox administration respectively (p<0.05; control n=7 and DNMP n=13). This was accompanied by a reduction in final palpable tumor number (Figure 2B) and in mammary tumor burden (Figure 2C) in DNMP mice upon sacrifice at 12 weeks old. There was also a reduction in the number of lung metastases with 60% of PyVT controls having surface lung metastases as compared with only 15% of DNMP (Figure 2D). Thus, inhibition of NF-κB within the mammary epithelium throughout progression to metastasis has a significant impact on tumor burden.
Figure 2.
An increase in tumor latency and a reduction in tumor burden is observed in DNMP mice treated with dox (2g/L) from 4 to 12 weeks old. A) Mice were palpated twice weekly for formation of mammary gland tumors. Kaplan Meier curves of the time until tumor palpation (p<0.05, significantly different from control; control n= 7, DNMP n=13). Day of dox administration was designated as day zero, day of tumor appearance was calculated from this point. B) Final palpable mammary gland tumor number at 12 weeks. *** indicates p<0.0001, significantly different from control; control n=8, DNMP n=13. C) All mammary glands were collected at 12 weeks and weighed to determine total gland mass (g). *** indicates p<0.0001, significantly different from control; control n=8, DNMP n=13. D) Number of mice per group with at least one surface lung tumor.
Tumor progression is impacted by inhibition of NF-κB activity in the absence of effects on postnatal development
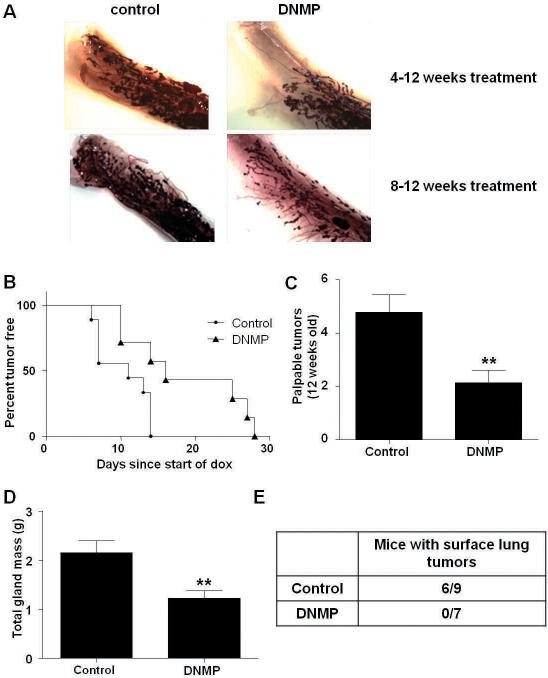
As NF-κB signaling could impact postnatal virgin ductal development we examined mammary gland whole mounts from DNMP and PyVT controls treated from 4 to 12 weeks with dox. This revealed reduced ductal invasion into the mammary fat pads in DNMP mice suggesting that the transgene may inhibit ductal development (Figure 3A). By 8 weeks of age virgin postnatal development is complete with ductal branching having extended throughout the mammary fat pad. Therefore, DNMP mice and PyVT controls were treated with dox from 8 to 12 weeks. No difference in ductal invasion was observed in DNMP mice versus control PyVT for this treatment time frame (Figure 3A).
Figure 3.
An increase in tumor latency and a reduction in tumor burden is observed in DNMP mice treated with dox (2g/L) from 8 to 12 weeks old. A) Whole mount analyses of DNMP and control mice. B) Mice were palpated twice weekly for formation of mammary gland tumors. Kaplan Meier curves show the time until tumor palpation (p<0.05, significantly different from control; control n= 9, DNMP n=7.) Day of dox administration was designated as day zero, day of tumor appearance was calculated from this point. C) Final palpable tumor number at 12 weeks. ** indicates p=0.0082, significantly different from control; control n=9, DNMP n=7. D) All mammary glands were collected at 12 weeks and weighed to determine total gland mass (g). ** indicates p=0.0093, significantly different from control; control n=7, DNMP n=9. E) Number of mice per group with at least one surface lung tumor.
Dox treatment from 8 to 12 weeks led to a significant increase in tumor latency in DNMP mice as compared to PyVT controls (Figure 3B). PyVT controls and DNMP mice developed palpable tumors with a median time of 11 days or 16 days from dox administration respectively (p<0.05; control n=9 and DNMP n=7). This was again accompanied by a decrease in mammary gland palpable tumor number (Figure 3C) and tumor burden (Figure 3D). An absence of metastasis was observed with no surface lung tumors found in DNMP mice (Figure 3E). This data suggests that the impact of NF-κB inhibition observed on tumor progression is not dependent on developmental effects.
Inhibition of NF-κB signaling leads to increased apoptosis during early stages of tumorigenesis
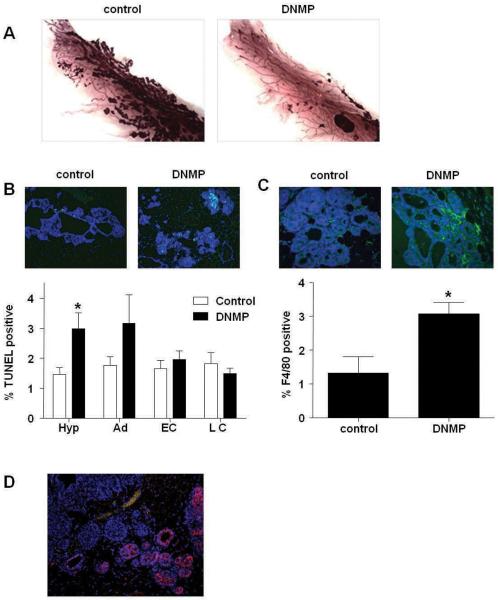
We treated DNMP and PyVT controls with dox from 8 to 9 weeks old to capture a period when signaling changes are occurring that would result in decreases in tumor burden at later stages. Whole mount analyses from DNMP and PyVT controls treated from 8 to 9 weeks of age demonstrate a dramatic reduction in tumor formation in DNMP mice (Figure 4A).
Figure 4.
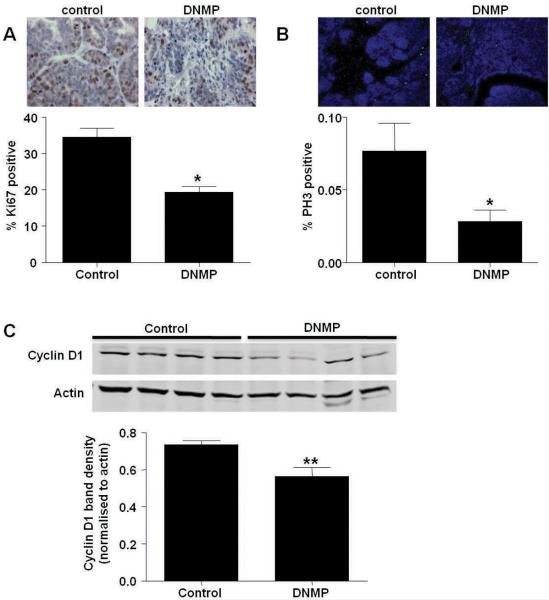
Inhibition of NF-κB signaling leads to increased apoptosis during early stages of tumorigenesis. PyVT controls and DNMP were treated with dox (2g/L) from 8 to 9 weeks old. A) Whole mount analyses. B) TUNEL immunofluorescence staining was performed and quantified according to tumor stage (Hyp = hyperplasia, Ad = adenoma, EC = early carcinoma, LC = late carcinoma). Representative images from hyperplastic lesions (20X) are shown: TUNEL (green) and DAPI (blue). C) F4/80 immunofluorescence staining was performed on hyperplastic lesions in PyVT control and DNMP sections and quantified according to tumor stage. Representative images (40X) are shown: F4/80 (green) and DAPI (blue). TUNEL and F4/80 positive cells were quantified (3 images from each stage) using metamorph software and expressed as a percentage of total cells, * indicates p<0.05, significantly different from control; n=6. D) Myc immunofluorescence staining was performed to detect transgene expression as recognized by the Myc tag. Representative image (20X) of mammary hyperplastic lesions: Myc tag (red) and DAPI (blue).
As NF-κB is known to up-regulate expression of anti-apoptotic proteins (Karin and Ben-Neriah, 2000) we performed TUNEL staining to determine if levels of apoptosis were changed at week 9 after a single week of transgene induction. Analysis of TUNEL staining by tumor stage revealed an increase in apoptosis in hyperplastic lesions in DNMP mice versus PyVT controls (Figure 4B). Quantification of numbers of macrophages in hyperplastic lesions by immunohistochemistry shows an increase in this population in DNMP mammary compared to controls (Figure 4C). It is likely that there is an increased infiltration of macrophages in order to phagocytose the apoptotic cells.
Since we had observed an effect on apoptosis during the early stages of tumorigenesis, but not during later stages, we investigated whether this effect was related to a differential expression of the transgene. We performed immunohistochemistry for myc-tagged transgene expression (Figure 4D). Analyses in DNMP mammary glands treated with dox from 8 to 9 weeks of age revealed a mosaic pattern of transgene expression. Interestingly, stronger staining was observed in areas of normal tissue and in adenomas than at later stages. The lack of significant effects on apoptosis during early and late carcinoma may be due to the pattern of transgene expression.
Inhibition of NF-κB signaling reduces proliferation and Cyclin D1 expression during mammary tumor progression
As NF-κB is known to regulate the expression of proteins involved in proliferation (Baud and Karin, 2009), the effect of DN-IκBα expression on proliferation was investigated in DNMP and PyVT control mammary glands treated with dox from 8 to 9 weeks. Immunohistochemistry and quantification of cells positive for the proliferation-associated antigen Ki67 revealed a significant reduction in proliferation in DNMP mammary (Figure 5A). To look more specifically at effects on mitosis, sections from DNMP and PyVT control glands from mice treated with dox from 8 to 9 weeks were stained for phosphohistone 3. A significant reduction in phospho-histone 3 was observed in DNMP glands as compared with controls indicating a reduction in cells undergoing mitosis (Figure 5B). The cell cycle regulatory protein Cyclin D1 is a key step in cell cycle progression and is up-regulated in human breast cancers (Roy and Thompson, 2006). Increased expression of Cyclin D1 is linked with up-regulation of NF-κB activity in the mammary gland (Cao and Karin, 2003). Western blot for Cyclin D1 in mammary glands from mice treated from 8 to 9 weeks old with dox revealed a significant decrease in Cyclin D1 protein in DNMP mice (Figure 5C). This data suggests that NF-κB signaling in the epithelium during tumor progression may regulate Cyclin D1 expression as one mechanism to effect proliferation.
Figure 5.
Inhibition of NF-κB signaling blocks proliferation and reduces Cyclin D1 expression during mammary tumorigenesis. PyVT controls and DNMP were treated with dox (2g/L) from 8 to 9 weeks old. A) Ki67 immunohistochemistry, representative images (63X) are shown. Ki67 positive cells were counted and expressed as a percentage of total cells, * indicates p<0.05; n=3. B) Phospho-histone H3 (PH3) immunofluorescence staining, representative images (20X) are shown: PH3 (green) and DAPI (blue). PH3 positive cells were quantified using Metamorph software and expressed as a percentage of total cells, * indicates p<0.05, significantly different from control; n=6. C) Expression of Cyclin D1 in whole cell mammary gland extracts. Protein expression was analyzed by western blot. Western blot for Actin was performed as a loading control. Densitometry was performed on Western blots and levels normalized to Actin loading control. Data are represented as mean ± S.E. band density. * indicates p<0.05, significantly different from control; n=4.
NF-κB activity has been linked with promotion of angiogenesis (Liu et al., 2009). We detected no significant differences between DNMP and PyVT control mammary glands treated with dox from 8 to 9 weeks old in levels of Von Willebrand Factor by immunohistochemistry or in mRNA levels of Vascular Endothelial Growth Factor (VEGF) by real time PCR (data not shown).
Treatment with the NF-κB inhibitor thymoquinone (TQ) inhibits mammary tumor growth
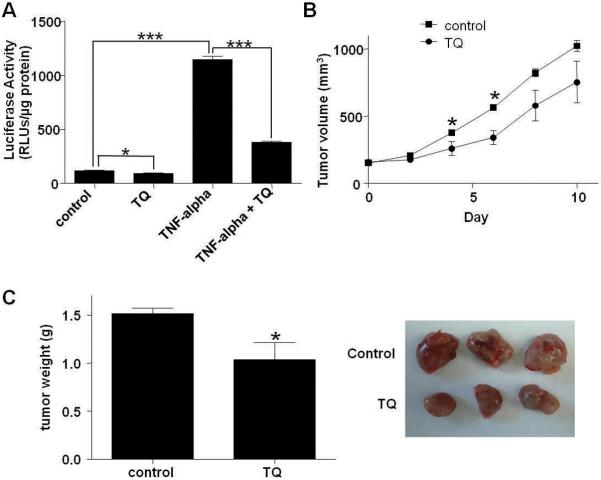
Our transgenic model shows that inhibition of NF-κB activity can inhibit mammary tumor progression. To extend the translational potential of our findings we investigated the impact of systemic NF-κB inhibition on tumor growth. We generated a cell line (PYG129) from a mammary tumor arising in a PyVT transgenic crossed with an NF-κB/luciferase NGL reporter transgenic (Everhart et al., 2006). These cells were injected via the tail vein into a wild type recipient and a second cell line (L129) was derived from the metastases that formed in the mouse lung. The plant derivative thymoquinone (TQ) has been shown to inhibit NF-κB activity and is being investigated as an anti-cancer treatment (Sethi et al., 2008; Jafri et al.). We treated L129 cells with 10ng/ml TNF-α in the presence or absence of 50μM TQ for 6 hours and measured NF-κB activity by luciferase assay. A significant reduction in NF-κB activity was observed in the presence of TQ (Figure 6A).
Figure 6.
TQ inhibits NF-κB activity and reduces growth of orthotopic tumors. A) L129 cells were treated with TNF-α (10 ng/ml) for 6 hours in the presence or absence of TQ (50μM). NF-κB activity was measured by luciferase assay. Data are represented as mean ± S.E. luciferase activity. * indicates p<0.05, *** indicates p<0.0001 significantly different; n=3. L129 cells (1 χ 106) were injected into the fat pads of wild type mice and mice were treated daily from day 10 post-injection with IP injections of TQ (40 mg/kg) or vehicle until harvest. B) Tumor volume was measured with calipers every second day. C) Mammary tumors were collected at 10 days after treatment began and weighed to determine total gland mass (g). Representative tumors from control and TQ treatment groups are shown. * indicates p<0.05, significantly different from control; control n=7, TQ n=6.
Orthotopic injection of 1 × 106 L129 cells into the mammary fat pad of wild type mice led to the formation of a palpable mammary tumor within approximately 7 days. Following detection of a palpable tumor, mice were treated daily by IP injection with 40mg/kg TQ or DMSO vehicle control. TQ treated tumor volumes were lower as compared with the control group (Figure 6B). This was accompanied by a significant reduction in final tumor weight in TQ treated mice (Figure 6C). Similar results were obtained with a second PyVT tumor cell line (data not shown). This data demonstrates that systemic treatment with an NF-κB inhibitor delays tumor expansion.
Discussion
The activation of NF-κB is observed in human breast cancers but whether this transcription factor functionally contributes to disease progression has not been extensively investigated. We have developed a new inducible transgenic mouse model in which NF-κB signaling can be inhibited in the mammary epithelium at specific times during tumor progression in the PyVT mouse mammary tumor model. This allows us to investigate whether NF-κB is an active contributor to tumor development. In initial studies we examined the effects of NF-κB inhibition from 4 until 12 weeks old encompassing the time period from which primary mammary tumors form to metastasis to the lungs in the PyVT model (Lin et al., 2003). We observed a significant inhibition of tumor progression. However, our data and other reports suggested that there may be an effect on development of the virgin gland (Brantley et al., 2001; Hennighausen and Robinson, 2005). To exclude developmental effects from those on tumorigenesis, we inhibited NF-κB from 8 to 12 weeks. Again we observed an increase in tumor latency and a decrease in tumor burden. This suggests that inhibition of NF-κB activity can impact mammary tumor progression independently of effects on development.
In both studies, reduced primary tumor burden was accompanied by a reduced endpoint number of lung metastases. We and others have previously shown that inhibition of NF-κB in the lung epithelium leads to a reduction in lung tumorigenesis (Stathopoulos et al., 2007; Meylan et al., 2009; Basseres et al. 2010) demonstrating that modulation of NF-κB can impact tumor formation in the lung microenvironment. NF-κB modulates expression of genes involved in metastasis such as matrix metalloproteinases 2 and 9 (Bond et al., 1998; Han et al., 2001) therefore an effect on metastasis could be predicted. However, as the current study demonstrates a significant decrease in primary tumor burden, it is possible that the observed decrease in the number of lung metastases stems from decreased seeding from a smaller primary tumor. We are unable to distinguish putative effects on metastasis from those on primary tumor, thus further investigation will be required to establish a direct effect on lung metastasis.
Investigation into the mechanisms responsible for the reduction in primary tumor growth revealed an increase in apoptosis in early stage tumors. This is in agreement with in vitro studies where NF-κB inhibition has been shown to promote apoptosis in human breast cancer cells (Singh et al., 2007). NF-κB activity promotes survival in HeLa cells after mitotic cell cycle arrest is induced by anti-microtubule drugs by blocking apoptosis (Mistry et al., 2004). Our data indicates that NF-κB may protect hyperplastic cells from apoptosis, allowing them to progress to carcinoma.
Mice in which Cyclin D1 expression is driven by the MMTV promoter develop mammary adenocarcinomas suggesting that elevated Cyclin D1 contributes to tumor development (Wang et al., 1994). Cyclin D1 is up-regulated in human breast cancers and regulates the transition from G1 to S phase (Fu et al., 2004; Roy and Thompson, 2006); therefore NF-κB may up-regulate Cyclin D1 and contribute to proliferation. Our model reveals that inhibition of NF-κB signaling leads to a reduction in Cyclin D1 accompanied by a decrease in proliferation. We also observed a reduction of phosphorylated histone 3 indicating less cells were undergoing mitosis. Inhibition of NF-κB in HeLa cells has been shown to delay mitotic entry (Cude et al., 2007), therefore inhibition of NF-κB may impact several stages of cell cycle progression.
In a recent study, a reduction in subcutaneous tumor growth, tumor microvascular density and VEGF was observed when NAFA murine mammary epithelial cells were stably transfected with a stabilized form of the NF-κB inhibitor IκBα (Liu et al., 2009). We did not observe effects on tumor vessel density or the expression of VEGF (data not shown). One explanation could be the differences between tumors growing in the microenvironment of the mammary gland compared with a subcutaneous tumor.
In a previous study, inhibition of NF-κB signaling with a dominant negative IKK1 protein did not impact tumor latency or number in the PyVT model but retarded tumor development in the MMTV-c-neu (Erb2/Her2) model (Cao et al., 2007). NF-κB activity has been linked with HER-2 positive tumors (Biswas and Iglehart, 2006) therefore further investigation is required in this subtype of tumors.
Pratt et al (Pratt et al., 2009) targeted mutant IκBα to mammary epithelium using the MMTV promoter. There are significant differences between their studies and the current study. They used chemical carcinogen treatment to induce tumor development while the current study focused on expression of an oncogenic signal, potentially more closely modeling an inherited genetic mutation. The previous study used constitutive mammary epithelial expression mediated via the MMTV promoter whereas our model is inducible, making feasible investigation of specific temporal windows of intervention. The ability to inhibit NF-κB at later stages during tumor progression allowed us to investigate effects after the stage of postnatal development. The general conclusion from both studies is that inhibition of NF-κB can impact breast tumor progression in a potentially therapeutic manner.
In addition to the experiments performed with the DNMP model, we investigated the effect of systemic treatment with the NF-κB inhibitor TQ on orthotopic mammary tumor progression. This data is in agreement with that of our transgenic model. To the best of our knowledge this is the first in vivo evidence suggesting that NF-κB inhibition by systemic treatment with TQ has potential for treatment of existing mammary tumors.
The current study suggests that NF-κB activity within mammary epithelium contributes to tumor progression in the murine mammary gland. The inhibition of NF-κB decreases primary tumor load and results in decreased numbers of lung metastases. The effect of NF-κB during tumor progression appears to be inhibition of apoptosis and promotion of proliferation via Cyclin D1 signaling. These results are relevant to current efforts aimed at developing inhibitors of NF-κB for treatment of cancer (Baud and Karin, 2009). They demonstrate that inhibition of NF-κB in vivo during primary mammary gland tumor development can be effective in blocking primary tumor progression with consequent effects on the extent of metastasis to the lung. The observation that inhibition of NF-κB signaling for a single week significantly decreases tumor load in a time frame where primary tumors have already developed as a result of a strong oncogenic stimulus is particularly interesting. As this models the clinical situation in which a patient is likely to present, we provide evidence that inhibition of NF-κB may prove to be an effective therapeutic strategy for treating patients with an existing breast tumor.
Materials and Methods
Isolation of PYG/L129 cells
Mammary tumors from PyVT mice crossed with NGL reporter mice (Everhart et al., 2006) on the FVB background were minced and washed with DMEM containing fungizone (Invitrogen, Carlsbad, CA) and gentamicin (Invitrogen). Tumor pieces were plated in DMEM with 10% FBS plus antibiotics and incubated at 37°C in 5% CO2 until tumor cells covered the dish. Single colonies were isolated and expanded. 1 × 106 cells were injected into the tail vein of an FVB mouse. After 6 weeks, lungs were harvested and a single tumor dissected out and L129 cells derived as above.
0.5 × 106 cells/well were plated in 6-well plates and incubated for 24 hours. Cells were treated with 10 ng/ml TNF-α (Pierce Biotechnology, Rockford, IL) in the presence or absence of 50μM Thymoquinone (MP Biomedicals, LLC, Solon, OH) for 6 hours. For TQ plus TNF-α treatment, cells were pretreated for 1 hr with TQ, medium removed and replaced with TQ plus TNF-α for the 6 hr treatment. Medium was removed and cells were washed with PBS before addition of Lysis Buffer (Promega, Madison, WI). The extracts were then centrifuged (13,793 × g, 4 °C, 5 min) and supernatants retained. Luciferase activity was measured as described (Connelly et al., 2007).
Mouse models
All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee. All mice were on an FVB strain background. MMTV-rtTA mice (Gunther et al., 2002) were crossed with the PyVT mouse mammary tumor model. MMTV-rtTA/PyVT mice were crossed with mice containing the NF-κB inhibiting (tet-O)7-IκB-αDN-Myc-His construct (Cheng et al., 2007; Connelly et al., 2010) to obtain mice carrying all three transgenes, these were termed DNMP. Littermates carrying the PyVT and DN-IκBα transgenes but lacking the MMTV-rtTA transgene were used as controls. DNMP mice (and controls) were treated with 2g/L freshly prepared doxycycline (dox; Sigma-Aldrich, St Louis, MO) in drinking water from 4 to 12 weeks, 8 to 12 weeks or 8 to 9 weeks old. Sucrose (5%) was added to decrease the bitter taste of dox water. A red bottle was used to prevent light-induced dox degradation and water was replaced twice weekly. DNMP mice (and controls) treated with dox from 4 to 12 weeks or 8 to 12 weeks were palpated twice weekly from 7 weeks of age to check for tumor formation. Tumor latency was determined as the day a tumor was first palpated from the start of dox treatment. At the time of sacrifice, palpable tumors were counted and mammary glands weighed to determine total gland mass (g).
Orthotopic injection of L129 cells and TQ treatment
Female FVB mice were injected in the right #4 inguinal mammary fat pad with 1 × 106 L129 cells in PBS. Daily IP injections of TQ (40 mg/kg) in DMSO or vehicle control were begun at day 10 post injection of cells when tumors were approximately 150mm3. On every second day tumor volume was measured with calipers. Body weight of each mouse was measured daily, no toxicity was observed. At sacrifice, tumors were weighed to determine mass (g).
Quantification of lung metastases
Lungs of mice treated from 4 to 12 weeks or 8 to 12 weeks of age with dox were inflated at the time of sacrifice with Bouin's fixative. Surface tumors were counted by two blinded readers under a dissecting microscope and counts averaged.
Whole mount analyses
Number 4 inguinal mammary glands of dox treated mice were collected, prepared and imaged as previously described (Connelly et al., 2007).
Immunohistochemistry and immunofluorescence
Number four inguinal mammary glands of mice treated from 8–9 weeks of age were fixed in 10% formalin overnight at 4°C. Glands were then dehydrated in a graded ethanol series followed by xylenes and embedded in paraffin. 5μm sections were prepared and stained for Ki67 expression (Vanderbilt University Medical Center Immunohistochemistry Core Laboratory). For Ki67 quantification, 10 images were captured per slide at 63× magnification using a Zeiss Axioplan 2 microscope and total Ki67 positive cells and total nuclei per field were counted by two blinded readers. Additional 5μm sections were assessed for apoptosis using the Deadend Fluorometric TUNEL system (Promega, Madison, WI). Staining was performed utilizing rabbit anti-Phospho-Histone 3 primary antibody (Upstate - Millipore, Danvers, MA) along with goat anti-rabbit Alexa Fluor 488 conjugated secondary antibody (Invitrogen), rat anti-F4/80 primary antibody (Invitrogen) along with goat anti-rat FITC conjugated secondary antibody (AbD Serotec, Raleigh, NC) and mouse anti-c-myc primary antibody (Sigma) along with goat anti-mouse Alexa Fluor 594 conjugated secondary antibody (Invitrogen). Slides were counterstained using DAPI (Sigma-Aldrich). For TUNEL quantification, 3 images from each of the 4 different stages of tumor progression were captured per slide using a Zeiss Axioplan 2 microscope at 20× magnification. Tumor stage was assessed using the criteria described by Lin et al (Lin et al., 2003). For F4/80 quantification, 3 images of hyperplastic lesions per section were captured at 40× magnification. For phospho-histone 3 quantification, 3 images of tumor area per section were captured at 20× magnification. Total nuclei (DAPI) and TUNEL, phospho-histone 3 and F4/80 positive cells were separately counted using Metamorph software and the average of the 3 images calculated.
Western blotting
Whole cell mammary gland extracts and nuclear extracts were prepared and western blot performed as previously described (Connelly et al., 2010). Primary antibodies: Cyclin D1, p42/44 MAPK, IκB and IκBα (Cell Signaling Technology, Danvers, MA), p65 (Santa Cruz Biotechnology, Santa Cruz, CA), TBP (Santa Cruz Biotechnology), Myc-tag (Sigma-Aldrich) and Actin (Santa Cruz Biotechnology).
Statistical analyses
Statistical analyses were performed using Graph Pad Prism (GraphPad Software Inc., San Diego, CA). Densitometric analyses were performed using Odyssey V3.0 software (Licor). All data are plotted graphically with vertical bars representing S.E. A Student's t test or Mann-Whitney test was used to assess differences between experimental conditions. Significance of data represented in the Kaplan-Meier curves was determined using log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests for statistical significance. For statistical analyses a probability (p) value of < 0.05 was taken as an appropriate level of significance.
Acknowledgments
This work was funded by NIH grant CA113734 awarded to F.E. Yull.
Footnotes
Conflict of Interest The authors have no potential financial interests or conflicts of interest to disclose.
References
- Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–6. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Basseres DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 2010;70:3537–46. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645–52. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101:10137–42. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Brantley DM, Chen CL, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, et al. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–55. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Karin M. NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003;8:215–23. doi: 10.1023/a:1025905008934. [DOI] [PubMed] [Google Scholar]
- Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci U S A. 2007;104:15852–7. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178:6504–13. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- Connelly L, Barham W, Pigg R, Saint-Jean L, Sherrill T, Cheng DS, et al. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J Cell Physiol. 2010;222:73–81. doi: 10.1002/jcp.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly L, Robinson-Benion C, Chont M, Saint-Jean L, Li H, Polosukhin VV, et al. A transgenic model reveals important roles for the NF-kappa B alternative pathway (p100/p52) in mammary development and links to tumorigenesis. J Biol Chem. 2007;282:10028–35. doi: 10.1074/jbc.M611300200. [DOI] [PubMed] [Google Scholar]
- Cude K, Wang Y, Choi HJ, Hsuan SL, Zhang H, Wang CY, et al. Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J Cell Biol. 2007;177:253–64. doi: 10.1083/jcb.200609166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–92. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–25. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010;29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Ju X, Willmarth NE, Casimiro MC, Ojeifo J, Sakamaki T, et al. Nuclear factor-kappaB enhances ErbB2-induced mammary tumorigenesis and neoangiogenesis in vivo. Am J Pathol. 2009;174:1910–20. doi: 10.2353/ajpath.2009.080706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P, Deacon K, Mistry S, Blank J, Patel R. NF-kappaB promotes survival during mitotic cell cycle arrest. J Biol Chem. 2004;279:1482–90. doi: 10.1074/jbc.M310413200. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr., Sledge GW., Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–39. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–69. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- Pratt MA, Tibbo E, Robertson SJ, Jansson D, Hurst K, Perez-Iratxeta C, et al. The canonical NF-kappaB pathway is required for formation of luminal mammary neoplasias and is activated in the mammary progenitor population. Oncogene. 2009;28:2710–22. doi: 10.1038/onc.2009.131. [DOI] [PubMed] [Google Scholar]
- Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–27. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–70. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, et al. Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther. 2007;6:1973–82. doi: 10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–60. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, et al. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:18514–9. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia MA, Gonzalez-Navarrete I, Dalmases A, Bosch M, Rodriguez-Fanjul V, Rolfe M, et al. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle. 2007;6:2284–92. doi: 10.4161/cc.6.18.4721. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]