Abstract
Glial-derived neurotrophic factor (GDNF) is a potent neuroprotective agent for multiple brain disorders, including Parkinson's disease. However, GDNF drug development is difficult because GDNF does not cross the blood-brain barrier (BBB). To enable future drug development of GDNF in mouse models, the neurotrophin was re-engineered as an IgG fusion protein to enable penetration through the BBB after intravenous administration. The 134-amino acid GDNF was fused to the heavy chain of a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR) designated the cTfRMAb. This antibody undergoes receptor-mediated transport across the BBB and acts as a molecular Trojan horse to ferry the GDNF into mouse brain. The cTfRMAb-GDNF fusion protein was expressed by stably transfected Chinese hamster ovary cells, affinity-purified, and the biochemical identity was confirmed by mouse IgG and GDNF Western blotting. The cTfRMAb-GDNF fusion protein was bifunctional and bound with high affinity to both the GDNF receptor α1, ED50 = 1.7 ± 0.2 nM, and the mouse TfR, ED50 = 3.2 ± 0.3 nM. The cTfRMAb-GDNF fusion protein was rapidly taken up by brain, and the brain uptake was 3.1 ± 0.2% injected dose/g brain at 60 min after intravenous injection of a 1-mg/kg dose of the fusion protein. Brain capillary depletion analysis showed the majority of the fusion protein was transcytosed across the BBB with penetration into brain parenchyma. The brain uptake results indicate it is possible to achieve therapeutic elevations of GDNF in mouse brain with intravenous administration of the cTfRMAb-GDNF fusion protein.
Glial-derived neurotrophic factor (GDNF) is a potential treatment for multiple brain disorders, including Parkinson's disease (PD), stroke, and addiction (Lapchak et al., 1997; Ron and Janak, 2005; Boado et al., 2008). However, GDNF does not cross the blood-brain barrier (BBB) in the mouse (Kastin et al., 2003) or the rhesus monkey (Boado and Pardridge, 2009). Consequently, the neurotrophin was administered by direct cranial infusion in patients with PD (Lang et al., 2006). However, the clinical trial was not successful, and subsequent studies showed limited penetration of GDNF into brain parenchyma after transcranial infusion (Salvatore et al., 2006). An alternative approach to GDNF drug development is the re-engineering of the neurotrophin as a fusion protein with a BBB molecular Trojan horse (Pardridge, 2008). The latter is a peptidomimetic monoclonal antibody (MAb) against an endogenous BBB peptide receptor transport system, such as the BBB insulin receptor or transferrin receptor (TfR). The MAb undergoes receptor-mediated transport across the BBB without interference of endogenous peptide transport. The MAb acts as a molecular Trojan horse to ferry a fused neurotherapeutic across the BBB after systemic administration of the fusion protein.
A fusion protein of GDNF and a genetically engineered MAb against the human insulin receptor (HIR) has been engineered (Boado et al., 2008), and the HIRMAb-GDNF fusion protein penetrates the primate BBB in vivo, whereas native GDNF does not cross the primate BBB (Boado and Pardridge, 2009). The HIRMAb-GDNF fusion protein retains high affinity binding to both the HIR and the GDNF receptor (GFR)-α1 and is equipotent with recombinant GDNF in GFRα1 receptor binding or bioassays in human neural cells (Boado et al., 2008). However, the HIRMAb-GDNF fusion protein cannot be tested in rodents because the HIRMAb part of the fusion protein is only active in humans and Old World primates, such as the rhesus monkey (Pardridge et al., 1995). There is no known MAb against the mouse or rat insulin receptor ectodomain that could be used as a BBB molecular Trojan horse. Therefore, a surrogate Trojan horse is used in rodents, which is a MAb against the TfR. The murine OX26 MAb against the rat TfR is used in rats (Pardridge et al., 1991); this MAb is not active against the mouse TfR (Lee et al., 2000). The rat 8D3 MAb against the mouse TfR is used for BBB drug delivery in the mouse (Lee et al., 2000). A chimeric form of the 8D3 TfRMAb has been engineered, in which the variable region of the heavy chain (VH) and the variable region of the light chain (VL) of the rat 8D3 TfRMAb were fused to the constant regions of the mouse IgG1 heavy chain and mouse κ light chain, respectively (Boado et al., 2009). The chimeric TfRMAb, designated cTfRMAb, is 85% mouse amino acid sequence, which allows for long-term administration in mouse models.
The purpose of the present study was to engineer, express, and validate a new IgG-GDNF fusion protein that would be active in mouse models of brain disease. The new fusion protein is composed of the cTfRMAb Trojan horse and GDNF, and this fusion protein is designated cTfRMAb-GDNF. Human GDNF was used because there is a 93% amino acid identity between human and mouse GDNF. The human GDNF monomer is fused to the carboxyl terminus of the heavy chain of the cTfRMAb, which places the neurotrophin in its native dimeric configuration (Fig. 1). After expression in stably transfected Chinese hamster ovary (CHO) cells, the cTfRMAb-GDNF fusion protein was examined with respect to binding to the mouse TfR and to the GFRα1. The plasma pharmacokinetics, metabolic stability, and brain uptake of the cTfRMAb-GDNF fusion protein in the C57BL/6 mouse were examined. The C57BL/6 mouse was investigated because this mouse strain is generally used in murine models of experimental PD (Alvarez-Fischer et al., 2008).
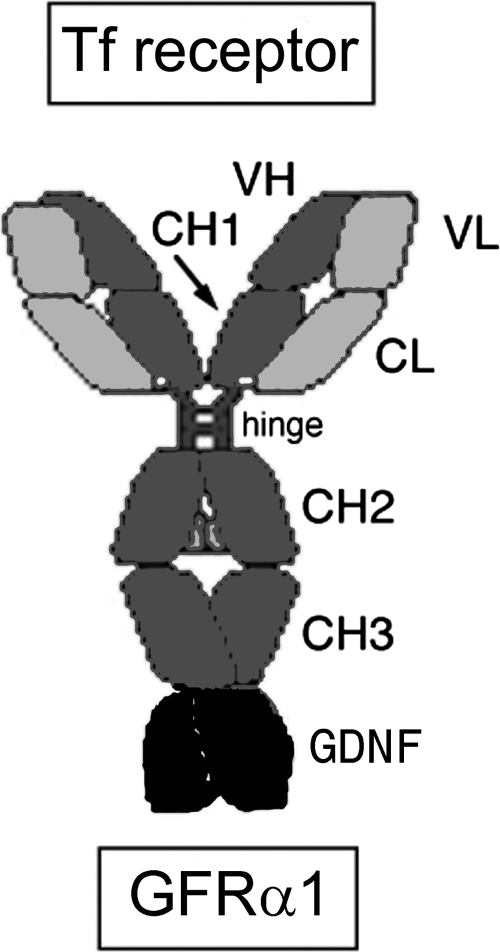
Fig. 1.
The cTfRMAb-GDNF fusion protein is composed of two HCs and two LCs. The HC is formed by fusion of the VH of the rat 8D3 MAb against the mouse TfR (mTfR) to the amino terminus of mouse IgG1 constant (C) region and fusion of human GDNF to the carboxyl terminus of the HC C-region. The LC is formed by fusion of the VL of the rat 8D3 MAb to the mouse κ LC C-region (CL). The HC C-region is composed of four domains: CH1, hinge, CH2, and CH3.
Materials and Methods
Production of CHO Line.
A tandem vector (TV) containing within a single plasmid DNA the expression cassettes encoding the cTfRMAb heavy chain (HC)-GDNF fusion protein, the cTfRMAb light chain (LC), and the murine dihydrofolate reductase (DHFR) was engineered similar to a TV described previously (Boado et al., 2010). The cDNA encoding the 134-amino acid (AA) human mature GDNF was amplified by polymerase chain reaction as described previously (Boado et al., 2008) and subcloned at the 3′-end of the cTfRMAb HC to form the pcTfRMAb-GDNF tandem vector shown in Fig. 2. The sequence of the TV was confirmed by bidirectional DNA sequencing performed at Eurofins MWG Operon (Huntsville, AL) using custom sequencing oligodeoxynucleotides synthesized at Midland Certified Reagent (Midland, TX). The TV was linearized, and DG44 CHO cells were electroporated, followed by selection in hypoxanthine-thymine-deficient medium and amplification with graded increases in methotrexate up to 80 nM in serum-free medium. The CHO line was dilutionally cloned at 1 cell/well, and high-producing clones were selected by measurement of medium mouse IgG concentrations by enzyme-linked immunosorbent assay (ELISA). The CHO line was stable through multiple generations and produced medium IgG levels of 5 to 10 mg/l in shake flasks at a cell density of 1 to 2 million cells/ml.
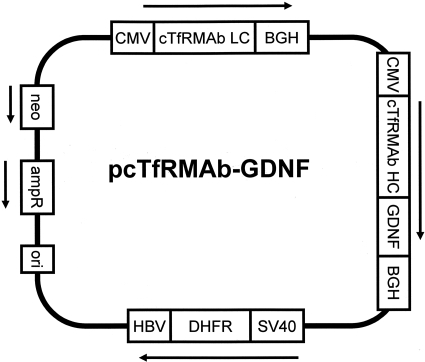
Fig. 2.
The TV expressing the cTfRMAb-GDNF fusion protein is composed of a single strand of DNA, which includes separate expression cassettes for the LC of the cTfRMAb, the HC gene formed by fusion of the cDNA encoding the GDNF to the 3′-end of the cDNA encoding the HC of the cTfRMAb, and murine DHFR, to allow for amplification of cell lines with methotrexate treatment. The HC and LC genes are 5′-flanked by the cytomegalovirus (CMV) promoter and 3′-flanked by the bovine growth hormone (BGH) polyA sequence; the DHFR gene is 5′-flanked by the SV40 promoter and 3′-flanked by the hepatitis B virus (HBV) polyA sequence. The plasmid also contains genes for neomycin (neo) resistance and ampicillin resistance (ampR).
Protein Purification.
The CHO cells were propagated in 1-liter bottles until 2.4 liters of conditioned serum-free medium was collected. The medium was ultrafiltered with a 0.2-μm Sartopore-2 sterile filter unit (Sartorius AG, Goettingen, Germany) and applied to a 25-ml protein G Sepharose 4 Fast Flow (GE Healthcare Bio-Sciences, Little Chalfont, Buckinghamshire, UK) column equilibrated in 25 mM Tris/25 mM NaCl/5 mM EDTA, pH 7.1. The column was washed with 25 mM Tris/1 M NaCl/5 mM EDTA, pH 7.1, and the fusion protein was eluted with 0.1 M glycine, pH 2.8. The acid eluate was pooled and neutralized to pH 5.5 with 1 M Tris base and concentrated with an Ultra-15 microconcentrator (Millipore Corporation, Billerica, MA) and stored sterile-filtered at 4°C.
SDS-Polyacrylamide Gel Electrophoresis and Western Blotting.
The homogeneity of the cTfRMAb-GDNF fusion protein was evaluated with SDS-polyacrylamide gel electrophoresis (PAGE) under reducing and nonreducing conditions using 12 and 7.5% Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA), respectively. Western blot analysis was performed with a goat anti-mouse IgG (H+L) antibody (Bethyl Laboratories, Montgomery, TX) for the mouse IgG Western blot and with a rabbit anti-human GDNF antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for the GDNF Western blot. The immunoreactivity of the cTfRMAb-GDNF fusion protein was compared with the cTfRMAb described previously (Boado et al., 2009) and recombinant human GDNF (PeproTech, Rocky Hill, NJ).
GFRα1-Binding ELISA.
The binding of the cTfRMAb-GDNF fusion protein to the GFRα1 was determined by ELISA as described previously (Boado et al., 2008). A mouse anti-human IgG (Invitrogen, Carlsbad, CA) was plated in 96-well plates overnight at 2 μg/well. After aspiration, washing, and blocking with 1% bovine serum albumin, the Fc fusion of the human GFRα1 extracellular domain (R&D Systems, Minneapolis, MN) was plated at 0.4 μg/well. After incubation at room temperature for 60 min, the wells were washed, and either the cTfRMAb-GDNF fusion protein or mouse IgG1κ (Sigma-Aldrich, St. Louis, MO) was plated for 2 h at room temperature. After washing, a goat anti-GDNF antibody (R&D Systems) was plated at 0.4 μg/well for 30 min at room temperature. After washing, a conjugate of alkaline phosphatase and a rabbit anti-goat IgG(H+L) (Vector Laboratories, Burlingame, CA) was plated, and detection at 405 nm was performed with an ELISA plate reader after color development with para-nitrophenylphosphate (Sigma-Aldrich). The ED50 of fusion protein binding to the GFRα1 was determined by nonlinear regression analysis using the BMDP2007e software (Statistical Solutions, Dublin, Ireland).
SK-N-MC Bioassay.
A bioassay of GDNF activity was performed as described previously (Boado et al., 2008) using human neural SK-N-MC cells, which were dual-transfected with the c-ret kinase and a luciferase reporter plasmid under the influence of the 5′-flanking sequence of the rat tyrosine hydroxylase (TH) gene (Tanaka et al., 2003). Addition of GDNF to the medium activates the TH promoter via signal transduction mediated by the GFRα1/c-ret kinase system, and this leads to increased luciferase expression by the cells. The cells were grown in collagen-coated 24-well dishes to 70% confluence, and 0.16 to 16 nM concentrations of the cTfRMAb-GDNF fusion protein were added to the medium. After a 24-h incubation at 37°C, the cells were extracted with 200 μl/well of Luciferase Reporter Lysis buffer (Promega, Madison, WI). After centrifugation, luciferase enzyme activity was measured in the lysate with a luminometer, and luciferase enzyme activity, reported as nanogram of luciferase, was normalized per milligram sample protein using the bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL). The luciferase activity (Act), nanogram of luciferase per milligram of protein, was plotted versus the concentration (S) of the cTfRMAb-GDNF fusion protein in the medium, and the data were fit to the following equation: Act = [(Amax)(S)/(EC50 + S)], where Amax is the maximal luciferase activity and ED50 is the concentration of fusion protein that produces a 50% increase in luciferase activity. The data were fit by nonlinear regression using the BMDP2007e statistical software (Statistical Solutions).
Mouse TfR Radio-Receptor Assay.
The binding of the cTfRMAb-GDNF fusion protein to the mouse TfR was determined with a radio-receptor assay described previously (Boado et al., 2009) using 125I-labeled rat 8D3 MAb to the mouse TfR as the binding ligand. The binding of the 125I-8D3 MAb to the mouse TfR, expressed on the plasma membrane of mouse fibroblasts, was displaced by increasing concentrations of either unlabeled 8D3 MAb or the cTfRMAb-GDNF fusion protein. For the 8D3 self-inhibition assay, the KD (nM), the maximal binding, Bmax (pmol/mg protein), and the nonsaturable binding (fmol/mg protein) were computed. For the cTfRMAb-GDNF cross-competition assay, the KI (nM) and the nonsaturable binding were computed. Parameter estimates were made by nonlinear regression analysis as described previously (Boado et al., 2009).
Radiolabeling of Fusion Protein.
The cTfRMAb-GDNF fusion protein, which was injected into mice for a pharmacokinetics analysis, was radiolabeled with [3H]N-succinimidyl propionate from American Radiolabeled Chemicals (St. Louis, MO), as described previously (Boado and Pardridge, 2009). The labeled protein was purified with a 1 × 28-cm Sephadex G-25 gel filtration column (GE Healthcare Bio-Sciences), with an elution buffer of 0.01 M sodium acetate/0.15 M NaCl, pH 5.5. The cTfRMAb-GDNF fusion protein was labeled to a specific activity of 0.40 μCi/μg and a trichloroacetic acid (TCA) precipitability of >99%.
Pharmacokinetics and Brain Uptake in the Mouse.
Adult male C57BL/6J mice, weighing 26 g, were obtained from The Jackson Laboratory (Bar Harbor, ME). All the procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the U.S. National Institutes of Health. Mice in groups of three were anesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg) and injected either intravenously in the tail vein or intraperitoneally with 0.1 ml (10 μCi) of [3H]cTfRMAb-GDNF fusion protein. The injection dose in each mouse of the cTfRMAb-GDNF fusion protein was 1.0 mg/kg. An aliquot (50 μl) of blood was collected at 0.25, 2, 5, 15, 30, and 60 min from three mice at each time point after injection of the fusion protein. The venous blood was sampled, in 50-μl aliquots, from a PE10 cannula inserted in the external jugular vein, which was maintained patent with heparinized saline. The blood was centrifuged for collection of plasma, which was analyzed for radioactivity. At 60 min after injection, the mice were euthanized, and the cerebral hemispheres were removed and weighed for each mouse. One hemisphere was used for total radioactivity after solubilization in Soluene-350 (PerkinElmer Life and Analytical Sciences, Waltham, MA), and one hemisphere was homogenized for capillary depletion analysis. Plasma and tissue samples were analyzed for 3H radioactivity with Optifluor-O (PerkinElmer Life and Analytical Sciences) and a liquid scintillation counter (Tricarb 2100TR; PerkinElmer Life and Analytical Sciences). Brain uptake data were expressed as a volume of distribution (VD), which is the ratio of the 60-min organ radioactivity (DPM/g) divided by the 60-min plasma radioactivity (DPM/μl), or as percentage of injected dose (ID)/g tissue. The plasma radioactivity that was precipitable with cold 10% TCA was determined at each time point. The plasma radioactivity, DPM/ml, was converted to percentage ID/ml, and the percentage ID/ml was fit to a biexponential equation:
The intercepts (A1, A2) and the slopes (k1, k2) were used to compute the pharmacokinetic parameters, including the median residence time, the central volume of distribution, the steady-state volume of distribution, the area under the plasma concentration curve (AUC), and the systemic clearance. Nonlinear regression analysis used the AR subroutine of the BMDP statistical software (Statistical Solutions). Data were weighted by 1/(%ID/ml)2. The organ clearance (μl/min/g), also called the BBB permeability-surface area product, is computed from the terminal brain uptake (%ID/g) and the 60-min plasma AUC (%IDmin/ml) as follows: PS product = [(%ID/g)/AUC] × 1000.
Capillary Depletion Method.
One cerebral hemisphere from each mouse was processed for the capillary depletion method as described previously (Triguero et al., 1990). Mouse brain, ∼0.2 g, was homogenized in a 4-fold volume of cold physiologic buffer with a glass tissue grinder. The solution was brought to a final dextran (60–80 kDa) (Sigma-Aldrich) concentration of 20% and centrifuged at 3200g at 4°C for 10 min. The fatty layer was transferred to a new tube, followed by removal of the postvascular supernatant, which was pooled with the fatty layer. The capillary pellet was resuspended in 1.0 ml of water. Aliquots of the initial dextran homogenate, the pooled postvascular supernatant, and the vascular pellet were analyzed for 3H radioactivity using Soluene-350 and Optifluor-O (PerkinElmer Life and Analytical Sciences) as described previously. The VD was determined for each of the three fractions from the ratio of total 3H radioactivity in the fraction, DPM per gram brain, divided by the 3H radioactivity in the 60-min terminal plasma, DPM per microliter. A high VD in the postvascular supernatant, compared with the VD in the capillary pellet, is evidence for transport of the protein through the BBB into brain parenchyma. The use of the capillary depletion method to assay BBB transcytosis in vivo is valid only when the study protein is bound with high affinity to the BBB receptor, as represented by a low nanomolar binding constant (Triguero et al., 1990).
Results
A tandem vector was engineered, which contained the expression cassettes for the HC fusion gene, the light gene, and the DHFR gene on a single strand of DNA. DNA sequence analysis showed the three expression cassettes spanned 6490 nucleotides. The LC was composed of 234 AA, which included a 20-AA signal peptide, a 108-AA VL of the cTfRMAb, and a 106-AA mouse κ LC constant region, which is 100% identical to AA 113 to 218 of BAA06141. The predicted molecular mass of the LC is 23,554 Da with a predicted isoelectric point of 5.73. The fusion protein of the cTfRMAb HC and the GDNF was composed of 597 AA, which included a 19-AA signal peptide. The predicted molecular mass of the HC, without glycosylation, is 64,018 Da with a predicted isoelectric point of 8.18. The domains of the fusion HC include a 118-AA VH of the cTfRMAb, a 324-AA mouse IgG1 constant region, which is 100% identical to AA 140 to 463 of BAC44885, a 2-AA linker (Ser-Ser), and the 134-AA human GDNF, which is 100% identical to AA 78 to 211 of NP_000505. The HC contains three N-linked glycosylation sites: one site in the constant region of the IgG HC and two sites in the GDNF domain.
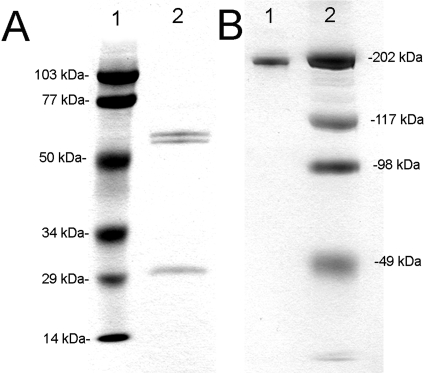
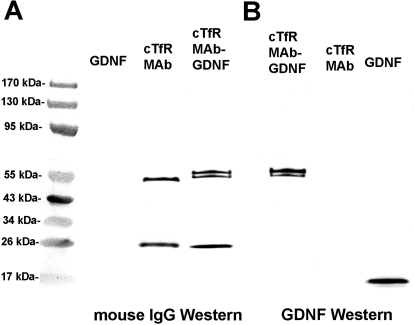
The CHO-derived cTfRMAb-GDNF fusion protein migrated as a doublet on reducing SDS-PAGE with a ∼65-kDa HC and a ∼30-kDa LC (Fig. 3A) and migrated as a 190-kDa protein on nonreducing SDS-PAGE (Fig. 3B). Western blot analysis with a primary antibody against mouse IgG detected both the HC and the LC of the cTfRMAb and the cTfRMAb-GDNF fusion protein but did not react with human GDNF (Fig. 4A). Western blot analysis showed a primary antibody against human GDNF reacted with both the HC of the cTfRMAb-GDNF fusion protein and with GDNF but did not react with HC of the cTfRMAb (Fig. 4B).
Fig. 3.
Reducing (A) and nonreducing (B) SDS-PAGE of molecular mass standards (lane 1) and the cTfRMAb-GDNF fusion protein (lane 2). The HC doublet reflects differential glycosylation, which is also seen on Western blotting (Fig. 4).
Fig. 4.
Western blotting with a primary antibody against mouse IgG (A) or against GDNF (B). The anti-mouse antibody reacts with the HC and LC of both the cTfRMAb and the cTfRMAb-GDNF fusion protein but does not react with GDNF. The anti-GDNF antibody reacts only with the HC of the cTfRMAb-GDNF fusion protein and with GDNF but does not react with the HC or LC of the cTfRMAb.
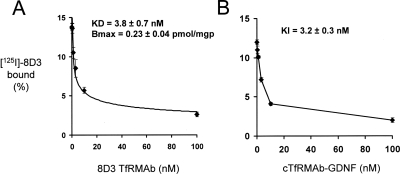
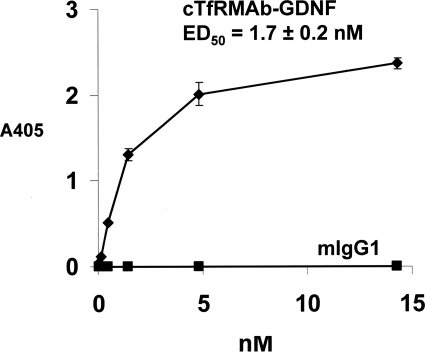
The 8D3 MAb was radio-iodinated and used as the ligand for the mouse TfR radio-receptor assay with mouse fibroblasts, and a self-inhibition curve was saturable and characterized by a KD of 3.8 ± 0.7 nM and a Bmax of 0.23 ± 0.04 pmol/mg protein (Fig. 5A). The KI of inhibition of binding of the 125I-8D3 MAb by unlabeled cTfRMAb-GDNF fusion protein was 3.2 ± 0.3 nM (Fig. 5B), which was comparable with the KD of 8D3 binding to the mouse TfR. The cTfRMAb-GDNF fusion protein retained high affinity binding to the recombinant human GFRα1 with an ED50 of 1.7 ± 0.2 nM; conversely, there was no binding of mouse IgG1 to the GFRα1 (Fig. 6). The cTfRMAb-GDNF fusion protein exhibited GDNF biological activity and stimulated luciferase gene expression in SK-N-MC neural cells cotransfected with the c-ret kinase and the luciferase gene under the influence of the TH promoter (Fig. 7). The ED50 in the bioassay, 1.4 ± 0.7 nM (Fig. 7), approximated the ED50 of binding in the GFRα1 ELISA, 1.7 ± 0.2 nM (Fig. 6).
Fig. 5.
Radio-receptor assay of the mouse TfR uses mouse fibroblasts as the source of the mouse TfR and 125I-8D3 as the binding ligand. Binding is displaced by unlabeled 8D3 MAb (A) or the cTfRMAb-GDNF fusion protein (B). The KD of 8D3 self-inhibition and the KI of cTfRMAb-GDNF cross-inhibition were computed by nonlinear regression analysis.
Fig. 6.
Binding of the cTfRMAb-GDNF fusion protein to the GFRα1 extracellular domain is saturable. The ED50 of cTfRMAb-GDNF fusion protein binding was determined by nonlinear regression analysis. There is no binding to the GFRα1 by mouse IgG1 (mIgG1).
Fig. 7.
The cTfRMAb-GDNF fusion protein activates luciferase gene expression in cultured SK-N-MC neural cells, which are cotransfected with the c-ret kinase and the luciferase gene under the influence of the TH promoter. Data are mean ± S.E. (n = 4 wells/point). The Amax and ED50 were determined by nonlinear regression analysis.
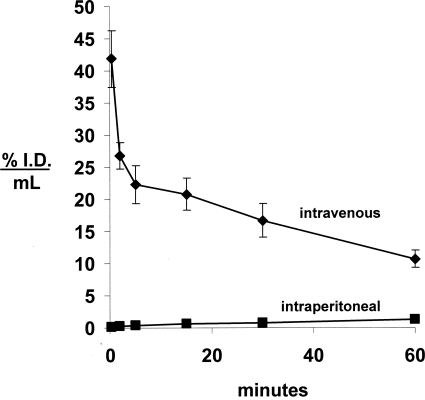
The cTfRMAb-GDNF fusion protein was radiolabeled with the 3H-N-succinimidyl propionate reagent (see Materials and Methods) and injected into adult male C57BL/6J mice via either intravenous or intraperitoneal administration. The clearance of the [3H]cTfRMAb-GDNF fusion protein from plasma after intravenous administration, as well as the appearance of plasma radioactivity after intraperitoneal administration of the fusion protein, is plotted in Fig. 8. The fusion protein entered the blood compartment poorly after intraperitoneal administration (Fig. 8). For the intravenous injection studies, the plasma radioactivity decay curve was fit to a biexponential equation to yield the pharmacokinetic parameters shown in Table 1. The [3H]cTfRMAb-GDNF fusion protein was metabolically stable after intravenous administration, because the plasma radioactivity that was precipitable by TCA was 98.2 ± 0.1, 97.1 ± 0.1, 96.7 ± 0.8, 96.3 ± 0.7, 95.4 ± 0.1, and 92.5 ± 0.8, respectively, at 0.25, 2, 5, 15, 30, and 60 min after intravenous injection.
Fig. 8.
Plasma concentration, expressed as percentage of ID/ml, of the [3H]cTfRMAb-GDNF fusion protein after intravenous or intraperitoneal injection in the mouse. Data are mean ± S.E. (n = 3 mice/point).
TABLE 1.
Pharmacokinetic parameters of cTfRMAb-GDNF in the mouse
The injection dose was 1.0 mg/kg, and the body weight of the mice was 0.026 kg.
| Parameter | Units | cTfRMAb-GDNF |
|---|---|---|
| A1 | %ID/ml | 22.7 ± 2.8 |
| A2 | %ID/ml | 24.7 ± 0.9 |
| k1 | min−1 | 1.08 ± 0.29 |
| k2 | min−1 | 0.014 ± 0.001 |
| MRT | min | 72 ± 5 |
| Vc | ml/kg | 81 ± 4 |
| Vss | ml/kg | 154 ± 4 |
| AUC60 min | %ID · min/ml | 1031 ± 19 |
| AUCss | %ID · min/ml | 1822 ± 90 |
| Cl | ml/min/kg | 2.11 ± 0.11 |
MRT, mean residence time; Vc, plasma volume; Vss, steady-state volume of distribution; AUC60 min, area under the curve first 60 min; AUCss, steady-state AUC; Cl, clearance from plasma.
The uptake of the [3H]cTfRMAb-GDNF fusion protein by brain was determined at 60 min after intravenous injection and was 3.1 ± 0.2% ID/g brain, which is high compared with the brain uptake of a brain blood volume marker in the mouse, such as the OX26 MAb to the rat TfR (Fig. 9A). The BBB permeability-surface area product of the cTfRMAb-GDNF fusion protein, which is equal to the ratio of the 60-min %ID/g (Fig. 9A) and the 60-min plasma AUC (Table 1), is 3.0 ± 0.2 μl/min/g. The capillary depletion analysis showed the VD of the cTfRMAb-GDNF fusion protein in the brain homogenate was high, 244 ± 19 μl/g (Fig. 9B), which is 23-fold higher than the brain VD for the OX26 MAb in the mouse, 10.7 ± 0.6 μl/g (Lee et al., 2000). The VD of the fusion protein in the postvascular supernatant, 147 ± 33 μl/g (Fig. 9B), is 60% of the homogenate VD, indicating the majority of the cTfRMAb-GDNF fusion protein had completed transcytosis through the BBB by 60 min after intravenous injection. The radioactivity in the postvascular brain supernatant that was precipitable by TCA was 98.1 ± 0.1% at 60 min after intravenous injection, showing the product appearing in the brain parenchyma was intact cTfRMAb-GDNF fusion protein.
Fig. 9.
A, brain uptake, expressed as percentage of ID/g brain, for the cTfRMAb-GDNF fusion protein, compared with the mouse brain uptake for the OX26 MAb against the mouse TfR, which is a brain blood volume marker in the mouse (Lee et al., 2000). B, VD of the cTfRMAb-GDNF fusion protein in the brain homogenate (H), the postvascular supernatant (S), and the vascular pellet (P). Data are mean ± S.E. (n = 3 mice).
Discussion
The results of these studies are consistent with the following conclusions. First, the genetic engineering of the tandem vector (Fig. 2) allowed for the isolation of a high producing, stably transfected CHO line that secretes the heterotetrameric cTfRMAb-GDNF fusion protein in serum-free medium. Second, the secreted cTfRMAb-GDNF fusion protein is correctly processed by the CHO cells based on SDS-PAGE and mouse IgG and human GDNF Western blotting (Figs. 3 and 4). Third, the cTfRMAb-GDNF fusion protein is a bifunctional protein with dual-receptor specificity, as shown by binding assays for both the mouse TfR and the GFRα1 (Figs. 5 and 6) and neural cell bioassay (Fig. 7). Fourth, the brain uptake of the cTfRMAb-GDNF fusion protein is high, 3.1 ± 0.2% ID/g brain (Fig. 9A), and the capillary depletion analysis indicates the majority of the cTfRMAb-GDNF fusion protein taken up by brain has penetrated the BBB and entered brain parenchyma (Fig. 9B).
There are multiple potential approaches to the genetic engineering of an IgG-GDNF fusion protein. GDNF, similar to many other neurotrophins, is produced within the cell as a preproprotein, and the amino terminal half of the preproprotein is cleaved to produce the 134-AA mature GDNF. Fusion of the prepropeptide to the amino terminus of the HC or LC of the cTfRMAb would enable the initial folding of the GDNF precursor within the host cell. However, the TfR binding sites on the antibody chains are near the amino terminus. Previous work has shown that fusion of therapeutic peptides to the amino terminus of the MAb results in a loss of affinity of the MAb for the target BBB receptor (Boado and Pardridge, 2010). In the present study, the mature 134-AA GDNF was fused to the carboxyl terminus of the HC of the cTfRMAb (Fig. 1), which is confirmed by the mouse IgG and GDNF Western blotting (Fig. 4). Fusion of the mature GDNF to the carboxyl terminus of the HC of the cTfRMAb places the GDNF in a dimeric configuration (Fig. 1), which mimics the GDNF dimer that binds the GFRα1 (Parkash et al., 2008). The re-engineering of GDNF as the IgG fusion protein shown in Fig. 1 allows for preservation of high affinity binding of GDNF to its cognate receptor. The ED50 of either cTfRMAb-GDNF fusion protein binding to the GFRα1, 1.7 ± 0.2 nM (Fig. 6), or fusion protein activation of the GFRα1/c-ret kinase system in neural cells, 1.4 ± 0.7 nM (Fig. 7), is in the low nanomolar range. These ED50 values for the cTfRMAb-GDNF fusion protein approximate the ED50 of GDNF binding to the GFRα1 or GDNF activation of the GFRα1/c-ret kinase in neural cells, as reported previously (Boado et al., 2008).
The cTfRMAb part of the cTfRMAb-GDNF fusion protein also retains high affinity binding to the mouse TfR with a KI of 3.2 ± 0.3 nM (Fig. 5B), and this high affinity enables rapid penetration into brain via transport on the BBB TfR in the mouse in vivo. The plasma pharmacokinetics and brain uptake of the cTfRMAb-GDNF fusion protein was examined in mice in this study. The cTfRMAb-GDNF fusion protein was tritiated and administered by either intraperitoneal or intravenous injection. The fusion protein distributes to the blood compartment poorly after intraperitoneal administration but is rapidly removed from plasma after intravenous injection (Fig. 7). The circulating cTfRMAb-GDNF fusion protein remains intact during the first hour after intravenous injection, as the plasma radioactivity was 95 and 93% precipitable by TCA at 30 and 60 min (see Results). The systemic clearance of the cTfRMAb-GDNF fusion protein, 2.11 ± 0.11 ml/min/kg (Table 1), is higher than the systemic clearance of either the rat 8D3 TfRMAb (Lee et al., 2000) or the genetically engineered cTfRMAb (Boado et al., 2009) in the mouse. The higher systemic clearance of the cTfRMAb-GDNF fusion protein may be related to the cationic charge of the GDNF, because this neurotrophin is rapidly removed from blood after intravenous injection (Boado and Pardridge, 2009).
The brain uptake of the cTfRMAb-GDNF fusion protein is high, 3.1 ± 0.2% ID/g brain (Fig. 9A), and is comparable with the high brain uptake of a fusion protein of the cTfRMAb and a single chain Fv antibody, which is 3.5 ± 0.3% ID/g brain (Boado et al., 2010). In contrast, the brain uptake in the mouse of a conjugate of paclitaxel and angiopep-2, a putative ligand for the BBB low-density lipoprotein receptor-related protein 1, is 10-fold lower, 0.34% ID/g brain (Thomas et al., 2009). The high mouse brain uptake of the cTfRMAb fusion proteins is attributed to rapid BBB transport in vivo. The rapid BBB transport correlates with the low nanomolar constant of cTfRMAb-GDNF fusion protein binding to the mouse TfR (Fig. 5). Binding of the cTfRMAb-GDNF fusion protein to the BBB TfR triggers transcytosis through the BBB and penetration of brain parenchyma. This activity is shown by the capillary depletion method, which shows the VD of the fusion protein in the postvascular supernatant is 60% of the total homogenate VD (Fig. 9B). This finding indicates the majority of the cTfRMAb-GDNF fusion protein that has bound the BBB TfR has crossed the BBB and entered brain.
The ID of the cTfRMAb-GDNF fusion protein in this study was 1.0 mg/kg (see Materials and Methods). Because the brain uptake of the fusion protein at this dose is 3.1% ID/g, the brain concentration of the cTfRMAb-GDNF fusion protein is 750 ng/g brain. The GDNF moiety constitutes 17% of the fusion protein based on AA composition (see Results). Therefore, the brain concentration of exogenous GDNF is 125 ng/g after an intravenous injection of the cTfRMAb-GDNF fusion protein (1.0 mg/kg). This concentration of cerebral GDNF represents a substantial increase in brain GDNF compared with the endogenous concentration. Endogenous GDNF has been measured in brain by ELISA after preparation of a brain homogenate. In the human brain, the GDNF concentration is 0.39 and 0.68 ng/g in the cortex and striatum, respectively (Michel et al., 2008). In the mouse, there are more disparate measurements of endogenous GDNF in the striatum, which vary from 0.3 ng/g (Griffin et al., 2006) to 8 ng/g (Airavaara et al., 2004). In either case, it is possible to achieve a substantial increase in brain GDNF after the systemic administration of the cTfRMAb-GDNF fusion protein. Therapeutic effects of GDNF in experimental PD are achieved with only a modest, e.g., 4-fold, increase in exogenous GDNF in the brain (Eslamboli et al., 2005). Such elevations in brain GDNF could be achieved with relatively small doses of the cTfRMAb-GDNF fusion protein considerably reduced from the dose, 1.0 mg/kg, used in the present study.
In conclusion, the present work describes the genetic engineering, expression, and validation of a novel IgG-GDNF fusion protein, which binds with high affinity both to the GFRα1, to induce neuroprotection in the brain, and to the mouse TfR, to induce transport across the mouse BBB. The transport of the cTfRMAb-GDNF fusion protein across the mouse BBB is rapid, which generates high concentrations of biologically active, exogenous GDNF in the brain. Future work on the therapeutic and toxicologic effects of the cTfRMAb-GDNF fusion protein in the mouse, including mouse models of PD, will complement parallel drug development of the human homolog, the HIRMAb-GDNF fusion protein (Boado et al., 2008).
Acknowledgments.
We thank Winnie Tai and Phuong Tram for technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS065917].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.031534.
- GDNF
- glial-derived neurotrophic factor
- PD
- Parkinson's disease
- BBB
- blood-brain barrier
- MAb
- monoclonal antibody
- TfR
- transferrin receptor
- HIR
- human insulin receptor
- HIRMAb
- engineered monoclonal antibody against the human insulin receptor
- GFR
- glial-derived neurotrophic factor receptor α1
- VH
- variable region of the heavy chain
- VL
- variable region of the light chain
- cTfRMAb
- chimeric monoclonal antibody against the mouse transferrin receptor
- CHO
- Chinese hamster ovary
- TV
- tandem vector
- HC
- heavy chain
- LC
- light chain
- DHFR
- dihydrofolate reductase
- AA
- amino acid
- ELISA
- enzyme-linked immunosorbent assay
- PAGE
- polyacrylamide gel electrophoresis
- TH
- tyrosine hydroxylase
- TCA
- trichloroacetic acid
- VD
- volume of distribution
- ID
- injected dose
- AUC
- area under the curve
- DPM
- disintegrations per minute
- BAA06141
- mouse immunoglobulin gamma-1 light chain
- BAC44885
- mouse immunoglobulin gamma-1 heavy chain.
References
- Airavaara M, Planken A, Gäddnäs H, Piepponen TP, Saarma M, Ahtee L. (2004) Increased extracellular dopamine concentrations and FosB/ΔFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci 20:2336–2344 [DOI] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Höglinger GU, Oertel WH, Hartmann A. (2008) Characterization of the striatal 6-OHDA model of Parkinson's disease in wild type and α-synuclein-deleted mice. Exp Neurol 210:182–193 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. (2009) Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Drug Metab Dispos 37:2299–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. (2010) Genetic engineering of IgG-glucuronidase fusion proteins. J Drug Target, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. (2009) Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng 102:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. (2008) GDNF fusion protein for targeted-drug delivery across the human blood-brain barrier. Biotechnol Bioeng 100:387–396 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhou Q-H, Lu JZ, Hui E-W, Pardridge WM. (2010) Pharmacokinetics and brain uptake of a genetically engineered fusion antibody targeting the mouse transferring receptor. Mol Pharm, 7:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. (2005) Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci 25:769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Boger HA, Granholm AC, Middaugh LD. (2006) Partial deletion of glial cell line-derived neurotrophic factor (GDNF) in mice: effects on sucrose reward and striatal GDNF concentrations. Brain Res 1068:257–260 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC: [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. (2003) Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett 340:239–241 [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, et al. (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466 [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Miller PJ, Collins F, Jiao S. (1997) Glial cell line-derived neurotrophic factor attenuates behavioural deficits and regulates nigrostriatal dopaminergic and peptidergic markers in 6-hydroxydopamine-lesioned adult rats: comparison of intraventricular and intranigral delivery. Neuroscience 78:61–72 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. (2000) Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292:1048–1052 [PubMed] [Google Scholar]
- Michel TM, Frangou S, Camara S, Thiemeyer D, Jecel J, Tatschner T, Zoechling R, Grünblatt E. (2008) Altered glial cell line-derived neurotrophic factor (GDNF) concentrations in the brain of patients with depressive disorder: a comparative post-mortem study. Eur Psychiatry 23:413–420 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (2008) Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem 19:1327–1338 [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Buciak JL, Friden PM. (1991) Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 259:66–70 [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. (1995) Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res 12:807–816 [DOI] [PubMed] [Google Scholar]
- Parkash V, Leppänen VM, Virtanen H, Jurvansuu JM, Bespalov MM, Sidorova YA, Runeberg-Roos P, Saarma M, Goldman A. (2008) The structure of the glial cell line-derived neurotrophic factor-coreceptor complex: insights into RET signaling and heparin binding. J Biol Chem 283:35164–35172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Janak PH. (2005) GDNF and addiction. Rev Neurosci 16:277–285 [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, Gerhardt GA, Gash DM. (2006) Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol 202:497–505 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Xiao H, Hirata Y, Kiuchi K. (2003) A rapid assay for glial cell line-derived neurotrophic factor and neurturin based on transfection of cells with tyrosine hydroxylase promoter-luciferase construct. Brain Res Brain Res Protoc 11:119–122 [DOI] [PubMed] [Google Scholar]
- Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, et al. (2009) Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res 26:2486–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. (1990) Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem 54:1882–1888 [DOI] [PubMed] [Google Scholar]