Abstract
Throughout most of history, serious burns occupying a large percentage of body surface area were an almost certain death sentence because of subsequent infection. A number of factors such as disruption of the skin barrier, ready availability of bacterial nutrients in the burn milieu, destruction of the vascular supply to the burned skin, and systemic disturbances lead to immunosuppression combined together to make burns particularly susceptible to infection. In the 20th century the introduction of antibiotic and antifungal drugs, the use of topical antimicrobials that could be applied to burns, and widespread adoption of early excision and grafting all helped to dramatically increase survival. However the relentless increase in microbial resistance to antibiotics and other antimicrobials has led to a renewed search for alternative approaches to prevent and combat burn infections. This review will cover patented strategies that have been issued or filed with regard to new topical agents, preparations, and methods of combating burn infections. Animal models that are used in preclinical studies are discussed. Various silver preparations (nanocrystalline and slow release) are the mainstay of many approaches but antimicrobial peptides, topical photodynamic therapy, chitosan preparations, new iodine delivery formulations, phage therapy and natural products such as honey and essential oils have all been tested. This active area of research will continue to provide new topical antimicrobials for burns that will battle against growing multi-drug resistance.
Keywords: Burn infection, multi-drug resistance, animal models, bioluminescence imaging, nanocrystalline silver, antimicrobial peptide, skin substitute, chitosan, photodynamic therapy
1. INTRODUCTION
1.1. History
Despite various current topical treatment regimens designed to eradicate the bacterial load within the burn wound, sepsis remains the leading cause of death in burn units around the world [1]. Advances in resuscitation, surgical management, control of infection, control of the hyper-metabolic response and rehabilitation have resulted in dramatic improvements in burn mortality and morbidity in the last 60 years [2]. Infection is a major complication of burns. Infection is linked to impaired resistance from disruption of the skin’s mechanical integrity and generalized immune suppression. The skin barrier is replaced by eschar. This moist, protein rich avascular environment encourages microbial growth. Migration of immune cells is hampered, and there is a release of intermediaries that impede the immune response. Eschar also restricts distribution of systemically administered antibiotics because of its avascularity.
1.2. Physiology of Burn
Burns are classified by their depth and severity as 1st, 2nd, 3rd and 4th degree Fig (1). First-degree burns are usually limited to redness (erythema), a white plaque and minor pain at the site of injury. These burns involve only the epidermis. Second-degree burns manifest as erythema with superficial blistering of the skin, involving the superficial (papillary) dermis and may also involve the deep (reticular) dermis layer. Third-degree burns occur when the epidermis is lost with damage to the subcutaneous tissue. Burn victims will exhibit charring and extreme damage of the epidermis, and sometimes hard eschar will be present. Fourth-degree burns damage muscle, tendon, and ligament tissue, thus result in charring and catastrophic damage of the subcutaneous tissue.
Fig. 1. Degrees of burn.
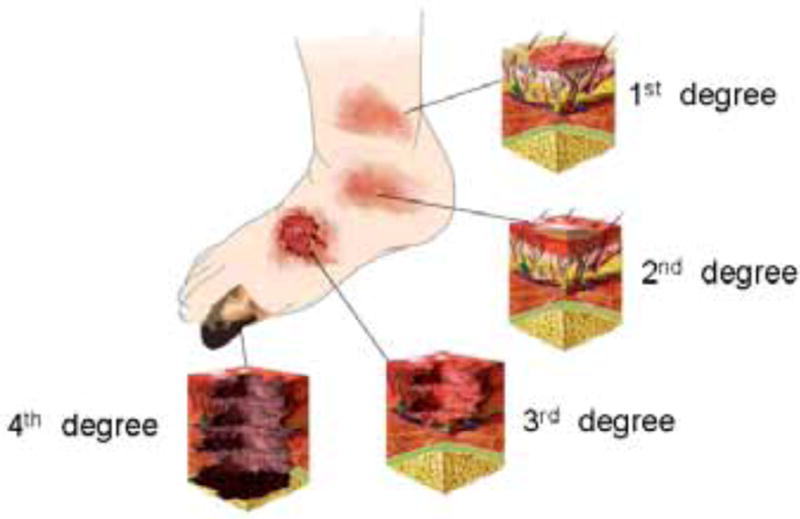
Relative depths of 1st, 2nd, 3rd, and 4th degree burns illustrated on a foot.
Burns can also be assessed in terms of total body surface area (%TBSA), which is the percentage affected by partial thickness or full thickness burns. The rule of nines is used as a quick and useful way to estimate the affected TBSA. More accurate estimation can be made using Lund & Browder charts which take into account the different proportions of body parts in adults and children [3]Fig. (2). Burns greater than 10% in children or 15% in adults are potentially life-threatening injuries (because of the risk of hypovolemic shock) and should have formal fluid resuscitation and monitoring in a burns unit.
Fig. 2. Lund & Browder charts.
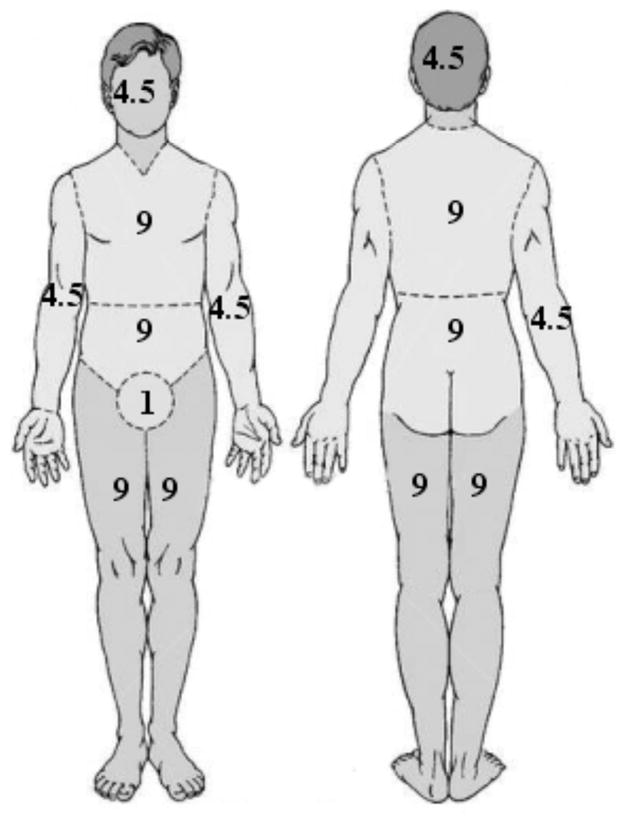
These are used to calculate the % of body surface area affected by burns and determine the prognosis and susceptibility to infection.
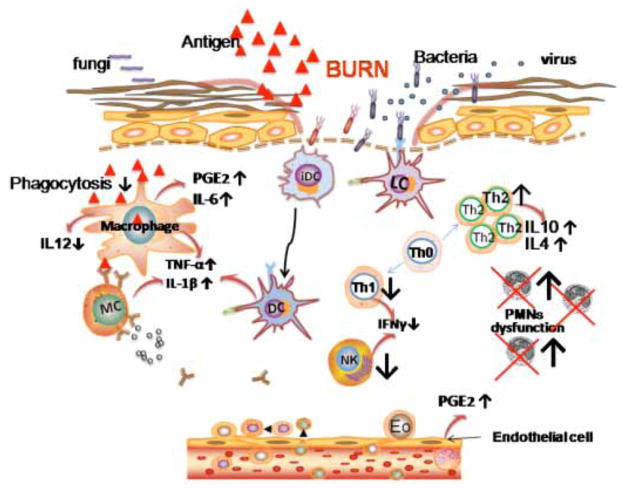
Severely burned skin ceases to perform its natural protective and barrier role and allows a dramatic increase in water loss and can become a portal for bacterial invasion. The activation of a pro-inflammatory cascade after a burn appears to be important in the development of subsequent immune dysfunction, bacterial translocation from the gut, susceptibility to sepsis and multiple organ failure. A local response to burning involves not only direct tissue coagulation but also burn tissue conversion, a process where the damaged cells, rather than recover, progress to cell death, extending the depth and severity of the original injury [4]. The systemic response to burning is driven by the loss of the skin barrier and release of vasoactive mediators from the wound and from subsequent infection. When the burn size exceeds about 25% of the body surface, interstitial edema develops in distant organs and soft tissues, mainly secondary to a combination of wound-released mediators and hypoproteinemia [5]. Thermal injury causes a massive release of proinflammatory cytokines, chemical mediators including histamine, complement, arachidonic acid, products of the coagulation cascade, and oxygen free radicals, which produce an increase in vascular permeability leading to hypovolemia and acute renal failure [5]. This may be complicated by systemic inflammatory response syndrome and marked immune suppression. Subsequent wound infection and bacterial translocation from the gastrointestinal tract promotes sepsis [6]. This adds to the final common pathway to multi-organ failure and death Fig. (3).
Fig. 3. Inflammatory signaling after burn that causes immunosuppression.
Interleukin-1 (IL-1) and tumor necrosis factor alpha (TNFα) are produced by a wide variety of cells. The production of prostaglandin E2 (PGE2) and IL6 are upregulated by endothelial cells and macrophages, while the latter secrete decreased amounts of IL-12. T helper cells begin to preferentially differentiate into Th-2 cells, which produce the anti-inflammatory cytokines IL-4 and IL-10. Neutrophil dysfunction occurs despite higher numbers after significant thermal injuries, while macrophage phagocytosis is lower.
1.3. Microbiology of Burn Infection
Table 1 [7–15] gives a listing of the microbial species responsible for invasive burn wound infection [16]. The incidence of antibiotic resistance amongst these species is also given.
Table 1.
Microbiology of Pathogens Responsible for Burn Infections and the Occurrence of Drug Resistance
| Group | Species | Drug Resistance |
|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | |
| Methicillin-resistant S. aureus | By definition | |
| Coagulase-negative staphylococci | MRSE increasing [7] | |
| Enterococcus sp. | ||
| Vancomycin-resistant enterococci | By definition | |
| Gram-negative bacteria | Pseudomonas aeruginosa | High innate resistance |
| Escherichia coli | ESBL increasing | |
| Klebsiella pneumoniae | ESBL increasing | |
| Serratia marcescens | increasing | |
| Enterobacter sp. | ESBL increasing [8] | |
| Proteus sp. | ESBL increasing [9] | |
| Acinetobacter sp. | Very common | |
| Bacteroides sp. | uncommon | |
| Fungi | Candida sp. | Azole resistance and efflux pumps increasing [10] |
| Aspergillus sp. | Increasing [11] | |
| Fusarium sp. | Common in agriculture [12] | |
| Alternaria sp. | Common in agriculture ]13] | |
| Rhizopus sp. | Azole resistance [14] | |
| Mucor sp. | Azole resistance [15] | |
| Viruses | Herpes simplex virus | |
| Cytomegalovirus | ||
| Varicella-zoster virus |
A consensus conference of the American Burn Association laid down guidelines on the distinction between colonization (virtually all burns) vs. infection (likely to produce disease) in burn wounds [17]. The most serious microbial infections are classified as “invasive”. This property is a function of the seriousness of the burn and the virulence of the microbial species or strain. Virulence is a combination of a variety of factors including the ability to colonize the burn (including adhesion to host cells and tissues), the expression of proteolytic enzymes, the ability to obtain nutrition from the host, and the ability to evade the host’s immune response.
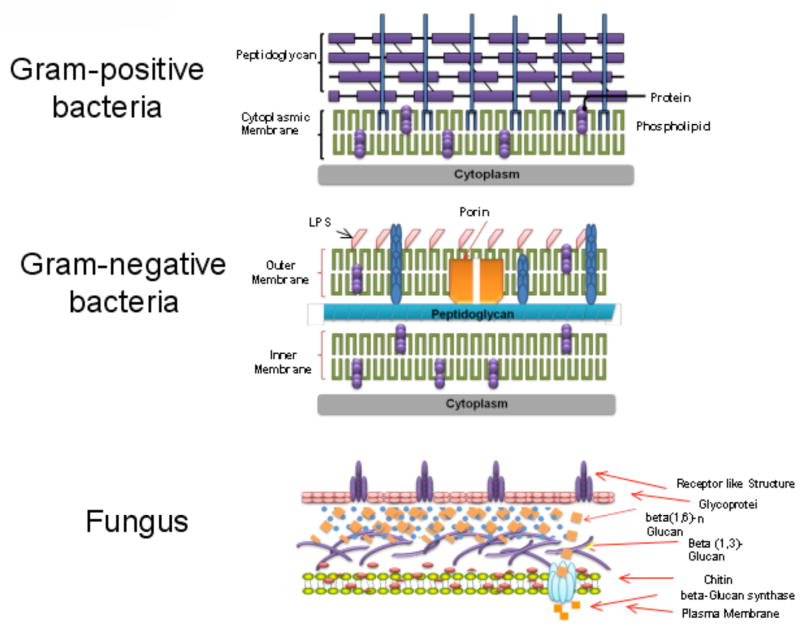
There is a fairly predictable time course of the microbial species that colonize or infect burns [18]. Gram-positive bacteria that survive the thermal insult, such as staphylococci located deep within sweat glands and hair follicles, heavily colonize the wound surface within the first 48 hours. Eventually (after an average of 5–7 days), burns are colonized with other microbes derived from the host’s normal gastrointestinal and upper respiratory flora or from the hospital environment. These pathogens include Gram-negative bacteria, while yeasts and fungi tend to be the latest colonizers. The cell wall structures of these three classes of pathogenic micro-organisms are shown in Fig. (4). The architectural differences largely govern the relative effectiveness of many antimicrobial agents that induce disruption of the integrity of the cell wall and subsequently the cell membrane.
Fig. 4. Cell wall structure.
Gram-positive bacteria, Gram-negative bacteria and fungi have different cell wall structures that govern their susceptibility to different antimicrobial agents.
1.4. Physiology of Burn Infection
Many physiological properties of the burn environment predispose to infection and inhibit traditional treatment by systemic antibiotics. The destruction of the microvasculature by the thermal damage means that the burn is considered to be non-vascularized tissue. Proteolysis and lipolysis is up-regulated in burns [19]. A marked increase in matrix metalloproteinases (and reduction in inhibitors) leads to breakdown of proteins thus providing more nutrients for microbes and making microbial penetration into the tissue easier.
Immunosuppression plays a big role in predisposing to infection in burns. Both innate and adaptive immune systems are involved in immunosuppression. Innate immunity is triggered immediately after microbial invasion in response to highly conserved structures present in large groups of microorganisms, while adaptive immunity is a function of T and B cells, a small number of which are activated selectively by particular antigens to expand clonally, a process that takes at least three to five days to become evident. Alterations in the innate immune system following thermal injury include neutrophils, macrophages, monocytes, basophils, NK-cells, and complement. In contrast, Thermal injury is associated with many mediators originating from the cytokine network, the endocrine system, and the arachidonic acid cascade which mediates immunosuppression. Initially, the immunologic response to severe burn injury is pro-inflammatory but later becomes predominately anti-inflammatory in an effort to maintain homeostasis and restore normal physiology. The anti-inflammatory response and the subsequent immunosuppression following burn injury are characterized by a set of opposing cell types and cytokines. Many of the changes in cytokine levels represent alterations of the adaptive immune system following burn injury, more specifically within the T-lymphocyte population.
Bacterial translocation is another physiological response caused by a combination of burns with a microbial insult and predisposes to development of sepsis [18]. The term ‘bacterial translocation’ is used to describe the passage of viable resident bacteria from the gastrointestinal tract to normally sterile tissues such as the mesenteric lymph nodes and other internal organs. Many systemic stresses can destroy the mucosal barrier of the intestines such as elective abdominal surgery, organ donors and those with intestinal obstruction, colorectal cancer, ischemia-reperfusion injury shock and pancreatitis. It has been shown [20] in a rat model that a two-hit injury consisting of burn and Enterococcus faecalis infection increased intestinal permeability and Gram-negative sepsis.
1.5. Antibiotic Resistance
The world-wide problem of growing antibiotic resistance shows no sign of ameliorating and the present time has been termed the “end of the antibiotic era” [21]. Commentators have asserted that if nothing is done, hitherto trivial infections that were easily treated with a simple course of antibiotics, could in future become essentially untreatable as in the days before antibiotics were discovered [22]. Resistance has been attributed to the widespread availability of antibiotics over the counter, wrong prescription practices (for instance giving antibiotics for respiratory viral infections), poor patient compliance leading to stopping treatment too early and overuse of antibiotics in livestock feedstuffs [23]. As shown in Table 1 resistance is also becoming common in burn pathogens.
1.6. Topical Antimicrobials
Because of the factors discussed above, topical antimicrobial preparations have been particularly applied to prevent and treat burn infections compared to other traumatic, surgical and medical indications which could be susceptible to infection. Non-healing chronic wounds such as diabetic, vascular and pressure ulcers are another similar indication [24]. Many of the agents are designed to be used prophylactically to prevent infection developing, while others are designed to kill the actually microbial cells that are proliferating within the burn when an infection has developed. Table 2 [25–80] gives a listing of topical antimicrobial therapy agents used for burns that forms the bulk of this review. Since many of these topical agents are applied onto the surface of the burn, if they are designed to be actively microbicidal, consideration must be given to the degree which the agents will penetrate into the burned infected tissue to reach the microbial cells that have invaded. Stephanides et al. [81] measured the extent to which several agents (gentamicin sulfate, mafenide acetate, nitrofurazone, povidone-iodine, silver nitrate, and silver sulfadiazine) penetrated through burn eschar. Studies [82, 83] have been carried out investigating the ability of “penetration enhancing agents” such as (glycerin, saline, sodium dodecyl sulfate, ethanol, hexane: ethanol, ethyl acetate:ethanol dimethyl sulfoxide, glycine and terpenes) to increase the penetration of diverse antimicrobial agents into the eschar of 3rd degree burns. Another consideration that is important in the field of topical antimicrobials is the selectivity towards microbial cells versus cytotoxicity to host cells and tissue. Many antimicrobial agents such as topical antibiotics [84], antiseptics [85], silver preparations [86, 87], antimicrobial peptides [88], antimicrobial photodynamic therapy [4, 89] have been investigated for possibility toxicity towards skin and other human cells.
Table 2.
Summary of Topical Antimicrobial Therapy Agents used for Burns Covered in this Review
| Agent Class | Specific Agent | Application | Ref |
|---|---|---|---|
| Skin Substitutes | Duoderm | Clinical 2nd degree burns | [25] |
| Trans-Cyte | Clinical 2nd degree burns | [26] | |
| Omniderm | Clinical 2nd degree burns | [27] | |
| Suprathel | Clinical 2nd degree burns | [28] | |
| Epigard | Clinical 2nd degree burns | [29] | |
| SYSpur-derm | Clinical 2nd degree burns | [30] | |
| Biobrane | Clinical 2nd degree burns | [31] | |
| Xenoderm | Clinical 2nd degree burns | [32] | |
| Topical antibiotics | Mafenide acetate | Clinical 2nd/3rd degree burns | [33] |
| Bacitracin | Clinical 2nd/3rd degree burns | [34] | |
| Mupirocin | Clinical 2nd/3rd degree burns | [35] | |
| Neosporin | Clinical 2nd/3rd degree burns | [36] | |
| Polymyxin B | Clinical 2nd/3rd degree burns | [37] | |
| Nitrofurazone | Clinical 2nd/3rd degree burns | [38] | |
| Nystatin | Clinical 2nd/3rd degree burns, fungal infections | [35] | |
| Silver | Silver nitrate | Clinical 2nd/3rd degree burns | [39] |
| Silver sulfadiazine | Clinical 2nd/3rd degree burns | [40] | |
| Silver foams (Contreet, Allevyn) | Clinical 2nd/3rd degree burns | [41] | |
| Flammacerium | Clinical 2nd/3rd degree burns | [42] | |
| Acticoat 7 | Clinical 2nd/3rd degree burns | [43] | |
| Aquacel-Ag | Clinical 2nd/3rd degree burns | [44] | |
| Silvercel | Clinical 2nd/3rd degree burns | [45] | |
| Silver amniotic membrane ( | Clinical 2nd/3rd degree burns | [46] | |
| Iodine | Povidone-iodine | Clinical 2nd/3rd degree burns | [47] |
| Cadexomer iodine (Iodosorb) | Clinical chronic wounds | [48] | |
| Liposomal iodine, Repithel | Clinical 2nddegree burns | [49] | |
| Iocide | Oral infections | [50] | |
| Photodynamic therapy | TBO | Mouse wound infections | [51] |
| PEI-ce6 | Mouse burn infections (A. baumannii) | [52] | |
| XF73 | In vitro, ex vivo skin infection | [53] | |
| BB6 | In vitro, mouse wound infection | [54] | |
| Sylsens B | Mouse burn infection S. aureus | [55] | |
| DP/hemin | Guinea pig burn infections S. aureus | [56] | |
| Cat PC | In vitro, veterinary infections | [57] | |
| EtNBSe | In vitro | [58] | |
| Chitosan | hydrogel | Clinical 2nd degree burns | [59] |
| film | 2nd degree burns in rabbits | [60] | |
| bandage | Mouse burn infections (Psuedomonas, Proteus) | [61] | |
| Antimicrobial peptide | Defensins | In vitro | [62] |
| Demegel | Pseudomonas infected rat burns | [63] | |
| Histone H1.2 | Pseudomonas infected rat burns | [64] | |
| Cecropin B | Pseudomonas infected mouse wounds | [65] | |
| rBPI | Clinical trial 2nd degree burns | [66] | |
| Ceragenins | In vitro | [67] | |
| Miscellaneous | Chlorhexidine (LM Miller) | Infected rat burns, clinical trial | [68, 69] |
| Superoxidized water | In vitro | [70] | |
| BCTP nanoemulsion | In vitro | [71] | |
| FPQC | Mice burns, Pseudomonas | [72] | |
| Acidified nitrite | In vitro | [73] | |
| Phage therapy | Mice burns, Pseudomonas | [74] | |
| p38MAPK inhibitor | Rat burns, Pseudomonas | [75] | |
| Probiotics, Lactobacillus | Clinical trial, 2nd, 3rd degree | [76] | |
| Honey | Clinical trial, 2nd degree | [77] | |
| Essential oils | In vitro | [78] | |
| MEBO | Clinical trial, 2nd degree | [79] | |
| Papaya | Clinical trial, 2nd, 3rd degree | [80] |
2. ANIMAL MODELS OF BURN INFECTIONS
The use of animals as models for burn infections has been a fundamental part of burn infection research for many decades. By establishing a burn infection similar to that observed in humans, the reasons for the development of infection can be researched, and ultimately, the goal is to seek approaches by which the infection can be thwarted. Animal species that have been used for burn models include mouse, rat, rabbit, as well as pig with the means of inflicting the burn injuries covering diverse techniques such as boiling water, ethanol bath, heated brass blocks, etc.
The severity of burn infections in animals and whether the animals develop sepsis and die is determined by the following factors (among others): the number of bacteria applied to the wound, the virulence of the particular bacterial strain, the size of the wound expressed as % of total body-surface area (TBSA), whether the bacteria are applied to the surface or injected into or beneath the burn, and the length of time the heated object or liquid is in contact with the skin (which determines whether a full thickness or partial thickness burn is delivered).
2.1. Mason-Walker Burn Model
About 40 years ago in 1968, Mason and Walker developed a burn model of rat using boiling water to inflict the thermal injury [90]. It was the first animal model burn for research and was used as a standard scald burn model by the U.S. Army Surgical Research Center in San Antonio TX for various studies [90]. The burning device was made of a thin metal half-cylinder with an aperture of calculated size cut from the central portion of the half-cylinder. The dimensions of the aperture were selected to provide the size of burn desired in animal of known weight (the upper limit of burn size permitting by this approach was approximately 30% of TBSA). The anesthetized animal was shaved on the back and then placed supine in the burning device. The exposed area was immersed in boiling water. It was reported that 10 seconds exposure produced a full-thickness burn, and 3 seconds a partial-thickness burn. The burns per se did not interfere greatly with mobility and the animals ate and drank easily. This model has been used widely in the studies on burn infections, including bacterial translocation in burn infections [91], development of antimicrobial peptides for eliminating multi-drug resistant bacteria in burns [92], development of wound dressing for preventing burn infections [93], etc.
2.2. Stieritz-Holder Burn Model
Stieritz and Holder reported an ethanol bath burn model in mouse [94]. In this model, female CF1 mice weighing 22–24 g were used. Mice were anesthetized and shaved on the back. An asbestos board with a window accounting for approximately 30% of TBSA was pressed firmly against the back of mice. Ethanol was evenly spread over the area outline by the window, ignited, and allowed to burn for 10 seconds. The infections were initiated by an immediate subcutaneous injection of 100 CFU of Pseudomonas aeruginosa in the burned area. The infections turned out to be rapidly fatal and the animals appeared moribund 20 hours after infection.
2.3. Other Burn Models
Stevens et al. [95] developed a mouse model of full-thickness burn by applying two pre-heated brass blocks (92–95 °C) to the opposing sides of an elevated skin folder on the backs of shaved mice for 5 seconds. This procedure is illustrated in Fig. (5). Six-week old male CD1 mice weighing 25–30 g were used. The combined brass block area was 1.8 cm × 1.8 cm, corresponding to a 5% of TBSA. After the infliction of burns, the eschars were immediately injected intradermally with 10–106 CFU of P. aeruginosa. Survival at 10 days was 100% with burn injury alone and 60% with infected burns. P. aeruginosa (consistently 108 CFU/g-tissue) were recovered from the unburned muscle by 24 hours for all the different inocula of bacteria.
Fig. 5. Mouse model of third degree burn infection.
Two pre-heated brass blocks are pressed to the opposing sides of an elevated skin fold on the backs of shaved mice, followed by topical application of a suspension of bacteria in saline.
A model of a partial thickness burn was developed by Bahar et al. [96] using male Wistar rats weighing 250–300 g. After the animal was anesthetized, a 2.5 cm × 2.5 cm, 5 mm-thick piece of absorbent lint cloth that had been immersed in boiling water (100 °C) was placed over the dorsum. The hot cloth was applied for different time intervals. This method produced reliable superficial and deep dermal burns, as confirmed by the histological features.
Suzuki et al. [97] described a burn model of rat based on skin contact with a glass chamber through which water circulates at a predetermined temperature. This allows application at a constant pressure of 10 g/cm2. The great advantage of this model is the possibility of varying temperature and exposure time, as required by the researcher, and also of applying higher or lower contact pressure.
2.4. Bioluminescence Imaging of Infection
Contag et al. [98] developed a method to monitor bacterial numbers and viability in real-time in infected wounds of living animals using genetically engineered bacteria that emit luminescence together with a sensitive low-light imaging camera. The rate of luciferase enzyme turnover in the presence of substrate allows for real-time measurements, and the enzyme is active at the body temperature of mammals. This method is a significant improvement on the traditional use of survival or body fluid sampling and subsequent plating and colony counting. The first method suffers from the disadvantage of being wasteful of animals, and does not really address the question of where the bacteria are in the animal, while the second method suffers from the disadvantage that tissue sampling introduces another source of experimental error, is laborious and does not give real-time results.
The imagining system Fig. (6a) used in our laboratory [99] consists of an ICCD photon-counting camera (Hamamatsu Photonics, Bridgewater, NJ) mounted in a light-tight specimen chamber, fitted with a light-emitting diode, a setup that allowed for a background gray-scale image of the entire mouse to be captured. By accumulating many images containing binary photon information (an integration time of 2 minutes was used), a pseudo-color luminescence image was generated. Superimposition of this image onto the gray-scale background image yielded information on the location and intensity in terms of photon number Fig. (6b). The camera was also connected to a computer system through an image processor (Argus-50, Hamamatsu Photonics). Argus-50 control program (Hamamatsu Photonics) was used to acquire images and to process the image data collected. This entire wound photon count was quantified as relative luminescence units (RLU) and was displayed in a false color scale ranging from pink (most intense) to blue (least intense). Another popular bioluminescence camera is the In Vivo Imaging System (IVIS) made by Caliper Life Sciences (Hopkinton, MA) [100].
Fig. 6. Use of bioluminescent imaging of genetically engineered bacteria and fungi in burn infections.
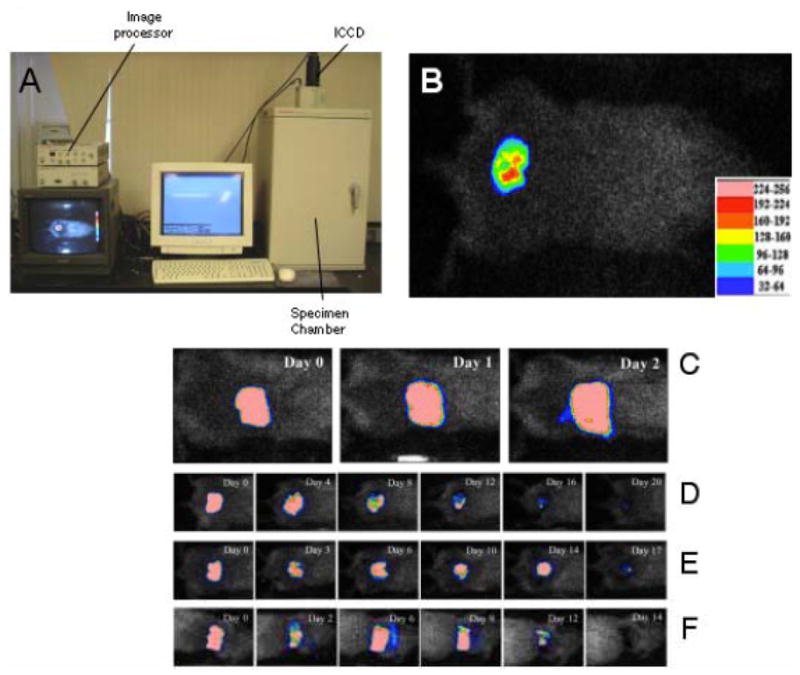
(A) Picture of the low-light camera suitable for in vivo bioluminescence imaging of small animals. (B) Example of 3rd degree burn on mouse back infected with bioluminescent P. aeruginosa showing color look-up-table for photon intensity. Successive bioluminescence images of representative mouse burns infected with bioluminescent strains of (C) P. aeruginosa, (D) Acinetobacter baumannii, (E) Staphylococcus aureus, and (F) Candida albicans, respectively.
Figure (6C) displays the successive bioluminescence images of representative mouse burns infected with bioluminescent strains of P. aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, and Candida albicans, respectively. For P. aeruginosa infection, which was acute and lethal, the mouse died on day 3 post-infection. For A. baumannii, S. aureus, and C. albicans infections, which were chronic, the infections sustained in the mice for 20, 17, and 14 days, respectively.
3. SKIN SUBSTITUTES
In the 1980’s the critical need to address the problem of early coverage of extensive burn injuries in F73patients with inadequate sources of autologous skin for grafting prompted the emergence of skin substitutes primarily as an experimental therapy [101]. The preponderance of studies and trials demonstrates that the use of skin substitutes facilitates better healing in burns, and minimizes the risk of infections. It should be emphasized that these products are not active antimicrobial agents but rather serve as biocompatible barriers to prevent infection [102]. Nevertheless they are frequently compared with the standard antimicrobial agent AgSD. Their use results in a measured improvement in elasticity and flexibility of the healed burn, lessened scar contracture a reduction in the number of scar contracture procedures and plastic surgical reconstructions required long term
Most pediatric burns are small-sized burns of intermediate depth and warrant only local treatment. The use of skin substitutes eliminates the need of changing dressings and repeated visits to the hospital clinics for that purpose. This helps in avoiding the pain, emotional trauma and promotes a cost effective method of management whereby any further treatment is unnecessary and the child returns to its daily activities unrestricted.
Synthetic skin substitutes include Duoderm (polyurethane and hydrocolloids) Opsite (polyurethane film), Omiderm (acrylamide film and hydroxyethylmethyl-metha-crylate with polyurethane). Biosynthetic skin substitutes (biological materials like collagen in combination with synthetic materials) include Biobrane (silicone film, nylon, collagen -derived peptides) and Transcyte (polymer with fibroblasts). Biological skin substitutes include homologous skin, porcine skin, collagen derivatives, human amniotic membrane and cultured allografts.
3.1. Duoderm
Duoderm is a hydrocolloid preparation that provides a moist environment that enhances the wound healing process and prevents infection at the site patented in 1985 [25]. Martin et al. [103] studied the effect of Duoderm on partial-thickness scalds in the pediatric age group Two hundred forty-eight pediatric burns were analyzed and the Duoderm-managed patients demonstrated a significantly lower graft rate.
3.2. Omniderm
Omniderm (ITG Laboratories, Redwood City, CA) consists of a water-vapor permeable polyurethane film [27]. Eldad et al. [104] examined the use of Omniderm as an interface in burn wounds requiring skin grafts, particularly on problematical areas and wounds that were heavily contaminated with bacteria. They concluded that about 75 per cent improvement was achieved in comparison with other dressings under similar conditions.
3.3. Suprathel
Suprathel® (PolyMedics Innovations Gmbh, Stuttgart, Germany) is an absorbable, synthetic wound dressing with properties of natural epithelium [28]. It consists of a copolymer of polylactide, trimethylene carbonate and epsilon-caprolactone. It becomes transparent upon application which allows close wound monitoring. Schwarze and others [105] evaluated the impact on wound healing of Suprathel in partial-thickness burn injuries. Thirty patients suffering from second-degree burn injuries were included in the study, with a mean of age 40.4 years. Burn injuries were randomly selected, partly treated with Omiderm and partly treated with Suprathel. They concluded that Suprathel showed a good impact on wound healing and pain reduction in partial-thickness burn injuries and also provided a significant increase of patient comfort.
3.4. Epigard and SYSpur-Derm
Epigard is a two-layered, non-medicated wound dressing which approximates the function of human skin [29]. The synthetic skin substitute SYSpur-derm is a bilaminar poly-urethane foam substance [30]. Mahnke and others [106] evaluated the efficacy of SYSpur-derm and Epigard by examining 85 skin biopsies taken from 23 pigs with experimental 3rd-degree burns. It was concluded that both the materials demonstrated an exceptional wound healing, showed good adhesion to the wound surface and had no toxic or antigenic effect.
3.5. Biobrane
Biobrane (Smith and Nephew and UDL Laboratories Inc.) was patented in 1989 [31] and consists of a silicone film, bonded to a cross-linked nylon fabric. Both layers are covered with a layer of hydrophilic collagen peptides which makes the dressing hydrophilic and tissue-compatible. Blood/sera clot in the nylon matrix, thereby enabling firm adherence of the dressing to the wound until epithelialization occurs. Barret et al. [107] hypothesized that the treatment of second-degree burns with Biobrane is superior to topical treatment. They concluded that the treatment of partial-thickness burns with Biobrane is superior to topical therapy with 1% silver sulfadiazine. It also markedly reduced pain, pain medication requirements, wound healing time, and length of hospital stay. Hassan and Shaw reported [108] punctate scarring from use of porous Biobrane which makes the use of this particular product questionable.
3.6. Transcyte and Dermagraft
TransCyte (Smith and Nephew and Advanced Tissue Sciences) is a human fibroblast-derived temporary skin substitute composed of a polymer membrane and neonatal human fibroblast cells cultured under aseptic conditions in vitro on a nylon mesh. The nylon mesh is coated with porcine dermal collagen and bonded to a polymer membrane (silicone), which provides a transparent synthetic epidermis upon application. As fibroblasts proliferate within the nylon mesh, they secrete human dermal collagen, matrix proteins and growth factors. Following freezing, no cellular metabolic activity remains; however, the tissue matrix and bound growth factors are left intact. It provides a temporary protective barrier and is transparent which allows direct visual monitoring of the wound bed. Amani et al. [26] undertook a study to determine if Transcyte reduces the length of hospital stay for partial thickness burns of any size or etiology. Significant difference was found between patients who were treated with dermabrasion and Transcyte compared with a population receiving standard therapy. The authors suggest that this modality is more efficacious and markedly reduces length of stay compared to traditional management.
Dermagraft (Advanced BioHealing) is a cryopreserved human fibroblast-derived dermal substitute; it is composed of fibroblasts, extracellular matrix, and a bioabsorbable scaffold patented in 1990 [109]. Dermagraft is manufactured from human fibroblast cells derived from newborn foreskin tissue seeded onto a bioabsorbable polyglactin mesh scaffold. The fibroblasts proliferate to fill the interstices of this scaffold and secrete human dermal collagen, matrix proteins, growth factors, and cytokines to create a three-dimensional human dermal substitute containing metabolically active, living cells.
3.7. Xenoderm and Alloderm
Xenoderm and Alloderm (LifeCell, Inc) are acellular matrices derived from porcine (Xenoderm) or human (Alloderm) skin. The tissue goes through a cell removal process while retaining the important biochemical and structural components [32]. Hosseini et al. [110] studied the outcomes of Xenoderm dressing compared with 1% silver sulfadiazine (SSD) in partial-thickness burns taking into account the wound infection, length of hospital stay, number of dressings and doses of analgesics used. 78 patients were considered for the study of which 37 were treated with daily washing along with application of topical SSD dressing and 39 with Xenoderm. The study concluded that Xenoderm was significantly more effective.
3.8. Other Skin Substitutes
Apligraf (Organogenesis Inc) is supplied as a living, bi-layered skin substitute: the epidermal layer is formed by human keratinocytes and has a well-differentiated stratum corneum; the dermal layer is composed of human fibroblasts in a bovine Type I collagen lattice [111]. Epicel (Genzyme Biosurgery) is a sheet of skin cells ranging from 2 to 8 cell layers thick. The grafts are grown or cultured from a postage stamp sized sample of patient’s own healthy skin, which is sent to GenzymeBiosurgery for processing. The cells within the epidermis of the skin sample are separated and grown by tissue culture. During this process, irradiated mouse 3T3 cells, are used to promote cell growth and to ensure that there will be a sufficient number of grafts available as soon as possible for treatment [112]. OrCel (Ortec International Inc) is a bilayered cellular matrix in which normal human allogeneic skin cells (epidermal keratinocytes and dermal fibroblasts) are cultured in two separate layers into a Type I bovine collagen sponge. Donor dermal fibroblasts are cultured on and within the porous sponge side of the collagen matrix while keratinocytes, from the same donor, are cultured on the coated, non-porous side of the collagen matrix [113].
4. TOPICAL ANTIBIOTICS
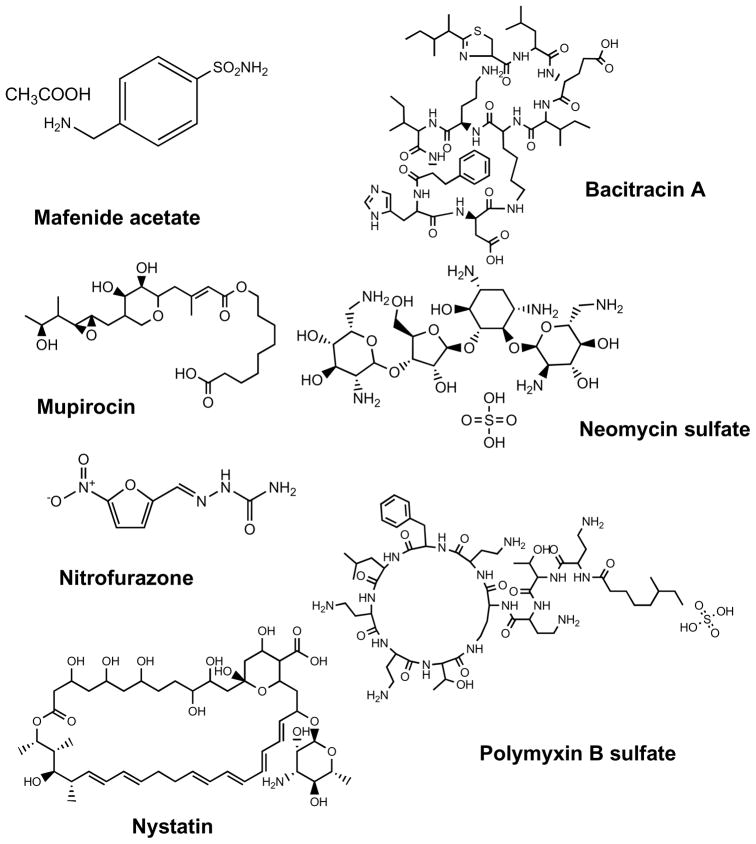
Widespread application of effective topical antimicrobial agents reduced the microbial load on the burn wound surface and reduced the risk of infection [33, 114]. Selection of topical antimicrobial therapy should be based on the agent’s ability to inhibit the microorganisms recovered from burn wound surveillance cultures. The chemical structures of the antibiotics that are particularly used for topical application in burns are shown in Fig. (7). Current patents describe the potential applications of these agents for wound dressings, bio-membrane, and wound coating material, postoperative adherent and cosmetic material applications. Burn units may rotate the use of various topical antimicrobial preparations to decrease the development of antibiotic resistance [115, 116].
Fig. 7. Chemical structures of antibiotics used in topical antimicrobial therapy of burns.
Mafenide acetate, bacitracin, mupirocin, neomycin, polymyxin B, nitrofurazone, nystatin.
4.1. Mafenide Acetate
Mafenide acetate (Sulfamylon) cream has a broad spectrum of activity against gram-negative bacteria, particularly Pseudomonas aeruginosa, but has little activity against gram-positive aerobic bacteria such as Staphylococcus aureus [33]. Mafenide acetate 0.5% cream (mafenide) is a methylated topical sulfonamide compound. Since topical mafenide acetate cream can be used without dressings, so it allows open burn wound therapy and regular examination of the burn wound surface [117]. Mafenide was introduced prior to silver sulfadiazine and was widely used for the treatment of burns. A 5% solution must be applied to saturate gauze dressings. The solution appears to be as effective as the cream preparation when used in this way [118]. This agent should be used in combination with nystatin to prevent this complication due to its limited antifungal activity [119]. In burn patients with concomitant respiratory acidosis, the use of mafenide acetate over a large surface area of this compound can be fatal [120], because it’s converted to p-sulfamylvanzoic acid, causing metabolic acidosis in the burn patient [33].
4.2. Bacitracin
Bacitracin is a mixture of related cyclic polypeptides produced by organisms of the licheniformis group of Bacillus subtilis var Tracy, isolation of which was first reported in 1945 [34]. It is a good alternative to silver sulfadiazine (Silvadene) for burn patients with a sulfa allergy. Bacitracin zinc is effective to promote regenerative healing when topically applied to wounds in concentrations from about 5% to 8%. The bacitracin zinc can be applied in a hydrophilic or hydrophobic carrier, and is advantageously impregnated in an absorbent pad [121].
4.3. Mupirocin
Mupirocin is a fermentation product of Pseudomonas fluorescens [35], which was a potent inhibitor against gram-positive skin flora such as coagulase-negative staphylococci and Staphylococcus aureus [122, 123]. Pharmaceutical compositions comprising mupirocin and chlorhexidine are of use in treating topical bacterial infections, in particular infected burn injuries [124]. Mupirocin has also increasingly been used as a burn wound topical agent in North America, where MRSA has become a problem [125, 126]. Various topical antibiotic preparations, including 1% silver sulfadiazine, 2% mupirocin, and 2% fusidic acid, were recently compared for their antibacterial effect in an MRSA-infected rat burn model [125]. Therefore, hospitals where MRSA is a problem may rotate the use of topical mupirocin in combination with these other agents to decrease the development of resistance.
4.4. Neosporin
Neosporin is a broad spectrum antibiotic ointment containing three different antibiotics: bacitracin, neomycin, and polymyxin B, in a petroleum jelly base. A study in total of 1053 patients with superficial burn injury, in which qualitative analysis showed Staphylococcus aureus and Pseudomonas spp. to be the most common infecting organisms. Quantitatively, fewer patients showed infection on the 7th and 18th day post-treatment in the povidone iodine plus neosporin group (PVP + N) than in silver sulphadiazine group (SSD). Similarly, healing times were also better with PVP + N, with a maximum number of patients having healed within 15 days. However, the mortality rates were not much different between the two groups [36].
4.5. Polymyxin B
Polymyxin B is a simple, basic peptide antibiotic that is generally available in an ointment base (white petrolatum). Interaction of polymyxin B with phospholipids allows penetration and disruption of bacterial cell walls and therefore affects membrane permeability [37]. It was reported that Polymyxin B inhibited the ability of PMNs to destroy ingested microorganisms, which may suppress the ability of the wound to restrain bacterial proliferation [127]. Considering the side effects, the absorption of topically applied polymyxin B is negligible, and systemic toxicity is rare and is often related to use over a large area for an extended period of time. If with bacitracin, polymyxin B encourages healing by containing surface bacteria.
4.6. Nitrofurazone
Nitrofurazone is a topical anti-infective agent effective against gram-negative and gram-positive bacteria. Treatment of nitrofurazone cream for patients with invasive Nitro-bacteria cloacae burn wound sepsis resulted in an overall 66% survival compared with an 86% mortality reported in the literature [38]. A nitrofurazone-impregnated catheter was introduced exclusively for use in burn patients [128]. The nitrofurazone-impregnated catheter was effective in reducing the incidence of catheter-associated urinary tract infection in a regional Burn Center. Use of these catheters may play an important role in minimizing the risk of urinary tract infection in burn patients. In an era when concern about bacterial resistance to many anti-infective agents is growing, the nitrofurans have continued to be active against organisms that have developed resistance to antibacterials [129].
4.7. Nystatin
Nystatin is an effective fungicide against Candida. Nystatin is produced by Streptomyces noursei and has its potent antifungal effect by binding to the sterols in the fungal cell membrane [35]. A lower concentration of this agent inhibits Candida albicans, but a higher concentration is needed to inhibit other fungi [130]. The nystatin can be encapsulated within a liposome, preferably a stable multilamellar vesicle, which broadly comprises one or more lipids one or more of phosphomonoglyceride, phosphatidic acid and sphingolipid [131]. However, since nystatin has no activity against bacteria, it should be used in combination with a topical agent that has activity against the broad spectrum of pathogenic bacteria that cause burn wound colonization and infection [33]. Furthermore, a Nystatin formulation was described as having reduced toxicity in a new patent [132].
5. TOPICAL SILVER PREPARATIONS
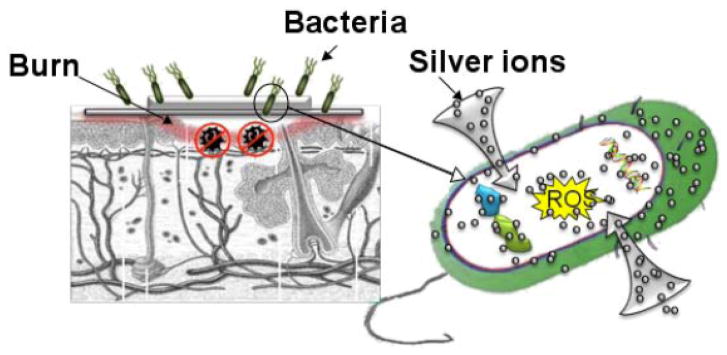
The medical uses of silver date back to at least the time of Hippocrates (the “father of modern medicine” [133]), who in his writings discussed silver’s role in wound care. By the late nineteenth and early twentieth century, surgeons routinely used silver sutures to reduce the risk of postoperative inflammation and infection [134]. During World War I, soldiers used silver leaf to limit and treat battle wound infections [135]. Silver fell into disuse around World War II due to the advent of antibiotics. Interest in the antimicrobial properties of silver was only reinvigorated in the year 1965 by Moyer who published a paper on silver nitrate’s antibacterial effects [39]. Today, silver has reemerged as a clinical treatment for the prevention and treatment of infections encountered in wounds, ulcers, and burns [136].
Many different silver containing antibacterial agents exist in the market, including topical creams, ointments, and solutions: silver nitrate, silver sulfadiazine (SSD), SSD with cerium nitrate, and newer sustained release silver products [136]. Silver requires ionization to fully exhibit its antimicrobial effect; positively charged silver ions readily bind to negatively charged proteins within the wound fluid, inhibiting delivery of silver ions to the wound bed. Indeed, a major problem with topical silver agents lies in their inability to penetrate deeply into the tissue and the low levels of silver release into the target area. In the last few years, several silver products have been introduced to the market in order to address these issues [137]. Fig. (7) graphically illustrates the use of silver as a topical antimicrobial agent in burns.
5.1. Silver Nitrate
Colloidal silver solutions consist of submicroscopic positively charged silver particles suspended in liquid base. These were commonly used up until Moyer’s article was published in the 1960’s [39], following which silver salts replaced colloidal silver solutions in clinical use. Silver salts consist of positively charged silver ions coupled to negatively charged ions (AgNO3), an arrangement that is much more stable than that of colloidal solutions. Silver nitrate is the most commonly used silver salt, exerting an antibacterial action (in vitro and in vivo) at a concentration of 0.5% and a significant toxic effect at a concentration greater than 1% [138]. Silver nitrate is most commonly administered topically by soaking gauzes, which are then applied to severe burns. Patents have been issued for silver nitrate gels, which are claimed to be easier to administer [139–141].
The use of silver nitrate in burn patients remains controversial [40]. Nitrate is toxic to tissues and wounds, and decreases wound healing. Reduced nitrate impairs wound re-epithelialization, thereby partially offsetting the benefits of silver. (Interestingly, a patent was issued to exploit silver nitrate’s toxicity as an intrauterine cauterizing agent [142]).
Other clinical uses of silver nitrate, aside from burns, exist. One percent silver nitrate drops were traditionally administered to newborns to prevent ophthalmia neonatorum (neonatal conjunctivitis). Many hospitals, however, have opted to use antibiotics (such as erythromycin drops) instead, due to fears of chemical irritation (and associated chemical conjunctivitis) following silver nitrate administration. Despite this well-founded concern, Zanoni et al. [143] found silver nitrate drops to be more effective in the prevention of ophthalmia neonatorum.
5.2. Silver Sulfadiazine
As discussed above, the use of silver fell into disuse with the advent of antibiotics. The renewed interest in silver comes as a consequence of the rapid development of bacterial resistance. In the 1970s, Fox [144] decided that it was unnecessary to choose between silver and antibiotics; he combined silver nitrate with the antibiotic sodium sulphadiazine. This formulation, known as silver sulfadiazine (FlammazineR SilvadeneR), benefits from the inhibitory effect of silver and the antibacterial action of sulphadiazine [136]. Silver sulfadiazine (SSD) was initially formulated as an ointment, but was later incorporated into a hydrophilic cream [40]. Although SSD remains the gold standard in topical burn treatment, recent studies indicate that (similar to silver nitrate) SSD may delay wound healing. Cho Lee et al. [145] found that epidermal growth factor (EGF) could significantly counter the deleterious effects of SSD on wound healing, and concluded that future preparations of SSD may come coupled with EGF. Another problem associated with SSD remains bacterial resistance to the sulphadiazine component [146]. SSD-resistant Pseudomonal species have been reported; a patent was issued for a formulation to combat these species, a combination of SSD and sodium piperacillin (the latter of which is very active against pseudomonas) [147]. Other patents have been issued for various SSD delivery systems, including animal tissue dressings [148], water-dispersible hydrophilic carriers [149], and topical spray preparations [150].
5.3. Cerium Nitrate-SSD
In 1976, Monafo et al. added cerium nitrate to SSD in the treatment of burn wounds, and concluded that the two ingredients had a synergistic effect [42]. Since that time, cerium nitrate-SSD (Flammacerium) has been routinely used in wound care. However, the efficacy of this combined formulation is disputed [40]. In 2005, Garner et al. conducted a review of pertinent literature and concluded that cerium nitrate-SSD reduces mortality and morbidity in severe burns when immediate excision and closure is not possible [151]. However, contrary to the hypothesis furthered by Monafo, Garner et al. concluded that cerium nitrate possesses minimal antimicrobial action, and gains its benefit from its action on burn eschar. Burned skin produces a lipid protein complex that causes immunosuppression in the burn patient, which leads to increased susceptibility to infection (and subsequent morbidity and mortality). Cerium nitrate binds to and then denatures this lipid protein complex, thereby preventing immunosuppression [151]. Patents have been issued for various delivery systems for cerium nitrate-SSD, including a creamlike water-dispersible hydrophilic composition [152].
5.4. Sustained Silver Releasing Systems
Silver nitrate, SSD, and cerium nitrate-SSD are considered the old guard in silver products. These are solutions, salts, or compounds that must be applied to gauze. Silver-based dressings are newer products that incorporate silver in the dressing itself [43]. In the last few years, there has been a proliferation of such products, accompanied by aggressive marketing campaigns by manufacturers. Patents have been issued for various silver dressings, including such products as Acticoat™, Aquacel-Ag™, Silvercel™, and Contreet™.
Acticoat-7 (Smith & Nephew, Hull, United Kingdom) dressing utilizes nanotechnology: nanocrystalline (<20 nm diameter) silver crystals are released from the dressing to the burn wound [43]. The silver crystals are released as an initial large-bolus, followed by a sustained release. Fong et al. [43] cautiously concluded that nanocrystalline (NC) silver not only reduces wound infection and promotes wound healing, but decreases the frequency of dressing changes, reduces pain levels, and cuts cost. A meta-analysis by Gravante et al. [153] confirmed these findings, and found NC silver treated patients to have a significantly lower incidence of infections than those treated with the older silver products (silver nitrate and SSD). It was concluded on this basis that NC silver possessed a significantly stronger antimicrobial action than older silver formulations. Numerous patents [154–157] have been issued for various NC silver dressings and topical preparations that were developed by Nucryst Pharmaceuticals Corp. which was subsequently taken over by Smith and Nephew. Despite the encouraging results of NC silver products, concern remains over toxicity of nanocrystalline products in general which has been demonstrated in vitro (but not in vivo) [43].
Several other sustained silver releasing products exist, such as Aquacel Ag® (ConvaTec, Princeton, NJ, USA). Ionic silver is incorporated into the hydrofiber wound dressing, which turns into a gel upon contact with wound tissue. Barnea et al. [44] presented a review of literature, and determined that Aquacel Ag is both effective and safe in acute and chronic wounds, exhibiting a broad antimicrobial profile with no delay in wound healing.
Silvercel® (Johnson and Johnson) is another sustained release product, which combines the antimicrobial effects of silver with alginate’s benefits [45]. Alginate is a polysaccharide found in the cell walls of algae that is able to absorb 200–300 times its weight in water, a capability that makes it particularly useful in exudate management of wounds. Bell et al. (2007) [158] compared Silvercel with Aquacel Ag, and found the former superior in its ability to manage exudate, hardly a surprising finding considering alginate’s powerful absorptive capabilities. Silvercel was able to maintain its shape and strength even in profusely wet wounds, unlike Aquacel Ag. On the other hand, Aquacel Ag-treated wounds were less likely to suffer from the adverse effects of excessive dressing adherence to wound tissues.
A patent was issued in 2003 for silver alginate foam [159]. Foams have emerged as another delivery system for sustained silver release, providing a simple and more convenient method of application. Jorgensen et al. [42] found Contreet Foam (sustained silver release foam, Coloplast) to be superior to Allevyn (hydrocellular foam dressing without added silver, Smith and Nephew), resulting in 45% greater reduction in ulcer area as compared to 25% in Allevyn.
Patents have been issued for numerous sustained silver release delivery preparations [160–163], and such formulations remain an area of active research and development.
5.5. Silver-Impregnated Biological Material
During the 1970’s, human amniotic membrane emerged as a useable dressing in burn patients. Sawhney [46] concluded that this biological dressing was superior to conventional dressing, as it promoted rapid re-epithelialization and healing of wounds. Since 1978, silver has been incorporated into amniotic membrane, producing significantly better results than amniotic membrane alone [164, 165]. A patent was issued for a wound dressing consisting of animal tissue (including amniotic membrane) incorporated with silver sulfadiazine; it was claimed that more silver was imparted onto the tissue than would be with other silver containing dressing [166]. The incorporation of silver into amniotic based membrane adds another dimension to the proliferation of silver containing products. EZ Derm (Brennen Medical, Inc) is modified pigskin impregnated with a soluble silver compound intended for treatment of burns. Originally developed by Genetic Laboratories. A patent describes the silver that EZ-Derm uses [167].
6. IODINE PREPARATIONS
6.1. Povidone-Iodine
Iodine and its antibacterial properties have been used for the prevention or management of wound infections for over 150 years [168]. However, the use of solutions (tincture) of iodine has been replaced by the widespread use of povidone-iodine, a water-soluble compound, which is a combination of molecular iodine and polyvinylpyrrolidone [169]. Povidone- iodine’s antiseptic action is due to the available iodine present in the complex. This solution is active against a wide variety of bacteria, fungi, protozoa, and viruses [170]. A patent on wound- healing compositions containing povidone iodine describes admixtures of an antifungal/antibacterial agent such as povidone iodine, sugar and a suitable carrier as substantially non allergenic, having excellent healing properties when applied to burns or open wounds and serves as an effective barrier to growth of healing tissues in gauze or similar dressing [47]. A device has been described in a recent patent [171] for the in-situ preparation of a pharmaceutical composition comprising povidone-iodine and a steroid or non-steroidal anti- inflammatory.
6.2. Liposomal Iodine (Repithel)
The combination of povidone-iodine (PVP-I) and liposomes unites the microbicidal activity of the antiseptic substance with the tolerability and lack of immunogenicity of liposomes; in addition, liposomes provide a moist molecular film for the wound environment. An animal study on the efficacy and tolerability of different formulations of a hydrogel with PVP-I liposomes in deep dermal burn wounds has indicated a good quality of wound healing with smooth granulation tissue, less inflammation, less wound contraction and no hyperkeratotic reactivity, especially with the 3% PVP-I liposome formulation [49]. Repithel (Mundipharma Research GmbH & Co. KG, Limburg/Lahn, Germany) is a new liposomal hydrogel formulation of PVP-I developed to provide a moist wound-healing environment with anti-infective and debriding properties. The pronounced positive effect of Repithel in burns in smokers (since smoking and excessive inflammation have a similar impeding effect on wound healing) [172]. There is a patent [173] describing a dry liposomal PVP-I composition which was directed towards a storage stable package.
6.3. Cadexomer Iodine (Iodosorb)
Cadexomer iodine (Iodosorb, Smith and Nephew) is a hydrophilic starch powder containing iodine, which is a suitable dressing for granulating wounds such as venous ulcers. Formulations of iodophors or “iodine carriers,” however, release low levels of iodine over a longer period. Cadexomer iodine is a slow-release antimicrobial capable of absorbing excess wound exudates while offering a sustained level of iodine in the wound bed. Cadexomer iodine also has established effectiveness in vivo against S. aureus and MRSA. Iodine needs moisture to be activated, similar to silver ion-containing products. A total of 61 outpatients with chronic venous ulcers participated in a randomized optional crossover trial using cadexomer iodine or a standard dressing for their ulcers. Both treatments were highly effective, but the epithelium of ulcers dressed with cadexomer iodine grew again significantly faster [174]. A recent patent describes an improved method for the preparation of cadexomer iodine [175].
6.4. Other Iodine Preparations
A patent describes iodine-containing derivatives, including, free iodine and iodoform for otic and nasal infections [176]. lodoform is a potent germicidal agent and the composition also contains one or more anti-inflammatory agents and one or more natural or synthetic compounds which provide analgesic benefits. Another patent (3M Innovative Properties Company, Saint Paul, MN) [177] describes liquid antiseptic compositions containing iodine and a sugar and/or sugar alcohol intended primarily for tissue antisepsis. Iocide™ (Biomedical Development Corporation, San Antonio, TX) is composed of a monobasic iodide salt, an organic acid having up to eight carbon atoms (such as citric acid, ascorbic acid or oxalic acid), an oxidizing agent (such as sodium percarbonate or sodium perborate) and an aqueous buffer [50]. Mixing these ingredients together generates iodine in a biologically compatible antimicrobial formulation. Another patent [178] described a stable, aqueous, iodine-based germicidal composition including iodine, a non-ionic surfactant (e.g. polyoxyethylene, polyoxypropylene block copolymers) and an iodine-solubilizing halide ion (chloride or bromide).
7. PHOTODYNAMIC THERAPY
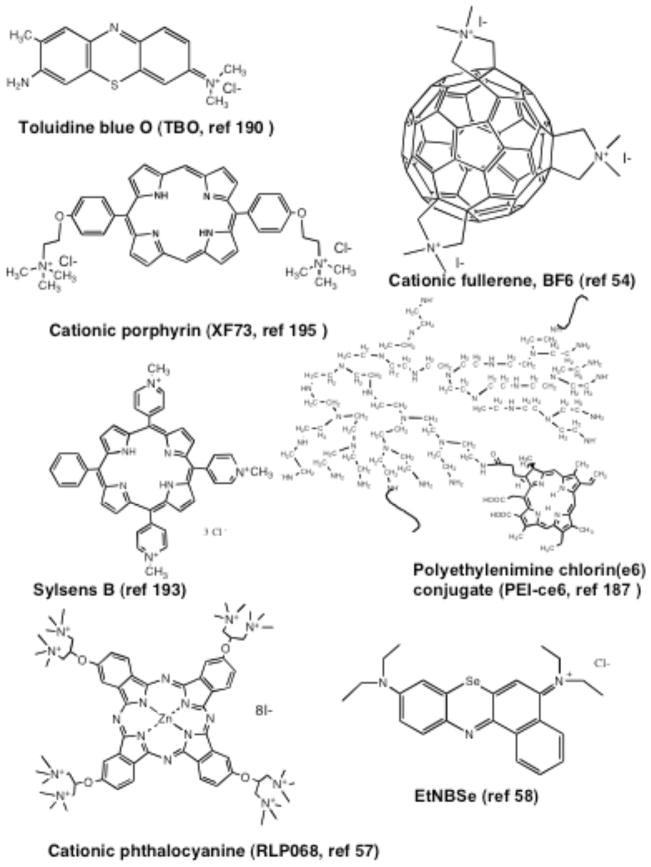
Photodynamic therapy (PDT) [179] was discovered over 100 years ago by observing the killing of microorganisms when harmless photosensitizers (PS) and visible light were combined in vitro [180]. Light of the correct wavelength excites the PS to its excited singlet state that can then undergo intersystem crossing to the long-lived excited triplet state. In the presence of the oxygen, the triplet state PS transfers energy to ground state molecular oxygen (a triplet) producing reactive oxygen species (such as singlet oxygen) that are able to kill microbial cells [181]. Fig. (9). graphically illustrates the mechanism of action of antimicrobial PDT and emphasizes its broad spectrum activity due to the ability to effectively kill or inactivate all known classes of microbial pathogen. Since its discovery PDT has primarily been developed as a treatment for cancer, ophthalmologic disorders and in dermatology [179]. However, in recent years interest in the antimicrobial effects of PDT has revived due to the inexorable increase in drug resistance among many classes of pathogen [182]. Numerous investigations have been performed [183] to develop and refine the PS with particular molecular properties that are able to selectively inactivate target microbial cells with minimal damage to host cells. The molecular structures of several of these PS that are discussed in the present section are shown in Fig. (10). Most of the powerful antimicrobial PS have an overall positive or cationic charge that may be provided by quaternized nitrogen atoms (with a constitutive cationic charge) or by basic amino groups with a pKa that allows them to be positively charged at the pH values that prevail in bacteria or in infected tissue.
Fig. 9. Concept of antimicrobial photodynamic therapy.
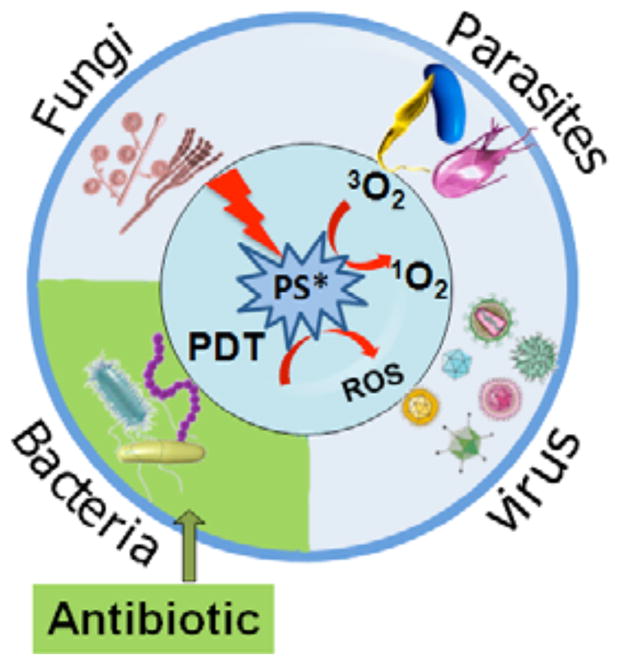
The photosensitizer is excited to its triplet state by light of the correct wavelength and then transfers its energy to molecular oxygen forming reactive oxygen species that are able to kill all classes of pathogenic micro-organisms.
Fig. 10. Chemical structures of photosensitizers used in antimicrobial applications.
These molecules possess either constitutive cationic charges or basic amino groups. Toluidine blue O (TBO), cationic fullerene (BF6), cationic porphyrin (XF73), meso-mono-phenyl-tri(N-methyl-4-pyridyl)-porphyrin (Sylsens B), polyethylenimine chlorin(e6) conjugate (PEI-ce6), cationic phthalocyanine (RLP068), selenium benzo(A)phenoxazinium chloride (EtNBSe).
7.1. Patents on Antimicrobial PDT
Nifantiev et al. [184] patented molecular conjugates with photosensitizers (PS) which can bind selectively to the surface of a microorganism bringing about photo-destruction upon irradiation. The key to these conjugates is a special spacer connecting at least one PS to a microorganism receptor (vector). Spacers having hydrophilic structure, such as ethylene glycol units and amino carboxyl end capped ethylene glycol units, are used for linking the vector to the PS. Bacterial species S. aureus, P. aeruginosa, and E. coli were used to test the effectiveness of the conjugates. The conjugates were found to be very effective in inactivating these bacterial species in the real patient-related environments where blood, serum and other body fluids are always present.
Hasan et al. patented [185] another group of PS conjugates for antimicrobial PDT. These involved covalently attaching a PS to a “non-pair member” (NPM) moiety. This NPM can be a linear, branched, or cyclic polypeptide including a small anti-microbial peptide (AMP) (see later section of this review). Histatins, defensins, cecropins, magainins, Gram positive bacteriocins, and peptide antibiotics can be attached to PS. Moreover the polypeptide can also be a poly-L-lysine, polyornithine or polyarginine molecule whose length and substitution ratio can vary [186, 187]. A further variation on the NPM can be polymers that are basic but not composed of amino-acids such as polyethylenimine (PEI, see Fig. 10). These conjugates were originally proposed for oral anti-bacterial applications (PDT for periodontitis [188]), but subsequent studies showed that PEI-ce6 was highly effective for treating burn infections in a mouse model [52].
Hamblin et al. [54] patented PS compounds based on functionalized fullerenes in antimicrobial PDT. In this invention, fullerene molecules, higher fullerenes, and their functionalized derivatives are modified to include a variety of properties needed for application of PDT to microbial cells. This is achieved by controlling hydrophobicity, molecular charge, and water solubility of the carbon nanomaterial specifically to target microbial species preferentially over other types of cells for PDT. A positive charge allows the fullerenes to bind to cells and overcome microbial permeability barriers. Cationic fullerenes in particular performed better as antimicrobial PS than the widely employed antimicrobial PS toluidine blue O. Accordingly, cationic fullerenes may find significant application for PDT of a wide variety of conditions, such as for example, localized infections in wounds, burns, and mucus membranes.
In another patent, Hamblin and Tegos [189] described the use of phenothiaziniums and microbial multi-drug resistance inhibitors to inactivate microbial cells. It has now been shown that phenothiaziniums are substrates for microbial multidrug resistance pumps (“MDR Pumps”). Inactivation of the microbial MDR pumps by inhibitors (“MDR inhibitors”) increases the amount of phenothiazinium that can be retained by the microorganism, thereby increasing efficacy upon photoactivation of the phenothiazinium by irradiation.
Biel [190] filed a patent utilizing a combined solution of a PS (e.g., methylene blue or toluidine blue) and a surfactant compound (e.g. polymyxin B or SDS) for PDT against bacterial or fungal infections, or for cancer cell eradication. Surfactants are used to affect the permeability of the membranes of the target cells. The treatable pathogens include S. aureus, C. albicans, E. coli, Enterococcus, Streptococcus, P. aeruginosa, Hemophilus influenza, and Clostridia.
Hamblin and Foley [58] patented a group of PS composed of chalcogen analogs of benzo(A)phenoxazinium dyes that are effective antimicrobial PS. In particular when the chalcogen is selenium (EtNBSe) the activity is maximized (Fig 10).
Albrecht et al. [191] disclosed a method of destroying bacteria in a treatment area of a patient by introducing a composition comprising Erythrosin B as a PS in a gel to a treatment area on a biological surface, allowing a predetermined time period to elapse to allow Erythrosin B to couple with bacteria in the treatment area, and applying radiation of a preselected wavelength to the treatment area to activate the PS and thus stimulating a photodynamic reaction to destroy bacteria.
In another patent, Albrecht et al. [192] described the use of liposomal formulations for improved efficacy of antimicrobial PDT. The formulations comprise a hydrophobic porphyrin PS, a monosaccharide, and one or more synthetic phospholipids, which are stable in storage especially through freeze-drying process and reconstitution.
Van Der Haas et al. [193] patented a method of preparing quaternized porphyrin derivatives suitable for antimicrobial PDT. A further patent [194] described the use of these porphyrins typified by Sylsens B (Fig 10) for carrying out PDT of fungal skin infections including burns.
Roncucci et al. patented [57] a series of phthalocyanine derivatives Fig. (10) having photosensitizing characteristics and high solubility in water, useful for photodynamic treatment of bacterial infections, in particular infections generated by Gram-negative bacteria.
In another patent, Bommer and Jori [195] proposed the use of porphyrins that are characterized by the presence of up to four positive charges in the peripheral substituent, and at least one hydrophobic tail comprising between 6 and 22 carbons inclusive, originating at one or more of the charged sites. In vitro test was carried out to evaluate the photosensitizing efficacies of the compounds. It was observed that a 4-log10-unit inactivation of E. coli was achieved upon 15 minutes irradiation in the presence of 1 μM porphyrin.
Love and his colleagues [53] patented a method of using porphyrin derivatives containing cationic groups for selective photodynamic inactivation of target microbial cells with minimal damage to host cells. It was claimed that the porphyrin derivatives typified by XF73 Fig. (10). can be used as selective PDT agents, i.e., for selectively killing microorganisms, and can also be used in combination with conventional antibiotics.
7.2. PDT for Burn Infections
The first paper describing PDT for burn infection was by Orenstein et al. [56]. These workers used deuteroporphyrin-hemin complex as an agent for PDT of burn wounds infected with a multiple-drug resistant strain of Staphylococcus aureus. The in vivo experiments by application of the above porphyrins in combination to infected burn wounds in guinea pigs was an effective way to reduce dramatically the contaminating S. aureus. Reduction of more than 99% of the viable bacteria was noted after the porphyrin mixture was dropped on the eschar or injected into the eschar, an effect that lasted for up to 24 hours.
Lambrechts et al. [55] used PDT mediated by meso-mono-phenyl-tri(N-methyl-4-pyridyl)-porphyrin (Sylsens B, Fig. 10) to treat burn wounds in mice with established bioluminescent S. aureus infections. PDT was applied after one day of bacterial growth by adding a 25% DMSO/500 microM PS solution to the wound followed by illumination with red light and periodic imaging of the mice using a sensitive camera to detect the bioluminescence. More than 98% of the bacteria were eradicated after a light dose of 210 J cm2 in the presence of PTMPP. However, bacterial re-growth was observed.
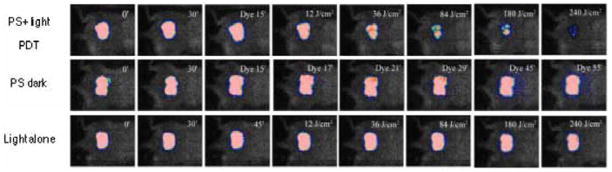
Dai et al. [52] evaluated the effectiveness of PDT for A. baumannii burn infections using mouse models. Burns were created on the backs of shaved mice by using two heated brass blocks (95 °C) with contact time between the blocks and mouse skin 10 seconds. Bioluminescent A. baumannii were inoculated to the burns, followed by addition of poly-ethylenimine-chlorin e6 conjugate and subsequent illumination with non-coherent red light at 660±15 nm.
Figure 11 shows the PDT dose-response of the bacterial luminescence of a representative mouse burn infected with A. baumannii and treated with PDT at 30 minutes after infection, a mouse burn treated with PS only (dark control), and a mouse burn treated with light only (light control). After 240 J/cm2 red light had been delivered (40 minutes irradiation time at an irradiance of 100 mW/cm2), PDT induced an approximately 3-log-unit reduction in bacterial luminescence from the mouse burn, while during the same period of time, only modest reduction of bacterial luminescence was observed in the dark control and the light control. When PDT was performed at 30 minutes after infection, over 3-log10 inactivation of A. baumannii was achieved, as quantified by luminescent imaging analysis. When PDT was performed 24 hours after infection (the time when the infections were fully established), over 1 log10 inactivation was achieved.
Fig. 11. Use of PDT to treat mouse burn infection caused by bioluminescent A. baumannii.
Bacterial luminescence of a representative mouse burn infected with A. baumannii and treated with PDT at 30 minutes after infection (top row), a mouse burn treated with PS only (dark control, middle row), and a mouse burn treated with light only (light control, bottom row). After 240 J/cm2 red light had been delivered (40 minutes irradiation time at an irradiance of 100 mW/cm2), PDT induced an approximately 3-log-unit reduction in bacterial luminescence from the mouse burn, while during the same period of time, only modest reduction of bacterial luminescence was observed in the dark control and the light control.
PDT did not delay wound healing in A. baumannii infected burns.
8. CHITOSAN PREPARATIONS
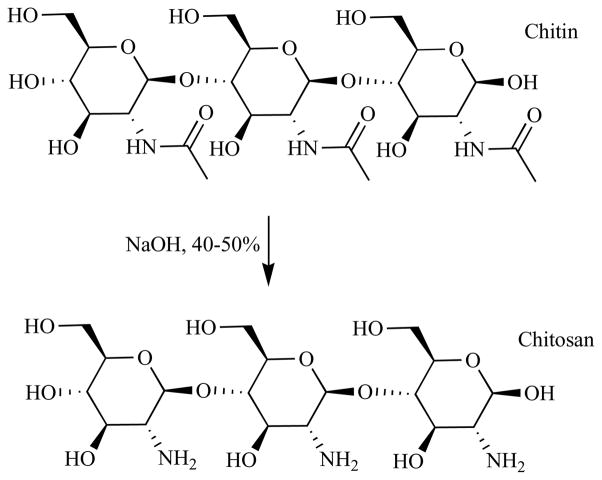
Chitosan is a polycationic polymer with a specific structure and properties. Chitosan molecules contain more than 5000 glucosamine units and the substance is obtained commercially from shrimp and crabshell chitin (a N-acetyl-glucosamine polymer) by alkaline deacetylation (NaOH, 40–50%) as shown in Fig. (12). Chitosan preparations of various molecular weights, degrees of deacetylation (DD), and with further molecular derivatization patterns have attracted much attention because of their potentially beneficial biological properties. Chitosan’s properties allow it to rapidly clot blood, and has recently gained approval in the United States for use in bandages and other hemostatic agents. Chitosan is a polymer with a number of basic amino groups and hence possesses an overall cationic charge especially at acidic pH. In common with many polycationic polymers it has pronounced antimicrobial effects due to destabilization of the outer membrane and pemeabilization of the plasma membrane [196].
Fig. 12. Chemical structure of chitin and chitosan.
Treatment of chitin (poly-N-acetyl glucosamine) with hot sodium hydroxide partially hydrolyzes the amide bonds to form chitosan (poly-glucosamine).
8.1. Patents for Antimicrobial Chitosan Preparations
A large number of patents have been filed or issued concerned with the preparation and use of various chitosan preparations as antimicrobial burn treatments or dressings. In this review we can only cite a few of them.
One of the first patents was by Sparkes et al. [197] who described a wound dressing comprising a blend of gelatin and chitosan. Yura and colleagues patented [198] a functional chitosan derivative comprising chitosan, incorporating a photo-reactive functional group, an amphipathic group, e.g. a polyoxyethylene alkyl ether, and a glycosaminoglycan and that has solubility in a neutral medium, self-crosslinking ability, and able to act as a wound dressing. Baker and Wiesmann [199] filed a patent for chitosan or chitosan derivatives such as chitosan-arginine and chitosan-acid amines, that exhibit bactericidal activity against bacterial pathogens, e.g., drug resistant bacteria such as Methicillin-resistant S. aureus (MRSA). Scherr patented [200] a foamed gel containing chitosan that may be layered onto a suitable backing for use as a wound dressing, or the gel may be directly applied to wounds to effect hemostatic activity as a result of the action of the chitosan. The composition of the foamed chitosan gel can reduce the risk of microbial infections in wounds. Muzarelli patented [201] a methyl pyrrolidinone chitosan derivative that could be used as a wound dressing material in the form of a freeze-dried material, powder, film, non-woven fabric, bandage, adhesive tape etc.
8.2. Chitosan for Burn Infections
Alsarra [202] investigated the formulation of chitosan gel for improving wound healing of burns in rats. High molecular weight chitosan with a 2,000,000 MWt and 92% DD, medium molecular weight chitosan with a 750,000 MWt and 75% DD, and low molecular weight chitosan with a 70,000 MWt and 63% DD were tested. It was shown that chitosan samples with high molecular weight and high degree of deacetylation had the best effect for the healing of burns.
Aoyagi et al. [203] described a chitosan dressing composed of chitosan film and minocycline hydrochloride. Chitosan with DD of 67%, 83% and 96% (mol/mol) were used, respectively. Various formulations were applied to burns in rats. Chitosan with DD of 83% containing 2 mg minocycline hydrochloride showed an excellent effect.
Boucard et al. [204] developed a bi-layer physical hydrogel, which consisted of chitosan and water. The first layer was a rigid protective gel that ensured good mechanical properties and gas exchanges, and the second soft and flexible layer allowed the material to follow the geometry of the wound and ensured a good superficial contact. A chitosan with 2.6% DD and molecular weight of 540,000 g/mol was used. This material was tested for skin regeneration after third-degree skin burns on the pig back. The results showed that chitosan materials were well-tolerated and promoted good tissue regeneration.
Cardenas [205] made several types of chitosan composite films by adding several additives to acetic acid chitosan solutions, such as: glycerol, oleic acid and linoleic acid in different proportions. They used chitosan acetate films, which were prepared using shrimp chitosan of low and high molecular weight (MWt = 68,000 g/mol and MWt = 232,000 g/mol). The films exhibited different physical properties depending upon the additives and/or mixture of them. The addition of glycerol to composite improves the elasticity of the films. In-vitro tests were carried out using the chitosan composite solution against S. aureus, P. aeruginosa, and A. baumannii, showing that the molecular weight has an influence on the bactericidal properties of the chitosan composite films and on its effect against Gram positive and Gram negative bacteria. Medical applications of the composite films were studied in patients with burns, ulcers and injuries, the films containing glycerol showed good adhesion in comparison with those without it. Excellent results in skin recovery were obtained after 7–10 days.
Ribeiro et al. [59] proposed a chitosan hydrogel for wound healing of burns. By preparing this hydrogel, chitosan solution 4% (w/w) was dispersed in lactic acid 2% (v/v). In vitro studies showed that chitosan-hydrogel was able to promote cell adhesion and proliferation, and are non-cyto-toxic to cells. In vivo studies using a rat burn model revealed that chitosan-hydrogel stimulated the wound healing, and was locally and systemically biocompatible based on the evidence that there was no granulomatous inflammatory reaction in burns treated with chitosan-hydrogel and no pathological abnormalities in the organs.
Sezer et al. [206] developed a chitosan film containing fucoidan and investigated its effectiveness for treating burns in rabbits. Fucoidan is a sulfated polysaccharide commonly obtained from seaweeds and shows significant gel contraction-promoting, integrin expression-enhancing, and heparin activities. It was found that chitosan film containing fucoidan induced significant wound contraction, and accelerated the wound closure and healing process. The authors also prepared a fucoidan-chitosan hydrogel and investigated its treatment efficiency on dermal burns in rabbits [60]. Similar results were obtained to those with chitosan film containing fucoidan.
Damour et al. [207] reported a clinical use of a dermal substrate made of collagen--GAG--chitosan grafted immediately after early excision of severe burns, then epidermalized either with autologous meshed autograft or with autologous cultured epidermis. The dermal substrate replaces the excised dermis by adhering to the underlying tissue, promoting fibrovascular ingrowth. In comparison to homograft, this dermal substrate is always available, can be stored, and is exempt from micro-organism transmission.
Peh et al. [208] investigated the mechanical, bioadhesive strength, and water vapor permeability of chitosan films prepared using two different solvents, acetic acid (chitosan-AA) and lactic acid (chitosan-LA). The properties of the two films were compared with those of a commercial preparation, Omiderm®. In addition, biological studies were conducted to investigate the skin irritation and systemic toxicity of the films. It was found that the three preparations differed significantly in terms of mechanical and bioadhesive strength properties. Chitosan-LA was more soft, flexible, pliable, and bioadhesive than chitosan-AA. Furthermore, chitosan-LA was non-allergic and non-toxic. As a result, the author concluded that chitosan-LA is more suitable to be used in the management of wound healing and skin burn.
Dai et al. [61] investigated the use of a freeze-dried chitosan acetate bandage (HemCon™) that was originally developed as a hemostatic dressing. They applied the dressing to 3rd degree burns in mice that had been infected with bioluminescent P. aeruginosa and Proteus mirabilis,
Figure (13). shows the successive bioluminescence images from day 0 to day 3 after P. aeruginosa infection of an untreated burn, a silver dressing-treated burn, and a chitosan acetate-treated burn, respectively. In the untreated burn, the bacteria multiplied approximately 1000 fold from day 0 to day 3, while in both the silver dressing and chitosan acetate bandage-treated burns, a decrease in bioluminescence signal was seen at day 1 as the dressing immediately quenched the light. There was a detectable increase in signal from the silver-dressing burn at day 2 and 3 (compared to day 1) that was not seen in the chitosan-acetate burn. The inset showing a silver-treated burn at day 3 was taken after the silver dressing was removed from a dead mouse and shows a vigorous bacterial proliferation underneath the dressing.
Fig. 13. Use of a chitosan acetate bandage to treat mouse burn infection.
The successive bioluminescence images from day 0 to day 3 after P. aeruginosa infection of an untreated burn (top row), a silver dressing-treated burn (middle row), and a chitosan acetate-treated burn (bottom row).
In the case of P. aeruginosa infections, the survival rate of mice treated with the chitosan acetate bandage was 73.3%, whereas the survival rate of mice treated with a nano-crystalline silver dressing was 27.3% (p = 0.0055) and that of untreated mice was 13.3% (p < 0.0002). For P. mirabilis infections, the comparable survival rates were 66.7%, 62.5%, and 23.1% respectively. Quantitative bioluminescent signals showed that the chitosan acetate bandage effectively controlled the growth of bacteria in the burn and prevented the development of systemic sepsis, as shown by blood culture.
9. ANTIMICROBIAL PEPTIDES
The term ‘antimicrobial peptides’ refers to a large number of peptides found among all classes of life possessing both antibacterial and antifungal activities. They have been shown to kill Gram-negative and Gram-positive bacteria (including strains that are resistant to conventional antibiotics), mycobacteria (including Mycobacterium tuberculosis), enveloped viruses, and fungi. Besides acting as endogenous antibiotics AMPs also participate in multiple aspects of immunity (inflammation, wound repair, and regulation of the adaptive immune system). So unlike the majority of conventional antibiotics the antimicrobial peptides also have the ability to enhance immunity by functioning as immunomodulators. AMPs are divided into heterogeneous groupings on the basis of their primary and secondary structures, their antimicrobial potential, their effects on host cells, and the regulation of their expression. Most AMPs are small (12–50 aminoacids), have a positive charge provided by Arg and Lys residues, and an amphipathic structure that enables them to interact with bacterial membranes [209]. Fig. (14). illustrates the selectivity obtained by AMPs that have a higher affinity for microbial membranes compared to the membranes of host cells and therefore preferentially lyse the pathogenic, microorganisms.
Fig. 14. Antimicrobial action of AMPs.
Schematic illustration of the selectivity exhibited by antimicrobial peptides that allow them to lyse microbial cell membranes but not those of host cells.
These natural antimicrobials are a part of the body’s natural defense mechanisms, and any loss of these compounds will obviously lead to a reduced ability to combat infection and predispose to sepsis. There are many reports of the association of AMPs with different skin diseases. Overexpression of AMPs can lead to increased protection against skin infections as seen in patients with psoriasis and rosacea; inflammatory skin-diseases which rarely result in superinfection. In other skin diseases, e.g. in patients with acne vulgaris, increased levels of AMPs are often found in inflamed or infected skin areas indicating a role of these peptides in the protection from infection. In addition, decreased levels of AMPs are associated with burns and resulting sepsis is often fatal [210]. This observation suggests a possible therapeutic role for AMPs in the management of burn wounds.
9.1. Defensins
Defensins are a family of cationic antimicrobial peptides exhibiting bactericidal, fungicidal and antiviral activity [211–213]. They are part of a non-specific chemical defense system secreted by non-lymphoid cells [62]. When released into the extracellular milieu, they exert their antimicrobial activity directly by attacking the microbial membrane, and contribute to the oxygen-independent killing of phagocytosed microorganisms. Furthermore, defensins are mediators in the crosstalk between the innate and adaptive immune systems [214]. Two human beta-defensins have been identified. Human B-defensin-1 has been detected in urogenital, gastrointestinal and pulmonary epithelia and most recently in skin [215–217]. Studies have shown decreased levels of AMPs mainly defensin associated with burns [210].which facilitates infection and subsequent sepsis. This reduction in defensins may also alter functions of T- and B-lymphocytes, neutrophils, macrophages, and complement, thereby contributing to the pathophysiology of burn-related systemic inflammatory responses [218]. The broad antimicrobial properties of defensins, their chemical resistance and their lack of antigenicity suggest that they may also be useful as topical or systemic agents for the prophylaxis and treatment of infections [62]. They may also protective role in preventing infection within burn wounds as altered expression of these peptides could promote local overgrowth of micro-organisms.
9.2. Demegel
Some of the synthetic AMPs have increased potency, enhanced specificity, and reduced toxicity relative to natural defensins. These peptides are quite resistance to the effects of high solute levels, and demonstrate greater antibiotic activity than natural peptides. One such synthetic peptide is Demegel D2A21 (Demegen, Inc. Pittsburgh, PA), which has had patents issued [219, 220]. This peptide has shown significant promise with in vitro studies against a large number of pathogens [221]. Chalekson et al. [63] demonstrated use of D2A21 in an infected burn model in improving survival and recovery. Later they compared the effect of this peptide on survival in an infected wound model with that of traditionally used wound therapies, silver sulfadiazine and sulfamylon and reported D2A21 demonstrated significant superior activity as compared with controls and standard therapy [222].
9.3. Histone H1.2
Histones are the most abundant proteins bound to DNA in eukaryotic cells and among the most evolutionary conserved proteins known. They are small basic proteins with a molecular weight between 11 and 20 kDa and they contain a high percentage of positively charged amino acids (circa 20%) such as lysine and arginine. Eukaryotic cells contain mainly five types of histones: histone linker H1 and core histones H2A, H2B, H3, and H4. Histone H1.2 is a member of the histone H1 family [223] which has a potential role in innate antimicrobial defense [224]. It has been demonstrated that histone H1.2 has good in vitro and in vivo (infected rat burn model with P. aeruginosa) activity against a number of burn wound infection pathogens [64]. A patent was issued for the recombinant production of H1.2 [225].
9.4. Cecropin B
Cecropin B and related peptides have been patented as antibacterial agents [226]. The antibacterial effect of cecropin B on P. aeruginosa infection of wounds in mice was investigated and it was observed application of cecropin B could reduce the mortality of the mice [65]. HB-107, a peptwide fragment derived from cecropin B, lacks antimicrobial activity yet showed up to 64% improvement in wound closure at day13 when compared to either scrambled peptide or vehicle controls in murine model of aseptic full-thickness excisional injury. This effect was comparable to that seen after treatment with currently accepted therapy. Histological evaluation of wounds treated with HB-107 displayed keratinocyte hyperplasia and increased leukocyte infiltration compared to controls. In two different rat models used by Ghiselli et al. [227], beta-lactams even increased plasma endotoxin and TNF-alpha levels, whereas the combination with cecropin B was shown to be the most effective treatment, with the highest survival rates (more than 90%), the highest antimicrobial activities, and the strongest.
9.5. Recombinant Bactericidal/Permeability-Increasing Protein (rBPI21)
This recombinant peptide derived from a human neutrophil sequence was patented as an antimicrobial agent [228]. Early treatment with rBPI21may be effective in attenuating multiple organ damage resulting from gut-origin endotoxin translocation in case of thermal injury. This might be associated with the down-regulation effects of tissue lipopolysaccharide-binding protein and CD14 gene expression by the use of rBPI21. Currently, there is an ongoing clinical trial investigating therapeutic effects of rBPI21 in burn patients [66].
9.6. Ceragenins
Ceragenins, or cationic steroid antibiotics (CSAs), are synthetically produced small molecule chemical compounds consisting of a sterol backbone with amino acids and other chemical groups attached to them. They were described in a series of patents [229–231]. While CSAs have a mechanism of action that is also seen in antimicrobial peptides, which form part of the body’s innate immune system, they avoid many of the difficulties associated with their use as medicines. Several of these derivatives exhibit antimicrobial activity against a broad range of bacteria. Although some forms of ceragenins are effective against both Gram-negative and Gram-positive bacteria, they are generally more potent against Gram-positive bacteria. Ceragenins have the unusual property of forming complexes with phospholipids. Thus ceragenins are a class of agents with many properties to make them favorable for application as antiinfective agents [67].
9.7. Other AMPs
A patent [232] described the house-fly (Musca domestic) pupa natural AMP as a product with broad-spectrum antibiotic activity and no hemolytic activity to blood erythrocytes of mice and was characterized by high temperature resistance, acid and alkali resistance and good stability.
Another patent related to multimeric forms of antimicrobial peptides, for example, defensin peptides that possess antimicrobial activity and may be formulated into antimicrobial compositions for coating medical devices and the like. The invention also relates to the use of these multimeric forms of peptides for inhibiting and/or reducing the growth of microorganisms in infections including burns [233].
Antimicrobial bolisin peptides were originally extracted from bovine thymus glands and were described in a patent [234]. Other patents have been issued based on antimicrobial peptides from histatin [235], silkworm hemolymph [236], heparin-binding protein or human neutrophil elastase [237–239].
10. MISCELLANEOUS TOPICAL ANTIMICROBIALS
Topical chlorhexidine has been studied in several formulations to combat burn infection. Macmanus et al. [68] studied chlorhexidine diphosphanilate 2% cream (CHP) in two rat models of fatal burn wound infection (20% scalds infected with Pseudomonas aeruginosa or Proteus mirabilis). It performed as well as silver sulfadiazine. Miller et al. [69] compared different concentrations of CHP in a clinical trial in 29 burn patients, each with two similar burns which could be separately treated. One burn site was treated with each of four different CHP concentrations, from 0.25 per cent to 2 per cent while the other site was treated with AgSD cream. There was a direct relationship between CHP concentration and patients’ ratings of pain on an analogue scale. The 0.25 per cent CHP cream was closest to AgSD in pain tolerance. A number of patents have been issued covering the use of chlorhexidine as a topical antimicrobial agent for use in burns [124, 240–242]
Superoxidized water is based on hypochlorous acid obtained by the electrochemical treatment of a saline solution, and was disclosed in a US patent [70]. Preferably, the pH of the super-oxidized water is in a range of 4 to 7, and the water has a redox potential of > 950 mV. Medicaments based on the super-oxidized water may be in liquid or gel form and can be applied to control the microbial population within the wound or burns and at the same time permit cell proliferation.
An antimicrobial nanoemulsion was disclosed in another US patent [243]. It is composed of an oil-in-water nanoemulsion consisting of about 5% by volume of a surfactant such as polyoxyethylenesorbitan monolaurate or polyoxyethyl-enesorbitan monooleate with 8% ethanol, 64% oil, 1% cetylpyridinium chloride and distilled water. Burn infections were among other applications proposed.
FPQC (1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-pipera-zinyl]-quinoline-carboxylic acid) [72] is a topical agent that has been tested in burned mice infected with resistant Pseudomonas strains; mortality in groups receiving topical therapy with FPQC was 0%, but 80% to 100% with sulfadiazine silver and 100% without treatment. Similar results were obtained in burned rats.
Acidfied nitrite has been shown to be an effective antimicrobial treatment for various skin pathogens including C. albicans and S. aureus [73]. It works by releasing nitric oxide and other reactive nitrogen intermediates locally in a phenomenon known as “nitrosative stress” [244].
Phage therapy was discovered as long ago as the 1920s [245], and has been considered to be a possible approach to burn infections. The disadvantage has been considered to be the narrow specificity of phages to particular species and strains of bacteria. McVay et al. [74] found that a cocktail of 3 phages could significantly decrease the mortality of thermally injured, P. aeruginosa-infected mice. Malik and Chhibber [246] demonstrated the protective effect of K. pneumoniae-specific bacteriophage KO1 isolated from the environment in a mouse model of burn wound infection induced by K. pneumoniae. A substantial decrease in the bacterial load of blood, peritoneal lavage, and lung tissue was noted following treatment with the bacteriophage preparation. A patent was issued for a cocktail of anti- P. aeruginosa phages for topical application [247].
Ipaktchi et al. [75] used topical application of an inhibitor of p38 mitogen activated proein kinase (MAPK) in a rat burn model infected with P. aeruginosa. They found that bacterial wound growth was significantly attenuated in animals treated with topical p38 MAPK inhibitor. They concluded that attenuation of excessive burn wound inflammatory signaling might prevent secondary damage of the dermal barrier and reduce the growth of opportunistic pathogens.
Probiotic therapy uses “good” bacteria to out-compete and in some cases actively kill harmful pathogenic bacteria. Lactobacillus sp. produce “lantibiotics”, which are antimicrobial proteins belonging to the general class of bacteriocins. A patent was issued for a lantibiotoiic in combination with mupirocin as a topical antimicrobial [248]. A preparation consisting of Lactobacillus plantarum was equivalent to silver sulfadiazine in a clinical trial of patients with infected second- and third-degree burns [76].
Honey has long been known to be a naturally occurring antimicrobial preparation [249]. It is even considered to be a “folk therapy” for burns [250]. Its antibacterial properties are due to a combination of its high osmolarity, and the production of hydrogen peroxide that is formed in a slow-release manner by the enzyme glucose oxidase present in honey. It becomes active only when honey is diluted by wound fluid. The non-peroxide antimicrobial activity is due to methylglyoxal and an unidentified synergistic component. In a clinical trial [77] honey dressing was compared with boiled potato peel dressings as a cover for fresh partial-thickness burns in two groups of 50 randomly allocated patients. In the 50 patients treated with honey, 90 per cent of wounds were rendered sterile within 7 days, while the 50 patients treated with boiled potato peel dressings had persistent infection for 7 days. Of the wounds treated with honey, 100 per cent healed within 15 days as against 50 per cent in the wounds treated with boiled potato peel dressings. A number of patents have been issued related to topical application of honey as an antimicrobial dressing [251–255].
Another alternative burn therapy has been proposed to be plant essential oils [78]. Citricidal is a grapefruit seed extract that was patented as an antimicrobial perfume ingredient [256]. Patchouli, tea tree, geranium, lavender essential oils and Citricidal were compared against S. aureus in a burn dressing in vitro. Citricidal, geranium oil and tea tree oil were the most effective in combination.
Moist exposed burn ointment (MEBO) was developed from traditional Chinese medicine [257]. It contains sesame oil, beta-sitosterol, berberine, and other small quantities of plant ingredients. Berberine has long been known to be an antimicrobial compound [258]. MEBO has been tested in clinical trials for partial-thickness thermal burns covering less than 40% of body surface area and shown to be equivalent to conventional therapy [257].
Due to the limited resources for the management of burns in most regions of Africa there is a significant role for many aspects of traditional African medicine. Carica papaya [80] is currently used in The Gambia as the major component of burns dressings, where the pulp of the papaya fruit is mashed and applied daily to full-thickness and infected burns. It appears to be effective in desloughing necrotic tissue, preventing burn wound infection, and providing a granulating wound suitable for the application of a split thickness skin graft. Possible mechanisms of action include the activity of proteolytic enzymes chymopapain and papain, as well as an antimicrobial activity.
11. CURRENT & FUTURE DEVELOPMENTS
There are a large number of diverse topical antimicrobial agents that have been approved, tested or proposed for the prevention and/or treatment of burn infections. The particular pathophysiology of burns does predispose them to infection with a variety of pathogens, and experience has shown that topical therapies produce better outcomes than traditional systemic antibiotics. Moreover the ever-increasing spread of multidrug resistance only serves to underline the importance of effective and safe topical therapies. The development of these topical antimicrobial approaches has been a fertile field for both small and large biotechnology companies as may be judged by the number of patents covered in this review. We believe that research in the area of topical antimicrobial agents will only continue to produce ever more effective hi-tech products for advanced societies as well as affordable and traditional preparations for the third world.
Fig. 8. Use of silver as a topical antimicrobial agent in burns.
Silver has multiple mechanisms of action as an antimicrobial agent including permeabilization of bacterial cell walls and generation of intracellular reactive oxygen species.
Acknowledgments
Research in the Hamblin laboratory is supported by the NIH (grant RO1AI050875), US Air Force MFEL program (contract FA9550-04-1-0079), Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), Congressionally Directed Medical Research Program (W81XWH-09-1-0514). T. Dai was supported by the Bullock-Wellman Postdoctoral Fellowship Award.
Footnotes
CONFLICT OF INTEREST
None
References
- 1.Sharma BR, Harish D, Singh VP, Bangar S. Septicemia as a cause of death in burns: an autopsy study. Burns. 2006 Aug;32(5):545–9. doi: 10.1016/j.burns.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Monafo WW. Then and now: 50 years of burn treatment. Burns. 1992;18(Suppl 2):S7–10. doi: 10.1016/0305-4179(92)90062-y. [DOI] [PubMed] [Google Scholar]
- 3.Malic CC, Karoo RO, Austin O, Phipps A. Resuscitation burn card--a useful tool for burn injury assessment. Burns. 2007;33(2):195–9. doi: 10.1016/j.burns.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12(10):1127–35. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robins EV. Burn shock. Crit Care Nurs Clin North Am. 1990;2(2):299–307. [PubMed] [Google Scholar]
- 6.Macintire DK, Bellhorn TL. Bacterial translocation: Clinical implications and prevention. Vet Clin North Am Small Anim Pract. 2002;32(5):1165–78. doi: 10.1016/s0195-5616(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 7.Ben Saida N, Ferjeni A, Benhadjtaher N, Monastiri K, Boukadida J. Clonality of clinical methicillin-resistant Staphylococcus epidermidis isolates in a neonatal intensive care unit. Pathol Biol (Paris) 2006;54(6):337–42. doi: 10.1016/j.patbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Daoud Z, Moubareck C, Hakime N, Doucet-Populaire F. Extended spectrum beta-lactamase producing Enterobacteriaceae in Lebanese ICU patients: Epidemiology and patterns of resistance. J Gen Appl Microbiol. 2006;52(3):169–78. doi: 10.2323/jgam.52.169. [DOI] [PubMed] [Google Scholar]
- 9.Migliavacca R, Migliavacca A, Nucleo E, Ciaponi A, Spalla M, De Luca C, et al. Molecular epidemiology of ESbetaL producing P. mirabilis strains from a long-term care and rehabilitation facility in Italy. New Microbiol. 2007;30(3):362–6. [PubMed] [Google Scholar]
- 10.Joseph-Horne T, Hollomon DW. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149(2):141–9. doi: 10.1111/j.1574-6968.1997.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 11.Perlin DS. Antifungal drug resistance: do molecular methods provide a way forward? Curr Opin Infect Dis. 2009;22(6):568–73. doi: 10.1097/QCO.0b013e3283321ce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YJ, Yu JJ, Zhang YN, Zhang X, Cheng CJ, Wang JX, et al. Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum. Mol Plant Microbe Interact. 2009;22(9):1143–50. doi: 10.1094/MPMI-22-9-1143. [DOI] [PubMed] [Google Scholar]
- 13.Avenot HF, Sellam A, Karaoglanidis G, Michailides TJ. Characterization of mutations in the iron-sulphur subunit of succinate dehydrogenase correlating with Boscalid resistance in Alternaria alternata from California pistachio. Phytopathology. 2008;98(6):736–42. doi: 10.1094/PHYTO-98-6-0736. [DOI] [PubMed] [Google Scholar]
- 14.Morera-Lopez Y, Torres-Rodriguez JM, Jimenez-Cabello T. In vitro susceptibility of fungal and yeast clinical isolates to itraconazole and voriconazole. Rev Iberoam Micol. 2005;22(2):105–9. doi: 10.1016/s1130-1406(05)70018-8. [DOI] [PubMed] [Google Scholar]
- 15.Khan ZU, Ahmad S, Brazda A, Chandy R. Mucor circinelloides as a cause of invasive maxillofacial zygomycosis: an emerging dimorphic pathogen with reduced susceptibility to posaconazole. J Clin Microbiol. 2009 Apr;47(4):1244–8. doi: 10.1128/JCM.02030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006 Apr;19(2):403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhalgh DG, Saffle JR, Holmes JHt, Gamelli RL, Palmieri TL, Horton JW, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28(6):776–90. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 18.Sharma BR. Infection in patients with severe burns: causes and prevention thereof. Infect Dis Clin North Am. 2007;21(3):745–59. ix. doi: 10.1016/j.idc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Neely AN, Brown RL, Clendening CE, Orloff MM, Gardner J, Greenhalgh DG. Proteolytic activity in human burn wounds. Wound Repair Regen. 1997;5(4):302–9. doi: 10.1046/j.1524-475X.1997.50404.x. [DOI] [PubMed] [Google Scholar]
- 20.Samonte VA, Goto M, Ravindranath TM, Fazal N, Holloway VM, Goyal A, et al. Exacerbation of intestinal permeability in rats after a two-hit injury: Burn and Enterococcus faecalis infection. Crit Care Med. 2004;32(11):2267–73. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 21.Poole MD. Are we facing the end of the antibiotic era? Ear Nose Throat J. 1993;72(6):433. [PubMed] [Google Scholar]
- 22.Davies J. Where have All the Antibiotics Gone? Can J Infect Dis Med Microbiol. 2006;17(5):287–90. doi: 10.1155/2006/707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections-State of the art. Photodiagnosis Photodyn Ther. 2009;6(3–4):170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis. 2009;49(10):1541–9. doi: 10.1086/644732. [DOI] [PubMed] [Google Scholar]
- 25.Pawelchak JM, Freeman FM. US4538603. Dressings, granules, and their use in treating wounds. 1985
- 26.Amani H, Dougherty WR, Blome-Eberwein S. Use of Transcyte and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns. 2006;32(7):828–32. doi: 10.1016/j.burns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Weiss J, Herman O, Rosenberg L, Shafir R. Use of meshed omiderm. Burns Incl Therm Inj. 1988;14(2):161. doi: 10.1016/0305-4179(88)90228-8. [DOI] [PubMed] [Google Scholar]
- 28.Hierlemann H, Planck H. EP1181941. Absorbable medical coating material and method for its manufacture. 2006
- 29.Murray DG. US4659572. Burn wound dressing material. 1987
- 30.Kiene S. Differential therapeutic indications for the useof Xenoderm and SYSpur-derm in burns and other skin defects. Zentralbl Chir. 1981;106(18):1201–3. [PubMed] [Google Scholar]
- 31.Woodruff EA. US4820302. Bio compatible and blood compatible materials and methods. 1989
- 32.Griffey ES, Livesey SA, Schiff CM, Boerboom LE. US7358284. Particulate acellular tissue matrix. 2008
- 33.Heggers JP, Hawkins H, Edgar P, Villarreal C, Herndon DN. Treatment of infections in burns. In: Herndon DN, editor. Total burn care. 3. London, England: Elsevier Health Sci; 2002. pp. 120–69. [Google Scholar]
- 34.Johnson BA, Anker H, Meleney FL. Bacitracin: A New Antibiotic Produced by a Member of the B. subtilis Group. Science. 1945;102(2650):376–7. doi: 10.1126/science.102.2650.376. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri TL, Greenhalgh DG. Topical treatment of pediatric patients with burns: a practical guide. Am J Clin Dermatol. 2002;3(8):529–34. doi: 10.2165/00128071-200203080-00003. [DOI] [PubMed] [Google Scholar]
- 36.Sinha R, Agarwal RK, Agarwal M. Povidone iodine plus neosporin in superficial burns--a continuing study. Burns. 1997;23(7–8):626–8. doi: 10.1016/s0305-4179(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 37.Brown MR, Wood SM. Relation between cation and lipid content of cell walls of Pseudomonas aeruginosa, Proteus vulgaris and Klebsiella aerogenes and their sensitivity to polymyxin B and other antibacterial agents. J Pharm Pharmacol. 1972;24(3):215–8. doi: 10.1111/j.2042-7158.1972.tb08967.x. [DOI] [PubMed] [Google Scholar]
- 38.Munster AM. Treatment of invasive Enterobacter cloacae burn wound sepsis with topical nitrofurazone. J Trauma. 1984;24(6):524. doi: 10.1097/00005373-198406000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Moyer CA, Brentano L, Gravens DL, Margraf HW, Monafo WW., Jr Treatment of large human burns with 0.5 per cent silver nitrate solution. Arch Surg. 1965;90:812–67. doi: 10.1001/archsurg.1965.01320120014002. [DOI] [PubMed] [Google Scholar]
- 40.Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26(2):131–8. doi: 10.1016/s0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen B, Price P, Andersen KE, Gottrup F, Bech-Thomsen N, Scanlon E, et al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: A randomised, controlled trial. Int Wound J. 2005;2(1):64–73. doi: 10.1111/j.1742-4801.2005.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monafo WW, Tandon SN, Ayvazian VH, Tuchschmidt J, Skinner AM, Deitz F. Cerium nitrate: A new topical antiseptic for extensive burns. Surgery. 1976;80(4):465–73. [PubMed] [Google Scholar]
- 43.Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1(4):441–9. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnea Y, Weiss J, Gur E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver-releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care. 2005;14(9):411–9. doi: 10.12968/jowc.2005.14.9.26835. [DOI] [PubMed] [Google Scholar]
- 46.Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns. 1989;15(5):339–42. doi: 10.1016/0305-4179(89)90015-6. [DOI] [PubMed] [Google Scholar]
- 47.Knutson RA. US4401651. Wound-healing compositions containing povidone-iodine. 1983
- 48.Johnson A. A combative healer with no ill-effect. Iodosorb in the treatment of infected wounds. Prof Nurse. 1991;7(1):60, 2, 4. [PubMed] [Google Scholar]
- 49.Reimer K, Fleischer W, Brogmann B, Schreier H, Burkhard P, Lanzendorfer A, et al. Povidone-iodine liposomes-an overview. Dermatology. 1997;195 (Suppl 2):93–9. doi: 10.1159/000246039. [DOI] [PubMed] [Google Scholar]
- 50.Siegel P, Bhatt B. US20070292360. Process and composition for oral hygiene. 2007
- 51.Wong TW, Wang YY, Sheu HM, Chuang YC. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49(3):895–902. doi: 10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53(9):3929–34. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love W, Brundish D, Rhys-Williams W, Feng XD, Pugin B. US7244841. Porphyrin derivatives and their use in photodynamic therapy. 2007
- 54.Hamblin MR, Wharton T, Gali H. WO2008136906. Photosensitizers for targeted photodynamic therapy. 2008
- 55.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4(7):503–9. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, et al. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol. 1997;19(4):307–14. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 57.Roncucci G, Chiti G, Dei D, Cocchi A, Fantetti L, Mascheroni S. US20090131392. Phthalocyanine derivatives, process for their preparation, pharmaceutical compositions containing them and their use. 2009
- 58.Hamblin MR, Foley JW. US20080015189. Antimicrobial photoinactivation using chalcogen analogs of benzo(A)phenoxazinium dyes. 2008
- 59.Ribeiro MP, Espiga A, Silva D, Baptista P, Henriques J, Ferreira C, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17(6):817–24. doi: 10.1111/j.1524-475X.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 60.Sezer AD, Cevher E, Hatipoglu F, Ogurtan Z, Bas AL, Akbuga J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol Pharm Bull. 2008;31(12):2326–33. doi: 10.1248/bpb.31.2326. [DOI] [PubMed] [Google Scholar]
- 61.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53(2):393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66(2):191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 63.Chalekson CP, Neumeister MW, Jaynes J. Improvement in burn wound infection and survival with antimicrobial peptide D2A21 (Demegel) Plast Reconstr Surg. 2002;109(4):1338–43. doi: 10.1097/00006534-200204010-00020. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12(20):1494–502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 65.Ren HT, Han CM, Zhang R, Xu ZJ, Meng ZQ, Weng HB, et al. The antibacterial effect of cecropin B on Pseudomonas aeruginosa infection of wounds in mice. Zhonghua Shao Shang Za Zhi. 2006;22(6):445–7. [PubMed] [Google Scholar]
- 66.Steinstraesser L, Koehler T, Jacobsen F, Daigeler A, Goertz O, Langer S, et al. Host defense peptides in wound healing. Mol Med. 2008;14(7–8):528–37. doi: 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epand RM, Epand RF, Savage PB. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 2008;21(6):307–11. doi: 10.1358/dnp.2008.21.6.1246829. [DOI] [PubMed] [Google Scholar]
- 68.McManus AT, Denton CL, Mason AD., Jr Topical chlorhexidine diphosphanilate (WP-973) in burn wound sepsis. Arch Surg. 1984;119(2):206–11. doi: 10.1001/archsurg.1984.01390140064011. [DOI] [PubMed] [Google Scholar]
- 69.Miller LM, Loder JS, Hansbrough JF, Peterson HD, Monafo WW, Jordan MH. Patient tolerance study of topical chlorhexidine diphosphanilate: a new topical agent for burns. Burns. 1990;16(3):217–20. doi: 10.1016/0305-4179(90)90044-w. [DOI] [PubMed] [Google Scholar]
- 70.Selkon JB. US7276255. Wound and ulcer treatment with super-oxidized water. 2007
- 71.Hamouda T, Hayes MM, Cao Z, Tonda R, Johnson K, Wright DC, et al. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J Infect Dis. 1999;180(6):1939–49. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- 72.Modak SM, Fox CL., Jr Sulfadiazine silver-resistant Pseudomonas in burns. New topical agents. Arch Surg. 1981;116(7):854–7. doi: 10.1001/archsurg.1981.01380190006002. [DOI] [PubMed] [Google Scholar]
- 73.Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol. 2001;90(4):648–52. doi: 10.1046/j.1365-2672.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- 74.McVay CS, Velasquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51(6):1934–8. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ipaktchi K, Mattar A, Niederbichler AD, Hoesel LM, Vollmannshauser S, Hemmila MR, et al. Topical p38 MAPK inhibition reduces bacterial growth in an in vivo burn wound model. Surgery. 2007;142(1):86–93. doi: 10.1016/j.surg.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peral MC, Martinez MA, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009;6(1):73–81. doi: 10.1111/j.1742-481X.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: A prospective randomized study. Burns. 1996;22(6):491–3. doi: 10.1016/0305-4179(96)00007-1. [DOI] [PubMed] [Google Scholar]
- 78.Edwards-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30(8):772–7. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Zhang HQ, Yip TP, Hui I, Lai V, Wong A. Efficacy of moist exposed burn ointment on burns. J Burn Care Rehabil. 2005;26(3):247–51. [PubMed] [Google Scholar]
- 80.Starley IF, Mohammed P, Schneider G, Bickler SW. The treatment of paediatric burns using topical papaya. Burns. 1999;25(7):636–9. doi: 10.1016/s0305-4179(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 81.Stefanides MM, Sr, Copeland CE, Kominos SD, Yee RB. In vitro penetration of topical antiseptics through eschar of burn patients. Ann Surg. 1976;183(4):358–64. doi: 10.1097/00000658-197604000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moghimi HR, Makhmalzadeh BS, Manafi A. Enhancement effect of terpenes on silver sulphadiazine permeation through third-degree burn eschar. Burns. 2009;35(8):1165–70. doi: 10.1016/j.burns.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Manafi A, Hashemlou A, Momeni P, Moghimi HR. Enhancing drugs absorption through third-degree burn wound eschar. Burns. 2008;34(5):698–702. doi: 10.1016/j.burns.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 84.Lineaweaver W, Howard R, Soucy D, McMorris S, Freeman J, Crain C, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120(3):267–70. doi: 10.1001/archsurg.1985.01390270007001. [DOI] [PubMed] [Google Scholar]
- 85.Damour O, Hua SZ, Lasne F, Villain M, Rousselle P, Collombel C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns. 1992;18(6):479–85. doi: 10.1016/0305-4179(92)90180-3. [DOI] [PubMed] [Google Scholar]
- 86.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118(3):407–13. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Supp AP, Neely AN, Supp DM, Warden GD, Boyce ST. Evaluation of cytotoxicity and antimicrobial activity of Acticoat Burn Dressing for management of microbial contamination in cultured skin substitutes grafted to athymic mice. J Burn Care Rehabil. 2005;26(3):238–46. [PubMed] [Google Scholar]
- 88.Jacobsen F, Mohammadi-Tabrisi A, Hirsch T, Mittler D, Mygind PH, Sonksen CP, et al. Antimicrobial activity of the recombinant designer host defence peptide P-novispirin G10 in infected full-thickness wounds of porcine skin. J Antimicrob Chemother. 2007;59(3):493–8. doi: 10.1093/jac/dkl513. [DOI] [PubMed] [Google Scholar]
- 89.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42(10):2595–601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8(6):1049–51. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Jones WG, 2nd, Minei JP, Barber AE, Rayburn JL, Fahey TJ, 3rd, Shires GT, et al. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg. 1990;211(4):399–405. doi: 10.1097/00000658-199004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, et al. Protegrin-1 enhances bacterial killing in thermally injured skin. Crit Care Med. 2001;29(7):1431–7. doi: 10.1097/00003246-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 93.Ulkur E, Oncul O, Karagoz H, Celikoz B, Cavuslu S. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and silver sulfadiazine 1% (Silverdin) for topical antibacterial effect in Pseudomonas aeruginosa-contaminated, full-skin thickness burn wounds in rats. J Burn Care Rehabil. 2005;26(5):430–3. doi: 10.1097/01.bcr.0000176879.27535.09. [DOI] [PubMed] [Google Scholar]
- 94.Stieritz DD, Holder IA. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: Description of a burned mouse model. J Infect Dis. 1975;131(6):688–91. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 95.Stevens EJ, Ryan CM, Friedberg JS, Barnhill RL, Yarmush ML, Tompkins RG. A quantitative model of invasive Pseudomonas infection in burn injury. J Burn Care Rehabil. 1994;15(3):232–5. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Bahar T, Bilezikci B, Maral T, Borman H. A modified partial-thickness burn model in rats. Burns. 2007;33:S52–S3. [Google Scholar]
- 97.Suzuki T, Hirayama T, Aihara K, Hirohata Y. Experimental studies of moderate temperature burns. Burns. 1991;17:443–51. doi: 10.1016/0305-4179(91)90069-s. [DOI] [PubMed] [Google Scholar]
- 98.Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA. Photonic detection of bacterial pathogens in living hosts. Mol Microbiol. 1995;18(4):593–603. doi: 10.1111/j.1365-2958.1995.mmi_18040593.x. [DOI] [PubMed] [Google Scholar]
- 99.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. JPhotochemPhotobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49(1):380–7. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33(8):946–57. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 102.Queen D, Evans JH, Gaylor JD, Courtney JM, Reid WH. Burn wound dressings--a review. Burns Incl Therm Inj. 1987;13(3):218–28. doi: 10.1016/0305-4179(87)90170-7. [DOI] [PubMed] [Google Scholar]
- 103.Martin FT, O’Sullivan JB, Regan PJ, McCann J, Kelly JL. Hydrocolloid dressing in pediatric burns may decrease operative intervention rates. J Pediatr Surg. 2010;45(3):600–5. doi: 10.1016/j.jpedsurg.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 104.Eldad A, Tuchman I. The use of Omiderm as an interface for skin grafting. Burns. 1991;17(2):155–8. doi: 10.1016/0305-4179(91)90141-3. [DOI] [PubMed] [Google Scholar]
- 105.Schwarze H, Kuntscher M, Uhlig C, Hierlemann H, Prantl L, Ottomann C, et al. Suprathel, a new skin substitute, in the management of partial-thickness burn wounds: Results of a clinical study. Ann Plast Surg. 2008;60(2):181–5. doi: 10.1097/SAP.0b013e318056bbf6. [DOI] [PubMed] [Google Scholar]
- 106.Mahnke PF, Rose E, Riedeberger J. A temporary synthetic skin subsitute: SYSpur-derm. Comparative histopathological examination. Zentralbl Chir. 1980;105(3):145–53. [PubMed] [Google Scholar]
- 107.Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105(1):62–5. doi: 10.1097/00006534-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Hassan Z, Shah M. Punctate scarring from use of porous Biobrane. Burns. 2006;32(2):258–60. doi: 10.1016/j.burns.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 109.Naughton GK, Naughton BA. US4963489. Three-dimensional cell and tissue culture system. 1990
- 110.Hosseini SN, Karimian A, Mousavinasab SN, Rahmanpour H, Yamini M, Zahmatkesh SH. Xenoderm Versus 1% Silver Sulfadiazine in Partial-thickness Burns. Asian J Surg. 2009;32(4):234–9. doi: 10.1016/S1015-9584(09)60400-0. [DOI] [PubMed] [Google Scholar]
- 111.Bell E. US4485096. Tissue-equivalent and method for preparation thereof. 1984
- 112.Allen-Hoffmann BL. US6964869. Method and composition for skin grafts. 2005
- 113.Ceres RA, Brown DJ, Lesnoy DC. US6500464. Bilayered collagen construct. 2002
- 114.Murphy KD, Lee JO, Herndon DN. Current pharmacotherapy for the treatment of severe burns. Expert Opin Pharmacother. 2003;4(3):369–84. doi: 10.1517/14656566.4.3.369. [DOI] [PubMed] [Google Scholar]
- 115.Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns. 2004;30(7):660–4. doi: 10.1016/j.burns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 116.Elliott TS, Lambert PA. Antibacterial resistance in the intensive care unit: mechanisms and management. Br Med Bull. 1999;55(1):259–76. doi: 10.1258/0007142991902240. [DOI] [PubMed] [Google Scholar]
- 117.Mcmanus A. US200402. Anti microbial mafenide-phosphanilate compound, pharmaceutical compositions and method of use therefor. 1993
- 118.Falcone PA, Harrison HN, Sowemimo GO, Reading GP. Mafenide acetate concentrations and bacteriostasis in experimental burn wounds treated with a three-layered laminated mafenide-saline dressing. Ann Plast Surg. 1980;5(4):266–9. doi: 10.1097/00000637-198010000-00003. [DOI] [PubMed] [Google Scholar]
- 119.Heggers JP, Robson MC, Herndon DN, Desai MH. The efficacy of nystatin combined with topical microbial agents in the treatment of burn wound sepsis. J Burn Care Rehabil. 1989;10(6):508–11. doi: 10.1097/00004630-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Monafo WW, West MA. Current treatment recommendations for topical burn therapy. Drugs. 1990p;40(3):364–73. doi: 10.2165/00003495-199040030-00004. [DOI] [PubMed] [Google Scholar]
- 121.Alvarez OM. US5061689. Zinc bacitracin containing wound dressing patent. 1991
- 122.Rode H, de Wet PM, Millar AJ, Cywes S. Bactericidal efficacy of mupirocin in multi-antibiotic resistant Staphylococcus aureus burn wound infection. J Antimicrob Chemother. 1988;21(5):589–95. doi: 10.1093/jac/21.5.589. [DOI] [PubMed] [Google Scholar]
- 123.Strock LL, Lee MM, Rutan RL, Desai MH, Robson MC, Herndon DN, et al. Topical bactroban (mupirocin): Efficacy in treating burn wounds infected with methicillin-resistant Staphylococci. J Burn Care Rehabil. 1990;11(5):454–9. [PubMed] [Google Scholar]
- 124.Drogemoller ML, Linley SL. US6214866. Composition comprising mupirocin and chlorhexidine. 2001
- 125.Embil JM, McLeod JA, Al-Barrak AM, Thompson GM, Aoki FY, Witwicki EJ, et al. An outbreak of methicillin resistant Staphylococcus aureus on a burn unit: Potential role of contaminated hydrotherapy equipment. Burns. 2001;27(7):681–8. doi: 10.1016/s0305-4179(01)00045-6. [DOI] [PubMed] [Google Scholar]
- 126.Meier PA, Carter CD, Wallace SE, Hollis RJ, Pfaller MA, Herwaldt LA. A prolonged outbreak of methicillin-resistant Staphylococcus aureus in the burn unit of a tertiary medical center. Infect Control Hosp Epidemiol. 1996;17(12):798–802. doi: 10.1086/647239. [DOI] [PubMed] [Google Scholar]
- 127.Hansbrough JF, Zapata-Sirvent RL, Cooper ML. Effects of topical antimicrobial agents on the human neutrophil respiratory burst. Arch Surg. 1991;126(5):603–8. doi: 10.1001/archsurg.1991.01410290079016. [DOI] [PubMed] [Google Scholar]
- 128.Darouiche RO, Raad I. US5624704. Antimicrobial impregnated catheters and other medical implants. 1999
- 129.Guay DR. An update on the role of nitrofurans in the management of urinary tract infections. Drugs. 2001;61(3):353–64. doi: 10.2165/00003495-200161030-00004. [DOI] [PubMed] [Google Scholar]
- 130.Mousa HA. Fungal infection of burn wounds in patients with open and occlusive treatment methods. East Mediterr Health J. 1999;5(2):333–6. [PubMed] [Google Scholar]
- 131.Lopez-Berestein G, Mehta R, Hopfer RL, Juliano RL. US4812312. Liposome-incorporated nystatin. 1989
- 132.Kwon GS, Yoo BK. US6413537. Nystatin formulation having reduced toxicity. 2002
- 133.Yapijakis C. Hippocrates of Kos, the father of clinical medicine, and Asclepiades of Bithynia, the father of molecular medicine. Review In vivo. 2009;23(4):507–14. [PubMed] [Google Scholar]
- 134.Alexander JW. History of the medical use of silver. Surg Infect (Larchmt) 2009;10(3):289–92. doi: 10.1089/sur.2008.9941. [DOI] [PubMed] [Google Scholar]
- 135.Borsuk DE, Gallant M, Richard D, Williams HB. Silver-coated nylon dressings for pediatric burn victims. Can J Plast Surg. 2007;15(1):29–31. doi: 10.1177/229255030701500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: Review of the literature. Burns. 2007;33(2):139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 137.Warriner R, Burrell R. Infection and the chronic wound: A focus on silver. Adv Skin Wound Care. 2005;18 (Suppl 1):2–12. doi: 10.1097/00129334-200510001-00001. [DOI] [PubMed] [Google Scholar]
- 138.Poon VK, Burd A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns. 2004;30(2):140–7. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 139.Kershaw D, DeBoorder B, Law SJ. US20100015208. Antibacterial wound dressing. 2010
- 140.Mason ADJ, Johnson AAJ, Ritchey CR. US4391799. Protective gel composition for treating white phosphorus burn wounds. 1983
- 141.Schmolka IR. US4376764. Silver ion gel compositions. 1983
- 142.Neuwirth RS. US2008895424. Intrauterine chemical cauterizing method and composition. 2008
- 143.Zanoni D, Isenberg SJ, Apt L. A comparison of silver nitrate with erythromycin for prophylaxis against ophthalmia neonatorum. Clin Pediatr (Phila) 1992;31(5):295–8. doi: 10.1177/000992289203100506. [DOI] [PubMed] [Google Scholar]
- 144.Fox CL, Jr, Modak SM. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother. 1974;5(6):582–8. doi: 10.1128/aac.5.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cho Lee AR, Leem H, Lee J, Park KC. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26(22):4670–6. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 146.Rosenkranz HS, Coward JE, Wlodkowski TJ, Carr HS. Properties of silver sulfadiazine-resistant Enterobacter cloacae. Antimicrob Agents Chemother. 1974;5(2):199–201. doi: 10.1128/aac.5.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fox CLJ, Modak SM. US4535078. Antibacterial composition comprising silver sulfadiazine and sodium piperacillin. 1985
- 148.Modak SM, Fox CLJ, Fox P. CA1219209. Wound dressing comprising silver sulfadiazine incorporated in animal tissue. 1987
- 149.Fox CLJ. US3761590. Silver sulfadiazine used in the treatment of burns. 1973
- 150.Chen JR. US6551577. Topical spray for burn treatment and anti-infection. 2003
- 151.Garner JP, Heppell PS. Cerium nitrate in the management of burns. Burns. 2005;31(5):539–47. doi: 10.1016/j.burns.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 152.Monafo WW. US4088754. Water-soluble cerium (cerous) salts in burn therapy. 1978
- 153.Gravante G, Caruso R, Sorge R, Nicoli F, Gentile P, Cervelli V. Nanocrystalline silver: A systematic review of randomized trials conducted on burned patients and an evidence-based assessment of potential advantages over older silver formulations. Ann Plast Surg. 2009;63(2):201–5. doi: 10.1097/SAP.0b013e3181893825. [DOI] [PubMed] [Google Scholar]
- 154.Ma RH, Yu YH. US7462753. Nano-silver wound dressing. 2008
- 155.Holladay RJ, Christensen H, Moeller WD. US7135195. Treatment of humans with colloidal silver composition. 2006
- 156.Gillis SH. US20060083792. Therapeutic treatments using the direct application of antimicrobial metal compositions. 2006
- 157.Gillis SH. US6989156. Therapeutic treatments using the direct application of antimicrobial metal compositions. 2006
- 158.Bell A, Hart J. Evaluation of two absorbent silver dressings in a porcine partial-thickness excisional wound model. J Wound Care. 2007;16(10):445–8. 50–3. doi: 10.12968/jowc.2007.16.10.27911. [DOI] [PubMed] [Google Scholar]
- 159.Scherr GH. US20030021832. Silver alginate foam compositions. 2003
- 160.Kim YS, Kim JY. EP1993620. Methods for producing silver-bonded antimicrobial moist wound dressings and moist wound dressings produced by the methods. 2008
- 161.Ma RH, Yu YH. US7462753. Nano-silver wound dressing. 2008
- 162.Mcknight JJ, Guldalian JJ. US3800792. Laminated collagen film dressing. 1974
- 163.Lerat YJ, Poncelet OJ. US7323614. Dressing and antiseptic agent containing silver. 2008
- 164.Haberal M, Oner Z, Bayraktar U, Bilgin N. The use of silver nitrate-incorporated amniotic membrane as a temporary dressing. Burns Incl Therm Inj. 1987;13(2):159–63. doi: 10.1016/0305-4179(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 165.Singh R, Kumar D, Kumar P, Chacharkar MP. Development and evaluation of silver-impregnated amniotic membrane as an antimicrobial burn dressing. J Burn Care Res. 2008;29(1):64–72. doi: 10.1097/BCR.0b013e31815f5a0f. [DOI] [PubMed] [Google Scholar]
- 166.Fox CLJ, Modak SM, Fox P. US4446124. Wound dressing comprising silver sulfadiazine incorporated in animal tissue. 1984
- 167.Capelli C. US6923990. Stabilized silver-ion amine complex compositions and methods. 2005
- 168.Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J. 2008;5(2):361–8. doi: 10.1111/j.1742-481X.2007.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Durani P, Leaper D. Povidone-iodine: use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J. 2008;5(3):376–87. doi: 10.1111/j.1742-481X.2007.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zamora JL. Iodine toxicity. Ann Thorac Surg. 1986;41(4):462–3. doi: 10.1016/s0003-4975(10)62714-2. [DOI] [PubMed] [Google Scholar]
- 171.Capriotti JA, Liang B, Samson CM, Stein J, Weise M. WO2009097123. Device for in situ generation of povidone-iodine compositions. 2009
- 172.Beukelman CJ, van den Berg AJ, Hoekstra MJ, Uhl R, Reimer K, Mueller S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel) for wound healing in vitro. Burns. 2008;34(6):845–55. doi: 10.1016/j.burns.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 173.Muhlau S, Fleischer W. US2004234589. Dry liposomal PVP-iodine compositions. 2004
- 174.Ormiston MC, Seymour MT, Venn GE, Cohen RI, Fox JA. Controlled trial of Iodosorb in chronic venous ulcers. Br Med J (Clin Res Ed) 1985;291(6491):308–10. doi: 10.1136/bmj.291.6491.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kota S, Adibhatla KS, Venkaiah CN. WO2008117300. Improved process for the preparation of cadexomer iodine. 2008
- 176.Larsen N. US2006280809. Anti-infective iodine based compositions for otic and nasal use. 2006
- 177.Scholtz MT. WO2009088826. Liquid antiseptic compositions containing iodine and A sugar and/or sugar alcohol. 2009
- 178.Foret C, Hemling TC. US5916581. Iodine antimicrobial compositions containing nonionic surfactants and halogen anions. 1999
- 179.Hamblin MR, Mroz P. Advances in photodynamic therapy: basic, translational and clinical. Norwood, MA: Artech House; 2008. [Google Scholar]
- 180.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23(5A):3591–600. [PubMed] [Google Scholar]
- 181.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1(4):279–93. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? 2004;3(5):436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Phoenix DA, Harris F. Light activated compounds as antimicrobial agents - patently obvious? Recent Pat Antiinfect Drug Discov. 2006;1(2):181–99. doi: 10.2174/157489106777452656. [DOI] [PubMed] [Google Scholar]
- 184.Nifantiev NE, Gitter B, Yashunsky DV. US2007016951. Anti-microbial photodynamic therapy. 2007
- 185.Hasan T, Hamblin MR, Soukos NS. US7268155. Photosensitizer conjugates for pathogen targeting. 2007
- 186.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, et al. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49(6):941–51. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 187.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50(4):1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Raghavendra M, Koregol A, Bhola S. Photodynamic therapy: a targeted therapy in periodontics. Aust Dent J. 2009;54 (Suppl 1):S102–9. doi: 10.1111/j.1834-7819.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 189.Hamblin MR, Tegos GP. US20080166304. Inactivation of Microorganisms with multidrug resistance inhibitors and phenothiaziniums. 2008
- 190.Biel MA. US7229447. Photodynamic therapy utilizing a solution of photosensitizing compound and surfactant. 2007
- 191.Albrecht V, Gitter B. US20050049228. Antimicrobial photodynamic therapy compound and method of use. 2005
- 192.Albrecht V, Fahr A, Scheglmann D, Gräfe S, Neuberger W. US7354599. Liposomal formulations of hydrophobic photosensitizer for photodynamic therapy. 2008
- 193.Van Der Haas HN, Lugtenburg J, Schuitmaker J, De Jong RL. US20090215851. Method of preparing a porphyrin derivative, a porphyrin derivative, use of said porphyrin derivative and a pharmaceutical composition containing said porphyrin derivative. 2009
- 194.Smijs GM, Schuitmaker JJ. US20060258635. Use of a porphyrin compound for the treatment of skin fungi. 2006
- 195.Love W, Brundish D, Rhys-Williams W, Feng XD, Pugin B. US7244841. Porphyrin derivatives and their use in photodynamic therapy. 2007
- 196.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4(6):1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 197.Sparkes BG, Murray DG. US4572906. Chitosan based wound dressing materials. 1986
- 198.Yura H, Saito Y, Ishihara M, Ono K, Saeki S. US7125968. Functional chitosan derivative. 2006
- 199.Baker S, Wiesmann WP. US20100056474. Methods and compositions for the prevention of and treatment of infections utilizing chitosan-derivative compounds. 2010
- 200.Scherr GH. US20070237811. Chitosan wound dressing. 2007
- 201.Muzzarelli R. US5378472. Methyl pyrrolidinone chitosan, production process and uses thereof. 1995
- 202.Alsarra IA. Chitosan topical gel formulation in the management of burn wounds. Int J Biol Macromol. 2009;45(1):16–21. doi: 10.1016/j.ijbiomac.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 203.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330(1–2):138–45. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 204.Boucard N, Viton C, Agay D, Mari E, Roger T, Chancerelle Y, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28(24):3478–88. doi: 10.1016/j.biomaterials.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 205.Cardenas G, Anaya P, von Plessing C, Rojas C, Sepulveda J. Chitosan composite films. Biomedical applications. J Mater Sci Mater Med. 2008;19(6):2397–405. doi: 10.1007/s10856-007-3275-3. [DOI] [PubMed] [Google Scholar]
- 206.Sezer AD, Hatipoglu F, Cevher E, Ogurtan Z, Bas AL, Akbuga J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: preparation and in vitro/in vivo evaluation. AAPS Pharm Sci Tech. 2007;8(2) doi: 10.1208/pt0802039. Article 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Damour O, Gueugniaud PY, Berthin-Maghit M, Rousselle P, Berthod F, Sahuc F, et al. A dermal substrate made of collagen-GAG-chitosan for deep burn coverage: first clinical uses. Clin Mater. 1994;15(4):273–6. doi: 10.1016/0267-6605(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 208.Peh K, Khan T, Ch’ng H. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 2000;3(3):303–11. [PubMed] [Google Scholar]
- 209.Mor A. Multifunctional host defense peptides: Antiparasitic activities. FEBS J. 2009;276(22):6474–82. doi: 10.1111/j.1742-4658.2009.07358.x. [DOI] [PubMed] [Google Scholar]
- 210.Schittek B, Paulmann M, Senyurek I, Steffen H. The role of antimicrobial peptides in human skin and in skin infectious diseases. Infect Disord Drug Targets. 2008;8(3):135–43. doi: 10.2174/1871526510808030135. [DOI] [PubMed] [Google Scholar]
- 211.Selsted ME, Szklarek D, Lehrer RI. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984;45(1):150–4. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lehrer RI, Szklarek D, Ganz T, Selsted ME. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985;49(1):207–11. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lehrer RI, Daher K, Ganz T, Selsted ME. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54(2):467–72. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pazgier M, Prahl A, Hoover DM, Lubkowski J. Studies of the biological properties of human beta-defensin 1. J Biol Chem. 2007;282(3):1819–29. doi: 10.1074/jbc.M607210200. [DOI] [PubMed] [Google Scholar]
- 215.Fulton C, Anderson GM, Zasloff M, Bull R, Quinn AG. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350(9093):1750–1. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 216.Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: A novel beta-defensin from human plasma. FEBS Lett. 1995;368(2):331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 217.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396(2–3):319–22. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 218.Bhat S, Milner S. Antimicrobial peptides in burns and wounds. Curr Protein Pept Sci. 2007;8(5):506–20. doi: 10.2174/138920307782411428. [DOI] [PubMed] [Google Scholar]
- 219.Strom RM, Brondsema PJ. US7091185. Periodic antimicrobial peptides. 2006
- 220.Strom RM, Brondsema PJ. US20050187151. Periodic antimicrobial peptides. 2005
- 221.Schwab U, Gilligan P, Jaynes J, Henke D. In vitro activities of designed antimicrobial peptides against multidrug-resistant cystic fibrosis pathogens. Antimicrob Agents Chemother. 1999;43(6):1435–40. doi: 10.1128/aac.43.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chalekson CP, Neumeister MW, Jaynes J. Treatment of infected wounds with the antimicrobial peptide D2A21. J Trauma. 2003 Apr;54(4):770–4. doi: 10.1097/01.TA.0000047047.79701.6D. [DOI] [PubMed] [Google Scholar]
- 223.Hadnagy A, Beaulieu R, Balicki D. Histone tail modifications and noncanonical functions of histones: perspectives in cancer epigenetics. Mol Cancer Ther. 2008;7(4):740–8. doi: 10.1158/1535-7163.MCT-07-2284. [DOI] [PubMed] [Google Scholar]
- 224.Rose FR, Bailey K, Keyte JW, Chan WC, Greenwood D, Mahida YR. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun. 1998;66(7):3255–63. doi: 10.1128/iai.66.7.3255-3263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pohlmeyer K, Behnke B, Wick RZ, Mayer G. US20030078204. Recombinant production of human histone 1 subtypes and their use for therapeutic purposes. 2003
- 226.Lai JS, Lee JH, Callaway JE. US5166321. Cecropin polypetides with activity against Gram-positive and Gram-negative bacteria. 1992
- 227.Ghiselli R, Giacometti A, Cirioni O, Mocchegiani F, Orlando F, D’Amato G, et al. Cecropin B enhances betalactams activities in experimental rat models of gram-negative septic shock. Ann Surg. 2004;239(2):251–6. doi: 10.1097/01.sla.0000108673.25385.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Horwitz A, Lambert LHJ, Little RG. US5578572. Anti-gram-positive bacterial methods and materials. 1996
- 229.Savage PB, Li C. US6486148. Steroid derived antibiotics. 2002
- 230.Savage PB, Li C. US6767904. Steroid derived antibiotics. 2004
- 231.Savage PB, Li C. US7598234. Steroid derived antibiotics. 2009
- 232.Dang X, Zhang W, Wang Y, Zheng X, Wang JX, Wang L. CN101392020. Musca domestic pupae natural antimicrobial peptide products and preparation method and use thereof. 2009
- 233.Beuerman RW, Liu S, Li J, Zhou L, Verma CS, Tan D. WO2009131548. Multimeric forms of antimicrobial peptides. 2009
- 234.Liepke C, Baxmann S, Adermann K. US2009149391. Novel antimicrobial bolisin peptides. 2009
- 235.Oppenheim FG, Xu T, Roberts FD, Spacciapoli P, Friden PM. US5912230. Anti-fungal and anti-bacterial histatin-based peptides. 1999
- 236.Hara S. US5646014. Peptide, antibacterial agent, peptide gene, recombinant DNA and method for preparing the peptide. 1997
- 237.Djurup R, Flodgaard HJ, Norris K. WO2004016653. Bactericidal, anti-apoptotic, pro-inflammatory and anti-inflammatory peptides of heparin-binding protein (Hbp) Or human neutrophil elastase. 2004
- 238.Schmidtchen A, Malmsten M. KR0027213. Improved antimicrobial peptides. 2009
- 239.Krieger TJ, Taylor R, Erfle D, Fraser JR, West MH, Mcnichol PJ. US6503881. Compositions and methods for treating infections using cationic peptides alone or in combination with antibiotics. 2009
- 240.Drake D, Marek CL. US20050191247. Chlorhexidine compositions. 2005
- 241.Warner PLJ, Kornegay GB, Redl G. US4666896. Chlorhexidine salts and compositions of same. 1987
- 242.Friedman J. US5002769. Compositions for the sustained-release of chlorhexidine. 1991
- 243.Baker JRJ, Hamouda T, Shih A, Myc A. US7655252. Antimicrobial nanoemulsion compositions and methods. 2010
- 244.Schlag S, Nerz C, Birkenstock TA, Altenberend F, Gotz F. Inhibition of Staphylococcal biofilm formation by nitrite. J Bacteriol. 2007;189(21):7911–9. doi: 10.1128/JB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Summers WC. Bacteriophage therapy. Annu Rev Microbiol. 2001;55:437–51. doi: 10.1146/annurev.micro.55.1.437. [DOI] [PubMed] [Google Scholar]
- 246.Malik R, Chhibber S. Protection with bacteriophage KO1 against fatal Klebsiella pneumoniae-induced burn wound infection in mice. J Microbiol Immunol Infect. 2009;42(2):134–40. [PubMed] [Google Scholar]
- 247.Soothill J, Hawkins C, Harper D. US20070190033. Bacteriophage-containing therapeutic agents. 2007
- 248.Walsh SM, Pittaway MC. US7517852. Compositions and methods for treating bacteria. 2009
- 249.Wahdan HA. Causes of the antimicrobial activity of honey. Infection. 1998;26(1):26–31. doi: 10.1007/BF02768748. [DOI] [PubMed] [Google Scholar]
- 250.Subrahmanyam M. Topical application of honey in treatment of burns. Br J Surg. 1991;78(4):497–8. doi: 10.1002/bjs.1800780435. [DOI] [PubMed] [Google Scholar]
- 251.Mousa MA. US5980875. Honey preparations. 1999
- 252.Mousa MA. US6171604. Honey preparations. 2001
- 253.Van Den Berg AJ, Hoekstra M. US20090304780. Wound dressings incorporating honey. 2009
- 254.Caskey PR. US20040127826. Honey in wound dressings. 2004
- 255.Molan P. US20040054313. Honey based wound dressing. 2004
- 256.Holzner G. US6479456. Antimicrobial perfume compositions. 2002
- 257.Ang ES, Lee ST, Gan CS, See P, Chan YH, Ng LH, et al. The role of alternative therapy in the management of partial thickness burns of the face-experience with the use of moist exposed burn ointment (MEBO) compared with silver sulphadiazine. Ann Acad Med Singapore. 2000;29(1):7–10. [PubMed] [Google Scholar]
- 258.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can Microbiol. 1969;15(9):1067–76. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]