Abstract
The characterization of the cross-reactive, or heterologous, neutralizing antibody responses developed during human immunodeficiency virus type 1 (HIV-1) infection and the identification of factors associated with their generation are relevant to the development of an HIV vaccine. We report that in healthy HIV-positive, antiretroviral-naïve subjects, the breadth of plasma heterologous neutralizing antibody responses correlates with the time since infection, plasma viremia levels, and the binding avidity of anti-Env antibodies. Anti-CD4-binding site antibodies are responsible for the exceptionally broad cross-neutralizing antibody responses recorded only in rare plasma samples. However, in most cases examined, antibodies to the variable regions and to the CD4-binding site of Env modestly contributed in defining the overall breadth of these responses. Plasmas with broad cross-neutralizing antibody responses were identified that targeted the gp120 subunit, but their precise epitopes mapped outside the variable regions and the CD4-binding site. Finally, although several plasmas were identified with cross-neutralizing antibody responses that were not directed against gp120, only one plasma with a moderate breadth of heterologous neutralizing antibody responses contained cross-reactive neutralizing antibodies against the 4E10 epitope, which is within the gp41 transmembrane subunit. Overall, our study indicates that more than one pathway leads to the development of broad cross-reactive neutralizing antibodies during HIV infection and that the virus continuously escapes their action.
The antibodies elicited by current human immunodeficiency virus (HIV) Env-based immunogens display very limited cross-neutralizing activities (reviewed in reference 15). The inability to elicit broad cross-reactive anti-HIV neutralizing antibodies (NAbs) by immunization is a major obstacle for the development of an effective vaccine against this virus. A better understanding of how cross-reactive NAbs develop during natural HIV infection, in particular the identification of factors that are associated with their development and the definition of the epitopes on the HIV Env that they recognize, may assist the design of more effective immunogens and facilitate the development of more appropriate immunization protocols.
The majority of NAbs generated by HIV type 1 (HIV-1)-infected subjects during the first months to a year following infection are capable of neutralizing the autologous virus but rarely exhibit cross-reactivity against heterologous isolates (27). In contrast, plasmas collected during chronic infection display various degrees of cross-neutralizing activities (6, 8, 16, 22), and a small subset of chronically HIV-1-infected individuals develop antibodies that neutralize a wide range of HIV isolates, including isolates from different clades (7, 10, 18, 20). Currently, very little is known about the factors that are linked or are conducive to the development of broad NAb responses during HIV infection or why very broad NAb responses are developed by only a small fraction of HIV-positive (HIV+) patients. Additionally, little is known about whether and how the broad NAb responses within individual patients evolve over time. Finally, it is unknown whether plasmas displaying limited, moderate, or broad NAb responses contain antibodies that recognize the same or different epitopes on the HIV Env.
Here we report that, in two cohorts of antiretroviral-naïve HIV+ patients with CD4+ T lymphocyte numbers of ≥250 cells/μl, a significant correlation was recorded between the breadth of the broad NAb responses in plasma and the time since infection, plasma viral load levels, and the binding avidity of anti-Env antibodies. Thus, the development of cross-reactive NAbs requires persistent HIV replication, which could lead to the maturation of antibodies against multiple conserved epitopes.
The epitopes targeted by plasma cross-reactive NAbs were located outside the variable regions of the HIV Env, irrespective of the breadth of the NAb responses. Antibodies to the CD4-binding site (CD4-BS) were present in all plasmas examined irrespective of the overall breadths of plasma NAb responses. However, only one subject was identified with exceptionally broad plasma NAb responses and whose anti-CD4-BS antibodies displayed cross-neutralizing activities. In the majority of cases studied in which the plasma displayed broad NAb responses, the epitope specificity of these responses was not precisely defined to known neutralization epitopes. Interestingly, we identified one subject, whose plasma displayed overall moderate breadth of cross-reactive NAbs, that developed cross-reactive NAbs that recognized the transmembrane gp41 Env subunit, specifically an epitope similar to that recognized by the broadly neutralizing monoclonal antibody (MAb) 4E10 (3, 36). Overall, our study indicates that more than one pathway leads to the development of broad NAb responses during HIV infection and that once infection is established, HIV continuously escapes from broadly cross-reactive NAbs.
MATERIALS AND METHODS
Cohorts.
HIV+ subjects from two cohorts were used, the UW/CFAR and the Vanderbilt/CFAR cohorts. We examined subjects with CD4+ T-cell counts of ≥250 per μl without antiretroviral therapy and with no AIDS-defining illness during the period of observation. In the Vanderbilt cohort (indicated by the prefix VC), 53 plasma samples from 21 subjects (Fig. 1) were studied, and in the CFAR cohort (indicated by the prefix CC), 43 plasma samples from 18 subjects were studied (Fig. 2). The VC cohort includes subjects with known times of seroconversion and with times since infection ranging from less than 1 year to more than 20 years. The VC cohort consists of 3 African American females and 18 males, 7 of which are African American, 10 of which are Caucasian, and 1 of which is of Asian descent. Subjects VC10014, VC10028, and VC10067 have secondary infections with hepatitis C virus. Subject VC10042 has secondary infections with both hepatitis C and hepatitis B viruses. In contrast, the time of seroconversion is unknown for the CC cohort, and only the time of enrollment is known. Further demographic information for the CC cohort currently is unavailable. For each subject in both cohorts, a minimum of two samples collected at different time points during infection were analyzed. Each sample was heat inactivated at 54°C for 1 h and centrifuged at 17,000 × g for 10 min prior to use in any assay.
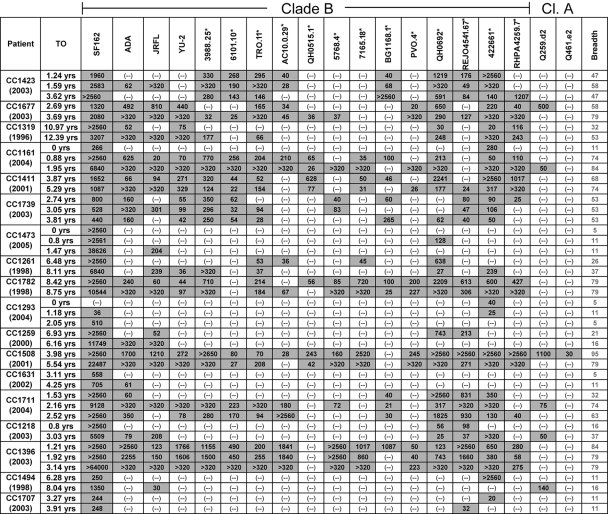
FIG. 1.
Cross-NAb responses in the Vanderbilt cohort. Values represent the plasma dilution at which 50% neutralization was detected. Plasma samples were tested in a dilution range of 1:20 to 1:2,560. Gray-shaded boxes indicate the neutralization of a particular isolate by the indicated plasma sample. (-), 50% neutralization was not recorded at a 1:20 plasma dilution; ETI, estimated time of infection; EY, estimated time (in years) since infection that the plasma sample was obtained and tested. Values listed in the % breadth column are the percentages of the isolates tested that each plasma neutralized (i.e., the breadth of neutralization). Clade B viruses that are part of a panel of viruses created to evaluate the anti-HIV neutralizing responses elicited during infection or during immunization (17) are indicated by an asterisk. Cl. A, clade A.
FIG. 2.
Cross-NAb responses in the UW/CFAR cohort. Values represent the plasma dilution at which 50% neutralization was detected. Plasma samples were tested in a dilution range of 1:20 to 1:2,560. Gray-shaded boxes indicate neutralization of a particular isolate by the indicated plasma sample. (-), 50% neutralization was not recorded at a 1:20 plasma dilution. The times of infection for this cohort are unknown. TO, time point (in years) after enrollment at which the plasmas were collected and tested for neutralizing activity. Values listed in the breadth column are the percentages of the isolates tested that each plasma neutralized (i.e., the breadth of neutralization). Clade B viruses that are part of a panel of viruses created to evaluate the anti-HIV neutralizing responses elicited during infection or during immunization (17) are indicated by an asterisk. Cl. A, clade A.
HIV-1 envelope-specific IgG titers.
Envelope-specific immunoglobulin G (IgG) titers were determined by enzyme-linked immunosorbent assay (ELISA). Fifty nanograms of soluble trimeric SF162 gp140 was absorbed onto each well of 96-well Immulon 2HB ELISA plates (Thermo) overnight in 0.1 M NaHCO3, pH 9.4, at room temperature. After blocking the plate in phosphate-buffered saline (PBS), 10% dry milk, 0.3% Tween-20, plasma samples diluted 1:1,000 in 10% dry milk, 0.03% Tween-20 in PBS in triplicate were titrated fivefold to a maximum dilution of 1:15,560,000 and incubated for 1 h at 37°C. Plates were washed in a plate washer, and bound antibodies were detected at 37°C for 1 h with goat anti-human IgG-horseradish peroxidase (HRP) (Bio-Rad) diluted 1:3,000. Plates were developed with 50 μl 1-Step Ultra TMB-ELISA solution (Pierce) and stopped with 50 μl 1N H2SO4, and the absorption at 450 nm was read on a Versamaxx microplate reader (Molecular Devices).
Antibody binding avidity determination.
The binding avidity index of plasma antibodies to HIV Env was determined as previously described, with slight modifications (34). Fifty nanograms of soluble trimeric SF162 gp140 was absorbed onto each well of 96-well Immulon 2HB ELISA plates in 0.1 M sodium bicarbonate, pH 9.0, overnight at room temperature. After being blocked in PBS, 10% dry milk, 0.3% Tween-20 and washed in PBS, 10% dry milk, 0.03% Tween-20, a single plasma dilution of 1:500 was incubated in each well for 2 h at room temperature. After being washed in a plate washer, ammonium thiocyanate was added in concentrations ranging from 10 to 4 M (in PBS) and incubated for 20 min at room temperature with shaking. Plates were washed again, and antibodies still bound to SF162 gp140 trimers were detected with goat anti-human IgG-HRP (Bio-Rad) as described above. The avidity index is reported as the concentration of NH4SCN required to remove 50% of antibodies bound to Env, which was calculated using a dose-response curve fit with nonlinear regression with GraphPad Prism 4.0 software (San Diego, CA).
Neutralization assays.
Neutralization assays were performed using Env pseudovirus variants in the TZM-bl cell-based assay as previously described (9, 33). Briefly, plasma samples at various dilutions were incubated for 90 min at 37°C in the presence of single-round-competent virions. The virus-plasma mixture was added for 72 h at 37°C to TZM-bl cells plated at a density of 3 × 103 cells per well in a 96-well plate 24 h prior to inoculation. The cell supernatants were removed, and 100 μl of SteadyLite plus (Perkin Elmer) was added to each well. Plates were incubated for 20 min at room temperature with gentle shaking to lyse the cells. Seventy-five microliters of the lysate was transferred to a microtiter plate, and the cell-associated luciferase activity (luminescence) for each well was determined on a Fluoroscan luminometer (Thermo). The neutralization values reported here are the plasma dilutions at which viral entry was inhibited by 50% compared to that in the absence of plasma (IC50). IC50s were calculated using a dose-response curve fit with nonlinear regression with GraphPad Prism 4.0 software (San Diego, CA). A plasma sample was scored as displaying neutralizing activity against a particular virus if at least 50% inhibition of infection was recorded at the lowest plasma dilution tested (1:20) in at least two independent neutralization assays.
Plasmas were tested for neutralization activity against 17 clade B and 2 clade A heterologous isolates (Tables 1 and 2) (4, 17). Most of the clade B viruses tested are part of a panel of viruses created to evaluate the anti-HIV-neutralizing responses elicited during infection or during immunization (17). We define the percentage (0% to 100%) of isolates neutralized by that sample out of the 19 isolates tested as the breadth of the cross-NAb response of a plasma sample. To test plasmas for non-HIV-specific neutralization activity, all plasmas initially were screened for neutralizing activity against the murine leukemia virus (MLV) envelope pseudotyped into the HIV backbone (data not shown). Subjects were excluded for further analysis if their plasma neutralized the pseudotyped MLV by more than 20% at a 1:20 dilution. The analysis of the autologous NAbs was not performed due to the current unavailability of viral Env clones from either cohort.
TABLE 1.
Factors correlated with the breadth of cross-NAb responses in plasma for the combined cohorts
| Covariate | Estimate | Univariate model result
|
|
|---|---|---|---|
| 95% CI | P valuea | ||
| Avg avidity | 1.72 | 1.53, 1.94 | <0.001 |
| Avg log(VL) | 1.91 | 1.56, 2.34 | <0.001 |
| Avg IgG | 2.35 | 1.84, 3.01 | <0.001 |
| Avg log(CD4) | 0.19 | 0.07, 0.49 | 0.001 |
All P values were statistically significant.
TABLE 2.
Factors correlated with the breadth of cross-NAb responses in plasma for the Vanderbilt cohort
| Covariates | Estimate | Model resultsa
|
|||
|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
||||
| 95% CI | P value | 95% CI | P value | ||
| Duration of Infection | 1.09 | 1.05, 1.13 | <0.001 | 1.03, 1.12 | 0.001 |
| Average Avidity | 1.93 | 1.63, 2.29 | <0.001 | 1.44, 2.06 | <0.001 |
| Average log(viral load) | 1.99 | 1.44, 2.76 | <0.001 | 1.24, 2.47 | 0.002 |
| Average IgG | 2.30 | 1.75, 3.01 | <0.001 | ||
| Average log(CD4) | 0.32 | 0.09, 1.16 | 0.084 | ||
| Average log(CD8) | 20.89 | 6.60, 66.04 | <0.001 | ||
Statistically significant P values are in boldface.
Peptide competition.
Peptide competition neutralization assays were performed as described previously (9), with slight modifications, using the following peptides: clade B consensus V1 (LMNATNTTNSSSGEK and NSSSGEKMEKGEIKN at 1:1) and V2 (SFNITTSIRDKVQKE, QKEYALFYKLDVVPI, and VPIDNDNTSSYRLIS at 1:1:1), V3 (TRPNNNTRKSIHIGP, KSIHIGPGRAFYTTG, and TTGEIIGDIRQAHCN at 1:1:1), 2F5 (EQELLELDKWASLWN), 4E10 (NWFDITNWLWYIRKKK), and 4E10 scrambled (FTNDWINWLWYIRKKK). Briefly, peptides were added at a final concentration of 10 μg/ml of each peptide to serially diluted plasmas and incubated at 37°C for 90 min. At this concentration of peptide and a concentration of antibody of 20 μg/ml, we estimated the peptide to be in excess of antibody by approximately 40-fold. At the final peptide concentration used during the peptide competition assays, the 4E10 and 4E10 scramble peptides did not interfere with virus infectivity. Pseudovirus was added to the plasma-peptide mixture for 37°C for 90 min. The plasma-virus-peptide mixture was added to TZM-bl cells, plated at a density of 3 × 103 cells/well, and incubated for 72 h at 37°C. After 72 h the medium was removed and the cells were lysed in 100 μl of SteadyLite (Perkin Elmer). The cell-associated luciferase activity was detected as described above. The percentage of neutralization at each plasma dilution (with or without peptide) was determined. At 70% neutralization in the absence of peptide (the linear portion of the neutralization curve), the percent reduction in neutralizing activity of plasma in the presence of peptide was determined.
Expression and purification of recombinant gp120.
SF162-derived gp120 was expressed by transiently transfecting 293EBNA (293E) cells with the pTT3 vector in Freestyle 293 expression medium (FS medium) (Invitrogen). High-density transfection (2 × 107 cells/ml) of 293E cells was carried out by incubating the cells in 25 μg/ml of plasmid DNA and 50 μg/ml of linear 25-kDa polyethylenamine (Polysciences) for 4 h with shaking at 37°C, as previously described (1). Following incubation, the transfected cells were seeded into a 10-liter WAVE (GE Healthcare) bag containing 4.75 liters of fresh FS medium and incubated for 96 h before the first harvest. The WAVE settings for expression are 5% CO2, 0.1 liter of airflow/min, and a 7° rocking angle, starting at 18 rpm for the first 24 h and then increasing to 23 rpm for the remainder of the expression.
Fresh Env supernatants were processed immediately following collection and spun at 500 × g for 10 min to pellet the cells. The concentration and buffer exchange of the cell supernatant was done using a tangential flow filtration system (Ultrasette Lab tangential flow device 30KD mwco sc) (Pall Life Sciences). Generally, the volume was reduced 20× and the buffer was exchanged into GNA loading buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, and 1 mM EGTA) for lectin affinity chromatography using GNA resin (Galanthus nivalis) (Vector laboratories). Following concentration and buffer exchange, the sample was centrifuged at 10,000 × g for 10 min to clarify the concentrated supernatant prior to chromatography. The sample was loaded at 3 ml/min onto a 30-ml GNA column packed in an XK26 column (GE Healthcare) equilibrated in GNA loading buffer using an ÄKTA 10/100 purifier (GE Healthcare). After being washed with approximately 5 to 8 column volumes, the column was eluted at 1 ml/min using GNA elution buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 0.75 M methyl-α-d-mannopyranoside, 1 mM EDTA, and 1 mM EGTA). Protein-containing fractions were pooled and subjected to DEAE anion-exchange chromatography. The sample was diluted fivefold in cold 20 mM Tris, pH 8.2, 1 mM EDTA, 1 mM EGTA in order to reduce the NaCl concentration to 20 mM before loading it onto the next column. The anion-exchange step was performed using a Tricorn 10/100 (GE Healthcare) column packed with 10 ml DEAE resin (GE Healthcare) equilibrated in DEAE buffer A (20 mM Tris, pH 8.0, 20 mM NaCl, 1 mM EDTA, 1 mM EGTA). The sample was loaded at 3 ml/min, and the flowthrough was collected. A linear NaCl gradient with DEAE buffer B (20 mM Tris, pH 8.0, 2 M NaCl, 1 mM EDTA, 1 mM EGTA) was run from 0 to 15% over 7.5 column volumes, and all fractions eluting from the column under 20 ms/cm were pooled with the flowthrough. The sample then was concentrated using 30-kDa Amicon ultracentrifugation concentrators (Millipore). This sample contains greater-than 95% pure gp120 according sodium dodecyl sulfate-polyacrylamide gel electrophoresis examination.
Adsorption of plasma antibodies to gp120.
Plasma anti-HIV Env antibodies were adsorbed on beads coated with HIV Env as previously described (18). Two different SF162 gp120 proteins were used, the wild-type (WT) and a CD4-BS mutant with a single amino acid substitution (D368R, based on the HXB2 Env numbering, which corresponds to position 364 in SF162). To generate the D368R mutant, mutagenesis was carried out using the primers SF162D364R (5′-CAATCCTCAGGAGGGCGCCCAGAAATTGTAATG-3′) and SF162D364R-r (5′-CATTACAATTTCTGGGCGCCCTCCTGAGGATTG-3′). The reaction mix was denatured at 95°C for 5 min, followed by 20 cycles of 95°C for 30 s, 55°C for 1 min, and 68°C for 7 min, followed by a final 10-min extension at 68°C. This amino acid substitution abrogates the binding of anti-CD4-BS antibodies to gp120 while retaining the ability to bind other MAbs (Fig. 3A).
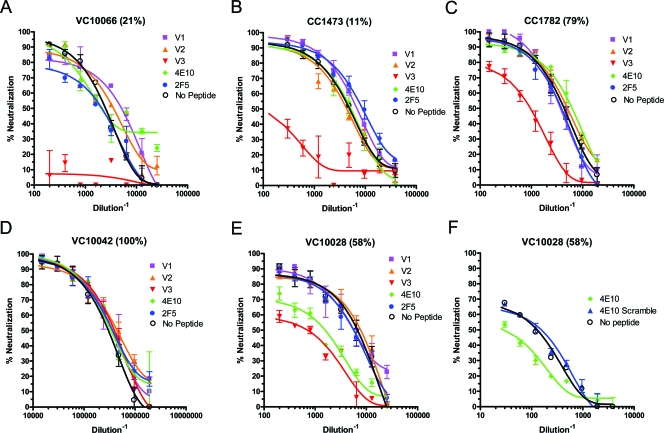
FIG. 3.
Peptide competition neutralization assays. (A to F) Serially diluted plasmas were preincubated with the consensus clade B peptides corresponding to various regions of the HIV Env before use in neutralization assays against the primary isolate SF162. (G) Competition of plasma VC10028 using the 4E10 and a scrambled 4E10 peptide with the JRFL virus. A reduction in neutralization after preincubation with peptides indicates that the plasma contains NAbs directed at the corresponding regions of the HIV Env. The breadth of neutralization is indicated in parenthesis after each plasma's code. Ab, antibody.
The recombinant SF162 gp120 proteins were coupled to MyOne Dynabeads Tosylactivated (Invitrogen) by following the manufacturer's instructions. Briefly, 40 mg of magnetic beads were reacted with 2 mg protein ligand overnight at 37°C with gentle rotation. After collecting the beads on a magnet, the supernatant was removed and the beads were incubated overnight at 37°C in PBS, 0.5% bovine serum albumin (BSA), 0.05% Tween 20. The magnetic beads were washed twice with PBS, 0.1% BSA, 0.05% Tween 20 and stored at 4°C in the same buffer, with the addition of 0.02% sodium azide. Bead-coupled Env proteins were tested for antigenic integrity by flow cytometry using the known MAbs b12, 447-52D, 2G12, and IgG-CD4, followed by detection with goat anti-human IgG-fluorescein isothiocyanate secondary antibody (data not shown). Mock adsorption/elution experiments using several anti-HIV Env MAbs at a concentration of 10 μg/ml in naïve plasma were performed as a positive control (data not shown). Six hundred microliters of plasma diluted 1:20 in Dulbecco's modified Eagle's medium-10% fetal bovine serum were incubated with 300 μl Env protein-coupled beads at room temperature for 120 min with gentle rotation. The samples were placed on a magnet, and the beads were isolated. Up to four serial adsorptions were performed for each plasma.
The antibodies bound to the bead-coupled Env proteins were eluted in a series of increasingly acidic solutions as previously described (18). The beads from each serial adsorption were combined and incubated in 0.1 M glycine-HCl, pH 2.7, for 30 s with vortexing. The beads were collected by brief centrifugation and held in place by a magnet. The supernatant was removed and adjusted to pH 7.5 with 1 M Tris (pH 9.0). The process was repeated with the beads in 0.1 M glycine-HCl, pH 2.3, and then again in 0.1 M glycine-HCl at pH 1.7. The final supernatants were buffer exchanged in PBS and washed over a 30-kDa Amicon ultracentrifugation concentrator (Millipore). Antibody concentrations were determined by bicinchoninic acid assay (Pierce). The depleted plasmas and the antibodies that were eluted from gp120 were used for ELISA and neutralization assays as described above. Both BSA-coupled beads and blank beads were used as negative controls in all assays.
Statistical analysis.
Logarithmic transformation was used for viral load numbers as well as CD4+ and CD8+ T-cell numbers. Initially, we computed the within-subject variation among the sampled time points for each immunological factor, including neutralizing breadth. We then computed the between-subject variance for each immunological factor and compared the between-subject variance to the within-subject variance using the F test. All of the F tests concluded that the within-subject variations were much smaller than the between-subject variations (P ≤ 0.001). Therefore, for each immunological factor examined, the within-subject average was computed and used for further statistical analyses. To assess what host factors influence the development of broadly neutralizing antibiotic responses, a logistic regression analysis was carried out using time-invariant variables for both the response and the predictors. Specifically, the response was the average breadth of cross-neutralization for each subject. First, univariate models were fitted one at a time with each of the predictors, and then all the potential predictors were added to a multivariate logistic regression model using the Vanderbilt cohort only. A manual backward variable selection procedure was carried out to remove one variable at a time using the Wald test and a significance level of 5%.
RESULTS
Factors correlated with the breadth of NAb responses in HIV+ plasma.
A wide range of NAb responses were recorded in both cohorts (Tables 1 and 2). While several plasmas displayed moderate degrees of breadth, neutralizing 30 to 70% of isolates tested (e.g., VC10002 and CC1423), other plasma samples displayed very narrow breadths (e.g., VC20011 and CC1293), neutralizing less than 10% of the isolates tested. Several plasmas were identified that exhibited broad breadth, neutralizing over 75% of the isolates tested (e.g., VC10014 and CC1508). Only one patient (VC10042) was identified with plasma (collected two decades after infection) that displayed extremely broad breadth, neutralizing all isolates tested.
We investigated the potential relationship between the breadth of the plasma NAb responses and the time since infection, blood CD4+ and CD8+ T lymphocyte numbers, plasma viral load levels, relative titers of Env-specific IgG, and the binding avidity index of these antibodies. A univariate model analysis showed that all predictor variables (except CD4+ T-lymphocyte numbers) were significantly positively associated (P ≤ 0.001) with the breadth of the NAb responses (Table 1 for combined cohorts and Table 2 for the Vanderbilt cohort, for which the time of infection is known). A multivariate model analysis (possible only with the Vanderbilt cohort) indicated that only the time since infection (P = 0.001), antibody binding avidity index (P < 0.001), and plasma viral load levels (P = 0.002) had significant impacts on the breadth of the NAb response. Under the multivariate model, the estimated odds of cross-neutralization increased by 7% annually since infection (95% confidence interval [CI], 3 to 12%; P = 0.001); the estimated odds of cross-neutralization increased by 72%, with a 1-U increase in the Env-binding avidity index (95% CI, 44 to 106%; P ≤ 0.001); and the estimated odds of cross-neutralization increased by 75% (95% CI, 24 to 147%; P = 0.002), with a 1-U increase in the log10 viral load.
Continuous evolution of broad NAb responses.
The availability of longitudinal plasma samples from several subjects allowed us to examine whether and how the breadth of the NAb response evolves over time. For subject VC10014 (Fig. 1), the breadth of the NAb response increased from less than 30% to more than 75% during a 3-year period of observation. Similarly, an increase in breadth was recorded for subject CC1711 from 32% to more than 60% during a single year of observation and for subject CC1218 from 16 to 37% during a 3-year observation (Fig. 2). In the majority of subjects, however, the breadth of NAb responses did not change significantly over time. Despite this, plasma samples collected longitudinally from certain subjects displayed different neutralization profiles. For example, plasma collected from VC10067 at 14.72 years postinfection efficiently neutralized isolates BG1168.1 and Q259d2.17 and not isolate 7165.18, but plasma collected a few months later no longer neutralized the first two isolates and weakly neutralized the third isolate (Fig. 1). During that period, the breadth of cross-neutralizing activity did not change significantly (from 37 to 32%). Similarly, plasma from VC10076 collected 5.29 years postinfection efficiently neutralized isolate BG1168.1 but not isolates 422661 and RPHA4259.7; plasma collected a year later no longer neutralized the first isolate but neutralized the other two isolates (Fig. 1). During that period, the breadth of cross-NAb responses changed from 21 to 26%. Therefore, it appears that a continuous and rapid evolution of the broad NAb response takes place during HIV infection.
NAbs targeting the variable regions of Env do not contribute to neutralizing breadth.
Peptide competition neutralization experiments were performed using peptides derived from the first, second, and third variable regions of the consensus clade B gp120 sequence (V1, V2, and V3 loops, respectively) to define the contribution of antibodies to these regions of the HIV Env in the overall cross-neutralizing potential of plasmas. The initial screening was conducted with the SF162 virus, which is known to be susceptible to neutralization by antibodies that bind to the variable regions of Env (9, 33). Indeed, SF162 was neutralized by all plasma samples tested (Fig. 1 and 2). The results are summarized in Fig. 4, and representative experiments are shown in Fig. 3.
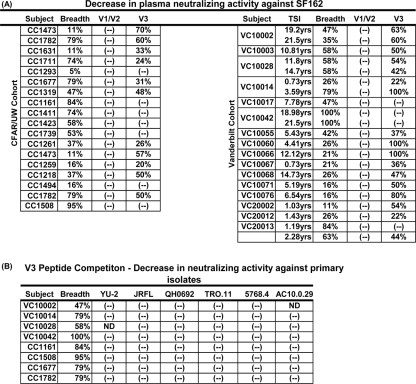
FIG. 4.
(A) Contribution of anti-V1, anti-V2, or anti-V3 antibody to the plasma's neutralizing activity against SF162. (B) Contribution of V3-directed antibodies to the plasma's overall neutralizing activity against six primary HIV-1 isolates. The values indicate the percent reduction of the plasma's neutralizing activity against the indicated isolates following the preincubation of the plasma with the indicated peptides. TSI, the time since infection (in years) that plasmas were collected in the Vanderbilt cohort; (-), no reduction in the plasma's neutralizing activity was recorded; ND, the plasma has no neutralizing activity against the indicated virus.
We found no evidence for the presence of cross-neutralizing anti-V1 or anti-V2 antibody against SF162 in these plasmas, irrespective of their overall breadth of cross-NAb responses (Fig. 4A and 3). In contrast, the majority of samples we studied contained V3-directed antibodies capable of neutralizing SF162. Interestingly, the anti-SF162-neutralizing activities of some plasmas with narrow neutralization breadths (CC1473, VC10060, and VC10066) were completely blocked by preincubating the plasmas with the consensus clade B V3-derived peptide, while the anti-SF162-neutralizing activities of plasmas with broad neutralizing potential (VC10042, CC1411, and CC1161) were unaffected by such treatment.
It is known, however, that most primary HIV-1 isolates are not susceptible to neutralization by anti-V3 antibodies (3, 17). To better assess the contribution of plasma anti-V3 antibodies in the overall cross-neutralizing activity of plasma, we performed V3 peptide competition experiments with several plasma samples exhibiting moderate to high breadth and several primary isolates: YU-2, JRFL, QH0692, TRO.11, 5768.4, and AC10.0.29. In all cases, the preincubation of plasmas with the consensus clade B V3 peptide had no effect on the overall neutralizing activities of plasmas against any of the isolates tested (Fig. 4B). Thus, plasma anti-V3 antibodies are not responsible for the neutralization of these primary isolates and contribute only minimally in defining the overall cross-neutralizing potential of plasmas collected from the HIV-infected subjects examined in these two cohorts.
NAbs targeting the MPER region contribute to the cross-neutralizing activity of certain HIV+ plasmas.
We next examined whether NAbs to the 2F5 and 4E10 epitopes, located in the membrane-proximal external region (MPER) of gp41, were present in these plasmas. These results are summarized in Fig. 6, and representative experiments are shown in Fig. 3. Here too we initially screened the plasma using SF612, since this virus is susceptible to MAb 2F5 and MAb 4E10 neutralization (19, 33). The preincubation of plasmas with a peptide representing the conserved 2F5 epitope did not decrease the anti-SF162-neutralizing activity of any of the plasmas (Fig. 6A). In contrast, the anti-SF162 neutralization activity of two longitudinal plasmas from subject VC10028 decreased by 29 and 38% when the plasmas were preincubated with a peptide representing the conserved E10 epitope (Fig. 6A and Fig. 3E), which is located in the MPER approximately four amino acids downstream from the 2F5 epitope.
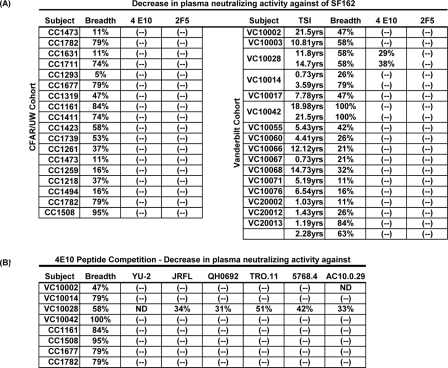
FIG. 6.
Presence of 2F5- or 4E10-like NAbs in plasma. (A) Values indicate the percent reduction in each plasma's neutralizing activity against SF162 following the preincubation of plasma with either r2F5 or 4E10 peptide. (B) The percent reduction in each plasma's neutralizing activity against six primary isolates following the preincubation of the plasma with the 4E10 peptide. (-), no reduction was recorded; ND, the plasma does not neutralize the indicated virus.
It is possible that the 4E10-like antibodies present in plasma VC10028 were active only against SF162 and other neutralization-sensitive isolates but not against neutralization-resistant primary isolates and, thus, do not contribute in defining the overall cross-neutralizing potential of plasma VC10028. We therefore examined whether these antibodies also could neutralize primary isolates other than SF162. In contrast to what we recorded with the anti-V3 NAbs (Fig. 4B), the 4E10-like NAbs contribute in defining the overall cross-neutralizing activities of plasma VC10028. The preincubation of this plasma with the 4E10 peptide resulted in a reduction of the plasma's neutralizing activity against those primary isolates that are neutralizable by the individual plasmas (Fig. 6B) (YU-2 is not neutralized by plasma VC10028, and therefore the preincubation of this plasma with the 4E10 peptide had no effect on the entry of YU-2 into target cells). It appears that a small number of subjects infected with HIV will develop 4E10-like antibodies that are cross-neutralizing.
As expected, the preincubation of other plasmas with the 4E10 peptide had no effect on their antiviral activities (Fig. 6B), since they did not contain anti-4E10 antibodies. Additionally, the preincubation of plasma VC10028 with a 4E10 scrambled peptide did not reduce the neutralizing activity of this plasma (Fig. 5F). These observations and the fact that the 4E10 peptide reduced the neutralizing activity of plasma from only 1 out of 39 subjects examined (Fig. 5E and F and 6) are in support of the presence of 4E10-like plasma VC10028.
FIG. 5.
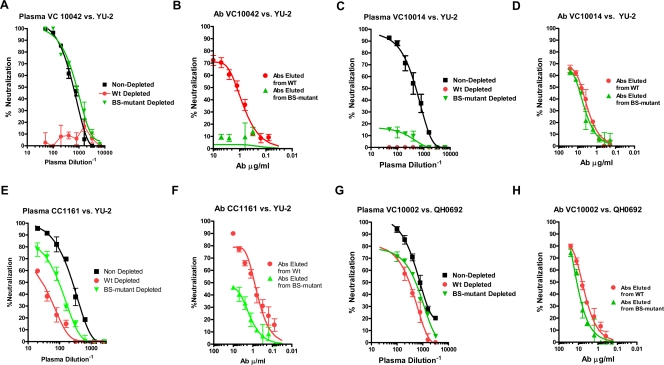
Contribution of anti-CD4-BS antibodies to plasma-neutralizing activities. Plasmas were incubated with beads coated with either WT gp120 or gp120 with a mutated CD4-BS. The flowthrough and the eluted antibodies were tested for neutralizing activity against HIV. (A and B) Sample from patient VC10042; (C and D) sample from patient VC10014; (E and F) sample from patient CC1161; (G and H) sample from VC10002. Flowthroughs (A, C, E, and G) and eluted antibodies (B, D, F and H) are shown. Nondepleted, neutralization curves obtained with unadsorbed plasmas; WT depleted, plasma flowthroughs from WT gp120; BS-mutant depleted, plasma flowthroughs from the CD4-BS mutant gp120.
Contribution of anti-CD4-BS antibodies to the overall cross-neutralizing potentials of HIV+ plasmas.
The overall cross-neutralizing activities of several of the most broadly neutralizing plasmas (more than 75% breadth; for example, VC10042, VC20013, CC1508, or CC1161) in either cohort were unaffected by preincubation with peptides derived from the variable regions of Env or with the 2F5 and 4E10 epitope-derived peptides (Fig. 4 and 6). Recent studies suggested that broadly cross-reactive antibody responses in plasma could be due to NAbs that bind to the CD4-BS of the HIV Env (10, 18). To define the contribution of anti-CD4-BS antibodies in the overall cross-neutralizing potential of plasma samples examined here, we performed adsorption experiments using a variant of gp120 containing a mutation in the CD4-BS that prevents the binding of anti-CD4-BS antibodies (10, 18).
Plasma samples from seven subjects displaying various degrees of neutralizing breadth were investigated. From the Vanderbilt/CFAR cohort we analyzed plasma from subject VC10042 collected 21.5 years postinfection, which neutralizes 100% of isolates tested, and its neutralizing activity is not affected by preincubation with peptides; plasma from subject VC10002 collected 22.51 years postinfection, which neutralizes 32% of the isolates tested; plasma from subject VC10014 collected 3.59 years postinfection, which neutralizes 79% of isolates tested, and its anti-SF162 neutralizing activity is completely eliminated when preincubated with the consensus V3-derived peptide; plasma from VC20011 collected 1.45 years postinfection, which neutralizes only SF162 and, very weakly, 5768.4; and plasma from VC20013 collected 2.28 years postinfection, which neutralizes 63% of the isolates tested. From the UW/CFAR cohort we analyzed plasma from CC1161, which neutralizes 84% of isolates, and plasma CC1508, which neutralizes 95% of isolates tested.
All plasma samples were incubated with WT gp120 or the CD4-BS mutant gp120 coupled to magnetic beads, and the corresponding flowthroughs as well as the eluted antibodies were tested for anti-HIV neutralizing activity against four isolates. The results are summarized in Table 3, and selected experiments are shown in Fig. 5.
TABLE 3.
Contribution of anti-CD4-BS antibodies to the cross-neutralizing activity of plasmasa
| Subject (% breadth) | Isolate | Plasma | % Neutralization of flowthrough
|
IC50 (μg/ml) of eluted antibodies
|
||
|---|---|---|---|---|---|---|
| WT gp120 | BS gp120 | WT gp120 | BS gp120 | |||
| VC10042 (100) | YU2 | 100 | (-) | 100 | 1.2 | (-) |
| QH0692 | 90 | 19 | 100 | 1.9 | (-) | |
| TORNO | 92 | 15 | 90 | 6.6 | (-) | |
| JRFL | 99 | 10 | 99 | 1.5 | (-) | |
| VC10014 (79) | YU2 | 92 | (-) | 15 | 6.5 | 8.1 |
| QH0692 | 52 | 12 | 28 | 14.5 | 19.1 | |
| TORNO | 70 | 25 | 45 | 12.3 | 14.6 | |
| JRFL | 95 | 5 | 35 | 7.3 | 10.3 | |
| CC1161 (84) | YU2 | 89 | 37 | 65 | 1.4 | (-) |
| QH0692 | 73 | 44 | 62 | 6.1 | (-) | |
| TORNO | 95 | 82 | 81 | 1.8 | 3.8 | |
| JRFL | 98 | 88 | 89 | 3.9 | 1.8 | |
| VC10002 (32) | YU2 | 40 | (-) | (-) | (-) | (-) |
| QH0692 | 85 | 53 | 60 | 7.2 | 10.8 | |
| TORNO | 72 | 52 | 57 | 8.9 | 15 | |
| JRFL | 90 | 47 | 55 | 8.6 | 13.4 | |
| CC1508 (95) | YU2 | 95 | 91 | 92 | (-) | (-) |
| QH0692 | 98 | 68 | 75 | 13.6 | 14.2 | |
| TORNO | 90 | 82 | 86 | 8.1 | 13.4 | |
| JRFL | 100 | 100 | 99 | (-) | (-) | |
| VC20013 (63) | YU2 | 27 | 12 | 22 | (-) | (-) |
| QH0692 | 78 | 78 | 72 | (-) | (-) | |
| TORNO | 73 | 73 | 72 | (-) | (-) | |
| JRFL | 70 | 63 | 58 | 8.4 | 9.7 | |
| VC20011 (5) | YU2 | (-) | (-) | (-) | (-) | (-) |
| QH0692 | (-) | (-) | (-) | (-) | (-) | |
| TORNO | (-) | (-) | (-) | (-) | (-) | |
| JRFL | (-) | (-) | (-) | (-) | (-) | |
The anti-HIV-neutralizing activities of the flowthrough from the WT gp120 and the CD4-BS mutant gp120 (BS gp120) and those of the antibodies eluted from the WT gp120 and CD4-BS mutant gp120 were determined as discussed in Materials and Methods. For the plasma and flowthroughs, the percent neutralization at a 1:50 dilution is shown. Significant neutralization (≥50%) is shown in boldface. For the eluted antibodies, the IC50s are shown. (-), neutralizing activity (50% neutralization) against the indicated virus was not recorded at a 1:50 dilution. The overall breadth of neutralization for each plasma is shown under each code identifier.
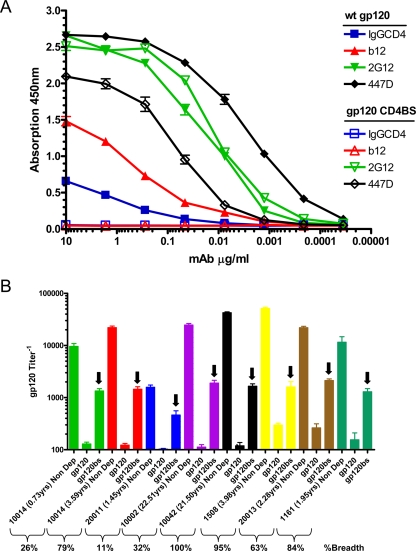
The CD4-BS mutant gp120 is not recognized by anti-CD4-BS antibodies, but it is recognized by antibodies directed to other regions of the HIV Env (Fig. 7). All plasma samples contained antibodies capable of recognizing the CD4-BS, as evidenced by the fact that the flowthrough from plasma adsorbed on the CD4-BS mutant gp120 contained antibodies capable of recognizing WT gp120 (Fig. 7B). In fact, very similar titers of anti-CD4-BS antibodies were present in plasmas with very different breadths of cross-NAb responses. In contrast, the adsorption of plasmas with WT gp120 was able to remove nearly all of the gp120-binding antibodies (Fig. 7B).
FIG. 7.
Detection of anti-CD4-BS antibodies in HIV+ plasmas. (A) Recognition of WT gp120 and CD4-BS mutant gp120 by specific anti-gp120 MAbs. IgG-CD4, chimeric molecule that binds to the CD4-BS of gp120; b12, an antibody that binds to an epitope that overlaps the CD4-BS; 447D, anti-V3 antibody; 2G12, an antibody that binds to a complex conformational epitope on gp120 formed by mannose residues. Closed symbols denote WT gp120 and open symbols denote CD4-BS mutant gp120 as the antigen in the ELISA. (B) Recognition of WT gp120 by the flowthroughs from WT gp120 and the CD4-BS mutant gp120 shown for seven HIV+ subjects with distinct breadths of NAb responses. Non Dep, relative end-point ELISA antibody titers in plasma; gp120, flowthrough following plasma's mixing with beads coated with WT gp120; and gp120bs, flowthrough following plasma's mixing with beads coated with CD4-BS mutant gp120. Arrows indicate the relative titers of antibodies binding to the CD4-BS of gp120.
The flowthrough from WT gp120 of plasma VC10042 had no neutralizing activity against any of the isolates tested, while the flowthrough from the CD4-BS mutant gp120 retained most of this plasma's neutralizing activity (Fig. 5A and Table 3). The antibodies eluted from WT gp120 displayed cross-neutralizing activity, while those eluted from the CD4-BS mutant gp120 did not (Fig. 5B and Table 3). Therefore, the exceptionally broad cross-neutralizing activity of this plasma is due exclusively to anti-CD4-BS antibodies.
The flowthrough from either WT gp120 or the CD4-BS mutant gp120 of plasma VC10014 did not display any neutralizing activity against any of the viruses tested (Fig. 5C and Table 3). The antibodies eluted from either gp120 had similar neutralizing activities (Fig. 5D and Table 3). Thus, although the neutralizing activity of this plasma targets the gp120 subunit exclusively, as is the case for VC10042, it appears that the cross-reactive NAbs in this plasma do not target the CD4-BS.
The flowthrough from WT gp120 of plasma CC1161 displayed reduced neutralizing activity compared to that of the nonadsorbed plasma against YU-2 and QH0692 but not against JRFL and TRO.11 (Fig. 5E and Table 3). The flowthrough from the CD4-BS mutant gp120 neutralized all four isolates tested (Fig. 5E and Table 3). The antibodies eluted from WT gp120 neutralized all isolates, but those eluted from the CD4-BS gp120 neutralized TRO.11 and JRFL but not YU-2 or QH0692 (Fig. 5F and Table 3). These results suggest that anti-CD4BS NAbs partially contribute in the neutralization of YU-2 and QH0692 but not in the neutralization of JRFL and TRO.11 by this plasma. Thus, it appears that plasma from CC1161 neutralizes distinct primary isolates through multiple neutralization targets on Env.
The neutralizing activity of the flowthrough from WT gp120 of plasma VC10002 was only minimally reduced compared to that of the nondepleted plasma against all isolates tested (Fig. 5G and Table 3) (YU-2 was not susceptible to neutralization by this plasma). Similar observations were made for the flowthrough from the CD4-BS mutant gp120 (Fig. 5G and Table 3). The eluted antibodies from both WT gp120 and the CD4-BS mutant gp120 displayed cross-neutralizing activities of similar breadth and potencies (Fig. 5H and Table 3). Overall, these results suggest that anti-CD4-BS NAbs do not contribute significantly in defining the overall cross-neutralizing activity of this plasma. NAbs that bind to gp41 or the oligomeric Env potentially are responsible for the neutralizing potential of this plasma.
For plasmas CC1508 and VC20013, the flowthroughs from both WT gp120 and the CD4-BS mutant gp120 displayed only a minimal reduction in neutralization potency and breadth compared to that of the nonadsorbed plasma, and the antibodies eluted from WT gp120 or the CD4-BS mutant gp120 exhibited various degrees of neutralizing activity against one or two, but not all, of the primary isolates tested (Table 3). These results indicate that the anti-gp120 NAbs in plasmas CC1508 and VC20013 contribute only minimally to the overall neutralizing potential of this plasma. As seen with plasma VC10002, anti-gp41 antibodies may constitute the majority of the cross-reactive NAbs in this plasma. However, it is noteworthy in the case of VC20013 that the antibodies eluted from either WT gp120 or the CD4-BS mutant gp120 neutralized JRFL and not any of the other isolates tested. In contrast, in the case of CC1508 the antibodies eluted from either WT gp120 or the CD4-BS mutant gp120 neutralized QH0692 and TORNO but not JRFL. This suggests that in the first case, the plasma contains anti-gp120 antibodies that bind to epitopes on the JRFL Env that are not present or exposed on the other isolates, while in the second case the plasma contains antibodies with different epitope recognition properties that recognize epitopes on QH0692 and TRO.11 but not JRFL. Therefore, in some cases different antibodies present in plasma may mediate neutralization depending on the viral Env target.
As expected, the flowthrough of the gp120-eluted antibodies from plasma of VC20011 did not display any neutralizing activity against the isolates tested (Table 3), a consequence of the very narrow cross-neutralizing activity of this plasma.
DISCUSSION
Our study indicates that in order for cross-reactive NAbs to develop during HIV infection, viral replication must be maintained for years. Most likely, and as suggested by the correlation between the binding avidity index of anti-Env antibodies and the breadth of plasma NAb responses, this continuous antigenic stimulation leads to the maturation of B cells and the emergence of antibodies that bind with high affinity to the viral Env. A prolonged viral replication is not, however, the sole factor associated with the emergence of cross-reactive NAbs. The epitope specificity of the NAbs that are gradually developed during infection also influences the breadth of the NAb responses.
Antibodies to the variable regions of HIV Env do not contribute significantly to the cross-neutralizing activity of plasmas, as others have also reported (10), although anti-V3 antibodies, which are generated by the majority of HIV-infected individuals we tested here irrespective of the overall breadths of NAb responses, can be effective against certain primary isolates with an exposed V3 loop, such as SF162. We assume that such viral variants are rapidly eliminated from the circulation. The reason why anti-V3 antibodies do not neutralize primary isolates may be due to the overall occluded nature of the V3 loop within the Env trimer (5, 31, 32, 35, 37, 38). It is possible that such antibodies neutralize some of the primary isolates once the virus binds to cell surface CD4 and undergoes conformational changes that expose the V3 loop. We did not test this possibility here. The observation that anti-V1 or anti-V2 antibodies with cross-neutralizing activities were not detected is not surprising either, since the V1-V2 region is extremely variable and diversifies continuously and very rapidly during infection (28, 30). However, it is very possible that anti-V1V2 antibodies contribute to the neutralization of autologous virus in these patients (29).
We note that the competition experiments performed with linear peptides may not permit the identification of antibodies that target the variable regions as part of a more complex conformational epitope. It is possible that some plasmas in our cohorts contain such antibodies. If the epitope of such antibodies is formed by variable regions, then most likely these antibodies will not display broad breadths of neutralization. An example of this is MAb 2909, which recognizes a quaternary epitope formed by the V2 and V3 loops that was isolated from an HIV-infected patient and neutralizes only SF162 (11). If, however, the epitopes of such antibodies are composed of both variable and constant regions, then they may display a broader neutralizing potential.
It is well known that anti-CD4-BS antibodies are generated after HIV infection (21, 23), and MAbs isolated from HIV+ subjects display different abilities to neutralize HIV. In fact, the only broadly cross-neutralizing anti-CD4-BS MAb known is b12 (3, 14, 25, 40). What is not yet well established is how prevalent anti-CD4-BS antibodies are during HIV infection and what their contributions are to the overall cross-neutralizing potential of plasma. In agreement with others (18), we observed that the broader and more potent cross-NAb responses in the two cohorts examined here were due to the presence of NAbs that bind to the CD4-BS of the HIV Env on the gp120 subunit (VC10042). However, HIV+ subjects with such exceptionally broad NAb responses are rare even among the antiretroviral-naïve, healthy individuals we examined here, some of whom were infected with HIV for more than a decade. In fact, plasmas displaying narrow or moderate breadths of NAb responses also contained anti-CD4-BS antibodies, but they were of limited cross-neutralizing potential. Therefore, the presence of anti-CD4-BS antibodies in plasma does not guarantee that the plasma will possess a broad cross-neutralizing capability. Different epitopes within the CD4-BS potentially are targeted by the B cells of individual HIV+ patients, and only rare individuals develop broadly neutralizing anti-CD4-BS antibodies. Mapping the precise epitope within the CD4-BS that these broadly cross-reactive anti-CD4-BS NAbs recognize may help us understand their broad cross-reactivity and could assist the design of more effective Env-based immunogens. The fact that the cross-reactive anti-CD4-BS NAbs in VC10042 neutralized viruses that are resistant to b12-mediated neutralization (such as 6101.1, TRO.11, and PVO [17]) suggests that their epitope(s) and that of MAb b12 differ.
Another HIV Env epitope identified as being recognized by plasma cross-reactive NAbs was that of MAb 4E10, currently the most broadly cross-neutralizing MAb known (3). Although several studies discussed the presence of binding anti-MPER antibodies in HIV+ plasmas (12, 24, 26), there has not been a report on the presence of antibodies that recognize the 4E10 epitope and that possess neutralizing activities. In fact, it was proposed that due to the cross-reactivity of MAb 4E10 with self antigens, such as certain membrane lipids, B cells that secrete 4E10-like antibodies are eliminated during B-cell differentiation (13). The identification in this study of a single HIV+ patient who developed NAbs that bind to the 4E10 epitope out of the 39 patients that were evaluated suggests that NAbs against this epitope are indeed rarely developed during HIV infection. This also suggests that although similar antibodies can be elicited by immunization, this is a very difficult task.
Since the only gp41-derived peptides we used here were those representing the epitopes recognized by MAbs 2F5 and 4E10, we do not know if the plasmas tested here contain NAbs that bind to other regions of the MPER, as a recent study discussed (2). It is also possible that in some cases the presence of high titers of antibodies not directed to the 2F5 or 4E10 epitope masks the presence of 4E10-like or 2F5-like NAbs in the peptide competition format. Although future studies will examine this possibility in more detail, we note that in the case of plasma VC10028 the presence of high levels of anti-V3 antibodies did not prevent the detection of 4E10-like antibodies, even against SF162 (Fig. 5E).
Plasma VC10028 exhibited moderate breadths of neutralization (∼60%) and was collected approximately 11 years postinfection (Fig. 1), with plasma viremia ranging from 103 to 104 RNA copies per ml. The fact that plasma VC10028, which contained 4E10-like NAbs, displayed only moderate breadths of NAb responses and failed to neutralize several isolates that are susceptible to neutralization by MAb 4E10 (for example, YU-2, 6101, QH0515.1, BG1168.1, and PVO) suggests that different amino acids within the 4E10 epitope are recognized by this MAb and the plasma 4E10-like antibodies. Alternatively, the titer of these antibodies in VC10028 is low and not sufficient to neutralize all isolates tested. If this is the case, then the fact that plasma isolated 11.8 years postinfection did not neutralize the QH0692 virus but plasma collected 14.7 years postinfection did neutralize it (Fig. 2) may be due to changes in the titers of these 4E10-like NAbs during this period. The eventual isolation and characterization of MAbs from this subject will be very useful in better understanding the epitope specificity and neutralization potential of such 4E10-like NAbs.
Several plasmas (such as VC10014) were identified that displayed broad NAb responses that were directed to gp120 but which we could not precisely map. At this point, we cannot completely eliminate the possibility that they bind some epitope overlapping the CD4-BS, since the CD4-BS mutant gp120 we used during the adsorption studies may still be recognized by certain anti-CD4-BS antibodies. Overall, however, our study and a recent one by Binley et al. (2) suggest that cross-reactive NAbs that bind to as-yet unidentified conserved epitope(s) are generated during HIV infection. Identifying such an epitope obviously is important for HIV Env-based immunogen design strategies.
Our observation that plasma samples collected from the same HIV+ subject within a few months of one another could neutralize different isolates, or the same isolates but with different potencies, is indicative of a continuous evolution of the NAbs and is the result of changes in both the titers and epitope specificities of these antibodies during short periods of time. Such changes may reflect the attempt of the immune system to counteract the continuous emergence of escape viral mutants (39). The subjects we examined here were asymptomatic and had stable CD4+ T lymphocyte numbers, and therefore their immune systems were capable of continuously adapting to the evolving viral Env. It will be important to examine, using longitudinal samples, whether the epitope specificities of the cross-reactive NAbs developed by individual patients remain the same or change over time. It also would be informative to assess whether the emergence of cross-reactive NAbs with particular epitope specificities impacts the levels of plasma viremia and whether cross-reactive NAbs of a particular epitope specificity have a greater impact on plasma viremia levels.
Overall, our results strongly suggest that more than one pathway leads to the development of broadly cross-neutralizing antibodies during HIV infection. From the point of vaccine development, this means that there is more than one way to elicit cross-reactive NAbs by immunization.
Acknowledgments
These studies were supported in part by NIH grant R01 AI47708 and a Collaboration for AIDS Vaccine Development Center grant from the Bill and Melinda Gates Foundation. We also acknowledge financial support by the M. J. Murdock Charitable Trust and the J. B. Pendleton Charitable Trust.
We also thank L. Corey, R. Strong, and W. Schief for the critical reading of the manuscript and many helpful suggestions.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Backliwal, G., M. Hildinger, V. Hasija, and F. M. Wurm. 2008. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng. 99721-727. [DOI] [PubMed] [Google Scholar]
- 2.Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 24 September 2008. Profiling the specificity of neutralizing antibodies in a large panel of HIV-1 plasmas from subtype B and C chronic infections. J. Virol. 8211651-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blish, C. A., R. Nedellec, K. Mandaliya, D. E. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21693-702. [DOI] [PubMed] [Google Scholar]
- 5.Bou-Habib, D. C., G. Roderiquez, T. Oravesz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 686006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, B. K., L. Wieczorek, E. Sanders-Buell, A. R. Borges, M. L. Robb, D. L. Birx, N. L. Michael, F. E. McCutchan, and V. R. Polonis. 2008. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 375529-538. [DOI] [PubMed] [Google Scholar]
- 7.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. J. Exp. Med. 332201-208. [DOI] [PubMed] [Google Scholar]
- 8.Cheng-Mayer, C., J. Homsy, L. A. Evans, and J. A. Levy. 1988. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc. Natl. Acad. Sci. USA 852815-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 808745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon, A. K., H. Donners, R. Pantophlet, W. E. Johnson, J. M. Decker, G. M. Shaw, F. H. Lee, D. D. Richman, R. W. Doms, G. Vanham, and D. R. Burton. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 816548-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorny, M. K., L. Stamatatos, B. Volsky, K. Revesz, C. Williams, X. H. Wang, S. Cohen, R. Staudinger, and S. Zolla-Pazner. 2005. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J. Virol. 795232-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, and L. Morris. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 816187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes, B. F., J. Fleming, E. W. St. Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 3081906-1908. [DOI] [PubMed] [Google Scholar]
- 14.Herrera, C., C. Spenlehauer, M. S. Fung, D. R. Burton, S. Beddows, and J. P. Moore. 2003. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J. Virol. 771084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, S. L., and L. Stamatatos. 2007. Prospects of HIV Env modification as an approach to HIV vaccine design. Curr. HIV Res. 5507-513. [DOI] [PubMed] [Google Scholar]
- 16.Kostrikis, L. G., Y. Cao, H. Ngai, J. P. Moore, and D. D. Ho. 1996. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J. Virol. 70445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Y., S. A. Migueles, B. Welcher, K. Svehla, A. Phogat, M. K. Louder, X. Wu, G. M. Shaw, M. Connors, R. T. Wyatt, and J. R. Mascola. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 131032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 783279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 17360-67. [DOI] [PubMed] [Google Scholar]
- 21.Moore, J. P., Y. Cao, D. D. Ho, and R. A. Koup. 1994. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J. Virol. 685142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 serotypes. J. Virol. 70427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, J. P., and D. D. Ho. 1993. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J. Virol. 67863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opalka, D., A. Pessi, E. Bianchi, G. Ciliberto, W. Schleif, M. McElhaugh, R. Danzeisen, R. Geleziunas, M. Miller, D. M. Eckert, D. Bramhill, J. Joyce, J. Cook, W. Magilton, J. Shiver, E. Emini, and M. T. Esser. 2004. Analysis of the HIV-1 gp41 specific immune response using a multiplexed antibody detection assay. J. Immunol. Methods 28749-65. [DOI] [PubMed] [Google Scholar]
- 25.Pantophlet, R., E. Ollmann Saphire, P. Poignard, P. W. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penn-Nicholson, A., D. P. Han, S. J. Kim, H. Park, R. Ansari, D. C. Montefiori, and M. W. Cho. 2008. Assessment of antibody responses against gp41 in HIV-1-infected patients using soluble gp41 fusion proteins and peptides derived from M group consensus envelope. Virology 372442-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritola, K., C. D. Pilcher, S. A. Fiscus, N. G. Hoffman, J. A. Nelson, K. M. Kitrinos, C. B. Hicks, J. J. Eron, Jr., and R. Swanstrom. 2004. Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J. Virol. 78:11208-11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong, R., F. Bibollet-Ruche, J. Mulenga, S. Allen, J. L. Blackwell, and C. A. Derdeyn. 2007. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 811350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybarczyk, B. J., D. Montefiori, P. R. Johnson, A. West, R. E. Johnston, and R. Swanstrom. 2004. Correlation between env V1/V2 region diversification and neutralizing antibodies during primary infection by simian immunodeficiency virus sm in rhesus macaques. J. Virol. 783561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattentau, Q. J., J. P. Moore, F. Vignaux, F. Traincard, and P. Poignard. 1993. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J. Virol. 677383-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 799069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava, I. K., E. Kan, Y. Sun, V. A. Sharma, J. Cisto, B. Burke, Y. Lian, S. Hilt, Z. Biron, K. Hartog, L. Stamatatos, R. H. Cheng, J. B. Ulmer, and S. W. Barnett. 2008. Comparative evaluation of trimeric envelope glycoproteins derived from subtype C and B HIV-1 R5 isolates. Virology 372273-290. [DOI] [PubMed] [Google Scholar]
- 35.Stamatatos, L., and C. Cheng-Mayer. 1993. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J. Virol. 675635-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum Retrovir. 171757-1765. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 694413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 724694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, M. Y., X. Xiao, I. A. Sidorov, V. Choudhry, F. Cham, P. F. Zhang, P. Bouma, M. Zwick, A. Choudhary, D. C. Montefiori, C. C. Broder, D. R. Burton, G. V. Quinnan, Jr., and D. S. Dimitrov. 2004. Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody. J. Virol. 78:9233-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]