Abstract
T cell recognition of minor histocompatibility antigens (mHags) underlies allogeneic immune responses that mediate graft-versus-host disease and the graft-versus-leukemia effect following stem cell transplantation. Many mHags derive from single amino acid polymorphisms in MHC-restricted epitopes, but our understanding of the molecular mechanisms governing mHag immunogenicity and recognition is incomplete. Here we examined antigenic presentation and T-cell recognition of HA-1, a prototypic autosomal mHag derived from single nucleotide dimorphism (HA-1H versus HA-1R) in the HMHA1 gene. The HA-1H peptide is restricted by HLA-A2 and is immunogenic in HA-1R/R into HA-1H transplants, while HA-1R has been suggested to be a “null allele” in terms of T cell reactivity. We found that proteasomal cleavage and TAP transport of the 2 peptides is similar and that both variants can bind to MHC. However, the His>Arg change substantially decreases the stability and affinity of HLA-A2 association, consistent with the reduced immunogenicity of the HA-1R variant. To understand these findings, we determined the structure of an HLA-A2-HA-1H complex to 1.3Å resolution. Whereas His-3 is accommodated comfortably in the D pocket, incorporation of the lengthy Arg-3 is predicted to require local conformational changes. Moreover, a soluble TCR generated from HA-1H-specific T-cells bound HA-1H peptide with moderate affinity but failed to bind HA-1R, indicating complete discrimination of HA-1 variants at the level of TCR/MHC interaction. Our results define the molecular mechanisms governing immunogenicity of HA-1, and highlight how single amino acid polymorphisms in mHags can critically affect both MHC association and TCR recognition.
Keywords: graft-versus-leukemia, stem cell transplantation, major histocompatibility complex
Stem cell transplantation (SCT) is used widely in the management of hematopoietic malignancies (1) and allogeneic T-cell immune responses are critical determinants of clinical outcome. Such donor-derived responses mediate the graft-versus-host disease observed in SCT, but are also responsible for the powerful graft-versus-leukemia (GvL) effect that underlies the clinical efficacy of allogeneic SCT (2–4). Both GvL and graft-versus-host disease depend on allogeneic transplantation and the presence of donor T cells in the graft (3, 4), and are therefore thought to result from alloreactive T-cell recognition. Because donor and recipient are typically MHC-matched, the targets of these responses are peptides known as minor histocompatibility antigens (mHags) that are presented by recipient but not donor HLA molecules (5). mHags originate from either genetic polymorphisms in autosomal genes that result in presentation of peptide species, or through presentation of epitopes derived from proteins encoded on the Y chromosome in female-into-male allografts (2).
The HA-1 antigen is the prototypic autosomal mHag and is encoded by the HMHA1 gene, which has 2 alleles, HA-1H and HA-1R (6). A single nucleotide polymorphism results in an amino acid dimorphism (His/Arg) in an HLA-A*0201 (HLA-A2)-restricted epitope, of which only one variant, VLHDDLLEA (VLH, encoded by HA-1H), is known to be immunogenic in this context. Expression of HA-1 is restricted primarily to hematopoietic tissue (2) and immune recognition of HA-1 on malignant cells is therefore expected to preferentially favor GvL. Indeed HA-1-specific T-cell responses have been closely correlated with curative GvL responses after donor lymphocyte infusion (DLI) (7, 8). Consequently, mHags such as HA-1 are of considerable clinical interest as potential tumor antigens for immunotherapeutic strategies, including HA-1 vaccination (9) and TCR gene transfer (10). A comprehensive understanding of the factors that govern immunogenicity of HA-1 and other immunodominant mHags is therefore likely to be important in the design of such approaches.
Despite their clinical importance, our understanding of the molecular mechanisms governing mHag immunogenicity, including that of HA-1, is currently incomplete. For several mHags, differential antigen processing, involving proteasomal generation or TAP transport, results in discordant cell-surface expression of polymorphic variant peptides, a mechanism that applies to presentation of HA-8 (11) and HA-3 (12). For other mHags, such as B7-HY, A1-HY, and HB-1, broadly equivalent cell-surface expression of polymorphic variants occurs, but discrimination between mHag-expressing cells and their negative counterparts is thought to occur at the TCR level (13–15). A third possibility is that mHag polymorphisms critically affect MHC binding, a mechanism that may apply to HA-1 (6). However, although differences in MHC binding-affinity between the immunogenic VLH and its polymorphic variant VLRDDLLEA (VLR, encoded by HA-1R) have been demonstrated, the lower reported affinity of the VLR peptide (6) is within the range of affinities for immunogenic cell-surface-expressed peptides (16, 17), and therefore additional effects on class I MHC presentation have not been excluded (6). Here we define the mechanism underlying HA-1 mHag immunogenicity by addressing the effects of the His>Arg change on HA-1 antigen processing, MHC binding, and TCR recognition. Our results show how this polymorphism critically affects MHC binding and, in addition, establishes TCR discrimination of single amino acid mHag variants as a plausible mechanism underlying mHag immunogenicity.
Results
Detection of VLH-Specific T-Cell Responses in a Chronic Myeloid Leukemia Patient After DLI.
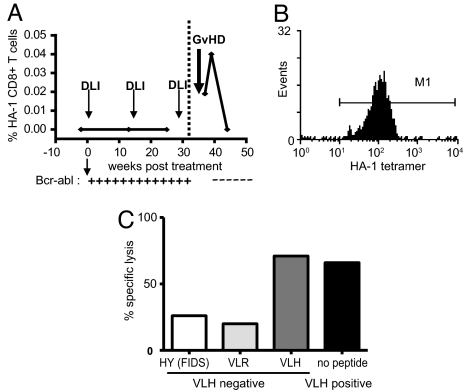
T-cell responses to the HA-1 mHag were detected in an HA-1H-positive patient diagnosed with chronic myeloid leukemia (CML) who had undergone SCT from a matched, unrelated donor homozygous for the HA-1R allele (Fig. 1). Following leukemic relapse, the patient received 3 escalating doses of DLI. After the third dose of DLI, VLH-specific CD8 T cells were detected within peripheral blood by binding to HLA-peptide tetramers, and their appearance correlated with elimination of the tumor. VLH-specific T cells were purified by magnetic selection and cloned by limiting dilution assay. The VLH-specific T-cell clone KP7 bound to VLH tetramer and was functionally specific for the VLH peptide presented by HLA-A2. It was also able to recognize naturally processed VLH epitope on VLH-positive LCL, in agreement with previously published data (7, 8) (see Fig. 1).
Fig. 1.
Detection and isolation of HA-1-specific cytotoxic T lymphocytes after SCT. (A) HLA-A2-VLH tetramers were used to detect HA-1-specific CD8 T-cell responses after donor lymphocyte infusion therapy for leukemic relapse following CML. DLI treatment was initiated 80 weeks after transplant (zero timepoint). Presence of tumor was assessed by quantitative RT-PCR for BCR-ABL transcripts (+/–). (B) The HA-1-specific T-cell clone KP7 stained with HLA-A2-VLH HA-1 tetramer. (C) The KP7 T-cell clone recognizes HA-1-positive lymphoblastoid cell line (LCL) cells presenting endogenously processed HLA-A2-VLH, and HA-1-negative HLA-A2+ LCL pulsed with VLH peptide. However, HA-1-negative LCL are not recognized in the absence of exogenously added peptide, or when pulsed with a control peptide (FIDS).
Proteasomal Generation and TAP Transport of VLH and VLR Variants Is Similar.
To understand the molecular basis of HA-1 mHag immunogenicity, we compared the relative propensities of VLH and VLR HA-1 peptides to undergo proteasomal generation and TAP transport. To determine proteasomal digestion directly, we synthesized extended (27 mer) peptides spanning the VLH/VLR epitope region and incubated these separately with 20S constitutive and immuno-proteasome preparations, before identification of the VLH/VLR species by mass spectroscopy (18). VLH and VLR nonamers, but not N-terminal-elongated precursors, were generated to a similar extent by the constitutive proteasome (within 2-fold), as indicated by appropriate cleavages before V(P1) and after A(P9) [supporting information (SI) Fig. S1A]. Interestingly however, neither the final epitopes nor any N-terminal-elongated precursors could be detected when either VLH or VLR 27 mers were incubated with immunoproteasome preparations, indicating a requirement for constitutive subunits for efficient epitope generation (see Fig. S1A). TAP transport was then assessed using a nonradioactive TAP binding assay based on fluorescence polarization (19). VLH and VLR each competed with the fluorescent test peptide similarly (Fig. S1B), indicating that proteasomal processing in the cytosol and transport into the endoplasmic reticulum by TAP is broadly equivalent in efficiency for the VLH and VLR peptides. These results therefore demonstrate that proteasomal cleavage and TAP transport are unlikely to substantially affect the relative immunogenicity of the two variants.
The VLR Polymorphic Variant Associates with HLA-A2 with Reduced Stability and Affinity.
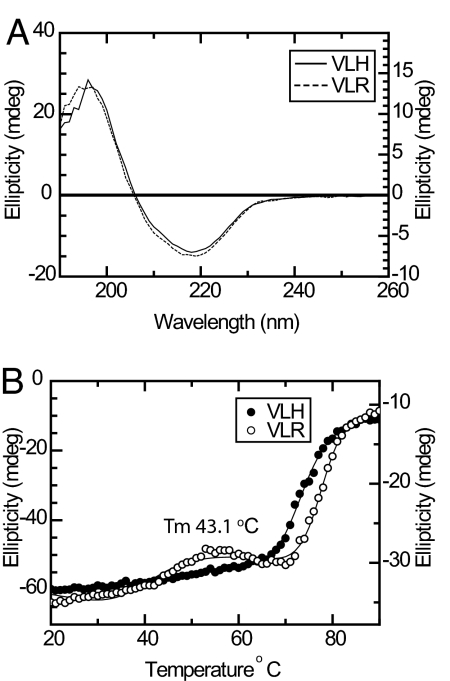
To address whether the His>Arg polymorphism could affect association with HLA-A2, we carried out circular dichroism (CD) measurements to assess the relative stability of the 2 complexes. HLA-A2-VLH/R complexes refolded with comparable yields at 4 °C and were each purified to a high level at 25 °C. Consistent with this, HLA-A2-VLH/R complexes yielded qualitatively identical CD spectra at 25 °C, confirming each protein had a similar overall fold (Fig. 2A). However, thermal denaturation measurements indicated substantial differences in the stability of the 2 complexes. Whereas HLA-A2-VLH yielded a single denaturation transition (Tm 73.5 °C), an additional folding transition was apparent for HLA-A2-VLR with a Tm 43.1 °C, consistent with dissociation of the complex (Fig. 2B). To assess the effect of the His/Arg alteration on MHC affinity, we then measured the binding of VLH/R peptides to HLA-A2 in a radioactive competition binding assay (20). VLH bound with a mean IC50 of 22 nM, broadly consistent with previous fluorescence-based affinity measurements (6). In contrast, multiple measurements indicated VLR bound extremely weakly to HLA-A2 (mean IC50 1,296 nM). These data indicate both variants can associate with HLA-A2. However, they also demonstrate that although the His>Arg change is not located at a primary anchor residue position, it has a substantial deleterious effect on peptide-MHC (pMHC) stability, which is manifest as a radical decrease in peptide affinity for HLA-A2.
Fig. 2.
CD analysis of HA-1 variant complexes. (A) Wavelength spectrum of HLA-A2-VLH (solid line) and HLA-A2-VLR (dashed line) complexes. (B) Thermal stability measurement of HLA-A2-VLR (open circles) indicates an additional folding transition relative to HLA-A2-VLH (solid circles).
Crystallographic Structure Determination of HLA-A2-VLH Complex.
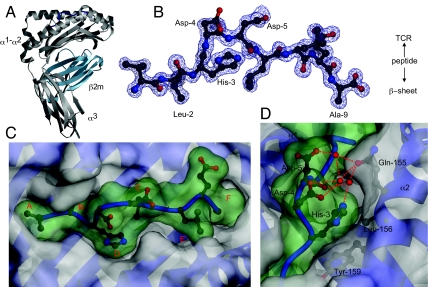
To address the molecular basis of the effect of the His>Arg dimorphism on HA-1 affinity for HLA-A2, we determined the crystal structure of the HLA-A2-VLH complex to 1.3Å resolution (Fig. 3 A and B, Table S1). As expected, the VLH peptide adopts an extended structure in the antigen binding groove, with retention of stabilizing interactions from MHC residues to the peptide N and C terminus. The canonical Leu-2 anchor residue is oriented in a standard position in the B pocket, forming extensive contacts with surrounding hydrophobic residues. In contrast, the C-terminal anchor residue is alanine, which is poorly represented at this position in HLA-A2 peptides (21) and forms only extremely scant contacts with the F pocket (Fig. 3C). The paucity of contacts with Ala-9 suggested that interactions mediated by secondary anchor residues, such as the polymorphic His-3, might substantially affect the overall stability of the pMHC complex.
Fig. 3.
Crystallographic structure of HLA-A2-VLH at 1.3Å. (A) Overall structure of HLA-A2-VLH complex, with heavy chain (gray), β2m (cyan), and VLH peptide (blue) shown. (B) 2Fo-Fc electron density for the VLH peptide, with primary anchors and P3 to P5 highlighted. (C) Structure of the VLH mHag in the HLA-A2-antigen binding groove, with antigen-binding pockets A to F indicated, and VLH peptide surface indicated in green. The structure highlights relatively poor contacts with pockets E and F. (D) Orientation of H3 in and around the D pocket. H3 packs snugly against the walls of the D pocket, maintaining van der Waal's contacts with Tyr-159, Leu-156, and Gln-155, and also to Asp-4 of the peptide. It is also participates in a hydrogen-bonding network to Gln-155, and peptide residues Asp-4 and Asp-5, via ordered water molecules. Semitransparent peptide surface shown in green.
Incorporation of Arg at the P3 Secondary Anchor Actively Destabilizes pMHC Association.
Analysis of the HLA-A2-VLH structure indicated how His-3 is accommodated in the antigen binding groove and suggested possible mechanisms to explain how Arg-3 might decrease complex stability. The His-3 side chain fits relatively snugly in the D pocket (see Fig. 3 C and D), forming van der Waals and hydrophobic interactions with Tyr-99, Tyr-159, and Leu-156 lining the pocket. In addition, His-3 mediates indirect contacts to Gln-155 and the peptide main chain at P5 via a single bridging water molecule. These contacts, and the fact that they are mediated by the imidazole ring (see Fig. 3D), raised the question of whether the His-3 side chain substantially stabilized the pMHC interaction relative to VLR. To address this issue, we generated a variant peptide with a His>Ala substitution at P3 (VLA). Interestingly, the affinity of VLH (22 nM) and mutant VLA (20 nM) peptides for MHC was extremely similar. Furthermore, HLA-A2-VLH/A complexes exhibited similar thermal stabilities [Tm 73.5 °C (VLH) versus 66.1 °C (VLA)] (Fig. S2 A and B). This indicates that the interactions mediated by His-3 make minimal energetic contributions and do not substantially stabilize the pMHC complex.
These considerations suggested the presence of the Arg side chain at P3 actively destabilized the pMHC complex relative to VLH. Based on inspection of the HLA-A2-VLH structure, 2 possible mechanisms were envisaged. First, substitution of Arg-3 could electrostatically destabilize the peptide conformation by introducing a positively charged side chain in relatively close proximity to Asp-4 and Asp-5 side chains. Alternatively, steric or charge effects could hinder the Arg side chain from being accommodated optimally in the D pocket. To test the first hypothesis, we carried out thermal stability measurements on HLA-A2 complexes containing VLR variant peptides with either one or both Asp residues substituted to Ala (VLRD4A, VLRD5A, VLRD45A). In each case, an early unfolding transition was evident (Tm ≈42–49 °C), indicating a thermal stability similar to that of the parental VLR peptide (Fig. S2 C and D). These data indicated the destabilizing effect of Arg-3 was not dependent on the presence of Asp-4 or Asp-5 side chains. In contrast, inspection of the HLA-A2/VLH structure indicated a strong possibility of steric and electrostatic clashes with D pocket residues (e.g., Tyr-99, Gln-155, Leu-156, Leu-160, Tyr-159) when modeling Arg at P3 (Fig. S3 A–D). The sole Arg-3 rotamer that did not clash substantially with the D pocket did involve major clashes with the peptide main chain in the P4-P5 region (Fig. S3E). This suggested that binding of VLR was sterically and electrostatically unfavorable, and was likely to require conformational adjustments in either the central region of the peptide or the HLA-A2 heavy chain itself (most likely in the α2 helix). Because His-3 in VLH is oriented in a similar position to that of many HLA-A2 structures, such limitations were hypothesized to disfavor Arg at P3 in other HLA-A2 peptides. Consistent with this, bioinformatic analyses of HLA-A2-restricted T-cell epitopes confirmed a disproportionately low frequency of Arg at P3 (Fig. S3F), strongly suggesting the effects evident from our analyses of HA-1 VLH/R peptides also apply to HLA-A2 peptides in general.
Complete Discrimination of VLH and VLR Variants by a VLH-Specific TCR.
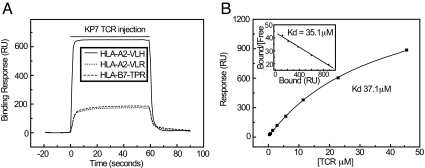
Despite a relatively low stability, the fact that the VLR peptide associated with HLA-A2 with an affinity similar to that of several low-affinity peptides eluted from cell-surface class I MHC molecules (16, 17, 22) suggested it may be presented at the cell surface at low levels in the steady state. This possibility raised the question of whether the VLH and VLR peptides could be discriminated by HA-1-specific T cells at the level of the TCR. To address this, we generated soluble HA-1-specific TCR heterodimer to characterize the molecular properties of HA-1 mHag recognition and assess how this was affected by His/Arg polymorphism. The TCR chains were amplified from the KP7 T-cell clone, which is functionally specific for the VLH peptide and was generated using standard methods, allowing analysis of KP7 TCR interaction with the HLA-A2-VLH/R complexes using surface plasmon resonance. The KP7 TCR bound HLA-A2-VLH with an affinity of 35 μM (Fig. 4, Table 1), somewhat lower than the typical values of 1–10 μM for human TCR/MHC interactions, most of which involve recognition of viral peptides (23). Consistent with this lower affinity, the KP7 TCR dissociated from HLA-A2-VLH with an extremely fast koff of 3s−1 (Fig. S4A) (23), suggesting TCR/MHC complexes are highly unstable at the cell surface. In addition, the thermodynamic profile of KP7/HLA-A2-VLH binding was similar to previously studied self-MHC-restricted TCR interactions (Fig. S4B, Table S2). These data suggested KP7 TCR recognition of HLA-A2-VLH was broadly similar to self-restricted TCR/MHC interactions, but involved a somewhat lower affinity and relatively faster dissociation kinetics compared to those previously measured.
Fig. 4.
Surface plasmon resonance analysis of TCR/HLA-A2-HA-1 interaction. (A) Specific binding of KP7 TCR to HLA-A2-VLH (solid line), with control (HLA-B7-TPR) and HLA-A2-VLR signals also shown (dashed and dotted lines, respectively). (B) Equilibrium affinity analysis of TCR/HLA-A2-VLH interaction. Scatchard plot is shown inset.
Table 1.
Molecular properties of TCR/HA-1-HLA-A2 interactions
Affinity and kinetics of KP7 TCR/HLA-A2-VLH interactions
| Kdeqm | 35 μM |
| kon | 8 × 104 M−1.s−1 |
| koff | 2.6 s−1 |
| t1/2 | 0.27 seconds |
| Kdcalc | 33 μM |
| Affinity of KP7 TCR for HA-1 variants | Kd (μM) |
| VLHDDLLEA | 35.8 |
| VLR | NB |
| V1A | 44.5 |
| H3A | 37.7 |
| D4A | NB |
| D5A | NB |
| L7A | 320.5 |
| E8A | NB |
Kdeqm, determined from equilibrium binding analysis.
Kdcalc, calculated from kinetic rate constants.
Kinetic rate constants were determined by global fitting to experimental data using BIAevaluation 3.1.
NB, no binding detected
To test the effect of the P3 His/Arg residue on TCR recognition of HA-1 nonamers, we directly compared binding of the KP7 TCR with HLA-A2-VLH and HLA-A2-VLR in parallel, at a temperature (25 °C) at which both complexes were stable (see Fig. 4A, Table 1). Whereas binding of TCR to HLA-A2-VLH complex was unambiguous, signals resulting from injection of KP7 over-flow cells containing immobilized HLA-A2-VLR were identical to those over control surfaces, indicating no interaction with the HLA-A2-VLR complex. To understand the energetic basis of this complete discrimination, we tested binding of KP7 to HLA-A2 presenting VLH peptide variants (see Table 1). Interestingly, substitution of Ala for His-3 resulted in an affinity similar to HLA-A2-VLH, suggesting the His-3 side chain itself is not essential for interaction with the KP7 TCR. In contrast, substitution of either Asp-4 or Asp-5 to Ala, both solvent-exposed, completely eliminated binding by the KP7 TCR. These results establish the importance of the central region of VLH for HA-1 recognition by the KP7 TCR and suggest that introduction of the lengthy and charged Arg at P3 is likely to substantially change the conformation and electrostatic nature of this energetically crucial interaction surface, thus providing a plausible mechanism for VLH/VLR discrimination by the TCR.
Discussion
Our results clarify the mechanisms underlying the immunogenicity of the HLA-A2-restricted HA-1 mHag, T-cell recognition of which is thought to mediate potent alloreactive T-cell immune responses following SCT. In doing so, we outline the molecular basis of 2 major mechanisms governing mHag immunogenicity that are likely to have more general applicability.
Proteasomal digestion analyses indicated that proteasomal generation of the HA-1 nonamer was unlikely to be influenced by the nature of the amino acid at P3. Interestingly, the HA-1 nonamer was generated only by the constitutive proteasome and not the immuno-proteasome. HA-1 is expressed in dendritic cells and it has been suggested that this presentation is critical in generation of primary HA-1-specific T cell responses in vivo (24). The chemotherapy regimens used for allogeneic SCT conditioning are associated with an inflammatory response that plays an important role in induction of alloreactive responses (25). As such, the immunoproteasome might have been expected to play an important role in HA-1-specific immunity, but our results indicate that constitutive generation in host tissue may be a critical determinant.
TAP transport was also broadly similar for the VLH/R peptides, whereas MHC association and TCR recognition discriminated the variants. We show that although the His>Arg change is not at a primary anchor residue position, it dramatically reduced the stability and affinity of pMHC association but did not eliminate binding completely. Comparison with other studies suggests this reduced affinity may be sufficient for cell-surface presentation (16, 17, 22), but it would be expected to result in substantially lower cell-surface density of VLR relative to VLH in the steady state, which would likely impact on relative immunogenicity. In contrast, despite the presence of a suboptimal C-terminal anchor residue, the affinity of the VLH variant is similar to that of other immunogenic epitopes, including those presented at >100 copies per cell (17). Consequently, our data permit a revised interpretation of HA-1 mHag immunogenicity, in which the VLR variant can bind MHC but has a greatly reduced cell-surface presentation, rendering it severely “immunorecessive” in comparison to the immunodominant VLH peptide. This interpretation is consistent with the lack of documented immunogencity of the VLR variant, but highlights the possibility that the VLR peptide is presented in the thymus of HA-1R individuals.
Consistent with the idea that the VLR variant may be presented in the thymus of homozygous HA-1R donors, our results show that HA-1-specific T cells induced after DLI during a curative GvL response can discriminate very efficiently between VLH and VLR HA-1 variants directly on the basis of their TCR. Discrimination of highly similar mHag variants presented at the cell surface by the TCR is a mechanism postulated to underlie responses to many minor mHags (13–15), but our data provides the first direct evidence for this in a naturally occurring mHag. Although an obvious caveat is that our results relate to a single T-cell clone (KP7), 3 observations suggest these properties are shared by TCRs expressed by other HA-1-specific T cells. First, the KP7 TCR Vβ region is identical to other clones of the same specificity (26, 27), suggesting similar modes of TCR/pMHC interaction. Second, other HA-1-specific T cells also fail to recognize VLR, even when pulsed onto the cell surface, consistent with the properties of KP7. Also, the pattern of reactivity to alanine-substituted variants exhibited by the KP7 TCR in direct-binding experiments is broadly similar to that of other HA-1-specific T-cell clones in cellular assays, consistent with a similar mode of TCR/MHC recognition (28). However, we cannot exclude the possibility that instead of resulting from central tolerance effects, discrimination of VLH from VLR variants by the KP7 TCR reflects a relatively conserved mode of VLH recognition that fortuitously depends on amino acid variation at P3 (26, 27). Either way, our results provide direct evidence that naturally generated mHag-specific T cells can discriminate absolutely between single amino acid mHag variants via the TCR.
Our study also clarifies key structural mechanisms affecting mHag presentation and recognition. In particular, structural data and thermal stability measurements suggest the drastic effect of the P3 change on MHC affinity is related to its role as a secondary anchor in the D pocket, whereby the His-3 side chain is tolerated and the lengthy Arg is likely to result in steric and electrostatic clashes with surrounding residues. Similarly dramatic changes in pMHC affinity have been noted previously, but based on alterations in primary anchor residues (29). However, the finding that secondary anchor alterations can be as drastic is important, as other peptide positions can act as secondary anchor residues for HLA-A2, implying there may be many alternative routes by which single amino acid changes in mHags can disrupt pMHC interaction. Clearly, the context in which such changes take place is likely to be very important, and it is notable that the C-terminal HA-1 Ala is a relatively uncommon primary anchor residue (21) that forms limited interactions with the F pocket, suggesting suboptimal energetic stabilization. In such a context, secondary anchor residue interactions are likely to assume particular significance.
The structural mechanisms that permit TCR discrimination of single amino acid mHag variants have previously remained unclear, and our results shed light on these. Crucially, the polymorphic His-3 side chain is located directly adjacent to a region of the peptide (comprising Asp-4 and Asp-5) that is energetically extremely important for TCR binding. However, our results argue against the side chain playing a critical direct role in TCR recognition, because the H3A mutant is recognized with a similar affinity by the KP7 TCR. Instead, they suggest the mechanism of discrimination for KP7 involves the Arg variant actively disrupting TCR interaction, possibly by altering the conformation in the adjacent energetically important peptide region, or alternatively by introducing steric or electrostatic clashes with CDR loops. It is possible that for other mHags, TCR discrimination involves direct interactions with the variant amino acid. Relevant to both mechanisms is the position of the amino acid change. The fact that the P3 side chain is oriented toward the central section of the peptide, a region that provides the most direct contacts for TCRs, is important.
Our results should facilitate therapeutic targeting of mHags by providing a molecular basis for understanding mHag immunogenicity and recognition. In particular, they improve understanding of HA-1, a target of vaccination (9), and also gene-transfer approaches aiming to boost GvL responses (10). In this regard, they are consistent with therapeutic strategies targeting VLH but not VLR, which is unlikely to be immunogenic in its native form at the cell surface. However, because our results highlight poor anchor residue interactions at the HA-1 C terminus, vaccination approaches may benefit from immunization with C-terminal anchor-modified HA-1 peptides to enhance responses to HA-1H, or even conceivably to induce responses to the HA-1R form. In addition, because discrimination of VLH from VLR peptides is observed to be intrinsic to the KP7 TCR, TCR transfer approaches with such reagents would be expected to retain this property. Secondly, they suggest that factors affecting the expression of constitutive vs. immuno-proteasome components are likely to impact upon responses to HA-1. Finally, they provide the first information on TCR/mHag-MHC interactions. In comparison to TCR/MHC interactions involving viral peptides, which relate primarily to T cells from the memory pool, HA-1 interactions with the KP7 TCR are relatively unstable and of low affinity. Because the stability of TCR/MHC interaction is a key factor both in productive immune synapse formation and in enabling transition to the memory compartment (30), these properties could adversely influence the effectiveness or longevity of HA-1-specific T-cell responses. Potentially, affinity modification of mHag-specific TCRs, such as KP7 in the context of gene transfer approaches, could overcome such difficulties.
Materials and Methods
T-Cell Cloning and Functional Assays.
Patient NC27PM (HA-1H+) received SCT for CML in 2001 from a matched-unrelated donor (HA-1RR). In 2002, the patient relapsed and received 3 doses of DLI. PBMC were collected before and after DLI infusions and monitored for VLH-specific cytotoxic T lymphocytes using HLA-A2-VLH tetramers (31). After the third DLI dose, a VLH peptide T cell line was set up using standard procedures, coinciding with molecular disease remission. After enrichment using VLH tetramer, cells were cloned by limiting dilution assay using allogeneic PBMC and LCL. Tetramer-staining clones were tested for lysis of VLH- and VLR-peptide-loaded HA-1R LCLs in standard chromium release assays (32).
Proteasome Digestion Analysis and TAP Transport Peptide Binding Assays.
Proteasomal generation of VLH and VLR nonamer species was tested by incubation of extended HA-1 peptide sequences (FAEGLEKLKECVLHDDLLEARRPRAHE and FAEGLEKLKECVLRDDLLEARRPRAHE) with purified 20S constitutive or immuno-proteasome preparations, as previously described (33). Peptide fragments were separated by capillary liquid chromatography and analyzed by mass spectrometry, with synthetic nonamer peptides VL(H/R)DDLLEA used as standards for relative quantification of epitope generation. Peptide binding to the human TAP, over-expressed in Sf9 insect cell microsomes as described (34), was measured using a nonradioactive fluorescence polarization assay (19), with relative TAP affinities of test peptides determined as the concentration required to inhibit 50% of specific TAP binding of the high affinity FITC-concjugated reporter peptide RRYNACTEL (IC50). Each peptide was tested in at least 2 independent experiments. Unlabeled, unsubstituted reporter peptide RRYNASTEL was included in each assay for normalization of results. Results are expressed as the ratio of the IC50 of the test peptide to the IC50 of unlabeled R9L.
MHC Affinity and Stability Experiments.
The affinity of VLH and VLR peptides for HLA-A2 was determined using an equilibrium assay that measures the ability of test peptides to compete with the radio-labeled FLPSDYFPSV standard peptide for binding to HLA-A2 (20). Purified class I molecules were incubated with test peptides and after 48 h, class I-peptide complexes separated from free peptide by gel filtration. Class I-bound and -free radioactivity was measured and the doses of test peptides yielding 50% inhibition of labeled peptide binding (IC50, nM) were calculated. Each peptide was tested in 2 to 4 independent experiments. CD experiments were carried out using a JASCO J-810 Spectropolarimeter, in 20 mM Hepes pH 7.4, 10 mM NaCl.
Class I MHC Complex Production, Crystallization and Structure Determination.
HLA-A2-VLH and HLA-A2-VLR complexes were produced by dilution refolding (35), and subsequently purified by gel filtration. Crystals of HLA-A2-VLH were obtained in 20% PEG 10K, 0.1M Hepes pH 7.5 using a Mosquito crystallization robot (TTP Labtech). Before collection of 1.3Å data at ID14-EH4 (ESRF, France), the crystal was soaked in precipitant buffer containing 15% ethylene glycol and flash frozen to 100K. Data were integrated using MOSFLM (36) and scaled using the CCP4 suite (37). The structure was solved by molecular replacement in CNS (38) using an HLA–A2 complex as a search model. The model was subjected to alternate cycles of model building [TURBO-FRODO, (39)] and refinement [CNS and SHELX (40)]. The final structure comprises residues 1–274 (heavy chain), 2–99 (β2M), 1–9 (peptide), and 336 water molecules, and has an Rfac of 16.9% and Rfree of 20.4% (see Table S1 for statistics). Atomic coordinates and structure factors have been deposited in the RCSB Protein Data Bank (accession code 3D25).
Soluble TCR Generation and Surface Plasmon Resonance Studies.
KP7 TCR genes (TRAV41, TRAJ28; TRBV7–9, TRBJ2–1) were amplified by Rapid Amplification of cDNA ends (RACE) PCR using 3′ primers annealing to the TCR α and β constant regions. For binding studies, TCR-leucine zipper fusion protein was produced in Escherichia coli, as previously described (41). KP7 TCR preparations were purified by gel filtration immediately before use to eliminate protein aggregates. Surface plasmon resonance experiments were performed using a BIAcore 3000 in HBS-EP, as described (29). Biotinylated class I MHC complexes were immobilized by injection over streptavidin-coupled surfaces (42). For kinetic measurements, high-flow rates (50–100 μl/min) and low class I MHC immobilization levels (250–500RU) were used to eliminate mass transport effects (42). Data analysis was carried out in BIAevaluation 3.1 and Microcal Origin. The extinction coefficient of KP7 was determined by amino acid analysis to be 65860 M−1·cm−1. For more information, please see SI Text.
Supplementary Material
Acknowledgments.
We thank Dr Raimond Ravelli for help with X-ray data collection. This work was supported by Medical Research Council funding (to B.E.W) and Leukaemia Research Fund support (to P.A.H.M.) F.M. is a Biotechnology and Biological Sciences Research Council postdoctoral research fellow. M.C. is a Medical Research Council Clinician Scientist. S.T and H.S. were supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 490 E6, Z3).
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the RCSB Protein Data Bank (accession code 3D25).
This article contains supporting information online at www.pnas.org/cgi/content/full/0900411106/DCSupplemental.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz MM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 4.Marmont AM, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 5.Falkenburg JH, van de Corput L, Marijt EW, Willemze R. Minor histocompatibility antigens in human stem cell transplantation. Exp Hematol. 2003;31:743–751. doi: 10.1016/s0301-472x(03)00190-5. [DOI] [PubMed] [Google Scholar]
- 6.den Haan JM, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–1057. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 7.Kircher B, et al. Induction of HA-1-specific cytotoxic T-cell clones parallels the therapeutic effect of donor lymphocyte infusion. Br J Haematol. 2002;117:935–939. doi: 10.1046/j.1365-2141.2002.03536.x. [DOI] [PubMed] [Google Scholar]
- 8.Marijt WA, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci USA. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambach L, et al. Human cytotoxic T lymphocytes specific for a single minor histocompatibility antigen HA-1 are effective against human lymphoblastic leukaemia in NOD/scid mice. Leukemia. 2006;20:371–374. doi: 10.1038/sj.leu.2404056. [DOI] [PubMed] [Google Scholar]
- 10.Mommaas B, et al. Adult and cord blood T cells can acquire HA-1 specificity through HA-1 T-cell receptor gene transfer. Haematologica. 2005;90:1415–1421. [PubMed] [Google Scholar]
- 11.Brickner AG, et al. The immunogenicity of a new human minor histocompatibility antigen results from differential antigen processing. J Exp Med. 2001;193:195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spierings E, et al. The minor histocompatibility antigen HA-3 arises from differential proteasome-mediated cleavage of the lymphoid blast crisis (Lbc) oncoprotein. Blood. 2003;102:621–629. doi: 10.1182/blood-2003-01-0260. [DOI] [PubMed] [Google Scholar]
- 13.Dolstra H, et al. A human minor histocompatibility antigen specific for B cell acute lymphoblastic leukemia. J Exp Med. 1999;189:301–308. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce RA, et al. Cutting edge: the HLA-A*0101-restricted HY minor histocompatibility antigen originates from DFFRY and contains a cysteinylated cysteine residue as identified by a novel mass spectrometric technique. J Immunol. 1999;163:6360–6364. [PubMed] [Google Scholar]
- 15.Wang W, et al. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 16.Cox AL, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 17.Engelhard VH, Brickner AG, Zarling AL. Insights into antigen processing gained by direct analysis of the naturally processed class I MHC associated peptide repertoire. Mol Immunol. 2002;39:127–137. doi: 10.1016/s0161-5890(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 18.Tenzer S, Schild H. Assays of proteasome-dependent cleavage products. Methods Mol Biol. 2005;301:97–115. doi: 10.1385/1-59259-895-1:097. [DOI] [PubMed] [Google Scholar]
- 19.Burgevin A, et al. A detailed analysis of the murine TAP transporter substrate specificity. PLoS ONE. 2008;3:e2402. doi: 10.1371/journal.pone.0002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buus S, Sette A, Colon SM, Miles C, Grey HM. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987;235:1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- 21.Peters B, et al. The design and implementation of the immune epitope database and analysis resource. Immunogenetics. 2005;57:326–336. doi: 10.1007/s00251-005-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo RT, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994;152:3913–3924. [PubMed] [Google Scholar]
- 23.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 24.van Lochem E, van der Keur M, Mommaas AM, de Gast GC, Goulmy E. Functional expression of minor histocompatibility antigens on human peripheral blood dendritic cells and epidermal Langerhans cells. Transpl Immunol. 1996;4:151–157. doi: 10.1016/s0966-3274(96)80009-8. [DOI] [PubMed] [Google Scholar]
- 25.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 26.Goulmy E, Pool J, van den Elsen PJ. Interindividual conservation of T-cell receptor beta chain variable regions by minor histocompatibility antigen-specific HLA-A*0201-restricted cytotoxic T-cell clones. Blood. 1995;85:2478–2481. [PubMed] [Google Scholar]
- 27.Verdijk RM, et al. Exclusive TCRVbeta chain usage of ex vivo generated minor histocompatibility antigen HA-1 specific cytotoxic T cells: implications for monitoring of immunotherapy of leukemia by TCRBV spectratyping. Hematol J. 2002;3:271–275. doi: 10.1038/sj.thj.6200197. [DOI] [PubMed] [Google Scholar]
- 28.den Haan JM, Mutis T, Blokland E, AP IJ, Goulmy E. General T-cell receptor antagonists to immunomodulate HLA-A2-restricted minor histocompatibility antigen HA-1-specific T-cell responses. Blood. 2002;99:985–992. doi: 10.1182/blood.v99.3.985. [DOI] [PubMed] [Google Scholar]
- 29.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 30.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 31.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 32.Murray RJ, et al. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176:157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterloh P, et al. Proteasomes shape the repertoire of T cells participating in antigen-specific immune responses. Proc Natl Acad Sci USA. 2006;103:5042–5047. doi: 10.1073/pnas.0509256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Endert PM, et al. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity. 1994;1:491–500. doi: 10.1016/1074-7613(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 35.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography. 1992 No. 26. [Google Scholar]
- 37.Collaborative Computational Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.Brünger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 39.Roussel A, Cambillau C. TURBO-FRODO. In: Silicon Graphics, editor. Silicon Graphics Geometry Partners Directory. Mountain View, CA: Silicon Graphics; 1991. pp. 86–89. [Google Scholar]
- 40.Sheldrick GM, Schneider TR. SHELXL: high resolution refinement. Methods Enzymol. 1997;277:319–343. [PubMed] [Google Scholar]
- 41.Willcox BE, et al. Production of soluble alphabeta T-cell receptor heterodimers suitable for biophysical analysis of ligand binding. Protein Sci. 1999;8:2418–2423. doi: 10.1110/ps.8.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willcox BE, et al. TCR binding to peptide-MHC stabilizes a flexible recognition interface. Immunity. 1999;10:357–365. doi: 10.1016/s1074-7613(00)80035-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.