Abstract
The molecular events mediating the complex interaction between exercise and cognition are not well-understood. Although many aspects of the signal transduction pathways mediate exercise induced improvement in cognition are understood, little is known about the molecular events interrelating physiological stress with synaptic proteins, following physical exercise. Small heat shock proteins (sHSP), HSP27 and α-B-crystallin are co-localized to synapses and astrocytes, but their role in the brain is not well-undersood. We investigated whether their levels in the hippocampus were modulated by exercise, using a well characterized voluntary exercise paradigm. Since sHSP are known to be regulated by many intracellular signaling molecules in other cells types outside the brain, we investigated whether similar regulation may be playing a role in the brain by measuring protein kinase B (PKB/Akt), pGSK3 and the mitogen activated protein (MAP) kinases, p38, phospho-extracellular signal-regulated kinase (pERK) and phospho-c-Jun kinase (pJNK). Results demonstrated exercise-dependent increases in HSP27 and α-B-crystallin levels. We observed that increases in sHSP coincided with robust elevations in the presynaptic protein, SNAP25 and the post-synaptic proteins NR2b and PSD95. Exercise had a differential impact on kinases, significantly reducing pAkt and pERK, while increasing p38 MAPK. In conclusion, we demonstrate four early novel hippocampal responses to exercise that have not been identified previously: the induction of (1) sHSPs (2) the synaptic proteins SNAP-25, NR2b, and PSD-95, (3) the MAP kinase p38 and (4) the immediate early gene product MKP1. We speculate that sHSP may play a role in synaptic plasticity in response to exercise.
Keywords: Western, HSP27, MAP kinases, Akt, MKP1, p38, exercise, post-synaptic protein
2. Introduction
Exercise has been shown to have benefits on cognitive function in humans (Angevaren et al., 2008; Kramer et al., 2006; Lautenschlager et al., 2008; Suominen-Troyer et al., 1986)and in rodents (Fordyce and Farrar, 1991; van Praag et al., 1999; van Praag, 2008; Vaynman et al., 2004). The beneficial impact of exercise on cognition is complex, depending on not only the intensity and duration, but also on the health of the animal. The complex relation between exercise and cognition has been compared to a concept in cellular biology, hormesis, described in 1854 by Virchow, relating to U-shaped dose-response curves, where low doses are stimulatory and high doses inhibitory (Calabrese, 2008).
Several steps in the molecular cascade(s), connecting exercise to cognition have been elucidated, using voluntary or mild exercise paradigms that improve cognition. Within 30 minutes after acute mild exercise, there is an induction of early immediate genes c-fos (Lee et al., 2003) in the hippocampus. BDNF is also induced early (Soya et al., 2007). Mechanistic studies have identified a role for IGF-1 (Ding et al., 2006a), calmodulin kinase II (CAMKII) (Vaynman et al., 2007), and the BDNF receptor trkB (Vaynman et al., 2004), but have raised questions that other factors may also play a role.
Other factors may play a role in the acute (adaptive) stress response to increased metabolic demand, which may also impact memory. The typical non-specific cellular response to stressors like environment, hyperthermia, toxins, infection, oxidative stress, or corticosteroids is known as the heat shock response. Voluntary exercise induces only transient increases of corticosteroids, while exhaustive forced exercise, causes sustained elevations in corticosteroids (Droste et al., 2003; Stranahan et al., 2008). Data from exercise studies suggests that long term or intense exercise may induce many heat shock proteins such as HSP70 and HSP72 (Horowitz and Robinson, 2007; Lancaster et al., 2004; Sumitani et al., 2002; Walters et al., 1998), HSP60 and HSP8 (hsc 71) (Ding et al., 2006b). There are no reports on small heat shock proteins (sHSP), which are part of the heat shock gene superfamily. There are 10 members of sHSP with their monomeric molecular mass of 15-42 kDa (Fontaine et al., 2003), and they share a common C-terminal motif, α-crystallin domain; however, their role in the CNS is poorly understood. The HSP27 and α-B-crystallin are expressed ubiquitously and are stress inducible. Under physiological conditions, their protein level in most tissues is low but levels can increase after heat shock, radiation, and oxidative stress. In the brain, α-B-crystallin is expressed primarily in oligodendrocytes and to a lesser degree in astrocytes, and play a role in protection of intermediate filament systems from abnormal aggregation (Iwaki et al., 1990). In retinal ganglion cells administration of BDNF suppresses HSP27 (Krueger-Naug et al., 2000).
HSPs appear to be induced via highly regulated signaling cascades, including the three major mitogen-activated protein kinases (MAPK) and protein kinase B (PKB/Akt) (Nadeau and Landry, 2007; van Ginneken et al., 2006; Wigmore et al., 2007). Both pathways can similarly be impacted by exercise (Chen and Russo-Neustadt, 2005; Shen et al., 2001). The MAPK element p38 has been reported to play a role in sHSP phosphorylation (Ito et al., 2005; Maizels et al., 1998), but the impact of exercise on p38 in the brain has not been evaluated. Although the association between sHSP and MAPK in the brain is poorly understood, the MAPK pERK protein (Muller et al., 2007; Shen et al., 2001) and mRNA (Molteni et al., 2002) are induced in the hippocampus by voluntary exercise, which may also impact sHSP.
In this study, we utilized our well-characterized seven day voluntary exercise paradigm, which has been shown to impact synaptic plasticity and cognitive function in rodents (Vaynman et al., 2006), to investigate the impact of exercise on sHSP and to explore their temporal interrelationship with synaptic proteins and the kinases thought to regulate sHSP in the hippocampus. Our intention is to provide a basic framework to start analyzing the relationship between adaptive stress and synaptic plasticity in response to exercise.
3. Results
3.1. Heat Shock response to exercise
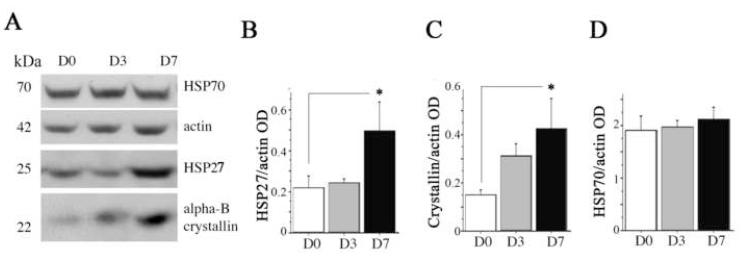
We first examined the overall impact of exercise on three heat shock proteins in the hippocampus that have been identified in the CNS: HSP27, α-B-crystallin and HSP70. We chose HSP70 because the levels of mRNA in the hippocampus were increased after exercise (Molteni et al., 2002). We chose HSP27 and α-B-crystallin, because of their susceptibility to be induced in the hippocampus by stressors (Akbar et al., 2001; Imura et al., 1999). Western blot analysis of HSP after β-actin normalization showed that 3 days of exercise promoted an increasing trend (p = 0.07) for α-B-crystallin levels compared to sedentary animals. By 7 days, both levels of HSP27 and α-B-crystallin were elevated (227 ± 28%, p < 0.05, and 287 ± 47%, p < 0.05; Fig. 1, A-C), respectively. There was no change in HSP70 levels (Fig. 1, A and D).
Fig.1. Impact of exercise on heat shock proteins (HSP) in the hippocampus.
(A) A representative Western immunoblot, showing levels of heat shock proteins and β-actin in sedentary rats (D0) or after 3 days (D3) or 7 days (D7) of voluntary exercise. The relative optical density (OD) of bands was calculated to determine the impact of exercise on brain levels of HSP27 (B), α-B-crystallin (C) and HSP70 (D). β–actin levels were measured to show equal loading and for normalization. Error bars indicate S.E.M. Treatment effects were analyzed by 1 ×1 ANOVA. For HSP27 and α-B-crystallin, values were log transformed to establish homogeneity of variance. Fisher’s LSD analysis was used for post-hoc test of planned comparisons. Asterisks indicate significant difference from D0 (p < 0.05).
3.2. HSP and their regulated intracellular signal molecules
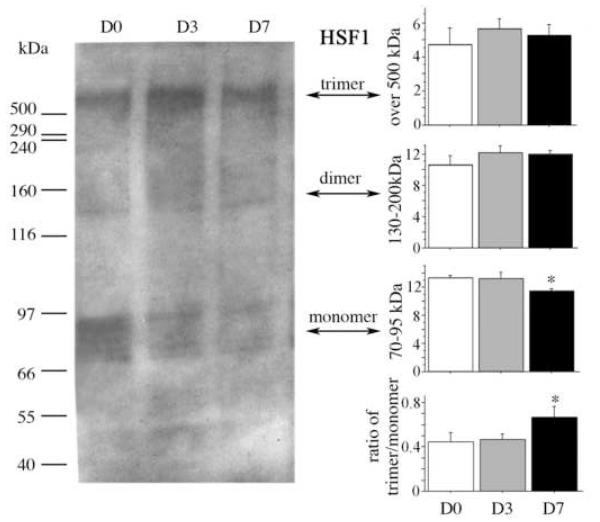
The transcriptional factor HSF1 activates HSPs, including sHSP, and the activation of HSF1 itself occurs after its phosphorylation, oligomerization, and translocation into the nucleus. We therefore investigated whether there was a change in its aggregation using non-reducing conditions (Fig. 2). Using a 3-15% gradient non-reducing gradient gel, several bands were visible, consistent with reports as monomer at 69-95 kDa, the exist of dimer 140 kDa, and 660 kDa trimer (Baler et al., 1993; Ito et al., 1997; Ito et al., 1998; Sarge et al., 1993; Westwood et al., 1991). There was no detectable evidence of oligomerization (trimers). Nevertheless, at 7 days, the HSF1 monomeric form was reduced and the ratio of trimer to monomer was increased (p < 0.05), but there was only a non-significant trend for percentage trimer to increase (not shown).
Fig. 2. Exercise-induced modulation of Heat Shock Factor-1 (HSF1) aggregates in the hippocampus.
Representative lanes of a Western immunoblot of HSF1 using a 3-15% non-reducing conditions, demonstrate multiple bands in sedentary (D0) rats or rats after 3 (D3) or 7 (D7) days of voluntary exercise. The top three graphs show the treatment means for the relative optical density of each set of bands, representing trimer, dimer and monomer. The bottom right graph shows the ratio of trimer to monomer as an index of aggregation. The ‘monomer’ data were log-transformed, and the ‘ratio’ data were square root-transformed data for homogeneity of variance prior to 1 × 1 ANOVA, and Fisher’s post-hoc analysis was used to determine difference between planned comparisons. Error bars represent S.E.M. Asterisks indicate significant difference from D0 (p < 0.05).
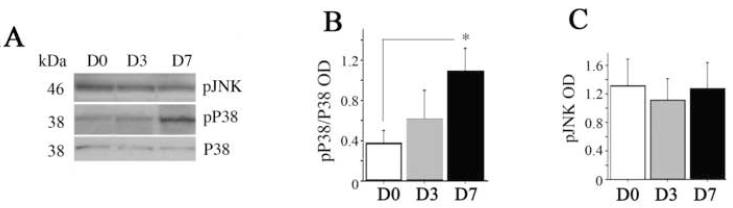
We then investigated whether levels of kinases known to regulate HSF-1 activation changed in response to exercise (Fig. 3-5). Densitometric analysis of levels of the active MAPK pP38 normalized to total p38 showed a significant increase by exercise day 7 (282 ±18%, 0.40 ± 0.27 vs 1.00 ± 0.33, p < 0.05, Fig. 3, A and B). There was no statistically significant change in phosphorylated JNK (pJNK) levels (Fig. 3, A and C).
Fig. 3. Exercise-induced changes in mitogen activatied protein (MAP) kinases.
(A) Representative lanes of a Western immunoblot for phosphorylated P38 (pP38), total P38, phosphorylated c-Jun kinase (pJNK). Bands of appropriate molecular weight are shown from hippocampi of sedentary (D0) rats or rats after 3 (D3) or 7 (D7) days of voluntary exercise. pP38 was stripped and re-probed with an antibody to total p38 for normalization. Treatment means for optical density (OD) of bands were calculated, and treatment effects were analyzed by 1×1 ANOVA. Graphs show (B) the ratio of pP38: P38 to reflect enzymatic activity (C) and pJNK levels. Equal loading was confirmed by Coomassie Blue (not shown). Error bars represent S.E.M. Asterisks indicate significant difference from D0 (p < 0.05).
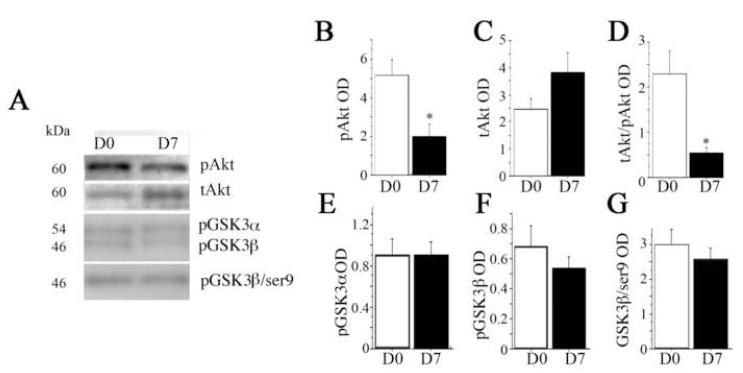
Fig. 5. Exercise effects on Akt and its down stream target GSK3.
(A) Representative lanes of a Western immunoblot for phosphorylated Akt (pAkt), total Akt (Akt), and active phosphorlated GSK3 α and β, and inactive phosphorylated GSK (pGSK3β-ser9) from hippocampi of sedentary (D0) rats or 7 (D7) days of voluntary exercise. pAkt was stripped and re-probed with an antibody to total Akt. GSK3 was run on separate blot. The relative optical density (OD) of bands was measured. Graphs show (B) treatment means for pAkt, (C) total Akt (tAkt) and (D) the ratio of pAkt to Akt as an index of enzymatic activity. (E-G) treatment means of active GSK3α/β tyr279/216 and inactive GSK3ser9. Treatment groups were evaluated by 1 × 1 ANOVA. Equal loading of protein was confirmed by Coomassie Blue (not shown). Error bars represent S.E.M. Asterisks indicate significant difference from D0 (p < 0.05).
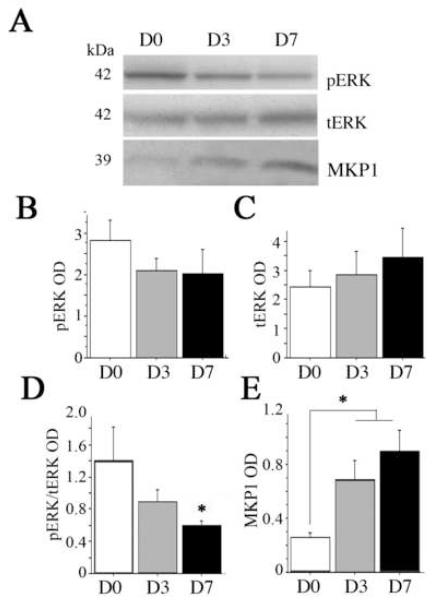
In contrast to the marked upregulation of pP38/P38, ERK phosphorylation (pERK) relative to total ERK was significantly down-regulated by day 7 (p < 0.05, Fig. 4, A and D). Total ERK showed a non-significant increase (Fig. 4, A and C). We then measured MKP1, the main phosphatase, which dephosphorylates pERK (Fig. 4, A and E). Compared to sedentary rats (day 0), exercise increased levels of MKP1 both at day 3 and day 7, but the differences did not quite reach statistical significance (p = 0.06) unless the two exercise groups were combined (p < 0.05, Fig. 4, B and C). The phosphatidyl inositol-3 (PI3) kinase Akt-GSK3 pathway has been reported be stimulated by 14 days after exercise (Chen and Russo-Neustadt, 2005). Since in the original group of animals, the more robust responses were observed at day 7, we chose this timepoint to examine exercise-dependent pAKT and GSK3 responses. Therefore, we measured phosphorylated Akt (pAkt ser473), total Akt, active GSK3α/βtyr279/216 and inactive GSK3βser9 (Fig. 5). To our surprise, the index of activity was suppressed (the ratio of pAkt to tAkt) by day 7 (p =0.02, Fig. 5, A, B, and D). Levels of tAkt were non-significantly increased (p < 0.10, Fig. 5C). There were no changes in GSK3 activity (Fig. 5, A and E-G).
Fig. 4. Exercise effects on extracellular related kinase (ERK) and its phosphatase mitogen activated kinase phosphatase 1 (MKP1).
(A) Representative lanes of a Western immunoblot for pERK, tERK, and MKP1 from hippocampi of sedentary (D0) rats or rats after 3 (D3) or 7 (D7) days of voluntary exercise. pERK was stripped and re-probed with an antibody to total ERK. MKP1 was run on separate blot. The relative optical density (OD) of bands was measured. Results were evaluated by 1 × 1 ANOVA for (B) phosphophorylated ERK (pERK), (C) total ERK (tERK), (D) the ratio of pERK/tERK as the index of enzyme activity and (E) MAP kinase phosphatase MKP1. Equal loading was confirmed by Coomassie Blue (not shown). Error bars represent S.E.M. Asterisks indicate significant difference from D0 (p < 0.05).
3.3. Synaptic proteins response to exercise
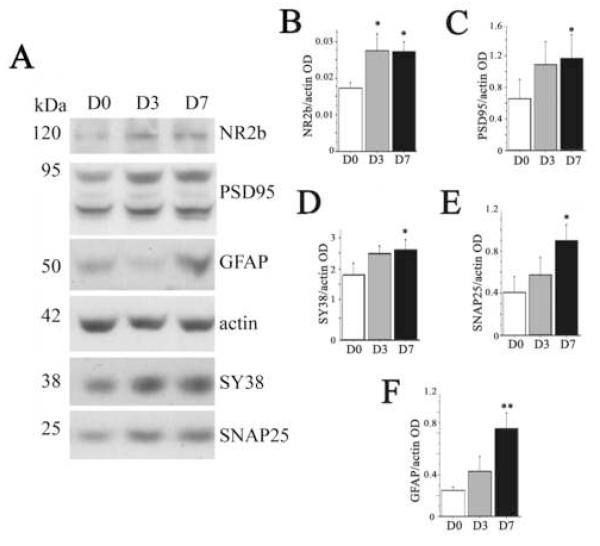
To investigate whether the HSP changes were associated with hippocampal synaptic changes, we measured pre- and post-synaptic markers including NR2b, PSD95, synaptophysin (SY38), and synaptosome-associated protein-25 (SNAP25 (Fig. 6, A-E). We also measured GFAP (Fig. 6, A and F), which is involved in synaptic plasticity and neurogenesis (Finch, 2003; Theodosis and Poulain, 1999) and is induced by exercise (Li et al., 2005; Uda et al., 2006). In this study, all these synaptic markers started to increase at day 3 after exercise but only NR2b was significant (165±18%, p < 0.05, Fig. 6B). Levels for all these markers increased significantly at exercise day 7: NR2b (158±9%, p < 0.05), PSD95 (181±32%, p = 0.05), synaptophysin (157±8%, p = 0.05, square root transformed data), SNAP25 (225±17%. p < 0.05,), and GFAP (320±18%, p = 0.01; Fig. 6, B-F, respectively).
Fig. 6. Exercise induced modulation of synaptic proteins and glial fibriallary acidic protein (GFAP).
(A) Representative lanes of a Western immunoblot for synaptic proteins and GFAP from hippocampi of sedentary (D0) rats or rats after 3 (D3) or 7 (D7) days of voluntary exercise. The relative optical density of bands was measured. Graphs show treatment means for (B) NR2b (C) post-synaptic density protein-95 (PSD95), (D) synaptophysin (SY38), (E) synaptosomal-associated protein-25 (SNAP25) and (F) GFAP, and treatment effects were evaluated by 1×1 ANOVA. Equal loading was confirmed by β-actin and for normalization. Error bars represent S.E.M. Asterisks indicate significant difference from D0 (*p < 0.05, **p = 0.01).
3.4. Correlation between sHSP and synaptic proteins or GFAP
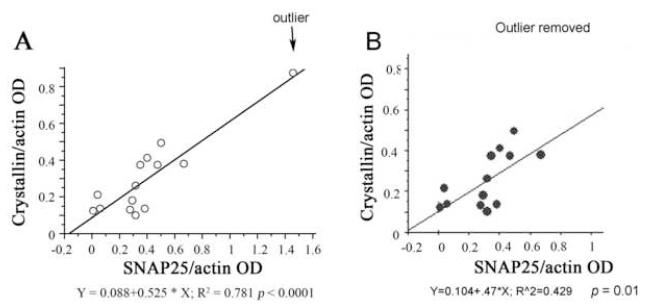
We did a linear regression analysis to assess a possible correlation between increased sHSP and increased synaptic proteins or GFAP for individual samples. The results showed that only α-B-crystallin correlated significantly with SNAP25 with (p < 0.0001, Fig. 7A) or without the high responder outlier (p = 0.01, Fig. 7B). sHSP did not correlate with PSD95, synaptophysin or GFAP or NR2b.
Fig. 7. Correlation between sHSP with selective synaptic proteins in the hippocampus of sedentary and exercising rats.
(A) α-B-crystallin levels correlated with SNAP25 levels (p < 0.0001), even when the outlier was removed (B) p < 0.01.
4. Discussion
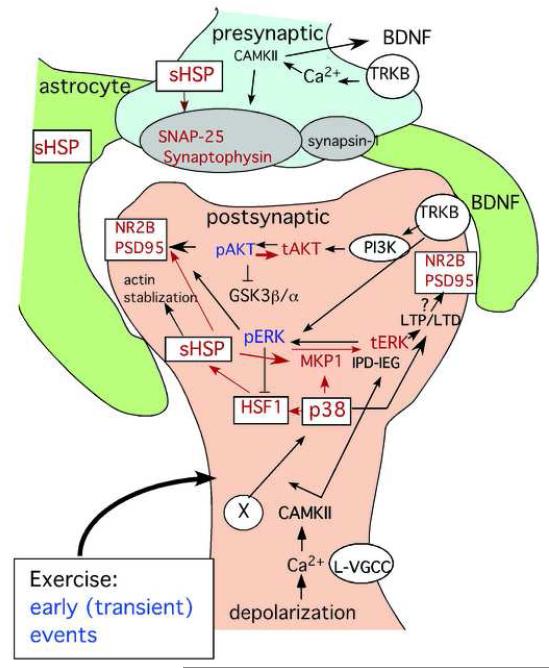
Our data demonstrate for the first time that voluntary exercise for seven days upregulates the small heat shock proteins HSP27 and α-B-crystallin. Also for the first time we describe exercise induced elevation of the pre-synaptic protein SNAP-25 and the post-synaptic protein PSD95, which coincides with the sHSP response. Of the kinases known to regulated sHSP in the periphery, only MAPK p38 was increased with sHSP. We have summarized our novel findings in a schematic diagram in blue, and how they might fit into what is known about the molecular events regulating memory (shown in black) after voluntary exercise (Fig. 8).
Fig. 8. Schematic diagram of possible effects of sHSP on the synapse in the hippocampus after exercise.
Molecular factors and pathways that are boxed with red letters and red arrows show our observed effects that we speculate are an early response to exercise modulating synaptic proteins; Letters in blue show our observed effects that are down-regulated; Letters in black are generally accepted pathways for synaptic plasticity; and letters in red show exercise-dependent upregulation. It is unclear whether the sHSP pathway precedes the induction of pERK, pAkt or BDNF. The circled “X” indicates the unknown factor triggering the heat shock cascade (eg. glucocorticoid receptor or temperature change or oxidative free radical). Even rats that ran minimum distances, still showed induction sHSP and synaptic proteins (not shown). Pre-synaptic proteins SNAP25 and synaptophysin were increased as well as the post-synaptic proteins PSD-95 and NR2b, but the localization of the sHSP is not known. The schematic diagram of the molecular pathway is illustrated in the post-synaptic density. However, sHSP can be induced in the astrocytes after hyperthermia and play a critical role in synaptogenesis. L-VGCC; L-Voltage-gated channels
4.1. Exercise and Heat shock proteins
Increased HSP27 or α-B-crystallin act as a chaperone, protecting cells from misfolded proteins. In neurodegenerative diseases where aberrant aggregation of proteins play a role in pathogenesis sHSP are thought to play a protective role, such as in Alzheimer’s disease (Wilhelmus et al., 2006), Parkinson disease (Outeiro et al., 2006; Renkawek et al., 1999), Alexander disease (Iwaki et al., 1989), Creutzfeldt-Jacob syndrome (Iwaki et al., 1992) and tauopathies (Kosik and Shimura, 2005). A mutation (S135F) in HSP27 is also associated with the inherited peripheral motor neuron disorders distal hereditary motor neuropathy type II and axonal Charcot-Marie-Tooth disease type 2L (Fontaine et al., 2006).
Similar to our previous study on proteomics (Ding et al., 2006b), we did not observe changes in HSP70 in response to voluntary exercise, although it has been reported to be increased after forced long-term exercise in mice (Sumitani et al., 2002).
Stress triggers a multi-step process of modification of heat shock transcription factors (HSFs) that results in DNA-binding to the heat shock element (HSE) in the heat shock protein (HSPs) promoter region, thereby activating transcription of HSP genes (Morimoto et al., 1997; Voellmy, 1994; Wu, 1995). The transcriptional factor HSF1 has been shown to activate HSPs, including sHSP. Given that HSF1 oligomerization is a key step for mediating transcription of HSP genes (Morimoto, 1993; Voellmy, 1994; Wu, 1995), we investigated possible changes in HSF1 aggregation with non-reducing gel. Although we did not see detectable increases in trimers, we cannot exclude a role for HSF1. The fact that monomer appears to be depleted with exercise may indicate some slight change in aggregation for which the assay was not sufficiently sensitive. Also a HSF1 response could be transient.
4.2. Signal Transduction Pathways: p38 induction
We measured MAPKs (ERK, JNK, P38) and Akt/GSK3 phosphorylation to see which intracellular signals corresponded with sHSP induction after exercise. P38 was initially characterized owing to its role in the response of cells to various stimuli such as heat shock (Rouse et al., 1994). Results showed a selective upregulation of p38, supporting a primary role for this kinase, which may argue against a major role of the other MAPKs or Akt/GSK3. The sHSP are phosphorylated by stress-activated kinase 2 (p38) and MAPKAP kinase 2/3 in a cell line of neurons (Geum et al., 2002), glia (Ito et al., 1997) as well as in other types of cells (Freshney et al., 1994; Guay et al., 1997). In glioma cells, p38 but not pERK or pJNK effectively phosphorylated HSP27 (Ito et al., 2005). In vivo, although similar responses are seen in skeletal muscle in response to exercise (van Ginneken et al., 2006), our results are the first to correlate p38 and sHSP in the brain with exercise.
These are consistent with a possible role for p38 in the sHSP response and the synaptic changes. P38 is highly expressed in brain regions that are important for learning and memory (Lee et al., 2000), and is required in hippocampal LTD that dependent on mGluR (Bolshakov et al., 2000) and NMDAR activation (Zhu et al., 2002).
4.3. Signal Transduction Pathways: Reduction in pERK
To our surprise, exercise caused a significant reduction in ERK activity and Akt activity. The reduction in pERK was surprising because voluntary exercise typically induces pERK (Ding et al., 2006a; Shen et al., 2001). There is little in the literature to explain this observation. We hypothesize that during the early days in exercise there are complex dynamic changes and feedback effects, preceding the sustained elevations in BDNF and pERK and pAKT. For example, unexpected suppression of c-fos mRNA, which is regulated by pERK, was observed after its induction by mild forced exercise (Soya et al., 2007). With repeated exercise (Lee et al., 2003), or repeated stress (Kwon et al., 2006), c-fos response is blunted. Voluntary exercise, can induce transient corticosteroids (Droste et al., 2003; Stranahan et al., 2008), which may transiently downregulate pERK during initial days of training (Kim et al., 2004; Yu et al., 2004). Consistent with the literature, we observed trends for increases in total ERK after 7 days exercise.
Given that ERK activity is involved in both the HS response and synaptic plasticity, the suppression in pERK was puzzling, but it is unlikely that our observation of pERK was artifactual or due to type II error, since we found significant increases in levels of one of its phosphatases, MKP1 (Price et al., 2007). MKP1 is one of the immediate early genes (IEG), and can be activated upon depolarization (Machado et al., 2008). This suggests that initial activation of MKP1 is present with increased neuronal activity, which occurs with exercise, and does not require phosphorylation of ERK.
Interestingly, p38, which we see increased after exercise, mediates heat shock induced MKP1 mRNA and protein (Wong et al., 2005). Further, increased HSF1 activity associated with HSP transfection, is mediated by increased MKP1 and reduced ERK activity (Seo et al., 2006). Also the in vivo heat shock response has been associated with reduced hippocampal pERK (Maroni et al., 2003). Reduced ERK activity causes dephosphorylation in HSF1-ser307, stabilizing HSF1 activity (Seo et al., 2006; Wigmore et al., 2007).
Although ERK activation mediates growth factor modulation of synaptic plasticity, there are still some other pathways that are independent of ERK activity, such as immediate early gene (IEG) induced preferentially by depolarization (IPD-IEG (Machado et al., 2008). A subset of IEG belong to IPD-IEG, including MKP1, and the Down syndrome critical region homolog 1 (Dscr1) has been found to play a role in LTP (Hoeffer et al., 2007) or LTD plasticity by inhibiting the calcineurin/NFAT pathway (Cano et al., 2005). Therefore, synaptic plasticity can be regulated at least partially without ERK participation. Neuronal response to stimuli, that is, neuronal plasticity and learning, are processes, which depend on rapid molecular adaptation (Morgan and Curran, 1989).
4.4. Signal Transduction Pathways: Reduction in pAKT
The phosphatidyl inositol-3 (PI3) kinase Akt-GSK3 pathway has been reported be stimulated by 14 days after exercise (Chen and Russo-Neustadt, 2005), but like pERK, we observed this pathway to be suppressed at 7 days. We had previously seen a slight non-significant reduction in pAkt, but the paradigm was 5 days voluntary exercise and 5 days Morris Water Maze swimming (Ding et al., 2006a). We do not know if, like pERK, there is a transient reduction in pAkt, prior to more sustained induction with longer exercise.
4.5. HSPs and the synapse
To our knowledge this is the first demonstration of increases in NR2b protein, PSD95 and SNAP25 in response to exercise. PSD95 is a post-synaptic scaffolding protein found in excitatory neurons and increased in enriched environment (Nithianantharajah et al., 2004). Transport of PSD95 to dendrites through PI3K-Akt signaling after NMDA receptor activation is induced by BDNF (Yoshii and Constantine-Paton, 2007). Increases in NR2b mRNA have been reported by our lab (Molteni et al., 2002) and others(Farmer et al., 2004), consistent with our protein results. SNAP25 is present in synaptic vesicles and the cytosolic portion of presynaptic membrane and forms a ternary complex with synaptobrevin, syntaxin where it is involved in the docking and subsequent exocytosis of synaptic vesicles. We were surprised to see an increase in SNAP25, since SNAP25 was reported to be unchanged after treadmill exercise (Liu et al., 2008) and since its affiliated protein syntaxin was not induced in response to voluntary exercise (Vaynman et al., 2006).
We observed a close correlation only between the pre-synaptic protein SNAP25 with the sHSP α-B crystallin. It is unclear whether this indicates a separate role for the different sHSP in the pre-synaptic site. Little is known regarding the role of sHSP or HSP in synaptic function. Hsc70, the constitutive form of HSP70 was found in synapses (Suzuki et al., 1999), and also has been observed to accumulate in the postsynaptic density in cerebral ischemia (Hu et al., 1998). In vitro in peripheral cells it is known that, HSP27 behaves as an actin cap-binding protein and can protect the actin cytoskeleton from destabilization caused by a variety of physiological stresses (Geum et al., 2002; Valentim et al., 2003). But it is not clear if HSP27 also affects the actin polymerization associated with synaptic plasticity. In the cerebellum sHSP are prominent in astrocytes and hypothesized to play a role in synaptic support (Bechtold and Brown, 2000).
HSP27 has also been found to be localized to synaptic sites as well as to peri-synaptic glial processes in rat cerebellum following hyperthermia (Bechtold and Brown, 2000). We observed an increase in the astrocyte marker glial fibrillary acidic protein (GFAP), similar to described in our proteomic study (Ding et al., 2006b), and exercise induced proliferation of astrocytes has been described by others (Uda et al., 2006). It is unknown if HSP27 in the hippocampus is similarly localized to astrocytes surrounding synapses. Thus the role of astrocytes in the sHSP response and synaptogenesis after exercise should be further investigated (Figure 8). Astrocytes may also play an indirect role in synaptogenesis since perivascular astrocytes or the “or the gliovascular unit” may increase vascularization (Wolburg et al., 2008) to facilitate increasing energy demands during synaptogenesis, but may also strengthens microvascular intensity by thickening basal lamina, thereby reducing cerebral permeability (Ding et al., 2006c).
4.6. Conclusion
In conclusion, we demonstrate four early novel hippocampal responses to exercise that have not been identified previously: the induction in (1) sHSPs (2) the synaptic proteins SNAP-25, NR2b, and PSD-95 and (3) the MAP kinase p38 and (4) the immediate early gene product MKP1. These data are consistent with a possible role for the p38 MAPK pathway in the induction of sHSP and synaptic proteins.
5. Experimental Procedures
5.1. Exercise paradigm
Rats (Male Sprague-Dawley rats, approximately 2 months of age, n =5 animals per group) were housed individually in separate standard polyethylene cages in a 12/12 h light/dark cycle at 22-24 °C, with food and water ad libitum, using the voluntary exercise paradigm to reflect the beneficial effect of moderate exercise on memory as described (Vaynman et al., 2006). Each individually housed rat in the exercise group was given access to its own wheel (diameter = 31.8 cm, width = 10 cm) that freely rotated against a resistance of 100 g attached to a receiver that monitored revolutions every hour (VitalViewer Data Acquisition System software, Mini mitter company, Inc., Sunriver, OR). The resistance has been introduced in our system to increase reliability of responses in cognition (Vaynman et al., 2006). In this paradigm, the rats’ distance run per night increases with time as their fitness levels increase. In this group rats ran a mean of (2.3 ± 0.18 km per night). These studies were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and were approved by UCLA Animal Research Committees.
5.2. Sample collection
To minimize the time between the end of the exercise bout and harvesting of tissue, 15 rats were sacrificed at approximately 8:00 AM by decapitation at 0 (sedentary), 3 or 7 days of exercise (n=5). Hippocampi were quickly removed, frozen on dry ice and stored at −70°C until processed for Western blot.
5.3. Quantitative Western blotting
The expression of synaptic markers, β-actin, GFAP, HSPs, HSF1, MAPK, Akt and GSK3 was assessed by standard immunoblotting techniques. The hippocampal tissue was homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris-HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. After centrifugation, the supernatants collected, and total protein concentrations were determined according to the Bio-rad DC protein assay (Bio-rad, Hercules, CA), using bovine serum albumin as standard.
Equal amounts (30μg) of protein samples were loaded and separated by electrophoresis on a 10% or 7.5-15% gradient polyacrylamide gel. For HSF1 trimer measurement, non-reducing samples were separated on 3-15% gradient gel. The gel was electro-transferred to a nitrocellulose membrane. Membranes were blocked with 10% nonfat milk, then incubated with primary antibodies at different dilutions (Table 1) followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. The dilution of secondary antibody (either goat-anti-rabbit or goat-anti-mouse) was 1:100,000 except 1:200,000 for HSF1 trimer. After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL or femto-ECL kit (Pierce, Rockford, IL) according to manufacturer’s instructions. The film signals were digitally scanned and quantified using densitometric image software (Molecular Analyst II, Bio-rad, Hercules, CA), normalized for β-actin level. Gels were also stained with Coomassie blue to ensure equal protein loading.
Table 1.
| Antigen | Host | Dilution | Company |
|---|---|---|---|
| HSP70 | mouse | 1/1000 | BD Biosciences. |
| HSP25 | rabbit | 1/1000 | Stressgen Biotechnologies Corp., Victoria, BC, CA |
| α-B-crystallin | rabbit | 1/1000 | Stressgen Biotechnologies Corp., Victoria, BC, CA |
| HSF1 | rabbit | 1/1000 | Stressgen Biotechnologies Corp., Victoria, BC, CA |
| NR2b | mouse | 1/500 | Biosource International, Camarillo, CA, USA |
| PSD95 | mouse | 1/1000 | Upstate, Lake Placid, NY, USA |
| synaptophysin | mouse | 1/10000 | Chemicon, Temecula, CA, USA |
| SNAP25 | mouse | 1/10000 | Sternberger, Lutheville, MD, USA |
| actin | mouse | 1/1000 | Chemicon, Temecula, CA, USA |
| GFAP | mouse | 1/1000 | Sigma, Saint Louis, MO, USA |
| p38 | rabbit | 1/500 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| pP38 | mouse | 1/500 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| MKP1 | rabbit | 1/200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| pJNK | rabbit | 1/1000 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| pAKT | rabbit | 1/1000 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| Akt | rabbit | 1/1000 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| pGSK3α/β | rabbit | 1/1000 | Biosource International, Camarillo, CA, USA |
| pGSK3βser9 | rabbit | 1/1000 | Cell Signaling Technology, Inc. Danvers, MA, USA |
| pERK | rabbit | 1/1000 | Biosource International, Camarillo, CA, USA |
| ERK | rabbit | 1/1000 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
5.4. Statistical analyses
Bartlett’s tests were performed to determine homogeneity of variance. If significant, data were log or square root transformed to establish homogeneity of variance. Then analysis of variance (1 × 1 ANOVA) was performed to determine treatment effects. If treatment effects were significant, then post-hoc analysis was performed to determine differences between planned comparisons, using the Fischer PLSD test. Statistical differences were considered significant at p < 0.05. The results of exercised rats were presented in bar figures, illustrating the mean and standard error of the mean (SEM). To evaluate association between HSP with synaptic markers or GFAP within animal, a linear regression analysis was performed.
Acknowledgments
This study was supported by AG021975 (SAF) and VA Merit (SAF) and NIH grant NS50465 (FGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Akbar MT, Wells DJ, Latchman DS, de Belleroche J. Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glial and neuronal cells compared to heat shock protein 70 and caspase 3 following kainate administration. Brain Res Mol Brain Res. 2001;93:148–63. doi: 10.1016/s0169-328x(01)00199-1. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005381.pub2. CD005381. [DOI] [PubMed] [Google Scholar]
- Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993;13:2486–96. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Brain Res Mol Brain Res. 2000;75:309–20. doi: 10.1016/s0169-328x(99)00323-x. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000;3:1107–12. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev. 2008;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Cano E, Canellada A, Minami T, Iglesias T, Redondo JM. Depolarization of neural cells induces transcription of the Down syndrome critical region 1 isoform 4 via a calcineurin/nuclear factor of activated T cells-dependent pathway. J Biol Chem. 2005;280:29435–43. doi: 10.1074/jbc.M506205200. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–93. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006a;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006b;24:1265–76. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006c;28:184–9. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–7. doi: 10.1016/s0197-4580(03)00051-4. discussion S131. [DOI] [PubMed] [Google Scholar]
- Fontaine JM, Rest JS, Welsh MJ, Benndorf R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones. 2003;8:62–9. doi: 10.1379/1466-1268(2003)8<62:tsodfp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine JM, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, Benndorf R. Abnormal small heat shock protein interactions involving neuropathy-associated HSP22 (HSPB8) mutants. Faseb J. 2006;20:2168–70. doi: 10.1096/fj.06-5911fje. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–33. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–49. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem. 2002;277:19913–21. doi: 10.1074/jbc.M104396200. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–68. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Dey A, Sachan N, Wong H, Patterson RJ, Shelton JM, Richardson JA, Klann E, Rothermel BA. The Down syndrome critical region protein RCAN1 regulates long-term potentiation and memory via inhibition of phosphatase signaling. J Neurosci. 2007;27:13161–72. doi: 10.1523/JNEUROSCI.3974-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Robinson SD. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res. 2007;162:433–46. doi: 10.1016/S0079-6123(06)62021-9. [DOI] [PubMed] [Google Scholar]
- Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J Neurosci. 1998;18:625–33. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Shimohama S, Sato M, Nishikawa H, Madono K, Akaike A, Kimura J. Differential expression of small heat shock proteins in reactive astrocytes after focal ischemia: possible role of beta-adrenergic receptor. J Neurosci. 1999;19:9768–79. doi: 10.1523/JNEUROSCI.19-22-09768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Kato K. Prostaglandins stimulate the stress-induced synthesis of hsp27 and alpha B crystallin. J Cell Physiol. 1997;170:255–62. doi: 10.1002/(SICI)1097-4652(199703)170:3<255::AID-JCP6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Kato K. Enhancement of expression of stress proteins by agents that lower the levels of glutathione in cells. Biochim Biophys Acta. 1998;1397:223–30. doi: 10.1016/s0167-4781(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Ito H, Iwamoto I, Inaguma Y, Takizawa T, Nagata K, Asano T, Kato K. Endoplasmic reticulum stress induces the phosphorylation of small heat shock protein, Hsp27. J Cell Biochem. 2005;95:932–41. doi: 10.1002/jcb.20445. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Kume-Iwaki A, Liem RK, Goldman JE. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander’s disease brain. Cell. 1989;57:71–8. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Kume-Iwaki A, Goldman JE. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990;38:31–9. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Wisniewski T, Iwaki A, Corbin E, Tomokane N, Tateishi J, Goldman JE. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am J Pathol. 1992;140:345–56. [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Ju JY, Kim JH, Kim TY, Yang BH, Lee YS, Son H. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027:1–10. doi: 10.1016/j.brainres.2004.07.093. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Shimura H. Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta. 2005;1739:298–310. doi: 10.1016/j.bbadis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–42. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Krueger-Naug AM, Hopkins DA, Armstrong JN, Plumier JC, Currie RW. Hyperthermic induction of the 27-kDa heat shock protein (Hsp27) in neuroglia and neurons of the rat central nervous system. J Comp Neurol. 2000;428:495–510. doi: 10.1002/1096-9861(20001218)428:3<495::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience. 2006;142:1281–92. doi: 10.1016/j.neuroscience.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–80. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lee SH, Park J, Che Y, Han PL, Lee JK. Constitutive activity and differential localization of p38alpha and p38beta MAPKs in adult mouse brain. J Neurosci Res. 2000;60:623–31. doi: 10.1002/(SICI)1097-4547(20000601)60:5<623::AID-JNR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lee TH, Jang MH, Shin MC, Lim BV, Kim YP, Kim H, Choi HH, Lee KS, Kim EH, Kim CJ. Dependence of rat hippocampal c-Fos expression on intensity and duration of exercise. Life Sci. 2003;72:1421–36. doi: 10.1016/s0024-3205(02)02406-2. [DOI] [PubMed] [Google Scholar]
- Li J, Ding YH, Rafols JA, Lai Q, McAllister JP, 2nd, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neurosci Lett. 2005;386:160–4. doi: 10.1016/j.neulet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, Liao PC, Jen CJ. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Machado HB, Vician LJ, Herschman HR. The MAPK pathway is required for depolarization-induced “promiscuous” immediate-early gene expression but not for depolarization-restricted immediate-early gene expression in neurons. J Neurosci Res. 2008;86:593–602. doi: 10.1002/jnr.21529. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Peters CA, Kline M, Cutler RE, Jr., Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem J. 1998;332(Pt 3):703–12. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni P, Bendinelli P, Tiberio L, Rovetta F, Piccoletti R, Schiaffonati L. In vivo heat-shock response in the brain: signalling pathway and transcription factor activation. Brain Res Mol Brain Res. 2003;119:90–9. doi: 10.1016/j.molbrainres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–62. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–10. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Muller AP, Cammarota M, de Oliveira Dietrich M, Rotta LN, Portela LV, Souza DO, Izquierdo I, Bevilaqua LR, Perry ML. Different Effect of High Fat Diet and Physical Exercise in the Hippocampal Signaling. Neurochem Res. 2007 doi: 10.1007/s11064-007-9530-7. [DOI] [PubMed] [Google Scholar]
- Nadeau SI, Landry J. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv Exp Med Biol. 2007;594:100–13. doi: 10.1007/978-0-387-39975-1_10. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol Learn Mem. 2004;81:200–10. doi: 10.1016/j.nlm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Klucken J, Strathearn KE, Liu F, Nguyen P, Rochet JC, Hyman BT, McLean PJ. Small heat shock proteins protect against alpha-synuclein-induced toxicity and aggregation. Biochem Biophys Res Commun. 2006;351:631–8. doi: 10.1016/j.bbrc.2006.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DM, Wloka MT, Chik CL, Ho AK. Mitogen-activated protein kinase phosphatase-1 (MKP-1) preferentially dephosphorylates p42/44MAPK but not p38MAPK in rat pinealocytes. J Neurochem. 2007;101:1685–93. doi: 10.1111/j.1471-4159.2007.04557.x. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–6. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–37. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HR, Chung DY, Lee YJ, Lee DH, Kim JI, Bae S, Chung HY, Lee SJ, Jeoung D, Lee YS. Heat shock protein 25 or inducible heat shock protein 70 activates heat shock factor 1: dephosphorylation on serine 307 through inhibition of ERK1/2 phosphorylation. J Biol Chem. 2006;281:17220–7. doi: 10.1074/jbc.M600062200. [DOI] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–29. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–7. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Mattson MP. Central Mechanisms of HPA Axis Regulation by Voluntary Exercise. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitani K, Miyamoto O, Yamagami S, Okada Y, Itano T, Murakami T, Negi T. [The influence of severe long-term exercise on the mouse hippocampus] Nippon Seirigaku Zasshi. 2002;64:152–8. [PubMed] [Google Scholar]
- Suominen-Troyer S, Davis KJ, Ismail AH, Salvendy G. Impact of physical fitness on strategy development in decision-making tasks. Percept Mot Skills. 1986;62:71–7. doi: 10.2466/pms.1986.62.1.71. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, Takagi H. Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res. 1999;816:99–110. doi: 10.1016/s0006-8993(98)01083-x. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–82. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Valentim LM, Rodnight R, Geyer AB, Horn AP, Tavares A, Cimarosti H, Netto CA, Salbego CG. Changes in heat shock protein 27 phosphorylation and immunocontent in response to preconditioning to oxygen and glucose deprivation in organotypic hippocampal cultures. Neuroscience. 2003;118:379–86. doi: 10.1016/s0306-4522(02)00919-3. [DOI] [PubMed] [Google Scholar]
- van Ginneken MM, de Graaf-Roelfsema E, Keizer HA, van Dam KG, Wijnberg ID, van der Kolk JH, van Breda E. Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-Jun NH2 terminal kinase, and heat shock protein 27 in equine skeletal muscle. Am J Vet Res. 2006;67:837–44. doi: 10.2460/ajvr.67.5.837. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and Exercise: Past and Future Directions. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–33. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–30. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4:357–401. [PubMed] [Google Scholar]
- Walters TJ, Ryan KL, Tehrany MR, Jones MB, Paulus LA, Mason PA. HSP70 expression in the CNS in response to exercise and heat stress in rats. J Appl Physiol. 1998;84:1269–77. doi: 10.1152/jappl.1998.84.4.1269. [DOI] [PubMed] [Google Scholar]
- Westwood JT, Clos J, Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991;353:822–7. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Wigmore SJ, Sangster K, McNally SJ, Harrison EM, Ross JA, Fearon KC, Garden OJ. De-repression of heat shock transcription factor-1 in interleukin-6-treated hepatocytes is mediated by downregulation of glycogen synthase kinase 3beta and MAPK/ERK-1. Int J Mol Med. 2007;19:413–20. [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2008 doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Wong HR, Dunsmore KE, Page K, Shanley TP. Heat shock-mediated regulation of MKP-1. Am J Physiol Cell Physiol. 2005;289:C1152–8. doi: 10.1152/ajpcell.00138.2005. [DOI] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–69. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–11. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Yu IT, Lee SH, Lee YS, Son H. Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro. Biochem Biophys Res Commun. 2004;317:484–90. doi: 10.1016/j.bbrc.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–55. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]