Abstract
Purpose
The aim of the study was to determine the feasibility of using a clinical optical breast scanner with molecular imaging strategies based on modulating light transmission.
Procedures
Different concentrations of single-walled carbon nanotubes (SWNT; 0.8–20.0 nM) and black hole quencher-3 (BHQ-3; 2.0–32.0 μM) were studied in specifically designed phantoms (200–1,570 mm3) with a clinical optical breast scanner using four wavelengths. Each phantom was placed in the scanner tank filled with optical matching medium. Background scans were compared to absorption scans, and reproducibility was assessed.
Results
All SWNT phantoms were detected at four wavelengths, with best results at 684 nm. Higher concentrations (≥8.0 μM) were needed for BHQ-3 detection, with the largest contrast at 684 nm. The optical absorption signal was dependent on phantom size and concentration. Reproducibility was excellent (intraclass correlation 0.93–0.98).
Conclusion
Nanomolar concentrations of SWNT and micromolar concentrations of BHQ-3 in phantoms were reproducibly detected, showing the potential of light absorbers, with appropriate targeting ligands, as molecular imaging agents for clinical optical breast imaging.
Keywords: Optical imaging, Molecular imaging, Breast, Transmission
Introduction
Optical breast imaging uses near-infrared light in the wavelength range of 600 to 1,000 nm to assess optical properties of tissue [1]. It can provide spectroscopic information about physiological and functional tissue parameters such as angiogenesis and tissue oxygenation by using intrinsic optical contrast [2, 3]. This technique could potentially provide added value to currently used breast imaging modalities: mammography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging, which all have drawbacks regarding sensitivity and specificity in the diagnosis, early detection, and treatment monitoring of breast cancer [4–7]. However, several optical imaging studies have shown that differences in intrinsic optical absorption are not always pronounced enough to detect breast masses against normal tissue background [8].
A possible solution is to enhance neoplastic lesion detection with imaging agents that change light transmission. In optical imaging, different strategies for contrast enhancement are possible, such as influencing the absorption, scattering, or fluorescence properties of tissue using specific imaging agents. Fluorescent breast imaging has already been demonstrated by several research groups [9–11]. Light scattering properties could potentially be influenced by administering particles such as used in Raman spectroscopy [12]. The focus of this study is using highly light-absorbing imaging agents that absorb sufficient amounts of light to be detected by the optical imaging system. Examples of such agents are small molecules like indocyanine green (ICG), methylene blue (MB), or larger agents such as nanotubes or nanocages. The use of ICG in humans was reported by Ntziachristos et al. and Intes et al. [13, 14]. ICG and MB were previously used as nontargeted optical contrast agents in a rat model by Cuccia et al. [15]. Carbon nanotubes were demonstrated as photoacoustic molecular imaging agents by our laboratory (photoacoustic imaging also being based on the absorption of the molecules) [16]. If one could specifically target cancer-associated molecules with the light-absorbing imaging agents and deliver sufficient amounts to the tumor site, molecular imaging of the breast could be achieved with optical transmission imaging.
In this study, we focused on light absorbers (not fluorescent agents) to change light transmission. Our aim was to determine the feasibility of using a clinical optical breast scanner with molecular imaging strategies based on modulating light transmission. Physical phantoms based on single-walled carbon nanotubes (SWNT) and black hole quencher-3 (BHQ-3) were studied. To our knowledge, this is the first report to test this new approach to molecular imaging.
Methods
Clinical Optical Breast Imaging System
A commercially available clinical optical breast imager, the SoftScan system (ART Advanced Research Technologies Inc.) was used for this study (Fig. 1). An earlier version of this system has been previously described [17]. This new version also uses a time resolved technique developed on a time correlated single photon counting technology. It uses pulsed laser diodes (PicoQuant GmbH, Berlin, Germany) each driven at 20 MHz and time multiplexed for simultaneous acquisition of all the wavelengths. The light is detected by a five-channel detector array using optical systems comprised of lens-coupled multimode fibers that form a “M”-shape constellation for collecting the photons. The detection array is placed opposite the emission fiber (transmission configuration), central detection fiber coaxial with the emission fiber. The fibers transfer the photons to fast photomultiplier (PMT) detectors (H7422-50 from Hamamatsu Corporation, Bridgewater, NJ, USA). The outputs of the PMTs are connected to time-correlated single photon modules (SPC-130 from Becker & Hickl GmbH, Berlin, Germany) that generate the temporal histogram of the photons. The emission and detection fibers are raster scanned simultaneously over the region of examination. The patient lies in a prone position and her breast is pending in a tank with two mobile glass walls that are used for stabilizing the breast during the scan. The tank is filled up with optical matching medium (OMM), containing 10% Liposyn II (Abbott Laboratories, Montreal, QC, Canada), demineralized water, and India ink (Idee Cadres, Laval, QC, Canada) in a previously described dilution that mimics the average absorption and scattering properties of a normal breast. Data are collected at four discreet wavelengths—684, 732, 781, and 827 nm—and consist of temporal point spread functions (TPSF) acquired with a 10-ps resolution within a 12.5-ns time window. Among the critical aspects of the time resolved systems are the drift (<5 ps/h) and the jitter (2 ps) [18].
Fig. 1.
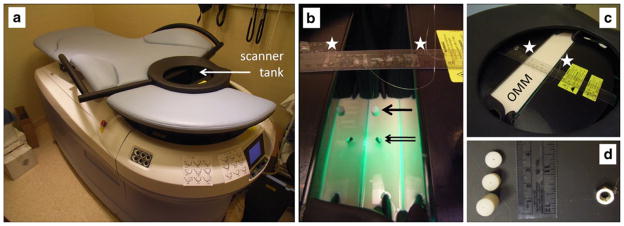
a The clinical optical breast imaging system (SoftScan, ART Advanced Research Technologies Inc.). b Positioning of the SWNT phantom (arrow) in the scanner tank hanging on a thin wire between the two stabilization plates (stars) with a weight at the bottom (double arrow) to keep the phantom in place, and c filling the tank completely with optical matching medium (OMM). d Examples of phantoms of three different sizes (200, 780, and 1,570 mm3).
Phantoms and Contrast Agents
Solid inclusions were made from polyurethane resin (Axson, Eaton Rapids, MI, USA). Titanium dioxide (TiO2; Alfa Aesar, Ward Hill, MA, USA) was added as scattering agent and ProJet 900NP (Avecia, Billingham, UK) as absorbing agent to mimic the optical properties of normal breast tissue: average scattering coefficient 1 mm−1 at 780 nm and minimum absorption coefficient 0.002 mm−1 at 780 nm. The details regarding the design and validation of phantoms are described in previous publications [19, 20]. The imaging agents to be tested, the SWNT and BHQ-3, were added at various concentrations. They were dissolved in dimethyl sulfoxide before mixing with polyurethane resin, and ultrasonication was used to ensure homogeneous phantoms. AP grade SWNTs were obtained from CarboLex Inc. (Lexington, KY, USA) with typical sizes of approximately 1 nm in diameter and 1 μm in length. BHQ-3 was obtained from Biosearch Technologies Inc. (Novato, CA, USA). Phantoms with no addition of imaging agent were fabricated as control phantoms.
Measurement Procedures
For the measurements, the solid inclusions were positioned in the system scanner tank, hanging on a thin fish wire between the glass stabilization plates, where normally a patient’s breast would be positioned (Fig. 1). The stabilization plates were positioned 60 mm apart, corresponding to a representative thickness expected for a breast. The volume of the tank was filled up with OMM. The solid inclusions were positioned in a central position within the scanner tank. Six concentrations of SWNTs (0.8, 1.6, 2.4, 4.0, 6.4, and 20.0 nM) were measured in specifically designed solid phantoms of 3 sizes (200, 780, and 1,570 mm3) on the clinical optical breast scanner using all four wavelengths. We first performed scans using only OMM to acquire the background signal. Then, SWNT absorption scans were done and compared to background absorption. Measurements were also repeated on 2 days at 4 weeks apart, to assess reproducibility. Five concentrations of BHQ-3 (2.0, 4.0, 8.0, 16.0, and 32.0 μM) were tested in an equivalent experimental setup using phantoms of two different sizes (200 and 780 mm3). These measurements were also repeated 4 weeks later.
Data for the tomographic view is acquired in transmission mode using five detection points for each illumination point positioned at angles optimized for maximizing the accuracy and sensitivity of the tomographic reconstruction. This 1×5 source-detector configuration is moved inside the field of view using a raster scan in step-and-shoot mode. The step size is adjustable in the range 0.5 to 10 mm with 0.1 mm accuracy. In the standard study workflow, data acquisition is performed in two steps: (1) a fast low precision scan is performed, which the results of this scan are used for power optimization of the lasers during the high precision scan to maximize signal to noise ratio (SNR), and (2) a high precision scan with optimized SNR is performed, using the step size and field of view selected by the user.
For this study, a scan resolution of 3×3 mm (i.e., scan step size of 3 mm in both x and y-directions) and a field of view of 54×54 mm were used for the high precision scan. For these settings, the scan duration was ~6 min for the high precision and less than 2 min for the low precision scan.
Image Analysis
The tomographic reconstruction and analysis of the images were performed using the SoftScan Review Workstation Software (ART Advanced Research Technologies Inc.). The software includes a diffuse optical tomography algorithm based on the diffusion equation [21], using the linearization of the heterogeneous time-domain diffusion equation within the first order of Rytov approximation [22]. Using the zeroth and first moments of the temporal distributions of photons and the Levenberg–Marquard minimization algorithm, the local differential scattering and absorption coefficients are evaluated for each of the four wavelengths. The thickness of the slice used for 3D reconstruction was 3 mm.
In this study, images of the absorption coefficient were used for the analysis of the contrast of the SWNT inclusions. Circular regions of interest (ROIs) of a constant size (10 mm diameter) were defined on the image of the slice that displays maximum absorption contrast for the inclusions. For the background scan, with OMM only, identical ROIs were placed at the same positions as for the inclusions. Average absorption was calculated by the software. Regression lines were fitted through the data sets using Microsoft Office Excel 2007. Reproducibility of measurements was determined using intraclass correlation coefficients. The software package SPSS 15.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical computations.
Results
All phantoms of different sizes and SWNT concentrations were detected by the system at all four wavelengths, with the best results obtained at 684 nm (Figs. 2 and 4). SWNT absorption was between 10% and 80% higher than background absorption, which was statistically significant (p<0.05; Fig. 4).
Fig. 2.
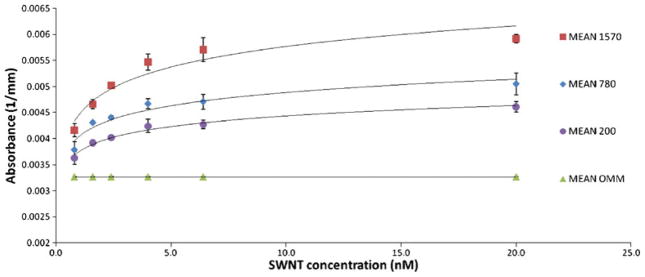
Average absorption measurements at 684 nm of phantoms of three sizes (200, 780, and 1,570 mm3) containing six concentrations of SWNTs (0.8, 1.6, 2.4, 4, 6.4, and 20 nM). Error bars represent the standard deviation of duplicate measurements.
Fig. 4.
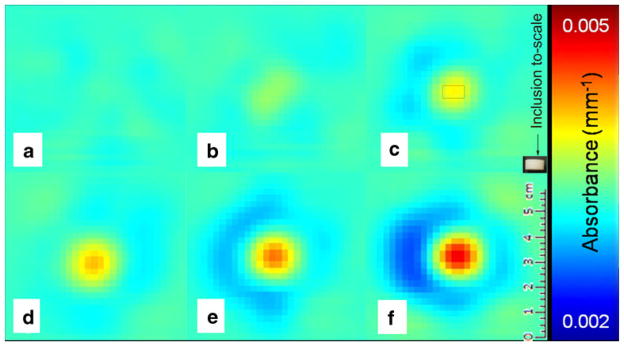
Optical absorption measurements at 684 nm: a optical matching medium only; b–f 200 mm3 phantom with different SWNT concentrations, i.e., 0.8 (b), 1.6 (c), 2.4 (d), 6.4 (e), and 20 nM (f). The rectangle drawn in c represents the to-scale inclusion for reference.
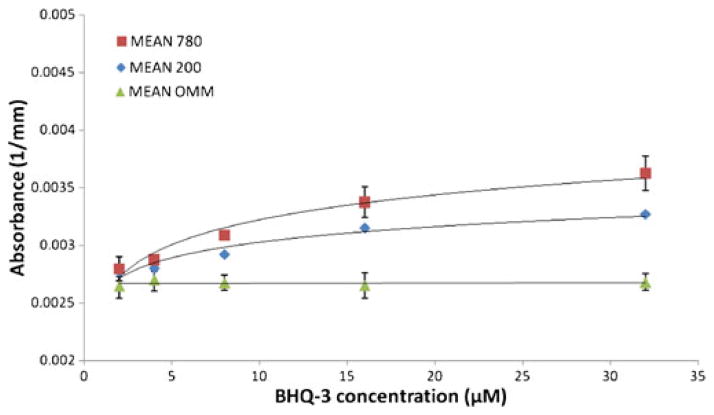
For the BHQ-3 phantoms, 684 nm was also the most sensitive wavelength (Fig. 3). Higher probe concentrations were needed in order to detect the BHQ-3 phantoms. Concentrations >8.0 μM gave an increase in absorption between 15% and 30% compared to background absorption; phantoms containing BHQ-3 in concentrations <8.0 μM were not visible on the optical images. To detect BHQ-3 at wavelengths other than 684 nm, higher concentrations of the imaging agent would be required, as can be expected from the absorbance spectrum of BHQ-3 [23].
Fig. 3.
Average absorption measurements at 684 nm of phantoms of two sizes (200 and 780 mm3) containing five concentrations of BHQ-3 (2.0, 4.0, 8.0, 16, and 32 μM). Error bars represent the standard deviation of duplicate measurements.
Optical absorption signal (y) was dependent on phantom size and SWNT concentration (x), e.g., for 200 mm3 at 684 nm: y=0.0003ln(x)+0.0038, R2=0.98. Similar size and concentration dependency was seen for the BHQ-3 phantoms, e.g., for 200 mm3 at 684 nm: y=0.0002ln(x)+0.0026, R2=0.94. See Supplementary Information for all results.
Absorption of the control phantoms that did not contain SWNT or BHQ-3 was approximately equal to the background (OMM) absorption (no statistically significant difference; p> 0.65). Reproducibility of measurements was excellent for both SWNT and BHQ-3 phantoms at all wavelengths with intraclass correlation coefficients ranging from 0.93 to 0.98 (Table 1).
Table 1.
Reproducibility of measurements for SWNT and BHQ-3 phantoms per wavelength
| Wavelength | Intraclass correlation coefficient for SWNTa (95% confidence interval) | Intraclass correlation coefficient for BHQ-3a (95% confidence interval) |
|---|---|---|
| 684 | 0.97 (0.91 to 0.99) | 0.93 (0.87 to 0.96) |
| 732 | 0.97 (0.91 to 0.99) | 0.93 (0.87 to 0.96) |
| 781 | 0.98 (0.94 to 0.99) | 0.94 (0.88 to 0.97) |
| 827 | 0.96 (0.89 to 0.98) | 0.94 (0.88 to 0.97) |
One-way random effects model where people effects are random (single measures)
Discussion
Detection of SWNT and BHQ-3 in phantoms in a clinical optical breast scanner is possible at nanomolar and micro-molar concentrations, respectively, showing the potential of using highly light-absorbing molecular imaging agents as optical imaging agents for breast disease. We found a positive relationship between the optical absorption signal and the concentration of the imaging agent. The reproducibility of the results was excellent.
The use of multiple wavelengths showed differences in absorption changes for the tested phantoms. For both SWNT and BHQ-3, 684 nm showed the most optimal changes in absorption; for SWNT, there were only small differences with the results at other wavelengths, but for BHQ-3, the same phantoms could not be detected at the higher wavelengths (see Supplementary Figs. 1, 2, 3, 4, 5, and 6). These results can be predicted from the absorbance spectra of the imaging agents: For BHQ-3, optical absorbance decreases rapidly at wavelengths higher than 700 nm [23], while for SWNT, absorbance spectra are more constant, displaying only a slow and small decrease for wavelengths longer than 680 nm [16, 24].
In this initial study, we tested a new approach to molecular imaging using a clinical optical breast scanner to detect changes in light transmission. We limited ourselves to one particular study setup, using phantoms that were positioned in the same way within the scanner tank each time, surrounded by optical matching media. This approach uses a relatively simple and homogeneous approximation of a patient’s breast and may be one of the limitations of our study. Results should therefore be validated in future studies in heterogeneous breast tissue, preferably in vivo. However, in our strategy, we regard the exogenous optical absorption as a relative change added on top of the existing endogenous optical absorption (determined in the first scan, before imaging agent administration). In this way, the tomographic reconstruction algorithm will take care of the heterogeneity of a breast, detecting the optical absorption changes after molecular imaging agent administration and thereby significantly reducing the impact of some well known limitations of diffuse optical tomography. Other limitations include the relatively poor spatial resolution of optical imaging and the complexities of its tomographic image reconstruction. The reconstruction models used by our system derive optical properties from the temporal histogram of the photons after propagation through diffuse media, the TPSF. When a TPSF is measured near a small inclusion with very high absorption contrast compared with the surrounding background, the inclusion will contribute to the TPSF in a way that can generate a transitory effect: the “ring-artifacts” around the inclusions as seen in Fig. 4 (in particular e–f).
The spatial resolution of the images is determined by the optical properties of the tissue, the breast thickness, and step size for each scan. The step size and acquisition time were optimized for a breast thickness ranging from 40 to 80 mm (between the stabilization plates) and the expected contrast between the optical properties of breast tumors in early stages and the normal surrounding tissue (a tumor volume of ~1 cm3 shows an absorption increase of approximately two times due to angiogenesis). The addition of the molecular imaging agent will allow detection of tumors with smaller volumes at even earlier stages of development (<1 cm3). Decreasing the step size would probably not influence the spatial resolution significantly due to the level of diffusion in the breast tissue. Due to this strong diffusion, the photons arriving at the detection point propagate through large volumes, much wider than the size of inclusions used in the study. This is equivalent to using a beam larger than the sample in classic spectroscopy in nonscattering media: A very large percentage of the photons could arrive to the detection point without sensing the absorption effects of the inclusions by traveling around it. The result is the so-called partial volume effect: a smaller contribution of the volume of interest to the overall absorption and by consequence a smaller contrast. When the absorption of the inclusions is close to that typical for breast tissue, those values are small enough that all of the absorber’s molecules (SWNT, BHQ, etc.) are still contributing to the overall absorption. In this case, the contrast obtained is linear with their concentration, as one can observe in Fig. 2 for the first two to three concentration values, before the partial volume effect and/or the higher concentrations are shifting it toward more logarithmic dependence. For higher concentrations, the absorption is dominated by the absorbers distributed near the surface, reducing the contribution of the inclusion’s core drastically. This shelf-shielding process reduces the effective absorption of the inclusion. Due to diffusion, its higher absorption is sensed at larger distances. This process generates some increase of the reconstructed size of the inclusion, visible in Fig. 4. The result of the two effects is a “dilution” of the contrast that is no longer linear with the concentration having an asymptotic trend toward a maximum value that corresponds to the moment when all photons incident on the surface of the inclusion are absorbed.
The detectability threshold could be reached by different combinations of volume and absorption. The contribution of a specific highly light-absorbing compound could significantly reduce the minimum volume visualized by the optical imaging system, possibly improving early detection of breast tumors. More investigations are needed to determine the minimum tumor size that can be detected for the largest amounts of dye (e.g., 1 μM SWNT) that can be delivered and accumulate at that site. In addition, more information is needed on relevant clinical imaging agent concentrations, i.e., how much of each imaging agent can realistically be delivered to the tumor site. De la Zerda et al. estimated concentrations of approximately 33.5 nM at the tumor site in their photoacoustic experiments [16]. Although SWNTs used in their study were about five times shorter than ours and in vivo delivery and accumulation depend on various factors, this gives some indication that the concentration ranges we were able to detect with the clinical optical breast scanner (0.8–20 nM) may be clinically relevant.
The results of our study are encouraging for optical transmission breast imaging as a modality since these data support opportunities for application of molecular breast imaging using highly light-absorbing imaging agents. With the use of relevant target-specific imaging agents, optical imaging could be a valid candidate for the earlier detection of breast cancer, e.g., in young women with dense breasts who are at increased breast cancer risk and for whom mammographic screening has very limited sensitivity [25, 26]. Other potential applications of this technique may be the selection of appropriate treatment, investigation of drug delivery, and early evaluation of response to treatment in breast cancer patients [27]. An important advantage of optical imaging is that it does not use any radioactive components or ionizing radiation and can thus be used more frequently, without restrictions for the timeline of the protocol. Because our strategy uses changes in light transmission (as opposed to fluorescence imaging), we do not encounter problems due to excitation light leaking through filters and causing high noise levels, an important problem in fluorescence optical imaging [28]. In addition, penetration depth with this technique (up to 15 cm) is more favorable than in other optical imaging techniques, such as optical coherence tomography or Raman spectroscopy (up to 5 mm) [12, 29].
Linking nanotubes to relevant peptides has already been demonstrated, and these target-specific molecular imaging agents have been imaged successfully in vivo [16]. In addition, preliminary toxicology studies showed no apparent toxicity which is encouraging for clinical translation [30]. We expect that each of the light-absorbing agents (from small molecules to larger nanoparticles) can be functionalized with selective targeting ligands, creating a spectrum of highly specific light-absorbing molecular imaging agents. Moreover, these agents could potentially be used as “theranostics”, combining the process of diagnosis and therapy [31].
In future studies, we plan to explore the effect of the position and size of the phantoms in the scanner, other potential light-absorbing molecular imaging agents (such as IRDye 800CW, gold and silver nanoparticles, etc.), and the influence of targeting ligands linked to these imaging agents (e.g., peptides targeting αvβ3 integrin, epidermal growth factor receptor, etc.), aiming for their eventual evaluation in a clinical setting.
Conclusion
We have shown that nanomolar concentrations of SWNT and micromolar concentrations of BHQ-3 in physical phantoms can reproducibly be detected by a clinical optical breast imager based on transmission optical imaging. This shows the potential of using highly light-absorbing molecular imaging agents, with appropriate targeting ligands, as optical imaging agents for breast disease using a commercially available clinical optical breast scanner.
Supplementary Material
Acknowledgments
This work was supported, in part, by funding from ART Advanced Research Technologies Inc., National Cancer Institute (NCI) In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747 (SSG), NCI Center of Cancer Nanotechnology Excellence (CCNE) CA119367 U54, and the Canary Foundation. We also thank Adam de la Zerda for helpful discussions.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11307-010-0356-3) contains supplementary material, which is available to authorized users.
References
- 1.Gibson AP, Hebden JC, Arridge SR. Recent advances in diffuse optical imaging. Phys Med Biol. 2005;50:R1–R43. doi: 10.1088/0031-9155/50/4/r01. [DOI] [PubMed] [Google Scholar]
- 2.Ntziachristos V, Chance B. Probing physiology and molecular function using optical imaging: applications to breast cancer. Breast Cancer Res. 2001;3:41–46. doi: 10.1186/bcr269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tromberg BJ, Shah N, Lanning R, et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters NH, Borel RI, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 5.Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management—follow-up and outcome. Radiology. 2008;248:773–781. doi: 10.1148/radiol.2483071786. [DOI] [PubMed] [Google Scholar]
- 6.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 7.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neo-adjuvant chemotherapy for palpable breast cancer. AJR Am J Roent-genol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 8.Leff DR, Warren OJ, Enfield LC, et al. Diffuse optical imaging of the healthy and diseased breast: a systematic review. Breast Cancer Res Treat. 2008;108:9–22. doi: 10.1007/s10549-007-9582-z. [DOI] [PubMed] [Google Scholar]
- 9.Godavarty A, Thompson AB, Roy R, et al. Diagnostic imaging of breast cancer using fluorescence-enhanced optical tomography: phantom studies. J Biomed Opt. 2004;9:488–496. doi: 10.1117/1.1691027. [DOI] [PubMed] [Google Scholar]
- 10.Corlu A, Choe R, Durduran T, et al. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt Express. 2007;15:6696–6716. doi: 10.1364/oe.15.006696. [DOI] [PubMed] [Google Scholar]
- 11.van de Ven S, Wiethoff A, Nielsen T, et al. A novel fluorescent imaging agent for diffuse optical tomography of the breast: first clinical experience in patients. Mol Imaging Biol. 2009;12:343–348. doi: 10.1007/s11307-009-0269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zavaleta CL, Smith BR, Walton I, et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc Natl Acad Sci USA. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntziachristos V, Yodh AG, Schnall M, Chance B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci USA. 2000;97:2767–2772. doi: 10.1073/pnas.040570597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intes X, Ripoll J, Chen Y, Nioka S, Yodh AG, Chance B. In vivo continuous-wave optical breast imaging enhanced with indocyanine green. Med Phys. 2003;30:1039–1047. doi: 10.1118/1.1573791. [DOI] [PubMed] [Google Scholar]
- 15.Cuccia DJ, Bevilacqua F, Durkin AJ, et al. In vivo quantification of optical contrast agent dynamics in rat tumors by use of diffuse optical spectroscopy with magnetic resonance imaging coregistration. Appl Opt. 2003;42:2940–2950. doi: 10.1364/ao.42.002940. [DOI] [PubMed] [Google Scholar]
- 16.De la Zerda A, Zavaleta C, Keren S, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nano-technol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Intes X. Time-domain optical mammography SoftScan: initial results. Acad Radiol. 2005;12:934–947. doi: 10.1016/j.acra.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Ntziachristos V, Chance B. Accuracy limits in the determination of absolute optical properties using time-resolved NIR spectroscopy. Med Phys. 2001;28:1115–1124. doi: 10.1118/1.1373674. [DOI] [PubMed] [Google Scholar]
- 19.Vernon ML, Freachette J, Painchaud Y, Caron S, Beaudry P. Fabrication and characterization of a solid polyurethane phantom for optical imaging through scattering media. Appl Opt. 1999;38:4247–4251. doi: 10.1364/ao.38.004247. [DOI] [PubMed] [Google Scholar]
- 20.Mincu N, Brunette J, Guilman O, et al. Diffuse optical tomography and spectroscopy performance assessment: phantoms and methodology. In: Nordstrom RJ, editor. Design and performance validation of phantoms used in conjunction with optical measurements of tissue. 1. SPIE; San Jose: 2008. pp. 68700O–68700O-10. [Google Scholar]
- 21.Contini D, Martelli F, Zaccanti G. Photon migration through a turbid slab described by a model based on diffusion approximation. I. Theory. Appl Opt. 1997;36:4587–4599. doi: 10.1364/ao.36.004587. [DOI] [PubMed] [Google Scholar]
- 22.Arridge SR, Schweiger M. Image reconstruction in optical tomography. Philos Trans R Soc Lond B Biol Sci. 1997;352:717–726. doi: 10.1098/rstb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black Hole Quencher® Dyes. Biosearch Technologies Inc; Novato, CA: [Accessed 19 Feb 2010]. http://www.biosearchtech.com/support/applications/dyes-from-biosearch-technologies/black-hole-quencher%C2%AE-dyes. [Google Scholar]
- 24.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 26.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 27.Mincu N, Djeziri S, Ichalalene Z, Leblond F, Khayat M. Sensitivity and repeatability of diffuse optical tomography: towards breast cancer neoadjuvant treatment monitoring. In: Chance B, Alfano RR, Tromberg BJ, Tamura M, Sevick-Muraca EM, editors. Optical tomography and spectroscopy of tissue VII. 1. SPIE; San Jose: 2007. pp. 64341D–64349D. [Google Scholar]
- 28.Godavarty A, Eppstein MJ, Zhang C, Sevick-Muraca EM. Detection of single and multiple targets in tissue phantoms with fluorescence-enhanced optical imaging: feasibility study. Radiology. 2005;235:148–154. doi: 10.1148/radiol.2343031725. [DOI] [PubMed] [Google Scholar]
- 29.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 30.Schipper ML, Nakayama-Ratchford N, Davis CR, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 31.Debbage P, Jaschke W. Molecular imaging with nanoparticles: giant roles for dwarf actors. Histochem Cell Biol. 2008;130:845–875. doi: 10.1007/s00418-008-0511-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.