Abstract
Pituitary adenoma tissues are hypovascular, and have a lower partial oxygen pressure compared with neighboring normal organs. In this study, we investigated whether hypoxia influences the cell invasiveness of the human pituitary adenoma cell line, HP-75. HP-75 cells were exposed to hypoxic (1–10% oxygen) or normoxic (21% oxygen) conditions for 24 hours. Gelatin and reverse zymogram assays were used to determine the enzyme activities of matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP). Cell adhesion and Matrigel cell invasion were examined with a Boiden chamber. Finally, the mRNA gene expression profiles of cells exposed to hypoxia or normoxia were examined by cDNA microarray and confirmed with real-time RT-PCR and flow cytometry. The gelatin and reverse zymograms revealed that the activities of MMP and TIMP were not significantly altered by hypoxia. Matrigel cell invasion and cell adhesion to Matrigel or collagen type IV were increased by hypoxia (3.8- and 4.8-fold, respectively). The cDNA microarray analysis revealed that laminin β2 chain mRNA was specifically up-regulated under hypoxic conditions (4.96-fold). Finally, real-time RT-PCR and flow cytometry verified the elevated expression of laminin β2 chain at the mRNA and protein levels under hypoxic conditions. RNA interference with siRNA targeting laminin β2 inhibited Matrigel invasion and adhesion to collagen type IV in a dose.dependent manner.
Collectively, these results suggested that hypoxia (1% oxygen) enhanced the cell invasion properties of a pituitary adenoma cell line in association with elevated expression of laminin β2 and enhanced binding to collagen type IV.
Key Words: cell invasion, hypoxia, laminin β2, pituitary adenoma, siRNA
Introduction
Hypoxia is a major crisis for eukaryotic cells, leading to necrosis. Highly aggressive, rapidly growing tumors tend to suffer from hypoxic or even anoxic conditions due to inadequate blood supply.1 Recently, several genes have been identified as hypoxia-responsive genes.2,3 Both hypoxia and subsequent hypoxia/reoxygenation exert a variety of influences on tumor cell biology, including activation of signal transduction pathways and gene regulatory mechanisms, induction of selection processes for gene mutations, tumor cell apoptosis and tumor angiogenesis.4 Most of these mechanisms contribute to tumor progression, indicating that tissue hypoxia may be a central factor for tumor aggressiveness.3,5 Thus, researchers have sought to investigate the hypoxic cell response in malignant tumors.1,6–9 However, hypoxia may also affect the cell-cell and cell-extracellular matrix (ECM) interactions involved in cell invasion; these changes are regarded as an initial step during tumor progression. Intracellular signaling cascades associated with hypoxia-induced apoptosis have been examined in several previous studies,9,10 but no previous work has investigated the effect of hypoxia on cell invasion. In addition, most of the studies to date have focused on malignant tumors rather than benign tumors, which also suffer from physiologically relevant hypoxic conditions.
As in all tumors, the growth of pituitary adenomas (a benign tumor type) requires adequate vascularization to supply oxygen to the tumor cells. However, in contrast to other tumors, pituitary adenomas are less vascularized than the surrounding normal tissues.11 Because an adequate blood supply enables tumor cells to proliferate, the reduced vascularity in pituitary adenomas is likely associated with their relatively slow growth. However, these tumors tend to be often invasive, with 25–35% of pituitary adenomas demonstrating invasion into neighboring normal structures, as shown by preoperative neuro-imagings.12 This invasiveness is associated with a high rate of recurrence; however, the molecular mechanisms associated with the invasiveness of this benign tumor type have not been previously clarified. Here, we examined the effects of hypoxia on the invasiveness of these cells and assessed possible mechanisms for these effects.
Material and Methods
Cell culture.
HP-75 cells were purchased from the ATCC (American Type Culture Collection, Manassas, VA) and routinely cultured in DMEM (BioWhittaker, Cambrex Corp., Nottingham, UK) containing 15% horse serum (TCS Cellworks, Buckingham, UK), 2.5% fetal calf serum (Life Technologies, Paisley, UK), 0.05% (w/v) glutamine, 100 µg/ml gentamicin, and 100 IU/ml penicillin (hereafter called culture medium) at 3°C in a humidified air atmosphere containing 5% carbon dioxide. The cell line was passaged three times before the following experiments.
Gelatin zymography and reverse zymography.
HP-75 (1 x 106) cells cultured in 60 mm plastic dishes (Sumitomo Bakelite, Tokyo, Japan) were exposed to hypoxia at 1, 3, 5 or 10% oxygen or normoxia at 21% oxygen for 24 hours. The activities of matrix metalloproteinase (MMP)-2 and MMP-9 were detected by gelatinase zymography. The supernatants were separated by 7.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; Minigel apparatus; BioRad, Hemel Hempstead, UK) using gelatin (0.1 mg/ml) and nonreducing conditions. Gels were washed twice with 2.5% (v/v) Triton X-100 and then incubated in zymography digestion buffer (200 mM NaCl, 50 mM Tris, 5 mM CaCl2, 1 µM ZnCl2, 0.02% (v/v) Brij-35, pH 7.6; all chemicals from Sigma, St. Louis, MO, USA) for 18 h at 37°C. The gels were then stained with 0.5% Coomassie Blue R250 in 30% methanol/10% glacial acetic acid in H2O for three hours at 23°C and destained to reveal discrete areas where the gelatin substrate was hydrolyzed by gelatinase activity. The activity of tissue inhibitors of metalloproteinases (TIMPs) was examined by reverse zymography. The cell supernatants were resolved as described above, and the gels were washed in 50 mM Tris, 5 mM CaCl2 and 2.5% (v/v) Triton X-100 for 2.5 hours at 23°C and then incubated in reverse zymography digestion buffer (50 mM Tris, 5 mM CaCl2, 100 mU/ml gelatinase A (Calbiochem, Cambridge, Mass., USA) for four hours at 37°C. Each gel was stained, predominantly the incorporated gelatin. The presence of TIMP activity was determined by discrete inhibition of MMP activity, visualized as a dark band on the lighter background.
Matrigel invasion.
Cell invasion was tested with BioCoat cell culture inserts in 24-well plates (Boyden chamber) (Becton Dickinson, Franklin Lakes, NJ, USA) using the reconstituted basement membrane Matrigel (Becton Dickinson). Briefly, 8-mm pore-sized polycarbonate filters were precoated with Matrigel (10 mg/filter) mixed with exogenous plasminogen (1 mg/filter). HP-75 cells (1x 105 cells/filter) were then added to the upper chamber and assembled with the lower chamber, which had been prefilled with serum-free MEM supplemented with 50 mg/ml of fibronectin as the chemoattractant. The chambers were incubated for eight hours with varying oxygen concentrations (1, 3, 5, 10 or 21%), and then the filters were fixed with 10% formalin. The cells on the upper surface of the filter were removed with a cotton swab, and cells that had invaded onto the lower surface of the membrane were stained with Diff-Quik (Sysmex, Kobe, Japan). The degree of invasion was measured by counting the number of cells in ten randomly selected areas of the lower surface of the filter at x40 magnification.
Cell adhesion assay.
Flat-bottom, 96-well plates (Nalge Nunc) were coated at 37°C for three hours with 100 µl of a 20 µg/ml solution of Matrigel, collagen type I, collagen type IV, fibronectin or vitronectin in PBS (pH 7.2). Noncoated wells were used as the negative control. This solution was then discarded, and nonspecific binding blocked with 100 µl 5% BSA in PBS for one hour at 37°C. The wells were rinsed twice with PBS and 1 x 104 cells in 100 µl nonserum DMEM were added to each well and allowed to spontaneously adhere for six hours at 1 or 21% oxygen. The nonadherent cells and medium were then aspirated, and the wells were washed three times with 100 µl PBS with vigorous shaking. Adhered cells were fixed with 3.7% PFA and stained with Diff-Quick. Subsequently, the cells were resolved in 1% SDS solution and absorbance (reflecting cell density) was recorded at 590 nm using a 96-well plate reader (BioRad, Tokyo, Japan).
cDNA microarray analysis for gene expression profile.
HP-75 (1 x 107) cells were plated on 60 mm plastic dishes (Sumitomo Bakelite, Tokyo, Japan) and exposed to 1% oxygen (hypoxia) or 21% oxygen (normoxia) for 24 hours. The cells were then washed with PBS and total RNA was immediately isolated with TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The RNA concentration was adjusted to 10 µg/10 µl, and the RNA quality was assessed on a 2% ethidium bromide-stained agarose gel. Poly (A) RNA was isolated from total cellular RNA (100 µg) using a MagExtractor (Toyobo, Tsuruga, Japan) according to the manufacturer's instructions, and 2 µg of mRNA was reverse transcribed to biotin-labeled probes using ReverTraAce (Toyobo) in the presence of a cDNA synthesis primer and biotin-16-deoxyuridine triphosphate. Gene Navigator human cancer cDNA filters (Toyobo) were prehybridized at 62°C for 30 minutes in 20 ml of hybridization solution (PerfectHyb Hybridization Solution, Toyobo,), and then the cDNA probes were denatured and hybridized to the filters overnight at 62°C. The membranes were washed three times with solution 1 (2 x SSC and 0.1% SDS) and three times with solution 2 (0.1 x SSC and 0.1% SDS) for five minutes each at 62°C. Specific signals on the filters were detected with a chemiluminescence detection kit (Imaging High, Toyobo), with CDP-Star (Gene Navigator cDNA Array System, Toyobo) as the chemiluminescent substrate. Images and quantitative gene expression data were obtained using a Fluor-S Multiimager system (Nippon BioRad Laboratories, Tokyo, Japan), and the ImaGene software (BioDiscovery, Los Angeles, CA, USA) was used to quantify the signal intensities. Intensity changes greater than 2.5-fold (increase) or 30% (decrease) were considered significant. These results were verified by real-time RT-PCR, and (in the case of laminin β2) flow cytometry.
Real-time RT-PCR.
Cells (5 x 106 in 10 ml culture medium) were seeded into T-75 flasks (Nalge Nunc), allowed to attach overnight, and then incubated in 1, 3, 5, 10 or 21% oxygen for 24 hours. Total RNA was prepared using the TRIZOL reagent (Gibco-BRL, Tokyo, Japan), and cDNA was synthesized using pd(N)6 Random Hexamer primers (Amersham Bioscience, Piscataway, NJ, USA). Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using the TaqMan® One-Step RT-PCR Master Mix Reagents Kit (Applied Biosystems, Tokyo, Japan) and a Gene Amp 5700 thermal cycler (Applied Biosystems). PCR amplification was performed using primers that spanned at least one intron, and monitored with FAM-labeled probes designed online (http://myscience.appliedbiosystems.com/index.jsp). Each amplification utilized cDNA derived from 20 µl mRNA solution (10 ng/µl), and the relative amount of PCR product was calculated as the threshold cycle (CT value) of the sample divided by that of human β-actin (TaqMan® Endogenous Controls, Applied Biosystems).
Flow cytometric analysis.
The expression of laminin β2 on the cell surface was determined by FACS analysis (FACS Calibur, Japan Becton Dickinson Bioscience, Tokyo, Japan). Aliquots of 2 x 106 cells in 10 ml culture medium were seeded into T-75 flasks (Nalge Nunc) and allowed to adhere overnight. After 24 hours exposure to 1, 3, 5, 10, or 21% oxygen, the cells were harvested by trypsinization and washed twice with phosphate buffer. The cells were washed twice with staining buffer (Mg++/Ca++-free PBS containing 1% heat-inactivated FCS, and 0.09% (w/v) sodium azide, pH 7.4), centrifuged at 250 x g, and resuspended in 500 µl of fixative (CellFix, BD Bioscience Pharmingen, San Diego, CA, USA). The cells were incubated for 20 minutes at 4°C for fixing, followed by centrifugation at 250 x g. The pellet was incubated with 1 ml staining buffer containing rabbit anti-human laminin β2 monoclonal antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 minutes at room temperature, washed twice with PBS and incubated with FITC-conjugated donkey anti-rabbit immunoglobulin (1:200, DAKO, Tokyo, Japan) for 30 minutes at room temperature. Cells not incubated with the fluorochrome-conjugated immunoglobulin were utilized as the negative control. The cells were washed twice in PBS and resuspended in staining buffer prior to flow cytometric analysis. The positive control was examined with non-immune rabbit immunoglobulin (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as a primary antibody.
Western blotting to detect laminin β2 in the supernatant.
Supernatants were collected when flow cytometric analysis was done as above. Briefly, supernatants under nonreducing conditions (without 2-mercaptoethanol) were assayed for protein content using the BCA protein assay kit (Bio-Rad, Hercules, CA, USA). Proteins (50µg) were separated by SDS-PAGE and blotted with primary antibodies. After transfer to PVDF membrane (Immun-Blot PVGF Membrane, Bio-Rad, Hercules, CA, USA), the bands were visualized with Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Gene silencing with short interfering ribonucleic acids (siRNA).
Briefly, beginning with the AUG start codon and scanning the length of the gene for AA sequences, the AA and the adjacent 19 nucleotides, potential siRNA target sites, were compared with potential sites, and any target sequence with significant homology to other human genes was eliminated. Each target sequence was selected to be as close as possible to the medial portions of the mRNA, making it theoretically more susceptible to siRNA degradation. The appropriate double stranded RNA pairs were synthesized (Dharmacon Research, Lafayette, CO), with an RNA duplex of Lamin A/C used as the positive control, and a scrambled RNA duplex used as the negative control that non-sequence-specificity was confirmed by BLAST.
The sequences utilized were:
laminin β2 (accession number, NM_002292):
target; 5′-TAAGTGGTCTGGAGCGAGATA
sense; 5′-TAAGTGGTCTGGAGCGAGATAdTdT
antisense; 5′-dTdTTATCTCGCTCCAGACCACTTA
Lamin A/C (accession number, NM_005572):
target; 5′-CTGGACTTCCAGAAGAACA
sense; 5′-CUGGACUUCCAGAAGAACAdTdT
antisense; 5′-dTdTGACCUGAAGGUCUUCUUGU
Scramble:
target; 5′-GCGCGCTTTGTAGGATTCG
sense; 5′-GCGCGCUUUGUAGGAUUCGdTdT
antisense; 5′-dTdTCGCGCGAAACAUCCUAAGC
To assess the transfection efficiency of each siRNA in the HP-75 cells, each sequence was labeled with the LabelT siRNA Tracker Intracellular Localization Kit (Mirus, Madison, WI), and subcellular localization of laminin β2, scramble, or Lamin A/C mRNA was monitored separately. In a sterile Eppendorf tube, 4 µl of TransIT-TKO Transfection Reagent was added dropwise to 200 µl serum-free medium (Opti-MEM, Invitrogen, Tokyo, Japan), mixed thoroughly by vortexing and incubated at room temperature for 20 minutes. To it was added 2.5, 5, or 10 µl of the siRNA targeting laminin β2, 5 µl of the positive control, or 5 µl of the scramble siRNA in 1X universal buffer (20 mM KCl, 6 mM HEPES, pH 7.5, 0.2 mM MgCl2), and the tube was incubated at room temperature for 20 minutes. Six-well plate onto which 1 x 105 cells in 1 ml of culture medium had been seeded on the previous day and allowed to adhere under normoxic conditions were washed twice with PBS and covered with 800 µl of complete growth medium, and the 200 µl of TransIT-TKO Reagent/ siRNA complex were added dropwise, making a final siRNA concentration of the concentration was adjusted to 25, 50, or 100 nM for laminin β2, 50 nM for the positive and the scramble siRNA. The cells were incubated at 37°C for 24 hours in a humidified CO2 chamber in 21% oxygen.
Immediately after mRNA interference as above mentioned, real-time RT-PCR was employed to detect expression of laminin β2 mRNA. Cell adhesion to collagen type IV and Matrigel cell invasion were examined for 4, 8, 12, 24, 36, or 48 hours after the gene silencing on laminin β2 mRNA. Protein level was detected with Western blotting. Briefly, the cells were lysed in 2 ml of RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% SDS, 1.0% Triton X-100, 1% deoxycholate, 5 mM EDTA) containing protease inhibitors, 1 mM phenylmethylsulfonyl fluoride, 10 mM benzamidine, and 2 µg/ml leupeptin (Cosmo Bio Co., Tokyo, Japan). Lysates (10 µg protein per sample) were resolved by 5–20% gradient SDS-PAGE (60 minutes at 100 volts) and transferred onto a PVDF membrane (Amersham International, Buckinghamshire, UK) for 60 minutes at 100 mA. The membrane was blocked overnight in 5% non-fat milk in TBS-T, and then incubated for two hours at room temperature with the appropriate primary antibody (0.2 µg/ml anti-laminin β2 or 0.2 µg/ml anti-β actin for internal standard; Santa Cruz Biotechnology Inc., Santa Cruz CA) in TBS-T. The blots were incubated for 1 hr in the second antibody (peroxidase conjugated swine anti-rabbit immunoglobulin 1:10,000; Santa Cruz Biotechnology Inc., Santa Cruz CA) in TBS-T and washed four times PBS and then developed using an ECL detection system (Santa Cruz Biotechnology Inc., Santa Cruz CA).
Data analysis.
Statistical analysis and graph plotting were performed with the GraphPad Prism 4 software package (Prism 4, San Diego, CA, USA). Means +/- standard deviations were compared using the Student's t-test. Statistical significance was defined as a p-value less than 0.05.
Results
The effects of hypoxia on cell invasion and adherence.
Aiming to examine enzyme activity of MMP and TIMP in gradient oxygen concentration between 1–21%, gelatin and reverse zymography were employed. They revealed that the activities of MMP-2, MMP-9 and TIMP-1 were not affected by incubation of HP-75 cells under hypoxic conditions. In contrast, when in vitro cell invasion through Matrigel in hypoxic condition was demonstrated, the cell invasion was significantly elevated in cells incubated with 1% oxygen versus those incubated with physiologically normal levels of 21% oxygen (per visual fields; 21%, 4.22 +/- 0.43; 10%, 4.50 +/- 0.43; 5%, 3.60 +/- 0.12; 3%, 4.50 +/- 0.22; 1%, 16.38 +/- 2.40 (p < 0.01)) (Fig. 1). The next, cell adhesion to Matrigel or type IV was elevated under hypoxic conditions. Cell adhesion was increased under hypoxic versus normoxic conditions, with O.D. values of 2.08 +/- 0.21 and 0.98 +/- 0.12, respectively, for Matrigel (p < 0.05) and values of 2.96 +/- 0.38 and 1.06 +/- 0.22, respectively, for collagen type IV (p < 0.01). In contrast, there were no significant hypoxic changes in cell adhesion to the uncoated control (21%, 0.24 +/- 0.02; 1%, 0.26 +/- 0.04) or wells coated fibronectin (21%, 2.08 +/- 0.21; 1%, 0.98 +/- 0.12), vitronectin (21%; 0.66 +/- 0.24; 1%, 0.72 +/- 0.08), or collagen type I (21%, 1.25 +/- 0.22; 1%, 1.54 +/- 0.22 (Fig. 2).
Figure 1.
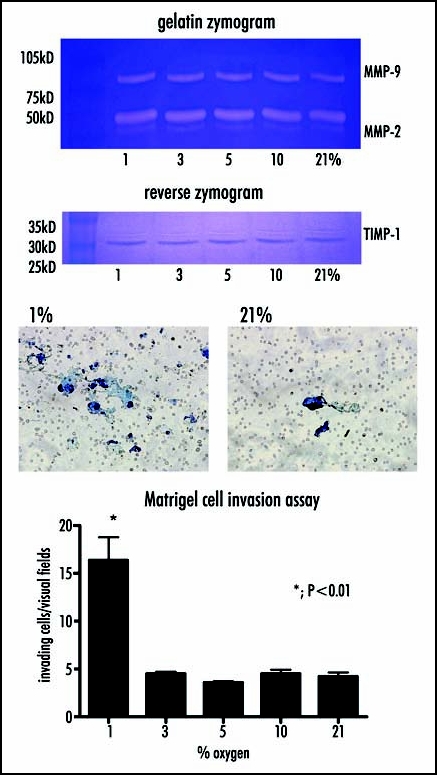
The activities of MMPs and TIMPs, and cell invasion through Mtrigel are examined in hypoxic condition. Left; Gelatin and reverse zymography reveal that the activities of MMP-2, MMP-9 and TIMP-1 are not affected by incubation of HP-75 cells under hypoxic conditions throughout 1–21% oxygen. Right; In contrast, cell invasion through Matrigel is significantly elevated in cells incubated with 1% oxygen versus those incubated with physiologically normal levels of 21% oxygen.
Figure 2.
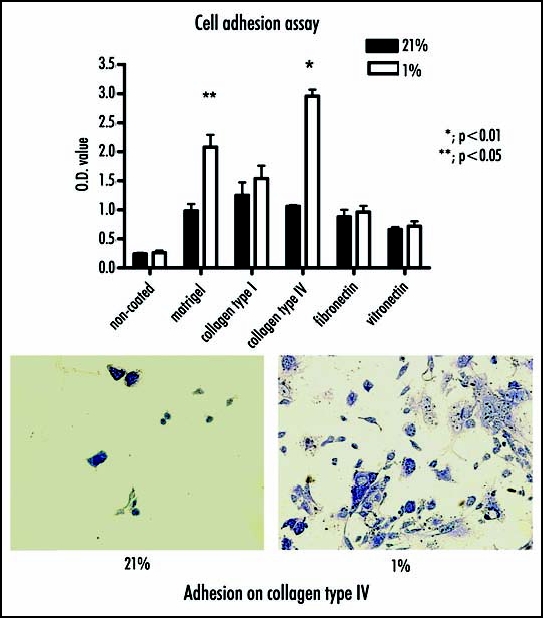
Cell adhesion to Matrigel, collagen type I or IV, fibronectin, vitornection are compared between 1% and 21% oxygen. (Top) Cell adhesion is increased under hypoxic versus normoxic conditions, respectively for Matrigel (p < 0.05) and for collagen type IV (p < 0.01). (Botton) There are significant elevated cell adhesion to collagen type IV at 1% oxygen.
cDNA microarray analysis and verification.
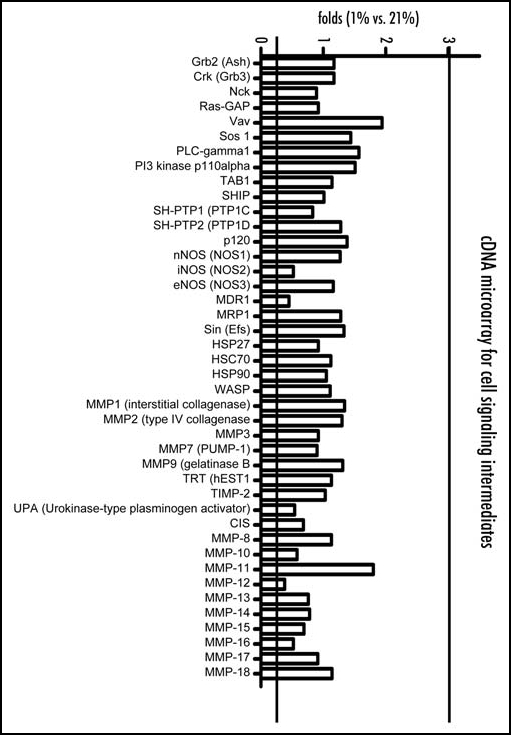
Of the examined 865 genes in microarray analysis, laminin β2 (an ECM gene) was significantly altered by hypoxia, showing a 4.96-fold elevation in cells incubated with 1% oxygen, meanwhile the other cell adhesion molecules involoving cadherin and integrin family (Fig. 3a), molecules relating cell signaling intermediates, involoving the MMP family (Fig. 3b) and were not significantly regulated by hypoxia at 1% oxygen.
Figure 3A.
Expressions of genes in hypoxia at 1% are compared with those at 21% oxygen by cDNA micro array analysis. Genes relating to cell adhesion molecules. Of the examined expression of laminin β2 (an ECM gene) is significantly elevated by hypoxia, showing a 4.96-fold elevation in cells after exposure at 1% oxygen.
Figure 3B.
Expressions of genes in hypoxia at 1% are compared with those at 21% oxygen by cDNA micro array analysis. Cell signaling intermediates. In particular, genes for matrix metalloproteinase (MMP) family are not altered at 1% oxygen.
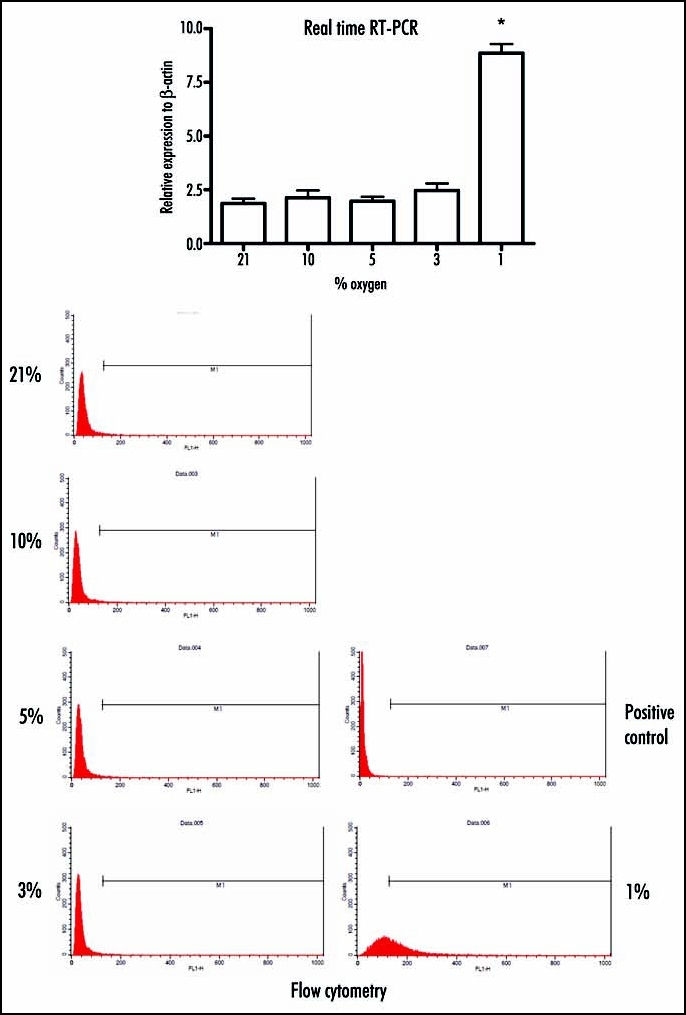
This up-regulation of laminin β2 under hypoxic conditions was firmly verified by real-time RT-PCR, which revealed that cells incubated with 1% oxygen showed a significant increase in laminin β2 versus those incubated under normoxic conditions (8.86 +/- 0.42 versus 1.86 +/- 0.23, respectively; p < 0.001). In contrast, incubation of cells with 3, 5 or 10% oxygen did not induce significant up-regulation of laminin β2 (2.46 +/- 0.34, 1.97 +/- 0.21 and 2.13 +/- 0.34, respectively). Flow cytometric analysis revealed that expression of laminin β2 on the cell surface was elevated following incubation of cells under hypoxic (1%) conditions versus normoxia (phase M1; 1%, 50.8% versus 2.0%, respectively). In this study, the positive control was examined with non-immune rabbit immunoglobulin. Western blotting indicated that the expression in culture supernatant is elevated along with decreasing oxygen concentration. (Fig. 4).
Figure 4.
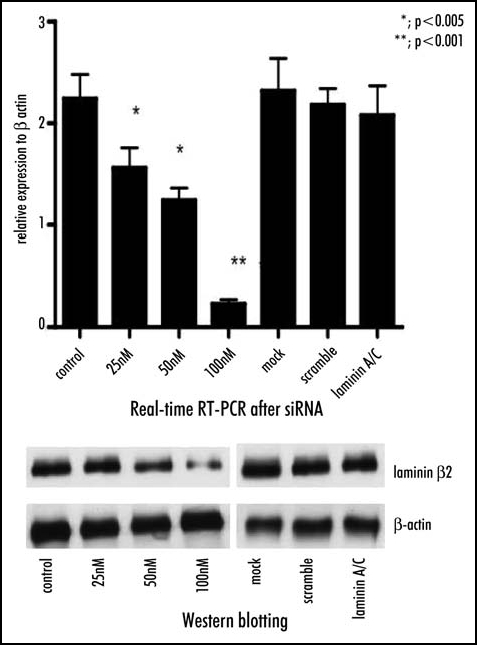
To verify the results obtained by cDNA microarray analysis, real-time RT-PCR and flowcytometric nalysis are performed. (Top) The up-regulation of laminin β2 under hypoxic conditions is verified by real-time RT-PCR, which reveals that cells incubated at 1% oxygen shows a significant increase in laminin β2 versus those incubated under normoxic conditions. However, incubation of cells at 3, 5, or 10% oxygen does not induce significant up-regulation of laminin β2. (Bottom) Flow cytometric analysis reveals that expression of laminin β2 on the cell surface is elevated following incubation of cells under hypoxic (1%) conditions versus normoxia demonstrates that expression of laminin β2 on the cell surface is significantly increased by incubation of cells only with at 1% oxygen.
Cell invasion and cell adhesion with siRNA targeting laminin β2 mRNA.
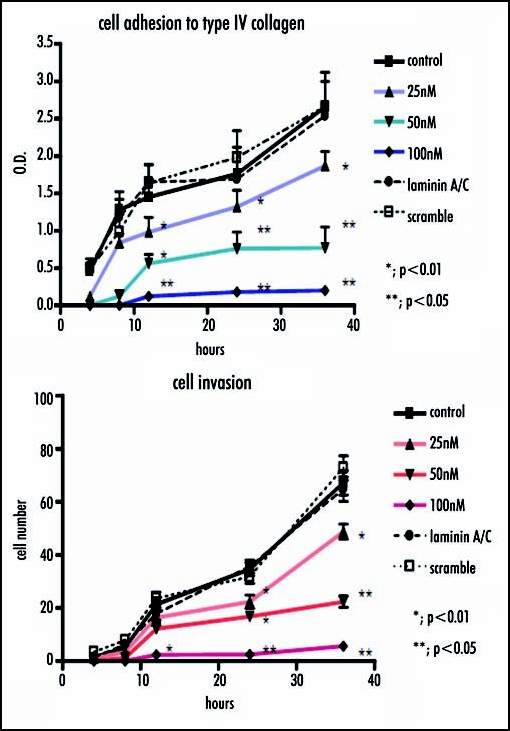
The inhibition of laminin β2 after siRNA method was verified by real-time RT-PCR, which showed a significant decrease in laminin β2 expression in a dose-dependent manner versus the control (25nM, p < 0.005; 50nM, p < 0.005; 100nM, p < 0.001), meanwhile the cells transfected Lamin A/C siRNA, or scramble siRNA did not show significant change in the expression of laminin β2. Similarly, Western blotting analysis indicated a significant decrease in laminin β2 expression in a dose-dependent manner versus the control, compared with the amounts of β-actin as internal standard (Fig. 5). Concomitantly, cell invasion through Matrigel and cell adhesion to collagen type IV after RNA interference with siRNA method was significantly reduced in a dose-dependent manner (Fig. 6).
Figure 5.
Laminin β2 mRNA is interfered by siRNA method and confirmed by real-time RT-PCR and Western blotting. (Top) Real-time RT-PCR shows a significant decrease in expression of laminin β2 mRNA by siRNA in a dose-dependent manner versus the control (25nM, p < 0.005; 50nM, p < 0.005; 100nM, p < 0.001), meanwhile the cells transfected Lamin A/C siRNA, or scramble siRNA does not. (Bottom) Western blotting analysis indicates a significant decrease in laminin β2 expression in a dose-dependent manner versus the control, compared with the amounts of β-actin as internal standard.
Figure 6.
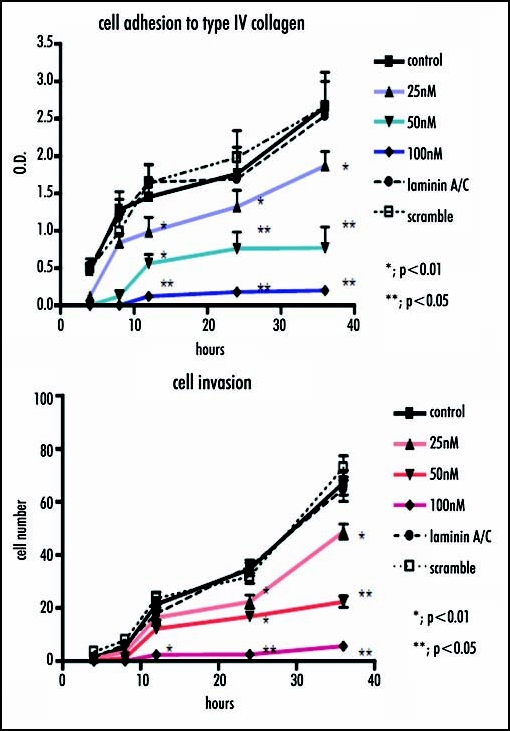
Cell invasion and adhesion are investigated when laminin β2 mRNA was interfered. Similarly, cell invasion through Matrigel and cell adhesion to collagen type IV are significantly reduced in a dose-dependent manner.
Discussion
The current study indicated that enzyme activity of MMP and TIMP in gradient oxygen concentration between 1–21% revealed that the activities of MMP-2, MMP-9 and TIMP-1 were not affected by incubation of HP-75 cells under hypoxic conditions. However, in vitro cell invasion through Matrigel in hypoxic condition was significantly elevated in cells incubated with 1% oxygen versus those incubated with 21% oxygen. Subsequently, cell adhesion to Matrigel or collagen type IV in hypoxia elevated under hypoxic condition. Meanwhile, there were no significant hypoxic changes in cell adhesion to the uncoated control or wells coated fibronectin, vitronectin, or collagen type I. In cDNA microarray analysis, it was disclosed that laminin β2 was significantly altered by hypoxia, showing a 4.96-fold elevation in cells incubated with 1% oxygen, meanwhile molecules relating cell signaling intermediates and the other cell adhesion molecules were not significantly regulated. The expression of laminin β2 in culture supernatant was elevated along with decreasing oxygen concentration (Fig. 4). Subsequently, the expression levels of the laminin β2 chain counterparts, i.e., the laminin alpha and gamma1 chains, in the results shown in Figure 3A did not alter throughout this study, then, it may indicate the possibility that the laminin β2 chain may exist as a beta2 chain monomer.
This up-regulation of laminin β2 under hypoxic conditions was firmly verified by real-time RT-PCR. Flow cytometric analysis revealed that expression of laminin β2 was elevated following incubation of cells under hypoxic (1%) conditions versus normoxia. Finally, when expression of laminin β2 mRNA was inhibited after siRNA, verified by real-time RT-PCR and Western blotting analysis, cell invasion through Matrigel and cell adhesion to collagen type IV were significantly suppressed.
Previous work has shown that hypoxic conditions affect the expression of oxygen-responsive genes, leading to up-regulation of angiogenic factors and down-regulation of angiogenesis inhibitors.13 This can lead to hypoxia-induced hematopoiesis, which subsequently increases the oxygen supply to the hypoxic organ or tumor. Studies have shown that this process involves the Erythropoietin (Epo),1 or hypoxia-inducible factor (HIF) transcription factors in many cancer cell lines.14,15 Expression of HIF family members has been found in every histotype of pituitary adenoma,16 but few previous studies have examined hypoxia-induced cell signaling in this tumor type.17 In contrast to the majority of solid tumors, Lloyd et al reported diminished expression of VEGF, the most significant angiogenic factor, in pituitary adenomas relative to the non-tumorous anterior pituitary lobe.18,19 This observation is consistent with the subnormal microvessel densities reported in various pituitary adenoma subtypes and supports the hypothesis that a lack of significant angiogenesis underlies the relatively slow pace of pituitary adenoma growth.20 Turner et al found significant differences in microvessel density between invasive and non-invasive PRL-producing adenomas but not in GH- and ACTH-producing adenomas.21 In contrast, Vidal et al. reported that the microvessel density was not significantly different in relation to invasiveness of pituitary tumors, even though their results demonstrated a tendency of invasive pituitary tumors to be more highly vascularized than noninvasive tumors. The authors concluded that factors other than microvessel density likely define the invasive potential in some pituitary tumors.22 To test one such possible mechanism, we herein examined whether hypoxic conditions similar to those found in minimally vascularized pituitary tumors might affect the invasiveness of a pituitary adenoma cell line. In our previous study, we investigated whether HIF 1-α protected HP75 cells, pituitary adenoma cell line from hypoxia induced apoptotic cell death. HP75 was transfected with siRNA targeting HIF 1-alpha mRNA sequences, followed by subjected to hypoxia (1% oxygen) for 24 hours, compared with normoxia (21%). This study strongly showed that HIF1-α down-regulated caspase-10, meanwhile laminin β2 was up-regulated in hypoxic condition mediated by HIF1-α.23
We found that pituitary adenoma-derived HP-75 cells exhibited enhanced in vitro invasiveness under severely hypoxic conditions (1% oxygen). This appears to be associated with increased cell adhesion to collagen type IV in the absence of changes in the activity of matrix metalloproteinases (MMPs) or tissue inhibitors of metalloproteinases (TIMPs). cDNA microarray, real-time PCR, immunofluorescent staining and flow cytometry revealed that laminin β2 was significantly up-regulated by severe hypoxia at 1% oxygen, while other adhesion, cell signaling and extracellular matrix genes remained unchanged.
A previous study in cultured pituitary adenoma cells showed that hypoxia reduced the expression of PRL mRNA and protein in the GH4C1 rat lactroph cell line, while the mRNA expression of VEGF164 and VEGF120 mRNA was up-regulated.24 Then, down-regulation of PRL by hypoxia in GH4C1 cells supports the notion that hypoxia is an important factor for the hormonal moiety of a prolactin-producing tumor. Thus, the previous study indicates that rat adenoma cells adapt to low oxygen conditions by modifying cell signaling via transcription of hypoxia-inducible genes. Here, we examined hypoxic responses in HP-75 cells derived from a human nonfunctioning pituitary adenoma. In 2003, Kristof et al studied the regional oxygen saturation (rSO2) of pituitary adenomas versus neighboring pituitary glands, using microspectrophotometric (MSP) measurements in a series of patients undergoing first-time transsphenoidal pituitary surgery with standard anesthesia.25 The authors concluded that the rSO2 of adenoma tissue was significantly lower than that of the pituitary gland, indicating differences in their blood supply and/or metabolism. Although the study did not differentiate among adenoma histotypes, these data provide strong evidence that pituitary adenoma cells may be exposed to hypoxic conditions, suggesting that hypoxia may be a major driving force favoring tumor progression in pituitary adenomas.
Laminin is a major cell-adherence protein that acts as a principle structural components of basement membranes. The laminin molecule initially extracted from rodent tumor extracellular matrices was a heterotrimer composed of three homologs later identified as comprising a number of structurally and functionally diverse laminin isoforms in humans, including five α, three β, and three γ chains that can potentially assemble into at least 30 different laminin heterotrimers.26–28 These isoforms associate at their carboxy (C)-termini to form a coiled-coil heterotrimeric molecule stabilized by disulfide linkages bound to the G domain. Recent studies have shown that each laminin chain has specific binding sites; for example, the G domain has an integrin-binding domain, while the β chain has a collagen-binding domain.28,29 Recent studies have shown that laminin family members not only function as mediators for cell adhesion to extracellular matrix, but also play crucial roles in cell signaling cascades for proliferation, migration, differentiation and apoptosis.30–32 Changes in the levels of the laminin β2 chain have been found in pathological conditions such as cancer, diabetes, and neuromuscular disorders.33–36 The cell adhesion to type IV collagen does not require any laminins. This cell adhesion is known as mediated by integrin α1 β1 or α2β1, while laminins bind cells through other integrins such as α6β1 and α3β1. In our study, the hypoxia-induced enhancement of laminin β2 expression in HP-75 cells is most likely related with the observed increase in the cell adhesion to collagen type IV, which is a major component of Matrigel. But the mechanism underlying enhanced cell adhesion specific to type IV collagen in hypoxic condition is still unknown. Future work will be required to clarify whether each of the β chain subtypes specifically bind different collagen subtypes.
The biological significance of the basement membrane has been extensively examined in many malignant tumors, and has been acknowledged in benign pituitary adenomas. A previous immunohistochemistry study revealed that the laminin β chain is localized in the pituitary stroma, particularly in the vascular basement membrane and sparse elements of the parenchymatous basement membrane.37 An in vitro study revealed that laminin inhibited prolactin secretion and cell proliferation in rat prolactinoma cells but not in normal pituitary cells. Furthermore, laminin showed a dynamic expression pattern during prolactinoma development, and reduced expression in pituitary hyperplasia and in normal pituitaries.38 However, while these previous studies indicate that laminin regulates hormone secretion, future work will be required to assess whether there are laminin subtype-specific signal cascades. In a corticotroph cell line (AtT-20 cells, ACTH production was inhibited in the presence of fibronectin, laminin, and collagen I, while cellular morphology was modified by changes in these extracellular matrix (ECM) molecules. In contrast, ACTH production and cellular morphology was not significantly altered in normal pituitary cells. These results indicated that ECM molecules control ACTH biosynthesis and cell proliferation in corticotroph tumor cells, suggesting that the ECM likely plays a role in corticotroph adenoma development.39 Collectively, these previous works indicate that ECM components may play important roles in mediating intracellular signal transduction and hormone production by pituitary adenomas.
Pituitary adenomas arise in adenohypophyseal cells and metastases are extremely rare. However, a large proportion of adenomas are invasive and spread to sellar and/or parasellar tissues.40 These invasions are not diagnostic of pituitary carcinoma; defined as metastatic tumors in the sellar region, they are exceedingly rare. Several studies have sought to identify features that could be used to predict the invasiveness of pituitary adenomas. Clinically significant invasion appears to be more frequent in macroadenomas than microadenomas,12,40,41 but no other consistent trends have been identified to date. Cell invasion includes three major steps: 1) cell locomotion to a targeting substrate such as the basal lamina of the vascular wall or the ECM, 2) cell adherence mediated by cell adhesion molecules, and 3) degradation of ECM by secreted proteinases such as MMPs or serine proteinases such as plasminogen and plasminogen activator. Thus, it is possible that cell invasion could be accelerated by up-regulation of cell adhesion molecule expression (i.e., that of laminin subtypes) even in the absence of MMP activity changes. Mitotic and MIB-1 labeling indices,42 expression levels of MMPs,43 p-27,42 p-53,44,45 and neural cell adhesion molecule (NCAM)46–48 have been proposed as predictors of pituitary tumor invasiveness, according to established knowledge for cell invasion in the other malignant tumors. However, most of these markers have proven inefficient for reliably predicting clinical invasiveness, since they are based on indirect methods such as immunohistochemistry. Thus, in vitro study of hypoxic versus normoxic responses could be clinically relevant if it is capable of accurately predicting cell invasion.
Here, we showed that hypoxia (1% oxygen) stimulated human nonfunctioning pituitary tumor (HP-75) cells to invade in vitro substrates, in association with increased laminin β2 expression. Further work will be required to assess the specific interactions between ECM components and cell signaling under these conditions, and it would be particularly useful to extend this work into cultured human pituitary adenoma cells. However, the present work provides new insight into the invasiveness of benign pituitary adenomas, and may form the basis for new inhibitory therapies.
Acknowledgements
This study was supported by a Grant in Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (No. 15390445 and No. 17591536) and by Grants in Aid from the Ministry of Health, Labour and Welfare, Japan, for Cancer Research (17–21).
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/abstract.php?id=4080
References
- 1.Lolkema MP, Gervais ML, Snijckers CM, Hill RP, Giles RH, Voest EE, Ohh M. Tumor suppression by the von Hippel-Lindau protein requires phosphorylation of the acidic domain. J Biol Chem. 2005 doi: 10.1074/jbc.M503220200. [DOI] [PubMed] [Google Scholar]
- 2.Heinl-Green A, Radke PW, Munkonge FM, Frass O, Zhu J, Vincent K, Geddes DM, Alton EW. The efficacy of a ‘master switch gene’ HIF-1{alpha} in a porcine model of chronic myocardial ischaemia. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi223. [DOI] [PubMed] [Google Scholar]
- 3.Kondo Y, Hamada J, Kobayashi C, Nakamura R, Suzuki Y, Kimata R, Nishimura T, Kitagawa T, Kunimoto M, Imura N, Hara S. Over expression of hypoxia-inducible factor-1alpha in renal and bladder cancer cells increases tumorigenic potency. J Urol. 2005;173:1762–1766. doi: 10.1097/01.ju.0000154343.35444.09. [DOI] [PubMed] [Google Scholar]
- 4.Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 5.Han XJ, Chae JK, Lee MJ, You KR, Lee BH, Kim DG. Involvement of GADD153 and cardiac ankyrin repeat protein in hypoxia-induced apoptosis of H9c2 cells. J Biol Chem. 2005 doi: 10.1074/jbc.M501095200. [DOI] [PubMed] [Google Scholar]
- 6.Mayer A, Hockel M, Wree A, Vaupel P. Microregional expression of glucose transporter-1 and oxygenation status: Lack of correlation in locally advanced cervical cancers. Clin Cancer Res. 2005;11:2768–2773. doi: 10.1158/1078-0432.CCR-04-2344. [DOI] [PubMed] [Google Scholar]
- 7.Nikinmaa M, Rees BB. Oxygen-dependent gene expression in fishes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1079–R1090. doi: 10.1152/ajpregu.00626.2004. [DOI] [PubMed] [Google Scholar]
- 8.Pescador N, Cuevas Y, Naranjo S, Alcaide M, Villar D, Landazuri MO, Del Peso L. Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem J. 2005 doi: 10.1042/BJ20042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Vaupel P, Mayer A. Hypoxia and anemia: Effects on tumor biology and treatment resistance. Transfus Clin Biol. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Viacava P, Gasperi M, Acerbi G, Manetti L, Cecconi E, Bonadio AG, Naccarato AG, Acerbi F, Parenti G, Lupi I, Genovesi M, Martino E. Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas. J Endocrinol Invest. 2003;26:23–28. doi: 10.1007/BF03345118. [DOI] [PubMed] [Google Scholar]
- 12.Bonneville JF, Bonneville F, Cattin F. Magnetic resonance imaging of pituitary adenomas. Eur Radiol. 2005;15:543–548. doi: 10.1007/s00330-004-2531-x. [DOI] [PubMed] [Google Scholar]
- 13.Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. Faseb J. 2005 doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 14.Hao P, Chen X, Geng H, Gu L, Chen J, Lu G. Expression and implication of hypoxia inducible factor-1alpha in prostate neoplasm. J Huazhong Univ Sci Technolog Med Sci. 2004;24:593–595. doi: 10.1007/BF02911365. [DOI] [PubMed] [Google Scholar]
- 15.Yu L, Liu Y, Cui Y. Expression of hypoxia inducible factor-1alpha and its relationship to apoptosis and proliferation in human laryngeal squamous cell carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2004;24:636–638. doi: 10.1007/BF02911379. [DOI] [PubMed] [Google Scholar]
- 16.Vidal S, Horvath E, Kovacs K, Kuroki T, Lloyd RV, Scheithauer BW. Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) in pituitary tumours. Histol Histopathol. 2003;18:679–686. doi: 10.14670/HH-18.679. [DOI] [PubMed] [Google Scholar]
- 17.Suliman M, Royds J, Cullen D, Timperley W, Powell T, Battersby R, Jones TH. Mdm2 and the p53 pathway in human pituitary adenomas. Clin Endocrinol (Oxf) 2001;54:317–325. doi: 10.1046/j.1365-2265.2001.01195.x. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr Pathol. 1999;10:229–235. doi: 10.1007/BF02738884. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd RV, Vidal S, Horvath E, Kovacs K, Scheithauer B. Angiogenesis in normal and neoplastic pituitary tissues. Microsc Res Tech. 2003;60:244–250. doi: 10.1002/jemt.10263. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol. 2005;288:H759–H768. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- 21.Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab. 2000;85:1159–1162. doi: 10.1210/jcem.85.3.6485. [DOI] [PubMed] [Google Scholar]
- 22.Vidal S, Kovacs K, Horvath E, Scheithauer BW, Kuroki T, Lloyd RV. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 2001;438:595–602. doi: 10.1007/s004280000373. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida D, Kim K, Noha M, Teramoto A. Anti-apoptotic action by hypoxia inducible factor 1-alpha in human pituitary adenoma cell-line, HP-75 in hypoxic condition. J Neurooncol. 2006;78:217–225. doi: 10.1007/s11060-005-9017-9. [DOI] [PubMed] [Google Scholar]
- 24.Cosio G, Jeziorski MC, Lopez-Barrera F, De La Escalera GM, Clapp C. Hypoxia inhibits expression of prolactin and secretion of cathepsin-D by the GH4C1 pituitary adenoma cell line. Lab Invest. 2003;83:1627–1636. doi: 10.1097/01.lab.0000098429.59348.36. [DOI] [PubMed] [Google Scholar]
- 25.Kristof RA, Aliashkevich AF, Hans V, Haun D, Meyer B, Thees C, Schramm J. The regional oxygen saturation of pituitary adenomas is lower than that of the pituitary gland: Microspectrophotometric study with potential clinical implications. Neurosurgery. 2003;53:880–885. doi: 10.1227/01.neu.0000083604.09901.f6. (discussion 5–6) [DOI] [PubMed] [Google Scholar]
- 26.Gersdorff N, Kohfeldt E, Sasaki T, Timpl R, Miosge N. Laminin gamma 3 chain binds to nidogen and is located in murine basement membranes. J Biol Chem. 2005 doi: 10.1074/jbc.M501875200. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson PJ, Rubio C, Lenander C, Auer G, Glimelius B. Tumour budding detected by laminin-5 {gamma}2-chain immunohistochemistry is of prognostic value in epidermoid anal cancer. Ann Oncol. 2005 doi: 10.1093/annonc/mdi179. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JL, Head SI, Morley JW. Synaptic plasticity in the dy(2J) mouse model of laminin alpha2-deficient congenital muscular dystrophy. Brain Res. 2005;1042:23–28. doi: 10.1016/j.brainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Amano T, Kwak O, Fu L, Marshak A, Shi YB. The matrix metalloproteinase stromelysin-3 cleaves laminin receptor at two distinct sites between the transmembrane domain and laminin binding sequence within the extracellular domain. Cell Res. 2005;15:150–159. doi: 10.1038/sj.cr.7290280. [DOI] [PubMed] [Google Scholar]
- 31.Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:1000–1009. doi: 10.1167/iovs.04-1185. [DOI] [PubMed] [Google Scholar]
- 32.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 33.Durkin ME, Jager AC, Khurana TS, Nielsen FC, Albrechtsen R, Wewer UM. Characterization of the human laminin beta2 chain locus (LAMB2): Linkage to a gene containing a nonprocessed, transcribed LAMB2-like pseudogene (LAMB2L) and to the gene encoding glutaminyl tRNA synthetase (QARS) Cytogenet Cell Genet. 1999;84:173–178. doi: 10.1159/000015249. [DOI] [PubMed] [Google Scholar]
- 34.Durkin ME, Nielsen FC, Loechel F, Albrechtsen R, Wewer UM. Regulation of laminin beta2 chain gene expression in human cancer cell lines. Eur J Biochem. 2001;268:3797–3806. doi: 10.1046/j.1432-1327.2001.02292.x. [DOI] [PubMed] [Google Scholar]
- 35.Wewer UM, Gerecke DR, Durkin ME, Kurtz KS, Mattei MG, Champliaud MF, Burgeson RE, Albrechtsen R. Human beta 2 chain of laminin (formerly S chain): cDNA cloning, chromosomal localization, and expression in carcinomas. Genomics. 1994;24:243–252. doi: 10.1006/geno.1994.1612. [DOI] [PubMed] [Google Scholar]
- 36.Wewer UM, Thornell LE, Loechel F, Zhang X, Durkin ME, Amano S, Burgeson RE, Engvall E, Albrechtsen R, Virtanen I. Extrasynaptic location of laminin beta 2 chain in developing and adult human skeletal muscle. Am J Pathol. 1997;151:621–631. [PMC free article] [PubMed] [Google Scholar]
- 37.Farnoud MR, Derome P, Peillon F, Li JY. Immunohistochemical localization of different laminin isoforms in human normal and adenomatous anterior pituitary. Lab Invest. 1994;70:399–406. [PubMed] [Google Scholar]
- 38.Kuchenbauer F, Theodoropoulou M, Hopfner U, Stalla J, Renner U, Tonn JC, Low MJ, Arzt E, Stalla GK, Paez-Pereda M. Laminin inhibits lactotroph proliferation and is reduced in early prolactinoma development. Mol Cell Endocrinol. 2003;207:13–20. doi: 10.1016/s0303-7207(03)00237-5. [DOI] [PubMed] [Google Scholar]
- 39.Kuchenbauer F, Hopfner U, Stalla J, Arzt E, Stalla GK, Paez-Pereda M. Extracellular matrix components regulate ACTH production and proliferation in corticotroph tumor cells. Mol Cell Endocrinol. 2001;175:141–148. doi: 10.1016/s0303-7207(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 40.Vieira JO, Jr, Cukiert A, Liberman B. Magnetic resonance imaging of cavernous sinus invasion by pituitary adenoma diagnostic criteria and surgical findings. Arq Neuropsiquiatr. 2004;62:437–443. doi: 10.1590/s0004-282x2004000300011. [DOI] [PubMed] [Google Scholar]
- 41.Couldwell WT. Transsphenoidal and transcranial surgery for pituitary adenomas. J Neurooncol. 2004;69:237–256. doi: 10.1023/b:neon.0000041886.61149.ab. [DOI] [PubMed] [Google Scholar]
- 42.Saeger W. Proliferation markers and cell cycle inhibitors in pituitary adenomas. Front Horm Res. 2004;32:110–126. doi: 10.1159/000079040. [DOI] [PubMed] [Google Scholar]
- 43.Knappe UJ, Hagel C, Lisboa BW, Wilczak W, Ludecke DK, Saeger W. Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol (Berl) 2003;106:471–478. doi: 10.1007/s00401-003-0747-5. [DOI] [PubMed] [Google Scholar]
- 44.Hentschel SJ, McCutcheon E, Moore W, Durity FA. P53 and MIB-1 immunohistochemistry as predictors of the clinical behavior of nonfunctioning pituitary adenomas. Can J Neurol Sci. 2003;30:215–219. doi: 10.1017/s0317167100002614. [DOI] [PubMed] [Google Scholar]
- 45.Musat M, Vax VV, Borboli N, Gueorguiev M, Bonner S, Korbonits M, Grossman AB. Cell cycle dysregulation in pituitary oncogenesis. Front Horm Res. 2004;32:34–62. doi: 10.1159/000079037. [DOI] [PubMed] [Google Scholar]
- 46.Aletsee-Ufrecht MC, Langley K, Gratzl O, Gratzl M. Differential expression of the neural cell adhesion molecule NCAM 140 in human pituitary tumors. FEBS Lett. 1990;272:45–49. doi: 10.1016/0014-5793(90)80445-o. [DOI] [PubMed] [Google Scholar]
- 47.Kleinschmidt-DeMasters BK, Conway DR, Franklin WA, Lillehei KO, Kruse CA. Neural cell adhesion molecule expression in human pituitary adenomas. J Neurooncol. 1995;25:205–213. doi: 10.1007/BF01053153. [DOI] [PubMed] [Google Scholar]
- 48.Rubinek T, Yu R, Hadani M, Barkai G, Nass D, Melmed S, Shimon I. The cell adhesion molecules N-cadherin and neural cell adhesion molecule regulate human growth hormone: A novel mechanism for regulating pituitary hormone secretion. J Clin Endocrinol Metab. 2003;88:3724–3730. doi: 10.1210/jc.2003-030090. [DOI] [PubMed] [Google Scholar]


