Abstract
Human exposure to bisphenol A (BPA) is widespread. Animal studies have demonstrated that BPA can alter endocrine function, but human studies are limited. For the present study, we measured urinary BPA concentrations and serum thyroid and reproductive hormone levels in 167 men recruited through an infertility clinic. BPA was detected in 89% of urine samples with a median (range) of 1.3 (<0.4 – 36.4) ng/mL. In multivariable regression models adjusted for potential confounders, BPA concentrations in urine collected on the same day as a blood sample were inversely associated with serum levels of inhibin B and estradiol:testosterone ratio (E2:T) and positively associated with follicle-stimulating hormone (FSH) and FSH:inhibin B ratio. Because BPA is metabolized quickly and multiple urine measures may better reflect exposure than a single measure, we also considered among a subset of the men the BPA concentrations in repeated urine samples collected weeks or months following serum sample collection. In these analyses, the effect estimates remained consistent for FSH and E2:T but were somewhat weakened for inhibin B; in addition, we observed inverse relationships between urinary BPA and free androgen index (ratio of testosterone to sex hormone binding globulin), estradiol, and thyroid stimulating hormone. Our results suggest that BPA exposure may be associated with altered hormone levels in men, but these findings need to be substantiated through further research.
INTRODUCTION
The monomer bisphenol A (BPA, 2,2-bis-(4-hydroxyphenyl)-propane; CAS Registry N. 80-05-7), synthesized as a synthetic estrogen in 1936 (1), is used to manufacture several polymeric materials that can be found in a wide variety of consumer products. These products include polycarbonate plastic baby and water bottles, epoxy resins which may be used as the lacquer lining of food and beverage cans, and some dental sealants and composites (2). As a result of dietary and other sources, there is widespread general population exposure to BPA. BPA was recently detected in 93% of urine samples from a reference population of 2517 US residents as part of NHANES 2003–2004 (3).
Hundreds of animal and in vitro studies have been conducted on the endocrine effects of BPA (4). While BPA has been known to possess estrogenic activity since the 1930’s, there is evidence that BPA is also an anti-androgen (5) that suppresses aromatase (6) and can alter thyroid hormone signaling (5,7) and prolactin release (8,9). Concerns surrounding BPA-induced health effects are heightened by the observation that the endocrine-modulating ability of BPA may occur at concentrations that are much lower than those currently measured in the general population (10). Human studies of BPA exposure and endocrine function are limited to small studies that have reported altered levels of gonadotropins or testosterone in relation to BPA concentrations in urine or serum (11–13). The present study was undertaken to assess the relationship between urinary BPA concentrations and serum thyroid and reproductive hormone levels in adult men.
METHODS
Subjects were recruited from an ongoing study on the relationship between environmental agents and reproductive health. Participating men were partners in subfertile couples seeking treatment from the Vincent Andrology lab at Massachusetts General Hospital (MGH). The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard School of Public Health, the Centers for Disease Control and Prevention, and the University of Michigan. After the study procedures were explained and all questions answered, subjects signed an informed consent. Men between the ages of 18 to 55 years without post-vasectomy status who presented to the Andrology Laboratory were eligible to participate. Of those approached, approximately 65% consented. Most men that declined to participate in the study cited lack of time on the day of their clinic visit as the reason for not participating.
Urinary BPA
A single spot urine sample was collected from each subject (n=167) on the day of their clinic visit in a sterile polypropylene cup. Because BPA is metabolized and excreted from the body rapidly, and a single urinary measure likely reflects an individual’s exposure in the hours to days leading up to urine sample collection (14), a second and third urine sample were collected from a subset of men (n=75 and n=4, respectively). These samples were generally collected between one week and two months following the original sample collection at a follow-up clinic visit. After measuring specific gravity (SG) using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), each urine sample was divided in aliquots and frozen at −80°C. Samples were shipped on dry ice overnight to the CDC, where the total urinary concentration of BPA (free plus conjugated species) was measured using online solid-phase extraction (SPE) coupled to isotope dilution–high performance liquid chromatography (HPLC) – tandem mass spectrometry (MS/MS) on a system constructed from several HPLC Agilent 1100 modules (Agilent Technologies, Wilmington, DE) coupled to a triple quadropole API 4000 mass spectrometer (Applied Biosystems, Foster City, CA) (15). First, 100 μL of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co., St. Louis, MO) to hydrolyze the BPA-conjugated species. BPA was then retained and concentrated on a C18 reversed-phase size-exclusion SPE column (Merck KGaA, Germany), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion-atmospheric pressure chemical ionization-MS/MS. The limit of detection (LOD) for BPA in a 0.1-mL urine sample was 0.4 μg/L. Low-concentration (~ 4 μg/L) and high-concentration (~ 20 μg/L) quality control materials, prepared with pooled human urine, were analyzed with analytical standards, reagent blanks, and unknown samples (15). For the presentation of the distribution of exposure levels, BPA concentrations were corrected for urine dilution by SG using the following formula: Pc = P[(1.024 − 1)/SG − 1)], where Pc is the SG-adjusted phthalate metabolite concentration (ng/ml), P is the measured BPA concentration, and SG is the specific gravity of the urine sample.
Serum hormones
One non-fasting blood sample was drawn between the hours of 9 am and 4 pm on the same day and time that the first urine sample was collected. Blood samples were centrifuged and the resulting serum was stored at −80 °C until hormone analysis at the MGH Reproductive Endocrinology Laboratory. Serum testosterone, estradiol, sex hormone binding globulin (SHBG), inhibin B, follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, free T4, total T3 and thyroid stimulating hormone (TSH) were measured using sensitive immunoassay methods described previously (16). The free androgen index (FAI was calculated as the molar ratio of total testosterone to SHBG. Free testosterone was also estimated using published methods (17). The testosterone:LH ratio, a measure of Leydig cell function, was calculated by dividing testosterone (nmol/L) by LH (IU/L). The ratios of FSH to inhibin B (FSH:inhibin B) and estradiol to testosterone (E2:T) were also calculated as measures of Sertoli cell function and aromatase activity, respectively.
Statistical analysis
Data analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics on subject demographics were tabulated, along with the distributions of urinary BPA concentrations and serum reproductive hormone levels. For BPA concentrations or hormone levels below the LOD, an imputed value equal to one-half the LOD was used. In preliminary data analysis, hormone and BPA concentrations were stratified by demographic categories to investigate the potential for confounding. Multivariable linear regression was used to explore relationships between urinary BPA and hormone concentrations. Serum concentrations of inhibin B, testosterone, free testosterone and estradiol closely approximated normality and were used in statistical models untransformed, while the distribution of FSH, LH, SHBG, prolactin, TSH, and all calculated hormone ratios were positively skewed and transformed by the natural logarithm for statistical analyses. Urinary BPA concentrations were also ln-transformed. Inclusion of covariates was based on statistical and biologic considerations (18). Specific gravity was included as a continuous variable in all models to adjust for urinary dilution. Age and BMI were also modeled as continuous variables, while race was categorized into four groups: white, African American, Hispanic, and other. Smoking status (current versus former or never smoker), the timing of collection of blood/urine samples by season (winter vs. spring, summer or fall) and by time of day (9:00 am – 12:59 pm vs. 1:00 pm – 4:00 pm) were considered for inclusion in the models as dichotomous variables. To improve interpretability, the regression coefficients were back transformed and expressed as a change in the dependent variable (i.e., serum hormone levels) for an interquartile range (IQR) increase in urinary BPA concentrations.
Four models were constructed: 1) using only urinary BPA concentrations from a single urine sample collected on the same day as the serum sample; 2) using the geometric mean urinary BPA concentration for each participant, where between one and three values were used to calculate each individual’s geometric mean (i.e. the geometric mean for men with only one value was equal to that single value); 3) using the geometric mean BPA concentration among only participants that contributed BPA data from at least two urine samples; and 4) using only the single urinary BPA measure collected on the same day as the serum sample among men with BPA data from at least two urine samples. The reason for including approach 4) was to assess the utility of collecting subsequent urine samples by comparing effect estimates between approaches 3) and 4). In a sensitivity analysis, the multivariable models were rerun after excluding men with highly concentrated or highly dilute urine samples (SG above 1.03 or below 1.01) (19). Models were also rerun using specific gravity-corrected BPA concentrations rather than using uncorrected urinary BPA concentrations but including specific gravity as a covariate. Finally, we assessed non-linear relationships between BPA concentrations and hormones by regressing the serum hormones levels on quartiles of urinary BPA concentrations.
RESULTS
After excluding nine men reporting use of medications that alter hormone levels (e.g., propecia, finasteride, cabergoline, clomid, GnRH, testosterone, or prednisone taper) and four men missing data on specific gravity, serum hormone levels and urinary BPA concentrations were available from 167 men. Of these men, a second urine sample was collected at a later date from 75 of them, and a third urine sample was collected from 4 men. Therefore, the total number of urine samples collected was 246. The time lapsed between the collection of consecutive urine samples ranged from 3 to 75 days, with a mean of 31 days and median (25th, 75th percentile) of 29 (27, 34) days. Most men were white (86%) and did not currently smoke (89%). The mean (SD) age and BMI were 37 (5.3) years and 27 (4.6), respectively. The distribution of urinary BPA concentrations for the 167 urine samples collected on the same day as the serum sample, along with the distribution of serum hormone levels, are available in the Supporting Information (Tables S1 and S2, respectively). Among all urine samples analyzed in the study, BPA was detected in 218 samples (89%). The geometric mean and median (25th, 75th percentile) BPA concentrations were both 1.3 ng/mL (0.7, 2.4 ng/mL), with a maximum concentration of 36.4 ng/mL. Among the 74 men from whom 2 urine samples were collected, BPA concentrations in the two samples were weakly correlated (Spearman r = 0.17; p-value = 0.14). Stratifying by the median duration between samples (29 days) made only a subtle difference (Spearman r = 0.20 for samples collected ≤ 29 days apart, r = 0.16 for sample collected ≥ 29 days apart).
FAI and prolactin were inversely associated with age (both Spearman correlation coefficients = −0.2; p-values <0.05). BMI was inversely associated with inhibin B, testosterone, and SHBG, but positively associated with FSH:inhibin B ratio, estradiol, FAI, E2:T ratio, and free T4 (all p-values <0.05). Current smokers had significantly lower median prolactin (p<0.05) and suggestively higher median free T3 and TSH levels (p-values = 0.06) than non-smokers. Median prolactin and free T4 levels were higher in samples collected in the afternoon compared to samples collected in the morning (p-values <0.05). Serum samples collected in the winter had median inhibin B and SHBG concentrations that were lower than those collected in spring, summer or fall. Samples collected in the winter also had a suggestively lower median concentration of SG-corrected BPA (1.3 ng/mL) than samples collected in spring, summer or fall (1.8 ng/mL, p=0.06). Finally, median uncorrected BPA concentration was higher among men whose urine sample was collected in the afternoon (1.4 ng/mL) versus men providing the urine sample in the morning (1.1 ng/mL; p<0.05), though this difference was not statistically significant (p=0.1) when comparing SG-corrected concentrations (1.7 ng/mL vs. 1.6 ng/mL).
All linear regression analysis results in Table 1 were adjusted for specific gravity, age, BMI, smoking status, and season and time of day blood/urine samples were collected. Crude regression results (not shown) were similar to the adjusted results presented in Table 1. In the adjusted models using only urinary BPA concentrations measured in the single urine sample collected on the same day as the serum sample (statistical approach 1, first column of regression coefficients in Table 1), there was a positive association between urinary BPA and serum FSH and a suggestive inverse association between BPA and inhibin B, where an IQR increase in BPA was associated with a 1.23 IU/L increase in FSH (95% confidence interval (CI) 1.10 to 1.40 IU/L; p-value = 0.0005) and a 16.8 pg/mL decrease in inhibin B (95%CI −33.8 to +0.22 pg/mL; p-value = 0.053). For the median level of FSH (7.75 IU/L) and inhibin B (156 pg/mL), this represents a 23.3% (95%CI 10.3% to 39.5%) increase in FSH and a 10.7% decrease (95%CI −21.6% to +0.1%) in inhibin B for an IQR increase in BPA (IQR 0.7 to 2.4 ng/mL). Urinary BPA was also positively associated with the FSH:inhibin B ratio and inversely associated with the E2:T ratio, where an IQR increase in BPA was associated with a 39.5% (95% CI 10.4% to 76.3%) increase in FSH:inhibin B ratio and a 12.7% decline (95%CI −21.8% to −1.2%) in E2:T ratio. In sensitivity analyses, effect estimates from the multivariable models were similar when including only men with SG ≥ 1.01 and ≤1.03 were included (n=137; results not shown).
Table 1.
Adjusted regression coefficients (95% confidence interval) for change in serum hormone level associated with an interquartile range increase in urinary BPA concentration. Adjusted for specific gravity, age, BMI, current smoking status, and season and time of day of blood/urine sample collection.
| 1) BPA measure from same day as serum hormone level only (n=167)a,d | 2) Geometric Mean BPA from all participants (n=167)b,d | 3) Geometric mean BPA from men with ≥2 BPA measures (n=75)c,d | 4) BPA from same day as serum among men with ≥2 BPA measures (n=75)a,d | |||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | p-value | β (95%CI) | p-value | β (95%CI) | p-value | β (95%CI) | p-value | |
| FSH (IU/L)d,f | 1.23 (1.10, 1.40) | 0.0005 | 1.18 (1.04, 1.34) | 0.01 | 1.13 (0.93, 1.36) | 0.21 | 1.31 (1.12, 1.50) | 0.0004 |
| LH (IU/L)d,f | 1.10 (0.99, 1.22) | 0.07 | 1.06 (0.95, 1.18) | 0.27 | 1.01 (0.85, 1.21) | 0.87 | 1.12 (0.98, 1.28) | 0.11 |
| Inhibin B (pg/mL)e | −16.8 (−33.8, 0.22) | 0.053 | −9.26 (−27.2, 8.71) | 0.31 | −11.3 (−36.9, 14.4) | 0.38 | −30.7 (−50.5, −10.8) | 0.003 |
| FSH:Inhibin B ratiod,f | 1.40 (1.10, 1.76) | 0.005 | 1.28 (1.00, 1.64) | 0.050 | 1.17 (0.82, 1.66) | 0.38 | 1.53 (1.16, 2.02) | 0.003 |
| Testosterone (ng/dL)e,g | 13.7 (−6.14, 33.6) | 0.17 | 9.96 (−11.0, 31.0) | 0.35 | −10.8 (−42.1, 20.5) | 0.49 | 4.28 (−20.1, 28.6) | 0.73 |
| SHBG (nmol/mL)d,f | 1.06 (0.99, 1.15) | 0.10 | 1.05 (0.98, 1.14) | 0.20 | 1.17 (1.04, 1.31) | 0.01 | 1.12 (1.02,, 1.23) | 0.03 |
| FAId,f | 1.00 (0.93, 1.06) | 0.90 | 0.99 (0.92, 1.07) | 0.86 | 0.89 (0.80, 0.98) | 0.02 | 0.94 (0.86, 1.01) | 0.12 |
| Free Testosterone (ng/dL)e | 0.31 (−0.17, 0.79) | 0.20 | 0.22 (−0.28, 0.73) | 0.38 | −0.31 (−1.04, 0.42) | 0.40 | −0.01 (−0.58, 0.58) | 0.99 |
| T:LH ratiod,f | 0.96 (0.85, 1.09) | 0.55 | 0.99 (0.87, 1.11) | 0.80 | 1.02 (0.85, 1.22) | 0.82 | 0.93 (0.80, 1.08) | 0.36 |
| Estradiol (pg/mL)e | −1.50 (−4.10, 1.11) | 0.26 | −2.26 (−5.02, 0.50) | 0.11 | −3.48 (−7.03, 0.06) | 0.054 | −2.16 (−5.00, 0.68) | 0.13 |
| E2:T ratiod,f | 0.87 (0.77, 0.99) | 0.03 | 0.86 (0.75, 0.97) | 0.01 | 0.79 (0.665, 0.97) | 0.02 | 0.85 (0.73, 0.99) | 0.04 |
| Prolactin (ng/mL)d,f | 0.98 (0.90, 1.06) | 0.61 | 0.95 (0.87, 1.04) | 0.26 | 0.93 (0.80, 1.07) | 0.30 | 1.01 (0.90, 1.15) | 0.84 |
| Free T4 (ng/dL)e | 0.001 (−0.05, 0.05) | 0.96 | 0.006 (−0.05, 0.06) | 0.82 | −0.01 (−0.05, 0.04) | 0.77 | −0.03 (−0.06, 0.01) | 0.19 |
| Total T3 (ng/mL)e | 0.004 (−0.03, 0.04) | 0.84 | 0.002 (−0.04, 0.04) | 0.91 | 0.04 (−0.01, 0.09) | 0.14 | 0.03 (−0.03, 0.06) | 0.32 |
| TSH (μIU/mL)d,f | 0.95 (0.83, 1.08) | 0.39 | 0.88 (0.76, 1.00) | 0.046 | 0.78 (0.60, 1.01) | 0.065 | 0.95 (0.77, 1.19) | 0.69 |
Among men with urine sample from same visit as blood (hormone) sample only. N=167.
Geometric mean of up to 3 repeated urine samples per subject, some of which were collected months after blood (hormone) sample. N=167.
Geometric mean value among men with at least 2 urine samples. N=75.
Ln-transformations of bisphenol A, FSH, LH, FSH:inhibin B, SHBG, FAI, T:LH, E2:T, prolactin, and TSH were used. Inhibin B, testosterone, free testosterone, estradiol, free T4, and total T3 were modeled untransformed.
Coefficient represents the change in hormone level for an IQR change in urinary BPA concentration after back-transformation of urinary BPA concentration. For an IQR change in BPA concentration, a coefficient equal to 0 indicates no change in hormone level, a coefficient < 0 indicates a decrease in hormone level, and a coefficient > 0 indicates an increase in hormone level.
Coefficient represents a multiplicative change in hormone level for an IQR change in BPA concentration after back-transformation of both hormone and BPA concentrations. For an IQR change in BPA concentration, a coefficient equal to 1.0 indicates no change in hormone level, a coefficient < 1.0 indicates a multiplicative decrease in hormone level, and a coefficient > 1.0 indicates a multiplicative increase in hormone level.
Models for testosterone also adjusted for ln-transformed SHBG.
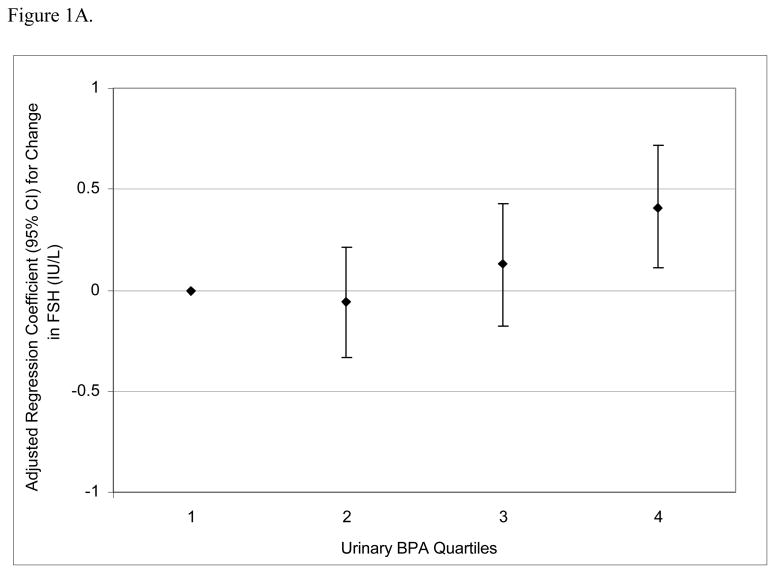
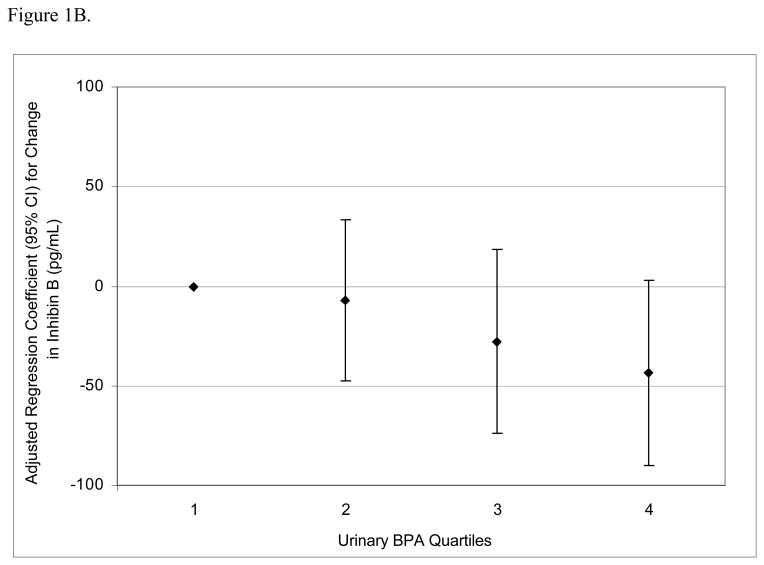
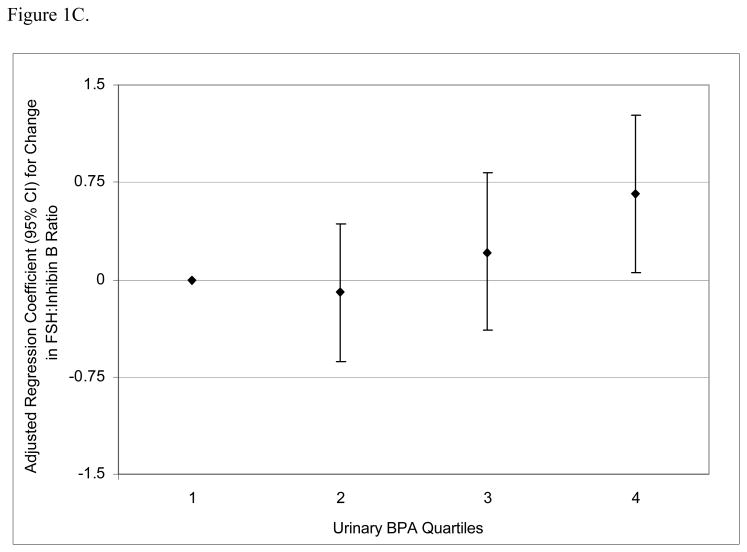
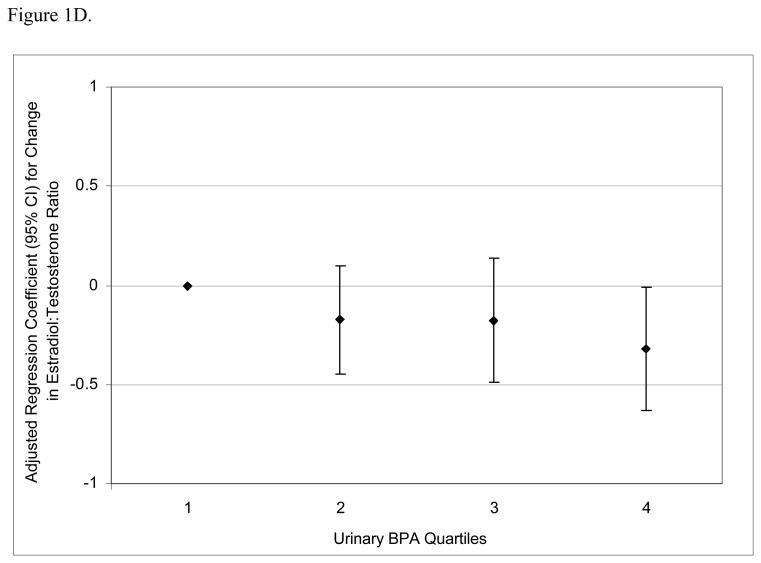
To assess the robustness of the associations between urinary BPA concentrations and serum hormone levels, and potential non-linear relationships, we divided BPA concentrations into quartiles. There was a positive dose-response trend between urinary BPA concentration quartiles and serum FSH levels (Figure 1A; p for trend = 0.002) and an inverse trend between urinary BPA quartiles and inhibin B (Figure 1B; p for trend = 0.04). There was a positive trend between urinary BPA quartiles and FSH:inhibin B ratio (Figure 1C; p for trend = 0.01) and a suggestive inverse trend between urinary BPA quartiles and E2:T ratio (Figure 1D; p for trend = 0.06).
Figure 1.
Adjusted regression coefficients for a change in hormone or hormone ratio associated with increasing quartiles of BPA (n=167). Adjusted for specific gravity, age, BMI, current smoking status, and season and time of day of blood/urine sample collection. A) FSH; B) inhibin B; C) FSH:inhibin B ratio; D) estradiol:testosterone (E2:T) ratio.
Results from the multivariable regression models using geometric mean BPA concentrations from multiple urine samples per participant, when available, differed somewhat from the models using only urine samples collected on the same day as the serum samples (Table 1, second and third columns of regression coefficients). When including all 167 subjects in this analysis (Table 1, approach 2, second column of regression coefficients), the inverse association between urinary BPA and the E2:T ratio remained similar and an inverse association between BPA and TSH was observed. The positive associations between BPA and FSH and FSH:inhibin B ratio also remained but were somewhat weakened, while the inverse association between BPA and inhibin B became less evident. When including only men (n=75) that contributed at least two urine samples in this analysis (Table 1, approach 3, third column of regression coefficients), the inverse relationship between urinary BPA and the E2:T ratio remained consistent. There were also inverse relationships between urinary BPA and FAI, estradiol, and TSH, as well as a positive relationship between BPA and SHBG. In these analyses an IQR increase in urinary BPA was associated with 11.1% (95%CI −20.1% to −2.3%), 13.4% (−27.0% to +0.2%), and 21.9% (−39.8% to +1.2%) declines in FAI, estradiol, and TSH, respectively. The relationships between urinary BPA and FSH, inhibin B and the FSH:inhibin B ratio were no longer statistically significant in this subset of participants. However, if among these same 75 men with at least 2 urine samples, we only used the sample collected on the same day as the serum sample (Table 1, approach 4, fourth column of regression coefficients), there were significant positive associations with FSH, FSH:inhibin B ratio, and SHBG, and inverse associations with inhibin B and E2:T ratio. The inverse associations with FAI and estradiol were statistically suggestive, while the inverse association with TSH was not.
DISCUSSION
In the present study, when using spot urine samples collected on the same day as a blood sample, urinary BPA concentrations were associated with serum levels of FSH, inhibin B, FSH:inhibin B ratio, and E2:T ratio. When one or two subsequent urine samples collected in the weeks or months following the collection of the serum sample were taken into consideration, the associations involving FSH and inhibin B weakened, but the inverse relationship with E2:T ratio remained consistent. In these analyses involving multiple urine samples, we also found associations between urinary BPA concentrations and SHBG, FAI, estradiol, and TSH. Based on our report of temporal within-individual variability in urinary BPA concentrations (14), the use of multiple urinary BPA measures may reflect an individual’s exposure level over time more adequately than a single measure. Therefore, while the subsequent urine samples were collected weeks or months after the collection of the serum samples used for hormone level measurement, we included these urine samples in the analysis. However, because multiple urinary BPA concentrations were not available for all participants, the additional samples were collected outside of the exposure time window likely to be of most interest (i.e. days or weeks leading up to measurement of serum hormone levels as opposed to after hormone levels were measured), and the number of samples and the time interval between their collection varied among the men with multiple BPA measures, we analyzed the data using four different approaches, as presented in Table 1.
The interpretation of the differences in effect estimates and confidence intervals between approaches 3) and 4) needs to be done cautiously because of the small sample size. Also, because of the large number of comparisons made some of the observed associations may be chance findings. Overall, the magnitude and direction of the effect estimates for both approaches were similar, though there were large differences in the magnitude of estimates for inhibin B (which also results in differences for the FSH:Inhibin B ratio). From a comparison of these analyses it is not possible to determine why there are differences in effect estimates. However, based on these results and our earlier work on urinary temporal variability in urinary BPA concentrations, we recommend collecting more than one sample per person to better estimate exposure, preferably within the most relevant time window of interest (e.g. prior to serum collection for the measurement of hormones as opposed to after serum collection).
Despite the variable findings obtained with these different statistical analysis approaches, our findings can be compared with the in vitro, animal, and limited human data on BPA exposure and endocrine function. In humans, serum BPA concentrations were associated with increased androgen levels, but not estradiol, LH, or FSH levels, among women in two small studies (n=41 and n=74) (12,13). In a study of 42 male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents and 42 male matched control workers, urinary BPA was inversely associated with plasma FSH levels, but not LH or free testosterone (11). These reports are inconsistent with our observation of a positive association between urinary BPA and FSH. However, our finding, along with the inverse association between BPA and inhibin B and the positive association between BPA and FSH:inhibin B ratio, may be consistent with animal and in vitro studies showing reduced testicular inhibin levels (20) and adverse effects on Sertoli cell function and sperm production in relation to BPA exposure (21–31). FSH and inhibin B are the two hormones most highly predictive of semen quality, where elevated levels of FSH and/or low levels of inhibin B are associated with poorer semen quality (16,32,33). FSH, a gonadotropin produced and secreted by the anterior pituitary, acts on Sertoli cells in the seminiferous tubules to initiate spermatogenesis. In addition to nurturing and protecting developing germ cells during spermatogenesis, Sertoli cells also produce and secrete inhibin B, a protein hormone, which then exerts negative feedback on the anterior pituitary to inhibit FSH secretion (34,35). Thus, BPA may be associated with adverse effects on the Sertoli cells or their FSH receptors to alter inhibin B production. This may, in turn, through reduced negative inhibin B feedback lead to increased pituitary FSH production and secretion, potentially resulting in reduced sperm production and semen quality. However, additional studies, including human studies with larger sample sizes and measures of semen and sperm quality, are needed to further examine this hypothesis.
Our finding of an inverse association between urinary BPA and the serum E2:T ratio using all four statistical analysis approaches is also consistent with previous reports. Since estradiol is produced through the aromatization of testosterone, a reduction in the E2:T ratio is considered a marker for declined aromatase activity. BPA has been shown to suppress aromatase in rat Leydig cells (29), rat ovarian granulosa cells (36), and placental JEG-3 cells (37,38). While our findings are consistent with BPA suppression of aromatase activity, an alternative explanation for a decreased E2:T ratio may involve differential effects of BPA exposure on testosterone and estradiol metabolism.
When considering multiple urinary BPA measures from individual participants, we found inverse associations between BPA and FAI, estradiol, and TSH. With regards to a reduced FAI, BPA has demonstrated anti-androgenic activity in a number of studies (5,6,20,29,39–43), although it was also found to be a strong SHBG ligand (44). The inverse association with circulating estradiol in this analysis was also consistent with a study involving both in vivo and in vitro experiments of rats and rat Leydig cells that reported declines in Leydig cell estradiol biosynthesis and circulating levels estradiol following environmentally relevant doses of BPA; these effects were attributed to BPA-induced inhibition of Leydig cell aromatase activity (29). Finally, perhaps consistent with our finding of an inverse relationship between BPA and TSH, with no corresponding positive association between BPA and free T4 or total T3, is a recent report showing that BPA suppresses TSH release from amphibian pituitary in a manner that is independent of both the thyroid hormone feedback mechanism and BPA’s estrogenic activity (9).
This study has several limitations. First, the present study is cross-sectional in nature due to the availability of only a single hormone measure from each participant, as well as only one urine sample from over half of the men. Thus, we cannot rule out reverse causation to explain our findings. For example, hormonal status could be associated with altered BPA metabolism and result in associations between hormone levels and urinary BPA concentrations (45). If this were the case, information on predictors of altered BPA metabolism would also be of great interest given the broad range of health concerns surrounding BPA exposure. Another limitation is the likelihood of exposure measurement error due to the high within-individual temporal variability in BPA exposure and the availability of multiple BPA measures from only a subset of participants. However, measurement error would be expected to be non-differential, which would tend to reduce the ability to detect associations between exposure and outcome. In addition, when using broad exposure categories (e.g. quartiles as presented in Figure 1), a single measure may adequately predict an individual’s exposure category over a longer period of time such as several months (14). Finally, because the present study was conducted among men recruited through an infertility clinic our ability to generalize the results to the general population may be limited. However, because to date no evidence exists that men from an infertility clinic are differentially affected by BPA exposure we expect our results to be generalizable.
In conclusion, human exposure to BPA may be associated with alterations in circulating hormone levels. Additional studies assessing the relationship between BPA exposure and endocrine function and downstream clinical implications are needed. These studies should be designed to utilize BPA exposure biomarkers from multiple time points in the exposure time-window that is most relevant to the health outcomes of interest.
Supplementary Material
Acknowledgments
Work supported by grants ES009718 and ES00002 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). The authors gratefully acknowledge Xiaoyun Ye, Amber Bishop, Tao Jia, and Jack Reidy (CDC, Atlanta, GA) for measuring the urinary concentrations of BPA. Disclaimer: The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.” Tables showing the distribution of urinary BPA and serum hormone concentrations appear in Supporting Information. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- 2.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the US. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH Publication No. 08 – 5994. National Toxicology Program. U.S. Department of Health and Human Services; Research Triangle Park, NC: 2008. [Accessed August 18, 2009]. NTP–CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. http://cerhr.niehs.nih.gov/chemicals/bisphenol/bisphenol.pdf. [Google Scholar]
- 5.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Bonefeld-Jorgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoeller RT. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid. 2007;17(9):811–7. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138(5):1780–6. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko M, Okada R, Yamamoto K, Nakamura M, Mosconi G, Polzonetti-Magni AM, Kikuyama S. Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. Gen Comp Endocrinol. 2008;155(3):574–80. doi: 10.1016/j.ygcen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6 Suppl):S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 11.Hanaoka T, Kawamura N, Hara K, Tsugane S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup Environ Med. 2002;59(9):625–8. doi: 10.1136/oem.59.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291(1):76–8. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51(2):165–9. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 14.Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116(2):173–8. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–13. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 16.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28(3):397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied regression analysis and other multivariate methods. 3. Brooks/Cole Publishing Company; Pacific Grove, CA: 1998. Selecting the best regression equation. [Google Scholar]
- 19.Teass AW, Biagini RE, DeBord DG, Hull RD. NIOSH Manual of Analytical Methods. National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. Application of biological monitoring methods; pp. 52–62. [Google Scholar]
- 20.Tohei A, Suda S, Taya K, Hashimoto T, Kogo H. Bisphenol A inhibits testicular functions and increases luteinizing hormone secretion in adult male rats. Exp Biol Med (Maywood) 2001;226(3):216–21. doi: 10.1177/153537020122600309. [DOI] [PubMed] [Google Scholar]
- 21.Hughes PJ, McLellan H, Lowes DA, Kahn SZ, Bilmen JG, Tovey SC, Godfrey RE, Michell RH, Kirk CJ, Michelangeli F. Estrogenic alkylphenols induce cell death by inhibiting testis endoplasmic reticulum Ca(2+) pumps. Biochem Biophys Res Commun. 2000;277(3):568–74. doi: 10.1006/bbrc.2000.3710. [DOI] [PubMed] [Google Scholar]
- 22.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, Aoki Y, Yonemoto J, Tohyama C. Bisphenol A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–190. [Google Scholar]
- 23.Al-Hiyasat AS, Darmani H, Elbetieha AM. Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci. 2002;110(2):163–7. doi: 10.1034/j.1600-0722.2002.11201.x. [DOI] [PubMed] [Google Scholar]
- 24.Tabuchi Y, Zhao QL, Kondo T. DNA microarray analysis of differentially expressed genes responsive to bisphenol A, an alkylphenol derivative, in an in vitro mouse Sertoli cell model. Jpn J Pharmacol. 2002;89(4):413–6. doi: 10.1254/jjp.89.413. [DOI] [PubMed] [Google Scholar]
- 25.Tabuchi Y, Takasaki I, Kondo T. Identification of genetic networks involved in the cell injury accompanying endoplasmic reticulum stress induced by bisphenol A in testicular Sertoli cells. Biochem Biophys Res Commun. 2006;345(3):1044–50. doi: 10.1016/j.bbrc.2006.04.177. [DOI] [PubMed] [Google Scholar]
- 26.Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185(1–2):119–27. doi: 10.1016/s0300-483x(02)00597-8. [DOI] [PubMed] [Google Scholar]
- 27.Tabuchi Y, Kondo T. cDNA microarray analysis reveals chop-10 plays a key role in Sertoli cell injury induced by bisphenol A. Biochem Biophys Res Commun. 2003;305(1):54–61. doi: 10.1016/s0006-291x(03)00708-3. [DOI] [PubMed] [Google Scholar]
- 28.Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315(1):119–24. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 29.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 30.Toyama Y, Suzuki-Toyota F, Maekawa M, Ito C, Toshimori K. Adverse effects of bisphenol A to spermiogenesis in mice and rats. Arch Histol Cytol. 2004;67(4):373–81. doi: 10.1679/aohc.67.373. [DOI] [PubMed] [Google Scholar]
- 31.Toyama Y, Yuasa S. Effects of neonatal administration of 17beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19(2):181–8. doi: 10.1016/j.reprotox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Jensen TK, Andersson AM, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Henriksen TB, Ernst E, Bonde JP, Olsen J, McNeilly A, Groome NP, Skakkebaek NE. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab. 1997;82(12):4059–63. doi: 10.1210/jcem.82.12.4456. [DOI] [PubMed] [Google Scholar]
- 33.Mabeck LM, Jensen MS, Toft G, Thulstrup M, Andersson M, Jensen TK, Giwercman A, Olsen J, Bonde JP. Fecundability according to male serum inhibin B--a prospective study among first pregnancy planners. Hum Reprod. 2005;20(10):2909–15. doi: 10.1093/humrep/dei141. [DOI] [PubMed] [Google Scholar]
- 34.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, Bremner WJ. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81(9):3341–5. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 35.Lo KC, Lamb DJ. The testis and male accessory organs. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5. Elsevier, Inc; Philadelphia, PA: 2004. pp. 367–87. [Google Scholar]
- 36.Zhou W, Liu J, Liao L, Han S. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283(1–2):12–8. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Nativelle-Serpentini C, Richard S, Seralini GE, Sourdaine P. Aromatase activity modulation by lindane and bisphenol-A in human placental JEG-3 and transfected kidney E293 cells. Toxicol In Vitro. 2003;17(4):413–22. doi: 10.1016/s0887-2333(03)00046-8. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Leung LK. Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol Lett. 2009;189(3):248–52. doi: 10.1016/j.toxlet.2009.06.853. [DOI] [PubMed] [Google Scholar]
- 39.Takao T, Nanamiya W, Nagano I, Asaba K, Kawabata K, Hashimoto K. Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 1999;65(22):2351–7. doi: 10.1016/s0024-3205(99)00502-0. [DOI] [PubMed] [Google Scholar]
- 40.Iida H, Maehara K, Doiguchi M, Mori T, Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol. 2003;17(4):457–64. doi: 10.1016/s0890-6238(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75(1):40–6. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem Toxicol. 2003;41(7):1035–44. doi: 10.1016/s0278-6915(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 43.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dechaud H, Ravard C, Claustrat F, de la Perriere AB, Pugeat M. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG) Steroids. 1999;64(5):328–34. doi: 10.1016/s0039-128x(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi T, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Fujiwara T, Takai Y, Momoeda M, Yano T, Taketani Y. Elevated serum bisphenol A levels under hyperandrogenic conditions may be caused by decreased UDP-glucuronosyltransferase activity. Endocr J. 2006;53(4):485–91. doi: 10.1507/endocrj.k06-032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.