Abstract
Fifteen years have passed since we published findings in the AJRCMB demonstrating that induction of early response fos/jun proto-oncogenes in rodent tracheal and mesothelial cells correlates with fibrous geometry and pathogenicity of asbestos. Our study was the first to suggest that the aberrant induction of signaling responses by crocidolite asbestos and erionite, a fibrous zeolite mineral associated with the development of malignant mesotheliomas (MMs) in areas of Turkey, led to altered gene expression. New data questioned the widely held belief at that time that the carcinogenic effects of asbestos in the development of lung cancer and MM were due to genotoxic or mutagenic effects. Later studies by our group revealed that proto-oncogene expression and several of the signaling pathways activated by asbestos were redox dependent, explaining why antioxidants and antioxidant enzymes were elevated in lung and pleura after exposure to asbestos and how they alleviated many of the phenotypic and functional effects of asbestos in vitro or after inhalation. Since these original studies, our efforts have expanded to understand the interface between asbestos-induced redox-dependent signal transduction cascades, the relationship between these pathways and cell fate, and the role of asbestos and cell interactions in development of asbestos-associated diseases. Of considerable significance is the fact that the signal transduction pathways activated by asbestos are also important in survival and chemoresistance of MMs and lung cancers. An understanding of the pathogenic features of asbestos fibers and dysregulation of signaling pathways allows strategies for the prevention and therapy of asbestos-related diseases.
Keywords: proto-oncogenes, mitogen-activated protein kinases, epidermal growth factor receptor, activator protein-1, nuclear factor-κB
CLINICAL RELEVANCE.
This review will help guide individuals in research on fiber carcinogenesis. The article also outlines therapeutic approaches for treatment of asbestos-related diseases that have been developed in the recent past.
Asbestos fibers are naturally occurring in rocks and soils and consist of six distinct types. The amphibole types of asbestos (crocidolite, amosite, anthophyllite, tremolite, and actinolite) are rod-like and more durable in the body than the only serpentine asbestos type, chrysotile (reviewed in Ref. 1). In the past, exposure to asbestos fibers in unregulated workplaces has given rise to pleural and lung fibrosis (asbestosis), lung cancer, and pleural and peritoneal malignant mesothelioma (MM) (2–4). Although not technically classified as “asbestos” fibers, exposure to Libby amphibole transition fibers (5) and erionite fibers (6) also have given rise to a spectrum of asbestos-associated diseases, suggesting that certain durable long (> 5 μm) fibers1 may have pathologic effects similar to those of asbestos. Asbestos may be a tumor promoter or co-carcinogen in the induction of lung cancers, as cigarette smoke appears to be a greater risk factor than asbestos exposure in these tumors (reviewed in Ref. 7). Moreover, the synergistic effects of asbestos and smoking in some cohorts implies that asbestos does not act in a manner similar to that of polycyclic aromatic hydrocarbons or other chemical carcinogens in cigarette smoke that are generally metabolized and/or interact directly with DNA.
The advent of new molecular technologies has fostered an explosion of knowledge, leading to new understandings of biological processes that initiate lung injury and repair or the development of different diseases by asbestos. Nonetheless, we still lack an understanding of the precise nature of all of the cellular targets that are perturbed by different types of asbestos fibers, how asbestos changes the intensity and duration of signaling through specific pathways, and how information from multiple signaling events is parsed and filtered to dictate phenotypic and functional endpoints in different cell types. As asbestos-related diseases have long latency periods, poor prognoses, and limited effective therapies, our focus remains on understanding the basic mechanisms of toxicity and lung or pleural remodeling that will inform translational studies on disease intervention and treatment.
OXIDANTS AND ASBESTOS
By the early 1990s, oxidants had been implicated in the activity of crocidolite and amosite, the most potent types of asbestos that are associated with the causation of MMs (reviewed in Ref. 8). The high iron content of these asbestos types appeared to be critical to the genesis of reactive oxygen species (ROS), including the highly DNA-damaging hydroxyl radical (OH·). In addition, it has been demonstrated that H2O2, the superoxide radical (O2−), and reactive nitrogren species are released from several types of asbestos fibers in cell-free solutions or in cells, especially alveolar or peritoneal macrophages, after phagocytosis of asbestos fibers in vitro or after inhalation. These reactive species may initiate cell signaling events both externally and within cells and act in a dose–response fashion to induce cell proliferation and injury (Figure 1).
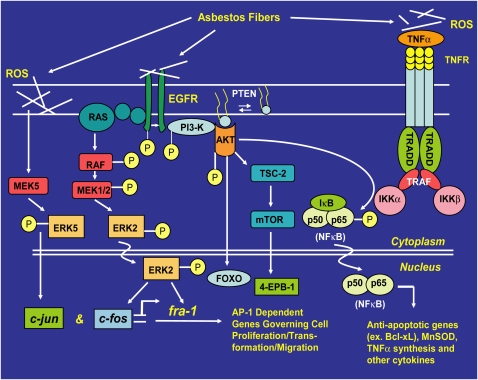
Figure 1.
Asbestos has pleiotropic effects on cell signaling pathways. Either through direct interactions with receptors or via the genesis of reactive oxygen species (ROS), asbestos activates cell signaling pathways that regulate gene expression and cell fate. Direct interaction with the epidermal growth factor receptor (EGFR) activates the Ras-Raf-extracellular signal–regulated kinase (ERK) pathway, which controls expression and transcriptional activity of the Fos family members of the activator protein-1 (AP-1) transcription factor. Asbestos also regulates c-Jun through activation of ERK5. Through AP-1, ERK1/2 and ERK5 govern outcomes that include cell proliferation, cell migration, and aspects of neoplastic transformation. Activation of the phosphoinositol-3 kinase (PI3K)/AKT pathway promotes cell survival through NF-κB. Recent work also indicates that asbestos fibers or ROS activate signaling through the TNF receptor. The diverse phenotypic outcomes of asbestos exposure depend on fiber type, fiber dose, and the signaling pathways resident in specific cell types. Moreover, the physical properties of asbestos tend to promote robust and persistent activation of signaling through ERK and other redox-responsive kinase cascades.
Asbestos also causes up-regulation of antioxidant enzymes such as manganese-containing superoxide dismutase (MnSOD, SOD2), catalase, and heme oxygenase in MMs and rodent lungs (9–12). Moreover, polyethylene glycol–conjugated catalase inhibited lung injury, inflammation, and asbestosis in a rodent inhalation model (13). At high cytotoxic concentrations, asbestos fibers caused formation of the pre-mutagenic lesion, 8-oxoguanine, that was rapidly repaired and/or released into cell medium by dying mesothelial cells (14, 15). While genomic alterations are an essential feature in the causation of cancer, large chromosomal deletions by asbestos appear to be clastogenic and associated with cell death (16).
We first showed that asbestos fibers, either directly or via production of reactive oxygen or nitrogen species, induced cell signaling events, including the production of diacylglycerol, hydrolysis of phosphatidylinositol, and activation of protein kinase C at the plasma membrane (17–19). These events were linked to increased expression of ornithine decarboxylase (ODC) (20), the enzyme responsible for the rate-limiting step in the synthesis of polyamines, a crucial step in cell proliferation induced by growth factors and phorbol ester tumor promoters. These studies led to speculation that the aberrant activation of diverse signaling cascades may be critical in asbestos-associated carcinogenic responses.
Fos/Jun, ACTIVATOR PROTEIN-1, AND ASBESTOS
The first evidence showing that asbestos fibers exert regulatory effects linked to aberrant transcriptional responses, cell proliferation, and cell transformation emanated from studies in which asbestos fibers caused induction of c-fos and c-jun proto-oncogene mRNA in pleural mesothelial cells and tracheo-bronchial epithelial cells in a dose–response fashion (21). This work also showed elevation of c-Fos and c-Jun proteins and increases in the ability of the activator protein-1 (AP-1) transcription factor to bind to DNA. AP-1 is composed of the Fos and Jun families of transcription factors. Whereas Jun members are capable of forming homodimers, binding DNA, and regulating transcription, all Fos family members (Fos, FosB, and Fra1) must form heterodimers with Jun family members (c-Jun, JunB, and JunD) to bind DNA. The activities of specific AP-1 complexes in gene expression have been studied extensively, but until the advent of chromatin immunoprecipitation techniques, it was not possible to document the interactions of various AP-1 subunits with specific gene promoters in the cell. Linking increased expression of AP-1 subunits such as Fos and Jun to specific endpoints remains a challenge, but increased expression of c-Jun by asbestos or hydrogen peroxide (H2O2) is critical to proliferation and transformation of tracheal epithelial cells (22). In contrast to H2O2 or the phorbol ester tumor promoter, 12-0-tetradecanoyl-13-phorbol acetate (TPA), which caused transient increases in c-fos and c-jun mRNA expression, the effects of asbestos were protracted, lasting for at least 24 hours. Most importantly, persistent induction of proto-oncogenes was dose related, occurred at subcytotoxic concentrations of asbestos fibers, and was most striking with crocidolite as compared with chrysotile asbestos at similar concentrations.
Our study published in the AJRCMB (Red Journal) (20) demonstrated that the ability of asbestos fibers to induce expression of c-fos and c-jun proto-oncogenes correlated with the carcinogenic potential of fibers, as riebeckite particles, which have the same chemical composition as crocidolite asbestos, were less potent in their ability to induce c-fos and c-jun expression, AP-1 DNA binding activity, and elevations in odc mRNA. Moreover, studies using N-acetyl-cysteine (NAC) (23) and protein kinase C inhibitors (19) clearly suggested that oxidative stress and kinase signaling were involved in asbestos-induced expression of c-fos and c-jun and in proliferative responses (23). Since crocidolite asbestos fibers are capable of inducing cell proliferation, cell cycle arrest, and apoptosis in various populations of mesothelial cells (24) and lung epithelial cells (25), presumably due to initial injury followed by compensatory proliferation of surrounding cells, our next goals were to identify the specific signaling cascades activated by asbestos fibers and how these are initiated. Moreover, we attempted to link specific signaling cascades to phenotypic endpoints such as proliferation, apoptosis, or cell transformation, and to determine critical changes in gene expression important in cell responses.
ASBESTOS FIBERS ACTIVATE THE EPIDERMAL GROWTH FACTOR RECEPTOR AND EXTRACELLULAR SIGNAL–REGULATED KINASES
The epidermal growth factor (EGF) receptor (EGFR) (HER) family of transmembrane proteins occurs on human mesothelial and lung epithelial cells, and many ligands (transforming growth factor-α, amphiregulin, heparin-binding EGF, and epiregulin) act on these receptors in a paracrine or autocrine fashion. The EGFR (HER1) can be phosphorylated (activated) by certain mutations, G protein–coupled receptors, cytokine receptors, and integrins (26–28). Through various downstream pathways, the EGFR plays a central role in survival, motility, attachment, and cell transformation.
We first demonstrated that the asbestos fibers, chrysotile and crocidolite, but not their nonfibrous analogs, antigorite and riebeckite, activated the EGFR in mesothelial cells, an event linked to activation of the downstream extracellular signal-regulated kinases (ERKs) (i.e., ERK1/2) (29). Since ERK1/2 regulates in part the transcriptional activity of c-fos, and both asbestos-induced c-fos and c-jun mRNA levels in distal bronchiolar epithelium are reduced in EGFR-null mice (30), these studies provide a link between activation of EGFR, ERK1/2, and AP-1–related gene expression. Crocidolite also caused up-regulation of EGFR mRNA and protein in mesothelial cells (31), suggesting a positive-feedback pathway that was later shown to be important in induction of matrix metalloproteinases by asbestos in lung epithelial cells (32). Others have demonstrated that phosphorylation of the EGFR occurs with other types of carcinogenic fibers and may be related to the generation of oxidants after incomplete phagocytosis of long fibers (33). Using an antibody specific to the external domain of the EGFR, we also showed using multi-fluorescence approaches that long (> 20 μm) crocidolite asbestos fibers deposited on the cell surface of immortalized human (MET5A) mesothelial cells were physically associated with the EGFR, suggesting that long fibers might cause dimerization of the EGFR (34). That EGFR phosphorylation by crocidolite asbestos was causally linked to transactivation of ERKs 1 and 2 was shown using a small molecule inhibitor of EGFR phosphorylation (AG1478) (25, 29, 35). However, asbestos-induced ERK 5 (also called Big MAP Kinase or BMK) phosphorylation was unaffected by pretreatment with AG1478, suggesting an EGFR-independent mechanism of ERK 5 activation in lung epithelial cells (35).
The ERK family of serine-threonine kinases also regulate expression of different members of the fos/jun family (and other genes) and appear to have different roles in transformation, proliferation, and cell motility (reviewed in Ref. 36). Because increased amounts of phosphorylated ERK1/2 protein are observed in small airway epithelial cells after inhalation of crocidolite asbestos (37, 38), and asbestos-induced proliferation of these cells in transgenic mice with a dominant-negative MEK1 construct linked to the CC10 promoter is curtailed (39), we have focused on this group of mitogen-activated protein kinases (MAPK). In lung epithelial cells, activation of ERK1/2 is required for expression of cyclin D1 and cell proliferation in response to serum growth factors (40). Oxidants prolong signaling through ERK1/2, which stabilizes c-Fos and induces cell cycle arrest by preventing the expression of cyclin D1. These observations supported earlier work showing that increased expression of c-Fos in response to activation of EGFR by asbestos is associated with apoptosis in mesothelial cells (31). Interestingly, ERK1/2-dependent stabilization of c-Fos by oxidative stress prevents expression of cyclin D1 by Fra-1 (40), which is expressed in a more protracted manner after initial elevations in c-Jun and c-Fos by asbestos. Fra-1 is required for AP-1–mediated mesothelial cell growth, migration, and transformation (41). Studies using an siRNA approach to knock down Fra-1 in rodent MM cells support the hypothesis that an ERK1/2-linked Fra-1 pathway is associated with expression of genes (c-met, cd44) (42) proven critical to invasion of human MMs (43). Recently, we have examined several significant increases and decreases in gene expression using global gene analysis (Affymetrix microarrays) on telomerase-immortalized human mesothelial cells (LP9/TERT-1) in vitro after exposures to equal surface area concentrations of crocidolite asbestos fibers or nonfibrous, nonmesotheliomagenic particles (platy talc, fine titanium dioxide, and glass beads) (44). In contrast to nonfibrous materials that caused no or transient expression of a few genes, asbestos fibers caused changes in expression of several AP-1–regulated and other genes linked to cell signaling, apoptosis, cell proliferation, and protein metabolism, and immune responses—alterations that increased with concentration and time of exposure. These studies suggest that gene profiling in target cells of lung disease may be predictive of the pathogenic potential of inhaled fibers or particles.
ASBESTOS ACTIVATES NF-κB AND THE TNF-α RECEPTOR
Shortly after our discovery that asbestos fibers stimulated an ERK/AP-1 pathway, we reported that another transcription factor, NF-κB, was activated by asbestos fibers in tracheal epithelial cells in vitro, mesothelial cells in vitro, and lungs of rats after inhalation of asbestos (45, 46). Moreover, asbestos fibers caused transcriptional activation of a number of NF-κB–dependent genes, including c-myc, another proto-oncogene through an oxidant-dependent pathway (45). Since activation of NF-κB is critical in up-regulating the expression of many genes linked to proliferation, apoptosis, and chemokine/cytokine production, it is undoubtedly a critical transcription factor in inflammation and responses in target cells of asbestos-related diseases. For example, we have recently shown that frustrated phagocytosis of asbestos fibers by human monocytes activates the NALP3 inflammasome that produces active IL-1β (47), an interleukin that binds to the IL-1 receptor 1. Upon ligand binding, intracellular adapters that include TNF receptor (TNFR)-associated factor 6 (TRAF6) are recruited to IL-1 receptor 1, potentially activating both the NF-κB and AP-1 pathways (48).
Alveolar macrophages are an early hallmark of inhalation of asbestos fibers. In response to asbestos fibers macrophages release TNF-α, which either binds to the TNFR or cooperatively interacts with oxidants to activate a Ras/MAPK/NF-κB pathway in lung epithelial cells (49). TNF-α causes both apoptosis and compensatory proliferation in mesothelial cells (24). The fact that IL-1β and TNF-α contribute to mesothelial cell transformation (50) suggests that their importance in multiple stages of tumorigenesis may be due in part to activation of AP-1– or NF-κB–dependent gene expression. Moreover, it has recently been reported that NF-κB is a constitutive survival factor in human mesothelial cells exposed to asbestos and is up-regulated in MM cells (51). Based on the observation that Bortezomib inhibits NF-κB activity in MMs and increases apoptosis, a phase II clinical trial has begun in Europe.
PRO-APOPTOTIC MECHANISMS OF ASBESTOS
In a number of in vitro models, asbestos fibers cause cell senescence, lytic cell death and apoptosis, primarily due to generation of ROS and/or physical interaction of fibers with the plasma membrane and cellular organelles. The induction of apoptosis by asbestos, an event that can lead to compensatory cell proliferation, and the importance of apoptosis in therapeutic approaches for eradication of MM and lung cancer cells have been widely studied and appear multi-faceted. For example, several pathways including: (1) an intrinsic or mitochondria-regulated pathway that may be p53 or protein kinase C dependent (52–54); (2) extrinsic pathways induced by death-receptor ligands such as TNF-α or FasL (55); and (3) cell signaling via AKT, prolonged activation of ERKs, and other MAPKs (40, 56–58) have been documented by asbestos and chemotherapeutic drugs in mesothelial cells, MM cells, and lung epithelial cells in vitro. In these studies, oxidative stress appears to be a central mechanism in the induction of apoptotic pathways triggered by asbestos fibers.
FUTURE GOALS AND TRANSLATIONAL APPROACHES TO INHIBIT ASBESTOS-INDUCED CELL SIGNALING PATHWAYS IN MMs
MMs are chemoresistant tumors with a poor prognosis and a long latency period that may be 30 or 40 years from initial exposure to asbestos. The fact that ERK activation occurs early after inhalation of asbestos and may escalate during the development of MM to instigate preneoplastic events, such as hyperplasia, motility/invasion, and tumor homeostasis, makes it an important target for tumor prevention and therapy. Since activation of ERK1/2 is increased endogenously in human MM tissues (59), and dysregulation of the Ras/Raf/MEK/ERK cascade occurs in many cancers, a variety of synthetic MEK and tyrosine kinase inhibitors may be useful in multi-drug therapeutic regimens. For example, we have recently shown that ERK 1/2 governs cell survival in lung epithelial cells via degradation of the pro-apoptotic, BH3-only protein, BimEL (58), which antagonizes the pro-survival Bcl-2 family member, Mcl-1, an effective target of a BH3 mimetic in B-RAF mutant human tumors (60). In these latter studies, use of a MEK inhibitor and the BH3 mimetic, ABT-737, caused synergistic effects on apoptosis and antitumor activity (60). Recent studies using small molecule inhibitors also link inhibition of ERK 1 and 2 to down-regulation of P-glycoprotein, the multidrug resistance gene 1 (mdr1) gene product (61). Moreover, it has been documented that EGFR-mediated activation of ERK 1 and 2 results in increased ABCG2 expression (breast cancer resistance protein, BCRP) and chemoresistance to the ABCG2 substrates, mitoxantrone and topotecan (62). Thus, activation of an EGFR-dependent ERK pathway also may be directly related to chemoresistance of MMs.
The redox-dependent signaling pathways instigated by asbestos or inflammation may also be critical players in MM. Protein oxidation transiently inactivates protein tyrosine phosphatase 1B (PTB1B) and the lipid phosphatase PTEN, which counteract signaling through the EGFR and the phosphoinsitol-3 kinase (PI3K) pathways, respectively. The PI3K/AKT (protein kinase B) pathway is frequently activated in MM and predicts sensitivity to certain therapeutics (56, 57). Moreover, a PI3K/MEK5/Fra-1 pathway governs proliferation by hepatocyte growth factor (HGF), that is, scatter factor, in human MMs (63). The fact that MMs exhibit features of “reactive-oxygen driven tumors,” including universal loss of the cyclin-dependent kinase inhibitor p16INK4A, activation of ERK1/2 and AKT, and activation of NF-κB survival pathways (64), suggests that targeting ROS production or metabolism may also emerge as an important therapeutic strategy in MMs.
As the EGFR inhibitor, Iressa, has proven ineffective in single-agent MM clinical trials, presumably because only 60% of patients with MMs express a functional EGFR receptor (65), other pathways to abrogate ERK/AP-1 and PI3K/AKT survival pathways initiated by asbestos and important in MM survival or chemoresistance may be merited. Crocidolite fibers are endocytosed in a process involving αvβ5 integrin receptors in pleural mesothelial cells (66, 67). Use of an integrin αvβ5 blocking monoclonal antibody, P1F6, reduces internalization of crocidolite fibers in lung carcinoma cells and is linked to depletion of cellular glutathione and increased oxidant stress (68). Integrin-mediated cell adhesion regulates gene expression via activation of transcription factors, including AP-1, through integrin-linked kinase (ILK), a serine-threonine protein kinase that interacts directly with the cytoplasmic domain of the β1/3 integrin subunits. ILK phosphorylation stimulates AP-1 transactivation via glycogen synthase kinase-3 (GSK-3) and subsequent regulation of c-Jun–DNA interactions (69). ILK is also an upstream effector of the PI3K-dependent regulation of AKT and is overexpressed in a number of cancers, including MMs (70, 71). Moreover, ILK expression in skin is regulated by erbB-2, a member of the EGFR family (72). A new ILK inhibitor, QLT0267, prevents EGF-induced phosphorylation of AKT and tumorigenicity (73), suggesting ILK as a potential therapeutic target. However, recent studies have linked ILK to the epithelial-mesenchymal transition in human ovarian carcinoma (74) and hepatoma cells (75). The latter studies showed that mesenchymal cell lines as opposed to epithelioid lines were more problematic in terms of both their prognosis and resistance to EGFR inhibitors. In addition, increased AKT activity was linked to increased activation of ILK that was validated with use of kinase-inactive ILK mutant lines.
CLINICAL SIGNIFICANCE
Studies above suggest that ILK is a novel target to overcome tumor resistance to EGFR inhibitors such as erlotinib (Tarceva), gefitimib (Iressa), and cetuximab. In a phase II clinical study in 63 patients with MM, erlotinib alone was ineffective in terms of median overall survival time, despite overexpression of EGFR in 75% of tumors, and immunohistochemical studies revealed activation of ERK and PI3K/AKT pathways in tumors, suggesting the importance of combination therapy to eradicate these survival pathways (76). Based on observations that vascular endothelial growth factor (VEGF) is produced by MM and other tumor cells, playing a critical role in establishment and maintenance of tumors, chemotherapeutic approaches using erlotinib and Bevacizumab, a humanized anti-VEGF monoclonal antibody, have been evaluated in phase II clinical studies in patients with recurrent or refractory non–small cell lung cancer (NSCLC) (77) and in previously treated patients with MM (78). Although the combination of drugs was tolerated reasonably in patients in both studies, and both progression-free and overall survival were increased in patients with NSCLC in comparison to use of either agent alone, no evidence of a favorable radiographic response was observed in patients with MM. These observations might be critical to both prevention and early treatment of asbestos-induced lung cancers and MMs.
CONCLUSIONS
Asbestos fibers initiate a number of signaling and survival pathways in mesothelial cells and lung epithelial cells, target cells of MMs and lung cancers (Figure 1). These same pathways are often up-regulated in MMs, where they contribute to tumor development, homeostasis, and resistance to chemotherapy.
These pathways may be activated by direct interaction of asbestos fibers with receptors on the cell surface and interaction with integrins or via elaboration of ROS generated catalytically on the fiber surface or after incomplete phagocytosis. Inflammation and interaction of asbestos fibers with other cell types such as macrophages may also play a role in cytokine elaboration and up-regulation of these pathways (47).
No single modality therapy has proven effective in the cure of MMs or lung cancers, presumably because of the multiplicity of survival and chemoresistance pathways in these tumors.
Small molecule inhibitors of EGFR, ERKs, and other kinases have been developed for cancer therapy. However, only a fraction of patients with lung cancers or MMs have EGFR mutations and/or are EGFR-dependent for growth and respond clinically to these agents. Other targets and approaches to circumvent crosstalk and redundancy between signaling pathways important in tumor cell survival and chemoresistance should be explored, especially because MMs associated with asbestos and other fibrous naturally occurring minerals (Libby amphibole, erionite) are still developing in the United States and other countries from past unregulated exposures.
Acknowledgments
The authors thank Jennifer Díaz for manuscript preparation and Maximilian B. MacPherson for illustrations. This review is dedicated to the memory of Johannes Janssen.
This research was supported by a grant from the Mesothelioma Applied Research Foundation (MARF) (B.T.M.), an STTR grant (R41CA126155) from the National Cancer Institute (Dr. Christopher Landry, PI and B.T.M., CoI), and a training grant (T32ES007122) from the National Institute of Environmental Health Sciences (B.T.M.).
Conflict of Interest Statement: N.H.H. has received reimbursement for consultancies from McGuire Woods ($10,000–$50,000), and has served as an expert witness for Plexus Law, England ($10,001–$50,000), Piper Alderman, Australia ($5,001–$10,000) and Grippo and Elden, Chicago ($1,001–$5,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Footnotes
Note that fibers are defined by U.S. regulatory agencies as structures with a greater than 3:1 length to diameter ratio as opposed to nonfibrous particles (< 3:1). This definition is controversial within the mineralogic and geologic scientific community.
References
- 1.Guthrie GD, Mossman BT, Ribbe PH. Health effects of mineral dusts. Washington, DC: Mineralogical Society of America; 1993.
- 2.Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Asbestos: scientific developments and implications for public policy. Science 1990;247:294–301. [DOI] [PubMed] [Google Scholar]
- 3.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998;157:1666–1680. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591–1603. [DOI] [PubMed] [Google Scholar]
- 5.ATSDR. Health consultation on mortality from asbestos in Libby, Montana. Atlanta, GA: United States Department of Health and Human Services; 2000.
- 6.Artvinli M, Baris YI. Malignant mesotheliomas in a small village in the Anatolian region of Turkey: an epidemiologic study. J Natl Cancer Inst 1979;63:17–22. [PubMed] [Google Scholar]
- 7.Mossman BT, Gee JB. Asbestos-related diseases. N Engl J Med 1989;320:1721–1730. [DOI] [PubMed] [Google Scholar]
- 8.Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med 2003;34:1117–1129. [DOI] [PubMed] [Google Scholar]
- 9.Janssen YM, Marsh JP, Absher MP, Hemenway D, Vacek PM, Leslie KO, Borm PJ, Mossman BT. Expression of antioxidant enzymes in rat lungs after inhalation of asbestos or silica. J Biol Chem 1992;267:10625–10630. [PubMed] [Google Scholar]
- 10.Holley JA, Janssen YM, Mossman BT, Taatjes DJ. Increased manganese superoxide dismutase protein in type II epithelial cells of rat lungs after inhalation of crocidolite asbestos or cristobalite silica. Am J Pathol 1992;141:475–485. [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen YM, Marsh JP, Absher MP, Gabrielson E, Borm PJ, Driscoll K, Mossman BT. Oxidant stress responses in human pleural mesothelial cells exposed to asbestos. Am J Respir Crit Care Med 1994;149:795–802. [DOI] [PubMed] [Google Scholar]
- 12.Mossman BT, Surinrut P, Brinton BT, Marsh JP, Heintz NH, Lindau-Shepard B, Shaffer JB. Transfection of a manganese-containing superoxide dismutase gene into hamster tracheal epithelial cells ameliorates asbestos-mediated cytotoxicity. Free Radic Biol Med 1996;21:125–131. [DOI] [PubMed] [Google Scholar]
- 13.Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, et al. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis 1990;141:1266–1271. [DOI] [PubMed] [Google Scholar]
- 14.Fung H, Kow YW, Van Houten B, Mossman BT. Patterns of 8-hydroxydeoxyguanosine formation in DNA and indications of oxidative stress in rat and human pleural mesothelial cells after exposure to crocidolite asbestos. Carcinogenesis 1997;18:825–832. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Marsh J, Ames B, Mossman B. Detection of 8-oxo-2′-deoxyguanosine, a marker of oxidative DNA damage, in culture medium from human mesothelial cells exposed to crocidolite asbestos. Carcinogenesis 1996;17:2525–2527. [DOI] [PubMed] [Google Scholar]
- 16.Jaurand MC. Mechanisms of fiber-induced genotoxicity. Environ Health Perspect 1997;105:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perderiset M, Marsh JP, Mossman BT. Activation of protein kinase C by crocidolite asbestos in hamster tracheal epithelial cells. Carcinogenesis 1991;12:1499–1502. [DOI] [PubMed] [Google Scholar]
- 18.Sesko A, Cabot M, Mossman B. Hydrolysis of inositol phospholipids precedes cellular proliferation in asbestos-stimulated tracheobronchial epithelial cells. Proc Natl Acad Sci USA 1990;87:7385–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung H, Quinlan TR, Janssen YM, Timblin CR, Marsh JP, Heintz NH, Taatjes DJ, Vacek P, Jaken S, Mossman BT. Inhibition of protein kinase C prevents asbestos-induced c-fos and c-jun proto-oncogene expression in mesothelial cells. Cancer Res 1997;57:3101–3105. [PubMed] [Google Scholar]
- 20.Janssen YM, Heintz NH, Marsh JP, Borm PJ, Mossman BT. Induction of c-fos and c-jun proto-oncogenes in target cells of the lung and pleura by carcinogenic fibers. Am J Respir Cell Mol Biol 1994;11:522–530. [DOI] [PubMed] [Google Scholar]
- 21.Heintz NH, Janssen YM, Mossman BT. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci USA 1993;90:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timblin CR, Janssen YW, Mossman BT. Transcriptional activation of the proto-oncogene c-jun by asbestos and H2O2 is directly related to increased proliferation and transformation of tracheal epithelial cells. Cancer Res 1995;55:2723–2726. [PubMed] [Google Scholar]
- 23.Janssen YM, Heintz NH, Mossman BT. Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res 1995;55:2085–2089. [PubMed] [Google Scholar]
- 24.Goldberg JL, Zanella CL, Janssen YM, Timblin CR, Jimenez LA, Vacek P, Taatjes DJ, Mossman BT. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am J Respir Cell Mol Biol 1997;17:265–271. [DOI] [PubMed] [Google Scholar]
- 25.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol 2001;24:405–413. [DOI] [PubMed] [Google Scholar]
- 26.Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 2002;277:9405–9414. [DOI] [PubMed] [Google Scholar]
- 27.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008;128:1365–1374. [DOI] [PubMed] [Google Scholar]
- 28.Shepard HM, Brdlik CM, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Invest 2008;118:3574–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanella CL, Posada J, Tritton TR, Mossman BT. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res 1996;56:5334–5338. [PubMed] [Google Scholar]
- 30.Manning CB, Cummins AB, Jung MW, Berlanger I, Timblin CR, Palmer C, Taatjes DJ, Hemenway D, Vacek P, Mossman BT. A mutant epidermal growth factor receptor targeted to lung epithelium inhibits asbestos-induced proliferation and proto-oncogene expression. Cancer Res 2002;62:4169–4175. [PubMed] [Google Scholar]
- 31.Zanella CL, Timblin CR, Cummins A, Jung M, Goldberg J, Raabe R, Tritton TR, Mossman BT. Asbestos-induced phosphorylation of epidermal growth factor receptor is linked to c-fos and apoptosis. Am J Physiol 1999;277:L684–L693. [DOI] [PubMed] [Google Scholar]
- 32.Shukla A, Barrett TF, Nakayama KI, Nakayama K, Mossman BT, Lounsbury KM. Transcriptional up-regulation of MMP12 and MMP13 by asbestos occurs via a PKCdelta-dependent pathway in murine lung. FASEB J 2006;20:997–999. [DOI] [PubMed] [Google Scholar]
- 33.Faux SP, Houghton CE, Hubbard A, Patrick G. Increased expression of epidermal growth factor receptor in rat pleural mesothelial cells correlates with carcinogenicity of mineral fibres. Carcinogenesis 2000;21:2275–2280. [DOI] [PubMed] [Google Scholar]
- 34.Pache JC, Janssen YM, Walsh ES, Quinlan TR, Zanella CL, Low RB, Taatjes DJ, Mossman BT. Increased epidermal growth factor-receptor protein in a human mesothelial cell line in response to long asbestos fibers. Am J Pathol 1998;152:333–340. [PMC free article] [PubMed] [Google Scholar]
- 35.Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene 2004;23:805–813. [DOI] [PubMed] [Google Scholar]
- 36.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21–44. [DOI] [PubMed] [Google Scholar]
- 37.Robledo RF, Buder-Hoffmann SA, Cummins AB, Walsh ES, Taatjes DJ, Mossman BT. Increased phosphorylated extracellular signal-regulated kinase immunoreactivity associated with proliferative and morphologic lung alterations after chrysotile asbestos inhalation in mice. Am J Pathol 2000;156:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummins AB, Palmer C, Mossman BT, Taatjes DJ. Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis. Am J Pathol 2003;162:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning CB, Sabo-Attwood T, Robledo RF, Macpherson MB, Rincon M, Vacek P, Hemenway D, Taatjes DJ, Lee PJ, Mossman BT. Targeting the MEK1 cascade in lung epithelium inhibits proliferation and fibrogenesis by asbestos. Am J Respir Cell Mol Biol 2008;38:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Z, Taatjes DJ, Mossman BT, Heintz NH. The duration of nuclear extracellular signal-regulated kinase 1 and 2 signaling during cell cycle reentry distinguishes proliferation from apoptosis in response to asbestos. Cancer Res 2004;64:6530–6536. [DOI] [PubMed] [Google Scholar]
- 41.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res 2002;62:6065–6069. [PubMed] [Google Scholar]
- 42.Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT. Microarray analysis and RNA silencing link fra-1 to cd44 and c-met expression in mesothelioma. Cancer Res 2003;63:3539–3545. [PubMed] [Google Scholar]
- 43.Ramos-Nino ME, Blumen SR, Pass H, Mossman BT. Fra-1 governs cell migration via modulation of CD44 expression in human mesotheliomas. Mol Cancer 2007;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla A, MacPherson MB, Hillegass J, Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C, Mossman BT. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am J Respir Cell Mol Biol 2009;41:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen YM, Barchowsky A, Treadwell M, Driscoll KE, Mossman BT. Asbestos induces nuclear factor kappa B (NF-kappa B) DNA-binding activity and NF-kappa B-dependent gene expression in tracheal epithelial cells. Proc Natl Acad Sci USA 1995;92:8458–8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen YM, Driscoll KE, Howard B, Quinlan TR, Treadwell M, Barchowsky A, Mossman BT. Asbestos causes translocation of p65 protein and increases NF-kappa B DNA binding activity in rat lung epithelial and pleural mesothelial cells. Am J Pathol 1997;151:389–401. [PMC free article] [PubMed] [Google Scholar]
- 47.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 2008;223:20–38. [DOI] [PubMed] [Google Scholar]
- 49.Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol 1999;20:942–952. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Faux SP, Hallden G, Kirn DH, Houghton CE, Lemoine NR, Patrick G. Interleukin-1beta and tumour necrosis factor-alpha promote the transformation of human immortalised mesothelial cells by erionite. Int J Oncol 2004;25:173–178. [PubMed] [Google Scholar]
- 51.Sartore-Bianchi A, Gasparri F, Galvani A, Nici L, Darnowski JW, Barbone D, Fennell DA, Gaudino G, Porta C, Mutti L. Bortezomib inhibits nuclear factor-kappaB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res 2007;13:5942–5951. [DOI] [PubMed] [Google Scholar]
- 52.Shukla A, Jung M, Stern M, Fukagawa NK, Taatjes DJ, Sawyer D, Van Houten B, Mossman BT. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am J Physiol Lung Cell Mol Physiol 2003;285:L1018–L1025. [DOI] [PubMed] [Google Scholar]
- 53.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am J Respir Cell Mol Biol 2003;29:198–205. [DOI] [PubMed] [Google Scholar]
- 54.Panduri V, Surapureddi S, Soberanes S, Weitzman SA, Chandel N, Kamp DW. P53 mediates amosite asbestos-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Cell Mol Biol 2006;34:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simeonova PP, Luster MI. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-alpha response from alveolar macrophages. Am J Respir Cell Mol Biol 1995;12:676–683. [DOI] [PubMed] [Google Scholar]
- 56.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene 2005;24:6080–6089. [DOI] [PubMed] [Google Scholar]
- 57.Ramos-Nino ME, Vianale G, Sabo-Attwood T, Mutti L, Porta C, Heintz N, Mossman BT. Human mesothelioma cells exhibit tumor cell-specific differences in phosphatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther 2005;4:835–842. [DOI] [PubMed] [Google Scholar]
- 58.Buder-Hoffmann SA, Shukla A, Barrett TF, MacPherson MB, Lounsbury KM, Mossman BT. A protein kinase Cdelta-dependent protein kinase D pathway modulates ERK1/2 and JNK1/2 phosphorylation and Bim-associated apoptosis by asbestos. Am J Pathol 2009;174:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Melo M, Gerbase MW, Curran J, Pache JC. Phosphorylated extracellular signal-regulated kinases are significantly increased in malignant mesothelioma. J Histochem Cytochem 2006;54:855–861. [DOI] [PubMed] [Google Scholar]
- 60.Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 2008;118:3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther 2007;6:2092–2102. [DOI] [PubMed] [Google Scholar]
- 62.Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Volker U, Kroemer HK. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos 2006;34:524–533. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Nino ME, Blumen SR, Sabo-Attwood T, Pass H, Carbone M, Testa JR, Altomare DA, Mossman BT. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. Am J Respir Cell Mol Biol 2008;38:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res 2008;21:117–122. [DOI] [PubMed] [Google Scholar]
- 65.Engelman JA, Janne PA. Factors predicting response to EGFR tyrosine kinase inhibitors. Semin Respir Crit Care Med 2005;26:314–322. [DOI] [PubMed] [Google Scholar]
- 66.Boylan AM, Sanan DA, Sheppard D, Broaddus VC. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J Clin Invest 1995;96:1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J, Liu W, Koenig K, Idell S, Broaddus VC. Vitronectin adsorption to chrysotile asbestos increases fiber phagocytosis and toxicity for mesothelial cells. Am J Physiol Lung Cell Mol Physiol 2000;279:L916–L923. [DOI] [PubMed] [Google Scholar]
- 68.Pande P, Mosleh TA, Aust AE. Role of alphavbeta5 integrin receptor in endocytosis of crocidolite and its effect on intracellular glutathione levels in human lung epithelial (A549) cells. Toxicol Appl Pharmacol 2006;210:70–77. [DOI] [PubMed] [Google Scholar]
- 69.Yoganathan TN, Costello P, Chen X, Jabali M, Yan J, Leung D, Zhang Z, Yee A, Dedhar S, Sanghera J. Integrin-linked kinase (ILK): a “hot” therapeutic target. Biochem Pharmacol 2000;60:1115–1119. [DOI] [PubMed] [Google Scholar]
- 70.Younes MN, Kim S, Yigitbasi OG, Mandal M, Jasser SA, Dakak Yazici Y, Schiff BA, El-Naggar A, Bekele BN, Mills GB, et al. Integrin-linked kinase is a potential therapeutic target for anaplastic thyroid cancer. Mol Cancer Ther 2005;4:1146–1156. [DOI] [PubMed] [Google Scholar]
- 71.Watzka SB, Setinek U, Huber M, Cantonati H, Lax F, Watson S, Weigel G, Muller MR. Reactivity of integrin-linked kinase in human mesothelial cell proliferation. Interact Cardiovasc Thorac Surg 2008;7:107–110. [DOI] [PubMed] [Google Scholar]
- 72.Xie W, Li F, Kudlow JE, Wu C. Expression of the integrin-linked kinase (ILK) in mouse skin: loss of expression in suprabasal layers of the epidermis and up-regulation by erbB-2. Am J Pathol 1998;153:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Younes MN, Yigitbasi OG, Park YW, Kim SJ, Jasser SA, Hawthorne VS, Yazici YD, Mandal M, Bekele BN, Bucana CD, et al. Antivascular therapy of human follicular thyroid cancer experimental bone metastasis by blockade of epidermal growth factor receptor and vascular growth factor receptor phosphorylation. Cancer Res 2005;65:4716–4727. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol 2006;290:C1532–C1542. [DOI] [PubMed] [Google Scholar]
- 75.Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, Tanabe KK. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res 2008;68:2391–2399. [DOI] [PubMed] [Google Scholar]
- 76.Garland LL, Rankin C, Gandara DR, Rivkin SE, Scott KM, Nagle RB, Klein-Szanto AJ, Testa JR, Altomare DA, Borden EC. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 2007;25:2406–2413. [DOI] [PubMed] [Google Scholar]
- 77.Herbst RS, O'Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–4750. [DOI] [PubMed] [Google Scholar]
- 78.Jackman DM, Kindler HL, Yeap BY, Fidias P, Salgia R, Lucca J, Morse LK, Ostler PA, Johnson BE, Janne PA. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer 2008;113:808–814. [DOI] [PubMed] [Google Scholar]