Abstract
An unmet challenge of spinal cord injury research is the identification of mechanisms that promote regeneration of corticospinal motor axons. Recently it was reported that IGF-I promotes corticospinal axon growth during nervous system development. We therefore investigated whether IGF-I also promotes regeneration or survival of adult lesioned corticospinal neurons. Adult Fischer 344 rats underwent C3 dorsal column transections followed by grafts of IGF-I-secreting marrow stromal cell grafts into the lesion cavity. IGF-I secreting cell grafts promoted growth of raphespinal and coerulospinal axons, but not corticospinal axons, into the lesion/graft site. We then examined whether IGF-I-secreting cell grafts promote corticospinal motor neuron survival or axon growth in a subcortical axotomy model. IGF-I expression coupled with infusion of the IGF binding protein inhibitor NBI-31772 significantly prevented corticospinal motor neuron death (93% cell survival compared to 49% in controls, P<0.05), but did not promote corticospinal axon regeneration. Coincident with observed effects of IGF-I on corticospinal survival but not growth, expression of IGF-I receptors was restricted to the somal compartment and not the axon of adult corticospinal motor neurons. Thus, whereas IGF-I influences corticospinal axonal growth during development, its application to sites of adult spinal cord injury or subcortical axotomy fails to promote corticospinal axonal regeneration under conditions that are sufficient to prevent corticospinal cell death and promote the growth of other supraspinal axons. We conclude that developmental patterns of growth factor responsiveness are not simply recapitulated after adult injury, potentially due to post-natal shifts in patterns of IGF-I receptor expression.
INTRODUCTION
The corticospinal system is the principal motor pathway in primates and modulates fine motor movement in the rat. Injury of corticospinal motor neurons in humans and non-human primates results in severe and permanent loss of motor function. For this reason, it is essential to identify mechanisms whereby regeneration of corticospinal axons can be augmented. One potential means of identifying mechanisms for promoting axonal regeneration in the injured adult central nervous system (CNS) is to identify and apply conditions that support outgrowth of axons during neural development.
A recent study examined gene expression profiles of corticospinal motor neurons during development and implicated insulin-like growth factor I (IGF-I) signaling in the developmental regulation of corticospinal outgrowth (Arlotta et al., 2005). IGF-I is one of two small insulin-like growth factors (Rinderknecht and Humbel, 1978). It binds the type I insulin-like growth factor receptor (IGF-IR) with high affinity and, with a much lower affinity, the type II insulin-like growth factor receptor and insulin receptor (Baserga, 2000). The IGF axis signals through IRS-1/Akt, Shc/ERK and 14.3.3/Raf pathways to promote cell survival and differentiation (Baserga, 2000; Jones and Clemmons, 1995). IGF-I is essential for normal growth and central nervous system development (Baker et al., 1993; Liu et al., 1993). Studies utilizing mice with either a genetic ablation of or the mis-expression of Igf-I have also demonstrated mitogenic activity of IGF-I in the developing brain (Beck et al., 1995; Popken et al., 2004).
In vitro, IGF-I prevents apoptotic neuronal cell death in cortical cultures, organotypic and dissociated lower motor neuron cultures, and in cerebellar granule neuron cultures (Bilak and Kuncl, 2001; Heck et al., 1999; Vincent et al., 2004a; Vincent et al., 2004b; Yamada et al., 2001; Yoshida et al., 2004). Further, IGFs induce neurite outgrowth in both embryonic and adult sensory neurons and in brainstem motor neurons in vitro (Fernyhough et al., 1993; Jones et al., 2003; Kimpinski and Mearow, 2001; Recio-Pinto et al., 1986; Salie and Steeves, 2005) and in vivo (Caroni and Grandes, 1990; Kaspar et al., 2003; Li et al., 1994).
Recently, it was reported that cultures of FACS-purified early postnatal corticospinal motor neurons respond to IGF-I stimulation with robust axonal outgrowth in vitro, and that blockade of IGF-I signaling during post-natal periods in vivo led to defasciculation and discontinuation of corticospinal axonal growth (Ozdinler and Macklis, 2006). This finding led us to investigate the hypothesis that IGF-I would induce corticospinal axon regeneration in the injured adult spinal cord or following subcortical axotomy. We now report that IGF-I gene delivery promotes the survival of injured adult corticospinal neurons, but does not promote corticospinal axonal regeneration under conditions that succeed in eliciting growth of other injured axonal populations.
METHODS
IGF-I cloning and plasmid generation
The class 1, Ea isoform IGF-I coding sequence was isolated from adult female Fischer 344 rat kidney. mRNA was isolated using Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA) and cDNAs were generated using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). IGF-I cDNA was amplified using primers 5′-CCTCAGCAGGCATTCATTTCG-3′ and 3′-CGTGGCATTTTCTGTTCCTCG-5′. For generation of the lentiviral construct, nested primers were then used to amplify the coding region, including the signal sequence (5′-ACTGACCGGTATGTCGTCTTCACATCTCTT-3′, 3′-ACTGGCGGCCGCCTACATTCTGTAGGTCTTGTTT-5′). The PCR product was cloned into the AgeI and NotI cut sites on a lentiviral expression vector between the CAG promoter and woodchuck post-transcriptional regulatory element.
Animal subjects
Animal use in this research was approved by the Institutional Animal Use and Safety Committee. For all surgical procedures, animals were anesthetized with 2ml/kg of a 25mg/mL ketamine, 1.3mg/mL xylazine and 0.25mg/mL acepromazine cocktail.
Marrow stromal cell culture, viral production and transduction of syngeneic marrow stromal cells
Primary rat bone marrow stromal cells (MSCs) were isolated from adult (150–165g) female Fischer 344 rats as previously described (Lu et al., 2003). 293T cells were transiently transfected to produce self-inactivating lentiviral vectors encoding either rat IGF-I or GFP as described previously (Blesch, 2004; Zufferey et al., 1998). Viral titers determined by p24 ELISA of serial dilutions of concentrated virus were 260μg/mL for lenti-GFP and 215μg/mL for lenti-IGF-I. Ten microliters of 215ug p24/mL (equivalent to 3.3×107 infectious units of lenti-GFP) were used to infect 3×106 cultured syngeneic MSCs. MSC media was changed after 24 hours and cells were grown until confluent. MSC media was replaced with serum-freeα-MEM for 12 hours. The conditioned, serum-free media was filtered (0.22μm) and collected. IGF-I ELISA (R&D Systems, Minneapolis, MN) was used to determine levels of secreted IGF-I.
In vitro assay of IGF-I bioactivity
Cerebellar granule neurons (CGNs) were isolated from P7 rat pups and plated in poly-L-lysine [20ug/mL] coated 96-well plates at 105cells/well in DMEM/F-12 + B27. After 24hrs, media was replaced with MSC-conditioned serum-free media from GFP or IGF-I expressing cells. Recombinant mouse IGF-I (PeproTech, Inc., Rocky Hill, NJ) was added to GFP MSC-conditioned media at concentrations ranging from 10 to 1000ng/mL. A cell viability assay using 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) was performed 24hrs later. Briefly, following overnight incubation at 37°C, 5% CO2, cells were cultured for two hours with 500μg/mL MTT. Media was removed after two hours and replaced with 0.04N HCl in isopropanol with trituration and shaking for 30min; optical density measurements were read at 570nm with a 690nm correction. Levels of survival and were measured as absorbance in experimental conditions divided by absorbance in control DMEM/F-12 + B27. All conditions were run in triplicate on cells isolated from three pups.
In Vivo Lesion Model 1: Spinal Cord Injury
To assess sensitivity of lesioned spinal cord axons to IGF-I, adult Fischer 344 rats (150–165g) were anaesthetized and underwent C3 dorsal column lesions, as previously described (Weidner et al., 2001). Briefly, a Kopf wire knife (David Kopf Instruments, Tujunga, CA) was inserted 0.6mm lateral to midline and 1.1mm under the dorsal surface of the spinal cord; the knife was extruded 2.5mm and lifted to transect the dorsal columns, with coincident compression with a 28gauge blunt tip from above to ensure lesion completeness. One group of subjects then received cell suspension injections of 2×105 IGF-I-secreting MSCs into the lesion cavity (n=9 animals), and a control group received injections of control, GFP-expressing MSCs (n=10). Two weeks later, corticospinal axons were anterogradely traced using a 10% solution of biotinylated dextran amine (BDA, 104MW; Invitrogen, Carlsbad, CA). BDA was injected into the motor cortex, 21 sites/hemisphere, 150nL/site, as previously described (Lu et al., 2005). Animals were perfused two weeks after tracer injection with 4% paraformaldehyde. Spinal columns were removed and post-fixed in 4% PFA at 4°C overnight, then cryoprotected in 30% sucrose. Responses of corticospinal axons, raphaespinal axons and cerulospinal axons to IGF-I-secreting cell grafts were then examined, as described below.
In Vivo Lesion Model 2: Subcortical Axotomy
Lesion model 1 examined whether IGF-I gene delivery within a site of spinal cord injury would promote corticospinal tract axon regeneration. Previous studies indicate that lesions remote from the cell body may limit the retrograde injury response, thereby attenuating responses to growth-promoting experimental manipulations (Fernandes et al., 1999). The distance of axotomy from cell body may influence the corticospinal response to injury in particular, as subcortical axotomy results in corticospinal neuronal death (Giehl and Tetzlaff, 1996) whereas remote axotomy in the spinal cord results in more modest corticospinal neuron cortical degeneration. Thus, to determine whether more proximal lesions of the corticospinal projection provide a model in which sensitivity to IGF-I can be detected, 24 additional rats were examined after subcortical axotomy. Layer V corticospinal neurons were first identified by retrograde labeling with the tracer cholera toxin B subunit (CTB; LIST Biological Laboratories, Campbell, CA). 2ul of a 1% CTB solution was injected into the dorsal columns at C4 bilaterally (±0.2mm lateral to midline, 0.8mm ventral to the dorsal spinal cord surface). Two weeks later, a subcortical axotomy of corticospinal projections was made by aspirating white matter underlying the motor cortex between two craniotomies flanking the motor cortex, as previously described (Lu et al., 2001). The lesion cavity was then filled with a collagen graft matrix containing either: 1) IGF-I secreting MSCs (n=6 animals), 2) GFP-expressing MSCs (n=6 animals), 3) IGF-I-secreting MSCs plus intraventricular infusions of the IGF binding protein inhibitor NBI-31772 (1-(3,4-dihydroxybenzoyl)-3-hydroxycarbonyl-6, 7-dihydroxyisoquinoline (EMD Biosciences, Gibbstown, NJ) (n=4 animals), 4) GFP-expressing MSCs plus intraventricular infusions of the IGF binding protein inhibitor NBI-31772 (n=4 animals), or 5) IGF-I-secreting MSCs plus intraventricular infusions of the control substance artificial CSF with 1.67% DMSO (n=4 animals). IGF binding proteins were blocked in this lesion model to enhance sensitivity for detecting an IGF-I effect, as post-developmental upregulation of IGF binding proteins may act as a biological mechanism for controlling or attenuating adult IGF-I signaling (Jones and Clemmons, 1995). To retain cells in the subcortical lesion cavity, they were placed into a gelled collagen matrix prior to implantation, as previously described (Lu et al., 2001). After an additional two week survival period, subjects were transcardially perfused with 4% paraformaldehyde.
Sectioning and histology
Spinal cords were removed from the vertebrae and serially sectioned in the sagittal plane at 35μm intervals. Every seventh section was used for BDA label detection. Sections were washed three times in TBS, incubated for 15′ at room temperature in 0.6%H2O2 in methanol, washed two more times and incubated overnight at 4°C in TBST (TBS + 0.25% Triton-X100) with avidin: biotinylated enzyme complex (Vector Labs, Burlingame, CA). Sections were washed 3 times on the second day in TBS and developed in 3,3′-diaminobenzidine (DAB), in parallel. Following DAB development, sections were washed three times in TBS and incubated overnight at 4°C in TBST with 5% donkey serum and primary antibody, rabbit anti-GFAP (DAKO, Carpinteria, CA; [1:750]). The next day, sections were washed three times in TBS, incubated 2.5hrs at room temperature in Alexa Fluor 594 conjugated secondary antibody generated in donkey (Invitrogen, Carlsbad, CA; [1:250]), incubated at room temperature in 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; [1μg/mL]; Sigma-Aldrich) for 10 minutes, then washed three times in TBS. Fluorescent labeling of other axonal markers, goat anti-serotonin (5-HT; ImmunoStar, Inc., Hudson, WI; [1:500]) and rabbit anti-tyrosine hydroxylase (TH; Millipore; [1:500]), was performed as described above with Alexa Fluor 594 and 647 conjugated secondary antibodies generated in donkey (Invitrogen; [1:250]).
For analysis of survival and axonal regeneration of cortical neurons following subcortical axotomy, immunohistochemistry on transverse cortical sections was performed. 40μm thick cryostat sections were washed three times in TBS, endogenous peroxidases were quenched with 0.6% H2O2 in TBS for 15min, sections were rinsed two times in TBS and then blocked for one hour in TBST with 5% serum. Primary antibodies were incubated overnight at 4°C in TBST with 5% serum at the following concentrations: rabbit anti-NF200 [1:500], mouse anti-GAP43 [1:1000] (Millipore) or goat anti-CTB (LIST Biological Laboratories; [1:80,000; 3-day incubation]). The next day (3 days later for CTB immunohistochemistry), sections were washed three times in TBS then incubated at room temperature for one hour in TBS with biotin conjugated secondary antibodies generated in either donkey or horse (Jackson ImmunoResearch Laboratories, West Grove, PA; [1:250]). Following secondary antibody incubation, sections were washed three more times in TBS, then incubated in avidin:biotinylated enzyme complex (Vector Labs) for one hour at room temperature prior to development with DAB.
For IGF-IR immunodetection, sections were immunolabeled for either IGF-IRα (Santa Cruz Biotechnology, Santa Cruz, CA; sc-712 [1:50]) or β (Cell Signaling Technology, Danvers, MA; #3027 [1:50]) subunits per manufacturers’ protocol. Sections were incubated in biotinylated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories; [1:250]) in TBS for one hour at room temperature. IGF-IR staining was then developed in DAB. Sections stained with rabbit anti-IGF-IRα were also stained with goat anti-CTB (LIST Biological Laboratories; [1:10,000]) overnight in TBST with 5% donkey serum followed by a two and a half hour incubation in Alexa Fluor 488 conjugated donkey anti-goat antibody (Invitrogen).
IGF-I production in vivo
Seven additional animals underwent grafting of IGF-I-secreting (n=3) or control (n=4) MSCs to the C3 spinal cord lesion cavity. Four weeks later, subjects were perfused with ice-cold saline (IGF-I n=3, GFP n=4). The cervical enlargement was removed and the graft was excised and frozen over dry ice in a pre-chilled, pre-weighed 1.5mL tube. The tissue was homogenized using a sonicator in ice-cold PBS [15μg tissue/mL] with 1.44% w/v NaCl, 0.5% w/v BSA, 0.1mM PMSF, 0.9mM aprotinin, 5mM EDTA and 0.1% Triton X-100. Samples were centrifuged at 14krpm for 30min at 4°C and supernatants were assayed via ELISA for IGF-I levels.
Analysis of tissue sections
The host-graft interface was identified using GFAP immunolabeling and DAPI staining (most glial processes abruptly terminate at the host/lesion or host/graft interface; DAPI identifies changes in the uniformity of nuclear distribution, which is distinct when comparing graft versus spinal cord cell populations). BDA labeled corticospinal axons were examined in every 7th section and images were digitally acquired with PictureFrame software (Optronics, Goleta, CA). Fluorescent images were inverted and thresholded, and pixel density within dorsal column grafts was quantified using ImageJ (NIH, Bethesda, MD). Every 7th cortical section from animals that underwent subcortical lesions were analyzed for axonal penetration using NF200 or GAP43 immunolabeling. Stereological counts of CTB-labeled surviving corticospinal motor neurons in lesioned and intact hemispheres were made using Stereo Investigator software (MicroBrightField, Inc, Williston, VT). Random fields of view were sampled throughout the extent of the motor cortex (sampling fraction 50%). The top and bottom 12.5% of the sections were omitted to prevent potential double-counting of neurons in adjacent sections. For quantification of surviving corticospinal motor neurons following intracerebroventricular infusions, the total number of CTB-labeled corticospinal motor neurons over a 980μm distance (490μm rostral and 490μm caudal to the site of infusion) were quantified in the lesioned and contralateral intact hemispheres. All analyses were conducted in a blinded manner.
Statistics
Group differences in experiment 1 were assessed using unpaired, two-sided Student’s t-test, with a significance criterion of p<0.05. In experiment 2, group differences were assessed by ANOVA and post-hoc differences were assessed by Fisher’s PLSD, with a significance criterion of p<0.05.
RESULTS
IGF-I production and bioactivity
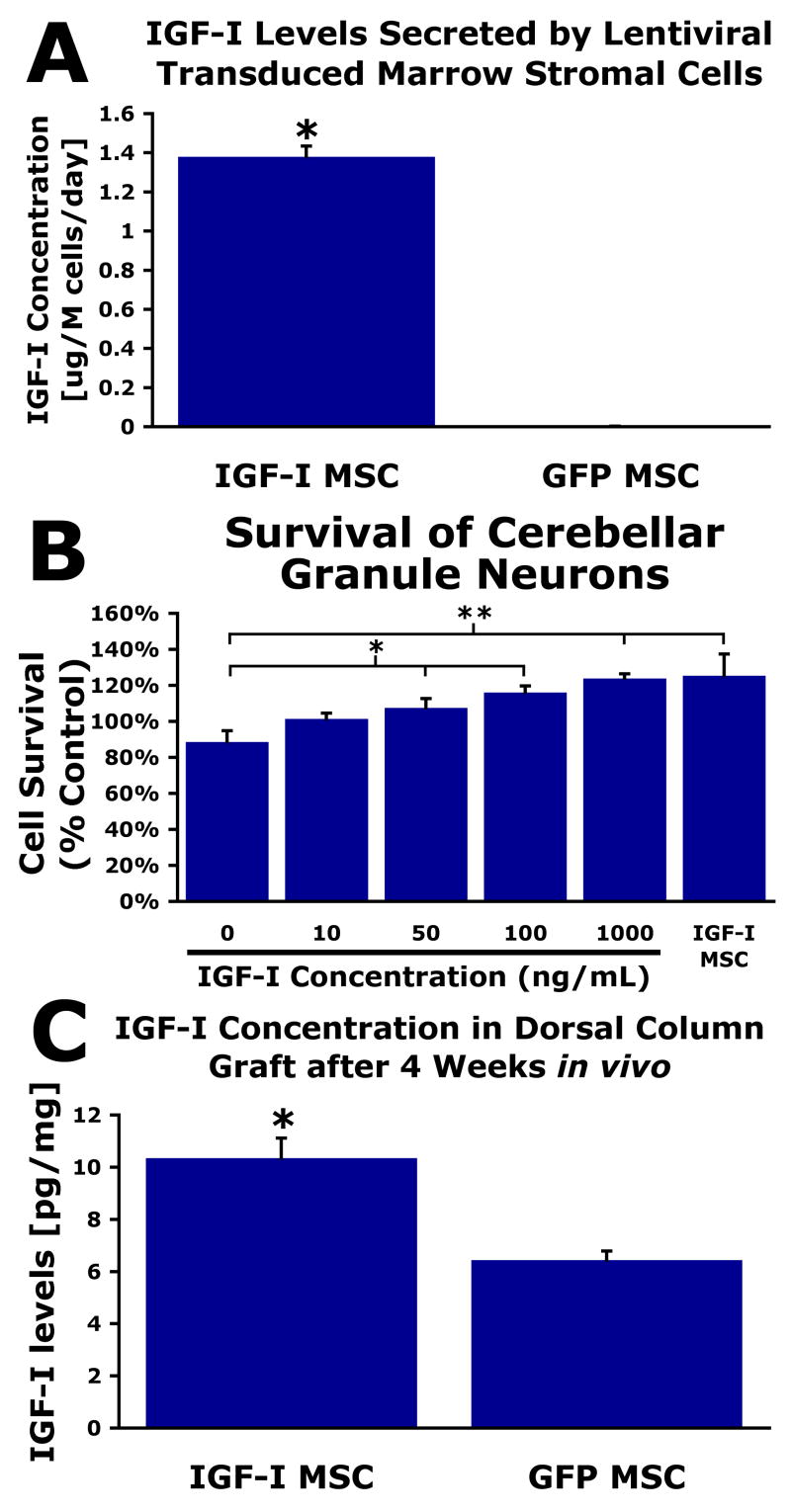
In vitro, lenti-IGF-I transduced MSCs secreted 1400±10ng IGF-I/106cells/day, while control, lenti-GFP transduced MSCs secreted no detectable amounts of IGF-I (Fig. 1, P=0.01). Bioactivity of secreted IGF-I was examined on cerebellar granule cells: 40% more cerebellar granule neurons survived when treated with IGF-I MSC-conditioned media survived compared to GFP MSC-conditioned media (P=0.02, Fig. 1), similar to effects of adding recombinant mouse IGF-I. Dissection of IGF-I-secreting MSCs four weeks after in vivo grafting to the spinal cord demonstrated a 61% increase in IGF-I levels compared to control grafted animals (P<0.01, Fig. 1). Thus, transduction to secrete IGF-I results in secretion of significantly elevated levels of bioactive IGF-I.
Figure 1.
Production and bioactivity of IGF-I. (A) IGF-I levels secreted into conditioned media by lentiviral-transduced MSCs, by ELISA (Mann Whitney *P<0.05). (B) Cerebellar granule neuron survival after 24hrs in conditioned medium from IGF-I-secreting cells, compared to GFP-transduced cells with known quantities of IGF-I protein added. Survival in presence of IGF-I-secreting cell medium is equal to that of the highest quantity of IGF-I protein (ANOVA P<0.005, post-hoc Fisher’s *P<0.05, **P<0.001). (C) MSC grafts transduced to express IGF-I exhibit significantly greater amounts of IGF-I protein after 4 weeks in vivo compared to naïve MSC grafts (Student’s t-test, *P=0.01). While naïve MSCs do not produce detectable levels of IGF-I in vitro prior to grafting, IGF-I is detectable in naïve grafts after 4 weeks in vivo, possibly due to migration of host cells into graft that express IGF-I.
IGF-I-secreting cells promote growth of supraspinal axons after SCI
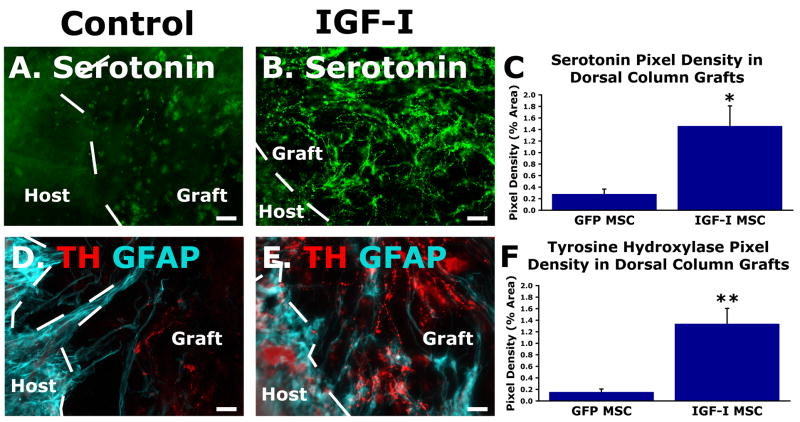
Growth of cerulospinal and raphaespinal axons was significantly greater into grafts of IGF-I-secreting MSC grafts compared to control, GFP-expressing MSC grafts four weeks after C3 spinal cord lesions (Fig. 2). The density of raphaespinal axons was 5-fold greater in IGF-I secreting grafts compared to GFP-producing grafts (P<0.02, Fig. 2C), and the density of TH-labeled cerulospinal axons was 8-fold greater (p<0.01, Fig. 2F). Thus, biologically active IGF-I secreted from transduced cell grafts significantly promotes the growth of descending axonal systems that modulate motor function in the rodent spinal cord.
Figure 2.
IGF-I promotes growth of raphaespinal and rubrospinal axons into IGF-I-secreting cell grafts in sites of spinal cord injury. (A) Few 5HT-labeled serotenorgic axons penetrate a GFP-expressing cell graft in the C3 lesion site, four weeks after placement of spinal cord lesion. (B) In contrast, 5HT-labeled axons extensively penetrate an IGF-I-secreting cell graft. (C) Quantification reveals a 5-fold increase in seroteonergic axon penetration into IGF-I-secreting cell grafts (two-tailed t-test *P<0.02). (D) Similarly, tyrosine hydroxylase-labeled cerulospinal axons modestly penetrate GFP-producing cell grafts in the lesion site, and (E) more extensively penetrate IGF-I-secreting cell grafts. (F) Quantification reveals an 8-fold increase in the axon penetration of IGF-I-secreting cell grafts (two-tailed t-test **P<0.01). Scale bar = 25μm.
IGF-I-secreting cells do not promote growth of corticospinal axons after SCI
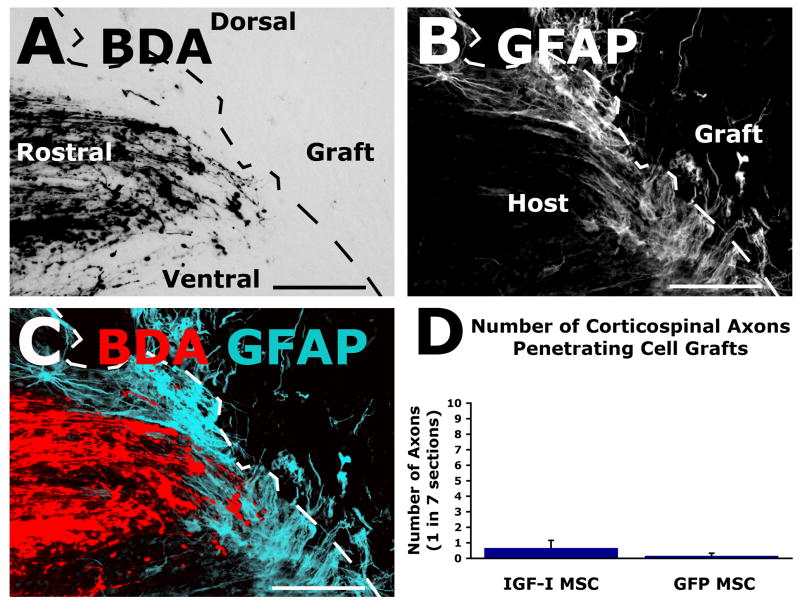
Unlike growth-promoting effects of IGF-I-secreting grafts on other motor control system in the spinal cord, no effects were detected on corticospinal axonal regeneration. Only rare BDA-labeled corticospinal axons were detected within IGF-I and control MSC grafts in the lesion cavities, and the extent of this growth did not differ between groups (P=0.4, Fig. 3). Thus, in the presence of quantities of IGF-I sufficient to promote the growth of other axonal systems, corticospinal axons exhibit no detectable response to IGF-I after spinal cord injury.
Figure 3.
Corticospinal axons do not regenerate into IGF-I-secreting cell grafts. (A) Light-level label demonstrates corticospinal tract, adjacent to lesion site. Sagittal section, left rostral, right caudal. No axonal penetration of IGF-I-secreting cell graft is evident. (B) GFAP labeling delineates host/graft interface. (C) Merge with color rendering demonstrates absence of corticospinal axon penetration of IGF-I graft. (D) Quantification reveals rare corticospinal axonal penetration of either graft type (IGF-I: 0.67±0.49, GFP: 0.17±0.17; P=0.4). Thus, while IGF-I-secreting cell grafts elicit regeneration of other supraspinal axonal populations that modulate motor function, they do not elicit growth of corticospinal axons under these conditions. Scale bar = 100μm.
IGF-I promotes survival but not growth of corticospinal motor neurons after subcortical lesions
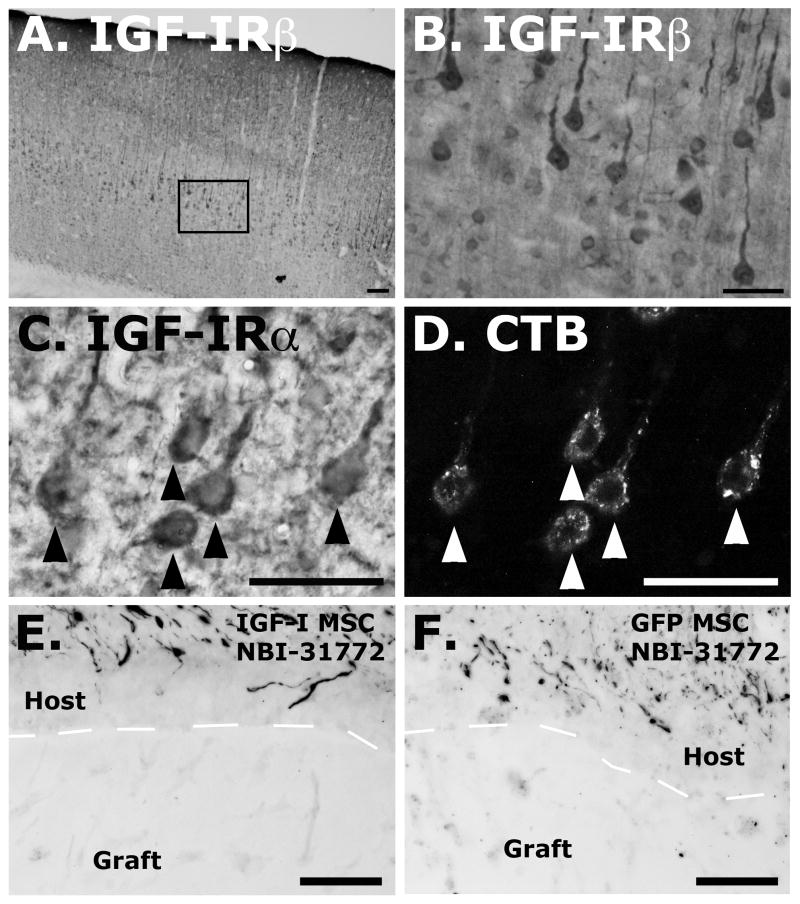
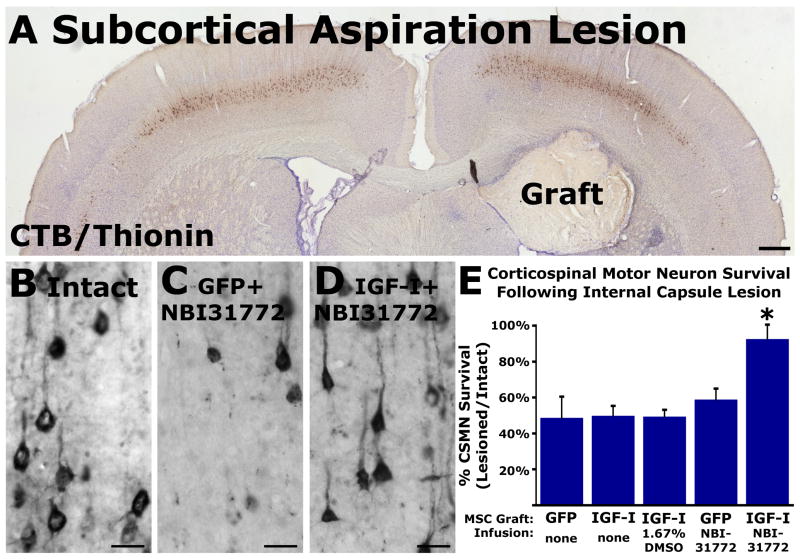
To determine whether IGF-I can promote cell survival or axon growth after axotomy close to the cell body, grafts of IGF-I-secreting cell grafts were placed in subcortical lesion sites. Both in the presence and absence of co-infusion of the IGF binding protein inhibitor NBI-31772, axons failed to penetrate IGF-I-secreting or control, GFP-expressing cell grafts in the lesion site (Fig. 5). Notably, IGF-I grafts combined with NBI-31772 infusions significantly ameliorated lesion-induced death of corticospinal motor neurons: 93% of corticospinal motor neurons survived the subcortical axotomy, compared to only 48% survival in control lesioned subjects (P<0.005; Fig. 5). Thus, IGF-I delivery combined with NBI-31772 infusion achieved sufficient in vivo levels to demonstrate biological efficacy in promoting corticospinal motor neuron survival, but failed to elicit corticospinal axonal regeneration into the lesion site, as assessed by heavy-chain neurofilament labeling (Fig. 5) and GAP-43 labeling (data not shown).
Figure 5.
Adult corticospinal motor neurons express the IGF-I receptor in the somal compartment. (A, B) IGF-I-receptor β subunit staining of large-diameter pyramidal neurons within layer V motor cortex. (C, D) IGF-I-receptor α subunit immunoloabeling in identified corticospinal motor neurons retrogradely labeled with CTB (arrowheads indicate co-labeled cells). In contrast, IGF-I receptor labeling is not detectable on lesioned or intact corticospinal motor axons in the cortex or spinal cord (not shown). (E) Heavy-chain neurofilament-immunoreactive axons do not penetrate subcortical grafts expressing IGF-I with NBI-31772 infusion (host-graft interface demarcated with dashed line), nor (F) control, GFP-producing subcortical grafts with NBI-31772 infusion. Scale bars, 100μm (A); 50μm (B–F).
Mechanisms underlying corticospinal responses to IGF-I: receptors are expressed in the somal but not axonal compartment
The differential effects of IGF-I overexpression on corticospinal neuronal survival but not axonal growth suggested potential differences in distribution of IGF-I receptors in neuronal compartments. Immunolabeling for IGF-I receptor confirmed the presence of both IGF-IRα and β subunits in identified layer V corticospinal somata (co-localized with retrograde CTB labeling; Fig. 5), and an absence of detectable expression in intact or lesioned CST axons in the spinal cord (not shown). Thus, differential distribution of IGF-I receptors is a candidate mechanism accounting for in vivo biological effects of this growth factor in the adult CNS.
DISCUSSION
An unmet challenge of spinal cord injury research is the identification of mechanisms for promoting regeneration of corticospinal motor axons. Signals modulating corticospinal axonal outgrowth during development represent candidate mechanisms for enhancing their growth after adult injury. A recent study by Ozdinler and Macklis reported that IGF-I is an important modulator of axonal outgrowth of early post-natal corticospinal motor neurons both in vitro and in vivo (Ozdinler and Macklis, 2006). Utilizing a gene delivery method that has previously resulted in the identification of growth factor sensitivity of other axonal systems after adult spinal cord injury (Grill et al., 1997; Lu et al., 2001; Tuszynski et al., 1994), we examined whether IGF-I over-expression in sites of spinal cord injury or subcortical axomtomy would promote corticospinal axonal regeneration or survival. While we find that IGF-I over-expression promotes the survival of corticospinal neurons after subcortical axotomy and promotes the regeneration of raphaespinal and cerulospinal axons after spinal cord injury, we did not detect regeneration of corticospinal axons. A candidate mechanism underlying the lack of corticospinal axonal sensitivity to IGF-I is the absence of detectable trafficking of the IGF-I receptor to the axonal compartment of corticospinal neurons.
There are challenges in interpreting outcomes of negative studies. The most important of these, in the present study, is whether levels of IGF-I expression sufficient to elicit growth were likely to have been achieved using our gene delivery methods. Amounts of IGF-I expression by transduced cells in vitro were sufficient to achieve survival of cerebellar granule neurons. Levels of IGF-I protein were significantly elevated in vivo in transduced cell grafts, and this elevation exceeded levels in control, GFP-expressing cell grafts by 61%. The in vivo augmentation of IGF-I levels in the spinal cord were sufficient to elicit a significant, 5-fold increase in growth of raphaespinal axons and a significant, 8-fold increase in growth of cerulospinal axons into the spinal cord lesion site. Thus, while we cannot conclude with certainty that still higher levels of IGF-I expression would not support corticospinal axonal regeneration, we can conclude that IGF-I expression at levels sufficient to support growth of other supraspinal systems that project to the spinal cord and that modulate motor function are not equally capable of supporting corticospinal axonal regeneration. The finding that the IGF-I receptor is not expressed on corticospinal axons in the spinal cord further supports the probability that even higher levels of IGF-I expression than those achieved in the current paradigm would not elicit a response from these axons. Notably, IGF-I supports corticospinal axonal outgrowth during development, as blockade of IGF-I function in the developing system terminates corticospinal growth (Ozdinler and Macklis, 2006). IGF-I receptor has been localized to developing corticospinal axons (Ozdinler and Macklis, 2006), thus the lack of corticospinal axonal regeneration in the adult likely results from developmentally-regulated shifts in growth factor receptor expression.
Also supporting the absence of a detectable effect of IGF-I on corticospinal axonal growth in adulthood, we found that subcortical grafts of IGF-I-secreting cells prevented corticospinal neuronal death when co-administered with an IGF binding protein inhibitor. Thus, sufficient in vivo levels of IGF-I were achieved to elicit effects on corticospinal neurons themselves (in a cell survival assay), but these levels were not sufficient to promote axonal regeneration into the same IGF-I-secreting subcortical grafts. The proximity of the IGF-I-secreting cell graft to corticospinal neuronal somata in the adjacent, overlying cortex likely provided the capability to activate survival mechanisms through somally-expressed IGF-I receptors.
The half-life of IGF-I protein in plasma is quite brief, on the order of 12 minutes (Guler et al., 1989). Yet grafts continuously secrete the growth factor, ensuring a continuous supply over the period of this experiment (confirmed by ELISA).
The results of this study correspond with previously published results regarding the effects of BDNF administration either by gene delivery or protein infusion in the CNS (Giehl and Tetzlaff, 1996; Lu et al., 2001). That is, both gene delivery and protein delivery of BDNF promoted survival of corticospinal motor neurons after subcortical axotomy (Giehl and Tetzlaff, 1996; Lu et al., 2001), but did not promote corticospinal axonal regeneration (Hiebert et al., 2002; Lu et al., 2001; Nakahara et al., 1996). Thus, similar to previous findings regarding BDNF, IGF-I exerts neurotrophic but not neurotropic effects on injured adult corticospinal axons. The lack of neurotropic effects in the adult may result from a shift in the role of the IGF-I axis from a developmental axonal outgrowth function to an adult somal support function, coinciding with a change in the intracellular compartmentalization of the IGF-I receptor.
Figure 4.
IGF-I prevents corticospinal motor neuron death after subcortical axotomy. (A) Nissl-stain section to illustrate lesion underlying motor cortex and graft in lesion site. Light brown staining present in layer V consists of corticospinal motor neurons retrogradely labeled with CTB. (B) Illustration of intact layer V motor cortex containing CTB-labeled corticospinal neurons. (C) Reductions in numbers of CTB-labeled motor neurons two weeks after subcortical axotomy, in subject that received GFP-producing cell graft with infusion of the IGF binding protein inhibitor NBI-31772. (D) Neurons remain largely labeled with CTB two weeks after subcortical axotomy in subjects that receive IGF-I-secreting cell grafts and infusions of NBI-31772. (E) Quantification indicates that IGF-I-secreting grafts combined with NBI-31772 infusion prevent axotomy-induced loss of CTB-labeled corticospinal motor neurons after subcortical axotomy (ANOVA P<0.005; * indicates significant differences on post-hoc Fisher’s). Scale bars, 500μm (A), 25μm (B–D).
Acknowledgments
We thank James Connor and Pouya Jamshidi for experimental assistance. Supported by NIH (R01 NS09881, NS42291), NIH/NIGMS Genetics Training Program (T32 GM08666), the Veterans Administration, The Spitzer Foundation and The Adelson Program in Neural Rehabilitation and Repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Bilak MM, Kuncl RW. Delayed application of IGF-I and GDNF can rescue already injured postnatal motor neurons. Neuroreport. 2001;12:2531–2535. doi: 10.1097/00001756-200108080-00048. [DOI] [PubMed] [Google Scholar]
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KJ, Fan DP, Tsui BJ, Cassar SL, Tetzlaff W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: differential regulation of GAP-43, tubulins, and neurofilament-M. J Comp Neurol. 1999;414:495–510. doi: 10.1002/(sici)1096-9861(19991129)414:4<495::aid-cne6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Research. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Tetzlaff W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Heck S, Lezoualc’h F, Engert S, Behl C. Insulin-like Growth Factor-1-mediated Neuroprotection against Oxidative Stress Is Associated with Activation of Nuclear Factor kappa B. J Biol Chem. 1999;274:9828–9835. doi: 10.1074/jbc.274.14.9828. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Khodarahmi K, McGraw J, Steeves JD, Tetzlaff W. Brain-derived neurotrophic factor applied to the motor cortex promotes sprouting of corticospinal fibers but not regeneration into a peripheral nerve transplant. Journal of Neuroscience Research. 2002;69:160–168. doi: 10.1002/jnr.10275. [DOI] [PubMed] [Google Scholar]
- Jones DM, Tucker BA, Rahimtula M, Mearow KM. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. Journal of Neurochemistry. 2003;86:1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Mearow K. Neurite growth promotion by nerve growth factor and insulin-like growth factor-1 in cultured adult sensory neurons: Role of phosphoinositide 3-kinase and mitogen activated protein kinase. Journal of Neuroscience Research. 2001;63:486–499. doi: 10.1002/jnr.1043. [DOI] [PubMed] [Google Scholar]
- Li L, Openheim R, Lei M, Houenou L. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. Journal of Neurobiology. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol. 2001;436:456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Experimental Neurology. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic fgf elicit differential responses in the adult spinal cord. Cell Transplantation. 1996;5:191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. European Journal of Neuroscience. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E, Rechler MM, Ishii DN. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986;6:1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- Salie R, Steeves JD. IGF-1 and BDNF promote chick bulbospinal neurite outgrowth in vitro. International Journal of Developmental Neuroscience. 2005;23:587–598. doi: 10.1016/j.ijdevneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts Genetically Modified to Produce Nerve Growth Factor Induce Robust Neuritic Ingrowth after Grafting to the Spinal Cord. Experimental Neurology. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, Imperiale MJ, Boulis NM. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Med. 2004a;6:79–85. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiology of Disease. 2004b;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Tanabe K, Wada K, Shimoke K, Ishikawa Y, Ikeuchi T, Koizumi S, Hatanaka H. Differences in survival-promoting effects and intracellular signaling properties of BDNF and IGF-1 in cultured cerebral cortical neurons. Journal of Neurochemistry. 2001;78:940–951. doi: 10.1046/j.1471-4159.2001.00497.x. [DOI] [PubMed] [Google Scholar]
- Yoshida E, Atkinson TG, Chakravarthy B. Neuroprotective gene expression profiles in ischemic cortical cultures preconditioned with IGF-1 or bFGF. Brain Res Mol Brain Res. 2004;131:33–50. doi: 10.1016/j.molbrainres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-Inactivating Lentivirus Vector for Safe and Efficient In Vivo Gene Delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]