Abstract
Chemotherapy resistance is an important problem often encountered during the course of breast cancer treatment. In order to design rational and efficacious therapies, the molecular mechanisms used by cells to develop resistance must be investigated. One mechanism employed by cancer cells is to alter cell signaling. This review examines the role of mitogen-activated protein kinases (MAPKs) and their endogenous negative regulators, mitogen-activated protein kinase phosphatases (MKPs), in chemotherapy resistance in breast cancer. MAPK signaling is activated in response to both growth factors and cellular stress. MKPs dephosphorylate MAPKs and are part of the dual-specificity family of phosphatases. MAPKs have been shown to be involved in resistance to tamoxifen, and MKPs have been linked to resistance to treatment with doxorubicin, mechlorethamine, paclitaxel, proteasome inhibitors, and oxidative-stress-induced cell death in breast cancer. The role of MKPs in tamoxifen resistance and the elucidation of the mechanisms involved with resistance to standard chemotherapy agents need to be investigated further. Growing evidence suggests that modulating MKP-1 activity could be a viable option to make breast cancer chemotherapy more effective.
Keywords: MKP-1, MAP kinase, Breast cancer, Endocrine therapy, Chemotherapy, Resistance
1 Introduction
It was estimated that the year 2008 would see 184,450 new cases of breast cancer and 40,930 deaths from breast cancer in the USA [1]. Breast cancer is a heterogeneous disease with several subtypes that have been identified through analysis of gene expression patterns [2]. The subtype of breast cancer dictates which course of treatment each patient will receive. Tamoxifen (Nolvadex®) is the most frequently utilized endocrine therapy for the treatment of breast cancers that express estrogen receptor (ER) [3]. Tamoxifen is a selective estrogen receptor modulator, acting as an estrogen antagonist in breast tissue and an estrogen agonist in uterine tissue [4, 5]. Estrogen is thought to induce cell growth by upregulating stimulatory growth factors such as transforming growth factor alpha [6]. The primary mechanism of action of tamoxifen is to bind to the ligand binding domain of the estrogen receptor and prevent the effects of estrogen [4, 5]. Endocrine therapies are thought to induce cell cycle arrest and apoptosis through the decreased expression of cell cycle regulators that promote cell cycle progression like c-myc and cyclin D1, induction of cell cycle inhibitors such as p21 and p27, and inhibition of antiapoptotic signaling pathways through decreased bcl-2 and increased Bax expression [4]. Adjuvant endocrine therapy provides around a 50% reduction in the chance that the patient’s breast cancer will recur [7]. Around 30% of ER-positive tumors do not respond to tamoxifen at the outset of treatment [4]. This is called de novo resistance. Acquired resistance, the development of resistance over the course of treatment, can come about through a variety of mechanisms and occurs in most tumors that initially respond to therapy [4]. Patients who present with metastatic breast cancer respond to first-line chemotherapies, which include anthracyclines and taxanes, at a rate of 30–70% [8–11]. The time to disease progression for these patients is approximately 6–10 months [8–11].
Altered cell signaling has long been recognized as a mechanism employed by cells in the development and progression of cancer [12]. The mitogen-activated protein kinase (MAPK) pathway is just one of the many signaling modules that have been implicated in this process. The three major branches of the MAPK family are involved in both cell growth and cell death, and the tight regulation of these pathways is paramount in determining cell fate [13]. Endogenously, MAPKs are negatively regulated by mitogen-activated protein kinase phosphatases (MKPs) [14]. MKPs belong to the dual-specificity family of protein tyrosine phosphatases [14, 15]. Both the MAPKs and MKPs have been shown to be involved in chemotherapy resistance in breast cancer [3–7, 16–20]. While several MKPs can be highly expressed in human cancers, this review will focus on MKP-1 because it is the most studied and best-characterized MKP at this time. In order to gain a better understanding of the role MAPK signaling plays in the broader context of breast cancer and the development of chemotherapy resistance, the contribution of MKPs to this process needs to be examined further. Evidence is accumulating that suggests that targeting MKPs, MKP-1 in particular, could have potential therapeutic benefit for patients by making chemotherapy and endocrine therapy in breast cancer more effective.
1.1 MAPK signaling
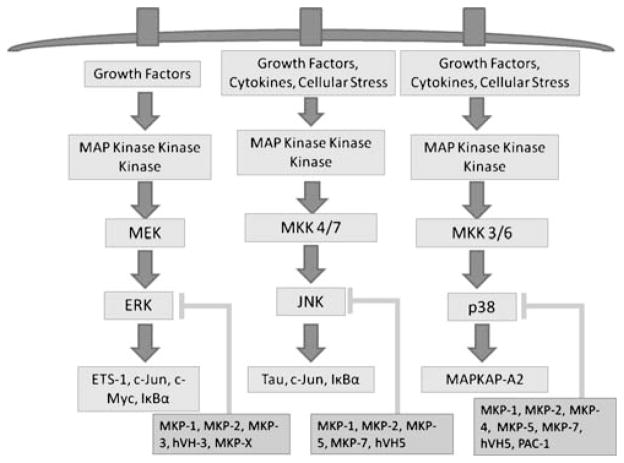
There are three major branches of the MAPK signaling pathway in mammalian cells: the extracellular signal regulated kinases (ERK), the c-Jun N-terminal kinases (JNK), and the p38 MAPKs (Fig. 1). ERK signaling is activated by growth factors, and it is generally involved in stimulating cell growth. JNK and p38 signaling is activated by growth factors, cytokines, and cellular stress. These two pathways can be responsible for both cell growth and cell death, depending on the activating stimuli and cellular context [13, 21, 22]. MAPK signaling follows a general paradigm in which the stimulus is received at the cell surface and is transmitted by a series of phosphorylation events to a MAP kinase kinase kinase (MKKK). Several enzymes can serve as MKKKs. For ERK, the MKKK is Raf [13]. JNK and p38 have several. For JNK, these include MEKK1, MEKK4, dual leucine zipper-bearing kinase, MLK1–4, leucine zipper-bearing kinase, TAK1, ASK1, and zipper sterile-α motif kinase. MLK2, MLK3, dual leucine zipper-bearing kinase, ASK1, map three kinase 1, and TAK1 are the MKKKs for p38 [13]. The MKKK then phosphorylates a specific MAP kinase kinase (MKK). The MKKs for ERK, JNK, and p38 are MEK, MKK 4/7, and MKK 3/6, respectively. These MKKs in turn phosphorylate ERK, JNK, and p38 [13]. MAPKs are dually phosphorylated on threonine and tyrosine residues in a TXY motif [14].
Fig. 1.
MAP kinase signaling. The three branches of the MAP kinase signaling family in mammalian cells are activated by stimuli at the cell surface. MAP kinase kinase kinases relay the signal to MAP kinase kinases, which activate ERK, JNK, and p38. The phosphorylation of their respective targets completes the cascade. MAP kinase phosphatases are endogenous negative regulators of MAP kinases. MKPs attenuate the signal by dephosphorylation and prevent MAPKs from carrying out their cellular functions
Once they are phosphorylated, the MAPKs are able to phosphorylate transcription factors, which then influence the transcription of their target genes. Downstream targets of ERK include ETS-1, c-Jun, and c-Myc. ETS-1 binds to DNA via an ETS domain and subsequently upregulates the transcription of p21, BID/BAX, which promote apoptosis, and matrix metalloproteinase (MMP)-1, MMP-3, MMP-9, and VEGF/VEGFR, which are involved in cell motility and angiogenesis [13, 16]. c-Jun, which dimerizes with c-Fos to form AP-1, has a variety of target genes. The genes affected depend on which isoforms of c-Jun and c-Fos make up AP-1 [13, 16]. c-Myc binds to E-box sequences in DNA after dimerizing with Max. Myc target genes include cyclin A, cyclin D, and cyclin E, which promote the cell cycle [13, 16]. ERK can also indirectly activate nuclear factor (NF)-κB by phosphorylating IκBα [16]. JNK signaling can be involved in both cell death and cell proliferation. One of the JNK target proteins is TAU, which is involved in the stabilization of microtubules through promotion of tubulin assembly [13]. Phosphorylation by JNK prevents this stabilization and can delay the cell cycle by inhibiting mitotic spindle formation. JNK can also phosphorylate c-Jun and IκBα, which tags them for ubiquitination and degradation by the proteasome [13]. The p38 pathway is the least characterized of all the MAPKs. One target that is known is mitogen-activated protein kinase activated protein kinase 2 (MAPKAP-K2). MAPKAP-K2 phosphorylates RNA binding effectors, such as tristetraprolin, heterogeneous ribonuclear protein A0, and poly(A) binding protein. These molecules are involved in reducing the stability of MKP-1, vascular endothelial growth factor, and MMP mRNA [13]. p38 phosphorylation prevents the effectors from carrying out their function, thereby increasing the stability of their target mRNAs [13].
1.2 MAP kinase phosphatases
MKP-1 is the founding member of the MKP family. MKPs are dual-specificity phosphatases (DUSPs) that recognize the TXY amino acid motif present in MAPK family members [14]. The active site signature motif of MKP family members is HCX5R [23]. MKP expression can be induced by factors that activate MAPKs, such as environmental stresses and growth factor stimulation [14, 24]. There are 11 MKP family members: MKP-1 (DUSP1), MKP-2 (DUSP4), MKP-3 (DUSP6), MKP-4 (DUSP9), MKP-5 (DUSP10), MKP-7 (DUSP16), MKP-X (DUSP7), PAC1 (DUSP2), hVH3 (DUSP5), hVH5 (DUSP8), and MK-STYX (DUSP24). These phosphatases can be grouped according to their subcellular localization. MKP-1, MKP-2, hVH3, and PAC1 are found in the nucleus. MKP-3, MKP-4, and MKP-X are found in the cytoplasm. MKP-5, MKP-7, and hVH5 are found in both the nucleus and the cytoplasm [14]. Substrate specificity differs slightly among the MKP family members. MKP-1 and MKP-2 can dephosphorylate all three MAPK family members. MKP-3, MKP-X, and hVH3 primarily target ERKs. MKP-5, MKP-7, and hVH5 inactivate JNK and p38, while MKP-4 and PAC-1 target ERK and p38 [14]. MKPs have a C-terminal catalytic domain and an N-terminal domain that contains two regions of sequence homology to the catalytic domain of the cdc25 phosphatase [25, 26].
The most frequently studied member of the MKP family is MKP-1. MKP-1 can dephosphorylate all three members of the MAPK family, but it has a much higher affinity for JNK and p38, with a much lower affinity for ERK [27]. There is a region in the MKP-1 gene that can bind p53, and it has been shown that p53 can activate the transcription of the MKP-1 gene in vivo [28, 29]. This binding can induce p53-dependent MKP-1 expression in response to oxidative stress [29]. It is thought that constitutive MKP-1 gene expression blocks G1-specific gene expression. Inhibition of phosphatase activity interferes with p53-mediated G1 arrest in response to growth factors. Thus, through upregulation of MKP-1, p53 may negatively regulate the cell cycle in response to growth factor stimuli [28].
1.3 MAPKs and endocrine therapy resistance
Studies have demonstrated that the MAPK pathway is involved in facilitating tamoxifen resistance [4, 7, 17–20]. ERK phosphorylation and increased activity have been shown to be associated with endocrine resistance and decreased survival in breast cancer patients [7, 17]. One possible mechanism for this is ERK phosphorylation of Ser 118 in the ER, which leads to ligand-independent activation of the ER [4, 19]. The role, however, of Ser 118 phosphorylation in endocrine therapy resistance is not completely understood. Increased Ser 118 phosphorylation can be associated both with better and worse survival outcomes in breast cancer patients. It has been suggested that Ser 118 phosphorylation is a favorable prognostic indicator prior to endocrine therapy and an unfavorable one after tamoxifen resistance is acquired [4].
Another possible mechanism of tamoxifen resistance involving the MAPK pathway is the overexpression of HER2. HER2 overexpression has been shown to result in the activation of the MAPK pathway in breast tumor cell lines, and exogenous inhibitors of HER2 signaling can partially restore sensitivity to antiestrogens [19]. Activation of HER2 seems to have a role in the growth of tamoxifen-resistant tumors, partly through nongenomic ER mechanisms, while genomic ER functions are suppressed [20]. Studies have shown that tamoxifen therapy slightly increases the expression of both EGFR and HER2 and that the expression of these molecules was greatly elevated in resistant tumors [1, 4, 20]. Clinical studies have demonstrated that patients whose breast cancers overexpress EGFR and/or HER2 are less likely to benefit from tamoxifen treatment [20]. It has also been shown that there is a strong correlation between HER2 expression and ERK and MKP-1 protein expression [13]. It is possible that EGFR and HER2 function to provide survival signals that override apoptotic programs in the cell. One mechanism that has been suggested is that HER2, by inducing the MAPK pathway, could protect ERK from inactivation by MKP-1, leaving it to deactivate JNK and p38 [13]. Another suggested mechanism involves the induction of cell cycle arrest following the activation of the MAPK pathway. In this situation, strong, sustained ERK activity leads to senescence or differentiation. Increased MKP activity may reduce this strong signal. The result is weak or transient ERK activity, which favors cell proliferation [13]. Increased p38 activity has also been observed in resistant tumors [4]. It is possible that activated p38 may promote resistance to tamoxifen because of its ability to phosphorylate the ER and enhance its nuclear functions [4].
1.4 MAPKs and chemotherapy resistance
Approximately 15% of breast cancers do not express ER, progesterone receptor (PR), or HER2 [30]. This subtype is called triple-negative breast cancer or basal-like breast cancer. The two terms are not interchangeable, but they are roughly equivalent [30]. The difference between these groups is not particularly important for this analysis, so the term triple-negative breast cancer will be used from hereon in. Triple-negative breast cancers have an early age of onset and are common in premenopausal African or Hispanic women [31]. Since these cancers do not express ER, PR, or HER2, they do not respond to targeted therapy [32]. Triple-negative breast cancers have been shown to have a higher chemosensitivity when compared to other breast cancers, but patients who did not achieve pathological complete response were more likely to relapse than those with other types of breast cancer [32, 33]. These patients also have a significantly shorter survival time following the development of metastasis compared to patients who do not have triple-negative breast cancer, with a peak risk of recurrence between the first and third years following diagnosis [34]. The majority of deaths in patients with triple-negative breast cancer occur within the first 5 years [34].
Research has shown that MAPK expression may be an underlying mechanism contributing to the generation of chemoresistance in triple-negative breast cancer [34]. Epidermal growth factor receptor, which is a receptor tyrosine kinase upstream from MAPK, has been shown to be overexpressed in up to 66% of triple-negative breast cancers [34]. Increased GF activity could lead to increased MAPK activity, which can contribute to an increase in cell growth. In addition, microarray data derived from primary tumor samples identified a cluster of genes associated with triple-negative breast cancer. Among these are genes that activate ERK, PI3K, AKT, p38, and NF-κB [2, 31]. Since triple-negative cancers do not respond to current targeted therapies aimed at the estrogen receptor and HER2, developing targeted agents aimed at the pathways that are activated in triple-negative breast cancer remains an important goal in cancer research. One potential target involved in chemoresistance may be MKP-1.
1.5 MKP-1 and chemotherapy resistance
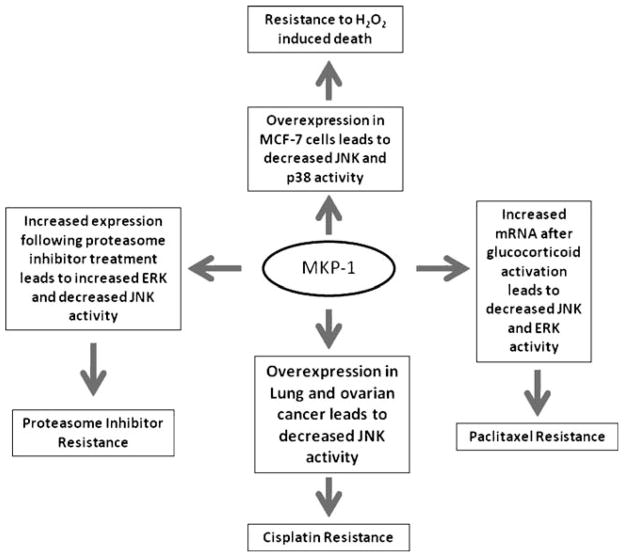
MKP-1 expression has been shown to be altered in a variety of human cancers, including breast, lung, prostate, ovarian, pancreatic, liver, and gastric cancer [14]. Growing evidence suggests that MKP-1 may play a role in chemotherapy resistance (Fig. 2). Overexpression of MKP-1 protected human lung cancer cells from cisplatin-induced death [35]. It was shown that MKP-1 targets JNK in response to cisplatin, leading to increased c-Jun activity and that MKP-1−/− mouse embryo fibroblasts (MEF) were more sensitive to cisplatin and etoposide (VePesid®) than MKP-1+/+ MEF cells. This study also demonstrated that activation of JNK is required for sensitizing cells to cisplatin [35]. It has also been shown that induction of MKP-1 after treatment with cisplatin is a general event in ovarian cancer cell lines, with knockdown of MKP-1 by siRNA increasing cisplatin-induced cell death [21].
Fig. 2.
MKP-1 and chemoresistance. Overexpression of MKP-1 plays a role in the development of resistance to chemotherapy in breast, lung, and ovarian cancers. In breast cancer, decreased JNK and p38 activity contributes to resistance to oxidative-stress-induced death. Treatment with proteasome inhibitors increases ERK and decreases JNK activity, leading to proteasome inhibitor resistance because of decreased levels of apoptosis. Activation of the glucocorticoid receptor increases MKP-1 mRNA and causes decreased JNK and ERK activity, which factors in to paclitaxel resistance. Cisplatin resistance in lung and ovarian cancer is caused in part by decreased JNK activity
MKP-1 is also overexpressed in a large proportion of breast cancers [36]. One study showed that there was an increase in JNK expression in malignant tissue samples from breast cancer patients, accompanied by an approximate 30% decrease in JNK activity in comparison to corresponding normal tissue. Further experiments in this same study showed a significant increase in MKP-1 and MKP-2 in the malignant tissue [36]. Overexpression of MKP-1 was able to protect breast cancer cells from chemotherapy-mediated apoptosis when they were treated with doxorubicin (Adriamycin®), mechlorethamine (Mustargen®), and paclitaxel (Taxol®) [37]. This is significant because many chemotherapy drugs, including anthracyclines, alkylating agents, and taxanes, use JNK activation to carry out their anticancer activity [22, 37]. Treatment of breast cancer cell lines with anthracyclines resulted in the repression of MKP-1 and increased phosphorylation of ERK [38]. Further silencing of MKP-1 with siRNA resulted in decreased ERK activation, but the mechanism for this remains unclear [38]. A similar study looked at MKP-1 overexpression in 30 tissue sections and found that MKP-1 was overexpressed in all in situ carcinomas (n=18) and in 50% of infiltrating carcinomas (n=30) [39]. This study also further characterized the effect of doxorubicin treatment ex vivo in 50 patient samples. MKP-1 expression was decreased in 39 samples while the other 11 samples showed a modest increase in MKP-1 levels [39]. In the 27 tumors that did not exhibit overexpression of MKP-1, treatment with doxorubicin further decreased MKP-1 levels, but there was no significant change in MKP-1 in the 23 tumors that did overexpress MKP-1 [39]. A decrease in MKP-1 activity could mean an increase in ERK signaling. Additionally, a study of 96 patients showed that those who harbored MKP-1 overexpression were more likely to experience relapse than those who did not [39]. Protea-some inhibition has been linked to the induction of MKP-1 expression [40]. This resulted in a decrease in ERK signaling, and further blockade of ERK led to an increase in proteasome-inhibitor-mediated apoptosis [40]. This induction of MKP-1, however, is thought to also limit the efficacy of proteasome inhibitors because of a subsequent decrease in JNK activity, resulting in decreased levels of apoptosis [41]. Knockdown of MKP-1 resulted in increased proteasome inhibitor sensitivity [41]. Interestingly, evaluation of a combination treatment with proteasome inhibitors and doxorubicin in breast cancer showed increased apoptosis, a decrease in MKP-1 levels, and an increase in JNK phosphorylation in vitro and resulted in delayed tumor growth in an in vivo xenograft model [41]. A combination of proteasome inhibitor treatment and p38 blockade also inhibited MKP-1 expression, increased JNK activity, and increased apoptosis in the A1N4-myc and BT474 breast cancer cell lines [42]. The activation of the glucocorticoid receptor can also inhibit paclitaxel-induced apoptosis by preventing it from inducing ERK and JNK activation [43]. Generally, glucocorticoids protect against apoptosis due to growth factor withdrawal in breast epithelial cells. The activation of the glucocorticoid receptor has been shown to lead to an increase in MKP-1 mRNA and using siRNA directed at MKP-1 decreases the antiapoptotic activity of glucocorticoids [43]. Inhibition of JNK and p38 signaling by overexpression of MKP-1 also increased resistance to H2O2-induced death in MCF7 breast cancer cells, with a correlation between MKP-1 induction and the disappearance of phosphorylated MAPKs, suggesting that MKP-1 might play a physiologic role in the inactivation of oxidative-damage-induced MAPK activities [22]. Furthermore, loss of MKP-1 sensitized cells to oxidative-damage-induced death [22].
The role that MKP-1 plays in breast cancer endocrine and chemotherapy resistance is an area that should be investigated further, keeping in mind the goal of translating research findings into clinical benefit for patients. The contribution of MKP-1 to tamoxifen resistance is not yet known, but a study done by Cui et al. demonstrated that MKP-3 was overexpressed 19-fold and eightfold, respectively, in MCF-7 and T47D cells that were resistant to tamoxifen compared to their parental lines [17]. Loss of MKP-1 activity has been shown to increase chemosensitivity in lung cancer and ovarian cancer [21, 35], but studies examining this effect in breast cancer are limited. Due to the fact that MKP-1 can dephosphorylate all three of the MAPK family members, there is an inherent complexity in this signaling pathway. It is probable that each of the branches of the MAPK family makes different contributions to therapy resistance. Whether overexpression or knockdown of MKP-1 is the necessary modulation to attempt to improve chemosensitivity will likely depend on which pathway is the most active in the cancer cells. Given that MKP-1 has a preference for dephosphorylating JNK and p38 over ERK and that these two pathways are exploited by a variety of chemotherapy drugs to facilitate apoptosis, it is probable that knockdown of MKP-1 will result in a decrease in therapy resistance. Further research in this area is necessary to illuminate whether or not this is indeed the case in breast cancer. An additional challenge that adds complexity to the problem of therapy resistance is that multiple signaling pathways can be activated simultaneously. A thorough investigation of the relationships between them is necessary to discover potential functional redundancies or mechanisms used to compensate for the blockade of a portion or the entirety of a particular pathway.
2 Conclusion
Chemotherapy resistance in breast cancer is a complex problem. There is growing evidence that MAPKs and their endogenous negative regulators, MKP, play a role in the development of resistance and that modulation of MKP-1 expression may improve chemosensitivity in cancer cells. Previous research in lung and ovarian cancer suggests that the loss of MKP-1 improves response to treatment in vitro. This sheds a light on the need to develop clinically relevant inhibitors to MKP-1. If successful, MKP-1 inhibitors could be used in combination with treatments already available to bring additional benefit to breast cancer patients. Further research must be conducted to ascertain an overall representation of the contribution of MAPKs and MKPs to therapy resistance in breast cancer, but previously conducted studies indicate that this is an important avenue to pursue.
Acknowledgments
Apologies are extended to those colleagues whose work could not be cited due to space limitations.
Financial support: Kelly K. Haagenson: Ruth L. Kirschstein National Research Service Award T32-CA009531. Gen Sheng Wu: National Institutes of Health Grant R01CA100073
Contributor Information
Kelly K. Haagenson, Graduate Program in Cancer Biology, Wayne State University School of Medicine, Detroit, MI, USA
Gen Sheng Wu, Email: wug@karmanos.org, Graduate Program in Cancer Biology, Wayne State University School of Medicine, Detroit, MI, USA. Program in Molecular Biology and Genetics, Department of Pathology, Karmanos Cancer Institute, Wayne State University School of Medicine, 110 E. Warren Ave, Detroit, MI 48201, USA.
References
- 1.National Cancer Institute. Cancer topics: Breast cancer. 2009 http://www.cancer.gov/cancertopics/types/breast.
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou JN, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. Journal of Clinical Oncology. 2005;23(11):2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 4.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Letters. 2007;256(1):1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn MB, Lipmann SM. Chemoprevention of cancer. In: Kufe DW, Pollack RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, et al., editors. Cancer medicine. Vol. 6. BC Decker; Hamilton: 2003. [Google Scholar]
- 6.Jordan VC. Estrogens and antiestrogens. In: Kufe DW, Pollack RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, et al., editors. Cancer medicine. Vol. 6. Hamilton: BC Decker; 2003. [Google Scholar]
- 7.Kurebayashi J. Resistance to endocrine therapy in breast cancer. Cancer Chemotherapy and Pharmacology. 2005;56(Suppl 1):39–46. doi: 10.1007/s00280-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 8.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treatment Reviews. 2008;34(4):378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Bonneterre J, Dieras V, Tubiana-Hulin M, Bougnoux P, Bonneterre ME, Delozier T, et al. Phase II multicentre randomised study of docetaxel plus epirubicin vs 5-fluorouracil plus epirubicin and cyclophosphamide in metastatic breast cancer. British Journal of Cancer. 2004;91(8):1466–1471. doi: 10.1038/sj.bjc.6602179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassilomanolakis M, Koumakis G, Barbounis V, Demiri M, Panopoulos C, Chrissohoou M, et al. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast. 2005;14(2):136–141. doi: 10.1016/j.breast.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, Shimizu S, Inaba M, Asaga T, Katayama K, Fukuda M, et al. A multicenter phase II study of docetaxel 60 mg/m2 as first-line chemotherapy in patients with advanced or recurrent breast cancer. Breast Cancer. 2004;11(4):374–379. doi: 10.1007/BF02968045. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 13.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacological Reviews. 2008;60(3):261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 14.Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer and Metastasis Reviews. 2007;26(3–4):579–585. doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- 15.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer and Metastasis Reviews. 2008;27(2):253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 16.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Advances in Enzyme Regulation. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Cui Y, Parra I, Zhang M, Hilsenbeck SG, Tsimelzon A, Furukawa T, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: A mechanism of tamoxifen resistance. Cancer Research. 2006;66(11):5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Research. 2006;66(24):11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Research. 2000;60(20):5887–5894. [PubMed] [Google Scholar]
- 20.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Research. 2008;68(3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Research. 2007;67(24):11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 22.Zhou JY, Liu Y, Wu GS. The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death. Cancer Research. 2006;66(9):4888–4894. doi: 10.1158/0008-5472.CAN-05-4229. [DOI] [PubMed] [Google Scholar]
- 23.NCBI. Dual specificity phosphatase 1. [Accessed 8 Apr 2009.];Homo sapiens. 2009 http://www.ncbi.nlm.nih.gov/protein/4758204?itemid=10&report=gpwithparts.
- 24.Hirsch DD, Stork PJ. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. Journal of Biological Chemistry. 1997;272(7):4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. Journal of Cell Science. 2006;119(Pt 22):4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 26.Keyse SM, Ginsburg M. Amino acid sequence similarity between CL100, a dual-specificity MAP kinase phosphatase and cdc25. Trends in Biochemical Sciences. 1993;18(10):377–378. doi: 10.1016/0968-0004(93)90092-2. [DOI] [PubMed] [Google Scholar]
- 27.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB Journal. 2000;14(1):6–16. [PubMed] [Google Scholar]
- 28.Li M, Zhou JY, Ge Y, Matherly LH, Wu GS. The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. Journal of Biological Chemistry. 2003;278(42):41059–41068. doi: 10.1074/jbc.M307149200. [DOI] [PubMed] [Google Scholar]
- 29.Liu YX, Wang J, Guo J, Wu J, Lieberman HB, Yin Y. DUSP1 is controlled by p53 during the cellular response to oxidative stress. Molecular Cancer Research. 2008;6(4):624–633. doi: 10.1158/1541-7786.MCR-07-2019. [DOI] [PubMed] [Google Scholar]
- 30.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: Therapeutic options. Lancet Oncology. 2007;8(3):235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 31.Kang SP, Martel M, Harris LN. Triple negative breast cancer: Current understanding of biology and treatment options. Current Opinion in Obstetrics and Gynecology. 2008;20(1):40–46. doi: 10.1097/GCO.0b013e3282f40de9. [DOI] [PubMed] [Google Scholar]
- 32.Stockmans G, Deraedt K, Wildiers H, Moerman P, Paridaens R. Triple-negative breast cancer. Current Opinion in Oncology. 2008;20(6):614–620. doi: 10.1097/CCO.0b013e328312efba. [DOI] [PubMed] [Google Scholar]
- 33.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clinical Cancer Research. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 34.Reis-Filho JS, Tutt AN. Triple negative tumours: A critical review. Histopathology. 2008;52(1):108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Research. 2006;66(17):8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- 36.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Letters. 2003;191(2):229–237. doi: 10.1016/s0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- 37.Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Research. 2007;67(9):4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 38.Small GW, Somasundaram S, Moore DT, Shi YY, Orlowski RZ. Repression of mitogen-activated protein kinase (MAPK) phosphatase-1 by anthracyclines contributes to their antiapoptotic activation of p44/42-MAPK. Journal of Pharmacology and Experimental Therapeutics. 2003;307(3):861–869. doi: 10.1124/jpet.103.055806. [DOI] [PubMed] [Google Scholar]
- 39.Rojo F, Gonzalez-Navarrete I, Bragado R, Dalmases A, Menendez S, Cortes-Sempere M, et al. Mitogen-activated protein kinase phosphatase-1 in human breast cancer independently predicts prognosis and is repressed by doxorubicin. Clinical Cancer Research. 2009;15(10):3530–3539. doi: 10.1158/1078-0432.CCR-08-2070. [DOI] [PubMed] [Google Scholar]
- 40.Orlowski RZ, Small GW, Shi YY. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. Journal of Biological Chemistry. 2002;277(31):27864–27871. doi: 10.1074/jbc.M201519200. [DOI] [PubMed] [Google Scholar]
- 41.Small GW, Shi YY, Edmund NA, Somasundaram S, Moore DT, Orlowski RZ. Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Molecular Pharmacology. 2004;66(6):1478–1490. doi: 10.1124/mol.104.003400. [DOI] [PubMed] [Google Scholar]
- 42.Shi YY, Small GW, Orlowski RZ. Proteasome inhibitors induce a p38 mitogen-activated protein kinase (MAPK)-dependent anti-apoptotic program involving MAPK phosphatase-1 and Akt in models of breast cancer. Breast Cancer Research and Treatment. 2006;100(1):33–47. doi: 10.1007/s10549-006-9232-x. [DOI] [PubMed] [Google Scholar]
- 43.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. Journal of Biological Chemistry. 2005;280(6):4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]