Abstract
Fragile X syndrome is the most common inherited form of cognitive deficiency in humans and perhaps the best-understood single cause of autism. A trinucleotide repeat expansion, inactivating the X-linked FMR1 gene, leads to the absence of the fragile X mental retardation protein. FMRP is a selective RNA-binding protein that regulates the local translation of a subset of mRNAs at synapses in response to activation of Gp1 metabotropic glutamate receptors (mGluRs) and possibly other receptors. In the absence of FMRP, excess and dysregulated mRNA translation leads to altered synaptic function and loss of protein synthesis-dependent plasticity. Recent evidence indicates the role of FMRP in regulated mRNA transport in dendrites. New studies also suggest a possible local function of FMRP in axons that may be important for guidance, synaptic development, and formation of neural circuits. The understanding of FMRP function at synapses has led to rationale therapeutic approaches.
Introduction
Geneticists first choose an interesting phenotype, then search for the responsible mutation and hope subsequent studies provide mechanistic insight into biology. Fragile X syndrome (FXS) and the FMR1 gene have proved how effective this paradigm can be. FXS is the most frequent form of inherited mental retardation and often presents as an autism spectrum disorder (Garber et al., 2008). This X-linked disorder was notable for its unusual inheritance pattern, referred to as the Sherman paradox. Sherman had shown in the 1980s the occurrence of nonpenetrant male carriers in fragile X syndrome families, an unusual observation for an X-linked disorder, and that these males could transmit their alleles to nonpenetrant daughters who could then have affected sons (Sherman et al., 1985). Moreover, a carrier female’s risk of having an affected son was related to her pedigree position relative to such nonpenetrant males, in essence showing increased penetrance of the fragile X mutation as the mutant gene passed to subsequent generations. Another feature stimulating interest was the poorly understood phenomenon of a “fragile site” or unstaining gap on the metaphase X chromosome among affected individuals that segregated with the mutant gene (Penagarikano et al., 2007). In 1991, the responsible gene was identified by positional cloning and named the Fragile X Mental Retardation 1 gene (Verkerk et al., 1991). The FMR1 gene resides precisely at the cytogenetic fragile X site and was the first example of a trinucleotide repeat mutation. Within the 5′-untranslated region of FMR1 is a polymorphic CGG repeat with the most common normal length of 30 triplets (Figure 1). Among individuals with FXS, this repeat is found to be expanded beyond 200 repeats, typically ~800 repeats, and is referred to as the full mutation. Alleles with an intermediate repeat length (55–200 repeats) are called premutations, typically found in FXS families, accounting for the nonpenetrant males observed by Sherman. Their daughters also are premutation carriers. However, premutation alleles are unstable in meiosis, particularly in female meiosis, often increasing in length from one generation to the next, with the chance of expansion into the full mutation range positively correlated with maternal premutation repeat length. Thus, the repeat length and instability accounts for the Sherman paradox (Fu et al., 1991). Although beyond the scope of this review, premutation alleles are themselves associated with phenotypes not found in FXS, such as an adult-onset, primarily male, neurodegenerative disorder and in female premutation carriers, primary ovarian insufficiency (Hagerman and Hagerman, 2007). The mechanism of premutation disease is likely RNA mediated (Swanson and Orr, 2007).
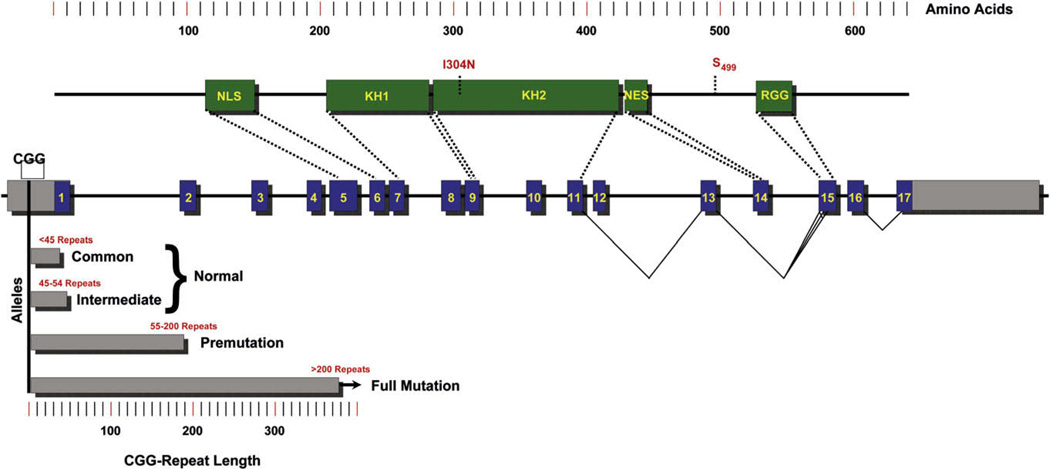
Figure 1. FMR1 Protein and Gene.
(Top) Protein domains (green) and key residues (red). NLS, nuclear localization signal; KH1 and KH2, RNA-binding domains; NES, nuclear export signal; RGG, RGG box, RNA binding. I304N, naturally occurring FXS mutation abrogating polysome association; S499, primary phosphorylated serine. (Middle) FMR1 gene, coding exons (blue) and untranslated regions (gray). Exons coding for major protein domains are indicated as well as alternative splicing. (Bottom) 5′ untranslated CGG-repeat alleles. The common and intermediate normal alleles (<55 repeats) are indicated, as are the premutation carrier alleles (55–200 repeats) and the full-mutation FXS alleles (>200 repeats).
In FXS, the full mutation allele is subjected to repeat-induced silencing by a poorly understood mechanism, leaving this locus heavily methylated with heterochromatic marks (Coffee et al., 1999; Sutcliffe et al., 1992). Hence, the full mutation allele is silenced, and the absence of the encoded protein, FMRP, results in FXS. It should be noted that, since repeat expansion is believed to be the most prevalent mutation in FMR1, conventional mutations of FMR1, such as nonsense and missense mutations, are not routinely screened for clinically, despite the full mutation being only one among many mutational mechanisms that give rise to a null mutation. Thus, more attention on the conventional mutations may lead to a broader phenotypic spectrum than is currently recognized for FXS and give us a better understanding of key FMRP residues.
The FMR Protein
The FMR1 gene codes for the fragile X mental retardation protein, FMRP, an mRNA-binding protein (Ashley et al., 1993) that is expressed in many tissues and is abundant in the brain, where it is believed to regulate a substantial mRNA population. Indeed, FMRP is found to selectively bind ~4% of the mRNA in the mammalian brain. FMRP is predominantly a 71 kDa protein, although several isoforms exist as a result of alternative splicing (Figure 1). The physiological role of distinct isoforms remains understudied and poorly understood. The protein is highly conserved (92% identity between human and chicken) and in mammals is one of three paralogous proteins (with the FXR1 and FXR2 proteins), whereas in Drosophila there is but a single orthologous protein in this small vertebrate gene family. FMRP contains several highly conserved domains. Both nuclear localization and nuclear export signals are found in FMRP, consistent with the observation that FMRP shuttles between the nucleocytoplasmic space (Eberhart et al., 1996; Sittler et al., 1996); however, the role of FMRP in the nucleus has received scant attention since these early reports (Fridell et al., 1996). The best-characterized motifs in FMRP are those that interact with RNA: two hnRNP-K-homology (KH) domains and an arginine-glycine-glycine (RGG box).
Although precise details and function have yet to be elucidated, a stem-G-quartet loop in RNA is recognized by the RGG box in vitro (Schaeffer et al., 2001), and such G-quartet structures are found on several FMRP-associated mRNAs (Brown et al., 2001; Darnell et al., 2001). FMRP-ligand mRNAs containing G quartets include those encoding MAP1b,amicrotubule-associated protein important for axonal development; SAPAP3/4, which are postsynaptic scaffolding proteins that bind PSD-95; NAP-22, a calmodulin-binding protein associated with lipid rafts; Munc13, a SNARE-associated protein involved in neurotransmitter release; Rab6-interacting protein; and the axon guidance factor, semaphorin 3F. Molecular interactions between FMRP and Fmr1, MAP1b and Sema3F mRNAs involving G-quartet/quadruplex structures have been validated using biophysical methods (Menon et al., 2008; Menon and Mihailescu, 2007; Schaeffer et al., 2001). The coimmunoprecipitation of FMRP with MAP1b mRNA in mouse brain has been confirmed by different laboratories (Lu et al., 2004; Zalfa et al., 2003) and is also observed in Drosophila between dFMRP and futsch, the MAP1b homolog (Zhang et al., 2001), suggesting that the interaction between FMRP and at least one target mRNA is evolutionarily conserved. More recently, reversible crosslinking-IP (CLIP) showed that FMRP binds directly to PSD-95 mRNA in situ (Zalfa et al., 2007). FMRP binding to the 3′ UTR of PSD-95 mRNA in vitro involves the C terminus of FMRP, which contains the RGG box. While the 3′ UTR of PSD-95 mRNA does contain a putative G quartet (Todd et al., 2003), FMRP binding to this structured G-rich region was not disrupted by lithium (Zalfa et al., 2007), which does disrupt G-quartet-dependent binding to FMRP (Darnell et al., 2001). Recently, FMRP was also shown to interact by co-IP with a guanine-rich, G-quartet-like sequence in amyloid precursor protein (APP) mRNA (Westmark and Malter, 2007). These studies indicate that FMRP can bind to structured G-rich regions, including the canonical G quartet (Table 1).
Table 1.
Validated FMRP Target mRNAs
The criteria for inclusion on this short list are evidence for an FMRP association in vitro or in vivo, evidence for dendritic mRNA localization or synaptic synthesis, and evidence for mGluR regulation. Sema3F does not meet these criteria and is included here as one example of an FMRP ligand that may play an important role outside of the mGluR theory for FMRP function. (+) Indicates positive while no symbol reflects lack of direct examination or unclear results. Several methods have been used to assess FMRP-target mRNA interactions, i.e., co-IP, CLIP, APRA, and direct in vitro methods (filter binding, gel-shift, affinity capture, UV crosslinking, biophysical). A number of targets contain a validated G-quartet structure or G-rich region that is necessary for FMRP binding. G quartets are determined by various approaches in the literature and are therefore referred to as G-quartet-like structures. Those mRNAs indicated to be lacking a perfect G-quartet-like structure may still have approximations of DWGG-N(0–5)DWGG-N(0–3)-DWGG-N(0–2)-DWGG, derived from Table 1 of Darnell et al. (2001). For example, the binding of FMRP to the PSD-95 3′ UTR involved a structured G-rich region, within a predicted G quartet, although binding occurred in the presence of lithium (Zalfa et al., 2007). Note also that other modes of FMRP interaction have been described, such as U-rich sequences (Dolzhanskaya et al., 2003) and kissing complex structures (Darnell et al., 2005). Several of the above FMRP-target mRNAs have been shown to be regulated by mGluR stimulation, either at the level of mRNA transport, stability, translation, or protein expression.
Several biochemical studies have clearly shown that FMRP is associated with actively translating polyribosomes in both cultured neuronal and nonneuronal cells, as well as in brain synaptoneurosomes (Feng et al., 1997b; Khandjian et al., 2004; Stefani et al., 2004). The KH domains appear crucial for the interaction of FMRP with polyribosomes. The importance of these domains becomes obvious given the observation of a rare FXS patient harboring a missense mutation in the second KH domain that interferes with ribosome interaction (Feng et al., 1997a); this has been recapitulated in a knockin mouse (Darnell et al., personal communication). However, the role of the KH domains in binding specific mRNAs and the precise RNA targets of these domains are unclear. Darnell et al. reported an in vitro selected RNA structure that binds tightly to the KH2 domain, the so-called kissing complex (Darnell et al., 2005). This RNA could compete FMRP off polyribosomes, something the G-quartet structure was unable to do. Nevertheless, despite considerable effort, there has been no similar structure reported in any naturally occurring RNA molecule. It is possible that the kissing complex approximates the structure of two distinct RNA molecules interacting together with the KH domain. One notion, based on the association of FMRP mRNP complexes with microRNAs and protein components of the microRNA pathway, is that there is an interaction between specific microRNAs and target mRNAs, which together could be recognized by the KH domain (Jin et al., 2004). Another possibility is an interaction between mRNAs and the BC1 RNA, which reportedly interacts with FMRP (Zalfa et al., 2003). However, recent studies failed to verify a specific interaction between BC1 and FMRP, calling this interaction into question (Iacoangeli et al., 2008a) and generating some controversy (Bagni, 2008; Iacoangeli et al., 2008b). This debate notwithstanding, the precise function of the FMRP KH domains and the interaction of FMRP with noncoding RNAs, including miRNAs, remain important avenues of inquiry into the role of FMRP in translational regulation.
Fragile X Phenotype Suggests FMRP Affects Dendritic mRNA function
A hallmark of the FXS neuroanatomical phenotype is the hyperabundance of dendritic spines with a long, thin, and otherwise immature morphology (Grossman et al., 2006; Irwin et al., 2000). An Fmr1 KO mouse model for FXS exhibits a similar excess of long, thin spines (Comery et al., 1997). Furthermore, Fmr1 KO mice display altered learning and behavior, greater susceptibility to seizures, and altered synaptic plasticity (Penagarikano et al., 2007). Since FMRP is associated with polyribosomes and localized to dendrites and spines (Antar et al., 2004; Feng et al., 1997b), it is hypothesized to regulate the local protein synthesis important for spine development and the synaptic plasticity so critical for learning and memory (Antar and Bassell, 2003; Bagni and Greenough, 2005; Grossman et al., 2006). The synaptic phenotypes in FXS are believed to result from impaired translational regulation. While the presence of polyribosomes within spines have driven studies to find numerous mRNAs that are selectively transported into dendrites and translated at synapses (Bramham and Wells, 2007), the identity of specific mRNA-binding proteins that play a direct role in mRNA transport or translational regulation remained elusive until recently (Kiebler and Bassell, 2006).
Although FMRP is an attractive candidate in mRNA localization and local protein synthesis, at the time of the original microarray analysis of FMRP targets in the brain (Brown et al., 2001), it was not known whether any of these were bona fide dendritically localized mRNAs. Since that time, subsequent fluorescence in situ hybridization (FISH) analysis demonstrated that FMRP colocalized with MAP1b mRNA in dendrites of cultured neurons (Antar et al., 2005). In addition, dendritic mRNA localization was observed for SAPAP3 (Kindler et al., 2004), already appreciated to be an FMRP ligand (Brown et al., 2001). Using an alternative microarray approach in cultured neurons to identify FMRP-associated mRNAs, a few were shown to be localized to dendrites, including the mRNA encoding regulator of G protein signaling, RGS5 (Miyashiro et al., 2003). A candidate-based approach revealed that the mRNAs encoding the α subunit of calcium/calmodulin-dependent protein kinase II, α-CaMKII, and the activity-regulated cytoskeletal-associated protein, Arc/Ar3.1, were both coprecipitated with FMRP in brain extracts (Zalfa et al., 2003). Since α-CaMKII and Arc/Arg3.1 are dendritically localized mRNAs that appear to be translated at synapses (Bramham and Wells, 2007), this provides further evidence that FMRP plays some role in dendritic mRNA regulation. More recently, PSD-95 mRNA was shown to be dendritically localized and directly associated with FMRP in vitro and in vivo (Muddashetty et al., 2007; Zalfa et al., 2007). Taken together, these studies are consistent with the possibility that FMRP might bind and regulate a subset of dendritic mRNAs.
While FMRP has been shown to associate with dendritic mRNAs, it does not appear to be necessary for the constitutive maintenance of their localization within dendrites. Analysis of dendritic mRNA localization in vivo using in situ hybridization failed to show any obvious differences in mRNA levels in dendrites between WT and Fmr1 KO brain (Steward et al., 1998). MAP2, α-CaMKII, and dendrin mRNAs, which are present constitutively in dendrites, were indistinguishable between WT and Fmr1 KO. Furthermore, the rapid transport of Arc/Arg3/1 mRNA into dendrites following seizures was not impaired in Fmr1 KO brain. A more recent study found no gross reduction in dendritic mRNA levels for α-CaMKII or PSD-95 in brain sections of cortex, hippocampus, or dentate gyrus (Muddashetty et al., 2007). Notably, quantitative FISH analysis of α-CaMKII and PSD-95 mRNA levels in dendrites of cultured neurons showed no significant differences (Muddashetty et al., 2007). However, one study reported that some APRA-identified FMRP ligands (i.e., RGS5) did display modestly reduced mRNA levels within dendrites of brain sections (Miyashiro et al., 2003). Combined, these data indicate that FMRP does not play a role in the steady-state maintenance or constitutive localization for the majority of FMRP-associated mRNAs present in dendrites, but this does not rule out the possibility that FMRP may influence the localization of a small subset of these. It is also possible that observations of modest reduction in dendritic mRNA localization may be due to reduced total mRNA levels, suggestive of a role for FMRP in mRNA stability (Miyashiro et al., 2003), which could modulate mRNA localization indirectly. With regard to PSD-95 mRNA, FMRP was found to modulate mRNA stability in hippocampal, but not cortical neurons (Zalfa et al., 2007). Total PSD-95 mRNA levels were dramatically reduced in hippocampus, but not cerebral cortex (Zalfa et al., 2007). In situ hybridization signal for PSD-95 mRNA within the molecular layer of hippocampus was modestly reduced in Fmr1 KO, although this result was not statistically significant (Zalfa et al., 2007). Nonetheless, reduced total levels of PSD-95 mRNA could explain the trend for reduced dendritic localization. Of interest, PSD-95 3′ UTR reporter mRNAs were less stable following transcriptional inhibition of FMRP-deficient hippocampal neurons. In contrast to the results in hippocampus (Zalfa et al., 2007), both Zalfa et al. (2007) and Muddashetty et al. (2007) reported that PSD-95 mRNA levels did not differ between wild-type and Fmr1 KO in cortex. Thus, further work is needed to understand a possible cell-type-specific role for FMRP in mRNA stability in hippocampus. In that mechanisms of mRNA stability can be linked to localization (Kiebler and Bassell, 2006), it will be interesting to assess a possible dual role for FMRP. However, it is likely that other mRNA-binding proteins are necessary for the steady state and/or maintenance of mRNA levels within dendrites, whereas FMRP may modulate localization, perhaps at the level of mRNA stability. More dynamic assays in live neurons are needed to rigorously assess a possible direct role for FMRP in the mechanism of dendritic mRNA transport (to be discussed in a later section).
FMRP Regulation of Local Translation: Excess and Dysregulated in Fragile X
FMRP can repress mRNA translation in vitro (Laggerbauer et al., 2001; Li et al., 2001), and a general consequence of FMRP deficiency in vivo is excess synthesis of specific proteins (Lu et al., 2004; Muddashetty et al., 2007; Zalfa et al., 2003). Synaptoneurosomes (SNS) from Fmr1 KO mice have increased levels of Map1b, α-CaMKII, and Arc protein, as well as higher levels of these mRNAs in polyribosomal fractions, suggesting excess translation at synapses (Zalfa et al., 2003). In a subsequent study, the Fmr1 KO phenotype was characterized by the excess translation of mRNAs at the basal state and loss of stimulus-induced translation in cortical synaptoneurosomes (Muddashetty et al., 2007). Quantitative real-time PCR analysis of α-CaMKII, PSD-95, and GluR1/2 mRNA levels in polysome gradient fractions from WT synaptoneurosomes showed that these mRNAs are normally recruited into actively translating polysomes in response to stimulation with DHPG, a Gp1 agonist of mGluRs. However, in Fmr1 KO mice, this stimulus-induced RNA shift is absent; rather, these mRNAs are elevated in polyribosomes at the basal state, essentially mimicking the phenotype observed in WT upon stimulation. Using metabolic labeling of SNS with 35S-methionine, this study showed that the DHPG-induced synthesis of PSD-95 and α-CaMKII protein was absent in Fmr1 KO mice. There was no difference in mRNA abundance in cortical synaptoneurosomes from WT and Fmr1 KO mice, consistent with quantitative FISH analysis of mRNA levels in the dendrites of cultured cortical neurons (Muddashetty et al., 2007). These findings (Muddashetty et al., 2007) serve to reconcile past observations of translational excess at steady state (Lu et al., 2004; Zalfa et al., 2003) with reports of deficient mGluR-induced protein synthesis (Todd et al., 2003; Weiler et al., 2004; Westmark and Malter, 2007) by showing that specific mRNAs have both characteristics in Fmr1 KO mice; namely, excess basal translation and loss of mGluR-induced translation. Thus, although total mRNA levels in dendrites and at synapses are not reduced in the absence of FMRP, these mRNAs are translationally dysregulated.
The prevalent view in fragile X syndrome is that the synaptic dysfunction and cognitive impairment are the result of excess protein synthesis at the synapse (Ronesi and Huber, 2008). While the excess translation is likely to be a major culprit, it may be of equal concern that there is a loss of stimulus-induced translation. The inability of synapses in the FXS brain to control where and when translation precisely occurs and the inability to augment translation in response to synaptic activity are likely to have consequences on long-term plasticity, which influences learning and memory. Indeed, a loss of protein synthesis-dependent plasticity has been observed, at least with regard to Gp1 mGluR LTD (Hou et al., 2006; Huber et al., 2002). Thus, the concept of translational dysregulation at FMRP-deficient synapses is likely to have important ramifications for the mechanism of synaptic dysfunction and cognitive impairment in FXS.
FMRP Function in Synaptic Plasticity and the mGluR Theory of FXS
A critical goal has been to investigate how FMRP-mediated translational regulation is involved in long-term synaptic plasticity. One form of synaptic plasticity with a now well-established link to FMRP is a type of long-term depression (LTD) that depends on activation of Gp1 metabotropic glutamate receptors (mGluRs) (Ronesi and Huber, 2008). mGluR-dependent LTD, which normally depends on the dendritic protein synthesis needed for the persistent internalization of AMPAR, is enhanced in Fmr1 KO mice and occurs independently of protein synthesis (Huber et al., 2002; Nosyreva and Huber, 2006; Ronesi and Huber, 2008). The mGluR theory of fragile X syndrome posits that FMRP normally acts as a negative regulator of translation downstream of Gp1 mGluRs, and in the absence of FMRP, there is runaway protein synthesis that leads to excess AMPAR internalization and exaggerated LTD (Bear et al., 2004). In addition, the mGluR theory suggests that many of the synaptic phenotypes in FXS may be directly attributed to exaggerated mGluR signaling, and therefore mGluR antagonists could be a useful therapy for FXS. There is solid evidence to support this theory: a number of phenotypes in animal models of FXS, including morphologic, physiological, and behavioral impairments, have been corrected either by administration of MPEP, an mGluR5 antagonist (McBride et al., 2005; Tucker et al., 2006; Yan et al., 2005), or genetic reduction of mGluR5 (Dolen et al., 2007; Bassell and Gross, 2008). FMRP-deficient neurons display excessive internalization of AMPAR at the basal state, but this defect can be corrected by MPEP (Nakamoto et al., 2007). Exaggerated Gp1 mGluR signaling is the cause of prolonged epileptiform discharges in hippocampal slices from Fmr1 KO (Chuang et al., 2005). MPEP reduces the excess protein synthesis in hippocampal slices from Fmr1 KO mice toward basal levels, suggesting a direct correlation between the translational impairment and FX phenotype (Dolen et al., 2007). In further support of this theory, stimulation of Gp1 mGluRs can induce dendritic spine elongation in vitro, reminiscent of the Fmr1 knockout mice (de Vrij et al., 2008; Vanderklish and Edelman, 2002), and excess filopodial-spines present in Fmr1 KO neurons in vitro are corrected by MPEP (de Vrij et al., 2008). Collectively, these data provide strong support for the mGluR theory. It will be interesting to assess the mechanism whereby specific translational responses may be normalized by MPEP treatment. One implication is that the excess protein synthesis has been lessened (Dolen et al., 2007). However, it is also possible that reduction of basal translation, using MPEP, has enabled stimulus-induced translation to now occur, either through Gp1 mGluRs (i.e., mGluR1) or other receptors. As discussed above, the loss of protein synthesis-dependent plasticity is a key feature of the synaptic impairment in fragile X syndrome and may represent the primary reason for cognitive impairment. Thus, for a therapeutic approach to improve cognition, learning, and memory in fragile X, there needs to be a restoration of stimulus-induced translation at synapses.
Is There an FMRP Connection to Arc Translation Needed for mGluR-LTD?
A major goal in the plasticity field has been identifying the specific locally synthesized proteins necessary for distinct forms of long-term synaptic plasticity. One well-studied gene with a revisited link to FMRP is the activity-regulated cytoskeleton-associated protein, Arc/Arg3.1, an immediate-early gene that is induced in response to numerous forms of activity, including those that induce long-term potentiation (Steward and Worley, 2001). Following synaptic activation, the mRNA encoding Arc/Arg3.1 is rapidly transported into dendrites and accumulates in regions of activated synapses in an NMDA receptor-dependent manner. Arc/Arg3.1 has also been shown to regulate AMPAR endocytosis through its interactions with dynamin and endophilin 2/3 (Castillo et al., 2008). Given that FMRP is known to associate with Arc mRNA and that synaptosomes from Fmr1 KO mice have excess Arc translation (Zalfa et al., 2003), one attractive model is that FMRP-mediated translation of Arc mRNA in dendrites may be necessary for the protein synthesis-dependent internalization of AMPAR underlying mGluR-LTD (Ronesi and Huber, 2008). However, Arc is neither known to be an LTD protein nor has it been linked to mGluR-dependent forms of synaptic plasticity.
Two exciting recent papers provide new evidence to support a model whereby Gp1 mGluRs stimulate the rapid translation of Arc necessary for AMPAR internalization and LTD. Park et al. (2008) show that mGluR-LTD is decreased albeit not eliminated in Arc/Arg3.1 KO mice (Park et al., 2008). In cultured hippocampal neurons from wild-type mice, the application of DHPG normally results in a rapid (5 min) and protein synthesis-dependent increase in Arc protein levels within the cell body and proximal dendrites, which is consistent with a model for local translation (Park et al., 2008). In the accompanying paper, Waung et al. (2008) provide three lines of evidence that bolster an argument for local Arc mRNA synthesis, including data showing that the local perfusion of DHPG onto severed dendrites results in a protein synthesis-dependent increase in Arc protein (Waung et al., 2008). Furthermore, acute blockade of new Arc synthesis prevents the persistent internalization of AMPAR in response to mGluR-LTD (Waung et al., 2008). The mechanism of mGluR-induced Arc translation appears to involve in part the general inhibition of local translation at the level of elongation by activation of eukaryotic elongation factor-2 kinase (eEF2K) (Park et al., 2008), although the mechanism by which that would favor the selective translation of Arc mRNA remains unclear.
The question becomes what is the role for FMRP in this model. Park et al. (Park et al., 2008) found that the rapid mGluR-dependent induction of Arc is absent in Fmr1 KO mice. These investigators also observed that mGluR-LTD was protein synthesis independent in Fmr1 KO mice, in keeping with a previous report, although curiously they did not observe the exaggerated LTD (Huber et al., 2002). Nonetheless, these data taken together with previous work showing that Arc mRNA may be an FMRP target suggest that elevated levels of Arc at synapses of FMRP-deficient neurons (Zalfa et al., 2003) could lead to persistent AMPAR internalization and altered LTD. Such a model can now be tested. It would be interesting to examine whether any defects in LTD or AMPAR trafficking in FMR1 KO mice could be rescued by genetic reduction of Arc expression (i.e., heterozygous mice). Further work is also needed to assess whether Arc synthesis in dendrites is impaired in Fmr1 KO, for example, by applying the indirect methods used by Waung et al. (2008) to examine dendritic synthesis of Arc in wild-type neurons. It is also important to determine that FMRP directly binds and represses Arc mRNA translation in dendrites, which may be translated in excess in FMRP-deficient neurons.
While dysregulated Arc expression may be a major underlying cause of the altered LTD and AMPAR trafficking defect in FXS, there are other candidates to consider who may play equivalent roles. It may not be a single protein whose dysregulation is responsible for the LTD/AMPAR phenotype. One candidate to consider is MAP1b, whose rapid synthesis has been recently linked to mGluR-LTD and AMPAR internalization (Davidkova and Carroll, 2007). MAP1b expression was shown earlier to be induced in hippocampal slices from WT, but not in Fmr1 KO, where its expression was elevated at basal state (Hou et al., 2006). Amyloid precursor protein, APP, is another FMRP-associated mRNA showing impaired DHPG-induced translation in Fmr1 KO (Westmark and Malter, 2007). Soluble forms of Aβ are elevated in Fmr1 KO mice (Westmark and Malter, 2007), and Aβ is known to promote AMPAR internalization during LTD (Hsieh et al., 2006). The FMRP target, PSD95 mRNA, which is translationally dysregulated in Fmr1 KO (Muddashetty et al., 2007) is believed to provide a scaffold necessary for AMPAR trafficking and LTD (Xu et al., 2008). Thus, future work is needed to identify these and other locally synthesized LTD proteins and assess their contribution to the defects in mGluR-dependent plasticity in Fmr1 KO mice (Ronesi and Huber, 2008).
Molecular Mechanism of FMRP-Mediated Repression and Derepression
A key question has been how FMRP-mediated translational repression is relieved or derepressed by activation of Gp1 mGluRs. Recent studies indicate that the phosphorylation status of FMRP, controlled by a PP2a/S6K1 signaling module, provides a dynamic mechanism to rapidly and bidirectionally regulate mRNA translation in neurons (Narayanan et al., 2007, 2008). Phosphorylated FMRP is known to associate with heavy (possibly stalled) polyribosomes, whereas dephosphorylated FMRP is run-off from heavy polysomes after azide treatment, suggesting a possible role for phosphorylation in ribosome stalling (Ceman et al., 2003). The primary phosphorylated FMRP residue, murine Ser 499, is conserved across all species from Drosophila to humans (human Ser 500; Drosophila Ser 406). A phospho-specific antibody to Ser 499 was used to demonstrate that the majority of FMRP in dendritic granules exists in a phosphorylated form (Narayanan et al., 2007) (Figure 2). Protein phosphatase 2A (PP2A) was identified as an FMRP phosphatase that can rapidly (<30 s) dephosphorylate FMRP in response to Gp1 mGluR activation. FMRP apparently begins to be rephosphorylated at 2 min, with much higher levels of phosphorylated FMRP noted at 10 min, although it is unclear whether it is the same FMRP molecules that are de- and rephosphorylated (Figure 3). FMRP rephosphorylation is known to partly involve mTOR-dependent PP2A suppression. It is believed that the rapid dephosphorylation of FMRP allows mRNAs to be translated, whereas rephosphorylation represses translation. Consistent with this model, total protein levels of PSD95 are modestly increased at 2 min and significantly elevated at 10 min following DHPG treatment. The PP2A inhibitor okadaic acid, but not other phosphatase inhibitors, blocked the rapid DHPG-induced dephosphorylation of FMRP and subsequent increase in protein levels for FMRP target mRNAs. Dendritic levels of phosphorylated FMRP are markedly reduced following a 30 s treatment with DHPG; this is blocked by okadaic acid. These data suggest that mGluR activation of PP2A and FMRP dephosphorylation provides a mechanism for rapid mRNA translation in dendrites, consistent with a model wherein FMRP inhibition of ribosome stalling is temporarily relieved (Narayanan et al., 2007). A prediction from these findings is that PP2A inhibition using okadaic acid may interfere with mGluR-LTD.
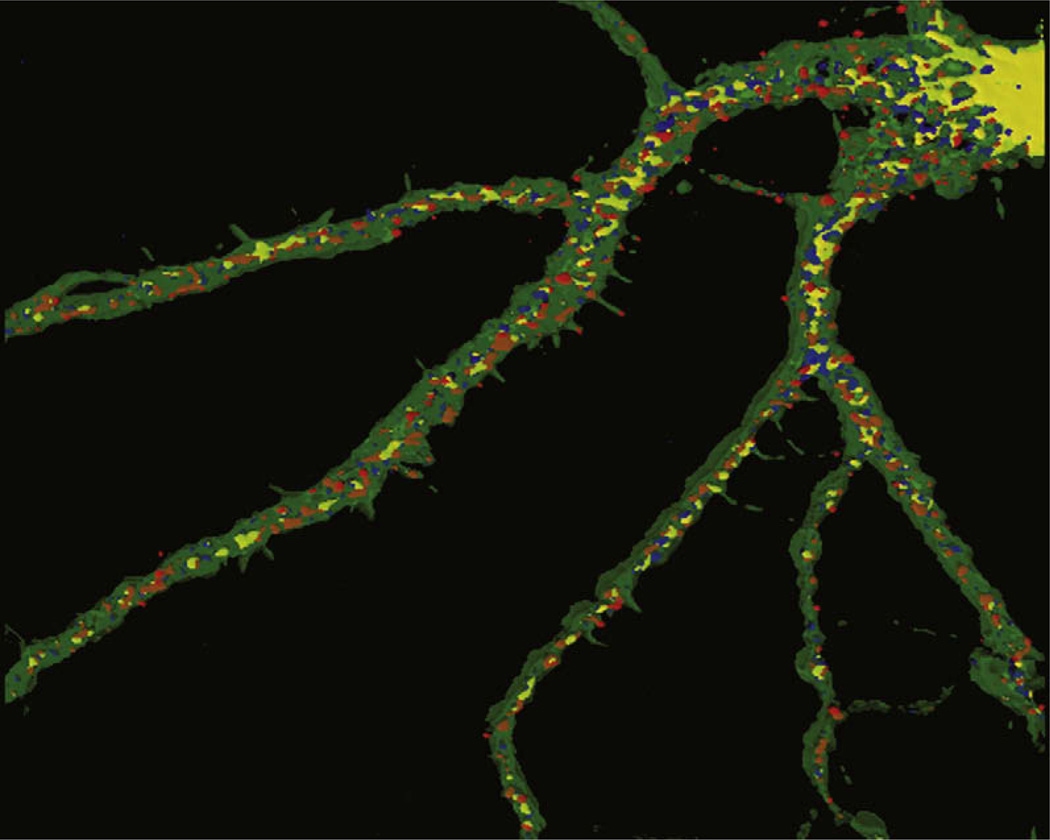
Figure 2. Visualization of Total and Phosphorylated FMRP in Dendrites and Spines.
Immunofluorescence and 3D reconstruction of a cultured hippocampal neuron labeled for phosphorylated FMRP (red), total FMRP (blue), and F-actin using phalloidin (green). Colocalization of FMRP and phospho-FMRP within granules is shown in white. FMRP is transiently dephosphorylated by PP2A in response to mGluR activation to allow translation of FMRP-bound mRNAs (Narayanan et al., 2007).
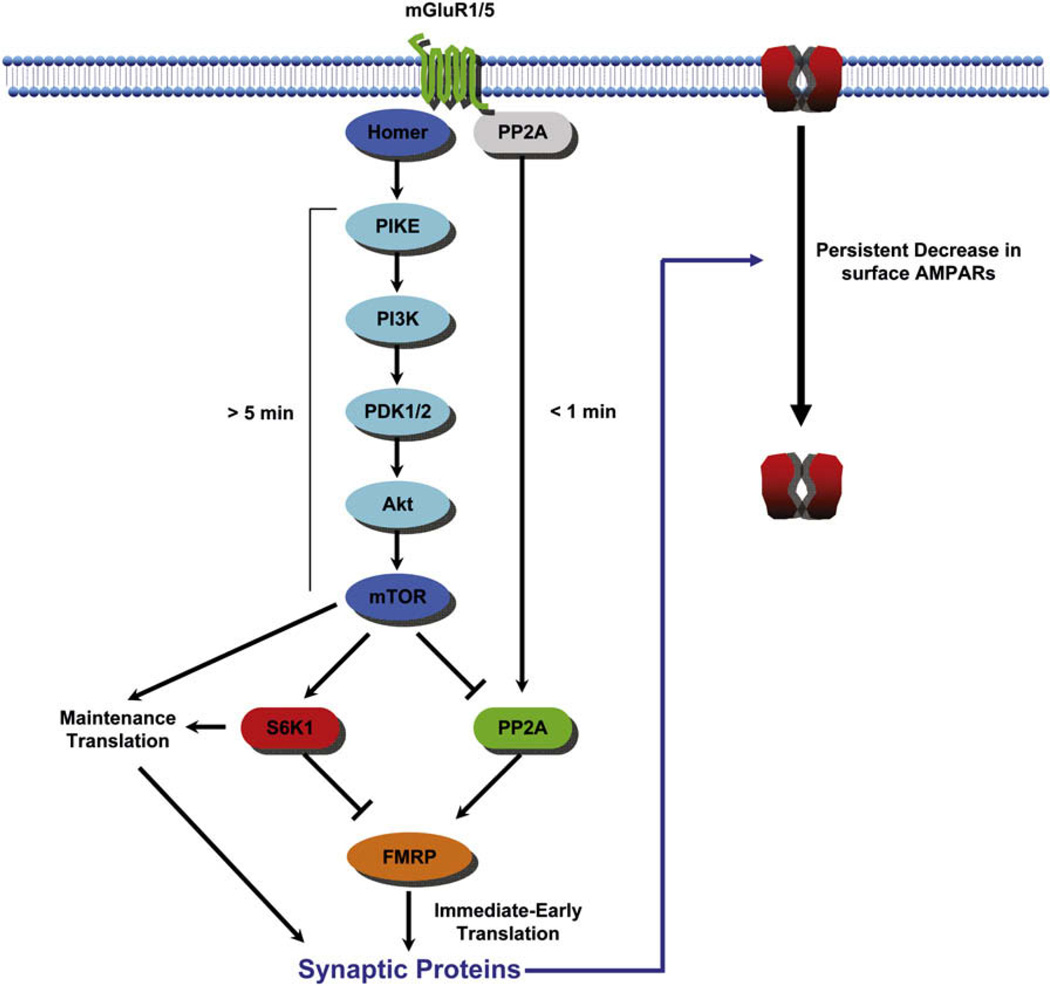
Figure 3. Postsynaptic FMRP Signaling Model.
Following stimulation of Gp1 mGluRs (green), inactive PP2A (gray) is immediately activated (green) and dephosphorylates FMRP (orange), rapidly allowing rapid translation of FMRP-associated mRNAs. Within 5 min, mTOR (blue) is activated via a homer cascade, inhibiting PP2A and activating S6K1 (red), leading to FMRP phosphorylation and possible translational inhibition of FMRP target messages. Simultaneously, mTOR activates translation in an FMRP-independent manner, triggering a sustained maintenance of translation. FMRP-dependent and -independent pathways control AMPA receptor internalization and other changes in synaptic function and spine morphology that contribute to mGluR-LTD. Based on Narayanan et al., 2007, 2008, and references therein.
In a subsequent report, ribosomal protein S6 kinase 1 (S6K1) was identified as an FMRP kinase in vitro and in vivo (Narayanan et al., 2008). S6K1 KO mouse hippocampal lysates show an absence of FMRP phosphorylation and increased expression of SAPAP3, mimicking the elevated expression of SAPAP3 in Fmr1 KO and hinting at loss of FMRP-mediated translational repression. These recent studies demonstrate that mGluR signals can rapidly affect the levels of phosphorylated FMRP in dendrites and suggest a model whereby translational responses at synapses may be temporally controlled by the phosphorylation status of FMRP downstream of mGluRs. An interesting corollary, based on the identification of S6K1 as the major neuronal kinase responsible for phosphorylating FMRP, is that the absence of either the hamartin or tuberin proteins (TS1 and TS2, respectively), responsible for another autistic spectrum disorder known as tuberous sclerosis (TS), leads to constitutive activation of the mTOR pathway and hyperphosphorylation of S6K1 (Yates, 2006). Thus, we could predict that FMRP would be hyperphosphorylated in TS, leading to the reduced or dysregulated translation of FMRP mRNA targets. Two autism spectrum disorders could thereby be linked together in the postsynaptic space, a cellular locale long believed to be important in autism.
While the above studies are consistent with a model whereby FMRP phosphorylation regulates translation at the level of ribosome stalling (elongation), it is also possible that FMRP regulation occurs at the level of translational initiation, whereby a translationally repressed mRNP becomes derepressed. This would be consistent with FMRP being present in both mRNPs (Zalfa et al., 2003) and polysomes (Feng et al., 1997b) in brain. It is also conceivable that some mRNAs may be regulated by FMRP at the level of ribosome stalling, whereas others may be regulated at the level of initiation in mRNPs; thus, no single mechanism may occur exclusively. One well-studied model for translational derepression at synapses is the cytoplasmic polyadenylation element binding protein, CPEB, which binds to both a CPE in α-CaMKII mRNA and to maskin, an mRNA-specific eIF4E binding protein (Huang et al., 2002). When maskin is bound to CPEB, it is prevented from binding eIF4G, which is necessary for translation initiation, and the CPEB/maskin complex tethers the mRNA in a translationally repressed mRNP complex. In response to NMDA receptor stimulation, CPEB is phosphorylated, maskin affinity for CPEB is decreased, and its interaction with eIF4G occurs, resulting in polyadenylation and translation initiation (Huang et al., 2002). An exciting recent study has discovered that CYFIP1, an FMRP interacting protein (Schenck et al., 2001), has a noncanonical eIF4E interacting domain, which forms a translationally dormant mRNP complex with FMRP and associated mRNAs (Napoli et al., 2008). In response to BDNF or DHPG stimulation, CYFIP1 is dissociated from eIF4E at synapses, allowing for translation. Both translational repression and stimulus-induced derepression were dependent on the FMRP-CYFIP complex and their interaction with mRNAs. Thus, there are certain parallels with the CPEB-Maskin mechanism, although it is unclear yet whether FMRP phosphorylation status can regulate this mechanism. In that CYFIP1 is known to bind and regulate the Rac1 GTPase pathway, this may provide another means for translational regulation of FMRP at synapses (Schenck et al., 2003).
FMRP as a Kinesin Adaptor for Regulated Transport of mRNP Granules
While the vast majority of FMRP exists in polyribosomes (Khandjian et al., 2004; Stefani et al., 2004), FMRP is also present in messenger ribonucleoprotein complexes (mRNP) (Zalfa et al., 2003), and FMRP-mRNP complexes can associate with microtubules (Wang et al., 2008a). Further, the interaction of FMRP-mRNPs with microtubules is increased when mRNAs are released from polyribosomes. These data suggest a model whereby the dynamic association of FMRP with translationally repressed, microtubule-associated mRNP complexes and polyribosomes might be a mechanism linking mRNA transport in dendrites with translational regulation at synapses (Wang et al., 2008a).
High-resolution fluorescence imaging studies in cultured neurons have shown that FMRP traffics in the form of motile RNA granules, which have been described for several localized mRNAs and binding proteins (Kiebler and Bassell, 2006). mRNA transport granules are believed to be translationally repressed mRNP complexes. In that some granules contain ribosomes and some lack ribosomes, mRNAs can likely be repressed at the level of initiation or elongation (Kiebler and Bassell, 2006). Once localized to the appropriate sites, mRNAs are believed to be released from granules and subsequently translated in response to appropriate physiological stimuli (Krichevsky and Kosik, 2001). FMRP is localized in granules in the neurites of PC12 cells (De Diego Otero et al., 2002) and in the dendrites (Antar et al., 2004, 2005) and axons (Antar et al., 2006) of primary hippocampal neurons. In dendrites, FMRP granules colocalize with ribosomal components and MAP1b mRNA (Antar et al., 2005), a known FMRP ligand. Live-cell imaging of EGFP-FMRP has revealed the active dendritic transport of FMRP granules in a microtubule-dependent manner (Antar et al., 2005). In cultured hippocampal neurons, FMRP trafficking into dendrites was stimulated by neuronal activity and activation of Gp1 mGluRs (Antar et al., 2004); brief depolarization of neurons using KCl resulted in a marked rise in FMRP levels in dendrites, which was independent of protein synthesis. KCl-induced trafficking was sharply attenuated by MPEP, an mGluR5 antagonist, but not by antagonists of NMDA or AMPARs. The brief application of DHPG, a Gp1 mGluR agonist, also increased FMRP trafficking in dendrites, which was dependent on microtubules. The importance of regulated FMRP trafficking is further underscored by observations in vivo of rapid and dynamic dendritic FMRP localization in response to visual experience, and in this example, trafficking was dependent on NMDA receptors (Gabel et al., 2004). Taken together, these in vitro and in vivo studies implicate distinct mechanisms for FMRP trafficking, which are regulated by experience and activity and may contribute to synaptic plasticity.
While in vitro studies show that FMRP is a component of mRNA transport granules that traffic along microtubules in an mGluR-dependent manner (Antar et al., 2005), we did not know until recently whether FMRP was directly required for this process. FMRP may instead be a passive passenger within the RNA transport granule, which may control translation, but have no influence on the active process of mRNA transport. To assess whether FMRP might play a role in the regulation of mRNA transport, a new study has used FISH and quantitative digital imaging analysis to quantitatively analyze the levels of dendritic mRNAs in response to DHPG in cultured hippocampal neurons from WT and Fmr1 KO mice (Dictenberg et al., 2008). Consistent with previous reports, there were no differences in the localization of mRNAs at steady state for several FMRP-associated mRNAs (i.e., MAP1b, α-CaMKII, SAPAP4, RGS-5, and others). However, each of the mRNAs exhibited DHPG-induced trafficking in WT neurons, which was either absent or markedly attenuated in Fmr1 KO neurons. There was no DHPG-induced trafficking of β-actin mRNA, which is known to be bound by ZBP1 (Eom et al., 2003) and localized to dendrites upon NMDA receptor activation (Tiruchinapalli et al., 2003). These findings show that the localization of a subset of dendritic mRNAs can be augmented upon mGluR activation and that FMRP is a critical requirement for this regulated transport.
In Drosophila neurons, another recent report has demonstrated a role for dFMRP in mRNA transport using live-cell imaging of fluorescently tagged RNA reporter dynamics (Estes et al., 2008). Of interest, mRNA granules in dFmr1 mutant neurons were less motile and exhibited reduced directional movement, suggesting that dFMRP may influence the quality and efficacy of mRNA dynamics. These findings are quite complementary to the study by Dictenberg et al. (2008) and provide a striking example of how an evolutionarily divergent protein has conserved a critical developmental function, and both reports represent a major breakthrough in our understanding of the mechanism of mRNA transport and molecular defects contributing to FXS.
The study by Dictenberg et al. (2008) provides new insight into the molecular mechanism. FMRP was shown to facilitate mRNA transport in response to DHPG by acting as an adaptor for kinesin-1, via an interaction between the C terminus of FMRP that contains the RGG box and kinesin light chain (Dictenberg et al., 2008), the known cargo-binding subunit. Dominant-negative constructs to either KLC or FMRP impaired the DHPG-induced localization of mRNAs to dendrites. Importantly, live-cell imaging of α-CaMKII 3′UTR, visualized by the MS2-GFP tagging method, demonstrated sharply reduced RNA granule motility in DHPG-stimulated neurons from Fmr1 KO mice. In addition, several FMRP-ligand mRNAs (i.e., MAP1b, α-CaMKII, SAPAP4, and RGS5) showed reduced kinesin interaction in vivo in Fmr1 KO mice. Since mRNA localization was not impaired at basal levels, these data suggest the presence of other as yet unidentified mRNA-binding proteins, which may play a role in the constitutive localization or maintenance of these mRNAs in dendrites.
It remains unclear whether FMRP directly binds to KLC. Interactions between FMRP with kinesin heavy chain (Kif5b) have been reported in mice and Drosophila (Kanai et al., 2004; Ling et al., 2004); however, the preparations used could not rule out the possibility that kinesin light chain was present. It is also possible that FMRP may be an adaptor for multiple motors. An interaction between FMRP and Kif3C was observed via biochemical and ultrastructural methods (Davidovic et al., 2007), and a dominant-negative construct to Kif3C reduced the density of FMRP granules in distal dendrites. However, this study did not address whether an interaction between FMRP and Kif3C was involved in mRNA localization.
Microtubules infrequently enter the dendritic spine, which is an actin-rich compartment. This raises the attractive idea that mRNA localization into the spine may involve a myosin motor. Interestingly, myosin Va was shown to facilitate the localization of TLS, an mRNA binding protein, and its ligand mRNA into spines following activation of Gp1 mGluRs (Yoshimura et al., 2006). Future work may reveal similar FMRP-myosin interactions involved in mRNA delivery into spines.
More studies are needed to identify the molecular composition of FMRP-associated RNA transport granules. To date, there have been proteomics studies to analyze the composition of RNA granules purified using various methods (Kiebler and Bassell, 2006). These studies have revealed that RNA granules are quite heterogeneous in size and composition. We now recognize that, in addition to RNA transport granules, there are P bodies and stress granules that are also present in neuronal processes and may play roles in mRNA degradation and translational repression. The possibility that mRNAs shuttle between different forms of RNA granules and polysomes to influence mRNA regulation is a newly appreciated idea (Kiebler and Bassell, 2006). RNA granules can disassemble in response to neuronal activity, releasing component mRNAs into polysomes (Krichevsky and Kosik, 2001). With regard to FMRP, it is known to interact with P body components in Drosophila (Barbee et al., 2006) and also stress granules in cultured mammalian cells (Mazroui et al., 2002). FMRP has been hypothesized to act as a gate controlling the release of mRNAs from granules into polysomes (Aschrafi et al., 2005); the potential molecular mechanism behind this, however, is unknown. It will be a challenge to characterize the protein and RNA composition of distinct FMRP-associated granules and assess their function in mRNA transport, stability, and translational regulation in dendrites and synapses.
The Role of FMRP in Axonal Development and Presynaptic Function
An exciting recent study by Bureau and colleagues (Bureau et al., 2008) revealed transient defects in ascending axonal projections and their experience-dependent plasticity in barrel cortex during a critical postnatal period of development from Fmr1 KO mice. Laser-scanning photostimulation (LSPS) was used to focally uncage glutamate at different sites near presynaptic L4 neurons within barrels. EPSPs were then recorded in single postsynaptic L3 neurons to generate synaptic input maps. These ascending L4 → L3 projections from barrels in Fmr1 KO mice showed a 40% reduction in their average strength. Electrophysiological analyses demonstrated that the reduction in the strength of the axonal projections is due to reduced connection probability, rather than a reduction in synaptic strength. Fmr1 KO mice showed diffuse axonal arbors between L4 → L3 projections, as analyzed using biocytin labeling of individual neurons and histochemical visualization of arbors. In addition, Fmr1 KO mice showed lack of experience-dependent plasticity following sensory deprivation by whisker trimming. In another study, Hanson and Madison (Hanson and Madison, 2007) developed an in vitro system to assess synaptic connectivity in neuronal networks mosaic for FMRP expression; they show that the presynaptic neurons derived from Fmr1 KO have a lower connection probability than their WT counterparts. Analysis of FMRP in Drosophila has also provided critical new insight into how the protein functions in axon development and activity-dependent refinement of synaptic connections (Tessier and Broadie, 2008). dFMRP is a known requirement to limit axon growth and synapse formation (Pan et al., 2004), and more recently it was shown to facilitate activity-dependent pruning of axon branches (Tessier and Broadie, 2008). Collectively, these findings from mice and fly models of FXS represent major strides toward the characterization of axonal and presynaptic defects.
Given the importance of axonal protein synthesis in axon guidance and regeneration (Wang et al., 2007), it is necessary to assess whether FMRP functions locally within axons to influence guidance and synapse formation. In cultured neurons, FMRP is localized to developing axons and growth cones, and growth cones from Fmr1 KO mice have excess filopodia and impaired dynamics (Antar et al., 2006). FMRP can be detected in regenerating axons in vivo and is associated with RISC components in sciatic nerve preparation (Murashov et al., 2007). FMRP is colocalized with RISC components in axons and growth cones of cultured DRG neurons (Hengst et al., 2006). It will important to analyze whether FMRP may function downstream of receptors for axon guidance factors to regulate local translation and assess whether impairments in local translation, perhaps via altered regulation of miRNAs, could account for the observed axonal defects in FXS models. Although futsch (MAP1b) is one dFMRP target whose translational impairment is already known to account for the synaptic overgrowth phenotype in Drosophila (Zhang et al., 2001), it is unclear whether this is caused by loss of local translational regulation in axons.
A recent study by Price et al. (Price et al., 2007) reports on a very exciting and unexpected role for FMRP in nociceptive sensitization and behaviors involved in pain processing, which may result from the impaired regulation of local protein synthesis in axons. In a battery of paradigms, Fmr1 KO mice display decreased nociception due to dysregulated mGluR1/5 signaling involving both ERK and mTOR regulation of protein synthesis. A particularly interesting set of findings here suggests the presence of protein synthesis in nociceptive axons that is important for pain processing. Since the same investigators also showed that FMRP is localized to nociceptive sensory axons (Price et al., 2006), these new findings should open up a line of inquiry to identify FMRP targets in axons whose local translation may be required for nociceptive plasticity. Interest in pursuing this line of inquiry is intensified by a recent study showing that local mGluR-regulated translation in primary afferent fibers regulates nociception (Jimenez-Diaz et al., 2008). Further studies may provide important links between altered nociceptive sensitization in Fmr1 KO mice and the self-injurious behavior reported in some fragile X syndrome patients. Importantly, these studies further define the breadth of FMRP functions and the role of local protein synthesis regulation throughout the central and peripheral nervous system.
The Role of FMRP in the Regulation of GABA(A) Receptor Subunits
GABA(A) receptors provide the major form of inhibitory synaptic transmission in the brain; they are important for the synaptic plasticity associated with learning and memory, and they are affected in disease states, such as epilepsy and anxiety. GABA(A) receptors are present along proximal dendrites of glutamatergic neurons, often noted near the spine neck, thus allowing for the inhibitory regulation of glutamatergic signaling. Several subunits of GABA(A) are reduced in the brains of Fmr1 KO mice, most notably the delta subunit (D’Hulst et al., 2006). Interestingly, in another study the mRNA for the delta subunit was shown to be an FMRP ligand, also altered in its expression in dendrites (Miyashiro et al., 2003). Most recently, the mRNA encoding GABA(A) receptor delta subunit was shown to be dendritically localized in response to mGluR activation in WT, but not Fmr1 KO neurons (Dictenberg et al., 2008). This unexpected finding reveals a surprising coordinate regulation between mGluRs and GABA(A) receptors and suggests a possible homeostatic mechanism that may be altered in FXS. In view of the above evidence of links between FMRP and GABA(A) receptor subunit expression and localization, it will come as no surprise that recent reports have revealed impairments in GABA synaptic transmission (Centonze et al., 2008; Curia et al., 2008). In the Drosophila model of FXS, a chemical library screen identified three compounds implicated in GABAergic signaling, including GABA itself, which rescued several mutant phenotypes (Chang et al., 2008). We envision future research yielding fresh insight into the mechanisms whereby FMRP-mediated translation is involved in GABAergic synaptic transmission as well as the role of FMRP at the interface between excitatory and inhibitory signaling pathways regulating translation.
Summary and Perspectives
Collectively, recent studies on FMR1 phenotypes in mouse and fly models of FXS provide strong evidence of diverse roles for FMRP in the regulation of neuronal development and plasticity, in both the central and peripheral nervous system. As discussed above, FMRP not only plays important roles in the adult synaptic plasticity underlying learning and memory, but also appears to be involved in axonal development, synapse formation, and the development and wiring of neuronal circuits (see also Gibson et al., 2008). FMRP appears to act similarly in many settings to control or limit morphologic changes in both axons and dendrites that are involved in synapse development and plasticity. There may therefore be more similarities in the mechanisms underlying dendritic, axonal, and synaptic impairments in FMR1 KO than previously thought. Importantly, recent evidence suggests that FMRP may regulate local protein synthesis in axons, as well as dendrites.
Future studies are likely to take several different directions. Rigorous characterization of the molecular mechanism behind the FMRP-mediated regulation of mRNA transport and local translation is essential (see proposed model in Figure 4). One major avenue to explore is FMRP’s interaction with dendritic miRNAs (Kye et al., 2007), which have recently been shown to regulate dendritic protein synthesis important for dendritic spine development (Schratt et al., 2006). Recent analysis indicates dysregulation of several miRNAs across the autism spectrum (Abu-Elneel et al., 2008), and thus it is critical to identify and assess how FMRP interactions with specific dendritic miRNAs may be dysregulated. Research is needed to characterize the precise mechanism whereby FMRP phosphorylation regulates translation, at the level of elongation and/or initiation, and the role of noncoding mRNAs and specific FMRP mRNA binding domains. It will be interesting to determine whether phosphorylation of FMRP and translational regulation occurs downstream of other receptors besides mGluRs. Recent evidence implicates altered signaling through Gq-coupled, M1 muscarinic acetylcholine receptors (mAChRs) in Fmr1 KO (Volk et al., 2007). In addition to the discussed studies reporting altered GABA receptor-mediated synaptic transmission, there is also recent evidence of altered dopamine receptor signaling in Fmr1 KO (Wang et al., 2008b; Weinshenker and Warren, 2008). Future studies may position FMRP-mediated translation downstream of GABA and dopamine receptors, and maybe even receptors for neurotrophins and axon guidance factors.
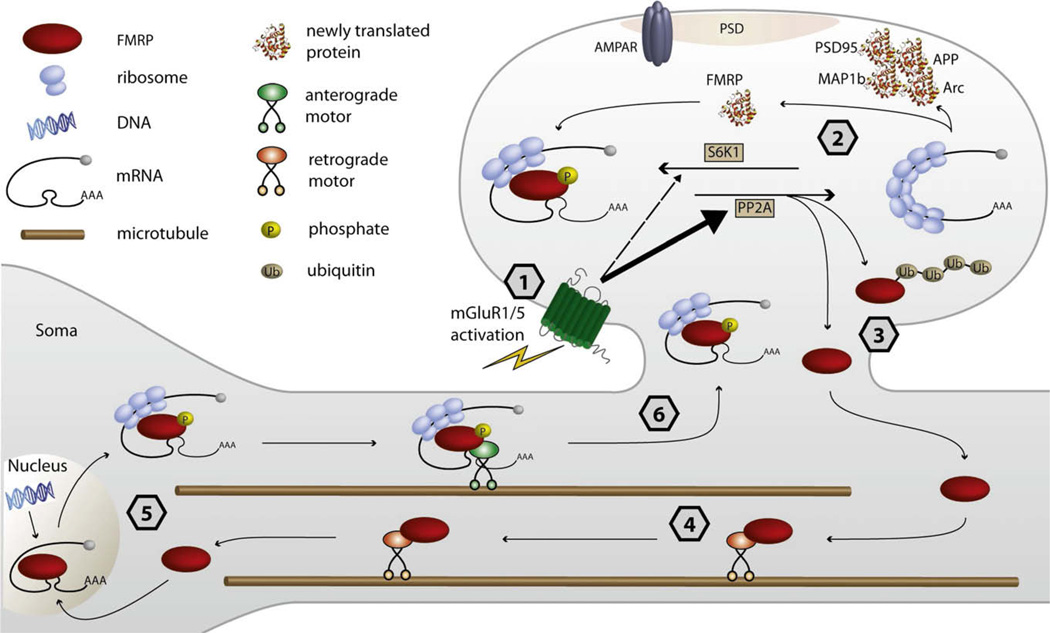
Figure 4. The Stimulated Travels and Function of FMRP throughout the Neuron.
FMRP is in a complex with several translationally arrested mRNAs at the synapse. Following mGluR stimulation, FMRP-target mRNAs are rapidly derepressed, allowing for local translation. A second phase of FMRP-dependent plasticity is shown that involves the subsequent transport of new mRNAs from the cell body into dendrites. The model shown here illustrates translational repression at the level of elongation, as suggested by Ceman et al. (2003). The translational activation and repression of mRNA is regulated by a PP2A/S6K1 signaling module (Narayanan et al., 2007, 2008) (see Figure 3). (1) Upon mGluR1/5 activation, PP2A is rapidly activated and dephosphorylates FMRP, thereby allowing for (2) local translation of proteins that affect AMPAR trafficking, i.e., PSD-95, Arc, Map1b, and App (Westmark and Malter, 2007; Todd et al.,2003; Muddashetty et al., 2007; Hou et al., 2006;Davidkova and Carroll, 2007; Waung et al., 2008; Park et al., 2008). Following mGluR activation, FMRP is rephosphorylated by S6K1 with slower kinetics, leading to translational repression. (3) FMRP can also be ubiquitinated following mGluR stimulation, and its proteosome-dependent degradation is necessary for mGluR-LTD (Hou et al., 2006). The local degradation of FMRP may contribute to local protein synthesis underlying mGluR-LTD. A mechanism of local FMRP degradation may be balanced by its synthesis. FMRP is synthesized in synaptoneurosomes upon mGluR activation (Weiler et al., 1997), which may provide a feedback mechanism to restore translational repression. Upon mGluR stimulation, (4) there may be a retrograde signal that leads to the transport of new FMRP-associated mRNAs from the soma. The active bidirectional transport of FMRP granules in dendrites has recently been described (Dictenberg et al., 2008). This model speculates that FMRP itself may traffic from the synapse to the cell body and/or nucleus, where it may complex with new target mRNAs, and return to the activated synapse. (5) In that FMRP can shuttle into the nucleus (Eberhart et al., 1996), it will be interesting to assess whether nucleocytoplasmic trafficking is regulated by mGluR signaling. (6) FMRP has recently been shown to be necessary for the transport of several mRNAs into dendrites, whereas neurons cultured from Fmr1 KO mice show impaired mRNA transport dynamics (Dictenberg et al., 2008). This model speculates that the trafficking population of FMRP is phosphorylated; however, future work is needed to assess whether FMRP phosphorylation may influence mRNA trafficking.
One critical goal for future work will be the attempt to match the specific mRNA ligands with specific FX phenotypes. For example, a number of FMRP ligands encode proteins important for the regulation of AMPAR trafficking, such as Arc, MAP1b, PSD-95, and APP (Ronesi and Huber, 2008) (see also Figure 4). We need to know whether any of these proteins are elevated at synapses of FMRP-deficient neurons, as suggested by Zalfa et al. (2003), and contribute to the excess internalization of AMPAR (Nakamoto et al., 2007). Further analysis of these and other critical FMRP ligands responsible for certain FX pheno-types, as well as the detailed molecular mechanisms involved in translational regulation, will pave the way to new therapies, which can perhaps be used in concert with drugs that act on Gp1 mGluRs. FX research has held out as a model of translational neuroscience, whereby the recent insights into FMRP function and strategies for the correction of phenotypes in FX model organisms offer hope for treating other autism spectrum disorders that affect learning and behavior.
ACKNOWLEDGMENTS
Supported in part by HD020521, MH080129, HD24064, and a Simons Foundation award to S.T.W. and NS051127 and a FRAXA award to G.J.B. The authors thank Christina Gross and Ravi Muddashetty for helpful comments, and Sharon Swanger for preparation of the model (Figure 4). S.T.W. discloses that he is the chair of the scientific advisory committee of Seaside Therapeutics.
REFERENCES
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of micro-RNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37:555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol. Cell. Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc. Natl. Acad. Sci. USA. 2005;102:2180–2185. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CT, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Bagni C. On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA. 2008;105:E19. doi: 10.1073/pnas.0801034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Gross C. Reducing glutamate signaling pays off in fragile X. Nat. Med. 2008;14:249–250. doi: 10.1038/nm0308-249. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendriticm RNA: transport, translation and function. Nat. Rev. Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Francesconi A, Carroll RC. The ups and downs of translation-dependent plasticity. Neuron. 2008;59:1–3. doi: 10.1016/j.neuron.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, Bagni C. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome pheno-types in Drosophila. Nat. Chem. Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 1999;22:98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex. 2008 doi: 10.1093/cercor/bhn159. in press. Published online September 11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19:903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic L, Jaglin XH, Lepagnol-Bestel AM, Tremblay S, Simonneau M, Bardoni B, Khandjian EW. The fragile X mental retardation protein is a molecular adaptor between the neurospecific KIF3C kinesin and dendritic RNA granules. Hum. Mol. Genet. 2007;16:3047–3058. doi: 10.1093/hmg/ddm263. [DOI] [PubMed] [Google Scholar]
- De Diego Otero Y, Severijnen LA, van Cappellen G, Schrier M, Oostra B, Willemsen R. Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol. Cell. Biol. 2002;22:8332–8341. doi: 10.1128/MCB.22.23.8332-8341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LB, Singer RH, Bassell GJ. A direct role for FMRP in activity dependent dendritic mRNA transport links filopodial spine morphogenesis to fragile x syndrome. Dev. Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhanskaya N, Sung YJ, Conti J, Currie JR, Denman RB. The fragile X mental retardation protein interacts with U-rich RNAs in a yeast three-hybrid system. Biochem. Biophys. Res. Commun. 2003;305:434–441. doi: 10.1016/s0006-291x(03)00766-6. [DOI] [PubMed] [Google Scholar]
- Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum. Mol. Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- Eom T, Antar LN, Singer RH, Bassell GJ. Localization of a b-actin mRNP complex with zipcode binding protein modulates the density of dendritic filopdia and filopodial synapses. J. Neurosci. 2003 doi: 10.1523/JNEUROSCI.23-32-10433.2003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes PS, O’Shea M, Clasen S, Zarnescu DC. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol. Cell. Neurosci. 2008;39:170–179. doi: 10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP and the the I304N mutation of severe fragile x syndrone abolishes this association. Mol. Cell. 1997a;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J. Neurosci. 1997b;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell RA, Benson RE, Hua J, Bogerd HP, Cullen BR. A nuclear role for the Fragile X mental retardation protein. EMBO J. 1996;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gabel LA, Won S, Kawai H, McKinney M, Tartakoff AM, Fallon JR. Visual experience regulates transient expression and dendritic localization of fragile X mental retardation protein. J. Neurosci. 2004;24:10579–10583. doi: 10.1523/JNEUROSCI.2185-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur. J. Hum. Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. An imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of Fragile X syndrome. J. Neurophysiol. 2008 doi: 10.1152/jn.90752.2008. in press. Published online September 10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J. Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome–an older face of the fragile X gene. Nat. Clin. Pract. Neurol. 2007;3:107–112. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J. Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies A beta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Jung MY, Sarkissian M, Richter JD. N-methyl-Daspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J. 2002;21:2139–2148. doi: 10.1093/emboj/21.9.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J. Neurosci. 2005;25:7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile x mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA. 2008a;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. Reply to Bagni: On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA. 2008b;105:E29. doi: 10.1073/pnas.0803737105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in Fragile X Mental Retardation Syndrome. Cereb, Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS ONE. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nat. Cell Biol. 2004;6:1048–1053. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Huot ME, Tremblay S, Davidovic L, Mazroui R, Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc. Natl. Acad. Sci. USA. 2004;101:13357–13362. doi: 10.1073/pnas.0405398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kindler S, Rehbein M, Classen B, Richter D, Bockers TM. Distinct spatiotemporal expression of SAPAP transcripts in the developing rat brain: a novel dendritically localized mRNA. Brain Res. Mol. Brain Res. 2004;126:14–21. doi: 10.1016/j.molbrainres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Kye MJ, Liu T, Levy SF, Xu NL, Groves BB, Bonneau R, Lao K, Kosik KS. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA. 2007;13:1224–1234. doi: 10.1261/rna.480407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck LA, Fischer Y. Evidence that FMRP is a negative regulator of translation. Hum. Mol. Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl. Acad. Sci. USA. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl. Acad. Sci. USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Menon L, Mihailescu MR. Interactions of the G quartet forming semaphorin 3F RNA with the RGG box domain of the fragile X protein family. Nucleic Acids Res. 2007;35:5379–5392. doi: 10.1093/nar/gkm581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon L, Mader SA, Mihailescu MR. Fragile X mental retardation protein interactions with the microtubule associated protein 1B RNA. RNA. 2008;14:1644–1655. doi: 10.1261/rna.1100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, Katwa LC, Van Scott MR. RNAi pathway is functional in peripheral nerve axons. FASEB J. 2007;21:656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc. Natl. Acad. Sci. USA. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E–BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates FMRP with the neuronal protein synthesis-dependent mTOR signaling cascade. J. Biol. Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Pan L, Zhang YQ, Woodruff E, Broadie K. The Drosophila fragile x gene negatively regulates neuronal elaboration and synaptic differentiation. Curr. Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu. Rev. Genomics Hum. Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F, Hargreaves KM. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 2006;141:2107–2116. doi: 10.1016/j.neuroscience.2006.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J. Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse. Sci. Signal. 2008;1:pe6. doi: 10.1126/stke.15pe6. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc. Natl. Acad. Sci. USA. 2001;98:8844–8849. doi: 10.1073/pnas.151231598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–898. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Sherman SL, Jacobs PA, Morton NE, Froster-Iskenius U, Howard-Peebles PN, Nielsen KB, Partington MW, Sutherland GR, Turner G, Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum. Genet. 1985;69:289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- Sittler A, Devys D, Weber C, Mandel JL. Alternative splicing of exon 14 determines nuclear or cytoplasmic localisation of fmr1 protein isoforms. Hum. Mol. Genet. 1996;5:95–102. doi: 10.1093/hmg/5.1.95. [DOI] [PubMed] [Google Scholar]
- Stefani G, Fraser CE, Darnell JC, Darnell RB. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J. Neurosci. 2004;24:9272–9276. doi: 10.1523/JNEUROSCI.2306-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc. Natl. Acad. Sci. USA. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Bakker CE, Willems PJ, Oostra BA. No evidence for disruption of normal patterns of mRNA localization in dendrites in FMR1 knockout mice. Neuroreport. 1998;9:477–481. doi: 10.1097/00001756-199802160-00022. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Dolzhanskaya N, Nolin SL, Brown T, Currie JR, Denman RB. The fragile X mental retardation protein FMRP binds elongation factor 1A mRNA and negatively regulates its translation in vivo. J. Biol. Chem. 2003;278:15669–15678. doi: 10.1074/jbc.M211117200. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum. Mol. Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Orr HT. Fragile X tremor/ataxia syndrome: blame the messenger! Neuron. 2007;55:535–537. doi: 10.1016/j.neuron.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. 2003;23:3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc. Natl. Acad. Sci. USA. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B, Richards RI, Lardelli M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum. Mol. Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J. Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev. Neurobiol. 2007;67:1166–1182. doi: 10.1002/dneu.20511. [DOI] [PubMed] [Google Scholar]
- Wang H, Dictenberg JB, Ku L, Li W, Bassell GJ, Feng Y. Dynamic association of the fragile X mental retardation protein as a messenger ribonucleoprotein between microtubules and polyribosomes. Mol. Biol. Cell. 2008a;19:105–114. doi: 10.1091/mbc.E07-06-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008b;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses. Proc. Natl. Acad. Sci. USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc. Natl. Acad. Sci. USA. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Warren ST. Neuroscience: fragile dopamine. Nature. 2008;455:607–608. doi: 10.1038/455607a. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Schluter OM, Steiner P, Czervionke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yates JR. Tuberous sclerosis. Eur. J. Hum. Genet. 2006;14:1065–1073. doi: 10.1038/sj.ejhg.5201625. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Fujii R, Watanabe Y, Okabe S, Fukui K, Takumi T. Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr. Biol. 2006;16:2345–2351. doi: 10.1016/j.cub.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat. Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila Fragile X-related gene regulates the MAP1b homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]