Abstract
The U.S. National Toxicology Program, the U.S. Environmental Protection Agency, and other national and international agencies are committing significant resources towards the development of alternative species to be used as replacements for mammalian models in toxicological studies. Caenorhabditis elegans is a well-characterized soil nematode that is becoming a useful model in the assessment of neurotoxicants. To determine the effects of potential neurotoxicants on C. elegans, four medium-throughput (feeding, growth, reproduction and locomotion) and two high-throughput (growth and reproduction) assays have been developed. Three of these assays use the COPAS Biosort, a flow cytometer capable of rapidly measuring thousands of nematodes in minutes. Medium-throughput feeding, growth, and reproduction assays were used to assess the toxicity of eight suspected neurotoxicants. For several of the neurotoxicants examined, significant effects were observed at similar concentrations between assays. High-throughput reproduction and growth assays were used to estimate the toxicity of thousands of chemicals in two libraries. These assays will prove useful in evaluating the role of alternative toxicological models in tiered toxicity testing of thousands of chemicals.
Introduction
Caenorhabditis elegans is a small (∼1.5 mm) free living soil nematode. It was originally chosen in 1963 by Sydney Brenner as a model organism to study the genetics of development and the nervous system of a simple metazoan. Today, it is likely one of the most thoroughly studied metazoans in terms of its cell biology, genetics, development, and behavior. C. elegans occur in two sexes: self-fertilizing hermaphrodites and males. Hermaphrodites contain 959 somatic cells, while the males have 1031 cells [36]. The difference in cell number is attributed to the specialized structures associated with mating and the male tail including muscles, neurons and neuronal support cells, proctodeal, and hypodermal cells [24]. Although there are a limited number of cells, C. elegans has highly differentiated digestive, reproductive, muscular, and nervous systems.
C. elegans develop from fertilized egg to gravid adult in about 3 days at 20°C [32,69]. A single hermaphrodite has the ability to produce approximately 300 offspring. Offspring mature through four larval stages, L1 - L4, growing in spurts between stages after molting old cuticles [14,41]. At two points in the lifecycle, if food is not available, nematode growth will arrest. Animals will survive for several days as starved hatchlings that arrest as L1's, or for several months when starved L2's develop into dauer larvae [42,52].
There is a wealth of knowledge available on C. elegans including its complete genomic sequence [59], the technology to quickly produce transgenic nematodes [44], and the ability to observe all of the cells in the living nematode by microscopy [32,65,66]. The available microscopy techniques in C. elegans have led to the development of an exceptionally detailed database on C. elegans anatomy, as well as on cell and developmental biology [2,32]. This database maps the development of every cell from the fertilized egg to the reproductive adult [57] and all of the neuronal pathways and connections [66].
A detailed map of the complete C. elegans nervous system, consisting of the physical location of all of the hermaphrodite neurons and all neuronal connections (neuron-neuron and neuromuscular), was originally described in 1986 by White, et al in “The Mind of the Worm” [66]. The adult C. elegans hermaphrodite contains 302 neurons in two independent nervous systems: 282 in the somatic nervous system and 20 in the pharyngeal nervous system [56,57,66]. There are approximately 6500 chemical synapses, 900 gap junctions, and 1500 neuromuscular junctions [32]. The pharyngeal nervous system is completely contained within the C. elegans pharynx (Figure 1, inset), with only four synapses between the pharyngeal and somatic nervous systems [1,8]. These nervous systems control locomotion, feeding, defecation, reproduction, and environmental sensing. C. elegans has the ability to sense differences in temperature, chemicals, odorants, and food. The male nervous system contains 473 neurons with the additional neurons involved in mating behavior [22,23,55]. Detailed descriptions of the C. elegans neuroanatomy can be found in [32], [66] and [2].
Figure 1. Effects of chlorpyrifos oxon on C. elegans feeding.
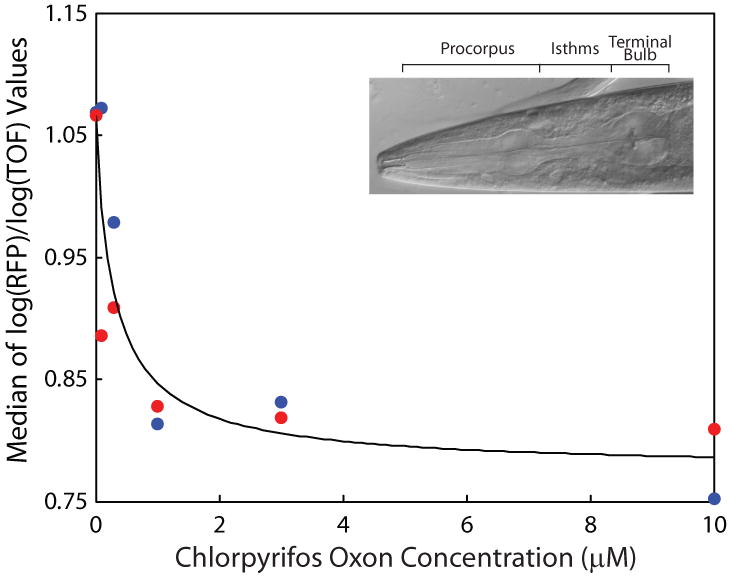
For each of two replicate experiments (shown as blue and red), groups of 25 adult C. elegans were exposed to one of five concentrations of chlorpyrifos oxon (plus controls) for 24 h. Medians of log(RFP)/log(TOF) values are plotted and the data fit to the Hill equation. inset, adult C. elegans pharynx.
One of the strengths of C. elegans as a model organism is the high degree of evolutionary conservation in its biological processes. In addition to evolutionary conservation in basic biological functions, such as intermediate metabolism, DNA, RNA and protein synthesis; orthologs for many of the proteins and regulatory pathways induced as part of the “stress response” in vertebrates have been identified in C. elegans [27,29,34,53,64,68].
In terms of neuropharmacology, C. elegans express many of the neurotransmitters and associated receptors that are found in higher eukaryotes. These include dopamine, acetylcholine, γ-aminobutyric acid (GABA), glutamate, and serotonin. In addition, several neuropeptides encoding genes have been identified including those encoding insulin-like, FMRFamide (Phe-Met-Arg-Phe-NH2)-like neuropeptides. Many of the neurons that express specific neurotransmitters have been identified [50].
Tools in robotics, image acquisition and analysis, gene knockout, and gene and protein expression measurements make it possible to study complex biological processes in C. elegans in medium- and high-throughput fashions. The defined nervous system, evolutionary conservation in neuronal structure and function, and the ability to utilize medium- and high-throughput technologies make C. elegans an excellent organism for the rapid detection of potential neurotoxicants in a non-mammalian organism.
Medium-Throughput Screening Assays
We have developed several semi-automated, medium-throughput assays that can assess the toxicity of approximately five compounds per week. These assays allow for the quantification of the effects of chemicals on growth and development, reproduction, feeding, and locomotion at different developmental stages. Feeding and locomotion, measured using adult nematodes, are two endpoints that directly assess neurotoxicity. Growth and development from C. elegans L1 larvae through 48 h post-hatch provides an indirect measurement of neurotoxicity. Reproduction, measured using young adult animals, provides an indication of the effects of a potential toxicant on development and indirectly, the neuromuscular activity of egg-laying.
Feeding, reproduction, and growth assays utilize the COPAS Biosort, a flow cytometer capable of sorting, dispensing, and measuring size and fluorescence parameters of individual nematodes [47]. In the Biosort, nematodes pass through a flow cell where axial length, extinction, and fluorescence are simultaneously measured using a pair of lasers. Axial length, or time of flight (TOF), is the length of time that a nematode interrupts the light beam. Extinction (EXT), an estimate of the nematode's optical density, is the amount of light that is blocked as a nematode passes in front of the laser. Both TOF and EXT are indicators of the size of an individual nematode, which increase as nematodes develop through larval stages into adults [47].
In these assays, between five and several hundred C. elegans are exposed to toxicants in liquid media [3] in 96-well plates. In addition to buffer and toxicant, each well contains E. coli OP50 as a food source [54]. After incubating nematodes at 20°C for 4 - 48 h, depending on the assay, they are aspirated from the wells and their behavior (feeding, locomotion) or size characteristics (growth, reproduction) are measured either by the Biosort or by microscopy. The following sections provide details and examples for each assay.
Feeding
Feeding in C. elegans is accomplished by the coordinated pumping of three sections of the pharynx (Figure 1, inset), which simultaneously push bacteria into the intestine and expel excess liquid out of the mouth [7]. The neuromuscular activity of feeding is regulated by a self-contained nervous system [1]. Feeding activity is affected by internal and external factors, including nutritional status and exogenous application of chemicals [6,37]. Feeding phenotypes have been described by directly observing and manually counting pharyngeal contractions of individual nematodes by microscopy [5], or by recording action potentials of pharyngeal muscles [48]. These methods provide useful information but, for practical reasons, only on few animals per test substance can be evaluated. In addition, these methods require specialized technical experience. As such they are not applicable towards the examination of the differential effects of chemical exposures on pharyngeal activity, in which many more measurements are needed to obtain sufficient statistical power for proper analyses.
We developed a feeding assay capable of measuring hundreds of individual nematodes in minutes using the Biosort [13]. In this assay, 25 synchronized adult nematodes (3d post-hatch) [54] are dispensed into each well of a 96-well plate containing varying concentrations of test toxicant. Following an incubation period of 24 h, red fluorescent microspheres (0.5 μm) are added to each well and nematodes are allowed to feed for 15 min. The amount of accumulated microspheres is determined from the level of fluorescence in each nematode. In addition to fluorescence, nematode sizes (TOF and EXT) are recorded. The feeding activity can then be expressed as size-corrected red fluorescence for cohorts of nematodes [13]. To model decreasing feeding activity with increasing dose, the Hill expression [35] , is subtracted from Vm+a0. EC50s are calculated by fitting the medians of the log(red fluorescence)/log(TOF) values for each cohort to the decreasing Hill equation as a function of dose (Figure 1):
| (eq. 1) |
Here, Vm+a0 denotes the red fluorescence for the non-treated nematodes and is the maximum value the expression can have. The value of the expression decreases to the lower asymptote, a0, as toxicant concentration increases. The lower asymptote represents a maximum possible decrease in red fluorescence. For the neurotoxicants and concentrations presented in this review, a0 ranged from 18% (chlorpyrifos) to 36% (cocaine base) of Vm+a0. The parameter, km, is the estimated concentration that reduces the red fluorescence to halfway between Vm+a0 and a0, which is, the EC50. Finally x is the shape parameter that governs the steepness of the decrease.
The effect of varying concentrations of chlorpyrifos oxon on feeding is presented in Figure 1. Here, the EC50 is estimated as 0.31 μM. A similar approach was used to test the effects of seven other known developmental neurotoxicants on C. elegans feeding (Table A). The EC50s for these neurotoxicants range from ∼0.14 μM (chlorpyrifos) to ∼122 μM (cadmium chloride).
Table A. Toxicity of Eight Developmental Neurotoxicants on C. elegans Feeding and Reproduction.
| Toxicant | EC50 (μM) | |
|---|---|---|
| Feeding | Reproduction | |
| chlorpyrifos | 0.141
(0.051, 0.23)a |
0.09
(0, 0.23) |
|
| ||
| chlorpyrifos oxon | 0.31
(0, 0.81) |
0.28
(0, 1700) |
|
| ||
| hexachlorophene | 16.6
(8.1, 25) |
6.3
(4.0, 8.5) |
|
| ||
| chlordiazepoxide | 68.6
(0, 290) |
49.9
(23, 77) |
|
| ||
| cocaine base | 94.5
(0, 5800) |
4.2
(2.5, 6.0) |
|
| ||
| methyl mercury | 112
(0, 270) |
1.42
(0.75, 2.1) |
|
| ||
| cadmium chloride | 122
(0, 406) |
18.0
(14, 22) |
|
| ||
| tebuconazol | 180
(76, 280) |
46.7
(11, 82) |
EC50, effective concentration that reduces feeding or reproduction activity to half the maximum activity
95% confidence interval
Reproduction
A common phenotype observed after exposure to chemicals or as a result of genetic mutations is a change in the number of offspring produced by a single nematode. The production of offspring may be affected at many stages including oocyte and sperm formation, fertilization, embryonic development, or egg-laying. Egg-laying behavior is regulated internally by signals to the vulva muscle (Figure 2, inset), which can be disrupted by environmental conditions such as exposure to chemicals [10,61,63]. C. elegans reproduction is routinely measured by manually counting the number of offspring as a function of time [58]. An alternative assay was developed that measures the amount of chitinase, a molecule released after egg hatching. This is an indirect measure of reproduction, but provides information on oocyte fertilization and ability to hatch [40].
Figure 2. Effects of hexachlorophene on C. elegans reproduction.
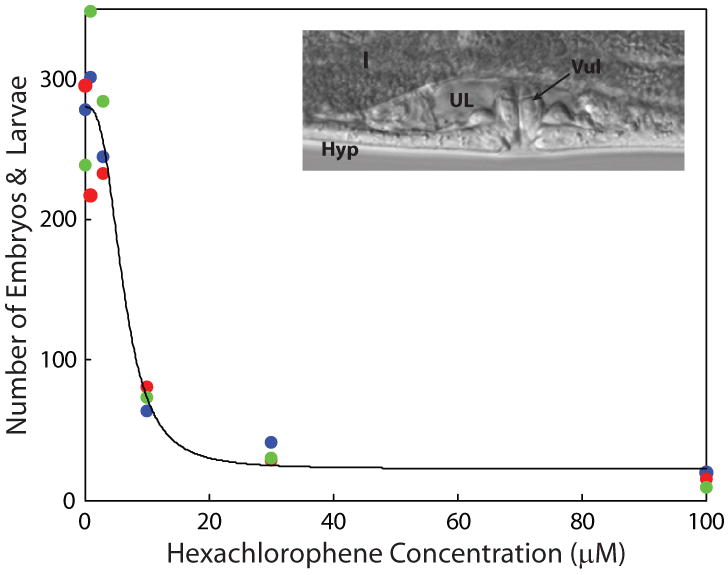
For each of three replicate experiments (shown as blue, red, and green), groups of five L4 nematodes were exposed to one of five concentrations of hexachlorophene for 48 h. The numbers of observations (larvae and embryos) were fit to the Hill equation. inset, region around an L4 C. elegans vulva: I, intestine; UL, uterine lumen; Hyp, hypodermis; Vul, vulva.
We developed a reproduction assay that uses the Biosort to directly count the number of offspring produced. In this assay, five age-synchronized L4 larvae are placed into each well of a 96-well plate containing varying concentrations of neurotoxicants. After incubating for 48 h, the size and stage of development of the adults and offspring are measured. The number of larvae produced during this time period is an indication of the effect of the toxicant on nematode fecundity. The numbers of nematodes are directly fit to the Hill expression used to analyze the feeding assay (eq. (1)). Over all assays, the maximum number of control offspring ranged from 268 to 318 per replicate.
The effect of varying concentrations of hexachlorophene on reproduction is presented in Figure 2. The EC50 is estimated as 6.3 μM. The effect of eight known developmental neurotoxicants on C. elegans reproduction was determined and EC50 values estimated using equation 1 (Table A). The rank order of EC50s in the reproduction assay was similar to those observed in the feeding assay with chlorpyrifos, chlorpyrifos oxon, and methyl mercury being the most toxic and cadmium chloride, chlordiazepoxide, and tebuconazol being the least toxic.
Growth
We developed a 48-h growth assay to measure nematode growth rates at several stages of development. In this assay, 50 synchronized L1 larvae are dispensed into each well of a 96-well plate containing varying concentrations of toxicant. Following incubation for 48 h at 20°C, the time necessary for untreated nematodes to develop to the final larval stage (L4), the size distribution of nematodes in each well is determined by measuring TOF and EXT.
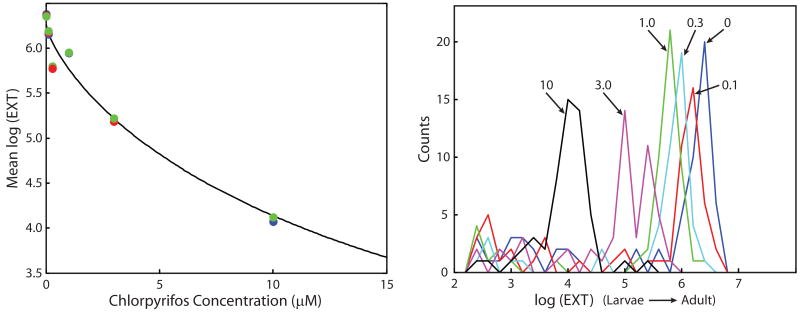
Using the log(EXT) measurements, growth is defined as the change in the distribution of sizes of nematodes measured when first loaded into the plates, compared to the distribution of sizes when measured after 48 h. Because the initial measurement on a specific nematode can not be matched with its final measurement, our method models the growth distribution of the initial cohort of nematodes. Briefly, the range of log(EXT) is divided into a number of bins. Each nematode is assumed to advance from its initial bin to larger log(EXT) measurement according to a uniform probability model. A weighted mixture of the lognormal distribution and the modeled nematode distribution is fit to the observed measurements. Using the estimated number of bins advanced, values are derived for the mean size (log(EXT)) and growth rates in terms of log(EXT)/h at each toxicant concentration. To estimate EC50 values, the decreasing Hill expression (eq. (1)) is fit as a function of toxicant concentration to both the estimated means and estimated growth rates. In cases where estimated means and growth rates are both observed at the lower asymptote, the estimated EC50s are very similar (Table B). Figure 3 illustrates the effects of 48 h exposures on the mean log(EXT) values at six concentrations of chlorpyrifos on C. elegans development. Based on the analysis of estimated log(EXT) means, an EC50 of 22.9 μM was calculated. For the growth assay, chlorpyrifos and chlorpyrifos oxon were the most toxic, while cadmium chloride and tebuconazol were the least (Table B).
Table B. Effects of Eight Developmental Neurotoxicants on C. elegans Growth.
| Toxicant | EC50 (μM) | |
|---|---|---|
| Means | Growth rates | |
| chlorpyrifos | 22.9
(13, 33)a |
3.9
(2.4, 5.4) |
|
| ||
| chlorpyrifos oxon | 8.8
(0, 65) |
5.0
(3.3, 6.7) |
|
| ||
| hexachlorophene | 18.2
(12, 24) |
19.3
(16, 23) |
|
| ||
| chlordiazepoxide | 64.8
(39, 91) |
66.3
(28, 105) |
|
| ||
| cocaine base | 34.6
(0, 86) |
72
(0, 180) |
|
| ||
| methyl mercury | 11.4
(10, 13) |
11.2
(9.5, 13) |
|
| ||
| cadmium chloride | 1131
(1097, 1166) |
502
(447, 557) |
|
| ||
| tebuconazol | 481
(284, 677) |
241
(179, 302) |
EC50, effective concentration that reduces mean growth or growth rate to half the maximum
95% confidence interval
Figure 3. Effects of chlorpyrifos on C. elegans growth.
For each of three replicate experiments (shown as blue, red, and green), groups of 50 L1 nematodes were exposed to one of five concentrations of chlorpyrifos for 48 h. Left panel, observations measured and estimated means fit to Hill equation. Right panel, modeled frequency histograms showing the effects of different concentrations of chlorpyrifos (0, 0.1, 0.3, 1.0, 3.0, and 10 μM) on nematode size distribution (log(EXT)) for one of the replicates.
Motion tracking
C. elegans locomotion is regulated by the same neurotransmitters as in mammals, with acetylcholine being the primary excitatory neurotransmitter [51]. C. elegans locomotive behavior has been studied on solid, agar surfaces and in liquid medium [17]. On solid surfaces, nematodes move forward or backward by bending the body, making sinusoidal waves [11].
Abnormal locomotive behavior, referred to as uncoordinated movement, is a commonly observed phenotype in C. elegans behavioral assays. Characterization of C. elegans movement has historically been made qualitatively by visual examination. Recently, different locomotive behaviors have been categorized using computer-aided visualization techniques. In these studies, single nematodes are observed using microscopes equipped with automated tracking systems [9,18,26,28,33,38]. Once identified, the single nematode can be tracked for several minutes to hours. Analysis of the nematodes can be quantified by over 60 movement parameters.
Another approach is to track tens to hundreds of nematodes simultaneously at low magnification [67]. Centroids of multiple nematodes are tracked using dark-field microscopy, where C. elegans appear as bright objects. The computer then tracks nematode movement in real time. Using this system, the effects of metals, ethanol, solvents, and organophosphate and carbamate pesticide exposures on movement have been quantified [3,4,12,15,20,30,45].
In our assay, we use a myo-2∷GFP strain of C. elegans (CB5584), which expresses high levels of green fluorescent protein in pharyngeal muscles. Motion tracking is performed on an inverted fluorescence microscope equipped with a CCD camera, motorized stage and an incubator; which maintains constant temperature and humidity throughout the image capture process. In this system, computer tracking monitors head movement rather than the centroid of the nematode. After exposing L4 C. elegans to neurotoxicants for 4 h, nematodes are transferred to agar pads. Each agar pad holds approximately 40 animals, which are tracked at low magnification for ∼35s (3.5 frames/sec). Motion tracking software then generates Computer Assisted Sperm Analysis-like statistics on each nematode [19,62], which can then be compared between treated and untreated nematodes. Figure 4 illustrates the effects of methyl mercury on C. elegans behavior after a 4 h exposure. Fitting the Hill equation (eq. (1)) to the average velocity yields an estimated EC50 of 10.0 μm/s. A similar analysis was performed on C. elegans exposed to chlorpyrifos with an estimated EC50 of 1.1 μm/s. Similar to feeding and reproduction, chlorpyrifos was more toxic than methyl mercury.
Figure 4. Effects of methyl mercury on C. elegans movement.
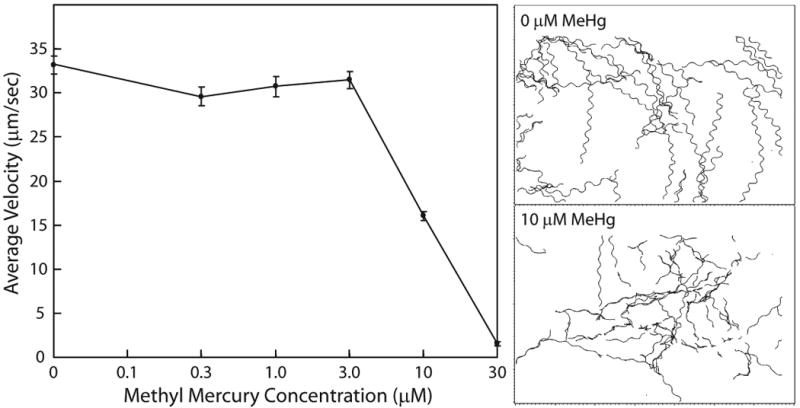
Groups of 40 L4 nematodes were tracked after exposure to one of five concentrations of methyl mercury for 4 h. Left panel, average velocity of C. elegans motion, mean ± s.e. Right panel, computer generated tracks for control nematodes (top) and those exposed to 10 μM methyl mercury (bottom).
High-Throughput Screening Assays
We have tested both reproduction and growth assays as high-throughput screening tools to estimate the potential toxicities using two chemical libraries: the National Toxicology Program's (NTP's) 1408 [70] and the EPA's ToxCast 320 pesticides actives [21]. Working stock solutions of the NTP or EPA chemical libraries were prepared in 96-well plates and dissolved in DMSO. Multiple 96-well plates with varying concentrations were prepared for each set of chemicals. For each plate, eight chemicals were diluted to the desired concentration in 1% DMSO for exposure plates and were replicated in three treatment wells per chemical at a single concentration. Each plate also contained four vehicle control wells and four positive control wells (1 and 4 % DMSO, respectively). Remaining wells were either rinse wells, to avoid carryover of nematodes between wells, or were empty.
Within the NTP library of 1408 chemicals, approximately 20% caused significant decreases in 48-h reproduction, compared to controls. However, the variability in the number of L4's loaded and the inherent biological variability of egg production and egg-laying led to wide variability in control groups.
In contrast, the 48-h growth assay showed much less variable from plate-to-plate and therefore the data were more consistent. The growth of control populations does vary from day-to-day, however, the change in log(EXT) is predictable over the 48-h exposure period. The 48-h growth assay was used to screen the EPA's ToxCast 320 at a single concentration of 200 μM. For approximately half of the chemicals, nematode growth was clearly reduced, while another quarter of the chemicals did not affect growth relative to control groups. Analytical techniques are being developed to determine the relative toxicity of the remaining chemicals that were not clearly positive or negative.
Discussion
Four medium- and two high-throughput assays are described that measure the toxicity of potential neurotoxicants using C. elegans as an alternative to mammalian model organisms. The sensitivity of the four endpoints (growth, feeding, reproduction, and locomotion) varied between chemicals. Similar EC50s were estimated for effects on feeding, reproduction, and growth for chlordiazepoxide and hexachlorophene (Tables A, B). Cocaine, cadmium chloride, and tebuconazol affected reproduction more than feeding and growth, while chlorpyrifos and chlorpyrifos oxon caused greater affects on feeding and reproduction than growth at similar concentrations. Locomotion after methyl mercury exposure was also affected at similar concentrations as reproduction and growth (Tables A, B and Fig. 4). Differences in EC50 values between endpoints may be a consequence of the mechanism of action of the neurotoxicant. For example, a neurotoxicant that specifically targets neuromuscular activity may have similar EC50s for feeding, motion and reproduction, but not growth. In contrast, a toxicant that affects cell differentiation or development may significantly inhibit growth and reproduction without affecting feeding or locomotion.
In the development of any medium- or high-throughput in vivo assay, classification of chemical toxicity is usually based on comparing treated individuals with animals under non-exposed or control conditions. Therefore, the assay with lower control variability is considered to be more reliable. Feeding, reproduction, and growth were examined as potential endpoints in high-throughput screening protocols. Feeding behavior varies greatly between isogenic individuals and is strongly influenced by environmental factors such as food availability and temperature [49]. Although environmental conditions are carefully controlled in all experiments, control feeding activity varies across experimental days and a large range of feeding activities are observed in control populations within the same day [13].
Variability between wells within a plate, plates within a day, and day-to-day was observed in the reproduction assay. Some of this variability is due to technical limitations of the COPAS Biosort. The Biosort dispenses nematodes into wells with ∼90% accuracy (Union Biometrica, personal communications). However, slight inaccuracies in the number of nematodes dispensed into wells can result in a large variability in the numbers of offspring within a well.
Day-to-day variability is also routinely observed in data from the 48-h growth assay. Some of this variability can be attributed to differences in individual growth rates. However, overall, the 48-h growth assay has the lowest variability among controls in the three assays. For this reason, the 48-h growth assay was selected for future high-throughput screening of chemical libraries.
Comparability to mammalian neurotoxicants
Many technical and statistical challenges in using C. elegans as an alternative test organism for in vivo medium- and high-throughput screening have been met. The next challenge is to use the data obtained using C. elegans to help in the prediction of toxicity in humans. To meet this challenge, the results obtained using C. elegans will have to be linked to data obtained using laboratory rodents and, where possible, from humans. Several studies have already demonstrated that changes in C. elegans following chemical exposures appear to be predictive of developmental shifts and/or neurological damage seen in laboratory studies using rodents [3,20,25,31,39,43,46,60,67]. Linking cell-based high-throughput screening data to in vivo screening results (C. elegans, Drosophila, zebrafish) to inform on the toxicity of different agents in humans is the ultimate goal of a collaborative research program established by the NTP, the EPA, and the National Institutes of Health Chemical Genomics Center (NCGC) [16].
Acknowledgments
The authors would like to thank Julie Rice and Daniel Snyder for technical assistance in generating the data presented in this manuscript. We would also like to thank Brooke Tvermoes for the C. elegans images. This work was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, and the National Toxicology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 2.Altun ZF, Hall DH. WormAtlas. 20022006 [Google Scholar]
- 3.Anderson GL, Boyd WA, Williams PL. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem. 2001;20:833–8. [PubMed] [Google Scholar]
- 4.Anderson GL, Cole RD, Williams PL. Assessing behavioral toxicity with Caenorhabditis elegans. Environ Toxicol Chem. 2004;23:1235–40. doi: 10.1897/03-264. [DOI] [PubMed] [Google Scholar]
- 5.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–70. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- 7.Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441–57. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avery L, Thomas JH. Feeding and defecation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor Laboratory Press; Plainview, NY: 1997. pp. 679–716. [PubMed] [Google Scholar]
- 9.Baek JH, Cosman P, Feng Z, Silver J, Schafer WR. Using machine vision to analyze and classify Caenorhabditis elegans behavioral phenotypes quantitatively. J Neurosci Methods. 2002;118:9–21. doi: 10.1016/s0165-0270(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 10.Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–9. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bargmann CI. Genetic and cellular analysis of behavior in C. elegans. Annual review of neuroscience. 1993;16:47–71. doi: 10.1146/annurev.ne.16.030193.000403. [DOI] [PubMed] [Google Scholar]
- 12.Boyd WA, Cole RD, Anderson GL, Williams PL. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem. 2003;22:3049–55. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- 13.Boyd WA, McBride SJ, Freedman JH. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: Evaluation of a novel, high-throughput screening assay. PLoS ONE. 2007 doi: 10.1371/journal.pone.0001259. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byerly L, Cassada RC, Russell RL. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol. 1976;51:23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- 15.Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 2004;194:248–56. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319:906–7. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croll NA. Components and Patterns in Behavior of Nematode Caenorhabditis Elegans. J Zoology. 1975;176:159–176. [Google Scholar]
- 18.Cronin CJ, Mendel JE, Mukhtar S, Kim YM, Stirbl RC, Bruck J, Sternberg PW. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 2005;6:5. doi: 10.1186/1471-2156-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis RO, Katz DF. Computer-aided sperm analysis (CASA): image digitization and processing. Biomaterials, artificial cells, and artificial organs. 1989;17:93–116. doi: 10.3109/10731198909118272. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan R, Dusenbery DB, Williams PL. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematode Caenorhabditis elegans. J Toxicol Environ Health A. 1999;58:451–62. doi: 10.1080/009841099157179. [DOI] [PubMed] [Google Scholar]
- 21.Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95:5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- 22.Emmons SW. Male development. WormBook. 2005:1–22. doi: 10.1895/wormbook.1.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emmons SW, Lipton J. Genetic basis of male sexual behavior. J Neurobiol. 2003;54:93–110. doi: 10.1002/neu.10163. [DOI] [PubMed] [Google Scholar]
- 24.Emmons SW, Sternberg PW. Male Development and Behavior. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor Laboratory Press; Plainview, N.Y.: 1997. p. 295. [PubMed] [Google Scholar]
- 25.Faber PW, Voisine C, King DC, Bates EA, Hart AC. Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:17131–6. doi: 10.1073/pnas.262544899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Z, Cronin CJ, Wittig JH, Jr, Sternberg PW, Schafer WR. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics. 2004;5:115. doi: 10.1186/1471-2105-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman JH, Slice LW, Dixon D, Fire A, Rubin CS. The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J Biol Chem. 1993;268:2554–2564. [PubMed] [Google Scholar]
- 28.Geng W, Cosman P, Berry CC, Feng Z, Schafer WR. Automatic tracking, feature extraction and classification of C elegans phenotypes. IEEE transactions on bio-medical engineering. 2004;51:1811–20. doi: 10.1109/TBME.2004.831532. [DOI] [PubMed] [Google Scholar]
- 29.Giglio AM, Hunter T, Bannister JV, Bannister WH, Hunter G. The copper/zinc superoxide dismutase gene of Caenorhabditis elegans. Biochem Mol Biol Int. 1994;33:41–44. [PubMed] [Google Scholar]
- 30.Graves AL, Boyd WA, Williams PL. Using transgenic Caenorhabditis elegans in soil toxicity testing. Arch Environ Contam Toxicol. 2005;48:490–4. doi: 10.1007/s00244-004-0031-2. [DOI] [PubMed] [Google Scholar]
- 31.Guven K, Power RS, Avramides S, Allender R, de Pomerai DI. The toxicity of dithiocarbamate fungicides to soil nematodes, assessed using a stress-inducible transgenic strain of Caenorhabditis elegans. J Biochem Mol Toxicol. 1999;13:324–33. doi: 10.1002/(sici)1099-0461(1999)13:6<324::aid-jbt6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Hall DH, Altun ZF. C elegans atlas. Editoin. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2008. [Google Scholar]
- 33.Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol. 2001;49:303–13. doi: 10.1002/neu.10014. [DOI] [PubMed] [Google Scholar]
- 34.Heschl MF, Baillie DL. The HSP70 multigene family of Caenorhabditis elegans. Comp Biochem Physiol [B] 1990;96:633–637. doi: 10.1016/0305-0491(90)90206-9. [DOI] [PubMed] [Google Scholar]
- 35.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (London) 1910;40:4–7. [Google Scholar]
- 36.Hodgkin J. Sexual Dimorphism and Sex Determination. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 243–279. [Google Scholar]
- 37.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–4. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 38.Hoshi K, Shingai R. Computer-driven automatic identification of locomotion states in Caenorhabditis elegans. J Neurosci Methods. 2006;157:355–63. doi: 10.1016/j.jneumeth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Hoss S, Henschel T, Haitzer M, Traunspurger W, Steinberg CE. Toxicity of cadmium to Caenorhabditis elegans (Nematoda) in whole sediment and pore water--the ambiguous role of organic matter. Environ Toxicol Chem. 2001;20:2794–801. [PubMed] [Google Scholar]
- 40.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–98. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 41.Knight CG, Patel MN, Azevedo RB, Leroi AM. A novel mode of ecdysozoan growth in Caenorhabditis elegans. Evolution & development. 2002;4:16–27. doi: 10.1046/j.1525-142x.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JA, Fleming JT. Basic culture methods. Methods Cell Biol. 1995;48:3–29. [PubMed] [Google Scholar]
- 43.Marroquin LD, Elyassnia D, Griffitts JS, Feitelson JS, Aroian RV. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics. 2000;155:1693–9. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- 45.Melstrom PC, Williams PL. Reversible AChE inhibitors in C. elegans vs. rats, mice. Biochem Biophys Res Commun. 2007;357:200–5. doi: 10.1016/j.bbrc.2007.03.122. [DOI] [PubMed] [Google Scholar]
- 46.Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:3264–9. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol Biol. 2006;351:275–86. doi: 10.1385/1-59745-151-7:275. [DOI] [PubMed] [Google Scholar]
- 48.Raizen DM, Avery L. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron. 1994;12:483–95. doi: 10.1016/0896-6273(94)90207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–82. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rand BR, Nonet ML. Neurontransmitter Assignment for Specific Neurons. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor Laboratory Press; Plainview, N.Y.: 1997. pp. 1049–1052. [Google Scholar]
- 51.Rand BR, Nonet ML. Synaptic Transmission. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor Laboratory Press; Plainview, N.Y.: 1997. pp. 611–643. [PubMed] [Google Scholar]
- 52.Riddle DL. The Dauer Larva. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 393–412. [Google Scholar]
- 53.Stringham EG, Jones D, Candido EP. Expression of the polyubiquitin-encoding gene (ubq-1) in transgenic Caenorhabditis elegans. Gene. 1992;113:165–173. doi: 10.1016/0378-1119(92)90392-3. [DOI] [PubMed] [Google Scholar]
- 54.Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 587–606. [Google Scholar]
- 55.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–76. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 56.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 57.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 58.Swain SC, Keusekotten K, Baumeister R, Sturzenbaum SR. C. elegans metallothioneins: new insights into the phenotypic effects of cadmium toxicosis. J Mol Biol. 2004;341:951–59. doi: 10.1016/j.jmb.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 59.The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2028. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 60.Treinin M, Chalfie M. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron. 1995;14:871–7. doi: 10.1016/0896-6273(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 61.Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–47. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vantman D, Koukoulis G, Dennison L, Zinaman M, Sherins RJ. Computer-assisted semen analysis: evaluation of method and assessment of the influence of sperm concentration on linear velocity determination. Fertil Steril. 1988;49:510–5. doi: 10.1016/s0015-0282(16)59782-9. [DOI] [PubMed] [Google Scholar]
- 63.Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–85. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weston K, Yochem J, Greenwald I. A Caenorhabditis elegans cDNA that encodes a product resembling the rat glutathione S-transferase P subunit. Nucleic Acids Res. 1989;17:2138. doi: 10.1093/nar/17.5.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White J. The Anatomy. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. pp. 81–122. [Google Scholar]
- 66.White JG, Southgate J, Thomson JN, Brenner FRS. The Structure of the nervous system of the nNematode Caenorhabditis elegans. Phil Trans Royal Soc London Series B, Biol Scien. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 67.Williams PL, Dusenbery DB. A promising indicator of neurobehavioral toxicity using the nematode Caenorhabditis elegans and computer tracking. Toxicol Ind Health. 1990;6:425–40. doi: 10.1177/074823379000600306. [DOI] [PubMed] [Google Scholar]
- 68.Wolf M, Nunes F, Henkel A, Heinick A, Paul RJ. The MAP kinase JNK-1 of Caenorhabditis elegans: location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J Cell Physiol. 2008;214:721–9. doi: 10.1002/jcp.21269. [DOI] [PubMed] [Google Scholar]
- 69.Wood WB. Introduction to C. elegans Biology. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1988. pp. 1–16. [Google Scholar]
- 70.Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, et al. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect. 2008;116:284–91. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]