Abstract
HIV-1 replication within macrophages of the CNS often results in cognitive and motor impairment, which is known as HIV-associated dementia (HAD) in its most severe form. IFN-β suppresses viral replication within these cells during early CNS infection, but the effect is transient. HIV-1 eventually overcomes this protective innate immune response to resume replication through an unknown mechanism, initiating the progression toward HAD. Here, we show that Suppressor Of Cytokine Signaling (SOCS) 3, a molecular inhibitor of IFN signaling, may allow HIV-1 to evade innate immunity within the CNS. We find that SOCS3 is elevated in an in vivo SIV/macaque model of HAD, and that the pattern of expression correlates with recurrence of viral replication and onset of CNS disease. In vitro, the HIV-1 regulatory protein transactivator of transcription (Tat) induces SOCS3 in both human and murine macrophages in a NF-κB-dependent manner. SOCS3 expression attenuates the response of macrophages to IFN-β at both proximal levels of pathway activation and downstream antiviral gene expression, and consequently overcomes the inhibitory effect of IFN-β on HIV-1 replication. These studies indicate that SOCS3 expression, induced by stimuli present in the HIV-1-infected brain such as Tat, inhibits antiviral IFN-β signaling to enhance HIV-1 replication in macrophages. This consequence of SOCS3 expression in vitro, supported by a correlation with increased viral load and onset of CNS disease in vivo, suggests that SOCS3 may allow HIV-1 to evade the protective innate immune response within the CNS, allowing the recurrence of viral replication, and ultimately promoting progression toward HAD.
Introduction
Even with the widespread use of highly active antiretroviral therapy (HAART), 50% of patients infected with HIV-1 will eventually experience neurologic symptoms, including both cognitive and motor dysfunction, known as HIV-associated neurocognitive disorder (HAND) (1). While only a small portion of these will go on to develop the severe and disabling dementia characteristic of HAD, mild cognitive impairment is common, and can be detrimental to a patient's quality of life (2), opportunity for employment (3), proper medication management (4), and survival (5). HAND is the result of HIV-1-induced neuronal injury and loss, predominantly in the basal ganglia and other subcortical regions of the brain where viral load is the most concentrated (6). Although neurons are not directly infected by HIV-1, viral replication within infiltrating macrophages and microglia, the resident macrophage of the brain, results in the production of viral proteins and activation-induced cytokines which can be damaging, or even toxic, to neurons (7).
Notably, while HIV-1 invades the CNS early following peripheral infection, neurological symptoms do not occur until later in the course of disease (8). This delay is due to the ability of the innate immune response to effectively suppress viral replication during the early stages of CNS infection (9). IFN-β, a member of the type I or ‘anti-viral’ family of IFNs, is a critical mediator of this response in the brain. In vitro, IFN-β inhibits HIV-1 (10) and SIV (11) replication in macrophages, the cellular initiators of CNS pathogenesis. In vivo, increased IFN-β expression correlates with suppression of CNS SIV replication in a SIV/macaque model of HAD (11), the most faithful model of human HAND available. Unfortunately, although the ability to express IFN-β persists through late stages of both HIV-1 (12) and SIV (13) disease, the virus eventually overcomes this antiviral effect through an unknown mechanism and replication resumes (8). Studies performed in a SIV/macaque model of HAD show that this resurgence of viral replication correlates with inflammatory dysregulation and progression to CNS disease (14). Therefore, it is critical that we understand the mechanism by which HIV-1 evades IFN-β's protective antiviral effect, in order to develop more potent therapies against CNS pathogenesis.
IFN-β inhibits viral replication by signaling through the JAK/STAT pathway to induce the expression of multiple antiviral target genes (15). In this pathway, IFN-β stimulates the cell-surface receptor subunits IFNAR1 and IFNAR2 to activate associated JAK family members JAK1 and TYK2. Activated JAKs then phosphorylate cytoplasmic STAT1 and STAT2, allowing them to hetero-dimerize, and subsequently enter the nucleus to induce transcription of IFN-stimulated genes (ISGs). Collectively, these antiviral genes can inhibit the replication of a broad range of viral pathogens, including HIV-1, at multiple stages of the viral life cycle. For example, the translation inhibitor PKR prevents reactivation of HIV-1 replication in latently infected cells (16), and ISG20, a 3′-5′ exonuclease that cleaves ssRNA, can delay the replication kinetics of HIV-1 (17). Functions such as these culminate in the terminal antiviral effects of IFN-β signaling.
The JAK/STAT signaling pathway is subject to negative regulation by a family of proteins known as SOCS. This family contains eight members: cytokine inducible SH2 domain containing protein and SOCS1-SOCS7 (18). Each use a central SH2 domain to bind specific phosphorylated tyrosine residues within the JAK/STAT signaling receptor complex. They subsequently inhibit pathway function either with a variable family member specific domain in the N-terminus, or the more conserved C-terminal SOCS box which targets associated proteins for proteosomal degradation. Classical SOCS upregulation is cytokine-dependent, creating a negative feedback loop to prevent excessive cytokine stimulation (18,19). However, many pathogens are capable of independently inducing SOCS expression to diminish the responsiveness of host cells to protective IFNs. For example, Listeria monocytogenes (20) and Mycobacterium avium (21), both macrophage-tropic intracellular pathogens, induce SOCS3 to prevent IFN-γ-mediated host clearance mechanisms. Toxoplasma gondii (22) achieves the same result through expression of SOCS1. Similarly, influenza A virus induces SOCS3 expression to evade IFN-β-mediated antiviral pathways (23), while herpes simplex virus type I has been reported to induce either SOCS1 (24) or SOCS3 (25) to evade IFN-γ and IFN-α responses, respectively. SOCS proteins have also been reported to be expressed at increased levels during HIV-1 infection (26,27). However, it is not currently known whether SOCS proteins are induced by stimuli present in the HIV-1-infected brain, or how they may affect protective IFN-β signaling.
One stimulus of particular interest in the HIV-1-infected brain is the multipotent HIV-1 protein Tat (28). Classically, Tat acts as a regulatory protein in the nucleus of HIV-1-infected cells, recruiting essential components of the transcriptional complex to the viral promoter to enhance HIV-1 transcription. However, Tat has a multitude of additional functions that contribute to HAND pathogenesis. Tat can be secreted to act on uninfected cells (29), either extracellularly through interactions with various cell surface receptors (30-32), or intracellularly following endocytosis (33). Acting distally, Tat can directly induce neuronal apoptosis (34), promote the expression of cytokines and chemokines (35-38), induce expression of the HIV-1 chemokine co-receptors CXCR4 and CCR5 (39), and inhibit the expression of important immune molecules such as MHC class I (40) and class II (41). By extension, further HAND promoting effects are likely. However, Tat has not yet been shown to affect critical IFN-β signaling within the HIV-1-infected brain.
In this study, we investigated whether SOCS proteins play a role in HIV-1 immune evasion in the CNS, thereby contributing to the development of HAND. We show that SOCS3 is expressed in a SIV/macaque model of HAD in a pattern that correlates with recurrence of viral replication and onset of CNS disease. SOCS3 is induced by HIV-1 Tat in macrophages and results in the inhibition of IFN-β signaling, ultimately preventing the ability of IFN-β to suppress HIV-1 replication. Collectively, these studies suggest that Tat-induced SOCS3 expression may promote progression to HAND by allowing HIV-1 to evade the innate immune response within the CNS.
Materials and Methods
Reagents
HIV-1 Tat1-72aa was provided by Dr. Avindra Nath (Johns Hopkins University, Baltimore, MD). Recombinant murine IFN-β, IFN-γ, and M-CSF, and recombinant human M-CSF were purchased from R&D Systems (Minneapolis, MN). Recombinant human IFN-β was purchased from PBL Biomedical Laboratories (Piscataway, NJ). BAY 11-7085 was purchased from Alexis Biochemicals (Farmingdale, NY). PMA was purchased from Calbiochem (La Jolla, CA). Antibodies against phospho-STAT1 (Tyr701 and Ser727), phospho-STAT3 (Tyr705 and Ser727), STAT3, and phospho-NF-κB p65 (Ser276) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against SOCS3, NF-κB p65, IκBα, STAT2, and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against STAT1 and phospho-STAT2 (Tyr689) were purchased from Upstate Biotechnology (Lake Placid, NY).
Cells
All murine studies were approved by the Animal Studies Committee at the University of Alabama at Birmingham. Primary cells were isolated from C57BL/6J wild-type mice unless otherwise specified. To generate primary murine bone marrow-derived macrophages (BMDM), bone marrow precursor cells were isolated from the femurs of adult mice, and macrophage differentiation induced by incubation with 20 ng/ml M-CSF for 5 days (42). Primary microglia (43), astrocytes (44), and cortical neurons (45) were prepared as previously described. The murine macrophage cell line RAW264.7 was maintained as previously described (46).
To generate primary human peripheral blood-derived macrophages (PBDM), PBMC from healthy donors were isolated by Ficoll Hypaque sedimentation, and macrophage differentiation induced by incubation with 10 ng/ml M-CSF for 7 days (47). To generate primary macaque PBDM, PBMC from healthy adult rhesus macaques were isolated by Ficoll Hypaque sedimentation, and macrophage differentiation induced by incubation with 100 U/ml each of M-CSF and GM-CSF for 3 to 5 days (48).
Human, monocytic THP-GFP reporter cells were derived from THP-1 cells and contain a stably integrated GFP reporter under the control of the HIV-1 NL4-3 LTR. THP-GFP-S3 cells were obtained by retroviral transduction of THP-GFP cells with a SOCS3 expressing retroviral vector, based on the murine stem cell virus (pMSCV-puro; Clontech, Mountain View, CA). All THP-1 derived cell lines were maintained in RPMI 1640 containing 10% FBS.
Ribonuclease protection assay and RT-PCR
Riboprobes for murine SOCS3, SOCS1, and GAPDH were prepared, and ribonuclease protection assay was performed as described previously (42). Briefly, 20 μg of total RNA was hybridized with the riboprobes at 42°C overnight. The hybridized mixture was treated with RNase A/T1 (1/200) and then analyzed by 5% denaturing (8 M urea) PAGE. mRNA expression values were calculated using ImageQuant software.
Alternatively, 1 μg of total RNA was reverse transcribed into cDNA and analyzed by PCR. Endpoint PCR was performed as previously described (49) with primers specific for the genes indicated. Quantitative real-time PCR was performed with Taqman (Applied Biosystems, Foster City, CA) probes according to the manufacturer's instructions. The data were then analyzed using the comparative CT method to obtain relative quantitation values (fold induction).
Immunoblotting
Cell lysates were separated by electrophoresis on SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with antibodies against the indicated proteins. 25 μg of lysate was sufficient for all proteins except SOCS3 which required 100 μg of lysate. Quantification of band density was performed using Quantity One software.
Luciferase assay
The murine SOCS3 promoter construct was generated as previously described (42), and the IKKβ dominant negative expression vector was provided by Dr. Randolph Noelle (Dartmouth Medical School, Lebanon, NH). Cells were transiently transfected using the Lipofectamine Plus method per manufacturer's instructions. Following treatment, the luciferase activity of each sample was measured using a luminometer and normalized to the protein concentration of each well.
SIV/macaque model and quantitative RT-PCR
Pigtailed macaques were infected with SIV for the times indicated, in accordance with federal guidelines and institutional policies, as previously described (50). Total RNA was collected from macaque basal ganglia, reverse transcribed as described above, and analyzed by quantitative real-time PCR with Taqman probes specific to human SOCS3 (Hs02330328_s1), macaque SOCS1 (Rh02914687_g1), and 18s (Hs99999901_s1).
Generation of SOCS3Δ/Δ Mice
Mice homozygous for a floxed (fl, flanked by loxP sites) SOCS3 allele (SOCS3fl/fl) (51) were serially bred with mice expressing Cre recombinase (Cre) under the control of the LysM promoter, to generate mice in which the conditional SOCS3 allele is excised specifically in macrophages and neutrophils (SOCS3Δ/Δ). BMDM were then collected as described above.
HIV-1 infection and flow cytometric analysis
THP-GFP or THP-GFP-S3 cells were incubated in the presence of 1 ng/ml PMA for 72 h to induce macrophage differentiation, followed by pretreatment with 0-30 U/ml of IFN-β for 24 h. Medium was removed and the cells were then infected with the molecular HIV-1 clone SG3 or the patient isolate CUCY. Three hours post viral inoculation, RPMI 1640/10% FBS containing the respective concentrations of IFN-β was re-added and cells incubated for an additional 24-72 h prior to collection. The percentage of GFP expressing cells was measured using a Guava EasyCyte flow cytometer.
Statistical analysis
Data was analyzed using GraphPad Prism software. Appropriate statistical tests were chosen for each experiment and are indicated in the respective figure legend.
Results
SOCS3 expression correlates with viral load and CNS disease in a SIV/macaque model of HAD
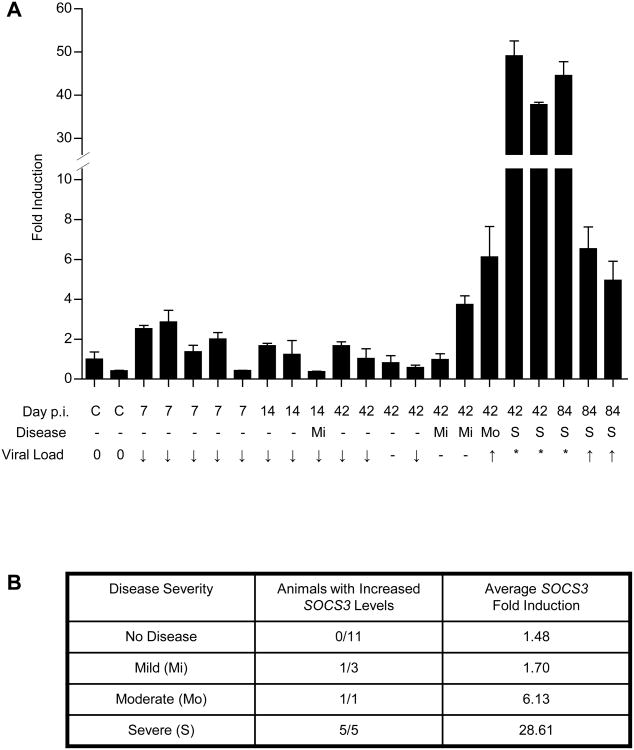
SOCS proteins are upregulated by a number of pathogens in order to evade protective host IFN responses. Specifically, SOCS1 and SOCS3 have been shown to prevent IFN-mediated inhibition of viral replication (23,24,52). To determine whether either of these molecules are induced in the brain during progression to HAND, we examined SOCS1 and SOCS3 mRNA levels in a SIV/macaque model of HAD. This model closely recapitulates the most severe form of human HAND, known as HAD, although in an accelerated fashion that consistently results in disease (11,14,50). Animals display an increase in CNS viral load shortly following infection, which then rapidly diminishes ∼10 days post-infection in response to an increase in IFN-β expression. Recurrence of viral replication within the brain occurs by 42 to 84 days post-infection, and correlates with onset of disease. We collected mRNA from the basal ganglia of either non-infected animals (C), or animals at a range of timepoints along the course of infection, and analyzed SOCS1 and SOCS3 expression by quantitative real-time PCR. SOCS3 mRNA expression was increased in SIV-infected animals in a pattern that correlated with increases in CNS viral load and onset of disease (Figure 1A). Collectively, 7 out of 9 animals with confirmed CNS disease (Mi, Mo, S) showed increased SOCS3 expression compared to uninfected controls, while animals without disease showed consistently low SOCS3 expression (Figure 1B). Additionally, the 6 most severely affected animals (Mo, S) showed the greatest increases in SOCS3 expression. SOCS1 mRNA expression was also detected in SIV-infected animals, but did not correlate with increases in viral load or CNS disease (data not shown). These data indicate that SOCS1 and SOCS3 mRNA are expressed within the brain during progression to HAD. Because SOCS3 expression correlated with recurrence of viral replication and onset of CNS disease, we concluded that it contributed more significantly to HAD progression and focused subsequent mechanistic studies on this protein.
Figure 1. SOCS3 expression correlates with viral load and CNS disease in a SIV/macaque model of HAD.
(A) RNA was collected from the basal ganglia of SIV-infected macaques at the times indicated post-infection (p.i.) and analyzed by quantitative RT-PCR with primers specific for SOCS3. Individual samples were normalized to 18S and displayed as fold increase above the non-infected control (c) with the highest levels of SOCS3 +/- standard error. Disease severity is indicated as mild (Mi), moderate (Mo) or severe (S). CNS viral load is indicated as no infection (0), <100,000 copies (↓), 100,000 to 1 million copies (-), 1 million to 10 million copies (↑), or >10 million copies (*). (B) Infected animals were grouped by disease severity. The number of animals with increased SOCS3 levels (defined as a 3-fold or greater increase above control levels), and average fold induction of SOCS3 expression for each group was determined.
HIV-1 Tat induces SOCS3 expression in macrophages and microglia
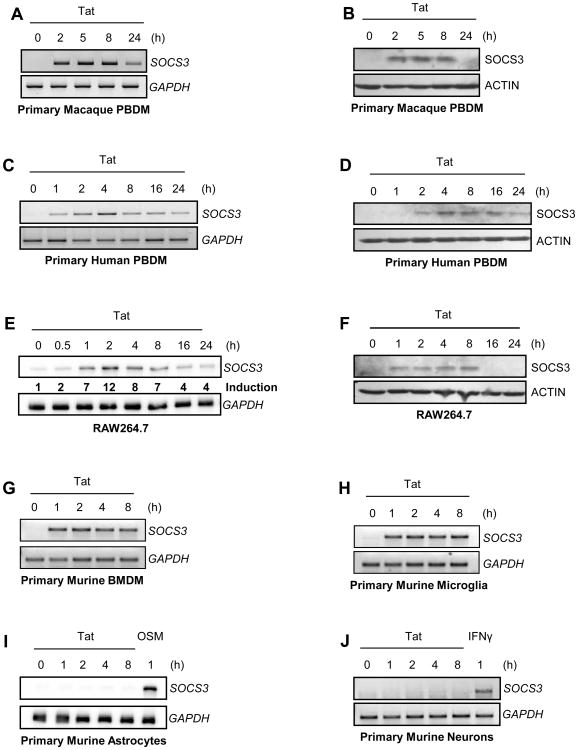
SOCS proteins are expressed in cell types throughout the CNS in a family member- and stimulus-specific manner (19,42,53,54). We hypothesized that SOCS3 must be expressed in macrophages, the most important source of viral replication within the CNS, to have a direct impact on viral replication. To determine whether macrophages could express SOCS3 in response to stimuli present in the HAD brain, we treated cells with HIV-1 Tat, a protein with multiple HAND promoting effects. Primary macaque PBDM were treated with HIV-1 Tat1-72aa for 0-24 h. A 10 nM concentration, close to the low physiologic concentration of Tat found in the sera of HIV-1-infected individuals (55), was sufficient to produce robust SOCS3 expression between 2 and 8 h, both at the level of mRNA (Figure 2A) and protein (Figure 2B). In order to assess whether Tat treatment of macaque macrophages in vitro also recapitulated the family member specificity seen in our in vivo model, we directly compared SOCS1 and SOCS3 expression by quantitative real-time PCR. Only negligible levels of SOCS1 mRNA expression were detected in comparison to SOCS3 (Supplemental Figure 1), indicating that the ability of Tat to induce SOCS expression is relatively specific for SOCS3 in vitro. Notably, SOCS3 mRNA was not elevated in primary macaque PBDM following a 4 h treatment with SIV alone (Supplemental Figure 1), suggesting that the early stages of virus infection itself are not sufficient to induce SOCS3 expression. To determine whether human macrophages act similarly, we exposed primary human PBDM to HIV-1 Tat1-72aa. Increased SOCS3 mRNA (Figure 2C) and protein (Figure 2D) levels were detected following Tat exposure, with maximal expression occurring 4 h after treatment. These data indicate that SOCS3 expression is induced by HIV-1 Tat, a stimulus present within the HAD brain, in both human and macaque macrophages.
Figure 2. HIV-1 Tat induces SOCS3 expression in macrophages and microglia.
Cells were treated with 10 nM HIV-1 Tat1-72aa for the times indicated. mRNA was analyzed by RT-PCR with primers specific for SOCS3 (A, C, G, H, J) or ribonuclease protection assay with a probe specific to SOCS3 (E, I). GAPDH mRNA was examined as a loading control. To calculate induction of SOCS3 mRNA (E), individual samples were normalized to GAPDH and expressed as fold increase above untreated cells. Protein was subjected to immunoblot analysis with antibodies specific for SOCS3 (B, D, F). Blots were stripped and reprobed for actin as a loading control. Data are representative of at least two independent experiments.
We then examined whether HIV-1 Tat could enhance SOCS3 expression in murine cells, which are a useful experimental tool for transfection and gene deletion studies. Although Tat is unable to enhance HIV-1 transcription in murine cells (56), it has been shown to successfully regulate host gene expression (57). RAW264.7 murine macrophages, chosen for their ability to induce SOCS3 in response to other stimuli (42), expressed SOCS3 in response to treatment with HIV-1 Tat1-72aa between 1 and 8 h at both the mRNA (Figure 2E) and protein (Figure 2F) level. Notably, treatment with a mutant form of Tat lacking critical core and basic regions (HIV-1 TatΔ31-61aa) did not induce a comparable increase in SOCS3 expression (data not shown). These results indicate that murine macrophages respond specifically to functional Tat by inducing SOCS3 expression, similarly to macrophages of other species. We then examined a range of primary murine CNS-relevant cells for their responsiveness to Tat treatment. Both primary murine BMDM (Figure 2G) and primary murine microglia (Figure 2H) displayed an increase in SOCS3 mRNA for the duration of HIV-1 Tat1-72aa exposure. In contrast, neither primary murine astrocytes (Figure 2I) nor primary murine cortical neurons (Figure 2J) expressed SOCS3 mRNA in response to HIV-1 Tat1-72aa treatment. Both of these cell types were capable of expressing SOCS3 in response to Oncostatin M (OSM; Figure 2I) or IFN-γ (Figure 2J) respectively, and have previously been shown to respond functionally to Tat (36,37). These data indicate that HIV-1 Tat-induced SOCS3 expression is restricted to cells of macrophage lineage within the CNS.
HIV-1 Tat-induced SOCS3 expression is transcriptionally regulated
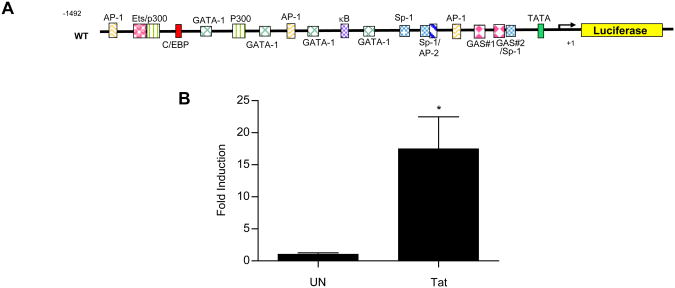
SOCS3 expression can be regulated either at the level of transcription or mRNA stabilization (58,59). To determine how HIV-1 Tat regulates SOCS3 expression in macrophages, we assessed activation of the SOCS3 promoter (Figure 3). RAW264.7 macrophages, chosen for their ease of transfection, were transiently transfected with a murine SOCS3 promoter-driven luciferase reporter construct (42) (Figure 3A). Cells were then treated with HIV-1 Tat1-72aa for 24 h, which was determined to be the optimal assay point. Tat induced a 17-fold increase in luciferase levels as compared to untreated cells (Figure 3B), a value consistent with Tat-induced increases in SOCS3 mRNA (Figure 2E). These data suggest that HIV-1 Tat regulates SOCS3 expression in macrophages at the level of transcription.
Figure 3. HIV-1 Tat-induced SOCS3 expression is transcriptionally regulated.
RAW264.7 cells were transiently transfected with 200 ng of a murine SOCS3 promoter-driven luciferase construct (A). Cells were then treated with 10 nM HIV-1 Tat1-72aa for 24 h and analyzed for luciferase production (B). Values are displayed as fold increase above untreated cells +/- standard error and represent three independent experiments performed in triplicate. *p<0.05 versus untreated cells as determined by unpaired t-test.
HIV-1 Tat signals through the NF-κB pathway to induce SOCS3 expression
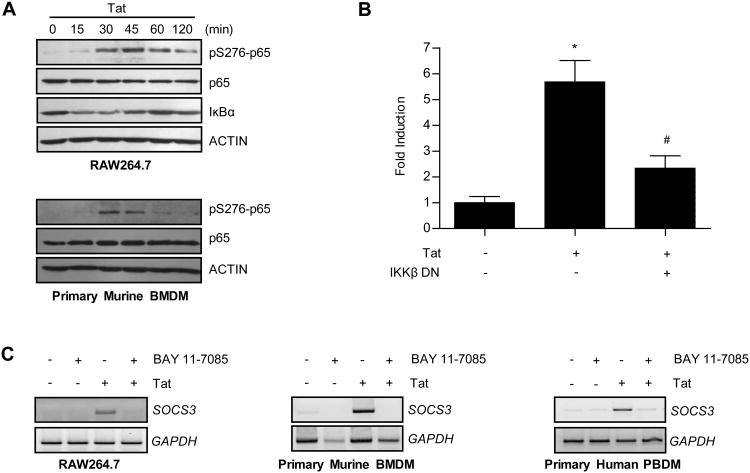
Different signaling pathways contribute to SOCS3 expression in a stimulus- and cell type-specific manner (19-21,23,42). Of these, the NF-κB pathway is frequently utilized by HIV-1 Tat to transcriptionally regulate gene targets (36,38). To determine whether Tat signals through this pathway to induce SOCS3 expression in macrophages, we first examined the kinetics of Tat-induced NF-κB pathway activation. RAW264.7 macrophages were exposed to HIV-1 Tat1-72aa for 0 to 120 min and analyzed for markers of NF-κB activation (Figure 4A). Phosphorylation of NF-κB p65 (serine 276) was detected at 30 min and remained elevated through 120 min. In addition, total levels of IκBα, a protein that sequesters NF-κB prior to activation, diminished noticeably between 15 and 45 min. HIV-1 Tat1-72aa treatment of primary murine BMDM showed a similar result, inducing phosphorylation of NF-κB p65 (serine 276) between 30 and 45 min (Figure 4A).
Figure 4. HIV-1 Tat signals through the NF-κB pathway to induce SOCS3 expression.
(A) Cells were treated with 10 nM HIV-1 Tat1-72aa for the times indicated. Protein was subjected to immunoblot analysis with antibodies specific to phosphorylated p65 (serine 276), total p65, and IκBα. Blots were then stripped and reprobed for actin as a loading control. Data are representative of two independent experiments. (B) RAW264.7 cells were transiently transfected with 50 ng of an IKKβ dominant negative (DN) construct or an empty pcDNA3 vector. Cells were also co-transfected with a murine SOCS3 promoter-driven luciferase reporter construct. Following 48 h of recovery, cells were treated with 10 nM HIV-1 Tat1-72aa for 24 h and analyzed for luciferase production. Values are displayed as fold increase above untreated cells +/- standard error. *p<0.001 versus untreated cells and #p<0.05 versus 10 nM Tat treatment alone as determined by one-way ANOVA followed by Bonferroni's multiple comparison test. (C) Cells were pretreated with 10 μM BAY 11-7085 for 2 h and then treated with 10 nM HIV-1 Tat1-72aa for 1 h. mRNA was analyzed by RT-PCR with primers specific for SOCS3. GAPDH mRNA was examined as a loading control.
We used two methods of pathway inhibition to determine whether the NF-κB pathway was necessary for HIV-1 Tat-induced SOCS3 expression. First, RAW264.7 macrophages were assayed for Tat-induced SOCS3 promoter activation in the absence or presence of an IKKβ dominant negative construct. This construct prevents the phosphorylation, and subsequent degradation of IκB, resulting in inhibition of NF-κB activation (60). While HIV-1 Tat1-72aa stimulation resulted in a nearly 6-fold increase in luciferase expression, inclusion of the IKKβ dominant negative construct reduced this expression by ∼65% (Figure 4B). Next, RAW264.7 macrophages, primary murine BMDM, or primary human PBDM were analyzed for endogenous Tat-induced SOCS3 expression following pre-treatment with a pharmacologic inhibitor of the NF-κB pathway. Cells were pre-treated with 10 μM BAY 11-7085, a concentration determined to prevent phosphorylation of p65, and then exposed to HIV-1 Tat1-72aa for 1 h. Exposure to BAY 11-7085 inhibited Tat-induced SOCS3 mRNA expression (Figure 4C). These data indicate that activation of the NF-κB signaling pathway is necessary for HIV-1 Tat-induced SOCS3 expression in macrophages.
Activation of the MAPK pathway (ERK, JNK, and p38) and the JAK/STAT pathway (STAT1 and STAT3) were also examined. While all three of the MAPK pathways were activated by HIV-1 Tat1-72aa with early kinetics (Supplemental Figure 2A), pharmacologic inhibition of these pathways did not prevent Tat-induced SOCS3 expression (Supplemental Figure 2B, C). By contrast, neither STAT1 nor STAT3 activation occurred with early kinetics following Tat treatment (Supplemental Figure 2D). These data suggest that the MAPK and JAK/STAT pathways are not necessary for HIV-1 Tat-induced SOCS3 expression in macrophages.
HIV-1 Tat-induced SOCS3 expression is not mediated by IL-10 or IFN-β expression
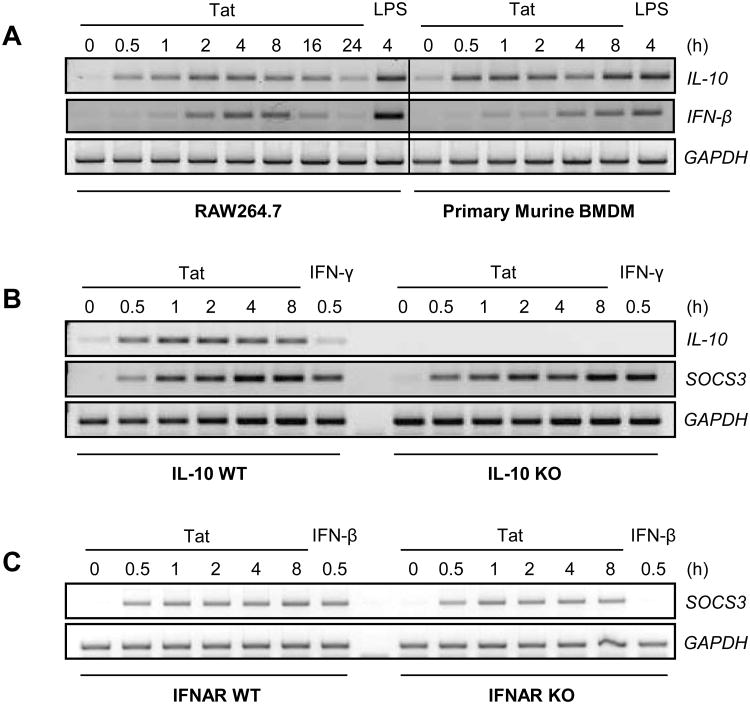
SOCS3 can be induced by a variety of stimuli, two of which are readily induced by HIV-1 Tat: IL-10 (61) and IFN-β (62). To determine whether either Tat-induced IL-10 or IFN-β are required intermediates for SOCS3 expression, the kinetics of their expression were first examined. RAW264.7 macrophages and primary murine BMDM were exposed to HIV-1 Tat1-72aa for the times indicated (Figure 5A). Both IL-10 and IFN-β mRNA expression were induced by Tat treatment, albeit with differential kinetics. To examine whether either of these cytokines were necessary for Tat-induced SOCS3 expression, macrophages deficient for IL-10 (Figure 5B) or the IFNAR (Figure 5C) were compared to wild-type macrophages following HIV-1 Tat1-72aa treatment. Neither deficiency altered HIV-1 Tat-induced SOCS3 mRNA expression, indicating that neither IL-10 nor IFN-β are intermediates in this process.
Figure 5. HIV-1 Tat-induced SOCS3 expression is not mediated by IL-10 or IFN-β expression.
(A) RAW264.7 cells or primary murine BMDM were treated with 10 nM HIV-1 Tat1-72aa, or 100 ng/ml LPS as a positive control, for the times indicated. mRNA was analyzed by RT-PCR with primers specific to IL-10 and IFN-β. A vertical line has been inserted to indicate the merge of separate gels. (B) Wild-type (WT) or IL-10 deficient (KO) primary murine macrophages were treated with 10 nM HIV-1 Tat1-72aa, or 100 ng/ml IFN-γ as a positive control, for the times indicated. mRNA was analyzed by RT-PCR with primers specific to IL-10 and SOCS3. (C) WT or IFNAR-deficient primary murine macrophages were treated with 10 nM HIV-1 Tat1-72aa, or 100 U/ml IFN-β as a positive control, for the times indicated. mRNA was analyzed by RT-PCR with primers specific to SOCS3. GAPDH was examined as a loading control in all experiments. Data are representative of two independent experiments.
HIV-1 Tat inhibits IFN-β-induced STAT activation and antiviral target gene expression
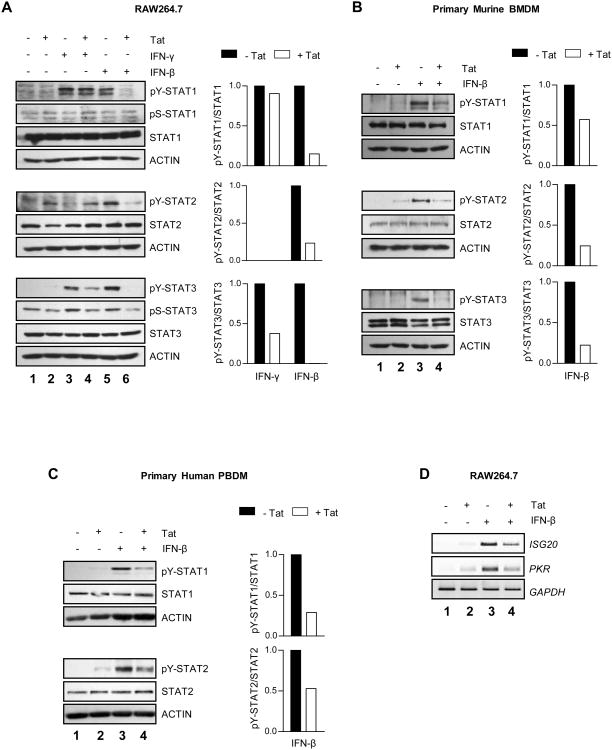
Pathogen-induced SOCS proteins can inhibit signaling downstream of both the type I and type II IFN receptors, thereby diminishing the host immune response (20-25). To determine whether HIV-1 Tat-induced SOCS3 expression is capable of inhibiting IFN signaling, RAW264.7 cells were pretreated with HIV-1 Tat1-72aa for 4 h to induce SOCS3 protein expression, and then exposed to IFN-β or IFN-γ for 15 min (Figure 6A). Treatment with IFN-β induced tyrosine phosphorylation of STAT1, STAT2, and STAT3 (lane 5), which was abolished by Tat pretreatment (lane 6). This inhibition was somewhat stimulus-specific, as IFN-γ-induced STAT1 and STAT3 tyrosine phosphorylation (lane 3) were affected to a much lesser extent by Tat pretreatment (lane 4). Although serine phosphorylation of STAT1 and STAT3 did not occur to a significant extent at this experimental timepoint, Tat pretreatment was sufficient to inhibit basal serine phosphorylation of STAT3 (lanes 2, 4, 6). Tat-induced inhibition of IFN-β signaling was also observed in primary murine BMDM (Figure 6B). IFN-β induced tyrosine phosphorylation of STAT1, STAT2, and STAT3 (lane 3), while pretreatment with HIV-1 Tat1-72aa inhibited this activation (lane 4).
Figure 6. HIV-1 Tat inhibits IFN-β-induced STAT activation and antiviral target gene expression.
RAW264.7 cells (A) or primary murine BMDM (B) were pretreated for 4 h with 10 nM HIV-1 Tat1-72aa or vehicle alone, and then exposed to 100 U/ml murine IFN-β or 100 ng/ml murine IFN-γ for 15 minutes. Protein was subjected to immunoblot analysis with antibodies specific to phosphorylated STAT1 (tyrosine 701 or serine 727), phosphorylated STAT2 (tyrosine 689), phosphorylated STAT3 (tyrosine 705 or serine 727), or total levels of these proteins. Blots were then stripped and reprobed for actin as a loading control. Quantification of band density shown to the right of blots represents pY-STAT levels normalized to total STAT levels, and is displayed as fold decrease below IFN treatment alone. Data are representative of at least two independent experiments. (C) Primary human PBDM were pretreated for 4 h with 10 nM HIV-1 Tat1-72aa or vehicle alone, and then exposed to 100 U/ml human IFN-β for 15 min. Antibodies specific to phosphorylated STAT1 (tyrosine 701), phosphorylated STAT2 (tyrosine 689), and total levels of these proteins were used for immunoblot analysis. Blots were then stripped and reprobed for actin as a loading control. Quantification of band density shown to the right of blots represents pY-STAT levels normalized to total STAT levels, and is displayed as fold decrease below IFN treatment alone. Data are representative of three independent experiments. (D) RAW264.7 cells were pretreated for 4 h with 10 nM HIV-1 Tat1-72aa or vehicle alone, and then exposed to 100 U/ml murine IFN-β for 4 h. mRNA was analyzed by RT-PCR with primers specific to murine ISG20 and PKR. GAPDH was examined as a loading control. Data are representative of two independent experiments.
This phenomenon was also observed in primary human PBDM. Human macrophages were pretreated with HIV-1 Tat1-72aa for 4 h to induce SOCS3 protein expression, and then exposed to IFN-β for 15 min (Figure 6C). IFN-β induced tyrosine phosphorylation of STAT1 and STAT2 (lane 3), but not STAT3, while pretreatment with Tat inhibited this activation (lane 4). Collectively, these data indicate that treatment with HIV-1 Tat, at timepoints sufficient to induce SOCS3 expression, inhibits IFN-β-induced STAT activation in both murine and human macrophages.
We also examined the ability of HIV-1 Tat to inhibit downstream IFN-β-induced antiviral gene expression. RAW264.7 cells were pretreated with HIV-1 Tat1-72aa for 4 h to induce SOCS3 protein expression, and then exposed to IFN-β for 4 h to induce target gene expression. IFN-β treatment induced mRNA expression of ISG20 and PKR (Figure 6D, lane 3), two important inhibitors of HIV-1 replication, while pretreatment with Tat attenuated this expression (lane 4). These data show that the inhibitory effect of HIV-1 Tat on IFN-β signaling results in decreased antiviral target gene expression.
SOCS3 mediates HIV-1 Tat-induced inhibition of IFN-β signaling
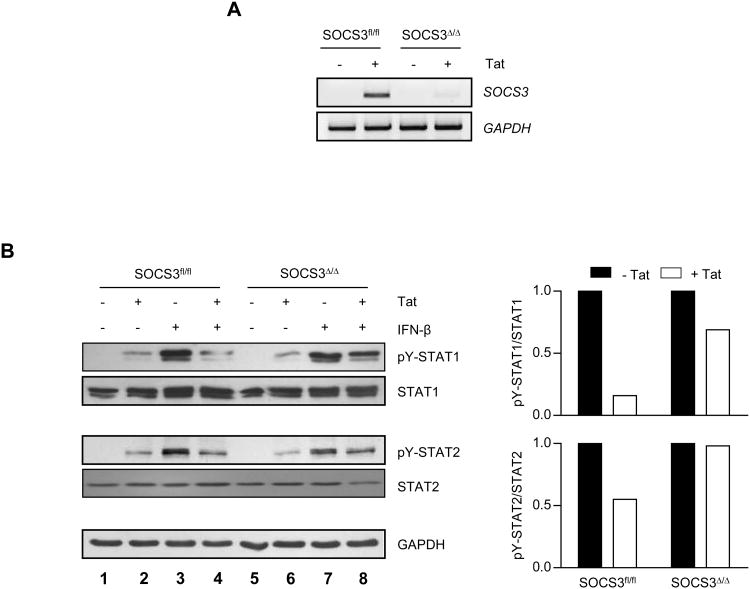
To determine whether the inhibitory activity of HIV-1 Tat is mediated by SOCS3, we evaluated the effect of SOCS3 deficiency on the ability of Tat to dampen IFN-β signaling. Primary murine BMDM were obtained from mice with a conditional SOCS3 allele that was either present and fully functional (SOCS3fl/fl), or excised specifically in macrophages (SOCS3Δ/Δ) (51). SOCS3 mRNA expression was abolished in SOCS3Δ/Δ macrophages in response to HIV-1 Tat1-72aa, as compared to SOCS3fl/fl macrophages (Figure 7A). Cells were then pretreated with HIV-1 Tat1-72aa for 4 h followed by treatment with IFN-β (Figure 7B). While macrophages containing a functional SOCS3 allele exhibited inhibition of IFN-β-induced STAT1 and STAT2 activation in response to Tat (lanes 3 and 4), SOCS3-deletion substantially attenuated this inhibition (lanes 7 and 8). These data indicate that SOCS3 expression mediates HIV-1 Tat-induced inhibition of IFN-β signaling.
Figure 7. SOCS3 mediates HIV-1 Tat-induced inhibition of IFN-β signaling.
(A) SOCS3fl/fl and SOCS3Δ/Δ primary murine BMDM were treated with 10 nM HIV-1 Tat1-72aa for 2 h, and mRNA analyzed by RT-PCR with primers specific for SOCS3. GAPDH mRNA was examined as a loading control. Data are representative of two independent experiments. (B) Cells were pretreated with 10 nM HIV-1 Tat1-72aa for 4 h, followed by treatment with 100 U/ml murine IFN-β for 30 min. Protein was subjected to immunoblot analysis with antibodies specific for phosphorylated STAT1 (tyrosine 701), phosphorylated STAT2 (tyrosine 689), or total levels of these proteins. Blots were then stripped and reprobed for GAPDH as a loading control. Quantification of band density shown to the right of blots represents pY-STAT levels normalized to total STAT levels, and is displayed as fold decrease below IFN treatment alone. Data are representative of two independent experiments.
SOCS3 prevents the ability of IFN-β to suppress HIV-1 replication
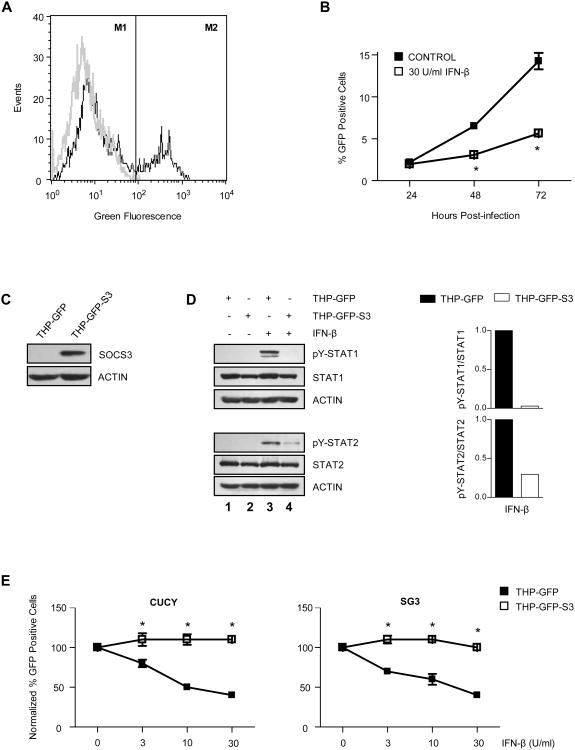
The most important functional consequence of IFN-β in the HIV-1-infected brain is inhibition of viral replication in macrophages. To determine whether the inhibitory effect of SOCS3 observed on upstream IFN-β signaling correlates with an inhibition of downstream antiviral function, we examined the effect of SOCS3 on HIV-1 replication. PMA differentiated THP-1 cells containing a stably integrated HIV-1 LTR-driven reporter construct (THP-GFP macrophages) were used to perform these experiments. In these cells, GFP expression is induced upon productive HIV-1 infection (Figure 8A), and can therefore be used to monitor viral replication. THP-GFP macrophages were infected with HIV-1 CUCY (R5/macrophage preference) in the absence or presence of IFN-β, and then incubated for 24-72 h (Figure 8B). A significant decrease in HIV-1 LTR-driven GFP expression was observed in the presence of IFN-β, indicating an inhibitory effect on HIV-1 replication in these cells. To evaluate the effect of SOCS3 on this process, THP-GFP macrophages containing an integrated SOCS3 expression construct (THP-GFP-S3, Figure 8C) were utilized rather than inducing SOCS3 with HIV-1 Tat, in order to avoid Tat's broader replication enhancing effects. In these cells, SOCS3 overexpression was sufficient to substantially diminish the robust STAT1 and STAT2 activation induced by IFN-β in parental THP-GFP macrophages (Figure 8D, lanes 3 and 4). THP-GFP and THP-GFP-S3 macrophages were then infected with HIV-1 CUCY or HIV-1 SG3 (X4/lymphocyte preference) in the presence of increasing amounts of IFN-β and incubated for 72 h prior to collection (Figure 8E). HIV-1 LTR-driven GFP expression generated in response to infection with either HIV-1 strain was suppressed ∼60% by the highest dose of IFN-β tested in THP-GFP macrophages, while IFN-β had no inhibitory effect in SOCS3 overexpressing THP-GFP macrophages. These results indicate that SOCS3 expression is sufficient to overcome the inhibitory effect of IFN-β and thereby enhance HIV-1 replication in macrophages.
Figure 8. SOCS3 prevents the ability of IFN-β to suppress HIV-1 replication.
(A) HIV-1 infection in THP-GFP macrophages was monitored by flow cytometric analysis of HIV-1 LTR-driven GFP expression. Background GFP expression levels were defined in uninfected cells (grey line, M1). Positive cells were defined by an increase in GFP expression above background levels (shift of black line into M2). (B) THP-GFP macrophages were infected with HIV-1 CUCY in the absence or presence of 30 U/ml of human IFN-β, and the percentage of GFP positive cells was measured 24-72 h post-infection. Values are displayed as mean +/- standard error. *p<0.001 versus control as determined by two-way ANOVA followed by Bonferroni's multiple comparison test. Data are representative of two independent experiments. (C) Protein from untreated THP-GFP and THP-GFP-S3 cells was subjected to immunoblot analysis with antibodies specific to SOCS3. Blot was stripped and reprobed for actin as a loading control. (D) THP-GFP and THP-GFP-S3 macrophages were treated with 30 U/ml of human IFN-β for 15 min. Protein was subjected to immunoblot analysis with antibodies specific to phosphorylated STAT1 (tyrosine 701), phosphorylated STAT2 (tyrosine 689), or total levels of these proteins. Blots were then stripped and reprobed for actin as a loading control. Quantification of band density shown to the right of blots represents pY-STAT levels normalized to total STAT levels, and is displayed as fold decrease below THP-GFP cells. Data are representative of two independent experiments. (E) THP-GFP and THP-GFP-S3 macrophages were infected with HIV-1 CUCY or HIV-1 SG3 in the presence of 0-30 U/ml of human IFN-β. The percentage of GFP positive cells was measured 72 h post-infection. Values are displayed as a fraction of control and represent an average of two independent experiments performed in triplicate +/- standard error. *p<0.01 versus THP-GFP macrophages as determined by two-way ANOVA followed by Bonferroni's multiple comparison test.
Discussion
IFN-β is a key mediator of the host immune response to HIV-1 infection within the CNS. Previous work has shown that IFN-β can inhibit HIV-1 replication within macrophages (10), the most important source of productive HIV-1 infection in the brain, and that increases in its expression correlate with early suppression of viral replication in a SIV/macaque model of HAD (11). Unfortunately, while the ability to express IFN-β persists through the late stages of disease, its antiviral effect is only transient (13). HIV-1 eventually overcomes this response to resume replication within the CNS, often leading to the cognitive and motor impairments which characterize HAND. In these studies, we describe a novel mechanism by which HIV-1 can evade IFN-β's antiviral effects in the CNS. We show that HIV-1 Tat-induced SOCS3 expression in macrophages diminishes IFN-β signaling, ultimately preventing the ability of IFN-β to inhibit HIV-1 replication. These studies suggest that SOCS3 may promote progression toward HAND by allowing HIV-1 to evade protective host immune responses in the CNS.
Although both SOCS1 and SOCS3 have been shown to prevent IFN-mediated inhibition of viral replication, our studies focused specifically on SOCS3. This decision was based primarily on our analysis of mRNA collected from a SIV/macaque model of HAD, which showed that increases in SOCS3 expression, but not SOCS1, correlated with recurrence of viral replication and onset of CNS disease. This model allowed us to evaluate SOCS expression throughout the course of disease progression from identically infected and harvested brains, providing us with an accurate kinetic analysis of SOCS expression during disease. Such well controlled data is difficult to obtain from human patients. Progression to HAD among a population of HIV-1-infected patients is much more variable in humans, as are the conditions under which post-mortem brain is collected. However, even with these limitations, available data does suggest that SOCS3 levels are increased in the human HAD brain. A microarray study performed on 24 basal ganglia samples by the National NeuroAIDS Tissue Consortium revealed that the signal intensity of a probe set for SOCS3 mRNA (227697_at) was increased significantly in post-mortem brains from patients with HAD when compared to control patients or HIV-1-infected patients without CNS disease (Dr. Benjamin Gelman, University of Texas Medical Branch, Galveston, TX; personal communication). Although variation between patients is substantial, and expected, these data support the conclusions of our study that SOCS3 may contribute to the progression toward HAND.
We determined that the HIV-1 protein Tat is sufficient to induce SOCS3 expression in macrophages, and therefore may be responsible for the most critical elevations in SOCS3 expression observed in the HAD brain. This attributes yet another HAND promoting effect to Tat, that of innate immune evasion within the CNS. Interestingly, induction of SOCS3 also reveals a concealed similarity between Tat and the HIV-1 accessory proteins, which are often responsible for inhibiting innate immunity (63). For example, Vif eliminates IFN-induced APOBEC3G, an antiviral effector capable of inducing virus-crippling hypermutation (64), while Vpu antagonizes another IFN-induced antiviral factor, tetherin, which prevents viral release from the cell membrane (65). Therefore, while the finding that Tat-induced SOCS3 can provide innate immune evasion within the CNS is novel, it is also supported by significant commonality with the current understanding of HIV-1 function.
In our studies, HIV-1 Tat directly induced high levels of SOCS3 expression. Because SOCS3 is thought to bind TYK2 (66), which is only present in the type I IFN receptor complex, Tat predictably inhibits type I IFN signaling (IFN-β) to a greater extent than type II IFN signaling (IFN-γ). However, HIV-1 Tat was not entirely specific for SOCS3 expression. Low levels of SOCS1 mRNA were also detected with delayed kinetics, suggesting an indirect relationship to Tat treatment. In addition, Cheng et al., showed that HIV-1 Tat induces SOCS2 expression in human monocytes (67), which we also detected in macrophages at very low levels. Because our data indicate that direct induction of SOCS3 by HIV-1 Tat is NF-κB-dependent, the absence of a NF-κB consensus site within the SOCS1 and SOCS2 promoters may account for their limited, and perhaps indirect, expression. Notably though, while several studies have reported a role for NF-κB in SOCS3 expression (23,68), none have shown NF-κB p65 binding to the SOCS3 promoter. Regardless of their relative expression levels, it remains possible that small quantities of these other SOCS family members could further augment the ability of Tat to inhibit IFN signaling within the HIV-1-infected brain.
In these studies we have clearly outlined an in vitro mechanism whereby HIV-1 Tat induces SOCS3 expression, thereby preventing the ability of IFN-β to suppress viral replication. While the correlation between SOCS3 expression and recurrence of viral replication in our in vivo model strongly supports this hypothesis, it does not preclude much greater complexity. First, although Tat is certainly one stimulus in the HIV-1-infected brain capable of inducing SOCS3, others are likely present. Cytokines with the ability to induce SOCS3 such as IL-6 and OSM are reportedly increased in the CNS during HIV-1 infection (36,69). Other soluble viral proteins in addition to Tat may also possess SOCS3-inducing activity. Second, while we have tested the ability of SOCS3 to inhibit the most well-known suppressor of HIV-1 replication in macrophages, IFN-β, it may have broader effects. Chemokines which bind the HIV-1 co-receptor CCR5, such as MIP-1α, MIP-1β, and RANTES (70), as well as other factors including macrophage-derived chemokine (MDC) (71) and IL-10 (72), have been shown to contribute to inhibition of HIV-1 replication in macrophages. Based on the inhibitory role that SOCS3 plays in multiple cytokine and chemokine signaling pathways, it is reasonable to expect that at least some of these additional inhibitory mechanisms may be affected by SOCS3 expression. Therefore, while our data strongly suggest that SOCS3 plays an important role in the progression to HAND, its mechanism of induction and inhibitory function in the HIV-1-infected brain may be broader than that defined by this study.
Finally, it is important to note that SOCS3 expression likely showed such a robust correlation with CNS disease progression because anti-HIV-1 immunity within the brain depends so heavily on the innate arm of the immune system. While HIV-1 infection in the periphery is controlled by the innate and adaptive immune systems working in collaboration, restricted access of adaptive effectors into the CNS (T cells, NK cells, and antibodies) shifts the immune burden to innate mechanisms. However, it is reasonable to expect that the SOCS3-mediated innate immune evasion observed in this study is transferable to HIV-1 replication in the periphery. Based on the increased importance that has recently been placed on innate immunity in the control of peripheral HIV-1 infection, specifically as mediated by type I IFN signaling pathways (i.e. APOBEC3G (64), TRIM (73)), it seems likely that the effect of SOCS3 on HIV-1 replication is not restricted to the CNS.
In summary, these studies describe a novel mechanism by which HIV-1, through the use of its regulatory protein Tat, can evade innate immune defenses. They specifically suggest a mechanism for progression toward HAND, but also more generally enhance our understanding of the complexities of HIV-1 immune evasion.
Supplementary Material
Acknowledgments
We thank Dr. Charles Elson (University of Alabama at Birmingham, Birmingham, AL) for providing IL-10-deficient mice, Dr. Chander Raman (UAB) for providing IFNAR-deficient mice, Dr. Warren Alexander (The Walter and Eliza Hall Institute, Victoria, Australia) for providing SOCS3-floxed mice, Dr. Phillip Smith (UAB) for providing human PBDM, Drs. George Shaw and Beatrice Hahn (UAB) for providing HIV-1 CUCY, Dr. John Kappes (UAB) and the UAB CFAR Virology Core for providing the HIV-1 LTR-driven GFP construct, Dr. Randolph Noelle (Dartmouth Medical School, Lebanon, NH) for providing the IKKβ dominant negative construct, and Dr. Avindra Nath (Johns Hopkins University, Baltimore, MD) for providing HIV-1 Tat1-72aa. We would also like the thank Dr. Phillip Smith, Dr. Avindra Nath, and Dr. Casey Morrow (UAB) for helpful advice and guidance on this project, and Julie Decker (UAB) for providing expert BSL3 training.
Footnotes
This work is funded in part by NIH grants NS45290 (E.N.B.), NS50665 (E.N.B.), NS57563 (E.N.B.), MH70306 (J.E.C.), NS47984 (J.E.C.), NS050028 (J.E.C.), NS055648 (J.E.C.), AI077457 (O.K.), and AI064012 (O.K.), by the National Multiple Sclerosis Society RG 3892-A-12 (E.N.B.), and by a GCE grant from the Bill and Melinda Gates Foundation (O.K.). L.N.A. is supported by the UAB Medical Scientist Training Program, and by NIH T32-AI-07493 and F30-NS-65600.
Abbreviations used in this paper: BMDM, bone marrow-derived macrophage; HAART, highly active antiretroviral therapy; HAD, HIV-associated dementia; HAND, HIV-associated neurocognitive disorder; ISG, IFN-stimulated gene; OSM, Oncostatin M; PBDM, peripheral blood-derived macrophage; SOCS, Suppressor Of Cytokine Signaling; Tat, transactivator of transcription.
References
- 1.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RM, Anderson JP, Patterson TL, McCutchan JA, Weinrich JD, Heaton RK, Atkinson JH, Thal L, Chandler J, Grant I. Validity of the Quality of Well-Being Scale for persons with human immunodeficiency virus infection. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1995;57:138–147. doi: 10.1097/00006842-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, Godfrey HP, Kirson DA, Grant I. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeux R, Stern Y, Tang MX, Todak G, Marder K, Sano M, Richards M, Stein Z, Ehrhardt AA, Gorman JM. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology. 1993;43:176–182. doi: 10.1212/wnl.43.1_part_1.176. [DOI] [PubMed] [Google Scholar]
- 6.Navia BA, Price RW. An overview of the clinical and biological features of the AIDS dementia complex. In: Gendelman HE, Grant I, Everall IP, Lipton SP, Swindells S, editors. The Neurology of AIDS. Oxford Univeristy Press; New York: 2005. pp. 339–356. [Google Scholar]
- 7.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 8.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 9.Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. The role of interferons in the control of HIV replication in macrophages. Clin Immunol Immunopathol. 1990;54:200–219. doi: 10.1016/0090-1229(90)90082-2. [DOI] [PubMed] [Google Scholar]
- 11.Barber SA, Herbst DS, Bullock BT, Gama L, Clements JE. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol. 2004;10(Suppl 1):15–20. doi: 10.1080/753312747. [DOI] [PubMed] [Google Scholar]
- 12.Minagawa T, Mizuno K, Hirano S, Asano M, Numata A, Kohanawa M, Nakane A, Hachimori K, Tamagawa S, Negishi M. Detection of high levels of immunoreactive human beta-1 interferon in sera from HIV-infected patients. Life Sci. 1989;45:iii–vii. doi: 10.1016/0024-3205(89)90147-1. [DOI] [PubMed] [Google Scholar]
- 13.Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV):steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- 15.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 16.Carpick BW, Graziano V, Schneider D, Maitra RK, Lee X, Williams BR. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J Biol Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 17.Espert L, Degols G, Lin YL, Vincent T, Benkirane M, Mechti N. Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J Gen Virol. 2005;86:2221–2229. doi: 10.1099/vir.0.81074-0. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 19.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoiber D, Stockinger S, Steinlein P, Kovarik J, Decker T. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J Immunol. 2001;166:466–472. doi: 10.4049/jimmunol.166.1.466. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez N, Greenwell-Wild T, Rekka S, Orenstein JM, Wahl SM. Mycobacterium avium-induced SOCS contributes to resistance to IFN-gamma-mediated mycobactericidal activity in human macrophages. J Leukoc Biol. 2006;80:1136–1144. doi: 10.1189/jlb.0306206. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J Immunol. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]
- 23.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, Ludwig S. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey KG, Ahmed CM, Dabelic R, Jager LD, Noon-Song EN, Haider SM, Johnson HM, Bigley NJ. HSV-1-induced SOCS-1 expression in keratinocytes use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J Immunol. 2009;183:1253–1262. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryo A, Tsurutani N, Ohba K, Kimura R, Komano J, Nishi M, Soeda H, Hattori S, Perrem K, Yamamoto M, Chiba J, Mimaya J, Yoshimura K, Matsushita S, Honda M, Yoshimura A, Sawasaki T, Aoki I, Morikawa Y, Yamamoto N. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc Natl Acad Sci U S A. 2008;105:294–299. doi: 10.1073/pnas.0704831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutsopoulos NM, Vazquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol. 2006;80:1145–1155. doi: 10.1189/jlb.0306142. [DOI] [PubMed] [Google Scholar]
- 28.Huigen MC, Kamp W, Nottet HS. Multiple effects of HIV-1 trans-activator protein on the pathogenesis of HIV-1 infection. Eur J Clin Invest. 2004;34:57–66. doi: 10.1111/j.1365-2362.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 32.Ghezzi S, Noonan DM, Aluigi MG, Vallanti G, Cota M, Benelli R, Morini M, Reeves JD, Vicenzi E, Poli G, Albini A. Inhibition of CXCR4-dependent HIV-1 infection by extracellular HIV-1 Tat. Biochem Biophys Res Commun. 2000;270:992–996. doi: 10.1006/bbrc.2000.2523. [DOI] [PubMed] [Google Scholar]
- 33.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 34.New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- 35.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 37.Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272:22385–22388. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Bosch I, Hofmann W, Sodroski J, Pardee AB. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman JD, Brown JA, Howcroft TK, Hwang J, Chawla A, Roche PA, Schiltz L, Nakatani Y, Singer DS. HIV-1 tat binds TAFII250 and represses TAFII250-dependent transcription of major histocompatibility class I genes. Proc Natl Acad Sci U S A. 1998;95:11601–11606. doi: 10.1073/pnas.95.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanazawa S, Okamoto T, Peterlin BM. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12:61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 42.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of LPS-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–5976. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen VT, Benveniste EN. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J Biol Chem. 2000;275:23674–23684. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- 44.Dong Y, Tang L, Letterio JJ, Benveniste EN. The Smad3 protein is involved in TGF-beta inhibition of class II transactivator and class II MHC expression. J Immunol. 2001;167:311–319. doi: 10.4049/jimmunol.167.1.311. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wu Y, Zhou Y. Modulation of inactivation properties of CaV2.2 channels by 14-3-3 proteins. Neuron. 2006;51:755–771. doi: 10.1016/j.neuron.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen VT, Walker WS, Benveniste EN. Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur J Immunol. 1998;28:2537–2548. doi: 10.1002/(SICI)1521-4141(199808)28:08<2537::AID-IMMU2537>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Maheshwari A, Smythies LE, Wu X, Novak L, Clements R, Eckhoff D, Lazenby AJ, Britt WJ, Smith PD. Cytomegalovirus blocks intestinal stroma-induced down-regulation of macrophage HIV-1 infection. J Leukoc Biol. 2006;80:1111–1117. doi: 10.1189/jlb.0306230. [DOI] [PubMed] [Google Scholar]
- 48.Flaherty MT, Hauer DA, Mankowski JL, Zink MC, Clements JE. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SJ, Qin H, Benveniste EN. Simvastatin inhibits IFN-gamma-induced CD40 gene expression by suppressing STAT-1alpha. J Leukoc Biol. 2007;82:436–447. doi: 10.1189/jlb.1206739. [DOI] [PubMed] [Google Scholar]
- 50.Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 52.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 53.Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- 54.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 55.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 56.Newstein M, Stanbridge EJ, Casey G, Shank PR. Human chromosome 12 encodes a species-specific factor which increases human immunodeficiency virus type 1 tat-mediated trans activation in rodent cells. J Virol. 1990;64:4565–4567. doi: 10.1128/jvi.64.9.4565-4567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim BO, Liu Y, Zhou BY, He JJ. Induction of C chemokine XCL1 (lymphotactin/single C motif-1 alpha/activation-induced, T cell-derived and chemokine-related cytokine) expression by HIV-1 Tat protein. J Immunol. 2004;172:1888–1895. doi: 10.4049/jimmunol.172.3.1888. [DOI] [PubMed] [Google Scholar]
- 58.Ehlting C, Lai WS, Schaper F, Brenndorfer ED, Matthes RJ, Heinrich PC, Ludwig S, Blackshear PJ, Gaestel M, Haussinger D, Bode JG. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178:2813–2826. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- 59.Gatto L, Berlato C, Poli V, Tininini S, Kinjyo I, Yoshimura A, Cassatella MA, Bazzoni F. Analysis of SOCS-3 promoter responses to interferon gamma. J Biol Chem. 2004;279:13746–13754. doi: 10.1074/jbc.M308999200. [DOI] [PubMed] [Google Scholar]
- 60.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 61.Cassatella MA, Gasperini S, Bovolenta C, Calzetti F, Vollebregt M, Scapini P, Marchi M, Suzuki R, Suzuki A, Yoshimura A. Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood. 1999;94:2880–2889. [PubMed] [Google Scholar]
- 62.Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181:3167–3176. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 65.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 66.Zeng B, Li H, Liu Y, Zhang Z, Zhang Y, Yang R. Tumor-induced suppressor of cytokine signaling 3 inhibits toll-like receptor 3 signaling in dendritic cells via binding to tyrosine kinase 2. Cancer Res. 2008;68:5397–5404. doi: 10.1158/0008-5472.CAN-07-6792. [DOI] [PubMed] [Google Scholar]
- 67.Cheng SM, Li JC, Lin SS, Lee DC, Liu L, Chen Z, Lau AS. HIV-1 transactivator protein induction of suppressor of cytokine signaling-2 contributes to dysregulation of IFN{gamma} signaling. Blood. 2009;113:5192–5201. doi: 10.1182/blood-2008-10-183525. [DOI] [PubMed] [Google Scholar]
- 68.Yang XP, Albrecht U, Zakowski V, Sobota RM, Haussinger D, Heinrich PC, Ludwig S, Bode JG, Schaper F. Dual function of interleukin-1beta for the regulation of interleukin-6-induced suppressor of cytokine signaling 3 expression. J Biol Chem. 2004;279:45279–45289. doi: 10.1074/jbc.M313072200. [DOI] [PubMed] [Google Scholar]
- 69.Ensoli F, Fiorelli V, DeCristofaro M, Santini MD, Novi A, Vannelli B, Thiele CJ, Luzi G, Aiuti F. Inflammatory cytokines and HIV-1-associated neurodegeneration oncostatin-M produced by mononuclear cells from HIV-1-infected individuals induces apoptosis of primary neurons. J Immunol. 1999;162:6268–6277. [PubMed] [Google Scholar]
- 70.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 71.Cota M, Mengozzi M, Vicenzi E, Panina-Bordignon P, Sinigaglia F, Transidico P, Sozzani S, Mantovani A, Poli G. Selective inhibition of HIV replication in primary macrophages but not T lymphocytes by macrophage-derived chemokine. Proc Natl Acad Sci U S A. 2000;97:9162–9167. doi: 10.1073/pnas.160359197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weissman D, Poli G, Fauci AS. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retroviruses. 1994;10:1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 73.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.