Abstract
G-protein-coupled receptors (GPCRs) are membrane proteins that convert extracellular information into intra-cellular signals. They are involved in many biological processes and therefore represent powerful targets to modulate physiological and pathological states. Recent studies have demonstrated that GPCR activity is regulated by several mechanisms. Among these, protein–protein interactions (and in particular interactions with other receptors leading to heteromerization) has been shown to have an important role in modulating GPCR function. This has expanded their repertoire of signaling and added a new level of regulation to their physiological roles. Emerging studies provide evidence for tissue-specific and disease-specific receptor heteromerization. This suggests that heteromers represent novel drug targets for the identification of selective compounds with potentially fewer side-effects.
Introduction
G-protein-coupled receptors (GPCRs) are involved in most (if not all) physiological processes. As such, GPCRs represent important drug targets, accounting for 40–50% of current drugs on the market. Advances in the knowledge of GPCR biology suggests that, as a result of being expressed in more than one organ, a given GPCR is usually involved in more than one physiological process. As a consequence, drugs targeting one receptor type in a specific pathological context are likely to act on the same receptor type in other tissues that are not involved in the disease, leading to undesired effects of the drug. For instance, Mu opioid receptor (MOR) agonists are potent analgesic compounds, but also lead to constipation by acting on MOR in the myenteric plexus in the intestinal tract [1]. The type-1cannabinoid receptor (CB1R) antagonist rimonabant was originally developed as an anti-obesity drug [2,3]. However, rimonabant targets peripheral as well as central nervous system receptors, leading to side effects such as anxiety, mood changes and depressive disorders that prevent the therapeutic use of this compound [4,5].
In the last decade, several mechanisms that regulate the function and properties of GPCRs have been identified [6]. One such mechanism is crosstalk between distinct GPCRs. This often occurs as the result of protein–protein interaction between receptor types, or ‘heteromerization’ [7]. Heteromerization is emerging as an important process involved in the specialization of receptor function. Receptor heteromers exist in selected tissues and, in some instances, heteromerization has been reported to be regulated during pathological events [8]. These receptor complexes represent functional entities [9] that mediate distinct biological responses. These properties suggest that receptor heteromers can be used as novel drug targets because compounds that target specific heteromers are likely to have improved specificity and reduced undesirable effects.
In this review, we examine recent studies demonstrating that GPCR heteromerization alters the properties of individual receptors (i.e. modulation of receptor pharmacology by receptor–receptor association) [10–12]. We discuss examples of studies that demonstrate the contribution of receptor heteromers to the regulation of physiological processes, as well as studies that identify unique pathological states associated with alterations in receptor–complex formation. We also describe various current and emerging approaches and tools to study and target receptor heteromers.
GPCR heteromers: new receptor properties
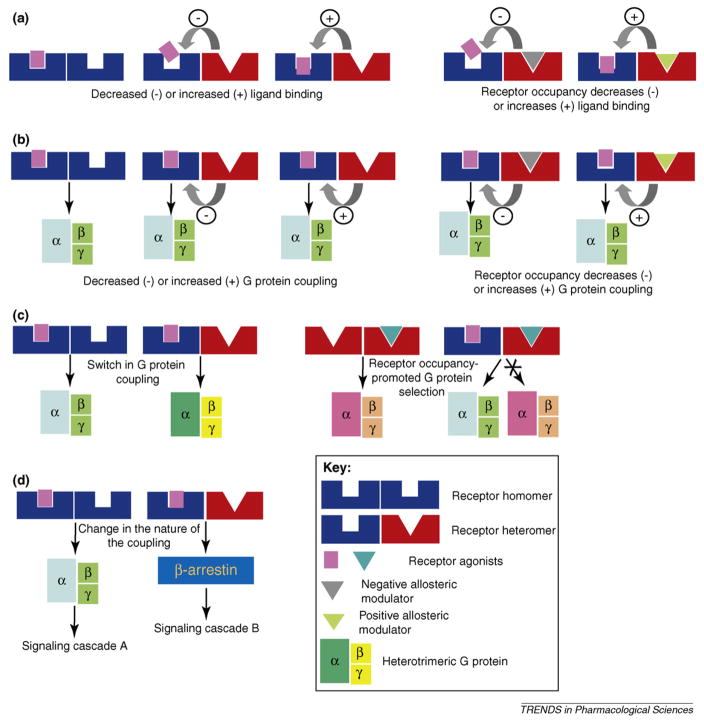
An emerging concept in GPCR research is the promotion of heteromer-specific signaling cascades that enable the diversification of receptor-mediated effects in a context-dependent fashion (Figure 1).
Figure 1. Modulation of receptor function by heteromerization (schematic).
(a) Heteromerization leads to the formation of an altered ligand binding site. Shown for DOR-KOR [15,37], DOR-MOR [14,17] and MOR-sst2A [54].
(b) Heteromerization leads to a decrease or increase in coupling to the G-protein. Decreased coupling was shown for the DOR-MOR heteromer [14]; increased coupling was shown for α2-AR-MOR [21], and AT1R-B2R [13].
(c) Heteromerization leads to coupling to a new G-protein. Shown for MOR-DOR heteromer coupling to Gαz [26,55]; heteromerization of two differently coupled receptors leads to preferential coupling to one G-protein upon co-stimulation. Shown for DOR-SNSR-4 [23].
(d) Heteromerization leads to a switch in receptor coupling, from G-protein to β-arrestin, and subsequent differential signaling and transcription factor activation. Shown for the MOR-DOR heteromer [28].
Changes in receptor pharmacology
Modulation of the binding properties of GPCRs by heteromerization has been described for several receptor pairs [13–16]. This is thought to be the consequence of an alteration in the binding pockets, revealing that some ligands may prefer the receptors in their homomeric or heteromeric conformation. For example, a decrease in the affinity and potency of receptor-selective agonists was observed in the case of Delta-Kappa opioid receptors (DOR-KOR) [15], DOR-MOR [17] and somatostatin sst2A-sst3 receptors [18]. In contrast, an increase in the affinity of β2-adrenergic receptor (AR) ligands was observed for β1-AR-β2-AR receptor pairs [19]. These studies indicate that heteromerization alters the ligand binding properties of the receptors, but the changes in receptor pharmacology do not seem to follow a common pattern because each receptor pair represents a unique alteration in receptor pharmacology. These changes may result from allosteric modulation of one protomer by ligand binding to the other protomer within the heteromer (positive or negative modulation depending on the receptor pair) [20].
Changes in receptor signaling
Studies have shown that receptor heteromerization leads to a change in G-protein coupling. In many cases, heteromerization leads to alterations in the extent of coupling to the same G-protein but recent findings show that, in some cases, there is a change in the nature of the coupled G-protein. A well-studied example of a change in the extent of G-protein coupling is the α2a-AR-MOR heteromer: morphine binding inhibits norepinephrine-mediated signaling to Gαi and the downstream MAP kinase cascade [21]. Förster resonance energy transfer (FRET) analyses revealed a conformational change that propagates from one receptor to the other, causing the rapid inactivation of the second receptor with subsecond kinetics [22]. These findings suggest that heteromerization induces a change in receptor conformation that is responsible for the new properties of the receptors.
Heteromerization can also result in a shift in the type of the coupled G-protein. Heteromerization between DOR and sensory neuron-specific receptor-4 was reported to lead to a switch from a Gαi/o- to a Gαq-mediated signaling pathway [23]. Heteromerization between D1-D2 dopamine receptors was found to lead to a switch from a Gαs/olf (D1R)- or Gαi (D2R)- to a Gαq/11-mediated pathway, and heteromerization between DOR and MOR was shown to lead to a switch from a Gαi- to a Gαz-mediated pathway [24–26].
Emerging studies also indicate that heteromer activation leads to a shift in the recruitment of distinct signaling molecules. In turn, this leads to specialized long-lasting downstream effects such as activation of distinct transcription factors and gene expression. Heteromerization of α2a-AR with α2c-AR reduces GRK-mediated receptor phosphorylation and β-arrestin recruitment, affecting downstream Akt phosphorylation [27]. Heteromerization of MOR with DOR leads to a switch from a predominantly G-protein-mediated to a β-arrestin-mediated pathway, in turn resulting in differential activation of transcription factors [28]. The mechanism underlying the activation of new signaling cascades by heteromers is incompletely understood. One protomer may function as a scaffold to recruit signaling molecules that promote distinct signaling of the other protomer, as proposed in the case of α2a-AR-MOR [21]. Such a mechanism has yet to be explored for other receptor pairs, but could represent a general means to diversify GPCR function.
GPCR heteromerization leads to qualitative as well as quantitative changes in coupling. This change can be from modulating the strength of an agonist-mediated response to switching to different G-protein coupling and/or associated signaling complexes. These novel properties highlight receptor heteromerization as a major mechanism that expands various GPCR-mediated effects, and contributes to the functional specification of receptors. This suggests that specific targeting of a receptor type within a heteromer could allow the activation of a discrete physiological pathway, with limitedeffects on the other processesinvolving the same receptor when it is not in a heteromeric complex.
GPCR heteromers in pathophysiology
In some cases, heteromers seem to constitutively exist in specific tissues where they participate to a given physiological process. In other cases, heteromers are disease-specific, making them attractive drug targets. Constitutive physiological heteromers have been reported to regulate cardiac contractility: angiotensin AT1 receptor (AT1R)-β2-AR heteromers regulate catecholamine-mediated control of heart rate [29]. β1-AR-β2-AR heteromers regulate sensitization to agonist stimulation and suppression of the spontaneous activity of β2-ARs in cardiac myocytes, optimizing β-adrenergic modulation of cardiac contractility [19]. Recent studies support a physiological role for post-synaptic cortical 5-HT2A-mGluR2 receptor heteromers because dysregulation of either component of the 5-HT2A–mGluR2 complex is thought to predispose schizophrenic patients to psychosis [30,31].
Disease-specific heteromers, through their novel signaling properties, can contribute to disease development. The best-described example is that of the AT1R-bradykinin B2 receptor (B2R) heteromer. The propensity of these two receptors to interact is controversial [32], but studies have demonstrated that upregulation of this receptor pair during pregnancy has a major role in Ang II–induced hypersensitivity in pre-eclampsia [8] and in the Ang II hyper-responsiveness of mesangial cells in experimental hypertension [33]. Another example is that of the prostaglandin EP1 receptor-β2-AR heteromer in airway smooth muscles; this heteromerization leads to reduced coupling of β2-AR to Gαs, thereby reducing the bronchodilatory potential of the β2-AR agonist isoproterenol. This regulation of adrenergic signaling by heteromerization could represent an additional mechanism contributing to asthma [34]. Another heteromer that could exhibit unique disease-specific signaling is the apelin receptor (APJ)-AT1R heteromer; apelin has been shown to inhibit angiotensin-mediated development of atherosclerosis [35].
Taken together, these examples indicate that heteromers could represent new drug targets. If heteromers are disease-specific, these complexes are formed or upregulated in a specific tissue only during disease. Compounds acting at a receptor complex could modulate a specific function of a receptor in a particular tissue or physiological process. For example, targeting the β1-AR-β2-AR complex instead of individual adrenergic receptors is likely to specifically affect cardiomyocytes. Targeting a heteromer that exists only or is upregulated during a disease could increase drug specificity. For example, AT1R-B2R- or APJ-AT1R-selective compounds could be used to specifically target the angiotensin receptors involved in asthma and atherosclerosis, respectively, without affecting the homomeric receptors that do not play a part in these diseases. Recent studies showing that a chaperone, RTP4, specifically increases the levels of DOR-MOR heteromers at the cell surface by protecting the complex from ubiquitination and degradation [36] suggest that factors regulating heteromer levels could serve as additional drug targets. Identification of proteins/factors involved in heteromer formation could open new avenues for the development of novel strategies to facilitate/prevent heteromer expression.
Targeting GPCR heteromers
Heteromer-specific ligands
As mentioned before, heteromerization is thought to lead to an alteration in ligand binding properties, suggesting that the altered binding pocket could accommodate compounds distinct from the corresponding protomer ligands. A proof of concept for such a drug is 6′-guanidinonaltrindole [37]. This acts as an opioid receptor agonist at the DOR-KOR heteromer but not at either homomer, suggesting that it represents a DOR-KOR heteromer-specific ligand. This drug has different effects according to administration site. Spinal (but not central) administration leads to analgesia, supporting the notion that selective tissue-specific targeting of GPCR heteromers can increase the local effects of drugs. Another example is that of SKF83959, a D1-like agonist [38] that reportedly binds the D1-D2 heteromer and activates the Gαq pathway [24] in the brain. These findings indicate that heteromer-specific ligands can also promote or block selected heteromer-mediated signaling pathways. This suggests that targeting heteromers allows for fine-tuned pharmacological intervention to modulate specific receptor function.
Bivalent ligands
Opioid receptor heteromers appear to play a part in the modulation of analgesic responses. Within DOR-MOR complexes isolated from spinalcord neurons [39], MOR responsiveness was increased by occupancy of the DOR-binding site by the DOR antagonist TIPPpsi, which was found to enhance morphine-induced analgesia [39], and to reduce development of tolerance to morphine [40]. This unique property of DOR-MOR heteromers suggests that targeting both receptors could yield increased analgesia while curbing the development of tolerance. MDAN-18, consisting of a MOR agonist (oxymorphone) and a DOR antagonist (NTI) tethered by a spacer, demonstrated improved anti-nociceptive activity compared with that achieved by co-administration of the two individual ligands [41], validating the bivalent ligand-based concept of targeting receptor heteromers.
Allosteric modulators
Compounds that work as allosteric modulators by acting at one receptor and causing an increased or decreased response at an associated receptor within a heteromer also represent potential new candidates to target heteromer-specific effects. Heteromerization of the adenosine 2A receptor (A2aR) and D2R leads to a reciprocal antagonistic effect between the two receptors [42]. As a consequence, A2aR antagonists potentiate the motor-activating effects of L-DOPA or D2 receptor agonists [43]; these represent promising allosteric modulators for the treatment of Parkinson’s disease. As mentioned above, DOR antagonists found to enhance morphine-induced analgesia [39] act as allosteric modulators of the DOR-MOR heteromer, and potentiate the anti-nociceptive potential of endogenous opioid peptides or improve the action of exogenous MOR agonists such as morphine.
Future perspectives
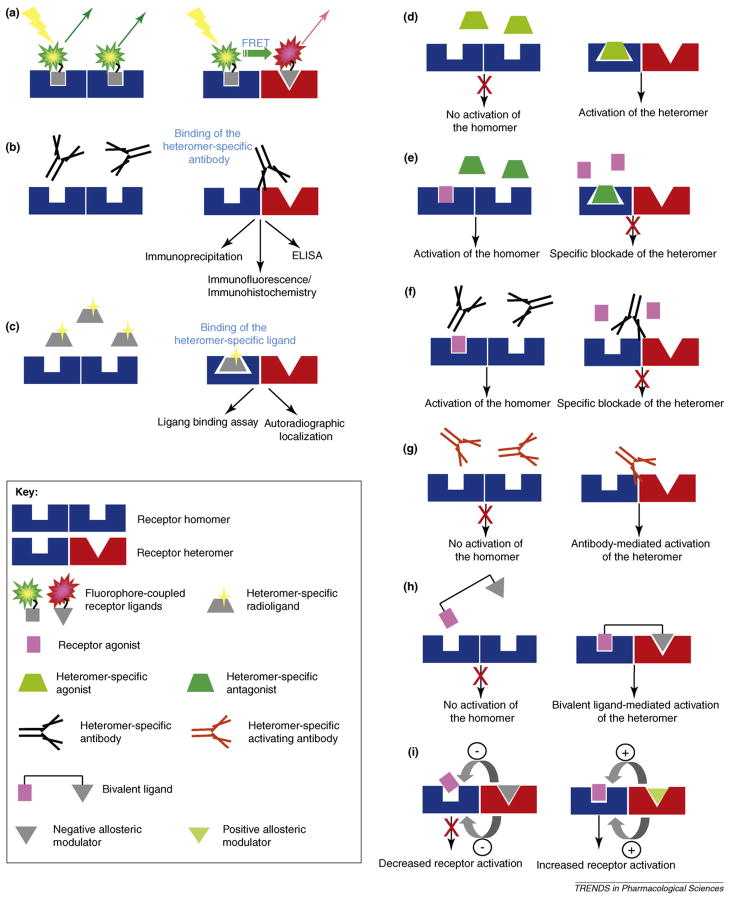
Identification of receptor heteromers in vivo and their involvement in pathophysiological processes is critical for their recognition as novel drug targets. Only a few techniques allow for the direct detection of GPCR complexes in native tissues (Figure 2). Fluorophore-coupled ligands have been developed [44,45] that could be used to carry out FRET analyses between interacting endogenous receptors. Antibodies specifically recognizing receptor heteromers [36,46] represent useful tools for the detection and analyses of GPCR complexes in vivo.
Figure 2. Emerging techniques to detect (a–c) and modulate (d–i) heteromers.
GPCR heteromers can be detected using three main approaches.
(a) Fluorophore-coupled ligand-mediated FRET. Fluorophore-coupled ligands for two receptors can be analyzed using FRET only if their two cognate receptors are in close proximity in a heteromeric complex.
(b) Heteromer-selective antibodies that recognize only heteromers can be used to study the localization and levels of heteromers.
(c) Heteromer-specific ligands can bind only to the altered receptor binding pocket formed in a heteromer.
GPCR heteromers can be modulated using six main approaches.
(d) Heteromer-specific agonists can activate a receptor only if in a heteromeric form.
(e) Heteromer-specific antagonists can block a receptor only if in a heteromeric form.
(f) Heteromer-specific antibodies can block receptor binding and/or signaling only if in a heteromeric form.
(g) Activating heteromer-specific antibodies can activate receptors only if in a heteromeric form.
(h) Bivalent ligands can bind and activate receptors only if their cognate receptors are in a heteromer.
(i) The ligand of one receptor can act as a negative or positive allosteric modulator of the interacting receptor in a heteromer. The allosteric modulator can alter the binding and/or signaling properties of the interacting receptor.
Elucidation of the role of a heteromer can be facilitated by the characterization and mutation of the residues or domains involved in receptor–receptor association. Several approaches, i.e. molecular modeling of receptor dimers [47,48], crosslinking, biophysical techniques [49] and transmembrane domain (TM) swapping [30] have been used to pinpoint such domains. These studies demonstrated the existence of multiple interaction interfaces, including TM1 and TM4 in the D2R homomer [49] and identified mGluR2 TM 4/5 as the dimerization interface with 5HT2aR in the 5HT2aR-mGluR2 complex [30]. Strategies aimed at preventing or disrupting receptor–receptor interaction using small molecules or peptides (such as a mGluR2 TM4 or mGluR2 TM5 homolog that would compete with the receptor for interacting with 5HT2aR) could yield alternative approaches to target heteromer activity.
Current and future challenges
Understanding the mechanisms governing GPCR heteromerization represent a outstanding challenge in all areas of biology, and is a prerequisite for the development of GPCR heteromer-targeted drugs. Design of simple and efficient high-throughput screening methods is hindered by the lack of basic knowledge about GPCR heteromer biology. For instance, the following questions must be answered:
If two receptors that can interact are expressed in a cell to equal extents, will they preferentially form heteromers?
How many receptors are present in an oligomer?
Are receptors in a heteromer in a stochiometric ratio? If not, in the context of a heteroligomer, what is the minimum proportion of one protomer required to change the other protomer’s properties?
Are there ways to ‘force’ receptors to associate that would increase the homogeneity (heteromeric form) of a sample?
Recent advances in GPCR research are starting to address the questions posed above. Studies analyzing receptor association using bioluminescence resonance energy transfer (BRET) determined the relative affinity of different receptors. The observation that β1-AR and β2-AR, or that DOR and KOR have the same propensity to form homo- or heteromers [50,51] suggest a binomial distribution of these receptors when they are co-expressed to the same extent. DOR or KOR have the same propensity to form homomers as compared with β2-AR-DOR, β2-AR-KOR or thyrotropin-releasing hormone receptor-KOR heteromers [51], suggesting that each receptor pair exhibits a distinct behavior. Other studies examining the multimeric nature of D2R provide evidence for an oligomeric rather than dimeric organization of GPCRs [49], in agreement with studies that estimate that the binding energy between only two protomers is too low to achieve stable interaction [52]. As mentioned before, emerging studies providing evidence for a chaperone-mediated increased formation of DOR-MOR heteromers [36] suggest that specific factors could facilitate heteromerization of specific receptor pairs. These and other recent studies help us to understand the basis of GPCR oligomerization, and are a prerequisite to efforts focusing on the development of screening of heteromer-based drugs.
Conclusion
Accumulating evidence showcases the critical role of GPCR heteromers in pathophysiological processes, and underscores the importance of these complexes as drug targets. Heteromers can be targeted by three main approaches. To date, heteromer-specific ligands number only a few compounds, highlighting the technical and methodological difficulty of identifying and testing these molecules. However, they represent a conceptually exciting approach because of the inherent specificity and selectivity of such compounds for tissue-specific and disease-specific receptor pairs. Bivalent ligands, consisting of two connected molecules ligands, are usually large compounds with properties that are not ‘drug-like’ (not following Lipinski’s rule of five [53]) and therefore are unlikely to be suitable in clinical settings. However, this approach is straightforward and has the advantage of using GPCR ligands that have already been identified and characterized. Allosteric modulation of one protomer by a ligand of the other protomer is an exciting approach, in particular those that target the allosteric site of the interacting partner. Identification of specific heteromers that are upregulated during pathological events and/or a heteromer-specific chaperone is likely to lead to novel strategies for pharmacological intervention.
Acknowledgments
We thank Ittai Bushlin and Dr. Dumaine Williams for careful reading of the manuscript and comments. This work was supported by NIH grants DA08862, DA19521 and 1P50GM071558-01A27398 (SBCNY) to LAD and AA017067 to RR.
References
- 1.Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr Opin Pharmacol. 2006;6:559–563. doi: 10.1016/j.coph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer FX, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 4.Christensen R, et al. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell PB, Morris MJ. Depression and anxiety with rimonabant. Lancet. 2007;370:1671–1672. doi: 10.1016/S0140-6736(07)61705-X. [DOI] [PubMed] [Google Scholar]
- 6.Alfaras-Melainis K, et al. Modulation of opioid receptor function by protein-protein interactions. Front Biosci. 2009;14:3594–3607. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre S, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbdAlla S, et al. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 9.Levac BA, et al. Oligomerization of opioid receptors: generation of novel signaling units. Curr Opin Pharmacol. 2002;2:76–81. doi: 10.1016/s1471-4892(02)00124-8. [DOI] [PubMed] [Google Scholar]
- 10.Franco R, et al. G-protein-coupled receptor heteromers: function and ligand pharmacology. Br J Pharmacol. 2008;153 (suppl 1):S90–98. doi: 10.1038/sj.bjp.0707571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Rios CD, et al. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 13.AbdAlla S, et al. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 14.Gomes I, et al. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocheville M, et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 17.George SR, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer M, et al. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A) J Biol Chem. 2001;276:14027–14036. doi: 10.1074/jbc.M006084200. [DOI] [PubMed] [Google Scholar]
- 19.Zhu WZ, et al. Heterodimerization of beta1- and beta2-adrenergic receptor subtypes optimizes beta-adrenergic modulation of cardiac contractility. Circ Res. 2005;97:244–251. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]
- 20.Rozenfeld R, et al. Heterodimers of G protein-coupled receptors as novel and distinct drug targets. Drug Discov Today: Ther Strat. 2006;3:437–443. [Google Scholar]
- 21.Jordan BA, et al. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 22.Vilardaga JP, et al. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 23.Breit A, et al. Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol Pharmacol. 2006;70:686–696. doi: 10.1124/mol.106.022897. [DOI] [PubMed] [Google Scholar]
- 24.Rashid AJ, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So CH, et al. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol Pharmacol. 2007;72:450–462. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]
- 26.Fan T, et al. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- 27.Small KM, et al. Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry. 2006;45:4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- 28.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barki-Harrington L, et al. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Maeso J, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sealfon SC, Gonzalez-Maeso J. Receptor pair for schizophrenia. Pediatr Res. 2008;64:1. doi: 10.1203/PDR.0b013e318180052a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen JL, et al. Lack of evidence for AT1R/B2R heterodimerization in COS-7, HEK293, and NIH3T3 cells: how common is the AT1R/B2R heterodimer? J Biol Chem. 2009;284:1831–1839. doi: 10.1074/jbc.M804607200. [DOI] [PubMed] [Google Scholar]
- 33.AbdAlla S, et al. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci. 2005;26:185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- 34.McGraw DW, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun HJ, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decaillot FM, et al. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldhoer M, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang ZJ, et al. The paradoxical effects of SKF83959, a novel dopamine D1-like receptor agonist, in the rat acoustic startle reflex paradigm. Neurosci Lett. 2005;382:134–138. doi: 10.1016/j.neulet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Gomes I, et al. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abul-Husn NS, et al. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferre S, et al. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferre S, et al. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- 44.Daly CJ, McGrath JC. Fluorescent ligands, antibodies, and proteins for the study of receptors. Pharmacol Ther. 2003;100:101–118. doi: 10.1016/j.pharmthera.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Albizu L, et al. Toward efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J Med Chem. 2007;50:4976–4985. doi: 10.1021/jm061404q. [DOI] [PubMed] [Google Scholar]
- 46.Gupta A, et al. Antibodies against G-protein coupled receptors: novel uses in screening and drug development. Comb Chem High Throughput Screen. 2008;11:463–467. doi: 10.2174/138620708784911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filizola M, et al. Prediction of heterodimerization interfaces of G-protein coupled receptors with a new subtractive correlated mutation method. Protein Eng. 2002;15:881–885. doi: 10.1093/protein/15.11.881. [DOI] [PubMed] [Google Scholar]
- 48.Filizola M, Weinstein H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2005;272:2926–2938. doi: 10.1111/j.1742-4658.2005.04730.x. [DOI] [PubMed] [Google Scholar]
- 49.Guo W, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercier JF, et al. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay D, et al. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem J. 2002;365:429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipinski CA, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer M, et al. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem. 2002;277:19762–19772. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- 55.Hasbi A, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]