Abstract
γ-secretase is a membrane protein complex associated with the production of Aβ peptides that are pathogenic in Alzheimer’s disease. We have characterized the activity of γ-secretase complexes under a variety of detergent solubilization and reconstitution conditions, and the structural state of proteoliposomes by electron microscopy. We found that γ-secretase activity is highly dependent on the physical state or integrity of the membrane bilayer - partial solubization may increase activity while complete solubization will abolish it. The activity of well-solubilzed γ-secretase can be restored to near native levels when properly reconstituted into a lipid bilayer environment.
Keywords: Membrane protein, aspartyl protease, Alzheimer's disease
1. Introduction
Production of Aβ peptides, that are pathogenic in AD (eg Aβ42, Aβ40) and derived from APP, is due to the proteolytic activity of the γ-secretase complex [1, 2]. This membrane protein complex consists of a core complex comprised of four integral membrane protein subunits, PS, Nct, APH-1 and Pen-2 [3–11], and an additional tightly bound membrane protein subunit, CD147 [12]. The core complex is necessary and sufficient for γ-secretase activity [8–10].
The γ-secretase complex is an unusual aspartyl protease able to cleave a variety of type I transmembrane proteins, such as Notch, E-cadherin, and the aforementioned APP, within their transmembrane regions. Therefore, in addition to its role in AD, γ-secretase has been found to participate in other biological processes, such as cell differentiation, cell-cell interaction and intracellular signaling [13–21]. Both extracellular and intracellular fragments are produced in the cleavage of γ-secretase substrates; while the amyloidogenic extracellular fragments of APP may be the most well known products, intracellular fragments have been found to be co-factors for biological activities as well. Most notably, the intracellular proteolytic fragment of Notch, NICD, is capable of inducing the transcription of many genes during development [22]
The establishment of protocols for the purification and reconstitution of active γ-secretase complexes, and the availability of robust in vitro activity assays, are critical for elucidating the molecular mechanism behind γ-secretase substrate processing. Unlike other membrane bound proteases, such as those of the rhomboid protease family [23–25] that retain function in the well-solubilized state, the functional activity of γ-secretase complexes requires the presence of a lipid bilayer environment [1,11,26,27]. Hence, it is logical to expect that the physical and chemical characteristics of the bilayer into which the complex is placed might affect functional activity.
Use of the detergent CHAPSO for reconstituting γ-secretase containing membranes with activity assay substrates has been broad-based [1, 11, 27, 28]. In some of these previous studies, CHAPSO-solubilized crude membrane or purified enzyme preparations, were mixed with test substrates (C100, a truncated form of its natural substrate APP, or a fluorogenic probe containing a small stretch of the APP transmembrane domain) and the total concentration of detergent was lowered from a starting value of 1% until activity could be detected, typically at levels around 0.25% or lower. This process was described as obtaining γ-secretase activity from samples in a “detergent-solubilized” state but was not found to be successful under similar conditions when using non-cholate detergents such as Triton X-100 or Brij35. Such findings led to suggestions that certain classes of detergents might maintain the enzyme in an active conformation while in a detergent-solubilized state, while others caused the solubilized proteins to assume an inactive conformation.
To gain insight into how the type of detergent used to solubilize cell membranes can affect the restoration of γ-secretase complex activity, we examined the activity of the reconstituted purified complexes and detergent-treated membrane preparations under a variety of detergent solubilization conditions, using detergents spanning a range of CMC values (CMC is the concentration above which detergents readily self-associate to form micelles), both native and extracted lipids, and reconstitution procedures involving dilution and detergent adsorption onto hydrophobic beads. For the activity assays, we used a fluorogenic peptide containing a transmembrane fragment of APP [27]. Through these studies we found that the activity of reconstituted γ-secretase complexes is critically dependent on the quality and extent of proteoliposome formation. More importantly, this work demonstrates that with appropriate reconstitution techniques this requirement can be met using complexes solubilized with detergents spanning a broad range of CMC values. While all the detergents evaluated in our study could be used to restore high levels of γ-secretase activity, they yielded differing degrees of reconstitution and vesicle sizes, resulting in a range of restored activity levels. These experiments also reveal how the detergent CHAPSO can actually increase the activity levels of γ-secretase containing cell membranes when applied at sub-solubilization levels.
2. Materials and Methods
2.1 γ-Secretase complex solubilization and reconstitution
Suspension HeLa cells were obtained from the National Cell Culture Center. Cells were manually homogenized on ice using a glass homogenizer; unbroken cells and cell debris were removed by low speed centrifugation (3000g for 10 minutes at 4°C); this was followed by a high speed centrifugation step (100,000g for 1.0 hour at 4°C) to collect cell membranes. HeLa cell membranes were solubilized on ice at a total protein concentration of about 10 mg/ml, using 2% CHAPSO, 1.0% FOS-CHOLINE-12, 0.6% FOS-CHOLINE-14, or 0.6% DDM in 50mM Tris buffer (pH 7.4) at 4°C for 1.0 hour. Solubilized membranes were centrifuged at 100,000 g for 1.0 hour to remove unsolubilized material, and supernatants were collected for reconstitution.
To reconstitute solubilized γ-secretase complexes into lipid vesicles, the concentration of solubilizing detergent in the protein-lipid-detergent mixtures was brought to 1/2 CMC or lower by dilution and removal of detergent overnight by adsorption onto hydrophobic beads (SM-2 Adsorbent, Bio-Rad). All samples were kept at 4°C during the reconstitution process.
2.2 γ-Secretase complex purification
γ-Secretase complex purification was performed as described previously [12]. Briefly, membranes isolated from 50-liter HeLa cell suspension cultures were solubilized with 0.6% FOS-CHOLINE-12 (Anatrace), in 50mM Tris buffer (pH 7.4) at 4°C for 1.0 hour and subjected to high-speed centrifugation (100,000g for 1.0 hour) to remove insoluble material. The supernatant was applied to a Q-Sepharose HP column (GE Healthcare). Bound proteins were eluted with a NaCl step gradient. Fractions containing γ-secretase were pooled and loaded onto a lentil lectin column (GE Healthcare). Bound proteins were eluted with methyl α-D-mannopyranoside. Eluted fractions were pooled, concentrated and applied to a calibrated molecular sieve column (GE Healthcare, Superdex 200). Under these conditions, the γ-secretase complex elutes at a molecular weight of about 250–300 kDa.
2.3 Fluorogenic substrate activity assay
Following the general approach of Farmery et al.[27] and the protocol supplied by the manufacturer (Calbiochem), reconstituted γ-secretease from detergent-solubilized HeLa cell membranes or purified γ-secretase complexes were incubated at 37°C in 150µl assay buffer containing 50mM Tris-HCl, pH 6.8, 2mM EDTA and 8µM fluorogenic γ-secretase substrate peptide (Calbiochem) for 16 hrs. As a negative control, 10µM of the γ-secretase inhibitor, L-685,458 (Calbiochem), was added to a subset of the reactions. After incubation, reactions were centrifuged at 15,000g for 15 min and placed on ice. Supernatants were transferred to 96 well plates (Maxisorp, Nunc), and the fluorescence from cleaved substrate measured using a plate reader (CytoFluor II) at an excitation wavelength of 355nm and an emission wavelength of 440 nm. Analysis of results (ANOVA) was carried out using spreadsheet software (Microsoft Excel).
2.4 Electron microscopy
Samples were applied to carbon-coated copper electron microscopy grids and negatively stained with 2% uranyl acetate. Images were collected on a Zeiss EM10 electron microscope over a magnification range of 1,000 to 10,000 ×.
3.0 Results
3.1 CHAPSO-treated HeLa cell membranes can yield activity greater than native membranes
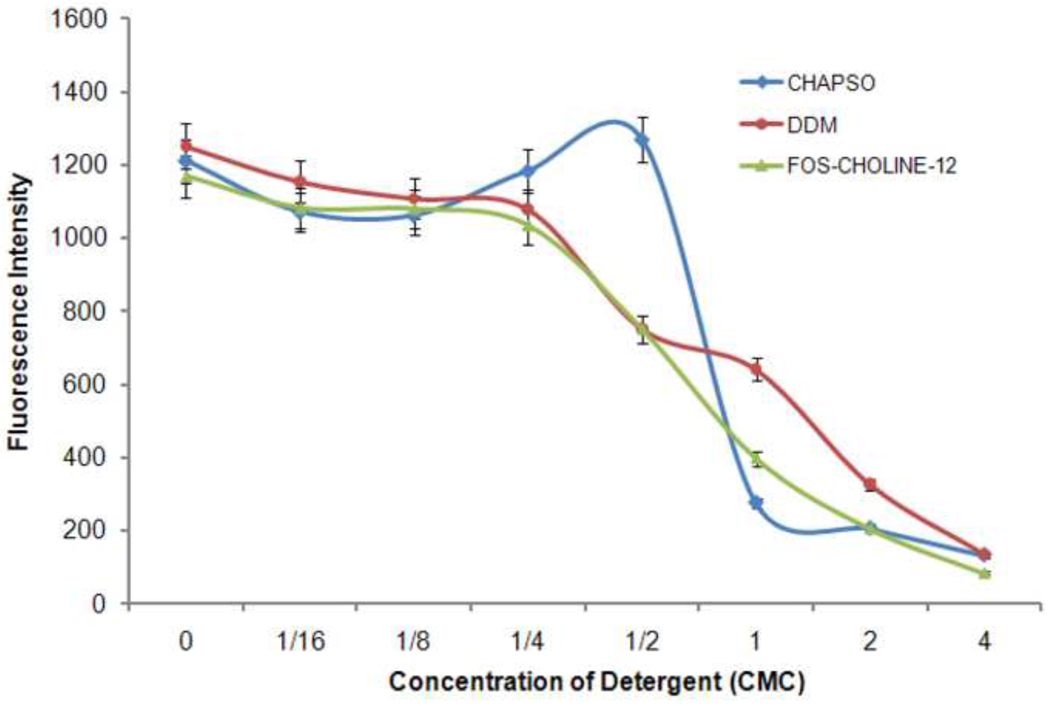
HeLa cell membranes were treated with a series of mild detergent solutions (CHAPSO, 8.00 mM CMC; DDM, 0.17 mM CMC or FOS-CHOLINE-12, 1.50 mM CMC) of increasing concentration (from 0 to 4 × CMC) and assayed for γ-secretase activity. As anticipated, activity steadily decreased with increasing levels of detergent and membrane solubilization (Figure 1). Although all of the detergents tested were highly effective in solubilizing the membranes, the use of CHAPSO at low concentrations uniquely led to an increase in activity over that observed for untreated membranes; an effect reported earlier [29]. After an initial decrease, the activity of CHAPSO-treated membranes trended upward with increasing detergent concentration until a maximum level was reached at a concentration of one-half the CMC (0.25%) (Figure 1). A similar increase in activity was not seen with the use of relatively low CMC detergents such as FOS-CHOLINE-12 and DDM, which instead yielded a continuous drop in activity beginning with the smallest amounts of detergent added.
Figure 1. Detergent titration of HeLa cell membranes containing γ-secretase complexes.
Treatment with low levels of CHAPSO (up to 0.5 CMC) can yield γ-secretase activity greater than that obtained with native membrane preparations. Use of FOS-CHOLINE-12 and DDM yield the anticipated drop in activity beginning with the smallest amounts added.
To investigate the basis for this increase in activity, electron microscopy of these CHAPSO-treated samples prepared in negative stain (2% uranyl acetate) was conducted to examine the structural states of the detergent treated membranes; the electron microscopy images showed that the isolated HeLa cell membranes (Figure 2a), when treated with relatively low levels of CHAPSO (up to 0.5 CMC), form smaller vesicles and lipid bilayer fragments while avoiding bulk solubilization of complexes (Figure 2b). These changes correlate with increased activity, suggesting that the smaller membranes are still capable of harboring the majority of γ-secretase complexes within a lipid bilayer environment, while allowing for increased substrate access.
Figure 2. Treatment of γ-secretase containing cell membranes with low levels (0.5 CMC) of CHAPSO.
Such treatment converts a) starting HeLa cell membranes into b) smaller bilayer fragments without significant solubilization of protein complexes. Size bars indicate 1 µm.
3.2 HeLa cell membranes, solubilized in high or low CMC detergents, can be reconstituted to yield near-native γ-secretase activity levels
Detergents covering a broad range of physical and chemical properties have been used to stably, but effectively, solubilize membrane proteins [30]. The high CMC detergent CHAPSO has been used extensively by several labs for the preparation of complexes for γ-secretase activity assays, whereas in our initial studies we found the intermediate CMC detergent FOS-CHOLINE-12, known for it’s effectiveness in membrane protein solubilization, to be also well-suited for this purpose. To assess the degree and conditions under which these and other detergents support the restoration of activity, we investigated the reconstitution of γ-secretase complex-containing cell membranes solubilized in one of four detergents covering an almost two orders of magnitude range in CMC.
For these experiments HeLa cell membranes were solubilized in either FOS-CHOLINE-14 (0.6% concentration), DDM (0.6%), FOS-CHOLINE-12 (1.0%) and CHAPSO (2.0%), maintaining a protein concentration of 10 mg/ml. The differences in the concentrations of detergents used reflects the fact that lower CMC detergents, such as DDM, require lower concentrations to achieve effective solubilization whereas higher CMC detergents, such as CHAPSO, can require significantly more. A subset of activity measurements were made using samples prepared in the presence of the γ-secretase inhibitor, L-685,458. Additionally, both reconstituted and solubilized samples were examined by electron microscopy of negatively-stained specimens.
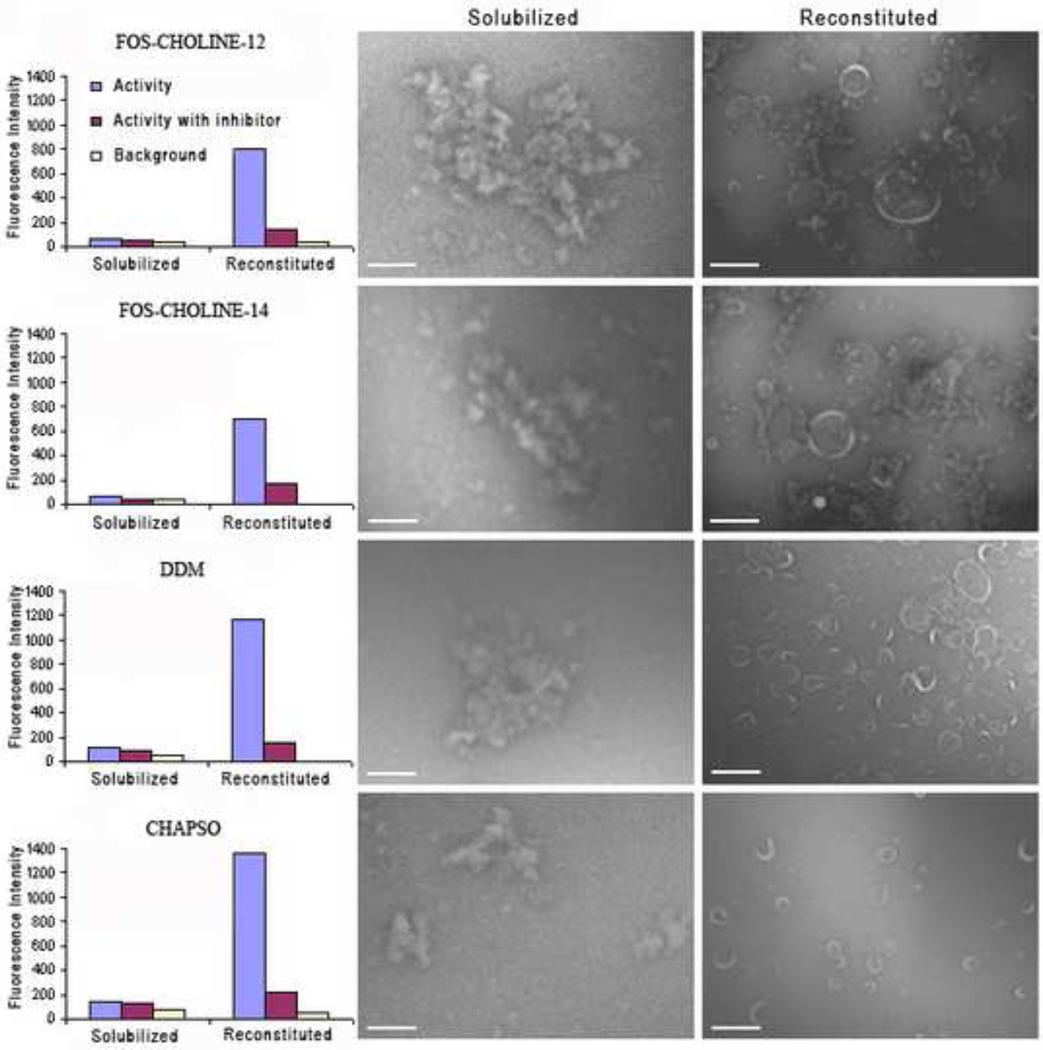
As expected, regardless of the detergent examined in these experiments, γ-secretase complexes were rendered effectively inactive in the detergent-solubilized state (Figure 3, left and middle panels). However, for each detergent evaluated it was found that activity could be restored if the solubilized complexes were reconstituted back into lipid bilayers (vesicles). Such results were consistently achieved by lowering solubilizing detergent levels to below their respective CMCs and, for low CMC detergents, by the additional use of detergent-binding beads [31].
Figure 3. Restoration of γ-secretase activity by reconstitution of detergent-solubilized complexes into lipid bilayers (vesicles).
HeLa cell membranes were solubilized in one of four different detergents, FOS-CHOLINE-12 and -14, DDM and CHAPSO. The charts of the left-hand panel show the relative activity levels of γ-secretase complexes in their detergent-solubilized and reconstituted forms with and without the presence of γ-secretase inhibitor L-685,458. The middle panel micrographs show aggregates of inactive detergent-solubilized membranes. Activity was restored upon reconstitution of γ-secretase complexes into lipid vesicles (right panel). Size bars indicate 1µm.
Electron microscopy of negatively-stained samples revealed the physical nature of the solubilized and reconstituted samples. The middle panel micrographs show aggregates of detergent-solubilized membranes. The aggregates formed as a result of sample dilution and staining during electron microscopy sample preparation. Solubilized membranes yielded little if any measurable activity. In each case, activity was restored to near native levels upon controlled reconstitution of γ-secretase complexes into lipid vesicles. Notably, differences were observed in the size of reconstituted vesicles and the levels of restored activity that correlated with the extent of reconstitution attained (Figure 3, right panel). Detergents facilitating more complete reconstitution (in this case, DDM and CHAPSO) yielded preparations with higher levels of activity. High CMC detergents like CHAPSO are readily removed and exchanged for lipids, typically yielding a very high percentage of proteins reconstituted into lipid bilayers (vesicles). Detergents with relatively low CMCs such as FOS-CHOLINE-14 are more difficult to remove; their use can easily result in a poorer degree of reconstitution and a higher percentage of aggregated membrane protein unless an appropriate detergent removal strategy is employed. All reconstituted samples showed a similar drop in activity in response to treatment with the γ-secretase inhibitor, L-685, 458.
3.3 Reconstitution of purified γ-secretase complex can restore activity to native-like levels
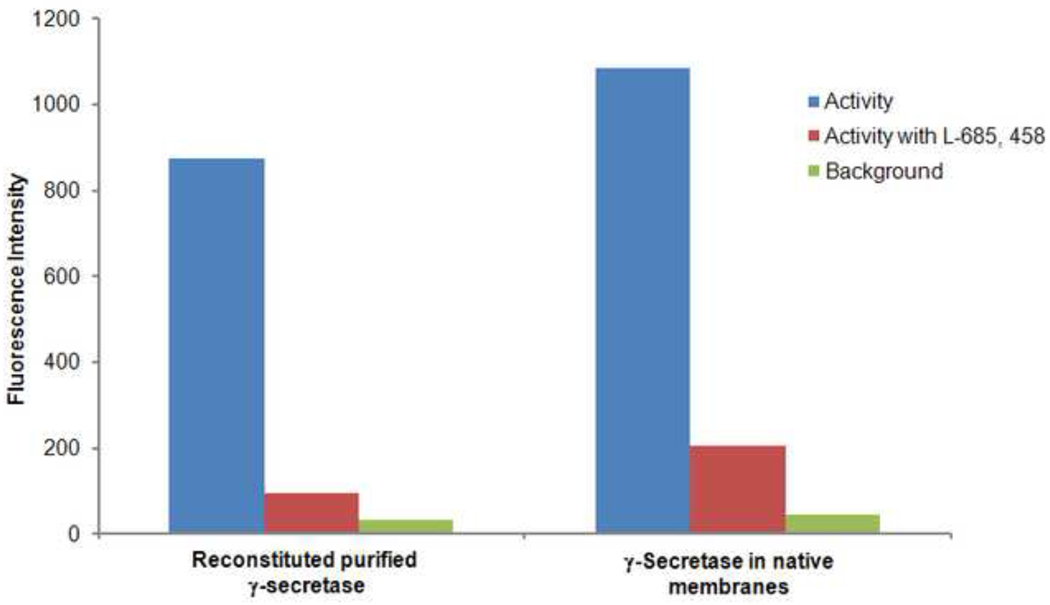
To ensure that the activity restored through the reconstitution of whole solubilized cell membranes was attributable to the successful reconstitution of the γ-secretase complex, experiments were also conducted with purified γ-secretase complexes solubilized in the detergent, FOS-CHOLINE-12, as previously described [12]. Purified γ-secretase was reconstituted into lipid vesicles by mixing the complex with brain total lipid extract, also solubilized with FOS-CHOLINE-12, using controlled dilution of the samples followed by removal of detergent through adsorption onto hydrophobic beads. Not surprisingly, purified γ-secretase complexes in the detergent-solubilized state showed no significant activity; activity was however restored upon reconstitution and, as with the complexes in native membranes, could be inhibited by the γ-secretase specific inhibitor, L-685,458 (Figure 4). These experiments demonstrate that when properly reconstituted, purified γ-secretase complexes solubilized using the non-cholate detergent, FOS-CHOLINE-12, can regain activity comparable to that observed for γ-secretase complexes in native cell membranes.
Figure 4. Purified γ-secretase complexes regain activity upon reconstitution into lipid bilayers.
γ-Secretase complexes were solubilized in FOS-CHOLINE-12, chromatographically purified and reconstituted using brain total lipid extract. Total γ-secretase complex levels in each sample were adjusted to be equal and confirmed by quantitative western blot. Reconstituted and native samples show high activity and give similar reductions in activity when treated with inhibitor L-685,458.
4. Discussion
The results of this work demonstrate that localization of the γ-secretase complex within a lipid bilayer environment is essential for its activity. We presented results showing that detergent-solubilized (FOS-CHOLINE-12) and chromatographically purified γ-secretase could be reconstituted into proteoliposomes to yield native-like activity levels. As other detergents have been used in reconstitution studies with varying degrees of success and resulting in a significant range in reported complex molecular weight (650 to 200 kDa), we further explored the process of reconstituting γ-secretase complexes using detergents spanning a two order of magnitude range in CMC (FOS-CHOLINE-12 and -14, DDM and CHAPSO). Through these experiments we found that γ-secretase activity could be restored to levels comparable to those observed with native membranes from samples solubilized in any of these detergents as long as the complexes were sufficiently-well reconstituted. This was consistently achieved through controlled dilution and removal of detergents by adsorption onto hydrophobic beads. Furthermore, differences in the levels of activity restored correlated with the extent of reconstitution attained. Detergents facilitating more effective reconstitution yielded higher levels of activity. While all detergents tested could be used to restore activity, some were more effectively applied; CHAPSO and DDM solubilized membranes allowed for more extensive reconstitution resulting in the restoration of higher levels of activity than we could obtain from membranes solubilized in FOS-CHOLINE-12 or FOS-CHOLINE-14.
In contrast to the other detergents we examined, and as reported by Li and colleagues [29], treatment of HeLa cell membranes with low levels of the detergent CHAPSO increased γ-secretase activity over that measured for native membranes. An explanation for this counter-intuitive behavior was obtained upon closer examination of samples prepared in this manner. From electron microscopy analysis of treated samples we determined that native membranes exposed to low levels of CHAPSO (up to 0.5 CMC) were broken into small lipid bilayer fragments without significant solubilization of complexes. These smaller membrane fragments continued to provide complexes with the necessary lipid bilayer environment while improving substrate access to the complexes by increasing the total bilayer perimeter.
As we have shown, γ-secretase activity requires the localization of γ-secretase complexes in the environment of well-formed lipid bilayers. Given this critical dependence it is not surprising that evidence, indicating that changes in the lipid composition of the cell membrane can substantially affect the function of the γ-secretase complex, has been mounting. γ-Secretase complexes and activity are reported to be localized largely within lipid raft regions of cell membranes [32–36]. Lipid rafts have been described as transient membrane microdomains, enriched in cholesterol and sphingolipids [37, 38]. The lipid composition of lipid rafts may be one reason we found the detergent CHAPSO facilitating the highest levels of γ-secretase activity restoration as CHAPSO has been shown to be especially good at preserving interactions between cholesterol, sphingolipids, and proteins, while solubilizing other lipids [39]. The enhanced presence of cholesterol and sphingolipids can also make lipid rafts thicker than other regions of the cell membrane. Changes in membrane thickness may spatially alter the transmembrane relationship between substrate and enzyme. In the case of γ-secretase such changes might affect the location of proteolytic cleavage along the transmembrane regions of substrates processed by the γ-secretase complex, helping to produce the range of Aβ peptides observed (i.e. Aβ-40, -42 and -49).
The underlying molecular mechanisms coupling lipid bilayer composition and Aβ peptide production, and accounting for the variability in substrate cleavage patterns by γ-secretase, have yet to be elucidated. Availability of active preparations of purified γ-secretase complexes reconstituted into well-defined lipid preparations will be essential for conducting the studies needed to address these questions. In the work presented here we have demonstrated that such preparations can be obtained in a straight-forward manner provided appropriate reconstitution methodologies are employed.
Research Highlights
-
➢
Partial solubilization of membranes with CHAPSO can increase γ-secretase activity.
-
➢
Completely solubilized γ-secretase is inactive.
-
➢
Purified γ-secretase regains activity after reconstitution into lipid bilayers.
-
➢
A broad range of detergents can be used to successfully reconstitute γ-secretase.
Acknowledgements
This work was supported by funding from the National Institutes of Health (B.K.J.) and by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
List of abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- PS
presenilin
- Nct
nicastrin
- APH-1
anterior pharynx-defective 1
- Pen-2
presenilin enhancer 2
- NICD
Notch intracellular domain
- DDM
dodecylmaltoside
- NTF
N-terminal fragment
- CTF
C-terminal fragment
- CMC
critical micelle concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or The Regents of the University of California.
References
- 1.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 2.Esler WP, Wolfe MS. A portrait of Alzheimer secretases-new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- 3.Periz G, Fortini ME. Functional reconstitution of gamma-secretase through coordinated expression of presenilin, nicastrin, Aph-1, and Pen-2. J. Neurosci. Res. 2004;77:309–322. doi: 10.1002/jnr.20203. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. doi: 10.1021/bi060799c. [DOI] [PubMed] [Google Scholar]
- 5.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 6.Ratovitski T, Slunt HH, Thinakaran G, Price DL, Sisodia SS, Borchelt DR. Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J. Biol. Chem. 1997;272:24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- 7.Levitan D, Lee J, Song L, Manning R, Wong G, Parker E, Zhang L. PS1 N- and C-terminal fragments form a complex that functions in APP processing and Notch signaling. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12186–12190. doi: 10.1073/pnas.211321898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 9.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nat. Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 10.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and characterization of the human γ-secretase complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid β-peptide production. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for the γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 14.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO. J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. J. Biol. Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent "γ-secretase" processing of deleted in colorectal cancer (DCC) J. Biol. Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 17.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 18.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 19.Kim DY, Ingano LA, Kovacs DM. Nectin-1, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. J. Biol. Chem. 2002;277:49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 20.Strooper BD, Annaert W. Presenilins and the intramembrane proteolysis of proteins: Facts and fiction. Nat. Cell Biol. 2001;3:E221–E225. doi: 10.1038/ncb1001-e221. [DOI] [PubMed] [Google Scholar]
- 21.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang D-S, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer L, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 22.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 23.Urban S, Lee JR, Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- 24.Urban S, Wolfe MS. Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1883–1888. doi: 10.1073/pnas.0408306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang Y, Ha Y. Crystal structure of a rhomboid family intramembrane protease. Nature. 2006;444:179–180. doi: 10.1038/nature05255. [DOI] [PubMed] [Google Scholar]
- 26.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Nasland J. Partial purification and characterization of γ-secretase from post-mortem human brain. J. Biol. Chem. 2003;278:24277–24284. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- 28.Franberg J, Welander H, Aoki M, Winblad B, Tjiernberg LO, Frykman S. Rat brain γ-secretase activity is highly influenced by detergents. Biochemistry. 2007;46:7647–7654. doi: 10.1021/bi0621258. [DOI] [PubMed] [Google Scholar]
- 29.Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walian P, Cross TA, Jap BK. Structural genomics of membrane proteins. Genome Biol. 2004;5:215. doi: 10.1186/gb-2004-5-4-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jap BK, Walian PJ. Two-dimensional crystallization of proteins. Encyclopedia of Life Sciences. Macmillan References Limited. 2001 [Google Scholar]
- 32.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urano Y, Hayashi I, Isoo N, Reid PC, Shibasaki Y, Noguchi N, Tomita T, Iwatsubo T, Hamakubo T, Kodama T. Association of active γ-secretase complex with lipid rafts. J Lipid Res. 2005;46:904–912. doi: 10.1194/jlr.M400333-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 2006;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 36.Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of γ-secretase and substrates in distinct membrane domains. J. Biol. Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 38.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 39.Hur JY, Welander H, Behbahani H, Aoki M, Frånberg J, Winblad B, Frykman S, Tjernberg LO. Active γ-secretase is localized to detergent-resistant membranes in human brain. FEBS J. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]