Full text
PDF
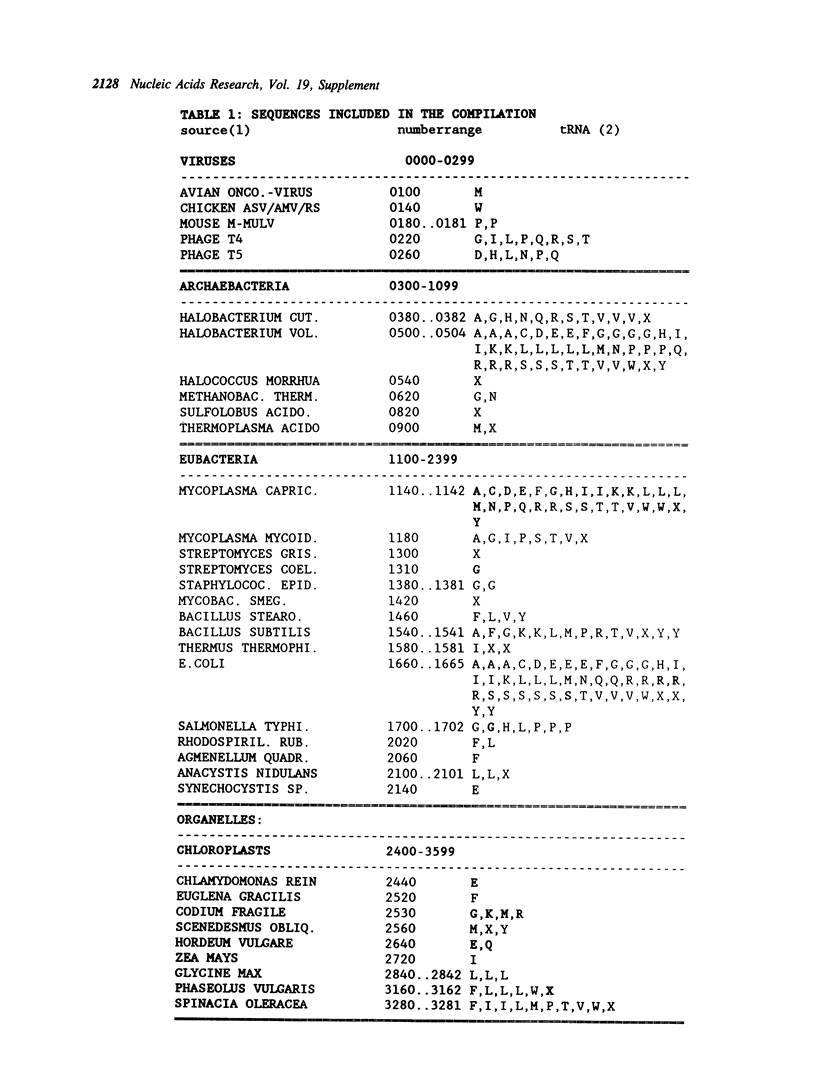
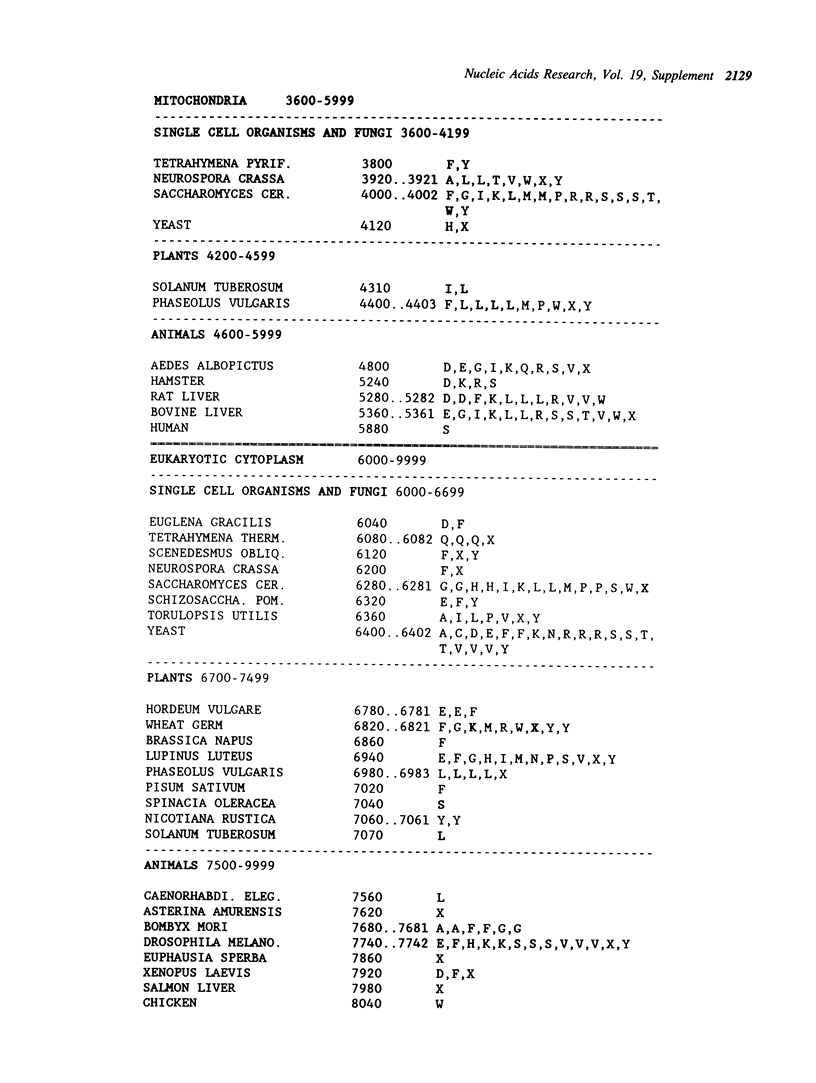
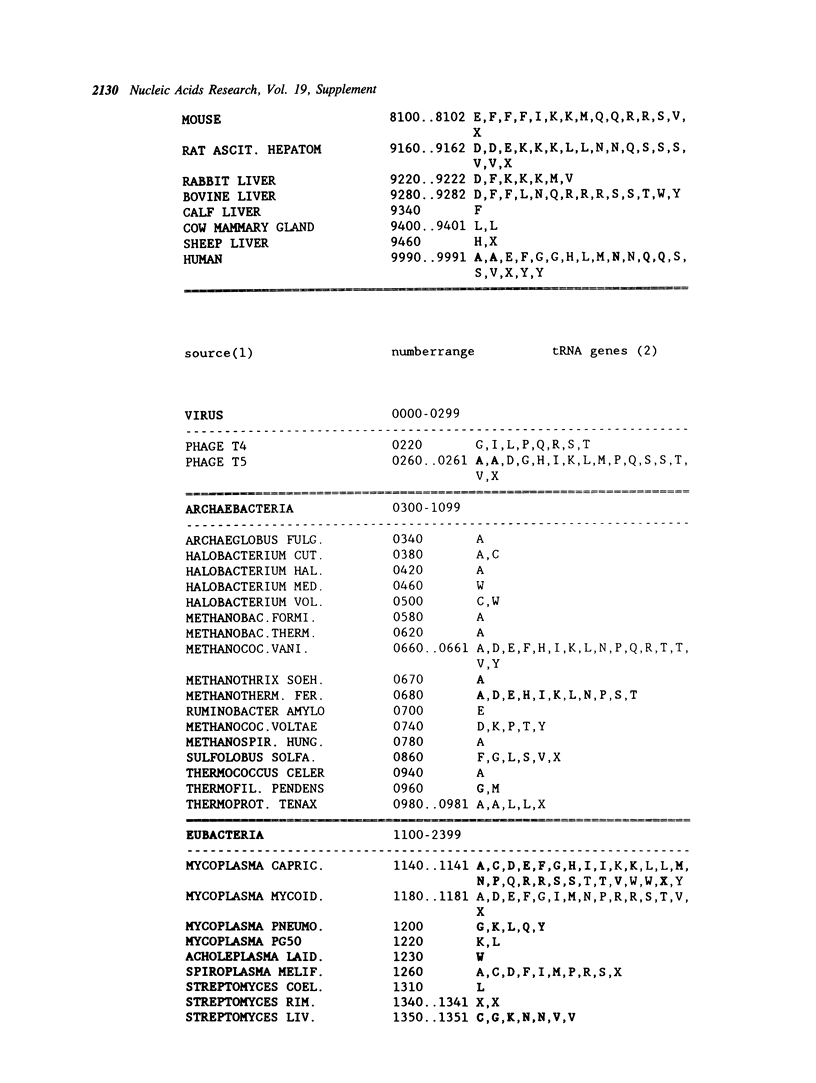
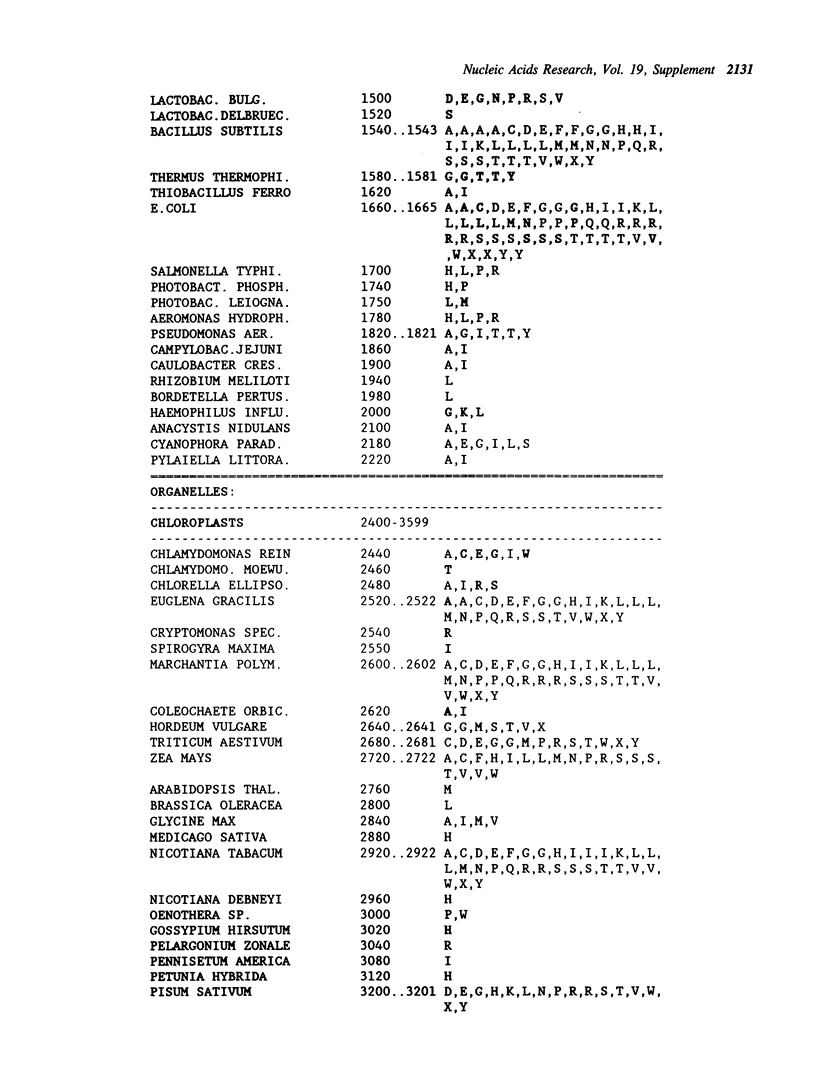
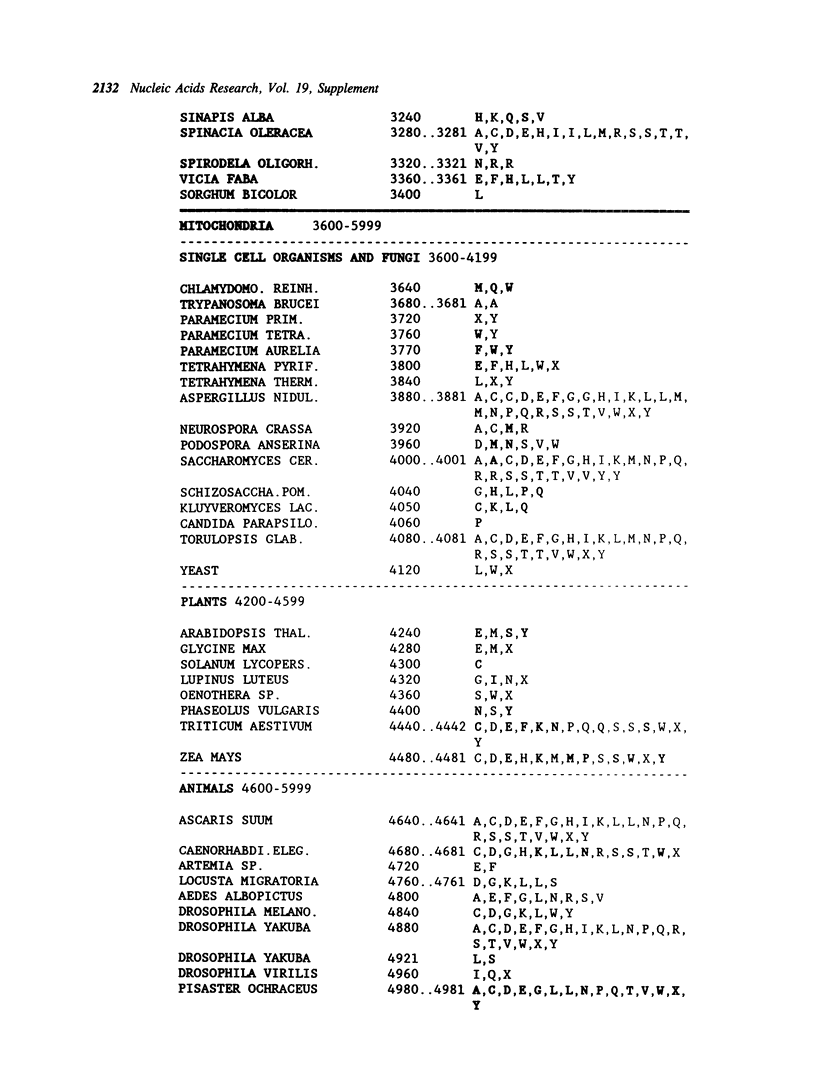
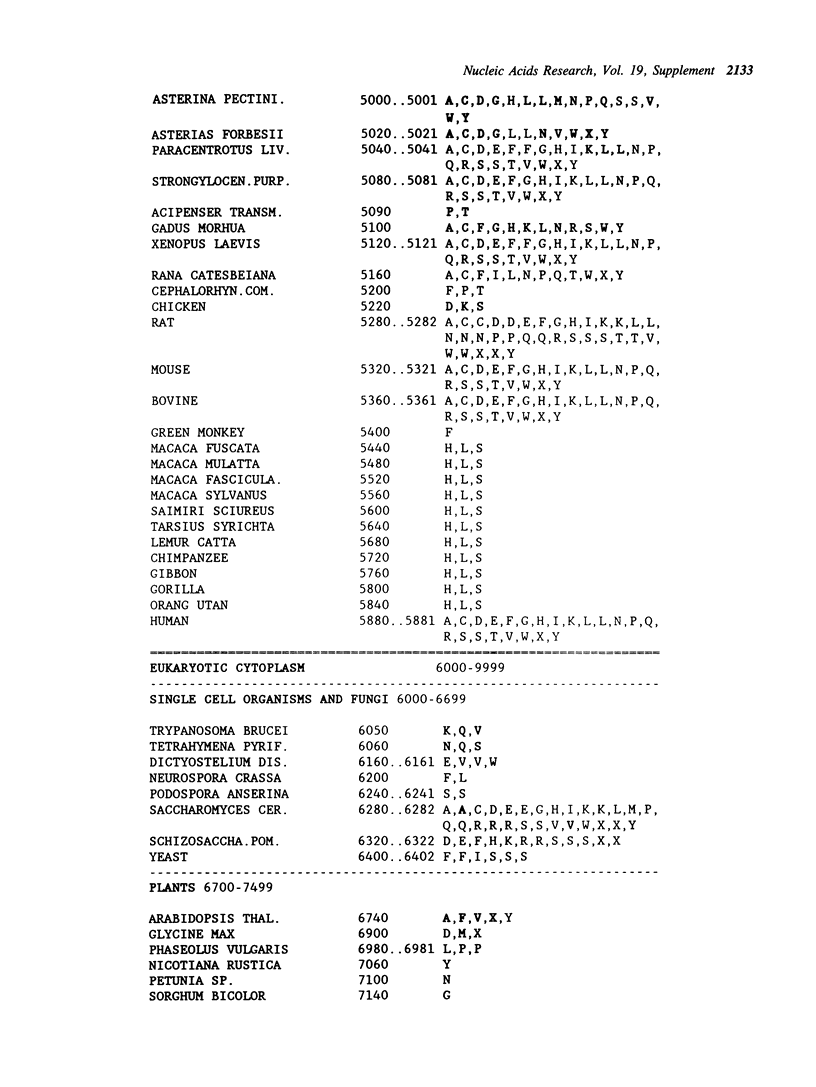
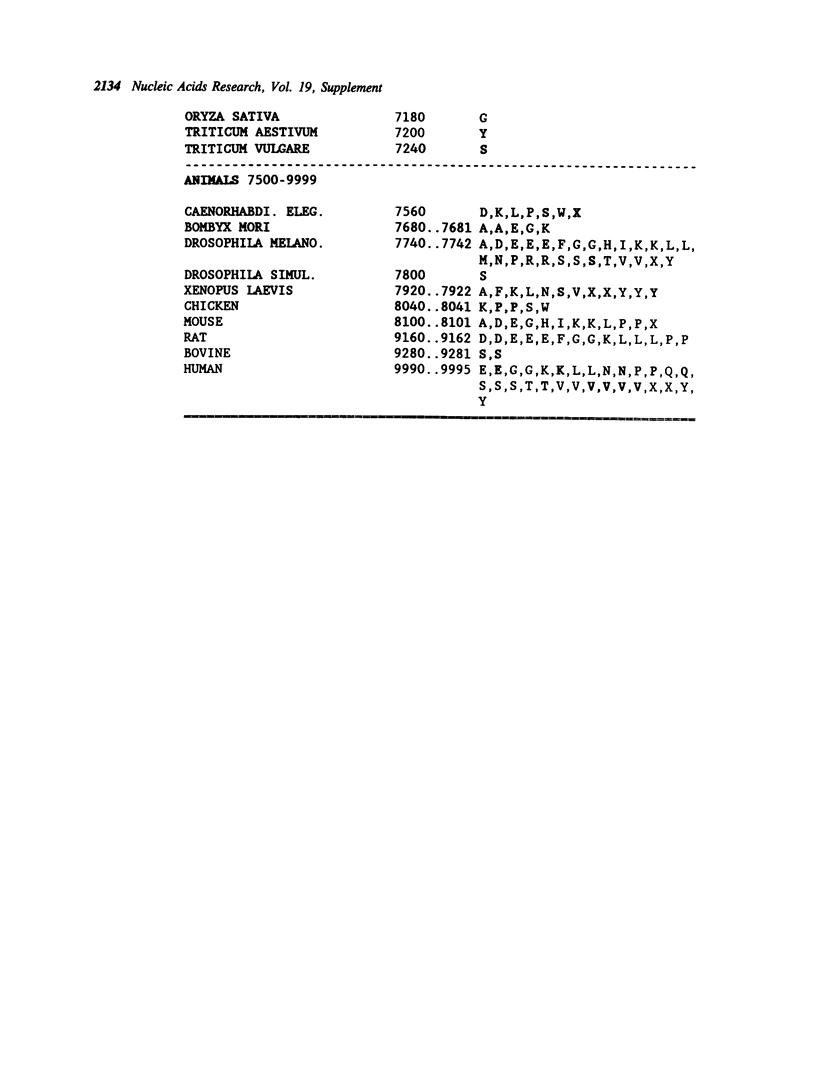





































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Woese C. R. The ribosomal gene spacer region in archaebacteria. Syst Appl Microbiol. 1988;10:211–214. doi: 10.1016/s0723-2020(88)80002-x. [DOI] [PubMed] [Google Scholar]
- Addison W. R., Astell C. R., Delaney A. D., Gillam I. C., Hayashi S., Miller R. C., Rajput B., Smith M., Taylor D. M., Tener G. M. The structures of genes hybridizing with tRNA4Val from Drosophila melanogaster. J Biol Chem. 1982 Jan 25;257(2):670–673. [PubMed] [Google Scholar]
- Addison W. R., Astell C. R., Delaney A. D., Gillam I. C., Hayashi S., Miller R. C., Rajput B., Smith M., Taylor D. M., Tener G. M. The structures of genes hybridizing with tRNA4Val from Drosophila melanogaster. J Biol Chem. 1982 Jan 25;257(2):670–673. [PubMed] [Google Scholar]
- Addison W. R., Gillam I. C., Hayashi S., Tener G. M. The nucleotide sequence of tRNAVal3a and tRNAVal3b from Drosophila melanogaster. Can J Biochem Cell Biol. 1985 Mar;63(3):176–182. doi: 10.1139/o85-025. [DOI] [PubMed] [Google Scholar]
- Agrawal H. P., Gupta R. C., Randerath K., Randerath E. The sequence of mitochondrial arginine tRNA (anticodon UCG) from a transplantable rat tumor, Morris hepatoma 5123D. FEBS Lett. 1981 Aug 3;130(2):287–290. doi: 10.1016/0014-5793(81)81141-6. [DOI] [PubMed] [Google Scholar]
- Agrawal H. P., Randerath K., Randerath E. Tumor mitochondrial transfer ribonucleic acids: the nucleotide sequence of Morris hepatoma 5123D mitochondrial tRNA GUC Asp. Nucleic Acids Res. 1981 Jun 11;9(11):2535–2541. doi: 10.1093/nar/9.11.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama K., Tanifuji S. Nucleotide sequence of a methionine initiator tRNA gene of Arabidopsis thaliana. Plant Mol Biol. 1989 Nov;13(5):599–600. doi: 10.1007/BF00027320. [DOI] [PubMed] [Google Scholar]
- Aldrich J., Cherney B. W., Merlin E., Christopherson L. The role of insertions/deletions in the evolution of the intergenic region between psbA and trnH in the chloroplast genome. Curr Genet. 1988 Aug;14(2):137–146. doi: 10.1007/BF00569337. [DOI] [PubMed] [Google Scholar]
- Aldrich J., Cherney B. W., Merlin E., Christopherson L. The role of insertions/deletions in the evolution of the intergenic region between psbA and trnH in the chloroplast genome. Curr Genet. 1988 Aug;14(2):137–146. doi: 10.1007/BF00569337. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Kubli E. The nucleotide sequence of histidine tRNA gamma of Drosophila melanogaster. Nucleic Acids Res. 1980 Aug 11;8(15):3259–3262. doi: 10.1093/nar/8.15.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzner-DeWeerd B., Hecker L. I., Barnett W. E., RajBhandary U. L. The nucleotide sequence of phenylalanine tRNA from the cytoplasm of Neurospora crassa. Nucleic Acids Res. 1980 Mar 11;8(5):1023–1032. doi: 10.1093/nar/8.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstutz H., Munz P., Heyer W. D., Leupoid U., Kohli J. Concerted evolution of tRNA genes: intergenic conversion among three unlinked serine tRNA genes in S. pombe. Cell. 1985 Apr;40(4):879–886. doi: 10.1016/0092-8674(85)90347-2. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Arcari P., Brownlee G. G. The nucleotide sequence of a small (3S) seryl-tRNA (anticodon GCU) from beef heart mitochondria. Nucleic Acids Res. 1980 Nov 25;8(22):5207–5212. doi: 10.1093/nar/8.22.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel'rod V. D., Kryukov V. M., Isaenko S. N., Bayev A. A. Nucleotide sequence in tRNA Val-2a from baker's yeast. FEBS Lett. 1974 Sep 1;45(1):333–336. doi: 10.1016/0014-5793(74)80874-4. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Eigel A., Vögtel D., Feldmann H. Nucleotide sequences of yeast genes for tRNA(2), tRNA(2) and tRNA(1): homology blocks occur in the vicinity of different tRNA genes. EMBO J. 1982;1(3):291–295. doi: 10.1002/j.1460-2075.1982.tb01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barciszewska M. Z., Keith G., Kubli E., Barciszewski J. The primary structure of wheat germ tRNAArg--the substrate for arginyl-tRNAArg:protein transferase. Biochimie. 1986 Mar;68(2):319–323. doi: 10.1016/s0300-9084(86)80029-3. [DOI] [PubMed] [Google Scholar]
- Barciszewska M., Barciszewski J., Kuchino Y., Nishimura S. The nucleotide sequences of two glycine tRNAs from Lupinus luteus seeds. Nucleic Acids Res. 1986 Dec 9;14(23):9525–9525. doi: 10.1093/nar/14.23.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barciszewska M., Dirheimer G., Keith G. The nucleotide sequence of methionine elongator tRNA from wheat germ. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1161–1168. doi: 10.1016/0006-291x(83)90684-8. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Sanger F. The sequence of phenylalanine tRNA from E. coli. FEBS Lett. 1969 Jun;3(4):275–278. doi: 10.1016/0014-5793(69)80157-2. [DOI] [PubMed] [Google Scholar]
- Bawnik N., Beckmann J. S., Sarid S., Daniel V. Isolation and nucleotide sequence of a plant tRNA gene: petunia asparagine tRNA. Nucleic Acids Res. 1983 Feb 25;11(4):1117–1122. doi: 10.1093/nar/11.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Sickinger H. D. The molecular basis for the differential translation of TMV RNA in tobacco protoplasts and wheat germ extracts. EMBO J. 1984 May;3(5):1091–1096. doi: 10.1002/j.1460-2075.1984.tb01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Bonitz S. G., Coruzzi G., Nobrega M., Tzagoloff A. Transfer RNA genes in the cap-oxil region of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5017–5030. doi: 10.1093/nar/8.21.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Bonitz S. G., Coruzzi G., Nobrega M., Tzagoloff A. Transfer RNA genes in the cap-oxil region of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5017–5030. doi: 10.1093/nar/8.21.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Pentella C., Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: isolation of mitochondrial transfer ribonucleic acid mutants and characterization of transfer ribonucleic acid genes of Saccharomyces cerevisiae. J Bacteriol. 1980 Mar;141(3):1086–1097. doi: 10.1128/jb.141.3.1086-1097.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Pentella C., Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: isolation of mitochondrial transfer ribonucleic acid mutants and characterization of transfer ribonucleic acid genes of Saccharomyces cerevisiae. J Bacteriol. 1980 Mar;141(3):1086–1097. doi: 10.1128/jb.141.3.1086-1097.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bird S., Duncker B., Garber P., Bonen L. Nucleotide sequence of the bean mitochondrial DNA region containing the tRNA(Asn) and tRNA(Tyr) genes. Nucleic Acids Res. 1989 Jun 12;17(11):4379–4379. [PMC free article] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Transfer RNA genes and the genetic code in Chlamydomonas reinhardtii mitochondria. Curr Genet. 1988 Dec;14(6):583–590. doi: 10.1007/BF00434084. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Transfer RNA genes and the genetic code in Chlamydomonas reinhardtii mitochondria. Curr Genet. 1988 Dec;14(6):583–590. doi: 10.1007/BF00434084. [DOI] [PubMed] [Google Scholar]
- Boesten B., Lenzen G., Danchin A., O'Gara F. Nucleotide sequence of a tRNA(leu)CAG gene from Rhizobium meliloti. Gene. 1987;55(1):153–156. doi: 10.1016/0378-1119(87)90259-9. [DOI] [PubMed] [Google Scholar]
- Boisnard M., Petrissant G. The nucleotide sequence of sheep liver histidine-tRNA (anticodon Q-U-G). FEBS Lett. 1981 Jun 29;129(1):180–184. doi: 10.1016/0014-5793(81)80785-5. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bonnard G., Weil J. H., Steinmetz A. The intergenic region between the Vicia faba chloroplast tRNA(CAALeu) and tRNA(UAALeu) genes contains a partial copy of the split tRNA(UAALeu) gene. Curr Genet. 1985;9(5):417–422. doi: 10.1007/BF00421614. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Ebel J. P., Shershneva L. P., Krutilina A. I., Venkstern T. V., Bayev A. A., Dirheirmer G. The corrected nucleotide sequence of valine tRNA from baker's yeast. Biochimie. 1974;56(9):1211–1213. doi: 10.1016/s0300-9084(74)80013-1. [DOI] [PubMed] [Google Scholar]
- Bordonné R., Dirheimer G., Martin R. P. Transcription initiation and RNA processing of a yeast mitochondrial tRNA gene cluster. Nucleic Acids Res. 1987 Sep 25;15(18):7381–7394. doi: 10.1093/nar/15.18.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsuk P., Sirko A., Bartnik E. A methionine tRNA gene from lupine mitochondria. Nucleic Acids Res. 1986 Sep 25;14(18):7508–7508. doi: 10.1093/nar/14.18.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L. The hisR locus of Salmonella: nucleotide sequence and expression. Mol Gen Genet. 1983;192(1-2):163–170. doi: 10.1007/BF00327662. [DOI] [PubMed] [Google Scholar]
- Bossi L. The hisR locus of Salmonella: nucleotide sequence and expression. Mol Gen Genet. 1983;192(1-2):163–170. doi: 10.1007/BF00327662. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Dron M., Loiseaux S., Mache R. Structure and transcription of the spinach chloroplast rDNA leader region. Nucleic Acids Res. 1982 Nov 11;10(21):6865–6878. doi: 10.1093/nar/10.21.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broida J., Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985 Oct 5;185(3):545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Broida J., Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985 Oct 5;185(3):545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Broida J., Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985 Oct 5;185(3):545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Broida J., Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985 Oct 5;185(3):545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. The Aspergillus nidulans mitochondrial genome. Curr Genet. 1985;9(2):113–117. doi: 10.1007/BF00436957. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Bull P., Thorikay M., Moenne A., Wilkens M., Sánchez H., Valenzuela P., Venegas A. The yeast tRNA(Phe) gene family: structures and transcriptional activities reveal member differences not explained by intragenic promoters. DNA. 1987 Aug;6(4):353–362. doi: 10.1089/dna.1987.6.353. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Two human tRNA(Ala) families are recognized by autoantibodies in polymyositis sera. Mol Biol Med. 1987 Feb;4(1):21–36. [PubMed] [Google Scholar]
- Burger G., Helmer Citterich M., Nelson M. A., Werner S., Macino G. RNA processing in Neurospora crassa mitochondria: transfer RNAs punctuate a large precursor transcript. EMBO J. 1985 Jan;4(1):197–204. doi: 10.1002/j.1460-2075.1985.tb02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Dirheimer G., Martin R. P. Yeast mitochondrial methionine initiator tRNA: characterization and nucleotide sequence. Nucleic Acids Res. 1980 Apr 11;8(7):1445–1457. doi: 10.1093/nar/8.7.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Weil J. H. The nucleotide sequences of the initiator transfer RNAs from bean cytoplasm and chloroplasts. Nucleic Acids Res. 1980 Mar 11;8(5):999–1008. doi: 10.1093/nar/8.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Weil J. H. The nucleotide sequences of the initiator transfer RNAs from bean cytoplasm and chloroplasts. Nucleic Acids Res. 1980 Mar 11;8(5):999–1008. doi: 10.1093/nar/8.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Morisco P., Rainaldi G., Gadaleta M. N., Saccone C. A novel gene order in the Paracentrotus lividus mitochondrial genome. Gene. 1987;53(1):41–54. doi: 10.1016/0378-1119(87)90091-6. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Morisco P., Rainaldi G., Gadaleta M. N., Saccone C. A novel gene order in the Paracentrotus lividus mitochondrial genome. Gene. 1987;53(1):41–54. doi: 10.1016/0378-1119(87)90091-6. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. The complete nucleotide sequence, gene organization, and genetic code of the mitochondrial genome of Paracentrotus lividus. J Biol Chem. 1989 Jul 5;264(19):10965–10975. [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Chakraburtty K., Steinschneider A., Case R. V., Mehler A. H. Primary structure of tRNA-Lys of E. coli B. Nucleic Acids Res. 1975 Nov;2(11):2069–2075. doi: 10.1093/nar/2.11.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Hecker L. I., Brum C. K., Schnabel J. J., Heckman J. E., Silberklang M., RajBhandary U. L., Barnett W. E. The nucleotide sequence of Euglena cytoplasmic phenylalanine transfer RNA. Evidence for possible classifications of Euglena among the animal rather than the plant kingdom. Nucleic Acids Res. 1981 Jul 10;9(13):3199–3204. doi: 10.1093/nar/9.13.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Kuo S., Hawkins E., Miller N. R. The corrected nucleotide sequence of yeast leucine transfer ribonucleic acid. Biochem Biophys Res Commun. 1973 Apr 16;51(4):951–955. doi: 10.1016/0006-291x(73)90019-3. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Pirtle I. L., Pirtle R. M. Nucleotide sequence and transcription of a human tRNA gene cluster with four genes. Gene. 1986;48(1):165–174. doi: 10.1016/0378-1119(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Pirtle I. L., Pirtle R. M. Nucleotide sequence and transcription of a human tRNA gene cluster with four genes. Gene. 1986;48(1):165–174. doi: 10.1016/0378-1119(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Wintz H., Weil J. H., Pillay D. T. Nucleotide sequence of chloroplast CF1-ATPase epsilon-subunit and elongator tRNAMet genes from Arabidopsis thaliana. Nucleic Acids Res. 1988 Nov 11;16(21):10372–10372. doi: 10.1093/nar/16.21.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. C., Wintz H., Weil J. H., Pillay D. T. Three mitochondrial tRNA genes from Arabidopsis thaliana: evidence for the conversion of a tRNAPhe gene into a tRNATyr gene. Nucleic Acids Res. 1989 Apr 11;17(7):2613–2621. doi: 10.1093/nar/17.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. Drosophila mitochondrial DNA: conserved sequences in the A + T-rich region and supporting evidence for a secondary structure model of the small ribosomal RNA. J Mol Evol. 1987;25(2):116–125. doi: 10.1007/BF02101753. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. Drosophila mitochondrial DNA: conserved sequences in the A + T-rich region and supporting evidence for a secondary structure model of the small ribosomal RNA. J Mol Evol. 1987;25(2):116–125. doi: 10.1007/BF02101753. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. Drosophila mitochondrial DNA: conserved sequences in the A + T-rich region and supporting evidence for a secondary structure model of the small ribosomal RNA. J Mol Evol. 1987;25(2):116–125. doi: 10.1007/BF02101753. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Bonitz S. G., Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Analysis of the nucleotide sequence and transcripts in the oxi1 region of yeast mitochondrial DNA. J Biol Chem. 1981 Dec 25;256(24):12780–12787. [PubMed] [Google Scholar]
- Coruzzi G., Bonitz S. G., Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Analysis of the nucleotide sequence and transcripts in the oxi1 region of yeast mitochondrial DNA. J Biol Chem. 1981 Dec 25;256(24):12780–12787. [PubMed] [Google Scholar]
- Cory S., Marcker K. A. The nucleotide sequence of methionine transfer RNA-M. Eur J Biochem. 1970 Jan;12(1):177–194. doi: 10.1111/j.1432-1033.1970.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Creusot F., Gaisne M., Verdière J., Slonimski P. P. A novel tRNA(Ala) gene and its adjacent sigma element downstream from the CYP1 (HAP1) gene in Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Mar 11;17(5):1865–1866. doi: 10.1093/nar/17.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs D. L., Gillam I. C., Tener G. M. Nucleotide sequences of three tRNA(Ser) from Drosophila melanogaster reading the six serine codons. J Mol Biol. 1987 Oct 5;197(3):389–395. doi: 10.1016/0022-2836(87)90552-3. [DOI] [PubMed] [Google Scholar]
- Cribbs D. L., Leung J., Newton C. H., Hayashi S., Miller R. C., Jr, Tener G. M. Extensive microheterogeneity of serine tRNA genes from Drosophila melanogaster. J Mol Biol. 1987 Oct 5;197(3):397–404. doi: 10.1016/0022-2836(87)90553-5. [DOI] [PubMed] [Google Scholar]
- Cribbs D. L., Leung J., Newton C. H., Hayashi S., Miller R. C., Jr, Tener G. M. Extensive microheterogeneity of serine tRNA genes from Drosophila melanogaster. J Mol Biol. 1987 Oct 5;197(3):397–404. doi: 10.1016/0022-2836(87)90553-5. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M. Sequence analysis of mitochondrial DNA from Podospora anserina. Pervasiveness of a class I intron in three separate genes. J Mol Biol. 1988 Dec 20;204(4):815–839. doi: 10.1016/0022-2836(88)90044-7. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Dalrymple B., Mattick J. S. Genes encoding threonine tRNAs with the anticodon CGU from Escherichia coli and Pseudomonas aeruginosa. Biochem Int. 1986 Oct;13(4):547–553. [PubMed] [Google Scholar]
- Daniels C. J., Gupta R., Doolittle W. F. Transcription and excision of a large intron in the tRNATrp gene of an archaebacterium, Halobacterium volcanii. J Biol Chem. 1985 Mar 10;260(5):3132–3134. [PubMed] [Google Scholar]
- Daniels C. J., Hofman J. D., MacWilliam J. G., Doolittle W. F., Woese C. R., Luehrsen K. R., Fox G. E. Sequence of 5S ribosomal RNA gene regions and their products in the archaebacterium Halobacterium volcanii. Mol Gen Genet. 1985;198(2):270–274. doi: 10.1007/BF00383005. [DOI] [PubMed] [Google Scholar]
- Das G., Henning D., Reddy R. One aspartic acid transfer RNA gene is present upstream of the U6 snRNA gene cluster in Drosophila melanogaster. Nucleic Acids Res. 1986 Oct 10;14(19):7816–7816. doi: 10.1093/nar/14.19.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLotto R., Schedl P. A Drosophila melanogaster transfer RNA gene cluster at the cytogenetic locus 90BC. J Mol Biol. 1984 Nov 15;179(4):587–605. doi: 10.1016/0022-2836(84)90157-8. [DOI] [PubMed] [Google Scholar]
- DeLotto R., Schedl P. A Drosophila melanogaster transfer RNA gene cluster at the cytogenetic locus 90BC. J Mol Biol. 1984 Nov 15;179(4):587–605. doi: 10.1016/0022-2836(84)90157-8. [DOI] [PubMed] [Google Scholar]
- DeLotto R., Schedl P. A Drosophila melanogaster transfer RNA gene cluster at the cytogenetic locus 90BC. J Mol Biol. 1984 Nov 15;179(4):587–605. doi: 10.1016/0022-2836(84)90157-8. [DOI] [PubMed] [Google Scholar]
- Debuchy R., Brygoo Y. Cloning of opal suppressor tRNA genes of a filamentous fungus reveals two tRNASerUGA genes with unexpected structural differences. EMBO J. 1985 Dec 16;4(13A):3553–3556. doi: 10.1002/j.1460-2075.1985.tb04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. M., Vaughan J., Weiss S. B. Identification and location of nine T5 bacteriophage tRNA genes by DNA sequence analysis. Nucleic Acids Res. 1986 May 27;14(10):4197–4205. doi: 10.1093/nar/14.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. M., Vaughan J., Weiss S. B. Identification and location of nine T5 bacteriophage tRNA genes by DNA sequence analysis. Nucleic Acids Res. 1986 May 27;14(10):4197–4205. doi: 10.1093/nar/14.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. M., Vaughan J., Weiss S. B. Identification and location of nine T5 bacteriophage tRNA genes by DNA sequence analysis. Nucleic Acids Res. 1986 May 27;14(10):4197–4205. doi: 10.1093/nar/14.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. M., Vaughan J., Weiss S. B. Identification and location of nine T5 bacteriophage tRNA genes by DNA sequence analysis. Nucleic Acids Res. 1986 May 27;14(10):4197–4205. doi: 10.1093/nar/14.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrès J., Keith G., Kuo K. C., Gehrke C. W. Presence of phosphorylated O-ribosyl-adenosine in T-psi-stem of yeast methionine initiator tRNA. Nucleic Acids Res. 1989 Feb 11;17(3):865–882. doi: 10.1093/nar/17.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingermann T., Bertling W., Pistel F., Amon E. Characterisation of a Dictyostelium discoideum DNA fragment coding for a putative tRNAValGUU gene. Evidence for a single transcription unit consisting of two overlapping class III genes. Eur J Biochem. 1985 Jan 15;146(2):449–458. doi: 10.1111/j.1432-1033.1985.tb08672.x. [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Durnford D. G. Nucleotide sequence of the genes for ribosomal protein S4 and tRNA(Arg) from the chlorophyll c-containing alga Cryptomonas phi. Nucleic Acids Res. 1990 Apr 11;18(7):1903–1903. doi: 10.1093/nar/18.7.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin H. J., RajBhandary U. L. Attempted expression of a human initiator tRNA gene in Saccharomyces cerevisiae. J Biol Chem. 1985 May 10;260(9):5596–5602. [PubMed] [Google Scholar]
- Drabkin H. J., RajBhandary U. L. Attempted expression of a human initiator tRNA gene in Saccharomyces cerevisiae. J Biol Chem. 1985 May 10;260(9):5596–5602. [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A. The nucleotide sequence of N-formyl-methionyl-transfer RNA. Partial digestion with pancreatic and T-1 ribonuclease and derivation of the total primary structure. Eur J Biochem. 1969 Mar;8(2):256–262. doi: 10.1111/j.1432-1033.1969.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Cleaves G. R., Timko K. D. Sequence and structure of a serine transfer RNA with GCU anticodon from mosquito mitochondria. J Mol Biol. 1984 Jun 25;176(2):251–260. doi: 10.1016/0022-2836(84)90423-6. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C. Sequence and structure of a methionine transfer RNA from mosquito mitochondria. Nucleic Acids Res. 1984 May 25;12(10):4185–4189. doi: 10.1093/nar/12.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Tillotson L. E. Mosquito mitochondrial transfer RNAs for valine, glycine and glutamate: RNA and gene sequences and vicinal genome organization. Curr Genet. 1986;10(9):701–707. doi: 10.1007/BF00410919. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Tillotson L. E. Mosquito mitochondrial transfer RNAs for valine, glycine and glutamate: RNA and gene sequences and vicinal genome organization. Curr Genet. 1986;10(9):701–707. doi: 10.1007/BF00410919. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Tillotson L. E. Mosquito mitochondrial transfer RNAs for valine, glycine and glutamate: RNA and gene sequences and vicinal genome organization. Curr Genet. 1986;10(9):701–707. doi: 10.1007/BF00410919. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Tillotson L. E. Mosquito mitochondrial transfer RNAs for valine, glycine and glutamate: RNA and gene sequences and vicinal genome organization. Curr Genet. 1986;10(9):701–707. doi: 10.1007/BF00410919. [DOI] [PubMed] [Google Scholar]
- Duester G., Campen R. K., Holmes W. M. Nucleotide sequence of an Escherichia coli tRNA (Leu 1) operon and identification of the transcription promoter signal. Nucleic Acids Res. 1981 May 11;9(9):2121–2139. doi: 10.1093/nar/9.9.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gewely M. R., Helling R. B., Dibbits J. G. Sequence and evolution of the regions between thr rrn operons in the chloroplast genome of Euglena gracilis bacillaris. Mol Gen Genet. 1984;194(3):432–443. doi: 10.1007/BF00425555. [DOI] [PubMed] [Google Scholar]
- El-Gewely M. R., Helling R. B., Dibbits J. G. Sequence and evolution of the regions between thr rrn operons in the chloroplast genome of Euglena gracilis bacillaris. Mol Gen Genet. 1984;194(3):432–443. doi: 10.1007/BF00425555. [DOI] [PubMed] [Google Scholar]
- Endoh H., Nagahashi S., Okada N. Tetrahymena pyriformis DNA fragment with a gene cluster for 3 putative serine tRNAs and an asparagine tRNA. Nucleic Acids Res. 1989 Dec 11;17(23):10122–10122. doi: 10.1093/nar/17.23.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard J. L., Kuntz M., Straus N. A., Weil J. H. A class-I intron in a cyanelle tRNA gene from Cyanophora paradoxa: phylogenetic relationship between cyanelles and plant chloroplasts. Gene. 1988 Nov 15;71(1):115–122. doi: 10.1016/0378-1119(88)90083-2. [DOI] [PubMed] [Google Scholar]
- Evrard J. L., Kuntz M., Straus N. A., Weil J. H. A class-I intron in a cyanelle tRNA gene from Cyanophora paradoxa: phylogenetic relationship between cyanelles and plant chloroplasts. Gene. 1988 Nov 15;71(1):115–122. doi: 10.1016/0378-1119(88)90083-2. [DOI] [PubMed] [Google Scholar]
- Feingold J., Bellofatto V., Shapiro L., Amemiya K. Organization and nucleotide sequence analysis of an rRNA and tRNA gene cluster from Caulobacter crescentus. J Bacteriol. 1985 Jul;163(1):155–166. doi: 10.1128/jb.163.1.155-166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold J., Bellofatto V., Shapiro L., Amemiya K. Organization and nucleotide sequence analysis of an rRNA and tRNA gene cluster from Caulobacter crescentus. J Bacteriol. 1985 Jul;163(1):155–166. doi: 10.1128/jb.163.1.155-166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felici F., Cesareni G. Structure of the Saccharomyces cerevisiae gene encoding tRNAIle (IAU). Nucleic Acids Res. 1987 Jan 12;15(1):364–364. doi: 10.1093/nar/15.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Hofstetter H., Birnstiel M. L. Some bacterial tRNA genes are transcribed by eukaryotic RNA polymerase III. Nucleic Acids Res. 1982 Nov 25;10(22):7153–7162. doi: 10.1093/nar/10.22.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A., Guérin M. A., Corlet J., Clarkson S. G. Structure and in vitro transcription of a glycine tRNA gene from Bombyx mori. EMBO J. 1984 Jul;3(7):1547–1552. doi: 10.1002/j.1460-2075.1984.tb02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M., Labouesse J., Dirheimer G., Fix C., Keith G. Primary structure of bovine liver tRNATrp. Biochim Biophys Acta. 1978 Nov 21;521(1):198–208. doi: 10.1016/0005-2787(78)90262-9. [DOI] [PubMed] [Google Scholar]
- Francis M. A., Dudock B. S. Nucleotide sequence of spinach cytoplasmic serine (IGA) tRNA. Nucleic Acids Res. 1989 Oct 11;17(19):7996–7996. doi: 10.1093/nar/17.19.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. A., Suh E. R., Dudock B. S. The nucleotide sequence and characterization of four chloroplast tRNAs from the alga codium fragile. J Biol Chem. 1989 Oct 15;264(29):17243–17249. [PubMed] [Google Scholar]
- Francis M. A., Suh E. R., Dudock B. S. The nucleotide sequence and characterization of four chloroplast tRNAs from the alga codium fragile. J Biol Chem. 1989 Oct 15;264(29):17243–17249. [PubMed] [Google Scholar]
- Francis M., Kashdan M., Sprouse H., Otis L., Dudock B. Nucleotide sequence of a spinach chloroplast proline tRNA. Nucleic Acids Res. 1982 Apr 24;10(8):2755–2758. doi: 10.1093/nar/10.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ftouhi N., Cedergren R. The nucleotide sequence of a small metT tRNA operon from Photobacterium leiognathi. Nucleic Acids Res. 1990 Jun 25;18(12):3662–3662. doi: 10.1093/nar/18.12.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafner J., Robertis E. M., Philippsen P. Delta sequences in the 5' non-coding region of yeast tRNA genes. EMBO J. 1983;2(4):583–591. doi: 10.1002/j.1460-2075.1983.tb01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamulin V., Mao J., Appel B., Sumner-Smith M., Yamao F., Söll D. Six Schizosaccharomyces pombe tRNA genes including a gene for a tRNALys with an intervening sequence which cannot base-pair with the anticodon. Nucleic Acids Res. 1983 Dec 20;11(24):8537–8546. doi: 10.1093/nar/11.24.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff J., Keith G., Ebel J. P., Dirheimer G. The primary structure of aspartate transfer ribonucleic acid from brewer's yeast. II. Partial digestions with pancreatic ribonuclease and T 1 ribonuclease and derivation of complete sequence. Biochim Biophys Acta. 1972 Jan 31;259(2):210–222. [PubMed] [Google Scholar]
- Garcia G. M., Mar P. K., Mullin D. A., Walker J. R., Prather N. E. The E. coli dnaY gene encodes an arginine transfer RNA. Cell. 1986 May 9;45(3):453–459. doi: 10.1016/0092-8674(86)90331-4. [DOI] [PubMed] [Google Scholar]
- Genbauffe F. S., Chisholm G. E., Cooper T. G. Tau, sigma, and delta. A family of repeated elements in yeast. J Biol Chem. 1984 Aug 25;259(16):10518–10525. [PubMed] [Google Scholar]
- Ghosh H. P., Ghosh K., Simsek M., RajBhandary U. L. Nucleotide sequence of wheat germ cytoplasmic initiator methionine transfer ribonucleic acid. Nucleic Acids Res. 1982 May 25;10(10):3241–3247. doi: 10.1093/nar/10.10.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. L., Brown J. R., O'Hara P. J., Buroker N. E., Beckenbach A. T., Smith M. J. Sequence of tRNA(Thr) and tRNA(Pro) from white sturgeon (Acipenser transmontanus) mitochondria. Nucleic Acids Res. 1988 Dec 23;16(24):11825–11825. doi: 10.1093/nar/16.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. L., Brown J. R., O'Hara P. J., Buroker N. E., Beckenbach A. T., Smith M. J. Sequence of tRNA(Thr) and tRNA(Pro) from white sturgeon (Acipenser transmontanus) mitochondria. Nucleic Acids Res. 1988 Dec 23;16(24):11825–11825. doi: 10.1093/nar/16.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Glew L., Lo R., Reece T., Nichols M., Söll D., Bell J. The nucleotide sequence, localization and transcriptional properties of a tRNALeuCUG gene from Drosophila melanogaster. Gene. 1986;44(2-3):307–314. doi: 10.1016/0378-1119(86)90195-2. [DOI] [PubMed] [Google Scholar]
- Gonos E. S., Goddard J. P. The nucleotide sequence of a human tRNA(Glu) gene. Nucleic Acids Res. 1990 Nov 25;18(22):6705–6705. doi: 10.1093/nar/18.22.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbulev V. G., Axel'rod V. D., Bayev A. A. Primary structure of baker's yeast tRNAVal 2b. Nucleic Acids Res. 1977 Sep;4(9):3239–3258. doi: 10.1093/nar/4.9.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Graf L., Kössel H., Stutz E. Sequencing of 16S--23S spacer in a ribosomal RNA operon of Euglena gracilis chloroplast DNA reveals two tRNA genes. Nature. 1980 Aug 28;286(5776):908–910. doi: 10.1038/286908a0. [DOI] [PubMed] [Google Scholar]
- Graf L., Kössel H., Stutz E. Sequencing of 16S--23S spacer in a ribosomal RNA operon of Euglena gracilis chloroplast DNA reveals two tRNA genes. Nature. 1980 Aug 28;286(5776):908–910. doi: 10.1038/286908a0. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Green G. A., Jones D. S. The nucleotide sequences of a cytoplasmic and a chloroplast tRNATyr from Scenedesmus obliquus. Nucleic Acids Res. 1985 Mar 11;13(5):1659–1663. doi: 10.1093/nar/13.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. A., Jones D. S. The nucleotide sequences of a cytoplasmic and a chloroplast tRNATyr from Scenedesmus obliquus. Nucleic Acids Res. 1985 Mar 11;13(5):1659–1663. doi: 10.1093/nar/13.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Grimm M. F., Goewert R. R., Collins R. A., Cole M. D., Lambowitz A. M., Heckman J. E., Yin S., RajBhandary U. L. Transcripts and processing patterns for the ribosomal RNA and transfer RNA region of Neurospora crassa mitochondrial DNA. J Biol Chem. 1981 Feb 25;256(4):2027–2034. [PubMed] [Google Scholar]
- Grisi E., Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. Nucleotide sequence of a region of the mitochondrial genome of Aspergillus nidulans including the gene for ATPase subunit 6. Nucleic Acids Res. 1982 Jun 11;10(11):3531–3539. doi: 10.1093/nar/10.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisi E., Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. Nucleotide sequence of a region of the mitochondrial genome of Aspergillus nidulans including the gene for ATPase subunit 6. Nucleic Acids Res. 1982 Jun 11;10(11):3531–3539. doi: 10.1093/nar/10.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Nicoghosian K., Haumont E., Söll D., Cedergren R. Nucleotide sequences of two serine tRNAs with a GGA anticodon: the structure-function relationships in the serine family of E. coli tRNAs. Nucleic Acids Res. 1985 Aug 12;13(15):5697–5706. doi: 10.1093/nar/13.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhl H., Feldmann H. The primary structure of a non-initiating methionine-specific tRNA from brewer's yeast. Eur J Biochem. 1976 Sep;68(1):209–217. doi: 10.1111/j.1432-1033.1976.tb10780.x. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gu X. R., Giroux S., Cedergren R. The nucleotide sequence of the argT locus of Aeromonas hydrophila. Nucleic Acids Res. 1988 Nov 25;16(22):10936–10936. doi: 10.1093/nar/16.22.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. R., Giroux S., Cedergren R. The nucleotide sequence of the argT locus of Aeromonas hydrophila. Nucleic Acids Res. 1988 Nov 25;16(22):10936–10936. doi: 10.1093/nar/16.22.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. R., Nicoghosian K., Cedergren R. J., Wong J. T. Sequences of halobacterial tRNAs and the paucity of U in the first position of their anticodons. Nucleic Acids Res. 1983 Aug 25;11(16):5433–5442. doi: 10.1093/nar/11.16.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. R., Nicoghosian K., Cedergren R. J., Wong J. T. Sequences of halobacterial tRNAs and the paucity of U in the first position of their anticodons. Nucleic Acids Res. 1983 Aug 25;11(16):5433–5442. doi: 10.1093/nar/11.16.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto J. M., Wintz H., Weil J. H., Grienenberger J. M. The genes coding for subunit 3 of NADH dehydrogenase and for ribosomal protein S12 are present in the wheat and maize mitochondrial genomes and are co-transcribed. Mol Gen Genet. 1988 Dec;215(1):118–127. doi: 10.1007/BF00331312. [DOI] [PubMed] [Google Scholar]
- Guelin E., Velours J., Guerin M. Cloning and sequencing of a fragment of the linear mitochondrial DNA of the yeast Candida parapsilosis supporting genes encoding subunit 8 of Fo ATP synthase and a putative t-RNA(Pro). Nucleic Acids Res. 1990 Jul 25;18(14):4267–4267. doi: 10.1093/nar/18.14.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemaut P., Keith G. Primary structure of bean chloroplastic tRNAPhe. Comparison with Euglena chloroplastic tRNAPhe. FEBS Lett. 1977 Dec 15;84(2):351–356. doi: 10.1016/0014-5793(77)80723-0. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Weil J. H. The nucleotide sequence of the maize and spinach chloroplast isoleucine transfer RNA encoded in the 16S to 23S rDNA spacer. Nucleic Acids Res. 1982 Mar 11;10(5):1653–1659. doi: 10.1093/nar/10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemaut P., Weil J. H. The nucleotide sequence of the maize and spinach chloroplast isoleucine transfer RNA encoded in the 16S to 23S rDNA spacer. Nucleic Acids Res. 1982 Mar 11;10(5):1653–1659. doi: 10.1093/nar/10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Roe B. A., Randerath K. The nucleotide sequence of human tRNAGly (anticodon GCC). Nucleic Acids Res. 1979 Oct 25;7(4):959–970. doi: 10.1093/nar/7.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta U., Gupta B. S. Caffeine differentially affects kinesthetic aftereffect in high and low impulsives. Psychopharmacology (Berl) 1990;102(1):102–105. doi: 10.1007/BF02245752. [DOI] [PubMed] [Google Scholar]
- Gupta U., Gupta B. S. Caffeine differentially affects kinesthetic aftereffect in high and low impulsives. Psychopharmacology (Berl) 1990;102(1):102–105. doi: 10.1007/BF02245752. [DOI] [PubMed] [Google Scholar]
- Guthrie C., McClain W. H. Rare transfer ribonucleic acid essential for phage growth. Nucleotide sequence comparison of normal and mutant T4 isoleucine-accepting transfer ribonucleic acid. Biochemistry. 1979 Aug 21;18(17):3786–3795. doi: 10.1021/bi00584a023. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Scholla C. A., Yesian H., Abelson J. The nucleotide sequence of threonine transfer RNA coded by bacteriophage T4. Nucleic Acids Res. 1978 Jun;5(6):1833–1844. doi: 10.1093/nar/5.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E. S., Brown J. W., Daniels C. J., Reeve J. N. Genes encoding the 7S RNA and tRNA(Ser) are linked to one of the two rRNA operons in the genome of the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1990 May 31;90(1):51–59. doi: 10.1016/0378-1119(90)90438-w. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Daniels C. J., Reeve J. N. Genes encoding 5S rRNA and tRNAs in the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1989 Apr 30;77(2):253–263. doi: 10.1016/0378-1119(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Harada F., Matsubara M., Kato N. Nucleotide sequences of two glutamine tRNAs from HeLa cells. Nucleic Acids Res. 1989 Oct 25;17(20):8371–8371. doi: 10.1093/nar/17.20.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Matsubara M., Kato N. Stable tRNA precursors in HeLa cells. Nucleic Acids Res. 1984 Dec 21;12(24):9263–9269. doi: 10.1093/nar/12.24.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Matsubara M., Kato N. Stable tRNA precursors in HeLa cells. Nucleic Acids Res. 1984 Dec 21;12(24):9263–9269. doi: 10.1093/nar/12.24.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C. M., Clark-Walker G. D. Nucleotide sequence of the structural genes for the mitochondrial cys, lys, gln and leu-tRNAs from the yeast Kluyveromyces lactis K8. Nucleic Acids Res. 1989 Feb 25;17(4):1762–1762. doi: 10.1093/nar/17.4.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C. M., Clark-Walker G. D. Nucleotide sequence of the structural genes for the mitochondrial cys, lys, gln and leu-tRNAs from the yeast Kluyveromyces lactis K8. Nucleic Acids Res. 1989 Feb 25;17(4):1762–1762. doi: 10.1093/nar/17.4.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Murao K., Ishikura H. The nucleotide sequence of proline tRNAmo5UGG from Bacillus subtilis. Biochem Int. 1985 Apr;10(4):663–671. [PubMed] [Google Scholar]
- Hatfield D. L., Dudock B. S., Eden F. C. Characterization and nucleotide sequence of a chicken gene encoding an opal suppressor tRNA and its flanking DNA segments. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4940–4944. doi: 10.1073/pnas.80.16.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke H. R., Gellissen G. Different mitochondrial gene orders among insects: exchanged tRNA gene positions in the COII/COIII region between an orthopteran and a dipteran species. Curr Genet. 1988 Nov;14(5):471–476. doi: 10.1007/BF00521271. [DOI] [PubMed] [Google Scholar]
- Haucke H. R., Gellissen G. Different mitochondrial gene orders among insects: exchanged tRNA gene positions in the COII/COIII region between an orthopteran and a dipteran species. Curr Genet. 1988 Nov;14(5):471–476. doi: 10.1007/BF00521271. [DOI] [PubMed] [Google Scholar]
- Haumont E., Nicoghosian K., Grosjean H., Cedergren R. J. The nucleotide sequence of mannosyl-Q-containing tRNAAsp from Xenopus laevis oocytes. Biochimie. 1984 Jul-Aug;66(7-8):579–582. doi: 10.1016/0300-9084(84)90154-8. [DOI] [PubMed] [Google Scholar]
- Hauser M. A., Scocca J. J. Location of the host attachment site for phage HPl within a cluster of Haemophilus influenzae tRNA genes. Nucleic Acids Res. 1990 Sep 11;18(17):5305–5305. doi: 10.1093/nar/18.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka K., Gojobori T., Horai S. Molecular phylogeny and evolution of primate mitochondrial DNA. Mol Biol Evol. 1988 Nov;5(6):626–644. doi: 10.1093/oxfordjournals.molbev.a040524. [DOI] [PubMed] [Google Scholar]
- Hecker L. I., Barnett W. E., Lin F. K., Furr T. D., Heckman J. E., RajBhandary U. L., Chang S. H. The nucleotide sequence of blue-green algae phenylalanine-tRNA and the evolutionary origin of chloroplasts. Nucleic Acids Res. 1982 Oct 25;10(20):6433–6440. doi: 10.1093/nar/10.20.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Alzner-Deweerd B., RajBhandary U. L. Interesting and unusual features in the sequence of Neurospora crassa mitochondrial tyrosine transfer RNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):717–721. doi: 10.1073/pnas.76.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Hecker L. I., Schwartzbach S. D., Barnett W. E., Baumstark B., RajBhandary U. L. Structure and function of initiator methionine tRNA from the mitochondria of Neurospora crassa. Cell. 1978 Jan;13(1):83–95. doi: 10.1016/0092-8674(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgcoth C., Hayenga K., Harrison M., Ortwerth B. J. Lysine tRNAs from rat liver: lysine tRNA sequences are highly conserved. Nucleic Acids Res. 1984 Mar 12;12(5):2535–2541. doi: 10.1093/nar/12.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen T. Y., Schnare M. N., Young P. G., Gray M. W. Rearranged coding segments, separated by a transfer RNA gene, specify the two parts of a discontinuous large subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1987 Feb 25;262(6):2879–2887. [PubMed] [Google Scholar]
- Heinonen T. Y., Schnare M. N., Young P. G., Gray M. W. Rearranged coding segments, separated by a transfer RNA gene, specify the two parts of a discontinuous large subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1987 Feb 25;262(6):2879–2887. [PubMed] [Google Scholar]
- Hellmund D., Metzlaff M., Serfling E. A transfer RNAArg gene of Pelargonium chloroplasts, but not a 5S RNA gene, is efficiently transcribed after injection into Xenopus oocyte nuclei. Nucleic Acids Res. 1984 Nov 12;12(21):8253–8268. doi: 10.1093/nar/12.21.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey N. D., Davidson N. Two drosophila melanogaster tRNAGly genes are contained in a direct duplication at chromosomal locus 56F. Nucleic Acids Res. 1980 Nov 11;8(21):4899–4910. doi: 10.1093/nar/8.21.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Steinhart W., Riddle D. L., Carbon J. The nucleotide sequence of the GGG-specific glycine transfer ribonucleic acid of Escherichia coli and of Salmonella typhimurium. J Biol Chem. 1973 Jun 25;248(12):4252–4262. [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Steinhart W., Riddle D. L., Carbon J. The nucleotide sequence of the GGG-specific glycine transfer ribonucleic acid of Escherichia coli and of Salmonella typhimurium. J Biol Chem. 1973 Jun 25;248(12):4252–4262. [PubMed] [Google Scholar]
- Himeno H., Masaki H., Kawai T., Ohta T., Kumagai I., Miura K., Watanabe K. Unusual genetic codes and a novel gene structure for tRNA(AGYSer) in starfish mitochondrial DNA. Gene. 1987;56(2-3):219–230. doi: 10.1016/0378-1119(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Hipskind R. A., Clarkson S. G. 5'-flanking sequences that inhibit in vitro transcription of a xenopus laevis tRNA gene. Cell. 1983 Oct;34(3):881–890. doi: 10.1016/0092-8674(83)90545-7. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hoe K. L., Hong H. J., Yoo S. H., Yoo O. J. The nucleotide sequence of a gene coding for human serine tRNA. Nucleic Acids Res. 1987 Dec 10;15(23):10045–10045. doi: 10.1093/nar/15.23.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Sequence of the genes for tRNACys and tRNAAsp from spinach chloroplasts. Nucleic Acids Res. 1983 Dec 20;11(24):8547–8554. doi: 10.1093/nar/11.24.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H. J., Yoo S. H., Yoo O. J. The nucleotide sequence of a human serine tRNA gene. Nucleic Acids Res. 1987 Jun 25;15(12):4987–4987. doi: 10.1093/nar/15.12.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosbach H. A., Silberklang M., McCarthy B. J. Evolution of a D. melanogaster glutamate tRNA gene cluster. Cell. 1980 Aug;21(1):169–178. doi: 10.1016/0092-8674(80)90124-5. [DOI] [PubMed] [Google Scholar]
- Hottinger H., Ohgi T., Zwahlen M. C., Dhamija S., Söll D. Allele-specific complementation of an Escherichia coli leuB mutation by a Lactobacillus bulgaricus tRNA gene. Gene. 1987;60(1):75–83. doi: 10.1016/0378-1119(87)90215-0. [DOI] [PubMed] [Google Scholar]
- Hovemann B., Sharp S., Yamada H., Söll D. Analysis of a drosophila tRNA gene cluster. Cell. 1980 Apr;19(4):889–895. doi: 10.1016/0092-8674(80)90080-x. [DOI] [PubMed] [Google Scholar]
- Howe C. J. The endpoints of an inversion in wheat chloroplast DNA are associated with short repeated sequences containing homology to att-lambda. Curr Genet. 1985;10(2):139–145. doi: 10.1007/BF00636479. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Cleaves G. R., Dubin D. T. Sequences of three transfer RNAs from mosquito mitochondria. Plasmid. 1983 Jul;10(1):55–65. doi: 10.1016/0147-619x(83)90057-4. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Cleaves G. R., Dubin D. T. Sequences of three transfer RNAs from mosquito mitochondria. Plasmid. 1983 Jul;10(1):55–65. doi: 10.1016/0147-619x(83)90057-4. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Kotin R. M., Dubin D. T. Sequences of the coding and flanking regions of the large ribosomal subunit RNA gene of mosquito mitochondria. Nucleic Acids Res. 1984 Oct 25;12(20):7771–7785. doi: 10.1093/nar/12.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Cote B. D., Lund E., Dahlberg J. E. Isolation and characterization of genomic mouse DNA clones containing sequences homologous to tRNAs and 5S rRNA. Nucleic Acids Res. 1983 Jul 25;11(14):4809–4821. doi: 10.1093/nar/11.14.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Cote B. D., Lund E., Dahlberg J. E. Isolation and characterization of genomic mouse DNA clones containing sequences homologous to tRNAs and 5S rRNA. Nucleic Acids Res. 1983 Jul 25;11(14):4809–4821. doi: 10.1093/nar/11.14.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Holton T. A., Whitfield P. R., Bottomley W. Spinach chloroplast rpoBC genes encode three subunits of the chloroplast RNA polymerase. J Mol Biol. 1988 Apr 20;200(4):639–654. doi: 10.1016/0022-2836(88)90477-9. [DOI] [PubMed] [Google Scholar]
- Hudson L., Rossi J., Landy A. Dual function transcripts specifying tRNA and mRNA. Nature. 1981 Dec 3;294(5840):422–427. doi: 10.1038/294422a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. A., Jones D. S. A fragment of the Pseudomonas aeruginosa genome contains five tRNA genes, four of which are linked to an EF-Tu gene. Nucleic Acids Res. 1988 Jul 25;16(14B):7193–7193. doi: 10.1093/nar/16.14.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui I., Dennis P. P. Characterization of the ribosomal RNA gene clusters in Halobacterium cutirubrum. J Biol Chem. 1985 Jan 25;260(2):899–906. [PubMed] [Google Scholar]
- Hui I., Dennis P. P. Characterization of the ribosomal RNA gene clusters in Halobacterium cutirubrum. J Biol Chem. 1985 Jan 25;260(2):899–906. [PubMed] [Google Scholar]
- Huiet L., Tyler B. M., Giles N. H. A leucine tRNA gene adjacent to the QA gene cluster of Neurospora crassa. Nucleic Acids Res. 1984 Jul 25;12(14):5757–5765. doi: 10.1093/nar/12.14.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z. K., Tartof K. D. Glutamate tRNA genes are adjacent to 5S RNA genes in Drosophila and reveal a conserved upstream sequence (the ACT-TA box). Nucleic Acids Res. 1982 Jul 24;10(14):4159–4172. doi: 10.1093/nar/10.14.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Clark B. F. The nucleotide sequence of a serine transfer ribonucleic acid from Escherichia coli. J Biol Chem. 1973 Oct 10;248(19):6663–6673. [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. The nucleotide sequence of a serine tRNA from Escherichia coli. FEBS Lett. 1971 Jul 15;16(1):68–70. doi: 10.1016/0014-5793(71)80688-9. [DOI] [PubMed] [Google Scholar]
- Izuchi S., Sugita M. Nucleotide sequence of a tomato mitochondrial tRNA(Cvs) (GCA) gene. Nucleic Acids Res. 1989 Feb 11;17(3):1248–1248. doi: 10.1093/nar/17.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Asakawa S., Araki T., Miura K., Smith M. J., Watanabe K. Conserved tRNA gene cluster in starfish mitochondrial DNA. Curr Genet. 1989 Mar;15(3):193–206. doi: 10.1007/BF00435506. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jank P., Riesner D., Gross H. J. Rabbit liver tRNA1Val:II. unusual secondary structure of T psi C stem and loop due to a U54:A60 base pair. Nucleic Acids Res. 1977 Jun;4(6):2009–2200. doi: 10.1093/nar/4.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen S., Guddal P. H., Johansen T. Organization of the mitochondrial genome of Atlantic cod, Gadus morhua. Nucleic Acids Res. 1990 Feb 11;18(3):411–419. doi: 10.1093/nar/18.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen S., Guddal P. H., Johansen T. Organization of the mitochondrial genome of Atlantic cod, Gadus morhua. Nucleic Acids Res. 1990 Feb 11;18(3):411–419. doi: 10.1093/nar/18.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen S., Guddal P. H., Johansen T. Organization of the mitochondrial genome of Atlantic cod, Gadus morhua. Nucleic Acids Res. 1990 Feb 11;18(3):411–419. doi: 10.1093/nar/18.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen S., Guddal P. H., Johansen T. Organization of the mitochondrial genome of Atlantic cod, Gadus morhua. Nucleic Acids Res. 1990 Feb 11;18(3):411–419. doi: 10.1093/nar/18.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Pirtle I. L., Pirtle R. M. The nucleotide sequence of tyrosine tRNAQ* psi A from bovine liver. Arch Biochem Biophys. 1985 Jan;236(1):448–453. doi: 10.1016/0003-9861(85)90647-2. [DOI] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Aspartate and asparagine tRNA genes in wheat mitochondrial DNA: a cautionary note on the isolation of tRNA genes from plants. Nucleic Acids Res. 1989 Oct 11;17(19):7865–7878. doi: 10.1093/nar/17.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Chloroplast-like transfer RNA genes expressed in wheat mitochondria. Nucleic Acids Res. 1989 Jul 25;17(14):5461–5476. doi: 10.1093/nar/17.14.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Nucleotide sequence of a second glutamine tRNA gene in wheat mitochondrial DNA. Nucleic Acids Res. 1989 Jun 26;17(12):4885–4885. [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. Structure of a spinach chloroplast threonine tRNA gene. J Biol Chem. 1982 Feb 10;257(3):1114–1116. [PubMed] [Google Scholar]
- Kashdan M. A., Pirtle R. M., Pirtle I. L., Calagan J. L., Vreman H. J., Dudock B. S. Nucleotide sequence of a spinach chloroplast threonine tRNA. J Biol Chem. 1980 Sep 25;255(18):8831–8835. [PubMed] [Google Scholar]
- Kato A., Takaiwa F., Shinozaki K., Sugiura M. Location and nucleotide sequence of the genes for tobacco chloroplast tRNAArg (ACG) and tRNALeu(UAG). Curr Genet. 1985;9(5):405–409. doi: 10.1007/BF00421612. [DOI] [PubMed] [Google Scholar]
- Kato A., Takaiwa F., Shinozaki K., Sugiura M. Location and nucleotide sequence of the genes for tobacco chloroplast tRNAArg (ACG) and tRNALeu(UAG). Curr Genet. 1985;9(5):405–409. doi: 10.1007/BF00421612. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Takemura S., Kondo T., Fukami T., Goto T. Chemical structure of a new modified nucleoside located in the anticodon of Bombyx mori glycine tRNA2. J Biochem. 1988 Jul;104(1):108–111. doi: 10.1093/oxfordjournals.jbchem.a122403. [DOI] [PubMed] [Google Scholar]
- Keith G., Dirheimer G. Evidence for the existence of an expressed minor variant tRNAPhe in yeast. Biochem Biophys Res Commun. 1987 Jan 15;142(1):183–187. doi: 10.1016/0006-291x(87)90468-2. [DOI] [PubMed] [Google Scholar]
- Keith G., Dirheimer G. Primary structure of Bombyx mori posterior silkgland tRNAPhe. Biochem Biophys Res Commun. 1980 Jan 15;92(1):109–115. doi: 10.1016/0006-291x(80)91526-0. [DOI] [PubMed] [Google Scholar]
- Keith G., Dirheimer G. Reinvestigation of the primary structure of brewer's yeast tRNA 3 Arg. Biochem Biophys Res Commun. 1980 Jan 15;92(1):116–119. doi: 10.1016/0006-291x(80)91527-2. [DOI] [PubMed] [Google Scholar]
- Keith G., Ebel J. P., Dirheimer G. The primary structure of two mammalian tRNAs Phe: identity of calf liver and rabbit liver tRNAs Phe. FEBS Lett. 1974 Nov 1;48(1):50–52. doi: 10.1016/0014-5793(74)81059-8. [DOI] [PubMed] [Google Scholar]
- Keith G., Picaud F., Weissenbach J., Ebel J. P., Petrissant G., Dirheimer G. The primary structure of rabbit liver tRNA Phe and its comparison with known tRNA Phe sequences. FEBS Lett. 1973 May 1;31(3):345–347. doi: 10.1016/0014-5793(73)80138-3. [DOI] [PubMed] [Google Scholar]
- Keith G., Pixa G., Fix C., Dirheimer G. Primary structure of three tRNAs from brewer's yeast: tRNAPro2, tRNAHis1 and tRNAHis2. Biochimie. 1983 Nov-Dec;65(11-12):661–672. doi: 10.1016/s0300-9084(84)80030-9. [DOI] [PubMed] [Google Scholar]
- Keith G., Roy A., Ebel J. P., Dirheimer G. The primary structure of tryptophan transfer ribonucleic acid from brewer's yeast. II. Partial digestion with pancreatic ribonuclease and derivation of complete sequence. Biochimie. 1972;54(11):1417–1426. doi: 10.1016/s0300-9084(72)80083-x. [DOI] [PubMed] [Google Scholar]
- Keith G. The primary structures of two arginine tRNAs (anticodons C-C-U and mcm5a2U-C-psi) and of glutamine tRNA (anticodon C-U-G) from bovine liver. Nucleic Acids Res. 1984 Mar 12;12(5):2543–2547. doi: 10.1093/nar/12.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keus R. J., Stam N. J., Zwiers T., de Heij H. T., Groot G. S. The nucleotide sequences of the genes coding for tRNAArgUCU, tRNAArgACG and tRNAAsnGUU on Spirodela oligorhiza chloroplast DNA. Nucleic Acids Res. 1984 Jul 25;12(14):5639–5646. doi: 10.1093/nar/12.14.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keus R. J., Stam N. J., Zwiers T., de Heij H. T., Groot G. S. The nucleotide sequences of the genes coding for tRNAArgUCU, tRNAArgACG and tRNAAsnGUU on Spirodela oligorhiza chloroplast DNA. Nucleic Acids Res. 1984 Jul 25;12(14):5639–5646. doi: 10.1093/nar/12.14.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter S., Fischer W., Sprinzl M. Sequences of three minor tRNAsArg from E. coli. Nucleic Acids Res. 1987 Apr 10;15(7):3184–3184. doi: 10.1093/nar/15.7.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of the tRNAMMet from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Sep 11;9(17):4387–4390. doi: 10.1093/nar/9.17.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Johnson J. Construction, expression, and function of a new yeast amber suppressor, tRNATrpA. J Biol Chem. 1988 May 25;263(15):7316–7321. [PubMed] [Google Scholar]
- Kimball M. E., Szeto K. S., Soll D. The nucleotide sequence of phenylalanine tRNA from Mycoplasma sp. (Kid). Nucleic Acids Res. 1974 Dec;1(12):1721–1732. [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Leffers H., Olesen T., Garrett R. A. A unique tRNA intron in the variable loop of the extreme thermophile Thermofilum pendens and its possible evolutionary implications. J Biol Chem. 1989 Oct 25;264(30):17834–17837. [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Irie T., Yoshida M., Takeishi K., Ukita T. The primary structure of yeast glutamic acid tRNA specific to the GAA codon. Biochim Biophys Acta. 1974 Oct 11;366(2):168–181. doi: 10.1016/0005-2787(74)90331-1. [DOI] [PubMed] [Google Scholar]
- Koch W., Edwards K., Kössel H. Sequencing of the 16S-23S spacer in a ribosomal RNA operon of Zea mays chloroplast DNA reveals two split tRNA genes. Cell. 1981 Jul;25(1):203–213. doi: 10.1016/0092-8674(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Yaginuma K., Taira M., Yoshida E., Imai M. Nucleotide sequence and evolution of the rat mitochondrial cytochrome b gene containing the ochre termination codon. Gene. 1982 Dec;20(2):177–185. doi: 10.1016/0378-1119(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Yaginuma K., Taira M., Yoshida E., Imai M. Nucleotide sequence and evolution of the rat mitochondrial cytochrome b gene containing the ochre termination codon. Gene. 1982 Dec;20(2):177–185. doi: 10.1016/0378-1119(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Yaginuma K., Taira M., Yoshida E., Imai M. Nucleotide sequence and evolution of the rat mitochondrial cytochrome b gene containing the ochre termination codon. Gene. 1982 Dec;20(2):177–185. doi: 10.1016/0378-1119(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Koski R. A., Clarkson S. G. Synthesis and maturation of Xenopus laevis methionine tRNA gene transcripts in homologous cell-free extracts. J Biol Chem. 1982 Apr 25;257(8):4514–4521. [PubMed] [Google Scholar]
- Krupp J. L., Shu H. H., Martin N. C. Human tRNASer gene organization and a tRNASer gene sequence. Nucleic Acids Res. 1988 Jan 25;16(2):770–770. doi: 10.1093/nar/16.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko V. N., Shlyapnikov M. G., Azbarov V. G., Garcia O., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment containing a distal part of tRNA gene region. Nucleic Acids Res. 1987 Jul 10;15(13):5480–5481. doi: 10.1093/nar/15.13.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko V. N., Shlyapnikov M. G., Azbarov V. G., Garcia O., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment containing a distal part of tRNA gene region. Nucleic Acids Res. 1987 Jul 10;15(13):5480–5481. doi: 10.1093/nar/15.13.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko V. N., Shlyapnikov M. G., Azbarov V. G., Garcia O., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment containing a distal part of tRNA gene region. Nucleic Acids Res. 1987 Jul 10;15(13):5480–5481. doi: 10.1093/nar/15.13.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko V. N., Shlyapnikov M. G., Azbarov V. G., Garcia O., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment containing a distal part of tRNA gene region. Nucleic Acids Res. 1987 Jul 10;15(13):5480–5481. doi: 10.1093/nar/15.13.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko V. N., Shlyapnikov M. G., Azbarov V. G., Garcia O., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment containing a distal part of tRNA gene region. Nucleic Acids Res. 1987 Jul 10;15(13):5480–5481. doi: 10.1093/nar/15.13.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Beier H., Akita N., Nishimura S. Natural UAG suppressor glutamine tRNA is elevated in mouse cells infected with Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1987 May;84(9):2668–2672. doi: 10.1073/pnas.84.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Tashiro F., Nishimura S. Tetrahymena thermophila glutamine tRNA and its gene that corresponds to UAA termination codon. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4758–4762. doi: 10.1073/pnas.82.14.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Ihara M., Yabusaki Y., Nishimura S. Initiator tRNAs from archaebacteria show common unique sequence characteristics. Nature. 1982 Aug 12;298(5875):684–685. doi: 10.1038/298684a0. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Nishimura S. Structure and transcription of the tRNAPro1 gene from Escherichia coli. Nucleic Acids Res. 1985 May 10;13(9):3213–3220. doi: 10.1093/nar/13.9.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Shindo-Okada N., Ando N., Watanabe S., Nishimura S. Nucleotide sequences of two aspartic acid tRNAs from rat liver and rat ascites hepatoma. J Biol Chem. 1981 Sep 10;256(17):9059–9062. [PubMed] [Google Scholar]
- Kuchino Y., Watanabe S., Harada F., Nishimura S. Primary structure of AUA-specific isoleucine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1980 May 13;19(10):2085–2089. doi: 10.1021/bi00551a013. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Yamamoto I., Nishimura S. Nucleotide sequence of Streptomyces griseus initiator tRNA. Nucleic Acids Res. 1982 Nov 11;10(21):6671–6674. doi: 10.1093/nar/10.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M., Evrard J. L., Weil J. H. Nucleotide sequence of the tRNA(Ser)GGA and tRNA(Gly)GCC genes from cyanelles of Cyanophora paradoxa. Nucleic Acids Res. 1988 Sep 12;16(17):8733–8733. doi: 10.1093/nar/16.17.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- LaRue B., Newhouse N., Nicoghosian K., Cedergren R. J. The evolution of multi-isoacceptor tRNA families. Sequence of tRNA Leu CAA and tRNA Leu CAG from Anacystis nidulans. J Biol Chem. 1981 Feb 25;256(4):1539–1543. [PubMed] [Google Scholar]
- Lawlor E. J., Baylis H. A., Chater K. F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev. 1987 Dec;1(10):1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- Learn G. H., Durbin M. L., Clegg M. T. A gene for tRNA-Ile(CAU) from chloroplasts of a monocot, Pennisetum americanum. Nucleic Acids Res. 1988 May 25;16(10):4734–4734. doi: 10.1093/nar/16.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Rajagopalan M., Kim Y. S., You K. H., Jacobson K. B., Hatfield D. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol Cell Biol. 1990 May;10(5):1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Rajagopalan M., Kim Y. S., You K. H., Jacobson K. B., Hatfield D. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol Cell Biol. 1990 May;10(5):1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Rajagopalan M., Kim Y. S., You K. H., Jacobson K. B., Hatfield D. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol Cell Biol. 1990 May;10(5):1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. H., Liaw L. L., Yung T. T., Lo S. J. Nucleotide sequence of the structural genes for the mitochondrial asp, lys, ser-tRNAs from chicken. Nucleic Acids Res. 1989 Nov 25;17(22):9477–9477. doi: 10.1093/nar/17.22.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. H., Liaw L. L., Yung T. T., Lo S. J. Nucleotide sequence of the structural genes for the mitochondrial asp, lys, ser-tRNAs from chicken. Nucleic Acids Res. 1989 Nov 25;17(22):9477–9477. doi: 10.1093/nar/17.22.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. H., Liaw L. L., Yung T. T., Lo S. J. Nucleotide sequence of the structural genes for the mitochondrial asp, lys, ser-tRNAs from chicken. Nucleic Acids Res. 1989 Nov 25;17(22):9477–9477. doi: 10.1093/nar/17.22.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmbeck J., Stummann B. M., Henningsen K. W. Sequence of two regions of pea chloroplast DNA, one with the genes rps14, trnfM and trnG-GCC, and one with the genes trnP-UGG and trnW-CCA. Nucleic Acids Res. 1987 Apr 24;15(8):3630–3630. doi: 10.1093/nar/15.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmbeck J., Stummann B. M., Henningsen K. W. Sequence of two regions of pea chloroplast DNA, one with the genes rps14, trnfM and trnG-GCC, and one with the genes trnP-UGG and trnW-CCA. Nucleic Acids Res. 1987 Apr 24;15(8):3630–3630. doi: 10.1093/nar/15.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Leon P., Walbot V., Bedinger P. Molecular analysis of the linear 2.3 kb plasmid of maize mitochondria: apparent capture of tRNA genes. Nucleic Acids Res. 1989 Jun 12;17(11):4089–4099. doi: 10.1093/nar/17.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon P., Walbot V., Bedinger P. Molecular analysis of the linear 2.3 kb plasmid of maize mitochondria: apparent capture of tRNA genes. Nucleic Acids Res. 1989 Jun 12;17(11):4089–4099. doi: 10.1093/nar/17.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979 Sep;18(1):47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- Lin F. K., Furr T. D., Chang S. H., Horwitz J., Agris P. F., Ortwerth B. J. The nucleotide sequence of two bovine lens phenylalanine tRNAs. Possible activation of a new phenylalanine tRNA gene during differentiation of lens cells. J Biol Chem. 1980 Jul 10;255(13):6020–6023. [PubMed] [Google Scholar]
- Lin J. P., Aker M., Sitney K. C., Mortimer R. K. First position wobble in codon-anticodon pairing: amber suppression by a yeast glutamine tRNA. Gene. 1986;49(3):383–388. doi: 10.1016/0378-1119(86)90375-6. [DOI] [PubMed] [Google Scholar]
- Looney J. E., Harding J. D. Structure and evolution of a mouse tRNA gene cluster encoding tRNAAsp, tRNAGly and tRNAGlu and an unlinked, solitary gene encoding tRNAAsp. Nucleic Acids Res. 1983 Dec 20;11(24):8761–8775. doi: 10.1093/nar/11.24.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney J. E., Harding J. D. Structure and evolution of a mouse tRNA gene cluster encoding tRNAAsp, tRNAGly and tRNAGlu and an unlinked, solitary gene encoding tRNAAsp. Nucleic Acids Res. 1983 Dec 20;11(24):8761–8775. doi: 10.1093/nar/11.24.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J. H., Bogorad L. Nucleotide sequence containing the maize chloroplast proline (UGG) and tryptophan (CCA) tRNA genes. Nucleic Acids Res. 1988 Jun 10;16(11):5192–5192. doi: 10.1093/nar/16.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J. H., Bogorad L. Nucleotide sequence containing the maize chloroplast proline (UGG) and tryptophan (CCA) tRNA genes. Nucleic Acids Res. 1988 Jun 10;16(11):5192–5192. doi: 10.1093/nar/16.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. P., Doebley J. Nucleotide sequence of the split tRNAleu(UAA) gene from Sorghum bicolor chloroplasts. Gene. 1986;43(1-2):169–174. doi: 10.1016/0378-1119(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Ma D. P., Yang Y. W., Hasnain S. E. Nucleotide sequence of Chlamydomonas reinhardtii mitochondrial genes coding for subunit 6 of NADH dehydrogenase and tRNATrp. Nucleic Acids Res. 1988 Dec 9;16(23):11373–11373. doi: 10.1093/nar/16.23.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. P., Yang Y. W. Nucleotide sequence of a tRNA(Gly) gene from Sorghum bicolor. Nucleic Acids Res. 1988 Apr 25;16(8):3588–3588. doi: 10.1093/nar/16.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson J. M., Roy K. L. Two human tyrosine tRNA genes contain introns. Gene. 1986;42(1):101–106. doi: 10.1016/0378-1119(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Madison J. T., Boguslawski S. J. Partial digestion of a yeast lysine transfer ribonucleic acid and reconstruction of the nucleotide sequence. Biochemistry. 1974 Jan 29;13(3):524–527. doi: 10.1021/bi00700a019. [DOI] [PubMed] [Google Scholar]
- Makowski D. R., Haas R. A., Dolan K. P., Grunberger D. Molecular cloning, sequence analysis and in vitro expression of a rat tRNA gene cluster. Nucleic Acids Res. 1983 Dec 20;11(24):8609–8624. doi: 10.1093/nar/11.24.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart J. R., Palmer J. D. The gain of two chloroplast tRNA introns marks the green algal ancestors of land plants. Nature. 1990 May 17;345(6272):268–270. doi: 10.1038/345268a0. [DOI] [PubMed] [Google Scholar]
- Manhart J. R., Palmer J. D. The gain of two chloroplast tRNA introns marks the green algal ancestors of land plants. Nature. 1990 May 17;345(6272):268–270. doi: 10.1038/345268a0. [DOI] [PubMed] [Google Scholar]
- Manhart J. R., Palmer J. D. The gain of two chloroplast tRNA introns marks the green algal ancestors of land plants. Nature. 1990 May 17;345(6272):268–270. doi: 10.1038/345268a0. [DOI] [PubMed] [Google Scholar]
- Manzara T., Hallick R. B. Nucleotide sequence of the Euglena gracilis chloroplast genes for serine and proline transfer RNAs and a functional open reading frame. Nucleic Acids Res. 1988 Oct 25;16(20):9866–9866. doi: 10.1093/nar/16.20.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Mignery R. E., Dudock B. S. Complete nucleotide sequence and properties of the major species of glycine transfer RNA from wheat germ. Biochemistry. 1977 Feb 22;16(4):797–806. doi: 10.1021/bi00623a036. [DOI] [PubMed] [Google Scholar]
- Marechal L., Runeberg-Roos P., Grienenberger J. M., Colin J., Weil J. H., Lejeune B., Quetier F., Lonsdale D. M. Homology in the region containing a tRNA(Trp) gene and a (complete or partial) tRNA(Pro) gene in wheat mitochondrial and chloroplast genomes. Curr Genet. 1987;12(2):91–98. doi: 10.1007/BF00434662. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Pham H. D., Underbrink-Lyon K., Miller D. l., Donelson J. E. Yeast mitochondrial tRNATrp can recognize the nonsense codon UGA. Nature. 1980 Jun 19;285(5766):579–581. doi: 10.1038/285579a0. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Schneller J. M., Keith G., Stahl A. J., Dirheimer G. Primary structure of yeast mitochondrial DNA-coded phenylalanine-tRNA. Nucleic Acids Res. 1978 Dec;5(12):4579–4592. doi: 10.1093/nar/5.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Sibler A. P., Dirheimer G. The primary structures of three yeast mitochondrial serine tRNA isoacceptors. Biochimie. 1982 Nov-Dec;64(11-12):1073–1079. doi: 10.1016/s0300-9084(82)80389-1. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Guillemaut P. Nucleotide sequence of bean mitochondrial tRNALeu4 and of its cytoplasmic counterpart. Re-examination of the modified nucleotide present at position 12 in bean mitochondrial and cytoplasmic tRNALeu1 sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11812–11812. doi: 10.1093/nar/16.24.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Guillemaut P. Nucleotide sequence of bean mitochondrial tRNALeu4 and of its cytoplasmic counterpart. Re-examination of the modified nucleotide present at position 12 in bean mitochondrial and cytoplasmic tRNALeu1 sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11812–11812. doi: 10.1093/nar/16.24.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Guillemaut P. Nucleotide sequence of bean mitochondrial tRNALeu4 and of its cytoplasmic counterpart. Re-examination of the modified nucleotide present at position 12 in bean mitochondrial and cytoplasmic tRNALeu1 sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11812–11812. doi: 10.1093/nar/16.24.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Guillemaut P. Nucleotide sequence of bean mitochondrial tRNALeu4 and of its cytoplasmic counterpart. Re-examination of the modified nucleotide present at position 12 in bean mitochondrial and cytoplasmic tRNALeu1 sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11812–11812. doi: 10.1093/nar/16.24.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Neuburger M., Guillemaut P., Douce R., Weil J. H., Dietrich A. A nuclear-encoded potato (Solanum tuberosum) mitochondrial tRNA(Leu) and its cytosolic counterpart have identical nucleotide sequences. FEBS Lett. 1990 Mar 26;262(2):170–172. doi: 10.1016/0014-5793(90)80181-h. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Neuburger M., Guillemaut P., Douce R., Weil J. H., Dietrich A. A nuclear-encoded potato (Solanum tuberosum) mitochondrial tRNA(Leu) and its cytosolic counterpart have identical nucleotide sequences. FEBS Lett. 1990 Mar 26;262(2):170–172. doi: 10.1016/0014-5793(90)80181-h. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal L., Guillemaut P., Grienenberger J. M., Jeannin G., Weil J. H. Sequence and codon recognition of bean mitochondria and chloroplast tRNAsTrp: evidence for a high degree of homology. Nucleic Acids Res. 1985 Jun 25;13(12):4411–4416. doi: 10.1093/nar/13.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massardo D. R. Nucleotide sequence of the genes encoding tRNA(his), tRNA(pro) and tRNA(gln) in the mitochondrial genome of Schizosaccharomyces pombe strain EF1. Nucleic Acids Res. 1990 Nov 11;18(21):6429–6429. doi: 10.1093/nar/18.21.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massardo D. R. Nucleotide sequence of the genes encoding tRNA(his), tRNA(pro) and tRNA(gln) in the mitochondrial genome of Schizosaccharomyces pombe strain EF1. Nucleic Acids Res. 1990 Nov 11;18(21):6429–6429. doi: 10.1093/nar/18.21.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massardo D. R. Nucleotide sequence of the genes encoding tRNA(his), tRNA(pro) and tRNA(gln) in the mitochondrial genome of Schizosaccharomyces pombe strain EF1. Nucleic Acids Res. 1990 Nov 11;18(21):6429–6429. doi: 10.1093/nar/18.21.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Ohta T., Kumagai I., Oshima T., Murao K., Hasegawa T., Ishikura H., Watanabe K. A thermostable Gm-methylase recognizes the tertiary structure of tRNA. J Biochem. 1987 May;101(5):1191–1198. doi: 10.1093/oxfordjournals.jbchem.a121983. [DOI] [PubMed] [Google Scholar]
- Mazabraud A. The nucleotide sequence of phenylalanine tRNA of Xenopus laevis. Biochimie. 1982 Oct;64(10):955–960. doi: 10.1016/s0300-9084(82)80359-3. [DOI] [PubMed] [Google Scholar]
- Mazzara G. P., Seidman J. G., McClain W. H., Yesian H., Abelson J., Guthrie C. Nucleotide sequence of an arginine transfer ribonucleic acid from bacteriophage T4. J Biol Chem. 1977 Nov 25;252(22):8245–8253. [PubMed] [Google Scholar]
- McClain W. H., Barrell B. G., Seidman J. G. Nucleotide alterations in bacteriophage T4 serine transfer RNA that affect the conversion of precursor RNA into transfer RNA. J Mol Biol. 1975 Dec 25;99(4):717–732. doi: 10.1016/s0022-2836(75)80181-1. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Hybrid transfer RNA genes in phage T4. Cell. 1984 Aug;38(1):225–231. doi: 10.1016/0092-8674(84)90544-0. [DOI] [PubMed] [Google Scholar]
- McCorkle G. M., Altman S. Large deletion mutants of Escherichia coli tRNATyr1. J Mol Biol. 1982 Feb 25;155(2):83–103. doi: 10.1016/0022-2836(82)90438-7. [DOI] [PubMed] [Google Scholar]
- McCoy J. M., Jones D. S. The nucleotide sequence of Scenedesmus obliquus chloroplast tRNAfMet. Nucleic Acids Res. 1980 Nov 11;8(21):5089–5093. doi: 10.1093/nar/8.21.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke A., Igloi G. L., Kössel H. Nucleotide sequence of tDNA(Cys)GCA and its flanking regions from Zea mays chloroplasts. Nucleic Acids Res. 1988 Jun 24;16(12):5696–5696. doi: 10.1093/nar/16.12.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall M. D., Leeds P., Fen H., Mathison L., Zwick M., Sleiziz C., Culbertson M. R. Frameshift suppressor mutations affecting the major glycine transfer RNAs of Saccharomyces cerevisiae. J Mol Biol. 1987 Mar 5;194(1):41–58. doi: 10.1016/0022-2836(87)90714-5. [DOI] [PubMed] [Google Scholar]
- Meng Y. B., Stevens R. D., Chia W., McGill S., Ashburner M. Five glycyl tRNA genes within the noc gene complex of Drosophila melanogaster. Nucleic Acids Res. 1988 Jul 25;16(14B):7189–7189. doi: 10.1093/nar/16.14.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichi B., Arnold H. H., Heyman T., Dirheimer G., Keith G. Primary structure of Bacillus subtilis tRNAsTyr. Biochem Biophys Res Commun. 1980 Jul 16;95(1):461–467. doi: 10.1016/0006-291x(80)90760-3. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C., Pham H. D., Donelson J. E. Sequence analysis of two yeast mitochondrial DNA fragments containing the genes for tRNA Ser UCR and tRNA Phe UUY. J Biol Chem. 1979 Nov 25;254(22):11735–11740. [PubMed] [Google Scholar]
- Miller D. L., Najarian D. R., Folse J. R., Martin N. C. A mutation in the tRNAAsp gene from yeast mitochondria. Effects on RNA and protein synthesis. J Biol Chem. 1981 Oct 10;256(19):9774–9777. [PubMed] [Google Scholar]
- Miller D. L., Sigurdson C., Martin N. C., Donelson J. E. Nucleotide sequence of the mitochondrial genes coding for tRNAglyGGR and tRNAvalGUR. Nucleic Acids Res. 1980 Mar 25;8(6):1435–1442. doi: 10.1093/nar/8.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Pirtle I. L., Dudock B. S., Pirtle R. M. The nucleotide sequence of arginine tRNACCG from bovine liver. Nucleic Acids Res. 1983 Apr 11;11(7):2013–2016. doi: 10.1093/nar/11.7.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Yokota T., Takebe Y., Nakamura M., Kaziro Y. A deletion mutant lacking three out of four transfer RNA genes upstream of the coding region of tufB. J Biochem. 1983 Apr;93(4):1101–1108. doi: 10.1093/oxfordjournals.jbchem.a134235. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Yokota T., Takebe Y., Nakamura M., Kaziro Y. A deletion mutant lacking three out of four transfer RNA genes upstream of the coding region of tufB. J Biochem. 1983 Apr;93(4):1101–1108. doi: 10.1093/oxfordjournals.jbchem.a134235. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Yokota T., Takebe Y., Nakamura M., Kaziro Y. A deletion mutant lacking three out of four transfer RNA genes upstream of the coding region of tufB. J Biochem. 1983 Apr;93(4):1101–1108. doi: 10.1093/oxfordjournals.jbchem.a134235. [DOI] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. Phylogenetic relationships and altered genome structures among Tetrahymena mitochondrial DNAs. Nucleic Acids Res. 1988 Jan 11;16(1):327–346. doi: 10.1093/nar/16.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morry M. J., Harding J. D. Modulation of transcriptional activity and stable complex formation by 5'-flanking regions of mouse tRNAHis genes. Mol Cell Biol. 1986 Jan;6(1):105–115. doi: 10.1128/mcb.6.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morry M. J., Harding J. D. Modulation of transcriptional activity and stable complex formation by 5'-flanking regions of mouse tRNAHis genes. Mol Cell Biol. 1986 Jan;6(1):105–115. doi: 10.1128/mcb.6.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munninger K. O., Chang S. H. A fluorescent nucleoside from glutamic acid tRNA of Escherichia coli K 12. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1837–1842. doi: 10.1016/0006-291x(72)90059-9. [DOI] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. Nucleotide sequence of valine tRNA mo5UAC from bacillus subtilis. Nucleic Acids Res. 1982 Jan 22;10(2):715–718. doi: 10.1093/nar/10.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A., Takemura S. Nucleotide sequence of leucine transfer RNA 1 from Candida (Torulopsis) utilis. J Biochem. 1978 Apr;83(4):1029–1038. doi: 10.1093/oxfordjournals.jbchem.a131991. [DOI] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Clarkson S. G. Nucleotide sequence of genes coding for tRNAPhe and tRNATyr from a repeating unit of X. laevis DNA. Cell. 1980 Feb;19(2):345–353. doi: 10.1016/0092-8674(80)90509-7. [DOI] [PubMed] [Google Scholar]
- Nagase T., Ishii S., Imamoto F. Differential transcriptional control of the two tRNA(fMet) genes of Escherichia coli K-12. Gene. 1988 Jul 15;67(1):49–57. doi: 10.1016/0378-1119(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Ozeki H., Shimura Y. Organization and structure of an E. coli tRNA operon containing seven tRNA genes. Cell. 1981 Jan;23(1):239–249. doi: 10.1016/0092-8674(81)90288-9. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Ozeki H., Shimura Y. Organization and structure of an E. coli tRNA operon containing seven tRNA genes. Cell. 1981 Jan;23(1):239–249. doi: 10.1016/0092-8674(81)90288-9. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Ozeki H., Shimura Y. Organization and structure of an E. coli tRNA operon containing seven tRNA genes. Cell. 1981 Jan;23(1):239–249. doi: 10.1016/0092-8674(81)90288-9. [DOI] [PubMed] [Google Scholar]
- Nelböck P., Stucka R., Feldmann H. Different patterns of transposable elements in the vicinity of tRNA genes in yeast: a possible clue to transcriptional modulation. Biol Chem Hoppe Seyler. 1985 Nov;366(11):1041–1051. doi: 10.1515/bchm3.1985.366.2.1041. [DOI] [PubMed] [Google Scholar]
- Netzker R., Köchel H. G., Basak N., Küntzel H. Nucleotide sequence of Aspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNAs and L-rRNA. Nucleic Acids Res. 1982 Aug 11;10(15):4783–4794. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzker R., Köchel H. G., Basak N., Küntzel H. Nucleotide sequence of Aspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNAs and L-rRNA. Nucleic Acids Res. 1982 Aug 11;10(15):4783–4794. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzker R., Köchel H. G., Basak N., Küntzel H. Nucleotide sequence of Aspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNAs and L-rRNA. Nucleic Acids Res. 1982 Aug 11;10(15):4783–4794. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzker R., Köchel H. G., Basak N., Küntzel H. Nucleotide sequence of Aspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNAs and L-rRNA. Nucleic Acids Res. 1982 Aug 11;10(15):4783–4794. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus H. Nucleotide sequence of the chloroplast genes for tRNA(Gln) and the 4 kD K polypeptide of photosystem II from mustard (Sinapis alba). Nucleic Acids Res. 1989 Jan 11;17(1):444–444. doi: 10.1093/nar/17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D., Pham H. D., Underbrink-Lyon K., Martin N. C. Characterization of tRNA genes in tRNA region II of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5007–5016. doi: 10.1093/nar/8.21.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen J., Link G. Nucleotide sequence of the mustard chloroplast genes trnH and rps19'. Nucleic Acids Res. 1990 Feb 25;18(4):1051–1051. doi: 10.1093/nar/18.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Leick V. Specific photoreactions between psoralens and yeast-tRNAPhe. Biochem Biophys Res Commun. 1982 May 14;106(1):179–185. doi: 10.1016/0006-291x(82)92075-7. [DOI] [PubMed] [Google Scholar]
- Nóbrega M. P., Nóbrega F. G. Mapping and sequencing of the wild-type and mutant (G116-40) alleles of the tyrosyl-tRNA mitochondrial gene in Saccharomyces cerevisiae. J Biol Chem. 1986 Mar 5;261(7):3054–3059. [PubMed] [Google Scholar]
- O'Neill G. P., Peterson D. M., Schön A., Chen M. W., Söll D. Formation of the chlorophyll precursor delta-aminolevulinic acid in cyanobacteria requires aminoacylation of a tRNAGlu species. J Bacteriol. 1988 Sep;170(9):3810–3816. doi: 10.1128/jb.170.9.3810-3816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. P., Schön A., Chow H., Chen M. W., Kim Y. C., Söll D. Sequence of tRNA(Glu) and its genes from the chloroplast genome of Chlamydomonas reinhardtii. Nucleic Acids Res. 1990 Oct 11;18(19):5893–5893. doi: 10.1093/nar/18.19.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden R. C., Beckman J. S., Abelson J., Kang H. S., Söll D., Schmidt O. In vitro transcription and processing of a yeast tRNA gene containing an intervening sequence. Cell. 1979 Jun;17(2):399–406. doi: 10.1016/0092-8674(79)90166-1. [DOI] [PubMed] [Google Scholar]
- Ohashi Ziro, Harada Fumio, Nishimura Susumu. Primary sequence of glutamic acid tRNA II from Escherichia coli. FEBS Lett. 1972 Feb 1;20(2):239–241. doi: 10.1016/0014-5793(72)80804-4. [DOI] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozaki K., Sugiura M. Structure and cotranscription of tobacco chloroplast genes for tRNAGlu(UUC), tRNATyr(GUA) and tRNAAsp(GUC). Nucleic Acids Res. 1985 Feb 25;13(4):1045–1056. doi: 10.1093/nar/13.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozaki K., Sugiura M. Structure and cotranscription of tobacco chloroplast genes for tRNAGlu(UUC), tRNATyr(GUA) and tRNAAsp(GUC). Nucleic Acids Res. 1985 Feb 25;13(4):1045–1056. doi: 10.1093/nar/13.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozaki K., Sugiura M. Structure and cotranscription of tobacco chloroplast genes for tRNAGlu(UUC), tRNATyr(GUA) and tRNAAsp(GUC). Nucleic Acids Res. 1985 Feb 25;13(4):1045–1056. doi: 10.1093/nar/13.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Okimoto R., Wolstenholme D. R. A set of tRNAs that lack either the T psi C arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990 Oct;9(10):3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Wolstenholme D. R. A set of tRNAs that lack either the T psi C arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990 Oct;9(10):3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins P. O., Jones D. S. Nucleotide sequence of Scenedesmus obliquus cytoplasmic initiator tRNA. Nucleic Acids Res. 1980 Feb 25;8(4):715–729. [PMC free article] [PubMed] [Google Scholar]
- Olson M. V., Page G. S., Sentenac A., Piper P. W., Worthington M., Weiss R. B., Hall B. D. Only one of two closely related yeast suppressor tRNA genes contains an intervening sequence. Nature. 1981 Jun 11;291(5815):464–469. doi: 10.1038/291464a0. [DOI] [PubMed] [Google Scholar]
- Orellana O., Cooley L., Söll D. The additional guanylate at the 5' terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol Cell Biol. 1986 Feb;6(2):525–529. doi: 10.1128/mcb.6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Almeida M. L., Guillemaut P., Keith G., Canaday J., Weil J. H. Primary structure of three leucine transfer RNAs from bean chloroplast. Biochem Biophys Res Commun. 1980 Jan 15;92(1):102–108. doi: 10.1016/0006-291x(80)91525-9. [DOI] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., 3rd, Timothy D. H. Identification of an aspartate transfer RNA gene in maize mitochondrial DNA. Curr Genet. 1985;9(6):517–519. doi: 10.1007/BF00434056. [DOI] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., Timothy D. H. Identification of two methionine transfer RNA genes in the maize mitochondrial genome. Plant Physiol. 1984 Dec;76(4):1079–1082. doi: 10.1104/pp.76.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., Timothy D. H. Identification of two methionine transfer RNA genes in the maize mitochondrial genome. Plant Physiol. 1984 Dec;76(4):1079–1082. doi: 10.1104/pp.76.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Gegenheimer P., Abelson J. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell. 1983 Feb;32(2):525–536. doi: 10.1016/0092-8674(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Peffley D. M., Sogin M. L. A putative tRNATrp gene cloned from Dictyostelium discoideum: its nucleotide sequence and association with repetitive deoxyribonucleic acid. Biochemistry. 1981 Jul 7;20(14):4015–4021. doi: 10.1021/bi00517a010. [DOI] [PubMed] [Google Scholar]
- Penswick J. R., Martin R., Dirheimer G. Evidence supporting a revised sequence for yeast alanine tRNA. FEBS Lett. 1975 Jan 15;50(1):28–31. doi: 10.1016/0014-5793(75)81033-7. [DOI] [PubMed] [Google Scholar]
- Pepe G., Holtrop M., Gadaleta G., Kroon A. M., Cantatore P., Gallerani R., De Benedetto C., Quagliariello C., Sbisà E., Saccone C. Non-random patterns of nucleotide substitutions and codon strategy in the mammalian mitochondrial genes coding for identified and unidentified reading frames. Biochem Int. 1983 Apr;6(4):553–563. [PubMed] [Google Scholar]
- Peterson R. C. Sequence and transcription of tRNAVal gene from Xenopus laevis. Biochim Biophys Acta. 1987 Jan 28;908(1):81–89. doi: 10.1016/0167-4781(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Pillay D. T., Guillemaut P., Weil J. H. Nucleotide sequences of three soybean chloroplast tRNAsLeu and re-examination of bean chloroplast tRNA2Leu sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2997–3001. doi: 10.1093/nar/12.6.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. W. The nucleotide sequence of a methionine tRNA which functions in protein elongation in mouse myeloma cells. Eur J Biochem. 1975 Feb 3;51(1):283–293. doi: 10.1111/j.1432-1033.1975.tb03928.x. [DOI] [PubMed] [Google Scholar]
- Piper P. W. The primary structure of the major cytoplasmic valine tRNA of mouse myeloma cells. Eur J Biochem. 1975 Feb 3;51(1):295–304. doi: 10.1111/j.1432-1033.1975.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Pirtle R., Calagan J., Pirtle I., Kashdan M., Vreman H., Dudock B. The nucleotide sequence of spinach chloroplast methionine elongator tRNA. Nucleic Acids Res. 1981 Jan 10;9(1):183–188. doi: 10.1093/nar/9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtle R., Kashdan M., Pirtle I., Dudock B. The nucleotide sequence of a major species of leucine tRNA from bovine liver. Nucleic Acids Res. 1980 Feb 25;8(4):805–815. [PMC free article] [PubMed] [Google Scholar]
- Pittet A. C., Hottinger H. Sequence of a hexameric tRNA gene cluster associated with rRNA genes in Lactobacillus bulgaricus. Nucleic Acids Res. 1989 Jun 26;17(12):4873–4873. [PMC free article] [PubMed] [Google Scholar]
- Pittet A. C., Hottinger H. Sequence of a hexameric tRNA gene cluster associated with rRNA genes in Lactobacillus bulgaricus. Nucleic Acids Res. 1989 Jun 26;17(12):4873–4873. [PMC free article] [PubMed] [Google Scholar]
- Pittet A. C., Hottinger H. Sequence of a hexameric tRNA gene cluster associated with rRNA genes in Lactobacillus bulgaricus. Nucleic Acids Res. 1989 Jun 26;17(12):4873–4873. [PMC free article] [PubMed] [Google Scholar]
- Pittet A. C., Hottinger H. Sequence of a hexameric tRNA gene cluster associated with rRNA genes in Lactobacillus bulgaricus. Nucleic Acids Res. 1989 Jun 26;17(12):4873–4873. [PMC free article] [PubMed] [Google Scholar]
- Pittet A. C., Hottinger H. Sequence of a hexameric tRNA gene cluster associated with rRNA genes in Lactobacillus bulgaricus. Nucleic Acids Res. 1989 Jun 26;17(12):4873–4873. [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Mahalingam R., Sable C. L., Venuti S. E., Cummings D. J. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990 Jan 11;18(1):173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Mahalingam R., Sable C. L., Venuti S. E., Cummings D. J. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990 Jan 11;18(1):173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Mahalingam R., Sable C. L., Venuti S. E., Cummings D. J. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990 Jan 11;18(1):173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrissant G., Boisnard M. Particularités structurales du méthionin-tRNAmMet de foie de lapin. Biochimie. 1974;56(5):787–790. doi: 10.1016/s0300-9084(74)80053-2. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Randerath E., Agrawal H. P., Randerath K. Rat liver mitochondrial lysine tRNA (anticodon U*UU) contains a rudimentary D-arm and 2 hypermodified nucleotides in its anticodon loop. Biochem Biophys Res Commun. 1981 Nov 30;103(2):739–744. doi: 10.1016/0006-291x(81)90511-8. [DOI] [PubMed] [Google Scholar]
- Randerath E., Gupta R. C., Chia L. L., Chang S. H., Randerath K. Yeast tRNA Leu UAG. Purification, properties and determination of the nucleotide sequence by radioactive derivative methods. Eur J Biochem. 1979 Jan 2;93(1):79–94. doi: 10.1111/j.1432-1033.1979.tb12797.x. [DOI] [PubMed] [Google Scholar]
- Randerath E., Gupta R. C., Morris H. P., Randerath K. Isolation and sequence analysis of two major leucine transfer ribonucleic acids (anticodon Mm-A-A) from a rat tumor, Morris hepatoma 5123D. Biochemistry. 1980 Jul 22;19(15):3476–3483. doi: 10.1021/bi00556a011. [DOI] [PubMed] [Google Scholar]
- Randerath K., Agrawal H. P., Randerath E. Tumor mitochondrial transfer RNAs: the nucleotide sequence of mitochondrial tRNALeuUAG from Morris hepatoma 5123D. Biochem Biophys Res Commun. 1981 May 29;100(2):732–737. doi: 10.1016/s0006-291x(81)80236-7. [DOI] [PubMed] [Google Scholar]
- Randerath K., Agrawal H. P., Randerath E. tRNA alterations in cancer. Recent Results Cancer Res. 1983;84:103–120. doi: 10.1007/978-3-642-81947-6_7. [DOI] [PubMed] [Google Scholar]
- Randerath K., Agrawal H. P., Randerath E. tRNA alterations in cancer. Recent Results Cancer Res. 1983;84:103–120. doi: 10.1007/978-3-642-81947-6_7. [DOI] [PubMed] [Google Scholar]
- Randerath K., Agrawal H. P., Randerath E. tRNA alterations in cancer. Recent Results Cancer Res. 1983;84:103–120. doi: 10.1007/978-3-642-81947-6_7. [DOI] [PubMed] [Google Scholar]
- Rashtchian A., Shaffer M. The nucleotide sequences of two tRNA genes from Campylobacter jejuni. Nucleic Acids Res. 1986 Jul 11;14(13):5560–5560. [PMC free article] [PubMed] [Google Scholar]
- Rashtchian A., Shaffer M. The nucleotide sequences of two tRNA genes from Campylobacter jejuni. Nucleic Acids Res. 1986 Jul 11;14(13):5560–5560. [PMC free article] [PubMed] [Google Scholar]
- Richard M., Bellemare G. Nucleotide sequence of Chlamydomonas moewusii chloroplastic tRNA(thr). Nucleic Acids Res. 1990 May 25;18(10):3061–3061. doi: 10.1093/nar/18.10.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos H., Lawrence G. H., Stewart T. S. The nucleotide sequence of bovine tRNA(Ser)UGA gene and its flanking sequences. Nucleic Acids Res. 1989 Mar 11;17(5):2139–2139. doi: 10.1093/nar/17.5.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Carbon J. Nucleotide sequence studies of normal and genetically altered glycine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5530–5541. [PubMed] [Google Scholar]
- Roberts R. J. Staphylococcal transfer ribonucleic acids. II. Sequence analysis of isoaccepting glycine transfer ribonucleic acids IA and IB from Staphylococcus epidermidis Texas 26. J Biol Chem. 1974 Aug 10;249(15):4787–4796. [PubMed] [Google Scholar]
- Robinson R. R., Davidson N. Analysis of a drosophila tRNA gene cluster: two tRNALeu genes contain intervening sequences. Cell. 1981 Jan;23(1):251–259. doi: 10.1016/0092-8674(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Rizi H. L., Weisz C., DiLauro M. N., Pike D., Chen C. Y., Chen E. Y. Comparison of rat liver and Walker 256 carcinosarcoma tRNAs. Nucleic Acids Res. 1979 Feb;6(2):673–688. doi: 10.1093/nar/6.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. D., Hendrick J. P., Jr, Lerner M. R., Steitz J. A., Reichlin M. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983 Feb 11;11(3):853–870. doi: 10.1093/nar/11.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Daniel V. Nucleotide sequence and transcription of a rat tRNA(Phe) gene and a neighboring Alu-like element. Gene. 1988 Sep 30;69(2):275–285. doi: 10.1016/0378-1119(88)90438-6. [DOI] [PubMed] [Google Scholar]
- Rosen A., Sarid S., Daniel V. Genes and pseudogenes in a reiterated rat tRNA gene cluster. Nucleic Acids Res. 1984 Jun 25;12(12):4893–4906. doi: 10.1093/nar/12.12.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Sarid S., Daniel V. Genes and pseudogenes in a reiterated rat tRNA gene cluster. Nucleic Acids Res. 1984 Jun 25;12(12):4893–4906. doi: 10.1093/nar/12.12.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Sarid S., Daniel V. Genes and pseudogenes in a reiterated rat tRNA gene cluster. Nucleic Acids Res. 1984 Jun 25;12(12):4893–4906. doi: 10.1093/nar/12.12.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Sarid S., Daniel V. Genes and pseudogenes in a reiterated rat tRNA gene cluster. Nucleic Acids Res. 1984 Jun 25;12(12):4893–4906. doi: 10.1093/nar/12.12.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. M., Looney J. E., Harding J. D. Nucleotide sequence of a mouse tRNALeu gene. Nucleic Acids Res. 1986 Jul 11;14(13):5567–5567. doi: 10.1093/nar/14.13.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Schold M., Larson G. P., Wallace R. B. Functional expression of a yeast ochre suppressor tRNA gene in Escherichia coli. Gene. 1982 Dec;20(3):423–432. doi: 10.1016/0378-1119(82)90211-6. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Cooke H., Buckland R. Nucleotide sequence of a segment of human DNA containing the three tRNA genes. Nucleic Acids Res. 1982 Nov 25;10(22):7313–7322. doi: 10.1093/nar/10.22.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Muskavitch K. M., Bogorad L. Location and sequence of the maize chloroplast gene for tRNAser (GCU); a third serine isoaccepting tRNA. Nucleic Acids Res. 1987 Jan 12;15(1):370–370. doi: 10.1093/nar/15.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo T., Costanzo F., Oliva A., Ammendola R., Duilio A., Esposito F., Cimino F. Structure and in vitro transcription of tRNA gene clusters containing the primers of MuLV reverse transcriptase. Eur J Biochem. 1986 Aug 1;158(3):437–442. doi: 10.1111/j.1432-1033.1986.tb09772.x. [DOI] [PubMed] [Google Scholar]
- Russo T., Duilio A., Ammendola R., Costanzo F., Cimino F. Nucleotide sequence of a mouse tRNA gene cluster. Nucleic Acids Res. 1987 Oct 26;15(20):8562–8562. doi: 10.1093/nar/15.20.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone C., Cantatore P., Gadaleta G., Gallerani R., Lanave C., Pepe G., Kroon A. M. The nucleotide sequence of the large ribosomal RNA gene and the adjacent tRNA genes from rat mitochondria. Nucleic Acids Res. 1981 Aug 25;9(16):4139–4148. doi: 10.1093/nar/9.16.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Borén T., Johansen T. I., Lustig F. Properties of a transfer RNA lacking modified nucleosides. J Biol Chem. 1988 Sep 25;263(27):13692–13699. [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S. B., Olson M. V. Insertion of a repetitive element at the same position in the 5'-flanking regions of two dissimilar yeast tRNA genes. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7674–7678. doi: 10.1073/pnas.79.24.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare A., Weil J. H., Grienenberger J. M. Nucleotide sequence of a maize mitochondrial tRNASer (UGA) gene. Nucleic Acids Res. 1989 Oct 11;17(19):7979–7979. doi: 10.1093/nar/17.19.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaré A., Lonsdale D., Weil J. H., Grienenberger J. M. Sequence analysis of the tRNA(Tyr) and tRNA(Lys) genes and evidence for the transcription of a chloroplast-like tRNA(Met) in maize mitochondria. Curr Genet. 1989 Sep;16(3):195–201. doi: 10.1007/BF00391477. [DOI] [PubMed] [Google Scholar]
- Sangaré A., Lonsdale D., Weil J. H., Grienenberger J. M. Sequence analysis of the tRNA(Tyr) and tRNA(Lys) genes and evidence for the transcription of a chloroplast-like tRNA(Met) in maize mitochondria. Curr Genet. 1989 Sep;16(3):195–201. doi: 10.1007/BF00391477. [DOI] [PubMed] [Google Scholar]
- Sangaré A., Lonsdale D., Weil J. H., Grienenberger J. M. Sequence analysis of the tRNA(Tyr) and tRNA(Lys) genes and evidence for the transcription of a chloroplast-like tRNA(Met) in maize mitochondria. Curr Genet. 1989 Sep;16(3):195–201. doi: 10.1007/BF00391477. [DOI] [PubMed] [Google Scholar]
- Santos T., Zasloff M. Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNAimet genes. Cell. 1981 Mar;23(3):699–709. doi: 10.1016/0092-8674(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. Phenylalanine and tyrosine transfer RNAs encoded by Tetrahymena pyriformis mitochondrial DNA: primary sequence, post-transcriptional modifications, and gene localization. Curr Genet. 1985;9(5):389–393. doi: 10.1007/BF00421610. [DOI] [PubMed] [Google Scholar]
- Schuster W., Brennicke A. Plastid, nuclear and reverse transcriptase sequences in the mitochondrial genome of Oenothera: is genetic information transferred between organelles via RNA? EMBO J. 1987 Oct;6(10):2857–2863. doi: 10.1002/j.1460-2075.1987.tb02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Grienenberger J. M., Weil J. H., Brennicke A. Comparison of tRNA(Trp) and tRNA(Pro) gene sequences in the chloroplast and mitochondrial genomes of oenothera. Nucleic Acids Res. 1988 Aug 11;16(15):7737–7737. doi: 10.1093/nar/16.15.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Grienenberger J. M., Weil J. H., Brennicke A. Comparison of tRNA(Trp) and tRNA(Pro) gene sequences in the chloroplast and mitochondrial genomes of oenothera. Nucleic Acids Res. 1988 Aug 11;16(15):7737–7737. doi: 10.1093/nar/16.15.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Grienenberger J. M., Weil J. H., Brennicke A. Comparison of tRNA(Trp) and tRNA(Pro) gene sequences in the chloroplast and mitochondrial genomes of oenothera. Nucleic Acids Res. 1988 Aug 11;16(15):7737–7737. doi: 10.1093/nar/16.15.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Z., Jolly S. O., Steinmetz A. A., Bogorad L. Overlapping divergent genes in the maize chloroplast chromosome and in vitro transcription of the gene for tRNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3423–3427. doi: 10.1073/pnas.78.6.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Böck A., Ott G., Sprinzl M., Söll D. The selenocysteine-inserting opal suppressor serine tRNA from E. coli is highly unusual in structure and modification. Nucleic Acids Res. 1989 Sep 25;17(18):7159–7165. doi: 10.1093/nar/17.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Sedlmeier R., Schmieger H. Nucleotide sequences of tRNA genes in Streptomyces lividans 66. Nucleic Acids Res. 1990 Jul 11;18(13):4027–4027. doi: 10.1093/nar/18.13.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlmeier R., Schmieger H. Nucleotide sequences of tRNA genes in Streptomyces lividans 66. Nucleic Acids Res. 1990 Jul 11;18(13):4027–4027. doi: 10.1093/nar/18.13.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Barrell B. G., McClain W. H. Five steps in the conversion of a large precursor RNA into bacteriophage proline and serine transfer RNAs. J Mol Biol. 1975 Dec 25;99(4):733–760. doi: 10.1016/s0022-2836(75)80182-3. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Comer M. M., McClain W. H. Nucleotide alterations in the bacteriophage T4 glutamine transfer RNA that affect ochre suppressor activity. J Mol Biol. 1974 Dec 25;90(4):677–689. doi: 10.1016/0022-2836(74)90532-4. [DOI] [PubMed] [Google Scholar]
- Seilhamer J. J., Cummings D. J. Altered genetic code in Paramecium mitochondria: possible evolutionary trends. Mol Gen Genet. 1982;187(2):236–239. doi: 10.1007/BF00331123. [DOI] [PubMed] [Google Scholar]
- Seilhamer J. J., Gutell R. R., Cummings D. J. Paramecium mitochondrial genes. II. Large subunit rRNA gene sequence and microevolution. J Biol Chem. 1984 Apr 25;259(8):5173–5181. [PubMed] [Google Scholar]
- Sekiya T., Kuchino Y., Nishimura S. Mammalian tRNA genes: nucleotide sequence of rat genes for tRNAAsp, tRNAGly and tRNAGlu. Nucleic Acids Res. 1981 May 25;9(10):2239–2250. doi: 10.1093/nar/9.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Mori M., Takahashi N., Nishimura S. Sequence of the distal tRNA1Asp gene and the transcription termination signal in the Escherichia coli ribosomal RNA operon rrnF(or G). Nucleic Acids Res. 1980 Sep 11;8(17):3809–3827. doi: 10.1093/nar/8.17.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Nishizawa R., Matsuda K., Taya Y., Nishimura S. A rat tRNA gene cluster containing the genes for tRNAPro and tRNALys. Analysis of nucleotide sequences of the genes and the surrounding regions. Nucleic Acids Res. 1982 Oct 25;10(20):6411–6419. doi: 10.1093/nar/10.20.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Nishizawa R., Matsuda K., Taya Y., Nishimura S. A rat tRNA gene cluster containing the genes for tRNAPro and tRNALys. Analysis of nucleotide sequences of the genes and the surrounding regions. Nucleic Acids Res. 1982 Oct 25;10(20):6411–6419. doi: 10.1093/nar/10.20.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Silberklang M., Hosbach H. A., Schmidt T., Kubli E., Gergen J. P., Wensink P. C., Söll D. The initiator tRNA genes of Drosophila melanogaster: evidence for a tRNA pseudogene. Nucleic Acids Res. 1981 Nov 25;9(22):5867–5882. doi: 10.1093/nar/9.22.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Silberklang M., Hosbach H. A., Schmidt T., Kubli E., Gergen J. P., Wensink P. C., Söll D. The initiator tRNA genes of Drosophila melanogaster: evidence for a tRNA pseudogene. Nucleic Acids Res. 1981 Nov 25;9(22):5867–5882. doi: 10.1093/nar/9.22.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., Dingermann T., Schaack J., Sharp J. A., Burke D. J., DeRobertis E. M., Söll D. Each element of the Drosophila tRNAArg gene split promoter directs transcription in Xenopus oocytes. Nucleic Acids Res. 1983 Dec 20;11(24):8677–8690. doi: 10.1093/nar/11.24.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Yamaki M., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster: comparison of nucleotide sequences of the gene for tRNALeu newly found in repeating units. J Biochem. 1985 Jun;97(6):1719–1725. doi: 10.1093/oxfordjournals.jbchem.a135230. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Yamaki M., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster: comparison of nucleotide sequences of the gene for tRNALeu newly found in repeating units. J Biochem. 1985 Jun;97(6):1719–1725. doi: 10.1093/oxfordjournals.jbchem.a135230. [DOI] [PubMed] [Google Scholar]
- Shindo-Okada N., Kuchino Y., Harada F., Okada N., Nishimura S. Biological and structural differences between tRNAVal species isolated from rat ascites hepatoma cells and normal rat liver. J Biochem. 1981 Aug;90(2):535–544. doi: 10.1093/oxfordjournals.jbchem.a133502. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov M. G., Kaliman A. V., Kazantsev S. I., Kryukov V. M., Bayev A. A. The nucleotide sequence of bacteriophage T5 glutamine transfer RNA. Biochim Biophys Acta. 1984 Jul 18;782(3):313–319. doi: 10.1016/0167-4781(84)90067-8. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov M. G., Kazantsev S. I., Kryukov V. M., Bayev A. A. The nucleotide sequence of bacteriophage T5 leucine tRNA. FEBS Lett. 1985 Nov 18;192(2):299–302. doi: 10.1016/0014-5793(85)80129-0. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov M. G., Ksenzenko V. N., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment which contains the gene for tRNAAsp. Eur J Biochem. 1986 Apr 15;156(2):285–289. doi: 10.1111/j.1432-1033.1986.tb09579.x. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov M. G., Ksenzenko V. N., Kryukov V. M., Bayev A. A. Nucleotide sequence of the bacteriophage T5 DNA fragment which contains the gene for tRNAAsp. Eur J Biochem. 1986 Apr 15;156(2):285–289. doi: 10.1111/j.1432-1033.1986.tb09579.x. [DOI] [PubMed] [Google Scholar]
- Shortridge R. D., Pirtle I. L., Pirtle R. M. Nucleotide sequence and transcription of a gene encoding human tRNAGlyCCC. Gene. 1985;33(3):269–277. doi: 10.1016/0378-1119(85)90234-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Bordonné R., Dirheimer G., Martin R. Structure primaire d'un tryptophane-tRNA de mitochondrie de levure capable de traduire le codon de terminaison U-G-A. C R Seances Acad Sci D. 1980 Mar 17;290(11):695–698. [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Nucleotide sequence of a yeast mitochondrial threonine-tRNA able to decode the C-U-N leucine codons. FEBS Lett. 1981 Sep 28;132(2):344–348. doi: 10.1016/0014-5793(81)81194-5. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. The primary structure of yeast mitochondrial tyrosine tRNA. FEBS Lett. 1983 Feb 21;152(2):153–156. doi: 10.1016/0014-5793(83)80368-8. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Yeast mitochondrial tRNAIle and tRNAMetm: nucleotide sequence and codon recognition patterns. Nucleic Acids Res. 1985 Feb 25;13(4):1341–1345. doi: 10.1093/nar/13.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibler A. P., Martin R. P., Dirheimer G. The nucleotide sequence of yeast mitochondrial histidine-tRNA. FEBS Lett. 1979 Nov 1;107(1):182–186. doi: 10.1016/0014-5793(79)80491-3. [DOI] [PubMed] [Google Scholar]
- Silverman S., Gillam I. C., Tener G. M., Söll D. The nucleotide sequence of lysine tRNA2 from Drosophila. Nucleic Acids Res. 1979 Feb;6(2):435–442. doi: 10.1093/nar/6.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S., Heckman J., Cowling G. J., Delaney A. D., Dunn R. J., Gillam I. C., Tener G. M., Söll D., RajBhandary U. L. The nucleotide sequence of the initiator tRNA from Drosophila melanogaster. Nucleic Acids Res. 1979 Feb;6(2):421–433. doi: 10.1093/nar/6.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau P., Wenzel R., Herrmann R., Hu P. C. Nucleotide sequence of a tRNA cluster from Mycoplasma pneumoniae. Nucleic Acids Res. 1990 May 11;18(9):2814–2814. doi: 10.1093/nar/18.9.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau P., Wenzel R., Herrmann R., Hu P. C. Nucleotide sequence of a tRNA cluster from Mycoplasma pneumoniae. Nucleic Acids Res. 1990 May 11;18(9):2814–2814. doi: 10.1093/nar/18.9.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau P., Wenzel R., Herrmann R., Hu P. C. Nucleotide sequence of a tRNA cluster from Mycoplasma pneumoniae. Nucleic Acids Res. 1990 May 11;18(9):2814–2814. doi: 10.1093/nar/18.9.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L., Boisnard M., Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974 Feb 22;247(5442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R. Histidine regulation in Salmonella typhimurium. 13. Nucleotide sequence of histidine transfer ribonucleic acid. J Biol Chem. 1972 May 25;247(10):2989–3000. [PubMed] [Google Scholar]
- Smardo F. L., Jr, Calvet J. P. Human glutamate tRNA forms stable hybrids in vitro with 28S ribosomal RNA. Nucleic Acids Res. 1987 Jan 26;15(2):661–681. doi: 10.1093/nar/15.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smardo F. L., Jr, Calvet J. P. Sequence analysis of the glutamate tRNA family: evidence for pseudogenes. Gene. 1987;57(2-3):213–220. doi: 10.1016/0378-1119(87)90124-7. [DOI] [PubMed] [Google Scholar]
- Southern S. O., Southern P. J., Dizon A. E. Molecular characterization of a cloned dolphin mitochondrial genome. J Mol Evol. 1988 Dec;28(1-2):32–42. doi: 10.1007/BF02143495. [DOI] [PubMed] [Google Scholar]
- Southern S. O., Southern P. J., Dizon A. E. Molecular characterization of a cloned dolphin mitochondrial genome. J Mol Evol. 1988 Dec;28(1-2):32–42. doi: 10.1007/BF02143495. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Hagenbüchle O., Zuniga M. C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977 Jul;11(3):561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J. Normal and mutant glycine transfer RNAs. Nat New Biol. 1971 Oct 27;233(43):274–277. doi: 10.1038/newbio233274a0. [DOI] [PubMed] [Google Scholar]
- Stahl S., Paddock G. V., Abelson J. Nucleotide sequence determination of bacteriophage T4 glycine transfer ribonucleic acid. Nucleic Acids Res. 1974 Oct;1(10):1287–1304. doi: 10.1093/nar/1.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Venegas A., Rutter W. J. Yeast tRNA3Leu gene transcribed and spliced in a HeLa cell extract. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5963–5967. doi: 10.1073/pnas.78.10.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier H. A gene for the major cytoplasmic tRNATyr from Nicotiana rustica contains a 13 nucleotides long intron. Nucleic Acids Res. 1986 Nov 11;14(21):8691–8691. doi: 10.1093/nar/14.21.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steege D. A. A nucleotide change in the anticodon of an Escherichia coli serine transfer RNA results in supD-amber suppression. Nucleic Acids Res. 1983 Jun 11;11(11):3823–3832. doi: 10.1093/nar/11.11.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Strittmatter G., Gozdzicka-Jozefiak A., Kössel H. Identification of an rRNA operon promoter from Zea mays chloroplasts which excludes the proximal tRNAValGAC from the primary transcript. EMBO J. 1985 Mar;4(3):599–604. doi: 10.1002/j.1460-2075.1985.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Feldmann H. Structure of a Saccharomyces cerevisiae gene encoding minor (AGY)tRNA(Ser). Nucleic Acids Res. 1988 Apr 25;16(8):3583–3583. doi: 10.1093/nar/16.8.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Shinozaki K., Sugiura M. Tobacco chloroplast tRNA(UUU) gene contains a 2.5-kilobase-pair intron: An open reading frame and a conserved boundary sequence in the intron. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3557–3561. doi: 10.1073/pnas.82.11.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter B., Altwegg M., Choffat Y., Kubli E. The nucleotide sequence of two homogeneic Drosophila melanogaster tRNATyr isoacceptors: application of a rapid tRNA anticodon sequencing method using S-1 nuclease. Arch Biochem Biophys. 1986 May 15;247(1):233–237. doi: 10.1016/0003-9861(86)90552-7. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Jenney F., Okawa N. Two transfer RNA sequences abut the large ribosomal RNA gene in Tetrahymena mitochondrial DNA: tRNA(leu) (anticodon UAA) and tRNA(met) (anticodon CAU). Curr Genet. 1987;11(4):327–330. doi: 10.1007/BF00355408. [DOI] [PubMed] [Google Scholar]
- Suyama Y. Nucleotide sequences of three tRNA genes encoded in Tetrahymena mitochondrial DNA. Nucleic Acids Res. 1985 May 10;13(9):3273–3284. doi: 10.1093/nar/13.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y. Nucleotide sequences of three tRNA genes encoded in Tetrahymena mitochondrial DNA. Nucleic Acids Res. 1985 May 10;13(9):3273–3284. doi: 10.1093/nar/13.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szweykowska-Kulinska Z., Beier H. Nucleotide sequences of two nuclear tRNA(Tyr) genes from Triticum aestivum. Nucleic Acids Res. 1990 Apr 11;18(7):1894–1894. doi: 10.1093/nar/18.7.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida E., Kobayashi M., Yaginuma K., Koike K. Tumor-associated mutations of rat mitochondrial transfer RNA genes. Nucleic Acids Res. 1983 Mar 25;11(6):1635–1643. doi: 10.1093/nar/11.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida E., Kobayashi M., Yaginuma K., Koike K. Tumor-associated mutations of rat mitochondrial transfer RNA genes. Nucleic Acids Res. 1983 Mar 25;11(6):1635–1643. doi: 10.1093/nar/11.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S., Murakami M., Miyazaki M. The primary structure of isoleucine transfer ribonucleic acid from Torulopsis utilis. Complete digestion with ribonucleases and construction of model of its secondary structure. J Biochem. 1969 Apr;65(4):553–566. doi: 10.1093/oxfordjournals.jbchem.a129049. [DOI] [PubMed] [Google Scholar]
- Takemura S., Ogawa K. The primary structure of alanine transfer ribonucleic acid 1 from Torulopsis utilis. II. Partial digestion with ribonuclease T1 and derivation of the complete sequence. J Biochem. 1973 Aug;74(2):323–333. [PubMed] [Google Scholar]
- Tanaka M., Wakasugi T., Sugita M., Shinozaki K., Sugiura M. Genes for the eight ribosomal proteins are clustered on the chloroplast genome of tobacco (Nicotiana tabacum): similarity to the S10 and spc operons of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6030–6034. doi: 10.1073/pnas.83.16.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Muto A., Osawa S. Nucleotide sequence of tryptophan tRNA gene in Acholeplasma laidlawii. Nucleic Acids Res. 1989 Jul 25;17(14):5842–5842. doi: 10.1093/nar/17.14.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]
- Thireos G., Penn M. D., Greer H. 5' untranslated sequences are required for the translational control of a yeast regulatory gene. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5096–5100. doi: 10.1073/pnas.81.16.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann H. U., Schmutzler C., Hüdepohl U., Blow M., Gross H. J. Genes, variant genes and pseudogenes of the human tRNA(Val) gene family. Expression and pre-tRNA maturation in vitro. J Mol Biol. 1989 Oct 20;209(4):505–523. doi: 10.1016/0022-2836(89)90590-1. [DOI] [PubMed] [Google Scholar]
- Tranquilla T. A., Cortese R., Melton D., Smith J. D. Sequences of four tRNA genes from Caenorhabditis elegans and the expression of C. elegans tRNALeu (anticodon IAG) in Xenopus oocytes. Nucleic Acids Res. 1982 Dec 20;10(24):7919–7934. doi: 10.1093/nar/10.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilla T. A., Cortese R., Melton D., Smith J. D. Sequences of four tRNA genes from Caenorhabditis elegans and the expression of C. elegans tRNALeu (anticodon IAG) in Xenopus oocytes. Nucleic Acids Res. 1982 Dec 20;10(24):7919–7934. doi: 10.1093/nar/10.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilla T. A., Cortese R., Melton D., Smith J. D. Sequences of four tRNA genes from Caenorhabditis elegans and the expression of C. elegans tRNALeu (anticodon IAG) in Xenopus oocytes. Nucleic Acids Res. 1982 Dec 20;10(24):7919–7934. doi: 10.1093/nar/10.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilla T. A., Cortese R., Melton D., Smith J. D. Sequences of four tRNA genes from Caenorhabditis elegans and the expression of C. elegans tRNALeu (anticodon IAG) in Xenopus oocytes. Nucleic Acids Res. 1982 Dec 20;10(24):7919–7934. doi: 10.1093/nar/10.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilla T. A., Cortese R., Melton D., Smith J. D. Sequences of four tRNA genes from Caenorhabditis elegans and the expression of C. elegans tRNALeu (anticodon IAG) in Xenopus oocytes. Nucleic Acids Res. 1982 Dec 20;10(24):7919–7934. doi: 10.1093/nar/10.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. D., Murgola E. J. Sequence analysis of the glyW region in Escherichia coli. Biochimie. 1985 Sep;67(9):1053–1057. doi: 10.1016/s0300-9084(85)80300-x. [DOI] [PubMed] [Google Scholar]
- Tucker S. D., Murgola E. J. Sequence analysis of the glyW region in Escherichia coli. Biochimie. 1985 Sep;67(9):1053–1057. doi: 10.1016/s0300-9084(85)80300-x. [DOI] [PubMed] [Google Scholar]
- Ulmasov T. N., Gulov M. K., Aliev K. A., Andrianov V. M., Piruzian E. S. Nucleotide sequences of the chloroplast psbA and trnH genes from cotton Gossypium hirsutum. Nucleic Acids Res. 1990 Jan 11;18(1):186–186. doi: 10.1093/nar/18.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Inokuchi H., Ohyama K., Ozeki H. Nucleotide sequence of Marchantia polymorpha chloroplast DNA: a region possibly encoding three tRNAs and three proteins including a homologue of E. coli ribosomal protein S14. Nucleic Acids Res. 1984 Dec 21;12(24):9551–9565. doi: 10.1093/nar/12.24.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Inokuchi H., Ohyama K., Ozeki H. Nucleotide sequence of Marchantia polymorpha chloroplast DNA: a region possibly encoding three tRNAs and three proteins including a homologue of E. coli ribosomal protein S14. Nucleic Acids Res. 1984 Dec 21;12(24):9551–9565. doi: 10.1093/nar/12.24.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel M., Weinberger A. J. Sequence of E. coli tRNA-Glu1 by automated sequential degradation. Nucleic Acids Res. 1975 Apr;2(4):469–475. doi: 10.1093/nar/2.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakharia V. N., Singhal R. P. The structure of aspartate transfer RNA from rabbit liver. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1072–1081. doi: 10.1016/0006-291x(82)91079-8. [DOI] [PubMed] [Google Scholar]
- Vani B. R., Ramakrishnan T., Kuchino Y., Nishimura S. Nucleotide sequence of initiator tRNA from Mycobacterium smegmatis. Nucleic Acids Res. 1984 May 11;12(9):3933–3936. doi: 10.1093/nar/12.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A., Gonzalez E., Bull P., Valenzuela P. Isolation and structure of a yeast initiator tRNAmet gene. Nucleic Acids Res. 1982 Feb 11;10(3):1093–1096. doi: 10.1093/nar/10.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A., Hevia E., Sánchez H. Sequence of two tRNA genes from a Thiobacillus ferrooxidans ribosomal operon. Nucleic Acids Res. 1988 Aug 25;16(16):8179–8179. doi: 10.1093/nar/16.16.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A., Hevia E., Sánchez H. Sequence of two tRNA genes from a Thiobacillus ferrooxidans ribosomal operon. Nucleic Acids Res. 1988 Aug 25;16(16):8179–8179. doi: 10.1093/nar/16.16.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Keith D. E., Jr, Buck M., McCloskey J. A., Pang H. Lysine tRNAs from Bacillus subtilis 168: structural analysis. Nucleic Acids Res. 1982 May 25;10(10):3125–3132. doi: 10.1093/nar/10.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Asai K., Oshima T., Kuchino Y. Chemical structure and thermal properties of initiator tRNA from Euphausia sperba in comparison with those of other eucaryotic initiator tRNAs. J Biochem. 1981 Nov;90(5):1259–1266. doi: 10.1093/oxfordjournals.jbchem.a133590. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kuchino Y., Yamaizumi Z., Kato M., Oshima T., Nishimura S. Nucleotide sequence of formylmethionine tRNA from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1979 Oct;86(4):893–905. doi: 10.1093/oxfordjournals.jbchem.a132621. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Miura K. Specific interaction between tRNA and its cognate amino acid as detected by circular dichroism and fluorescence spectroscopy. Biochem Biophys Res Commun. 1985 Jun 28;129(3):679–685. doi: 10.1016/0006-291x(85)91945-x. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Yang W. K., Mullin B. C., Nichols J. L. Purification of tryptophan transfer RNA from chick cells and its identity with "spot 1" RNA of Rous sarcoma virus. J Biol Chem. 1975 Aug 25;250(16):6627–6629. [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Weber F., Dietrich A., Weil J. H., Maréchal-Drouard L. A potato mitochondrial isoleucine tRNA is coded for by a mitochondrial gene possessing a methionine anticodon. Nucleic Acids Res. 1990 Sep 11;18(17):5027–5030. doi: 10.1093/nar/18.17.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Dietrich A., Weil J. H., Maréchal-Drouard L. A potato mitochondrial isoleucine tRNA is coded for by a mitochondrial gene possessing a methionine anticodon. Nucleic Acids Res. 1990 Sep 11;18(17):5027–5030. doi: 10.1093/nar/18.17.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill D., Heyman T. Nucleotide sequence of two proline tRNA (AGG and CGG) genes from chicken. Nucleic Acids Res. 1990 Oct 25;18(20):6134–6134. doi: 10.1093/nar/18.20.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Normal yeast tRNA(CAGGln) can suppress amber codons and is encoded by an essential gene. J Mol Biol. 1986 Dec 20;192(4):725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Kiraly I., Dirheimer G. Structure primaire des tRNA Thr 1a et b de levure de bière. Biochimie. 1977;59(4):381–391. doi: 10.1016/s0300-9084(77)80314-3. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Martin R., Dirheimer G. The primary structure of tRNAIIAgr from brewers' yeast. 2. Partial digestion with ribonuclease T1 and derivation of the complete sequence. Eur J Biochem. 1975 Aug 15;56(2):527–532. doi: 10.1111/j.1432-1033.1975.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Weisshaar M., Ahmadian R., Sprinzl M., Satoh M., Kushiro A., Tomita K. Sequences of four tRNA genes adjacent to the tuf2 gene of Thermus thermophilus. Nucleic Acids Res. 1990 Apr 11;18(7):1902–1902. doi: 10.1093/nar/18.7.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar M., Ahmadian R., Sprinzl M., Satoh M., Kushiro A., Tomita K. Sequences of four tRNA genes adjacent to the tuf2 gene of Thermus thermophilus. Nucleic Acids Res. 1990 Apr 11;18(7):1902–1902. doi: 10.1093/nar/18.7.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar M., Ahmadian R., Sprinzl M., Satoh M., Kushiro A., Tomita K. Sequences of four tRNA genes adjacent to the tuf2 gene of Thermus thermophilus. Nucleic Acids Res. 1990 Apr 11;18(7):1902–1902. doi: 10.1093/nar/18.7.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wich G., Jarsch M., Böck A. Apparent operon for a 5S ribosomal RNA gene and for tRNA genes in the archaebacterium Methanococcus vannielii. Mol Gen Genet. 1984;196(1):146–151. doi: 10.1007/BF00334107. [DOI] [PubMed] [Google Scholar]
- Wich G., Jarsch M., Böck A. Apparent operon for a 5S ribosomal RNA gene and for tRNA genes in the archaebacterium Methanococcus vannielii. Mol Gen Genet. 1984;196(1):146–151. doi: 10.1007/BF00334107. [DOI] [PubMed] [Google Scholar]
- Wich G., Leinfelder W., Böck A. Genes for stable RNA in the extreme thermophile Thermoproteus tenax: introns and transcription signals. EMBO J. 1987 Feb;6(2):523–528. doi: 10.1002/j.1460-2075.1987.tb04784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Nagel W., Roe B., Dudock B. Primary structure of E. coli alanine transfer RNA: relation to the yeast phenylalanyl tRNA synthetase recognition site. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1215–1221. doi: 10.1016/0006-291x(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Williamson S. E., Doolittle W. F. Genes for tRNAIle and tRNAAla in the spacer between the 16S and 23S rRNA genes of a blue-green alga: strong homology to chloroplast tRNA genes and tRNA genes of the E. coli rrnD gene cluster. Nucleic Acids Res. 1983 Jan 11;11(1):225–235. doi: 10.1093/nar/11.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S. E., Doolittle W. F. Genes for tRNAIle and tRNAAla in the spacer between the 16S and 23S rRNA genes of a blue-green alga: strong homology to chloroplast tRNA genes and tRNA genes of the E. coli rrnD gene cluster. Nucleic Acids Res. 1983 Jan 11;11(1):225–235. doi: 10.1093/nar/11.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. K., Brown T., Roe B. A. Nucleotide sequence of pheW; a third gene for E. coli tRNAPhe. Nucleic Acids Res. 1986 Jul 25;14(14):5937–5937. doi: 10.1093/nar/14.14.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., Mendenhall M. D., Cummins C. M., Culbertson M. R., Knapp G. Splicing of a yeast proline tRNA containing a novel suppressor mutation in the anticodon stem. J Mol Biol. 1986 Nov 5;192(1):49–63. doi: 10.1016/0022-2836(86)90463-8. [DOI] [PubMed] [Google Scholar]
- Winey M., Mendenhall M. D., Cummins C. M., Culbertson M. R., Knapp G. Splicing of a yeast proline tRNA containing a novel suppressor mutation in the anticodon stem. J Mol Biol. 1986 Nov 5;192(1):49–63. doi: 10.1016/0022-2836(86)90463-8. [DOI] [PubMed] [Google Scholar]
- Wintz H., Chen H. C., Pillay D. T. Nucleotide sequence of a soybean mitochondria tRNA Glu (TTC) gene. Nucleic Acids Res. 1987 Dec 23;15(24):10588–10588. doi: 10.1093/nar/15.24.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H., Chen H. C., Pillay D. T. Presence of a chloroplast-like elongator tRNAMet gene in the mitochondrial genomes of soybean and Arabidopsis thaliana. Curr Genet. 1988 Mar;13(3):255–260. doi: 10.1007/BF00387772. [DOI] [PubMed] [Google Scholar]
- Wintz H., Chen H. C., Pillay D. T. Presence of a chloroplast-like elongator tRNAMet gene in the mitochondrial genomes of soybean and Arabidopsis thaliana. Curr Genet. 1988 Mar;13(3):255–260. doi: 10.1007/BF00387772. [DOI] [PubMed] [Google Scholar]
- Wintz H., Grienenberger J. M., Weil J. H., Lonsdale D. M. Location and nucleotide sequence of two tRNA genes and a tRNA pseudo-gene in the maize mitochondrial genome: evidence for the transcription of a chloroplast gene in mitochondria. Curr Genet. 1988 Mar;13(3):247–254. doi: 10.1007/BF00387771. [DOI] [PubMed] [Google Scholar]
- Wintz H., Grienenberger J. M., Weil J. H., Lonsdale D. M. Location and nucleotide sequence of two tRNA genes and a tRNA pseudo-gene in the maize mitochondrial genome: evidence for the transcription of a chloroplast gene in mitochondria. Curr Genet. 1988 Mar;13(3):247–254. doi: 10.1007/BF00387771. [DOI] [PubMed] [Google Scholar]
- Wittig S., Wittig B. Function of a tRNA gene promoter depends on nucleosome position. Nature. 1982 May 6;297(5861):31–38. doi: 10.1038/297031a0. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Clary D. O. Sequence evolution of Drosophila mitochondrial DNA. Genetics. 1985 Apr;109(4):725–744. doi: 10.1093/genetics/109.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M., Goddard J. M. Nucleotide sequence of Rattus norvegicus mitochondrial DNA that includes the genes for tRNAile, tRNAgln and tRNAf-met. Gene. 1982 Nov;20(1):63–69. doi: 10.1016/0378-1119(82)90087-7. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M., Goddard J. M. Nucleotide sequence of Rattus norvegicus mitochondrial DNA that includes the genes for tRNAile, tRNAgln and tRNAf-met. Gene. 1982 Nov;20(1):63–69. doi: 10.1016/0378-1119(82)90087-7. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., McCutchan T., Kohli J., Söll D. The nucleotide sequence of the major glutamate transfer RNA from Schizosaccharomyces pombe. Nucleic Acids Res. 1979;6(6):2057–2068. doi: 10.1093/nar/6.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T. Nucleotide sequences of the Chlorella ellipsoidea chloroplast genes for tRNA(ArgACG) and tRNA(SerGCU). Nucleic Acids Res. 1989 Jun 12;17(11):4372–4372. doi: 10.1093/nar/17.11.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Shimaji M. Peculiar feature of the organization of rRNA genes of the Chlorella chloroplast DNA. Nucleic Acids Res. 1986 May 12;14(9):3827–3839. doi: 10.1093/nar/14.9.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Kuchino Y., Ishikura H. Nucleotide sequence of initiator tRNA from Bacillus subtilis. J Biochem. 1980 May;87(5):1261–1269. doi: 10.1093/oxfordjournals.jbchem.a132863. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Sequence relationship of three valine acceptor tRNAs from Escherichia coli. Nat New Biol. 1971 Sep 22;233(38):113–114. doi: 10.1038/newbio233113a0. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Folk W. R. The nucleotide sequences of the two glutamine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 May 10;250(9):3243–3253. [PubMed] [Google Scholar]
- Yarus M., Barrell B. G. The sequence of nucleotides in tRNA Ile from E. coli B. Biochem Biophys Res Commun. 1971 May 21;43(4):729–734. doi: 10.1016/0006-291x(71)90676-0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Davidson N. The gross anatomy of a tRNA gene cluster at region 42A of the D. melanogaster chromosome. Cell. 1980 Nov;22(1 Pt 1):137–148. doi: 10.1016/0092-8674(80)90162-2. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Inokuchi H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. IV. Amber suppressor Su+6 a double mutant of a new species of leucine tRNA. J Mol Biol. 1984 Aug 25;177(4):627–644. doi: 10.1016/0022-2836(84)90041-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Kimura M., Ohno M., Inokuchi H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. III. Ochre suppressors of lysine tRNA. J Mol Biol. 1984 Aug 25;177(4):609–625. doi: 10.1016/0022-2836(84)90040-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Kimura M., Ohno M., Inokuchi H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. III. Ochre suppressors of lysine tRNA. J Mol Biol. 1984 Aug 25;177(4):609–625. doi: 10.1016/0022-2836(84)90040-8. [DOI] [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Young R. A. Transcription termination in the Escherichia coli ribosomal RNA operon rrnC. J Biol Chem. 1979 Dec 25;254(24):12725–12731. [PubMed] [Google Scholar]
- Zachau H. G., Dütting D., Feldmann H. The structures of two serine transfer ribonucleic acids. Hoppe Seylers Z Physiol Chem. 1966;347(4):212–235. doi: 10.1515/bchm2.1966.347.1.212. [DOI] [PubMed] [Google Scholar]
- Zhang D. H., Spreitzer R. J. Nucleotide sequences of the Chlamydomonas reinhardtii chloroplast genes for tryptophan and glycine transfer RNAs. Nucleic Acids Res. 1989 Nov 11;17(21):8873–8873. doi: 10.1093/nar/17.21.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. H., Spreitzer R. J. Nucleotide sequences of the Chlamydomonas reinhardtii chloroplast genes for tryptophan and glycine transfer RNAs. Nucleic Acids Res. 1989 Nov 11;17(21):8873–8873. doi: 10.1093/nar/17.21.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T. The barley chloroplast DNA atpBE, trnM2, and trnV1 loci. Nucleic Acids Res. 1984 Mar 12;12(5):2549–2559. doi: 10.1093/nar/12.5.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T. The barley chloroplast DNA atpBE, trnM2, and trnV1 loci. Nucleic Acids Res. 1984 Mar 12;12(5):2549–2559. doi: 10.1093/nar/12.5.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúiga M. C., Steitz J. A. The nucleotide sequence of a major glycine transfer RNA from the posterior silk gland of Bombyx mori L. Nucleic Acids Res. 1977 Dec;4(12):4175–4196. doi: 10.1093/nar/4.12.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire 'dihydrouridine' loop and stem. EMBO J. 1983;2(8):1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire 'dihydrouridine' loop and stem. EMBO J. 1983;2(8):1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B. F., Mulder E., Brakenhoff J. P., Sloof P., Benne R. The variable region of the Trypanosoma brucei kinetoplast maxicircle: sequence and transcript analysis of a repetitive and a non-repetitive fragment. Mol Biochem Parasitol. 1988 Jan 1;27(1):71–82. doi: 10.1016/0166-6851(88)90026-6. [DOI] [PubMed] [Google Scholar]
- de Vries H., Haima P., Brinker M., de Jonge J. C. The Neurospora mitochondrial genome: the region coding for the polycistronic cytochrome oxidase subunit I transcript is preceded by a transfer RNA gene. FEBS Lett. 1985 Jan 7;179(2):337–342. doi: 10.1016/0014-5793(85)80547-0. [DOI] [PubMed] [Google Scholar]
- del Rey F. J., Donahue T. F., Fink G. R. sigma, a repetitive element found adjacent to tRNA genes of yeast. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4138–4142. doi: 10.1073/pnas.79.13.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey F., Donahue T. F., Fink G. R. The histidine tRNA genes of yeast. J Biol Chem. 1983 Jul 10;258(13):8175–8182. [PubMed] [Google Scholar]
- van Tol H., Stange N., Gross H. J., Beier H. A human and a plant intron-containing tRNATyr gene are both transcribed in a HeLa cell extract but spliced along different pathways. EMBO J. 1987 Jan;6(1):35–41. doi: 10.1002/j.1460-2075.1987.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Stutz E. The soybean chloroplast genome: nucleotide sequence of a region containing tRNA-Val (GAC) and 16S rRNA gene. Nucleic Acids Res. 1988 Feb 11;16(3):1200–1200. doi: 10.1093/nar/16.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


