Abstract
The bioactive lipid sphingosine-1-phosphate (S1P) is emerging as an important mediator of immune and inflammatory responses. S1P formation is catalyzed by sphingosine kinase (SK), of which the SK1 isoenzyme is activated by tumor necrosis alpha (TNF-α). SK1 has been shown to be required for mediating TNF-α inflammatory responses in cells, including induction of cyclooxygenase 2 (COX-2). Because TNF-α and COX-2 are increased in patients with inflammatory bowel disease (IBD), we investigated the role of SK1 in a murine model of colitis. SK1−/− mice treated with dextran sulfate sodium (DSS) had significantly less blood loss, weight loss, colon shortening, colon histological damage, and splenomegaly than did wild-type (WT) mice. In addition, SK1−/− mice had no systemic inflammatory response. Moreover, WT but not SK1−/− mice treated with dextran sulfate sodium had significant increases in blood S1P levels, colon SK1 message and activity, and colon neutrophilic infiltrate. Unlike WT mice, SK1−/− mice failed to show colonic COX-2 induction despite an exaggerated TNF-α response; thus implicating for the first time SK1 in TNF-α-mediated COX-2 induction in vivo. Inhibition of SK1 may prove to be a valuable therapeutic target by inhibiting systemic and local inflammation in IBD.—Snider, A. J., Kawamori, T., Bradshaw, S. G., Orr, K. A., Gilkeson, G. S., Hannun, Y. A., Obeid, L. M. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis.
Keywords: sphingosine-1-phosphate, tumor necrosis factor alpha, cyclooxygenase-2, inflammation, ceramide
Sphingolipids, a diverse family of biologically active lipids, are involved in numerous cellular and biological events. Ceramide regulates cellular responses to stress, including apoptosis (1) and senescence (2), whereas sphingosine induces cytoskeletal rearrangement (3) and inhibits PKC (4). Conversely, sphingosine-1-phosphate (S1P) induces prosurvival effects, induces proliferation and migration of endothelial and cancer cells (5, 6), is involved in endothelial barrier enhancement (7), and regulates immune cell trafficking (8,9,10,11). S1P binds to S1P receptors (SIPRs), a family of 5 G-protein coupled receptors, which are differentially expressed in tissues (12). Recently, S1P has been shown to play a vital role in immune function and inflammation. Specifically, S1P and S1P1R are essential for lymphocyte egress from peripheral lymphoid tissue and the thymus (9). It has also been demonstrated that FTY720, a sphingosine analog, can induce peripheral blood lymphopenia (13).
S1P is generated by phosphorylation of sphingosine by sphingosine kinases (SKs). Two isoforms of SK have been cloned—SK1 and SK2. Activation of SK2 can mediate apoptosis (14), whereas SK1 activation is involved in cellular proliferation and migration (15). Moreover, SK1 appears to be a highly regulated enzyme that is activated by many growth factors and cytokines; prominent among them is tumor necrosis factor alpha (TNF-α). TNF-α activates SK1, leading to S1P generation, which, in turn, appears to mediate many cellular TNF responses. TNF-α activation of SK1 in endothelial cells generates S1P and leads to endothelial NOS (eNOS) activation (16). Similarly, SK1 activation by TNF-α and consequent increased S1P production induce cyclooxygenase-2 (COX-2) expression and production of prostaglandin E2 (PGE2) (17, 18). In fact, the SK1/S1P pathway appears to be necessary to mediate the TNF-α inflammatory response in several cell types, including L929 fibroblasts, A549 lung adenocarcinoma cells, and HT-29 colon cancer cells (18, 19).
TNF-α has been implicated in diseases such as cancer, rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease (IBD). TNF-α levels are increased in patients with IBD (20), a spectrum of diseases which include ulcerative colitis (UC) and Crohn’s disease (CD), and is characterized by infiltration of activated leukocytes (21) and increased proinflammatory cytokines (22). Current therapies that block TNF-α-induced effects, with either an anti-TNF-α mAb or recombinant TNF-R, significantly improve disease states for both rheumatoid arthritis (23) and IBD (24).
In this study we examined the role of SK1/S1P in mediating the inflammatory activity of TNF-α in vivo. Human tissue from patients with UC exhibited expression of SK1. Moreover, in a well-established animal model of UC, namely, dextran sulfate sodium (DSS) -induced colitis, we demonstrate that SK1−/− mice are significantly protected from disease manifestations of IBD. In addition, we demonstrate for the first time that SK1 is downstream of TNF-α and is necessary for activation of COX-2 in vivo and that SK1 is necessary for the inflammatory infiltration in colons from DSS-induced colitis. The implications of these results for our understanding of IBD and possible novel therapeutics are discussed.
MATERIALS AND METHODS
Human tissue array
A human tissue array from Cooperative Human Tissue Network (CHTN), Colorectal Carcinoma Progression TMA (CHTN2003CRCprog) was used for immunohistochemistry. Anti-human SK1 antibody, used previously (19), was used to detect the presence or absence of SK1 in normal colon mucosa or tissue from patients with UC.
Mice
C57BL/6 wild type (WT) mice were purchased from Charles River Laboratories (Wilmington, MA, USA), and SK1 knockout mice (SK1−/−) mice from Dr. Rick Proia [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Bethesda, MD, USA] were backcrossed at least 5 generations to C57BL/6 (25). Animals were maintained under standard laboratory conditions. All animal procedures were approved by the Medical University of South Carolina (MUSC) Institutional Animal Care and Use Committee and followed the guidelines of the American Veterinary Medical Association.
Induction of colitis
Acute colitis was induced by adding 5% (w/v) DSS (MP Biomedicals, Inc., Solon, OH, USA) to drinking water for 3 or 5 days. DSS solutions were monitored to ensure equal consumption between WT and SK1−/− mice. Control mice were given regular drinking water.
Histology score
Colons were removed, opened longitudinally, and fixed with 10% formalin. Sections were embedded in paraffin, fixed to glass slides, and stained with hematoxylin and eosin (H&E). The entire colon was microscopically examined for damage, and the most severe point of damage scored in a masked fashion. Scores were assigned as described previously (26), as follows: 0, normal colon mucosa; 1, shortening of basal 1/3 of crypts with slight edema and lymphocytic infiltration; 2, loss of the basal 2/3 of crypts with moderate inflammation in the lamina propria; 3, total loss of crypts with severe inflammation in the lamina propria, but with surface epithelium still remaining; and 4, loss of all crypts and surface epithelium with severe inflammation in the mucosa, muscularis propria, and submucosa.
Lipid analysis
Advanced analyses of sphingosine and ceramide species were performed by the Lipidomics Core at MUSC on a Thermo Finnigan TSQ 7000, triple-stage quadrupole mass spectrometer (Thermo Finnegan, Waltham, MA, USA) operating in a multiple reaction monitoring (MRM) positive ionization mode, as described previously (27).
SK activity assay
SK activity was determined as described previously, with minor modifications (28). After sacrifice, colon tissue was harvested in SK1 buffer (containing 20 mM Tris-HCl, pH 7.4; 1 mM EDTA; 0.5 mM deoxypyridoxine; 15 mM NaF; 1 mM β-mercaptoethanol; 1 mM sodium orthovanadate; 40 mM β-glycerophosphate; 0.4 mM phenylmethylsulfonyl fluoride; 10% glycerol; 0.5% Triton X-100; and complete protease inhibitors). Tissue was homogenized using a rotor homogenizer. After brief sonication and protein concentration (determined by bicinchoninic acid protein assay), 30 μg of protein was incubated in 90 μl of reaction mixture containing sphingosine (50 μM, delivered in 4 mg/ml fatty acid-free bovine serum albumin), [γ-32P]ATP (5 μCi, 1 mM dissolved in 10 mM MgCl2), and SK1 buffer for 30 min at of 37°C. The reaction was terminated by the addition of 10 μl of 1 N HCl and 400 μl of chloroform/methanol/HCl (100:200:1, v/v/v). Subsequently, 120 μl of chloroform and 120 μl of 2 M KCl were added, and samples were centrifuged at 3000 g for 5 min. Then, 200 μl of the organic phase was transferred to new glass tubes and dried. Samples were resuspended in chloroform/methanol/HCl (100: 100:1, v/v/v). Lipids were then resolved on silica thin layer chromatography plates using 1-butanol/methanol/acetic acid/water (8:2:1:2, v/v/v/v) as a solvent system and visualized by autoradiography. The radioactive spots corresponding to S1P were scraped from the plates and counted for radioactivity. Background values were determined in negative controls in which sphingosine was not added to the reaction mixture.
Harvesting of RNA
Colon sections were placed in RNAlater (Ambion, Austin, TX, USA) at 4°C. Next, tissue was homogenized, and total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA, USA) per the manufacturer’s instructions. RNA yield and purity were assessed by spectroscopic analysis.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
RNA extracted from colon tissue was reverse-transcribed into cDNA using the SuperScript III First Strand cDNA synthesis system from Invitrogen (Carlsbad, CA, USA). cDNA was synthesized from 0.5 μg RNA using random hexamer primers and SuperScriptIII from Invitrogen. Real-time RT-PCR was performed on a Bio-Rad iCycler to quantify mRNA levels of SK1, COX-2, and TNF-α. The standard real-time RT-PCR reaction volume was 25 μl, including 12.5 μl SYBR Green PCR reagents (Bio-Rad, Hercules, CA, USA), 5 μl cDNA template, 1 μl forward primer (4 μM), 1 μl reverse primer (4 μM), and 5.5 μl water. The primers for real-time were as follows: SK1 forward primer 5′-CCGTACTTGGTTCATGTGCCA-3′ and reverse primer 5′-TCCCCGTCCACAGAAAACACT-3′; COX-2 forward primer 5′-TGAGTACCGCAAACGCTTCTC-3′ and reverse primer 5′-TGCAGCCATTTCTTTCTCTCCT-3′; TNF-α forward primer 5′-AATGGCCTCCCTCTCATCAGTT-3′ and reverse primer 5′-CCACTTGGTGGTTTCCTACGA-3′; and β-actin forward primer 5′-TAAGGCCAACCGTGAAAAGATG-3′ and reverse primer 5′-CTGGATGGCTACGTACATGGCT-3′. The RT-PCR steps were as follows: 2 min at 95°C, followed by cycles (n=40) consisting of a 15-s melt at 95°C, a 60-s annealing/extension at 60°C, and a final step of 1-min incubation at 60°C. All reactions were performed in triplicate. The data were analyzed using Q-Gene software (29) and expressed as fold-change mean normalized expression (MNE) from control value. MNE is directly proportional to the amount of RNA of the target gene relative to the amount of RNA of the reference gene, β-actin.
FACS analysis of surface receptor expression
Colon cells (2×107 cells/ml) were resuspended in sterile FACS buffer [PBS with 1% sodium azide (Sigma, St. Louis, MO, USA), and 0.1% FBS] and incubated for 20 min on ice with 1 μg anti-CD16/CD32 per 106 cells. FITC-labeled anti-GR1/Ly-6G (BD Pharmingen, San Jose, CA, USA) was added to 106 cells and incubated on ice for 20 min, protected from light. Following 2 washes in FACS buffer, fluorescence was detected with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Data analysis
Statistical analyses were performed using two-way ANOVA to test for species and treatment effects. Values of P < 0.05 were considered significant.
RESULTS
Patients with UC have increased expression of SK1 and COX-2
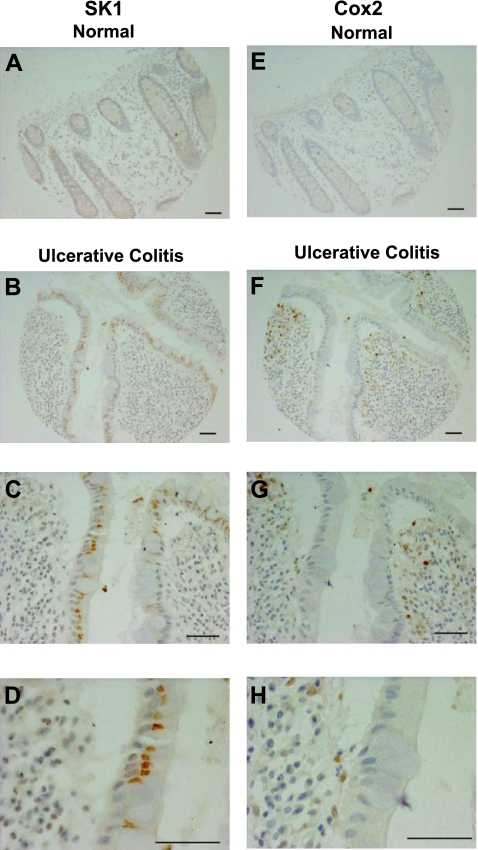
Because SK1 and its product S1P have a role in inflammation and SK1 has been implicated in COX-2 induction, we investigated whether SK1 and COX-2 levels were increased in human IBD. Expression in IBD was determined using human antibodies directed at SK1 and COX-2 on a tissue array of colon carcinoma progression from the CHTN. Six samples in this array were from patients with UC and 7 samples were from normal colon mucosa. Tissue from all 6 patients with ulcerative colitis had significantly greater expression of SK1 than normal colon mucosa (Fig. 1A–D). Expression of SK1 was increased in the epithelial cells as well as in the stromal cells from patients with UC (Fig. 1C, D). Likewise, and as described previously (30), COX-2 expression was increased in tissue from patients with UC when compared to normal colon mucosa (Fig. 1E–H). However, COX-2 expression was predominantly increased in the inflammatory mononuclear infiltrate and to a lesser extent in the epithelial cells (Fig. 1G, H). Increased expression of COX-2 and SK1 in colons of patients with UC suggests their involvement in disease.
Figure 1.
Immunohistochemistry for SK1 and COX-2 in human colon samples. A, E) Normal mucosa with SK1 (A) and COX-2 staining (E). B–D, F–H) Ulcerative colitis sample with SK1 (B–D) and COX-2 staining (F–H). Scale bars = 50 μm.
SK1−/− mice are partially protected from DSS-induced colon damage
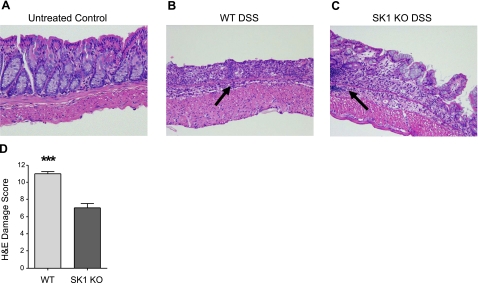
To determine whether SK1 expression was necessary for in vivo inflammation, we evaluated its role in IBD progression. We utilized a well-established mouse colitis model, DSS-induced colitis. WT and SK1−/− mice were administered 5% DSS in their drinking water for 7 days (control mice were given regular drinking water). Water intake was monitored to ensure equal DSS intake among animals, and intake was not significantly different among treatment groups (data not shown). Control mice from both groups exhibited no signs of disease or infection. Next, colons were harvested after sacrifice, and H&E sections were prepared for scoring the DSS-induced damage, as described in Materials and Methods. After 7 days with DSS, WT and SK1−/− mice had some blood in their colon, as indicated by visual examination of mouse colons on sacrifice (data not shown); however, there was decreased incidence of blood in the colon observed in SK1−/− mice. Control mice from both strains displayed a normal epithelial lining, with normal crypt length, an intact surface epithelium, and no histological evidence of damage (Fig. 2A). WT mice had severe inflammation throughout the entire colon, with partial loss of crypts in the proximal and medial portions of the colon and total loss of crypts and surface epithelium in the distal colon (Fig. 2B). Colons of SK1−/− mice had significantly less damage in the proximal and medial sections of the colon with some areas of damage in the distal colon (Fig. 2C). Whereas distal sections of colons from both WT and SK1−/− mice had more damage, WT mice had greater total area of severely damaged tissue. Unlike in colons of WT mice, colons from SK1−/− mice had sections of undisturbed tissue (Fig. 2C), including in the distal colon. Moreover, inflammatory infiltrate was visually more prevalent in the colons of WT mice (Fig. 2B, arrow). Inflammatory infiltration did occur in the colons of SK1−/− mice, but was patchy and significantly less severe (Fig. 2C, arrow). On overall scoring as described in the Materials and Methods, DSS-induced damage was significantly more severe in WT mice (Fig. 2D), indicating that absence of SK1 is protective against DSS-induced colitis.
Figure 2.
SK1−/− mice are partially protected against DSS-induced colitis. Tissue sections from WT and SK1−/− mice were H&E stained and graded for colonic damage as described in Materials and Methods. A–C) Representative sections from untreated mice (A), WT mice after 7 days of DSS treatment (B), and SK1−/− mice after 7 days of DSS treatment (C). Arrows indicate areas of inflammatory infiltrate. D) Damage score from WT and SK1−/− mice after 7 days of DSS. Data represent means ± se;n = 2 mice/group for no treatment, 6 mice/group for DSS treatment; ***P < 0.001.
SK1−/− mice are protected from pathobiologic DSS-induced parameters of disease
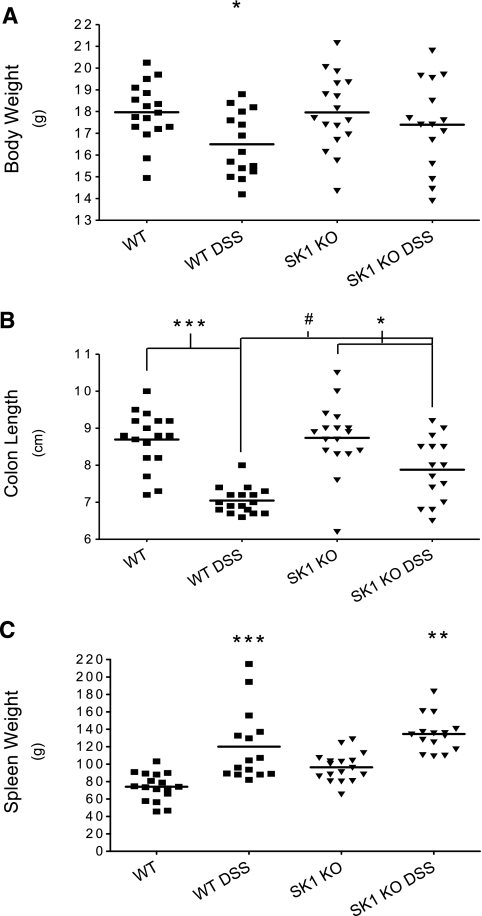
To characterize the pathobiologic mechanism by which SK1−/− mice were protected from DSS-induced colitis, earlier times of DSS treatment were examined. Mice were given DSS for 3 and 5 days and examined for common parameters of colitis including weight loss, shortening of the colon, and splenomegaly. WT and SK1−/− untreated control mice weighed the same amount after 5 days of regular drinking water. However, WT mice lost significantly more weight after 5 days of DSS treatment than did SK1−/− mice (Fig. 3A).
Figure 3.
Effects of DSS-induced colitis in WT and SK1−/− mice. WT and SK1−/− mice were administered 5% DSS in drinking water for 5 days and disease severity was assessed by weight loss (A), change in colon length (B), and spleen weight (C). Data represent means ± se;n > 10 mice/group; *P < 0.05, **P < 0.01, ***,#P < 0.001.
Next, colons were removed after sacrifice, opened longitudinally, and their length was measured. Colons from WT mice treated with DSS for 5 days had significant colon shortening when compared to WT untreated control animals (Fig. 3B). Whereas there was a difference in the mean colon length from SK1−/− untreated control and SK1−/− DSS-treated animals, it was not as significant as that observed between WT untreated control and DSS-treated mice. This disparity in colon shortening between WT and SK1−/− mice indicates that SK1−/− mice are significantly protected from DSS-induced colitis.
Of note is that spleens from WT mice that received DSS for 5 days were significantly enlarged when compared to WT untreated control mice. SK1−/− mice did exhibit some spleen enlargement, and spleens from untreated control SK1−/− mice were larger (on average) than untreated control WT mice (Fig. 3C). In summary, WT but not SK1−/− mice had significantly worse disease manifestation after 5 days of DSS treatment.
Circulating red blood cells (RBCs) are significantly decreased in WT mice with DSS-induced colitis
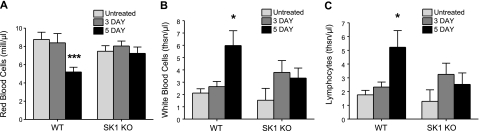
Significant increases in blood loss also indicate disease severity. Whole blood was obtained from untreated control animals and animals after 3 and 5 days of DSS treatment and complete blood counts were performed with Ani Lytics (Gaithersburg, MD, USA). Circulating RBCs were significantly decreased in the blood of WT mice compared to untreated control animals (Fig. 4A). Circulating RBCs from SK1−/− mice were not significantly altered in DSS-treated mice. Similarly, hemoglobin and hematocrit were decreased in WT mice after 5 days of DSS treatment (data not shown), but were not altered in SK1−/− mice; indicating and quantifying that WT mice had more significant bleeding after DSS treatment for 5 days.
Figure 4.
WT mice have significantly fewer circulating RBCs and increased circulating WBCs after DSS administration. Complete blood counts were performed on whole blood from untreated WT and SK1−/− mice, as well as mice after 3 and 5 days of DSS treatment. Blood was analyzed for RBCs (A), WBCs (B), and lymphocytes (C). Data represent means ± se;n = 10 mice/group; *P < 0.05, ***P < 0.001.
SK1−/− mice fail to mount a systemic inflammatory reaction
To determine the extent of the systemic inflammatory response in DSS-induced colitis, circulating white blood cells (WBCs) were measured after 3 and 5 days of DSS treatment. There was no significant difference in baseline circulating neutrophil and lymphocyte numbers between WT and SK1−/− mice. On treatment with DSS, circulating neutrophils and lymphocytes were significantly increased in WT mice at day 5 of DSS treatment (Fig. 4B, C). In contrast, SK1−/− mice showed slight, but not statistically significant, changes in circulating neutrophils and lymphocytes. This increase in circulating WBCs could indicate that WT mice had systemic inflammation whereas SK1−/− mice did not.
WT mice with DSS-induced colitis have increased circulating levels of S1P
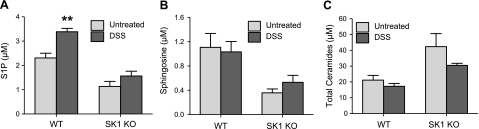
To investigate the role of lipids in the systemic inflammatory response to DSS-induced colitis, we measured blood lipids. Sphingolipids in whole blood were analyzed using HPLC-ESI-MS. As expected, blood S1P in control WT mice was higher than in control SK1−/− mice (Fig. 5A). Interestingly, S1P was significantly increased in the blood of WT mice after DSS treatment (Fig. 5A), whereas SK1−/− mice did not have significantly increased S1P. Examination of other sphingolipids revealed that sphingosine was higher basally and after DSS in WT mice compared to SK1−/− mice (Fig. 5B). Likewise, dihydro-S1P and dihydrosphingosine were also much higher basally in the blood of WT mice, compared to SK1−/− mice (data not shown). Interestingly, total ceramide was higher in SK1−/− mice than in WT mice, and decreased slightly but not significantly on DSS treatment (Fig. 5C). Taken together, these data indicate that increases in blood S1P occur in WT mice as disease severity increases, suggesting a possible link between S1P levels and systemic inflammation.
Figure 5.
S1P is elevated in the blood of WT mice. Whole blood was collected with anticoagulant from mice after 5 days of DSS administration. Samples were analyzed for sphingolipid content using HPLC-ESI-MS by the Lipidomics Core Facility at MUSC: S1P (A), sphingosine (B), and total ceramide in whole blood with or without DSS administration (C). Data represent means ± se;n = 5 mice/group; **P < 0.01.
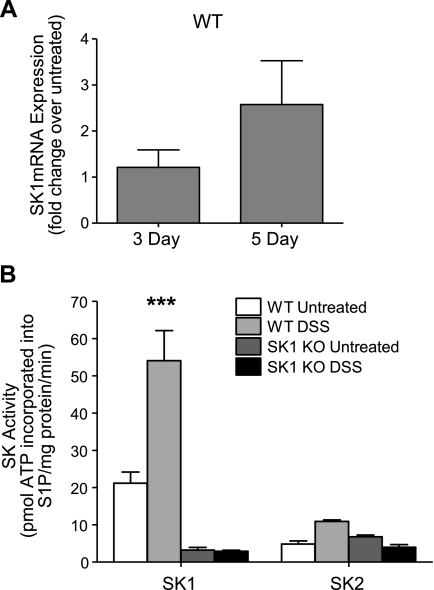
SK1 message levels and activity increase in response to DSS treatment in WT but not SK1−/− colon tissue
Next, to determine the role of SK1 in local inflammation, we measured SK1 in colon tissue in DSS-treated WT mice. Colon tissue from DSS-treated WT mice had more than a 2-fold increase in SK1 message (Fig. 6A). In addition, SK1 activity more than doubled in colon tissues of WT DSS-treated mice (Fig. 6B), whereas colons from SK1−/− mice had little to no SK1 activity either basally or after DSS treatment (Fig. 6B). A slight increase in tissue S1P was also observed in colon tissue from WT mice but not in SK1−/− mice. S1P increased from 0.55 ± 0.1 pmol/mg protein in colons from untreated WT mice to 0.65 ± 0.1 pmol/mg protein in colons from DSS-treated mice. S1P in untreated SK1−/− mice were basally lower than those of WT and remained essentially unchanged at 0.4 ± 0.05 and 0.4 ± 0.04 pmol/mg protein prior to and after DSS treatment, respectively. Of note, SK2 activity did not significantly change in colons from WT or SK1−/− mice on DSS treatment (Fig. 6B), indicating that SK1 activity is responsible for the increased S1P in colons from WT mice. Taken together, these data suggest that SK1—and not SK2 activity—is important in DSS-induced colitis.
Figure 6.
SK1 message and activity increase in WT mice with DSS colitis. A) Real-time RT-PCR was performed to determine message levels of SK1 in colon tissue from WT untreated mice and WT mice after 3 and 5 days of DSS treatment, using β-actin as a reference gene. B) SK activity in colon tissue from untreated and DSS-treated WT and SK1−/− mice. Data represent means ± se;n = 5 mice/group; ***P < 0.001.
SK1−/− mouse colons, but not WT mice, were protected from granulocyte infiltration
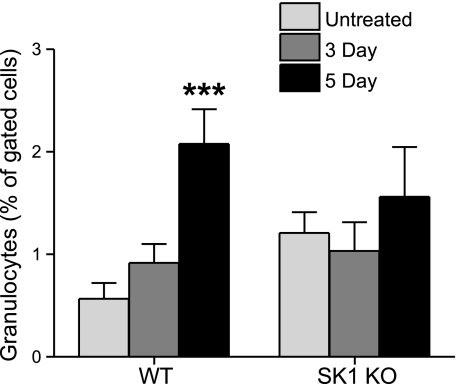
The SK1/S1P pathway has been implicated in neutrophil migration in vitro (31). To test whether the SK1/S1P pathway was necessary for neutrophil infiltration, and thus cellular manifestations of IBD, granulocyte infiltration into the colon was measured using flow cytometry. Colons from untreated control and DSS-treated mice were homogenized and subjected to flow cytometry as described in Materials and Methods. Colons from WT mice had a significant increase in granulocytic infiltration after 5 days of DSS treatment (Fig. 7). However, colons from SK1−/− mice exhibited no difference in granulocytic infiltration between untreated controls and DSS-treated mice although basal levels were higher. These data indicate for the first time that the SK1/S1P pathway is involved in tissue inflammatory infiltration in vivo.
Figure 7.
Colons from WT mice have significant granulocytic infiltration after DSS treatment. Colons from untreated and DSS-treated WT and SK1−/− mice were homogenized and stained for granulocyte marker, GR1. Flow cytometry was used for detection of granulocytes. Data represent means ± se;n = 5 mice/group; ***P < 0.001.
SK1 is necessary for COX-2 expression in vivo
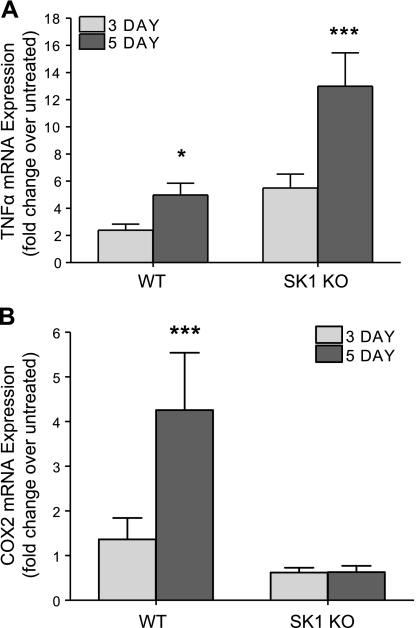
Next, we investigated molecular mechanisms by which SK1−/− mice were protected from DSS-induced colitis. Because SK1 and S1P have been shown to mediate TNF-α activation of COX-2 expression in cells, we next measured COX-2 in response to DSS treatment. Colons from WT mice had a 2.5-fold increase in TNF-α expression and a 5-fold increase in COX-2 expression (Fig. 8). However, COX-2 expression was not induced in SK1−/− mice, despite a 13-fold increase in TNF-α expression. These data demonstrate for the first time that SK1 and, likely, S1P are necessary for TNF-α induction of COX-2 in vivo. They also suggest that the SK1/COX-2 pathway may be involved in a negative feedback regulation of TNF-α.
Figure 8.
COX-2 induction, but not TNF-α, is prevented in SK1−/− mice administered DSS. Colon tissue was harvested from untreated WT and SK1−/− mice and mice treated with DSS for 3 and 5 days; mRNA was extracted and reverse transcribed into cDNA. Real-time RT-PCR was performed to determine relative levels of TNF-α (A) and COX-2 (B); β-actin was used as a reference gene. Data represent means ± se;n = 5–10 mice/group; *P < 0.05, ***P < 0.001.
DISCUSSION
TNF-α is a major inflammatory cytokine in IBD, and its expression is upregulated in colon tissue from patients with active IBD (32, 33). Several studies have linked TNF-α signaling to SK1 activation. Moreover, it has been shown that SK1 activation by TNF-α leads to numerous downstream effects, including COX-2 and PGE2 production (18), monocyte degranulation (34), and transcription of adhesion molecules (17). All together, these studies suggest numerous effects of inflammation induced by TNF-α are downstream of SK1.
To determine whether TNF-α exerts its effects through SK1 in IBD, we first examined SK1 expression in human tissue arrays from patients with IBD. SK1 expression is increased in patients with UC, not only in the colonic epithelial cells, but in the inflammatory infiltrate. This increased SK1 expression corresponds to increased COX-2 expression predominantly in the stromal mononuclear infiltrate. Next, we used the well-established DSS-induced colitis model in WT and SK1−/− mice. We demonstrate that SK1−/− mice are protected from several disease parameters including weight loss, colon shortening, colon damage, splenomegaly, anemia, and leukocytosis. Moreover, we demonstrate that blood S1P was elevated in WT but not in SK1−/− mice in response to DSS treatment. We also demonstrate that colons of WT but not SK1−/− mice had significant increases in inflammatory infiltrate, SK1 activity, and COX-2 expression. Collectively, these data indicate that the SK1/S1P pathway is likely involved in mediating many effects in IBD, most likely downstream of TNF-α and upstream of COX-2.
Increased SK1 expression in colons from patients with UC, not only in the colonic epithelial cells but also in the inflammatory infiltrate, is similar to that seen in lung tissues from lung cancer patients (35) and in colon tissues from patients with adenocarcinomas (19). Possibly, SK1 is activated in all cases in response to inflammatory stimuli, leading to S1P generation, recruitment of inflammatory cells, and activation of COX-2. The fact that our immunohistochemistry studies with human IBD samples show that SK1 is expressed in both epithelial and stromal cells, whereas COX-2 is predominantly seen in the stromal inflammatory infiltrate, lends support to the notion that activation of SK1 maybe necessary for the consequent inflammatory infiltration and COX-2 activation. Indeed, in colon tissues of DSS-treated SK1−/− mice, there was failure to recruit inflammatory infiltrate and to express COX-2, likely leading to protection from a damaging inflammatory process in DSS-treated mouse colons.
IBD is characterized by neutrophilic infiltration into the crypts and lamina propria of the colon. S1P has been shown to affect migration of leukocytes (36, 37) whereby leukocyte migration can be both positively and negatively regulated by S1P depending on the concentrations used. S1P treatment inhibited IL-8 and fMLP-induced chemotaxis and migration in human neutrophils at high doses (37). In addition, high doses of S1P led to inhibition of CHO cell migration, whereas lower doses increased CHO migration (38). Interestingly, inhibition of SK activity by dimethylsphingosine was shown to inhibit PMA-induced migration of human peripheral blood leukocytes (36), and to inhibit leukocyte infiltration in vitro and in vivo in a C5a-induced mouse peritonitis model (39). Of note is the study by Billich and co-workers (40) on neutrophil function of SK1−/− mice, which demonstrated that SK1−/− neutrophils behaved similarly to those from WT mice in their ability to migrate toward fMLP and C5a in vitro and to be recruited into the peritoneum (in response to the chemokines KC and MIP-2 or to lipopolysaccharide) in vivo. On the other hand, in our study we observed failure of granulocytic infiltration in colon tissues from DSS-treated SK1−/− mice compared to colons of WT mice. The tissue inflammatory infiltrate in WT mouse colons coincided with an increase in tissue SK1 activity and S1P in response to DSS, whereas increases in tissue SK1 activity and S1P were not seen in SK1−/− mice. It is therefore likely that the increase in tissue SK1 and S1P, but not the SK1 intrinsic to neutrophils, is required for the inflammatory infiltrate. Thus, S1P itself may act as a chemoattractant for granulocytes in colons of WT mice or it may be necessary for production of local chemoattractants. In either case, the results provide strong evidence that the SK1/S1P pathway is critical for the tissue inflammatory reaction. Of note is that SK2 activity did not increase in colons from WT mice, and was apparently unable to compensate for the loss of SK1 in SK1−/− mice.
The protection from DSS-induced colitis by loss of SK1 leads us to ponder a potential therapeutic approach for IBD by targeting the SK1/S1P pathway. This is especially important in light of the controversial role of targeting the COX-2 pathway in the treatment of IBD. Increased COX-2 expression is well documented in IBD (30, 41). However, it has been shown that knockout of COX-2 in vivo leads to increased severity of disease in colitis models. Moreover, nonspecific inhibition of cyclooxygenases with indomethacin enhances disease severity (42). More recently, specific inhibition of COX-2 has been shown to reduce colitis (43,44,45,46). Interestingly, in our study COX-2 expression in the colons of DSS-treated SK1−/− mice did not increase as it did in the colons of DSS-treated WT mice, despite a significantly enhanced TNF-α response in the SK1−/− mice colons compared to colons of WT mice. These results are intriguing, as they are the first in vivo evidence for the requirement of SK1 to mediate the TNF- α response on COX-2. In addition, these studies indicate that SK1 maybe a worthy therapeutic target for the treatment of IBD. In fact, Maines et al. (47) investigated the effect of SK inhibition on DSS-induced colitis in mice. They demonstrated that severity of DSS-induced colitis was inhibited with orally available inhibitors of SK1, thus corroborating our proposal that SK1 is an attractive target for anti-inflammatory therapy.
Another emerging target for anti-inflammatory therapy is the S1P receptor family. There is increasing evidence that S1P and its receptors are implicated in lymphocyte trafficking (9, 48, 49). In fact, it has been shown that S1P levels affect lymphocyte egress from the thymus and secondary lymph nodes (9), and S1P has also been shown to stimulate egress of T lymphocytes into the blood (50). The immunosuppressant drug FTY720, which induces lymphopenia by sequestering T lymphocytes in lymph nodes, is thought to act through its phosphorylation and action on S1P receptors (48, 49). Interestingly, neither in our studies nor in those of Billich and co-workers (40) or Allende et al. (25) was there evidence of peripheral lymphopenia or neutropenia in SK1−/− mice. This may be attributed to the fact that basal S1P blood levels were only modestly decreased in SK1−/− mice compared to WT mice. On DSS treatment, blood levels of S1P increased significantly in WT mice but not in SK1−/− mice. Concomitant with that finding, we observed an increase in peripheral blood leukocytosis in WT mice but not in SK1−/− mice, indicating that S1P maybe required for generating a circulating leukocyte reaction. This may be due to regulation of lymphocyte and neutrophil egress in response to an increase in circulating S1P. Notably, FTY720 has also been shown to improve IBD severity in 2,4,6-trinitrobenzene sulfonic acid and oxazolone-induced colitis models through manipulation of T-helper and dendritic cells (51, 52). Taken together, these data indicate that targeting S1P in blood and/or S1P receptors maybe therapeutically beneficial in IBD.
In summary, we demonstrate in this study that SK1 expression is increased in human IBD colons and that SK is activated and S1P is generated in a mouse model of IBD. Moreover, we demonstrate that loss of SK1 appears to protect against IBD severity in mice. This protection maybe in part mediated through lack of SK1 activity and lack of increases in S1P levels in colons of DSS-treated SK1−/− mice, leading to lack of inflammatory infiltration and lack of COX-2 expression. All together, these data suggest that modulation of the SK1/S1P pathway, and subsequently COX-2, could lead to better therapeutics for IBD, as seen in our DSS-induced colitis model.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant GM062887 and a Veterans Affairs (VA) Merit Award (L.M.O.) and by the American College of Rheumatology Research and Education Foundation’s Within Our Reach: Finding a Cure for Arthritis campaign (G.S.G.). Lipid analysis was conducted by the Lipidomics Core at MUSC and was supported by NIH grant C06 RR018823. We thank Dr. Richard Proia (NIDDK, Bethesda, MD, USA) for supplying the SK1−/− mice; George Washington, DeAnna Baker, Samer Maalouf, and Tatsuya Kaneshiro for technical help; Margaret H. Romano and the Core Histology Laboratory for their technical assistance; Chris Clarke for careful examination of the manuscript; and Kathy Wiita-Fisk for her administrative assistance.
References
- Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Programmed cell death induced by ceramide. Science (New York) 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Venable M E, Lee J Y, Smyth M J, Bielawska A, Obeid L M. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Seufferlein T, Rozengurt E. Sphingosine induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J Biol Chem. 1994;269:27610–27617. [PubMed] [Google Scholar]
- Lee S C, Kuan C Y, Wen Z D, Yang S D. The naturally occurring PKC inhibitor sphingosine and tumor promoter phorbol ester potentially induce tyrosine phosphorylation/activation of oncogenic proline-directed protein kinase FA/GSK-3alpha in a common signalling pathway. J Protein Chem. 1998;17:15–27. doi: 10.1023/a:1022582312954. [DOI] [PubMed] [Google Scholar]
- Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J. 2000;348:71–76. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Van Brocklyn J R, Hobson J P, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- Singleton P A, Dudek S M, Chiang E T, Garcia J G. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- Martino A. Sphingosine 1-phosphate as a novel immune regulator of dendritic cells. J Biosci. 2007;32:1207–1212. doi: 10.1007/s12038-007-0122-0. [DOI] [PubMed] [Google Scholar]
- Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol. 2006;3:11–19. [PubMed] [Google Scholar]
- Eigenbrod S, Derwand R, Jakl V, Endres S, Eigler A. Sphingosine kinase and sphingosine-1-phosphate regulate migration, endocytosis and apoptosis of dendritic cells. Immunol Invest. 2006;35:149–165. doi: 10.1080/08820130600616490. [DOI] [PubMed] [Google Scholar]
- Von Wenckstern H, Zimmermann K, Kleuser B. The role of the lysophospholipid sphingosine 1-phosphate in immune cell biology. Arch Immunol Ther Exp (Warsz) 2006;54:239–251. doi: 10.1007/s00005-006-0028-9. [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf D, Jakobs K H. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Rosen H, Sanna G, Alfonso C. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol Rev. 2003;195:160–177. doi: 10.1034/j.1600-065x.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Toman R E, Goparaju S K, Maceyka M, Nava V E, Sankala H, Payne S G, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait N C, Paugh S W, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pettus B J, Bielawski J, Porcelli A M, Reames D L, Johnson K R, Morrow J, Chalfant C E, Obeid L M, Hannun Y A. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson K R, Pettus B J, Bielawski J, Tanaka T, Wargovich M J, Reddy B S, Hannun Y A, Obeid L M, Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Breese E J, Michie C A, Nicholls S W, Murch S H, Williams C B, Domizio P, Walker-Smith J A, MacDonald T T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Popivanova B K, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juanatey C, Gonzalez-Gay M A. Rheumatoid arthritis and anti-TNF-alpha therapy. Atherosclerosis. 2005;181:209. doi: 10.1016/j.atherosclerosis.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Osterman M T, Lichtenstein G R. Current and future anti-TNF therapy for inflammatory bowel disease. Curr Treat Options Gastroenterol. 2007;10:195–207. doi: 10.1007/s11938-007-0013-3. [DOI] [PubMed] [Google Scholar]
- Allende M L, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane C A, Mandala S, Spiegel S, Proia R L. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Szulc Z M, Hannun Y A, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem. 1996;60:529–537. doi: 10.1002/(sici)1097-4644(19960315)60:4<529::aid-jcb9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Muller P Y, Janovjak H, Miserez A R, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Singer I I, Kawka D W, Schloemann S, Tessner T, Riehl T, Stenson W F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Lin C I, Chen C N, Chen J H, Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J Cell Biochem. 2006;99:1216–1232. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- Murch S H, Braegger C P, Walker-Smith J A, MacDonald T T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A D, Ayass M, Chensue S. Tumor necrosis factor and IL-1 beta expression in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993;16:241–246. doi: 10.1097/00005176-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Zhi L, Leung B P, Melendez A J. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208:109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- Johnson K R, Johnson K Y, Crellin H G, Ogretmen B, Boylan A M, Harley R A, Obeid L M. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- Kaneider N C, Djanani A, Fischer-Colbrie R, Wiedermann C J. Sphingosine kinase-dependent directional migration of leukocytes in response to phorbol ester. Biochem Biophys Res Commun. 2002;297:806–810. doi: 10.1016/s0006-291x(02)02304-5. [DOI] [PubMed] [Google Scholar]
- Kawa S, Kimura S, Hakomori S, Igarashi Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett. 1997;420:196–200. doi: 10.1016/s0014-5793(97)01516-0. [DOI] [PubMed] [Google Scholar]
- Kohno T, Igarashi Y. Attenuation of cell motility observed with high doses of sphingosine 1-phosphate or phosphorylated FTY720 involves RGS2 through its interactions with the receptor S1P. Genes Cells. 2008;13:747–757. doi: 10.1111/j.1365-2443.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Vlasenko L P, Melendez A J. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J Immunol. 2005;174:6456–6461. doi: 10.4049/jimmunol.174.10.6456. [DOI] [PubMed] [Google Scholar]
- Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol Lett. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Hendel J, Nielsen O H. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol. 1997;92:1170–1173. [PubMed] [Google Scholar]
- Tsubouchi R, Hayashi S, Aoi Y, Nishio H, Terashima S, Kato S, Takeuchi K. Healing impairment effect of cyclooxygenase inhibitors on dextran sulfate sodium-induced colitis in rats. Digestion. 2006;74:91–100. doi: 10.1159/000097657. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Serraino I, Dugo L, Centorrino T, Ciccolo A, Sautebin L, Caputi A P. Celecoxib, a selective cyclo-oxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur J Pharmacol. 2001;431:91–102. doi: 10.1016/s0014-2999(01)01403-0. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hirata I, Murano M. Effects of nimesulide, a cyclooxygenase-2 selective inhibitor, on colitis induced tumors. Inflammopharmacology. 2008;16:36–39. doi: 10.1007/s10787-006-1543-3. [DOI] [PubMed] [Google Scholar]
- Martin A R, Villegas I, Alarcon de la Lastra C. The COX-2 inhibitor, rofecoxib, ameliorates dextran sulphate sodium induced colitis in mice. Inflamm Res. 2005;54:145–151. doi: 10.1007/s00011-004-1337-2. [DOI] [PubMed] [Google Scholar]
- Maines L W, Fitzpatrick L R, French K J, Zhuang Y, Xia Z, Keller S N, Upson J J, Smith C D. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53:997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo C G, Cinamon G, Lesneski M J, Xu Y, Brinkmann V, Allende M L, Proia R L, Cyster J G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei G J, Card D, Keohane C, Rosenbach M, Hale J, Lynch C L, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science (New York) 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab S R, Cornelissen I, Pereira J P, Regard J B, Xu Y, Camerer E, Zheng Y W, Huang Y, Cyster J G, Coughlin S R. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science (New York) 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory N A, Zahn N, Schmidt R, Geisslinger G, Radeke H H, Stein J M. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44:3305–3316. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke H H, Stein J M. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]