Abstract
Objective
Ras homolog gene family member A (RhoA)/Rho-kinase-mediated Ca2+ sensitization is a critical component of constrictor responses. The present study investigates how angiotensin II activates RhoA.
Methods and Results
Adenoviral vectors were used to manipulate the expression of regulator of G protein signaling (RGS) domain containing Rho-specific guanine exchange factors (RhoGEFs) and proline-rich tyrosine kinase 2 (PYK2), a non-receptor tyrosine kinase, in primary rat vascular smooth muscle cells. As an evidence of RhoA activation, RhoA translocation and MYPT1 (the regulatory subunit of myosin light chain phosphatase) phosphorylation were analyzed by western blot. Results showed that over-expression of PDZ-RhoGEF, but not p115-RhoGEF or leukemia-associated RhoGEF (LARG), enhanced RhoA activation by angiotensin II. Knock-down of PDZ-RhoGEF decreased RhoA activation by angiotensin II. PDZ-RhoGEF was phosphorylated and activated by PYK2 in vitro, and knock-down of PDZ-RhoGEF reduced RhoA activation by constitutively active PYK2, indicating that PDZ-RhoGEF links PYK2 to RhoA.. Knock-down of PYK2 or PDZ-RhoGEF markedly decreased RhoA activation by A23187, a Ca2+ ionophore, demonstrating that PYK2/PDZ-RhoGEF couples RhoA activation to Ca2+.
Conclusions
PYK2 and PDZ-RhoGEF are necessary for angiotensin II-induced RhoA activation and for Ca2+ signaling to RhoA.
Keywords: Angiotensin II, RhoA, Ca2+ sensitization, PDZ-RhoGEF, PYK2
Constrictor responses in the vasculature are maintained through activation of heterotrimeric G proteins following ligand binding to membrane receptors. Subsequent to heterotrimeric G protein activation, the cytosolic Ca2+ concentration is elevated, resulting in the activation of myosin light chain kinase (MLCK).1,2 MLCK phosphorylates the 20 kDa regulatory myosin light chain (MLC) at Ser19 or Thr18, and consequently activates the myosin ATPase activity, leading to cross-bridge cycling and contraction.1 In contrast, MLC phosphatase (MLCP) regulates vascular tone through de-phosphorylation of phosphorylated MLC. Because MLCP activity appears to be independent of Ca2+, the MLCP-mediated regulation of vascular tone is known as Ca2+ sensitization. Ras homolog gene family member A (RhoA) and its downstream effecter, Rho-kinase, plays a critical role in regulation of MLCP activity through phosphorylation of MLCP regulatory subunit, MYPT1.2 Moreover, RhoA/Rho-kinase pathway has also been implicated in the pathophysiology of hypertension.3,4 However, how RhoA is activated upon binding of the vasoconstrictor to its receptor remains elusive.
RhoA activity is regulated by guanine exchange factors (GEFs),5 GTPase activating proteins (GAPs),5 and guanine nucleotide dissociation inhibitors (GDIs).6 A subset of Rho-specific GEFs (RhoGEFs), including PDZ-RhoGEF, p115RhoGEF and LARG, contains a regulator of G protein signaling (RGS) domain that is believed to bind activated Gα12/13, resulting in RhoGEF activation. RGS domain-containing RhoGEFs thereby may serve as a molecular bridge between heteromeric G proteins and RhoA.7 Our previous studies have shown increased mRNA expression of RGS domain-containing RhoGEFs in aorta of spontaneously hypertensive rats (SHR)8 and increased protein expression of PDZ-RhoGEF in small mesenteric arteries of angiotensin II-induced hypertensive rats.9 A fragment of PDZ-RhoGEF has also been shown to induce Ca2+ sensitization in permeabilized vascular smooth muscle.10 Together, these studies indicate that RGS domain-containing RhoGEF, in particular PDZ-RhoGEF, may play a role in vascular Ca2+ sensitization.
In addition to protein-protein interaction, the activation of PDZ-RhoGEF and LARG may also involve tyrosine phosphorylation.11 We recently demonstrated that salicylates relaxed blood vessels through inhibition of proline-rich tyrosine kinase 2 (PYK2)-mediated RhoA/Rho-kinase activation.12 PYK2 has previously been shown to co-immunoprecipitated with PDZ-RhoGEF.13 Therefore, in the present study, we tested the hypothesis that PYK2/PDZ-RhoGEF mediates RhoA activation upon stimulation with angiotensin II.
Methods
Cell culture
Primary rat aortic vascular smooth muscle cells (VSMCs) were prepared from the thoracic aortas of Sprague Dawley rats as previously described,14 and maintained in DMEM supplemented with 10% fetal bovine serum (Invitrogen), 100 μg/mL streptomycin, and 100 U/mL penicillin at 37°C under a 95% air/ 5% CO2 atmosphere. VSMCs with no more than 5 passages were used in all experiments.
Constructs
Rat PDZ-RhoGEF, p115RhoGEF and LARG cDNA were cloned from cultured rat aortic vascular smooth muscle cells by RT-PCR. Human PYK2 full-length cDNA was cloned from HEK293 cells by RT-PCR. These PCR products were inserted into pGEM-T. The inserts were verified by sequencing. To be easily measured, they were tagged with human influenza hemagglutinin (HA) at 5', and their over-expression adenoviral vectors expressing were generated using Adeno-X™ Expression Systems 2 (Clotech). HA-tagged constitutively active PYK2 composed of the whole kinase domain was generated with a similar method. Myc-tagged human PDZ-RhoGEF was a gift of Dr. Wedegaertner (Department of Microbiology and Immunology, Thomas Jefferson University). siRNA adenoviral vectors were generated using a system from Ambion according to the manufacturer's instruction. PYK2 siRNA target sequence was described previously.15 PDZ-RhoGEF siRNA target sequence was: sense strand, 5'-CCCCAUCAU UCCUCCACCA-3'; antisense strand, 5'-UGGUGGAGGAAUG AUGGGG-3'.
Transduction
Rat VSMCs, COS-7, or NIH3T3-L1 cells in 60mm dishes (around 90% confluent) were used to perform transduction. Briefly, cells were incubated with adenoviral vectors at a multiplicity of infection (MOI) of 40 PFU per cell for 4 hours, and then washed once with OptiMEM medium. Finally, 3 ml of culture medium was then added. After 40–48 hours, these cells were stimulated with angiotensin II or ionomycin, and cell lysates were then prepared.
Western blotting
Cells were grown to confluence in 60mm dishes, and then infected with adenoviral vectors. After 40–48 hours of infection, cells were treated with angiotensin II (100 nM) or ionomycin (1 μM), and then immediately placed on ice and washed twice with ice-cold PBS. All subsequent manipulations were performed on ice. To measure translocation, samples were homogenized as previously described.16 Otherwise, samples were lysed with 100 μl of ice-cold RIPA buffer (Upstate: 50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM β-glycerophosphate, 50 mM NaF, 1 mM EGTA, 1 mM Na3VO4, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 2 μg/ml pepstatin). Cells were scraped with a rubber policeman, rocked for 30 minutes at 4°C, and centrifuged at 14,000 g for 30 minutes at 4°C. The supernatants were then used for western blotting with standard techniques. The primary antibodies used in the present study were as follows: monoclonal anti-β-actin (Sigma), rabbit anti-PYK2 (Sigma), rabbit anti-PDZ-RhoGEF (Alpha Diagnostics), monoclonal anti-phosphotyrosine (Upstate), monoclonal anti-myc (Roche Applied Sciences), rat anti-human influenza hemagglutinin (anti-HA. Roche Applied Sciences), monoclonal anti-RhoA (BD Biosciences), rabbit anti-phosphoMYPT1 (Santa Cruz), monoclonal anti-MYPT1 (BD Biosciences).
In vitro phosphorylation
Myc-tagged PDZ-RhoGEF was over-expressed in HEK293T cells, purified by affinity chromatography (Roche Applied Science), and phosphorylated at 37°C in a total of 50 μl buffer containing 200 ng myc-tagged PDZ-RhoGEF, 1 unit constitutively active PYK2 (Cyclex), 60 mM HEPES-NaOH (pH 7.5), 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 10 μM ATP. Reaction was stopped by adding 50 μl staurosporine (20 μM).
GEF activity assay
GEF activity of PDZ-RhoGEF was measured with RhoGEF exchange assay Biochem Kit (Cytoskeleton) according to the manufacturer's instruction. RhoA activation assay: GTP bound RhoA in cell lines were measured with G-LISA™ Small G-protein Activation Assays Kit (Cytoskeleton) according to the manufacturer's instruction.
Statistical Analysis
The results are shown as mean ± SEM and n represents the number of animals/cell cultures used in the experiments. Statistical analyses were performed using one-way ANOVA or Student's t-test. Post hoc comparisons were performed using Bonferroni's test. Western blot data were analyzed by one-sample t test and the P value was computed from the t ratio and the numbers of degrees of freedom. Values of P<0.05 were considered statistically significant.
Results
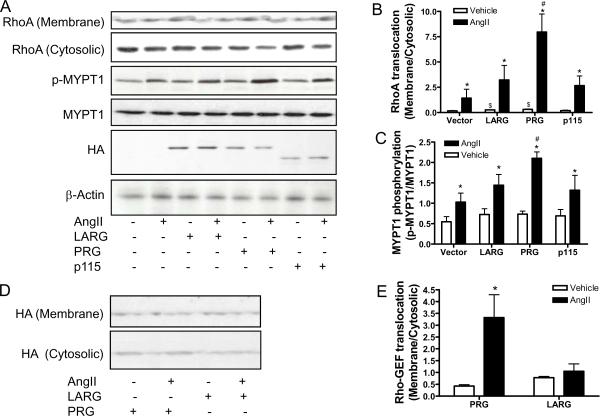
PDZ-RhoGEF (PRG), LARG and p115RhoGEF were over-expressed in primary rat VSMCs with respective adenoviral vectors to determine their contribution to RhoA activation, upon stimulation with angiotensin II. Figures 1A–C show that the over-expression of PDZ-RhoGEF, but not LARG or p115RhoGEF, significantly increased angiotensin II-induced RhoA translocation and MYPT1 phosphorylation. Notably, immunofluorescence analysis revealed that angiotensin II transiently induced translocation, from the cytosol to the membrane, of over-expressed PDZ-RhoGEF, but not the closest homolog, LARG (please see www.ahajournals.org). This result was then confirmed by western blot analysis (Fig. 1D and E).
Figure 1.
PDZ-RhoGEF mediates RhoA activation by angiotensin II. A-C, rat VSMCs were infected with control, LARG, PDZ-RhoGEF, or p115RhoGEF adenoviral vector. After 40 hours, these cells were stimulated with angiotensin II (100 nM) for 3 minutes, and then RhoA translocation or MYPT1 phosphorylation was analyzed by western blot. A representative result (A) and the summary (B and C) of at least four independent experiments are presented. *p<0.05 vs. Vehicle infected with the same adenoviral vector; #p<0.05 vs. AngII-Vector; $p<0.05 vs. Vehicle-Vector; student's t test with bonferroni correction. D and E, rat VSMCs were infected with LARG or PDZ-RhoGEF adenoviral vector. After 40 hours, these cells were stimulated with angiotensin II (100 nM) for 3 minutes, then the subcellular localization of over-expressed LARG or PDZ-RhoGEF is analyzed by western blot with anti-HA. n = 3. *p<0.05 vs. vehicle; student's t test with bonferroni correction.
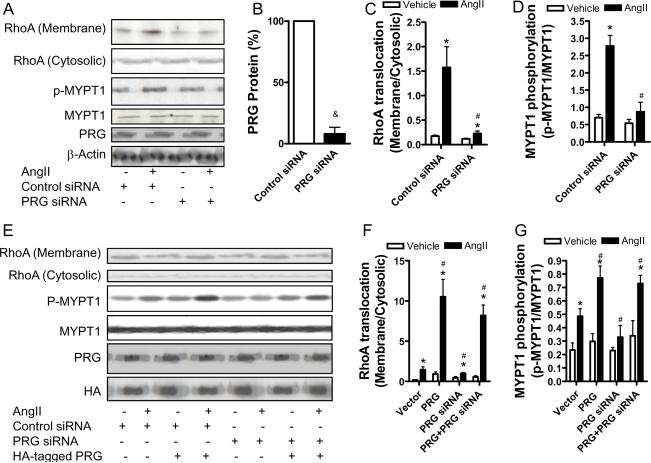
Since the over-expression study indicated that PDZ-RhoGEF mediated angiotensin II-induced RhoA activation, PDZ-RhoGEF siRNA adenoviral vector was then generated to investigate the role of endogenous PDZ-RhoGEF in angiotensin II-induced RhoA activation. The PDZ-RhoGEF siRNA vector reduced PDZ-RhoGEF protein expression by more than 90% in rat VSMCs (Fig. 2A and B), and markedly decreased angiotensin II-induced RhoA translocation and MYPT1 phosphorylation (Fig. 2A, C, and D), supporting that PDZ-RhoGEF is necessary in angiotensin II-induced RhoA activation. To verify the specificity of this PDZ-RhoGEF siRNA vector, a human PDZ-RhoGEF expression adenoviral vector was generated, which had distinct sequences at the target site (please see www.ahajournals.org). Co-infection of human PDZ-RhoGEF over-expression vector and rat PDZ-RhoGEF siRNA vector revealed that the over-expression of human PDZ-RhoGEF was not affected by rat PDZ-RhoGEF siRNA, and markedly restored angiotensin II-induced RhoA translocation and MYPT1 phosphorylation, clearly demonstrating the specificity of this PDZ-RhoGEF siRNA (Fig. 2E–G).
Figure 2.
Knockdown of PDZ-RhoGEF reduces RhoA activation by angiotensin II. A-C, rat VSMC were infected with control or PDZ-RhoGEF siRNA adenoviral vector. After 40 hours, these cells were stimulated with angiotensin II (100 nM) for 3 minutes, then RhoA translocation or MYPT1 phosphorylation was analyzed by western blot. A representative result (A) and the summary (B-D) of at least four independent experiments are presented. &p<0.05 vs. control siRNA; *p<0.05 vs. vehicle infected with the same adenoviral vector; #p<0.05 vs. AngII-Control siRNA; student's t test with bonferroni correction. E-G, rVSMC were infected with the indicated adenoviral vectors. After 40 hours, these cells were stimulated with angiotensin II (100 nM) for 3 minutes, then RhoA translocation or MYPT1 phosphorylation was analyzed by western blot. A representative result (E) and the summary (F and G) of at least three independent experiments are presented. *p<0.05 vs. vehicle infected with the same adenoviral vector; #p<0.05 vs. AngII-Control siRNA; student's t test with bonferroni correction.
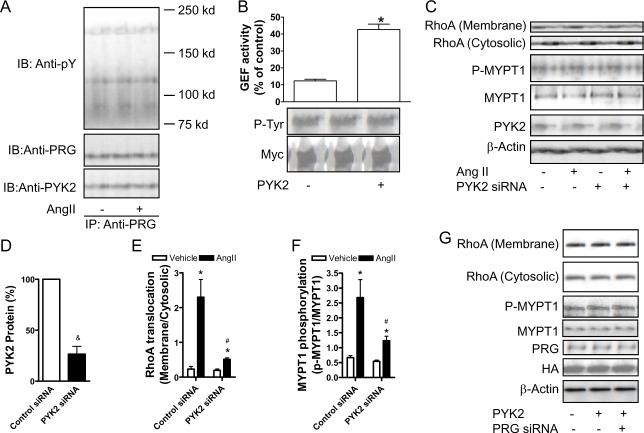
Tyrosine phosphorylation is involved in the activation of many RhoGEFs.17 To test whether tyrosine phosphorylation is involved in angiotensin II-induced RhoA activation, PDZ-RhoGEF was immuno-precipitated from angiotensin II-treated rat VSMCs. Figure 3A shows that angiotensin II markedly induced tyrosine phosphorylation of PDZ-RhoGEF. Notably, angiotensin II also evidently increased tyrosine phosphorylation of another protein with a molecular weight of around 130 Kd. A previous study showed that PDZ-RhoGEF was precipitated by anti-PYK2.13 After membrane stripping and re-visualization with anti-PYK2, results demonstrated that this protein was PYK2 (Fig. 3A). The co-immunoprecipitation of PDZ-RhoGEF and PYK2 was further confirmed by co-expression of myc-tagged PDZ-RhoGEF and HA-tagged PYK2 in HEK293T cells (please see www.ahajournals.org).
Figure 3.
PYK2 mediates RhoA activation through phosphorlating PDZ-RhoGEF. A, rat VSMCs were stimulated with angiotensin II (100 nM) for 3 minutes. PDZ-RhoGEF was then immuno-precipitated, and analyzed by western blot. A representative result of two independent experiments was presented. B, immuno-precipitated myc-tagged PDZ-RhoGEF was incubated with constitutively active PYK2 in the phosphorylation buffer for 1 hour at 37°C, and the tyrosine phosphorylation and the GEF activity of PDZ-RhoGEF were analyzed. n≥3. * p<0.05; student's t test. C-F, rat VSMCs were infected with PYK2 siRNA adenoviral vectors. After 40 hours, cells were stimulated with angiotensin II (100 nM) for 3 minutes, and RhoA translocation or MYPT1 phosphorylation was analyzed by western blot. A representative result (C) and the summary (D-F) of 3 independent experiments are presented. &p<0.05 vs. control siRNA; *p<0.05 vs. vehicle infected with the same adenoviral vector; #p<0.05 vs. AngII-Control siRNA; student's t test with bonferroni correction. G, rat VSMCs were infected with control, PYK2, or PYK2 combined with PDZ-RhoGEF siRNA adenoviral vector. After 40 hours, cells were harvested, and RhoA translocation or MYPT1 phosphorylation was analyzed by western blot.
To determine whether PYK2 regulates PDZ-RhoGEF activity through tyrosine phosphorylation, PDZ-RhoGEF was immuno-precipitated from rat VSMCs and then phosphorylated by constitutively active PYK2 in vitro. Figure 3B demonstrates that treatment with PYK2 markedly increased the tyrosine phosphorylation of PDZ-RhoGEF, in conjunction with a marked increase in the GEF activity. The essential role of tyrosine phosphorylation in PDZ-RhoGEF activation by PYK2 was further supported by results showing that without ATP, PYK2 did not increase either tyrosine-phosphorylation or GEF activity of PDZ-RhoGEF (please see www.ahajournals.org).
To examine the role of PYK2 in angiotensin II-induced RhoA activation, we also generated PYK2 siRNA adenoviral vector. Figures 3C–F show that the PYK2 siRNA vector reduced PYK2 protein expression by more than 75% (Fig.3C and D), and markedly decreased angiotensin II-induced RhoA translocation and MYPT1 phosphorylation (Fig.3C, E and F), supporting that PYK2 is also necessary for angiotensin II-induced RhoA activation.
To evaluate whether PYK2 activates RhoA via phosphorylation of PDZ-RhoGEF in living cells, constitutively active PYK2 expression adenoviral vector was also generated. The over-expression of this constitutively active PYK2 in rat VSMCs markedly induced RhoA translocation and MYPT1 phosphorylation, and these effects were markedly reduced by PDZ-RhoGEF siRNA (Fig. 3G). Taken together, these results strongly indicate that upon stimulation with angiotensin II, PYK2 phosphorylates PDZ-RhoGEF, resulting in RhoA activation in living cells.
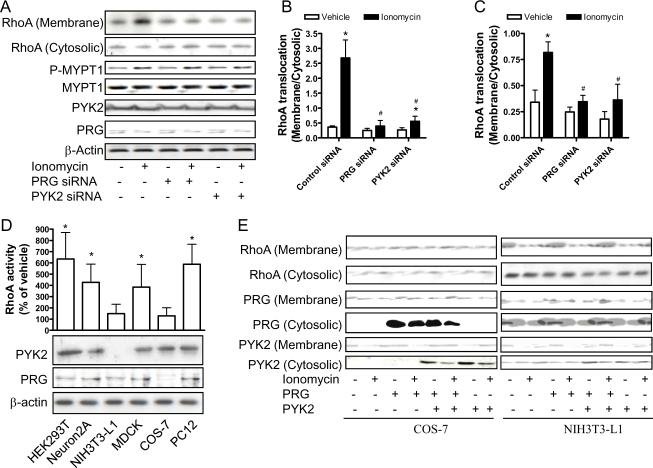
Previous studies demonstrated that Ca2+ signaling is necessary and even sufficient to activate PYK2.18 Therefore, we examined whether angiotensin II activated RhoA in a Ca2+-dependent manner. Results show that membrane-permeant Ca2+ chelator, AM-BAPTA completely abolished angiotensin II-induced RhoA activation and MYPT1 phosphorylation (please see www.ahajournals.org), indicating that RhoA activation by angiotensin II is also Ca2+-dependent. To test whether Ca2+ activates RhoA through PYK2/PDZ-RhoGEF pathway, we analyzed the effect of PYK2 or PDZ-RhoGEF siRNA on RhoA activation upon stimulation with ionomycin, a Ca2+ ionophore. Figures 4A–C show that knock-down of either PYK2 or PDZ-RhoGEF markedly decreased ionomycin-induced RhoA translocation and MYPT1 phosphorylation, supporting that Ca2+ activates RhoA through PYK2/PDZ-RhoGEF pathway.
Figure 4.
PYK2/PDZ-RhoGEF couples Ca2+ signaling to RhoA. A-C, rat VSMCs were infected with control, PYK2 or PDZ-RhoGEF siRNA adenoviral vectors. After 40 hours, cells were stimulated with ionomycine (1 μM) for 30 minutes, and RhoA translocation or MYPT1 phosphorylation was analyzed by western blot. A representative result (A) and the summary (B and C) of at least three independent experiments are presented. *p<0.05 vs. vehicle infected with the same adenoviral vector; #p<0.05 vs. AngII-Control siRNA; student's t test with bonferroni correction. D, The expression level of PYK2 and PDZ-RhoGEF in the indicated cell lines was analyzed by western blot. After stimulation with ionomycine (1 μM) for 30 minutes, the GTP bound RhoA was measured by G-LISA™ Small G-protein Activation Assays. The summary of three independent experiments is shown. *p<0.05; student's t test. E, COS-7 or NIH-3T3L1 cells were infected with the indicated adenoviral vectors. After 40 hours, RhoA translocation was measured by western blot. A representative result of two independent experiments is shown.
Because Ca2+ and RhoA are ubiquitous intracellular signal transducers, we finally tested whether PYK2/PDZ-RhoGEF linked Ca2+ signaling to RhoA in other cells. The expression levels of PYK2 and PDZ-RhoGEF in six different cell lines were examined by western blot analysis. Figure 4D demonstrates that except for NIH-3T3-L1 (low PYK2 and PDZ-RhoGEF) and COS-7 cells (low PYK2), PYK2 and PDZ-RhoGEF were expressed at comparable levels in other cells (HEK293T, MDCK, Neuron2A, and PC12). Accordingly, ionomycin activated RhoA in tested cells, except for NIH-3T3-L1 and COS-7 (Fig.4D). Expression of PDZ-RhoGEF in COS-7 cells or co-expression of PYK2 and PDZ-RhoGEF in NIH-3T3-L1 cells markedly restored RhoA activation by ionomycin(Fig. 4E), further supporting that PYK2 and PDZ-RhoGEF are necessary to link Ca2+ to RhoA activation.
Discussion
Angiotensin II is one of the most potent vasoconstrictors and its action is partially mediated by RhoA/Rho-kinase-induced Ca2+ sensitization.19–21 In the present study, we have identified specific signaling components that mediate angiotensin II-induced RhoA activation. Our results indicate that 1) PDZ-RhoGEF is necessary for RhoA activation by angiotensin II; 2) RhoA activation by angiotensin II also requires PYK2; 3) PYK2 activates RhoA through phosphorylation of PDZ-RhoGEF; 4) PYK2/PDZ-RhoGEF couples Ca2+ signaling to RhoA.
Because RGS domain can serve as a molecular bridge between heteromeric G proteins (primarily G12/13) and RhoGEF,7 RGS domain containing RhoGEFs, PDZ-RhoGEF, p115RhoGEF, and LARG, are believed to play a role in RhoA activation upon agonist binding to G protein coupled receptors (GPCRs). Our present data indicate PDZ-RhoGEF, but not p115RhoGEF and LARG, mediates angiotensin II-induced RhoA activation, as evidenced by the different effects on angiotensin II-induced RhoA activation when they were knocked-down or over-expressed. Moreover, treatment with angiotensin II induced translocation of PDZ-RhoGEF, but not LARG. Since cellular membrane is the primary location for RhoA activation,22 this result strongly supports that PDZ-RhoGEF plays a significant role in angiotensin II-induced RhoA activation.
Consistent with the role of PDZ-RhoGEF in angiotensin II-induced RhoA activation, our present data indicate that protein-protein interaction through RGS domain may not be involved in angiotensin II-induced RhoA activation. First, tyrosine phosphorylation is sufficient to activate PDZ-RhoGEF in vitro, suggesting that RGS domain-mediated protein-protein interaction is not necessary. Second, RhoA activation by angiotensin II is abolished by AM-BAPTA, indicating that it is Ca2+-dependent. While there is no evidence that RGS domain-mediated protein-protein interaction requires Ca2+,studies have shown that activation of PYK2 is Ca2+-dependent.18 Third, PYK2 siRNA markedly reduced RhoA activation by angiotensin II, indicating a essential role of PYK2 in angiotensin II-induced RhoA activation. Our results are also consistent with studies showing that although coupled to the same Gα subunit,23,24 GPCRs utilize distinct RGS domain-containing RhoGEFs.7,25 Taken together, these data indicate that mechanisms other than RGS domain-mediated protein-protein interaction is important in regulating RGS domain-containing RhoGEFs.
The present study shows that tyrosine phosphorylation by PYK2 is sufficient to activate PDZ-RhoGEF. Although tyrosine phosphorylation has been shown to regulate RhoGEFs, such as VAVs,26 this is, to our knowledge, the first study showing that PDZ-RhoGEF activity is regulated by tyrosine phosphorylation. In line with these results, angiotensin II induced PDZ-RhoGEF tyrosine phosphorylation in VSMCs,13 and genistein, a non-specific tyrosine kinase inhibitor, almost abolished angiotensin II-induced RhoA translocation in rat VSMCs (data not shown). While the mechanism remains unclear, tyrosine kinase inhibitors have been shown to attenuate constrictive responses to vasoconstrictors.27–29 Therefore, our results may also provide a molecular basis for these investigations.
PYK2 is regulated by various extracellular signals that activate GPCRs and/or elevated cytoplasmic Ca2+,18 such as angiotensin II.13,30 PYK2 deficiency markedly reduces RhoA activation by chemokines in macrophages, suggesting that PYK2 is involved in RhoA activation.31 The present study shows that knock-down of PYK2 markedly reduced angiotensin II-induced RhoA activation. This is consistent with previous studies showing that knock-down of PYK2 by anti-sense oligonucleotides abolished various angiotensin II-induced cellular responses.30,32 The essential role of PYK2 in angiotensin II-induced RhoA activation is also supported by that PYK2 activates PDZ-RhoGEF through tyrosine phosphorylation in vitro. Further, a complex containing both PYK2 and PDZ-RhoGEF is suggested by the co-immunoprecipitation. These results together indicate that subsequent to PYK2 activation upon stimulation with angiotensin II, PDZ-RhoGEF is phosphorylated by PYK2 and in turn activates RhoA. However, we can not exclude that other kinases are also involved in this signaling, in particular Src which can work as an amplifier during PYK2 activation.33
Consistent with that Ca2+ signaling is sufficient to activate RhoA in VSMCs,34 our results show that Ca2+ chelator abolished angiotensin II-induced RhoA activation. Moreover, knock-down of PYK2 or PDZ-RhoGEF markedly reduced RhoA activation by Ca2+ ionophore, indicating that PYK2/PDZ-RhoGEF couples Ca2+ signaling to RhoA. Due to the essential role of Ca2+ in vasoconstriction, these data indicate that PYK2/PDZ-RhoGEF may mediate RhoA activation upon stimulation with various constrictors. However, because cultured rat VSMCs did not response to many tested constrictors, it remains to be verified with other techniques, such as gene targeting.
In summary, we have identified PYK2 and PDZ-RhoGEF as the specific signaling components that mediate RhoA activation by angiotensin II. PYK2/PDZ-RhoGEF is sufficient to link Ca2+ signaling to RhoA, thereby providing a potential mechanism for constrictor responses.
Condensed Abstract.
The mechanism by which vasoconstrictors activate RhoA remains elusive. In the present study, we show that in response to angiotensin II, PYK2 is activated and subsequently phosphorylates PDZ-RhoGEF. The PYK2/PDZ-RhoGEF pathway is sufficient to couple Ca2+ signaling to RhoA, thus offering a mechanism involved in constrictor responses.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Wedegaertner (Department of Microbiology and Immunology, Thomas Jefferson University) for kindly providing us Myc-tagged human PDZ-RhoGEF expression vector.
SOURCES of FUNDING This work was supported by National Institutes of Health grants HL-71138 and HL-74167 (Dr. Webb).
Footnotes
DISCLOSUREs None
References
- 1.Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- 2.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 3.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 4.Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. Rho-kinase as a molecular target for insulin resistance and hypertension. Faseb J. 2006;20:169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 5.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying Z, Jin L, Dorrance AM, Webb RC. Increaseed expression of mRNA for regulator of G protein signaling domain-containing Rho guanine nucleotide exchange factors in aorta from stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2004;17:981–985. doi: 10.1016/j.amjhyper.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Hilgers RH, Todd J, Jr., Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens. 2007;25:1687–1697. doi: 10.1097/HJH.0b013e32816f778d. [DOI] [PubMed] [Google Scholar]
- 10.Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZ-RhoGEF, an activator of the Ca(2+) sensitization pathway in smooth muscle. Structure. 2004;12:1955–1965. doi: 10.1016/j.str.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 12.Ying Z, Giachini F, Tostes R, Webb C. Salicylates dilate blood vessels through inhibiting PYK2 mediated RhoA/Rho-kinase activation. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 14.Ying Z, Jin L, Palmer T, Webb RC. Angiotensin II up-regulates the leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), a regulator of G protein signaling domain-containing RhoGEF, in vascular smooth muscle cells. Mol Pharmacol. 2006;69:932–940. doi: 10.1124/mol.105.017830. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest. 2004;114:1782–1789. doi: 10.1172/JCI18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 17.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases--GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 19.Williams J, Bogwu J, Oyekan A. The role of the RhoA/Rho-kinase signaling pathway in renal vascular reactivity in endothelial nitric oxide synthase null mice. J Hypertens. 2006;24:1429–1436. doi: 10.1097/01.hjh.0000234125.01638.3b. [DOI] [PubMed] [Google Scholar]
- 20.Matrougui K, Tanko LB, Loufrani L, Gorny D, Levy BI, Tedgui A, Henrion D. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler Thromb Vasc Biol. 2001;21:1288–1293. doi: 10.1161/hq0801.093653. [DOI] [PubMed] [Google Scholar]
- 21.Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- 22.Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. J Biol Chem. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar M, Seasholtz TM, Buckmaster C, Toksoz D, Brown JH. A rho exchange factor mediates thrombin and Galpha(12)-induced cytoskeletal responses. J Biol Chem. 1999;274:26815–26821. doi: 10.1074/jbc.274.38.26815. [DOI] [PubMed] [Google Scholar]
- 24.Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, Huang S. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- 25.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 26.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Di Salvo J, Pfitzer G, Semenchuk LA. Protein tyrosine phosphorylation, cellular Ca2+, and Ca2+ sensitivity for contraction of smooth muscle. Can J Physiol Pharmacol. 1994;72:1434–1439. doi: 10.1139/y94-207. [DOI] [PubMed] [Google Scholar]
- 28.Laniyonu AA, Saifeddine M, Yang SG, Hollenberg MD. Tyrosine kinase inhibitors and the contractile action of G-protein-linked vascular agonists. Can J Physiol Pharmacol. 1994;72:1075–1085. doi: 10.1139/y94-150. [DOI] [PubMed] [Google Scholar]
- 29.Sabri A, Govindarajan G, Griffin TM, Byron KL, Samarel AM, Lucchesi PA. Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle. Circ Res. 1998;83:841–851. doi: 10.1161/01.res.83.8.841. [DOI] [PubMed] [Google Scholar]
- 30.Rocic P, Jo H, Lucchesi PA. A role for PYK2 in ANG II-dependent regulation of the PHAS-1-eIF4E complex by multiple signaling cascades in vascular smooth muscle. Am J Physiol Cell Physiol. 2003;285:C1437–1444. doi: 10.1152/ajpcell.00075.2003. [DOI] [PubMed] [Google Scholar]
- 31.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocic P, Griffin TM, McRae CN, Lucchesi PA. Altered PYK2 phosphorylation by ANG II in hypertensive vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H457–465. doi: 10.1152/ajpheart.00546.2001. [DOI] [PubMed] [Google Scholar]
- 33.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 34.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.