Abstract
Background
Growth, differentiation and regional specification of telencephalic domains, such as the cerebral cortex, are regulated by the interplay of secreted proteins produced by patterning centers and signal transduction systems deployed in the surrounding neuroepithelium. Among other signaling molecules, members of the fibroblast growth factor (FGF) family have a prominent role in regulating growth, differentiation and regional specification. In the mouse telencephalon the rostral patterning center expresses members of the Fgf family (Fgf8, Fgf15, Fgf17, Fgf18). FGF8 and FGF17 signaling have major roles in specification and morphogenesis of the rostroventral telencephalon, whereas the functions of FGF15 and FGF18 in the rostral patterning center have not been established.
Results
Using Fgf15-/- mutant mice, we provide evidence that FGF15 suppresses proliferation, and that it promotes differentiation, expression of CoupTF1 and caudoventral fate; thus, reducing Fgf15 and Fgf8 dosage have opposite effects. Furthermore, we show that FGF15 and FGF8 differentially phosphorylate ERK (p42/44), AKT and S6 in cultures of embryonic cortex. Finally, we show that FGF15 inhibits proliferation in cortical cultures.
Conclusion
FGF15 and FGF8 have distinct signaling properties, and opposite effects on neocortical patterning and differentiation; FGF15 promotes CoupTF1 expression, represses proliferation and promotes neural differentiation.
Background
Regional specification, growth and differentiation of telencephalic subdivisions, such as the cerebral cortex, are regulated by the interplay of secreted proteins produced by patterning centers and signal transduction systems deployed in the surrounding neuroepithelium. The dorsal telencephalic patterning center is the source for bone morphogenetic proteins (BMPs) and Wnts that regulate development of dorsal and caudal parts of the telencephalon, including the choroid plexus and hippocampus [1-3]. A putative ventral patterning center expresses sonic hedgehog (SHH); dorsoventral patterning of the telencephalon is controlled through the GLI3 repressor of the SHH pathway [4-6]. An additional putative patterning center at the cortical-subcortical junction (pallium-subpallium boundary (PSB)) expresses multiple signaling molecules, such as Tgfα, Neuregulin 1 and 3, Fgf7, the putative Wnt antagonist Sfrp2 [7,8] and Fgf15 (this paper). Finally, the rostral patterning center expresses members of the Fgf family (Fgf8, Fgf15, Fgf17, Fgf18) [9-13].
The mammalian fibroblast growth factor (FGF) family consists at least 22 members whose functions range from specifying regional and cell fate to promoting proliferation, differentiation and survival [14]. FGF ligand binding to their cognate tyrosine kinase receptors (FGFR1-4) activates two major phosphorylation cascades: the Ras/mitogen activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase/AKT pathways [14].
FGF signaling is implicated in early steps of neural induction [14]. As the neural plate matures, its rostral margin, known as the anterior neural ridge, expresses at least three members of the Fgf family, Fgf8, Fgf17 and Fgf18 [9-12]. Following neurulation, these genes continue to be expressed in the rostral midline of the forebrain. Fgf15 expression is also prominent in the early rostral forebrain [15-17]. Within the rostral patterning center, Fgf8 and Fgf18 are expressed in its core, whereas Fgf17 expression extends dorsally [18] and Fgf15 extends ventrally [13,16,17,19]. Fgf15 is also expressed at the PSB and in the caudal-most region of the ventral pallium [16,17,19,20].
FGF8 and FGF17 signaling have major roles in specification and morphogenesis of the rostroventral telencephalon. Within the cortex, FGF8 is essential for producing most of the frontal cortex [17,21,22], whereas FGF17 is essential for the development of the dorsomedial frontal cortex [17,18]. The functions of FGF15 and FGF18 in the rostral patterning center have not been established, although Fgf18 expression in the cortical plate is implicated in laminar patterning and neuronal migration [23].
FGF8 promotes proliferation and survival of telencephalic progenitor cells, and specifies rostral telencephalic fate through positively regulating expression of several transcription factors, including Erm, Er81, Foxg1, Nkx2.1, Pea3 and Sp8 [16,17,19,22,24,25]. In addition, FGF8 represses caudal telencephalic fate through reducing the expression of CoupTF1, Emx2 and Wnt8b [12,21,22,25-27]. Fgf17 expression is genetically downstream of Fgf8 (this paper) and it is important in local patterning within the rostral cortex [17,18].
FGF8, FGF17 and FGF18 belong to the same subfamily based on their amino acid sequence, whereas FGF15, known as FGF19 in humans, chickens and zebrafish, is part of a distinct subfamily [28,29].
Here we explore the functions of FGF15 in neocortical development. Previous loss of function studies in the mouse established its function in the development of the gall bladder, inner ear, and cardiac outflow tract [30-33]. The function of the zebrafish Fgf15 homologue (Fgf19) has been assessed in the developing brain using antisense (morpholino) oligonucleotides [34]. That study provided evidence that Fgf15(19) promotes proliferation, similar to known functions of other Fgfs. On the other hand, our analysis of Fgf15-/- mutant mice, and primary cortical cultures treated with recombinant FGF15, provides evidence that FGF15 suppresses proliferation and promotes differentiation in the developing telencephalon. Furthermore, we demonstrate that Fgf15 promotes expression of CoupTF1, a transcription factor that represses proliferation, and promotes differentiation and caudoventral fate. Thus, in vivo reduction of Fgf15 dosage has the opposite effect of reducing Fgf8 dosage. Finally, in primary cortical cultures, we demonstrate that FGF15 and FGF8 have distinct effects on phosphorylation of ERK (p42/44), AKT, and S6, providing a link between signaling differences and the distinct cellular effects of these proteins.
Results
Fgf15 expression is repressed by FGF8 and is promoted by SHH
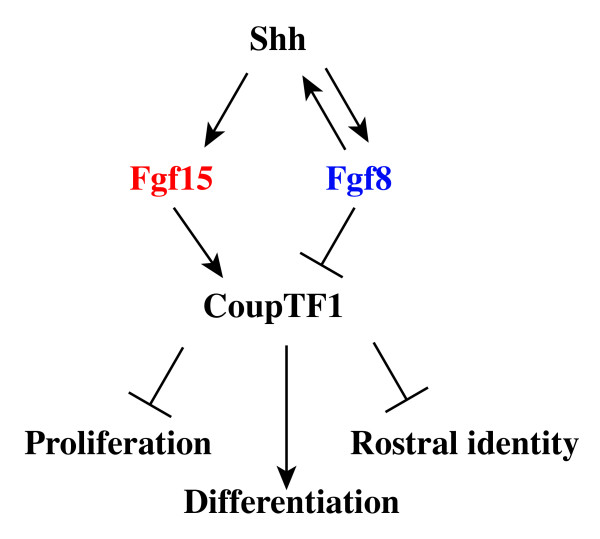
To assess the role of FGF15 in cortex development, we extended the analysis of Fgf15 expression in the developing telencephalon by in situ RNA hybridization, to complement previous reports [16,17,19]. At embryonic day 9.5 (E9.5), Fgf15 was expressed in the anterior forebrain neuroepithelium (Figure 1a), but was excluded from the anterior dorsal midline where Fgf8 was expressed (Figure 1a, b, arrowhead). At E12.5, Fgf15 was strongly expressed in the septum (Additional file 1g, arrow), preoptic area (Additional file 1h, arrow), and weakly expressed in the neuroepithelium of the PSB (Additional file 1g, arrowhead). In addition, Fgf15 was strongly expressed in the caudal ganglionic eminence (CGE; Additional file 1j, arrow).
Figure 1.
In situRNA hybridization on horizontal sections in wild-type,Fgf8Null/Neoand Fgf15-/-E9.5 embryos. (a, b) Fgf15 (a) and Fgf8 (b) expression in adjacent sections of a wild-type embryo. (c-d') Fgf15 expression in wild-type (c) and Fgf8Null/Neo embryos (d). Higher magnifications of (c, d) are shown in (c', d'). (e-n) Expression analysis in wild-type and Fgf15 mutant embryos showing adjacent sections of CoupTF1 (e, f), Fgf8 (g, h), Spry2 (i, j), Erm (k, l) and cMyc (m, n). Arrowheads in (a, b): rostral midline that is Fgf8+ and Fgf15-; the midline becomes Fgf15+ in Fgf8Null/Neo (d, d'). Arrow in (d): loss of Fgf15 expression in the midbrain of the Fgf8Null/Neo mutant. Arrows in (e, f): reduced CoupTF1in Fgf15-/-. Bar in (a) is 200 μm.
The overlapping and complementary expression of Fgf15 and Fgf8 in the dorsal midline suggests regulatory interactions between these factors. Gain-of-function experiments by Gimeno and Martinez [16] demonstrated that FGF8 protein can induce Fgf15 expression in the mesencephalon and prosencephalon. Thus, we analyzed the expression of Fgf15 in severely hypomorphic Fgf8 mutants (Fgf8Null/Neo) [22]. At E9.5, Fgf15 expression was strongly reduced at the midbrain/hindbrain boundary (Figure 1c, d, arrows), consistent with Gimeno and Martinez [16]. However, in the rostral forebrain, Fgf15 expression remained strong, and it was ectopically expressed in the anterior midline (Figure 1c, d, c', d', arrowheads). At E12.5, Fgf15 expression remained strong in the dysmorphic septal area and appeared to be increased in the CGE (Additional file 1e–j'). Thus, while Fgf15 is positively regulated by FGF8 in the midbrain, it is repressed by FGF8 in the prosencephalic midline.
There is evidence that SHH promotes Fgf15 expression in the diencephalon [35] and in the cortex [6,20]. Our analysis of the Shh-/- mutant extended these findings by demonstrating that Fgf15 expression in the rostral telencephalon requires SHH function (Additional file 2). Thus, while Fgf15 expression in the rostral telencephalon requires SHH, it remains strong in the severe Fgf8 hypomorph, despite the lack of telencephalic Shh expression in these mutants [22]. In addition, Gli3, a repressor of the SHH signal, may to be over-expressed in the Fgf15 mutants at E12.5 (Additional file 2q, q'), suggesting a positive feedback between FGF15 and SHH.
Opposite effects of FGF15 and FGF8 on patterning gene expression in the cortical primordium
To determine FGF15's functions in forebrain development we analyzed the Fgf15 null (Fgf15-/-) mutant mouse [32], and focused on the cortical phenotype. Histological analysis showed that the mutant cortex was thinner than the wild type (Figures 2 and 3; quantification below). The gross morphology of Fgf15 mutant embryos is similar to the wild-type cohort (Additional file 3).
Figure 2.
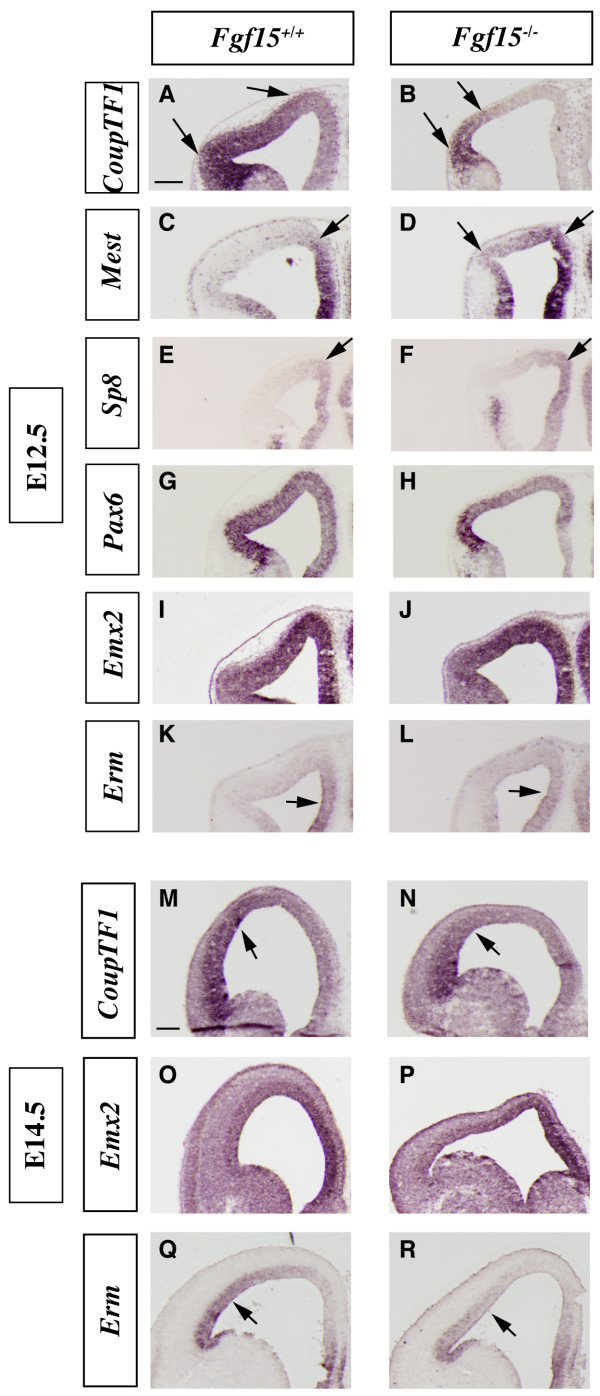
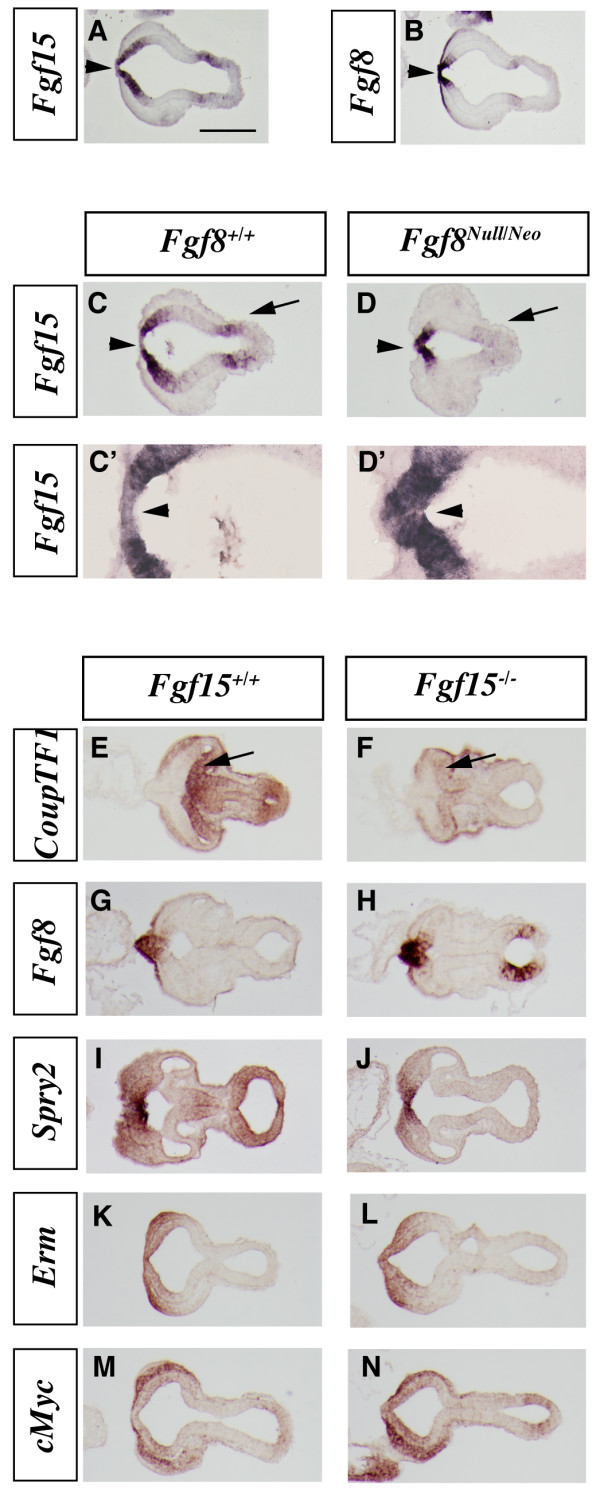
Analysis of the cortical patterning defects in the Fgf15 mutants. (a-r) In situ RNA hybridization on coronal sections of Fgf15+/+ (left column) and Fgf15-/- (right column) embryos at E12.5 (a-l) and E14.5 (m-r). CoupTF1 (a, b, m, n), Mest (c, d), Sp8 (e, f), Pax6 (g, h), Emx2 (i, j, o, p) and Erm (k, l, q, r). Arrows highlight the changes in the extent and/or intensity of expression. Bar in (a, m) is 200 μm.
Figure 3.
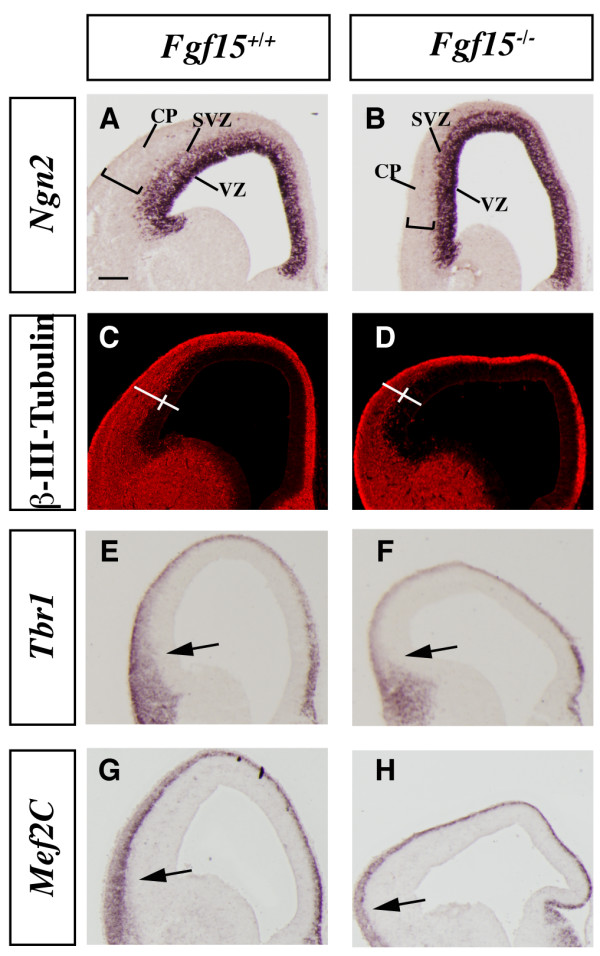
Analysis of the cortical neurogenesis defects in the Fgf15 mutants. (a-h) In situ RNA hybridization (a, b, e-h) and immunofluorescence (c, d) on coronal sections of Fgf15+/+ (left column) and Fgf15-/- (right column) embryos at E14.5. Ngn2 (a, b), β-III-Tubulin (c, d), Tbr1 (e, f), Mef2C (g, h). Arrows point out the reduced thickness of the cortical plate expression of Tbr1 and Mef2C. Brackets in (a-d) highlight the reduced thickness of the cortical plate. CP, cortical plate; SVZ, subventricular zone; VZ, ventricular zone. Bar in (a) is 200 μm.
We started the analysis at E9.5, soon after neurulation has generated the telencephalic vesicles, by studying mRNA expression of genes known to have important roles in cortical development or known to be markers of FGF signaling.
CouTF1 promotes caudoventral cortical identity [36-38]. While reducing Fgf8 dosage increased CoupTF1 expression [21,22], we observed that loss of Fgf15 strongly reduced its expression at E9.5 (Figure 1e, f). Subtle changes in expression are also observed, including increased Fgf8 expression (Figure 1g, h); however, establishing these changes would require more quantitative methods. On the contrary, we did not observe a major effect on the expression of two genes that are positively regulated by FGF signaling, Spry2 (Figure 1i, j) and Erm (Figure 1k, l). Expression of cMyc, a gene downstream of the Ras/MAPK signaling [39,40], may be slightly increased (Figure 1m, n).
Next, we interrogated FGF15 function by analyzing CoupTF1, Mest, Sp8, Pax6, Emx2 and Erm expression in the cortical ventricular and mantle zones at E12.5 and E14.5. As at E9.5, CoupTF1 expression was strongly reduced (Figure 2a, b and 2m, n), in contrast with its increase in Fgf8 mutants [21,22]. Fgf15 and Fgf8 mutants also showed the opposite effects on Mest and Sp8 expression (Figure 2c, d and 2e, f, respectively) [22,41].
On the other hand, Pax6, Erm and Emx2 showed subtle changes in the Fgf15 mutant. Pax6 cortical ventricular zone expression showed a modest decrease at E12.5 (Figure 2g, h) as well as Erm at E12.5 and E14.5 (Figure 2k, l and 2q, r). Emx2 expression may be increased in the dorsomedial cortical ventricular zone at E12.5 and E14.5 (Figure 2i, j and 2o, p). At this stage Fgf15 expression in the rostral patterning center does not appear to overlap with the cortical expression of the gene analyzed here. The changes in cortical expression could, however, result from the earlier patterning changes observed at E9.5 (Figure 1) and through diffusion of FGF15.
Zebrafish treated with morpholinos to Fgf19 (the name of zebrafish Fgf15) exhibit loss of subpallial molecular features [34]. We, however, did not detect a strong subpallial phenotype in the mouse Fgf15 mutant, although there was a suggestion of reduced GAD67,Nkx2.1 and Pax6 expression in the mantle zone of the ventral septum and piriform cortex (Additional file 4). There was, however, a clear reduction in the number of GAD67+cortical interneurons in the dorsomedial cortex (Additional file 4a, b). Reelin expression in this region appeared normal showing that differentiation of the marginal zone was not grossly disrupted (Additional file 4e, f).
In summary, reduced expression of Fgf15 and Fgf8 has opposite effects on the expression of several transcription factors that have prominent functions in regulating cortical regional fate, proliferation and differentiation. This was particularly clear for CoupTF1, which promotes caudoventral cortical fate [37,38], and which represses proliferation and promotes differentiation [38].
FGF15 promotes maturation of the cortical neural precursors in the ventricular and subventricular zones
The cortex in the Fgf15 mutants was thinner than the wild type at E14.5 (Figures 2 and 3), suggesting that FGF15 may regulate the balance between proliferation and differentiation. To explore this, we analyzed markers of differentiation and indices of proliferation.
Neurogenin2 (Ngn2) expression marks progenitor cells in the cortical ventricular zone (VZ) and subventricular zone (SVZ) and is largely excluded from the postmitotic neurons of the cortical plate [42] (Figure 3a). Fgf15 mutants maintained strong Ngn2 expression in the VZ, while its expression in the SVZ seemed to be reduced; however, the Ngn2 negative area was much thinner (Figure 3a, b), suggesting reduced cortical plate thickness. To confirm this observation, we studied expression of β-III-Tubulin, a marker of differentiating neurons. Indeed, the width of the cortical postmitotic zone was greatly reduced in the Fgf15 mutant (50% in ventral regions and 45% in dorsal regions; Figure 3c, d), while the width of the VZ was larger in the mutants (30% in ventral regions and 25% in dorsal regions; Figure 3c, d). The reduced thickness of the cortical plate was confirmed by analyzing expression of markers of early born cortical projection neurons, Tbr1 and Mef2C (Figure 3e–h). Tbr1 and Mef2C expressions were reduced in the cortical plate of the Fgf15 mutants (Figure 3e, f, g, h). Thus, Fgf15 mutants show a defect in the growth of the cortical plate, perhaps due to defect in neurogenesis.
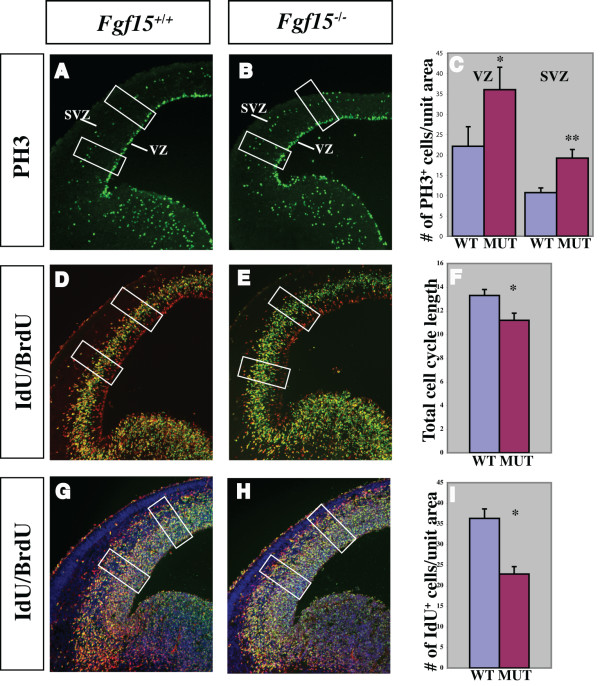
We explored the hypothesis that the cortical plate defect is caused because FGF15 may promote the switch between proliferation and differentiation. Thus, we assessed the mitotic index by immunostaining with phospho-histone H3 (PH3), a marker for M phase of the cell cycle. At E14.5, we observed a 37% increase in the density of PH3+ cells in the cortical VZ and a 42% increase in the SVZ (Figure 4a–c). This was already evident at E12.5 when the increase was 20% (Additional file 5a–c).
Figure 4.
Analysis of proliferation and cell cycle in the Fgf15 mutants. (a-i) Comparison of the rate of proliferation of the neuronal progenitors at E14.5 in coronal hemisections from Fgf15+/+ (left column) and Fgf15-/- (right column) embryos. The number of neuronal progenitors undergoing mitosis was evaluated by PH3+ immunofluorescence (a, b); quantification of the PH3+ cells in the VZ and SVZ is shown in (c) (n = 3; p = 0.0019, Student's t-test, indicated by * and **; see Materials and methods). The analysis of the cell cycle length (d, e) (quantification in (f)) and of the number of neuronal progenitors exiting the cell cycle (Q fraction) (f, g) (quantification in (i)) were performed at E14.5 by double labeling with IdU and BrdU (n = 3; p = 0.001, Student's t-test, indicated by *; see Materials and methods). The rectangles in a-b, d-e, g-h indicate the sampled bins. The error bars indicate the standard deviations. MUT, mutant; SVZ, subventricular zone; VZ, ventricular zone; WT, wild type.
The rate of proliferation of the cortical precursor cells is linked to the length of the cell cycle; in addition, the fraction of cells in a given phase of the cell cycle is directly proportional to the length of that specific phase, relative to the total length of the cell cycle [43,44]. Sequentially exposing proliferating cells to iodo-deoxyuridine (IdU) and bromo-deoxyuridine (BrdU) allows one to differentiate between defined populations of proliferating cells and then to calculate the cell cycle length (see Materials and methods). We compared the length of the cell cycle in E12.5 and E14.5 wild type and Fgf15 mutants using IdU/BrdU double labeling [45] (Figure 4d–f; Additional file 5d–f). Cell cycle length in the mutants was reduced to 11 hours, compared to the 13.5 hours of the wild type at E14.5 (Figure 4d–f), and 10 hours, compared to the 11 hours of the wild type at E12.5 (Additional file 5d–f).
Next, we analyzed the fraction of neural precursor cells exiting the cell cycle to become neurons (Q fraction) at E14.5. To identify cells exiting the cell cycle, we used differential labeling by IdU and BrdU [45]. The IdU/BrdU double labeling method allows one to distinguish between cells that are proliferating from cells that exit the cell cycle (see Materials and methods). Fgf15 mutants showed a 38% reduction in Q fraction (cells exiting the cell cycle) (Figure 4g–i).
Overall, these data provide evidence that reducing the dosage of Fgf15 reduces cell cycle length and cell cycle exit. Thus, FGF15 induces neurogenesis and this observation contrasts with the typical function of FGFs in promoting proliferation and maintenance of the neural progenitor state [14].
Analysis of the Wnt and the retinoic acid signaling pathways in the Fgf15 mutants
To investigate how FGF15 regulates cell cycle and neurogenesis, we studied signaling pathways that regulate the switch between proliferation and neurogenesis in Fgf15 mutants. First, we investigated the retinoic acid pathway, which promotes neural differentiation [46]. We introduced an allele that expresses LacZ under the control of retinoic acid receptor enhancer elements [47] into the Fgf15-/-background. At E12.5 and E14.5, there was a strong decrease in β-galactosidase activity in the mutants (Figure 5a–d), suggesting reduced retinoic acid signaling, consistent with the decrease in neuronal differentiation (Figure 3).
Figure 5.
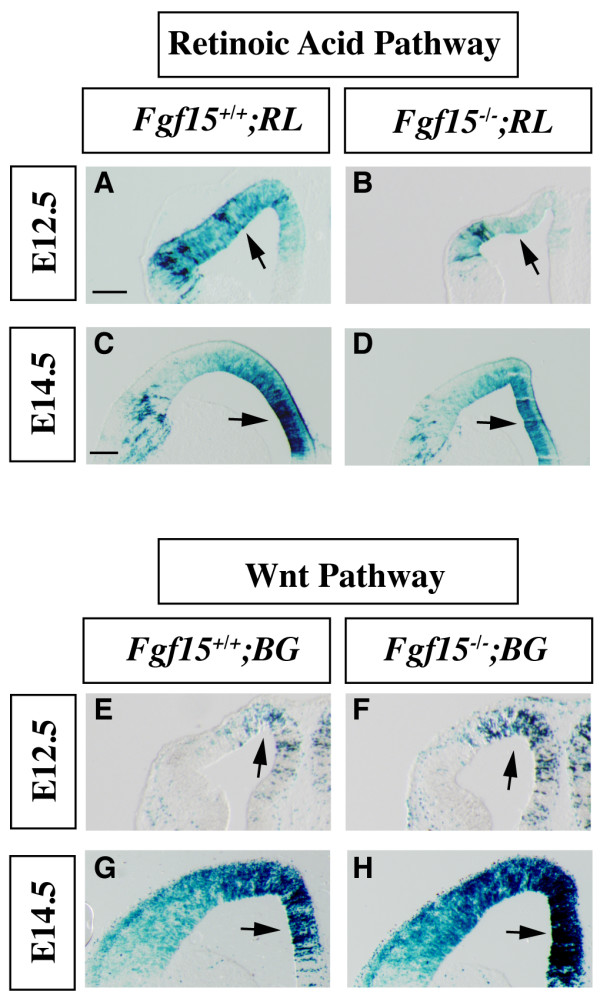
Activation of the Wnt/β-catenin and retinoic acid signaling pathways in the Fgf15 mutants.(a-d) Retinoic acid pathway activation was determined at E12.5 (a, b) and E14.5 (c, d) by β-galactosidase staining of coronal sections of Fgf15-/-; RL+ embryos (RL, RARE LacZ, retinoic acid reporter). (e-h) The Wnt/β-catenin pathway activation was revealed by β-galactosidase staining of coronal sections of Fgf15-/-; BG+ (BG, BATgal, Wnt/β-catenin reporter) at E12.5 (e, f) and E14.5 (g, h). Arrows indicate the regions that show the largest changes in the extent and/or intensity of expression. Three separate cases were analyzed for each genotype. Bar in (a, c) is 200 μm.
Wnt signaling through the β-catenin pathway promotes cortical proliferation [2,48-50] and represses differentiation of cortical neural progenitors [51]. We introduced a Wnt/β-catenin LacZ reporter allele [52] into the Fgf15-/- mutants and observed increased numbers of β-galactosidase+ cells in their cortex at E12.5 and E14.5 (Figure 5e–h), consistent with an increase in Wnt signaling, and the increase in proliferation (Figure 4).
Expression of FGF pathway effector genes in Fgf15 mutants
Towards understanding the mechanism of FGF15 function, we examined the effect of Fgf15 mutation on selected components of the FGF signal transduction pathway in the rostral telencephalon at E9.5, E12.5 and E14.5. We focused on expression of Fgf receptors (Fgfrs) and Sprouty2 (Spry2); Sprouty genes encode negative regulators of the FGF signaling pathway that are induced by FGF8 [24,53,54].
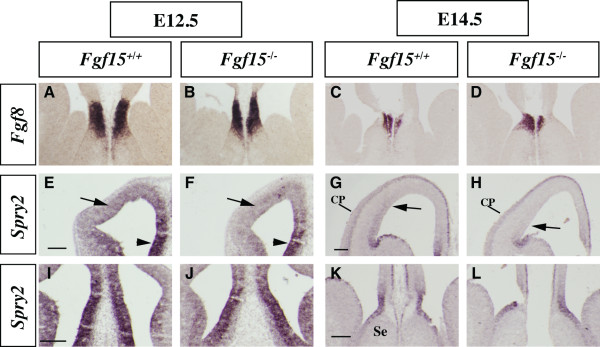
At E9.5 and E12.5, expression of Fgf8 and Spry2 in the rostral patterning center and the rostral midline (that is, anlage of the septum) appeared normal in the Fgf15 mutants (Figures 1g–j and 6a–l). At later stages, E12.5 and E14.5, Spry2 expression was reduced in the VZ of the ventrolateral cortex (Figure 6e–h, arrows) while its expression in the dorsomedial cortex was preserved (Figure 6e–f, arrowheads and Figure 6i–l).
Figure 6.
Analysis of FGF signaling components in the Fgf15 mutants. (a-l) In situ hybridization on coronal sections: Fgf8 at E12.5 (a, b) and E14.5 (c, d); Spry2 at E12.5 (e, f, i, j) and E14.5 (g, h, k, l). Arrows in (e-h) indicate the change in Spry2 gene expression in the lateral pallium; arrowheads in (e, f) indicate the change in the medial pallium. CP: cortical plate; Se: septum. Bars in (e, g, i, k) are 200 μm.
Next we examined expression of Fgfr1-4 at E12.5 and E14.5. Fgfr2 showed the largest change in expression: it increased in the VZ, particularly in the dorsolateral cortex (Additional file 6e–h); in the cortical plates its expression was lost. Fgfr1 showed subtle changes in the VZ (a slight increase), but was reduced in the cortical plate and intermediate zone (Additional file 6a–d). Fgfr3 expression was reduced in the dorsal cortex at E14.5 (Additional file 6i–l, arrows).
FGF15 preferentially binds to FGFR4 in vitro [33,55-57]. We confirmed that Fgfr4 expression was not detectable in the E12.5 cortex (data not shown), as previously published [58]. However, we did observe its expression in the superficial cortical plate at E14.5; this expression domain was preserved, but thinner in the Fgf15 mutants (Additional file 6m–n). Thus, Fgf15 mutants showed varied effects on the expression of several FGF receptors in cortical progenitors and in the cortical plate.
FGF15 and FGF8 differentially affect signaling pathways in cultured embryonic cortical cells
Toward establishing how FGF15 and FGF8 differentially affect cortical patterning proliferation and differentiation, we studied the effects of recombinant FGF15 and FGF8 proteins on dissociated E12.5 cortex; at this age most of the cortical cells are neuroepithelial progenitors.
Unlike mouse and human FGF2 and FGF8, which share 93% and 98% amino acid identity, mouse FGF15 shares only 51% identity with its human homolog FGF19 (data not shown) [28,29]. Thus, we compared the effect of purified mouse recombinant FGF15 protein with commercially available recombinant human FGF19 protein. Our results with FGF15 and FGF19 were indistinguishable (data not shown).
FGFs signal through two major pathways that when activated phosphorylate ERK (42/44) and the AKT [14,59]. These pathways can also activate other important kinases that regulate processes such as protein translation (S6 kinase) and Wnt signaling (GSK3 kinase).
First, we began titrating the FGFs to find concentrations that led to robust changes in the levels of pERK (42/44). We compared 5 ng/ml with 50 ng/ml and found that they led to similar results, with the higher concentration yielding easily detectable levels of pERK (42/44) (Figure 7a, b; Additional file 7c, d). We chose to focus on 50 ng/ml.
Figure 7.
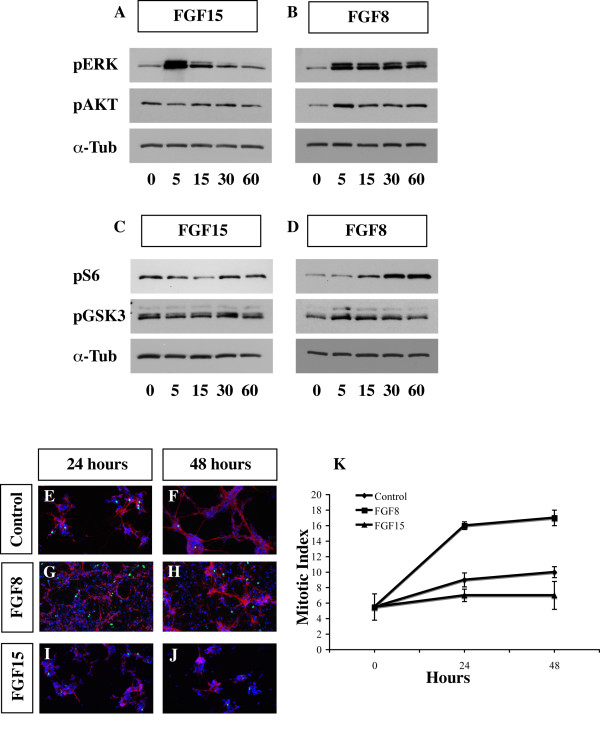
Activation of the FGF15 and FGF8 downstream cytosolic effectors. Comparison of phosphorylation levels of four proteins that are modified in response to FGF15 and FGF8. E12.5 primary cortical cultures were starved for 24 hours and then treated with recombinant FGF15 or FGF8. Cell lysates were analyzed after 0, 5, 15, 30 and 60 minutes by immunoblottingblotting to detect phosphorylated forms of pERK 42/44 (a, b, top panels), pAKT (a, b, middle panels) and pS6 (c, d, top panels), and pGSK3 (c, d, middle panels). The α-Tubulin (α-Tub) antibody (a-d, bottom panels) was used for normalization (these results are characteristic of what we observed the three times these experiments performed). The numbers under the bands indicate the fold-induction or reduction, with respect to T = 0, and are normalized with respect to the α-Tubulin level. (e-j) Immunofluorescence for β-III-Tubulin (red), phosphohistone-3 (PH3) (green) and Hoechst (blue) on E12.5 primary cortical cultures that were either not treated, treated with 50 ng/ml recombinant FGF8 or FGF15 for 24 and 48 hours (n = 4 for each experiment; p = 0.01, Student's t-test). (k) Graph showing the mitotic index calculated before starting the treatment (T0) and after 24 and 48 hours of treatment with recombinant FGF8 and FGF15. Non-treated cells were used as a control. The mitotic index was calculated by dividing the number of PH3+ cells with the total number of cells (Hoechst labeled nuclei). Bars in the graph represent the standard deviation. The average number of nuclei and PH3+ cells for each sample/350 μm2 were as follow. T0: 113.5 ± 5 nuclei and 6.25 ± 2.5 PH3+ cells. At 24 hours: control, 226.75 ± 4 nuclei and 20.5 ± 3.8 PH3+ cells; FGF8, 297.5 ± 12.5 nuclei and 48.3 ± 1.5 PH3+ cells; FGF15, 113 ± 10 nuclei and 8.25 ± 2.5 PH3+ cells. At 48 hours: control, 79.6 ± 3 nuclei and 8.25 ± 1 PH3+ cells; FGF8, 164 ± 11 nuclei and 29 ± 3 PH3+ cells; FGF15, 44 ± 3 nuclei and 3.4 ± 0.5 PH3+ cells.
We found several qualitative differences in the response to FGF8 and FGF15. First, while both ligands induced a rapid (5 minutes) increase in pERK, their levels decreased more rapidly in the FGF15-treated samples (Figure 7a, b). We used recombinant FGF2 as a control, because it is a known mitogen for cortical progenitors [60,61], and as an activator of the ERK kinase pathway (Additional file 7). FGF2 showed similar results as FGF8 (Figure 7; Additional file 7).
While both FGF8 and FGF2 induced the phosphorylation of AKT (pAKT; Figure 7b, and Additional file 7a, respectively), FGF15 did not (Figure 7a). Activation of ERK and AKT, through phosphorylation, results in the phosphorylation and subsequent inhibition of GSK3 activity [62,63]. FGF8 and FGF2 increased levels of pGSK3 (Figure 7d, and Additional file 7b, respectively), whereas FGF15 treatment did not increase pGSK3 (Figure 7c). As GSK3 is an inhibitor of the Wnt/β-catenin pathway [64], these results suggest that FGF2/8 may repress Wnt signaling more than FGF15 through this mechanism.
FGFs promote protein translation and cell growth through the mTor/S6 pathway [65,66]. FGF8- and FGF2-treated cells increased phosphorylation of S6 protein, albeit with slower kinetics than pERK and pAKT (Figure 7d, and Additional file 7b, respectively). On the contrary, FGF15 did not show this increase; in fact, we observed a reduction of S6 phosphorylation in the first 15 minutes (Figure 7c).
Finally, we compared the effects of FGF15 and FGF8 recombinant proteins on proliferation and differentiation of cortical progenitors in vitro. We treated primary E12.5 cortical cultures grown in vitro for 24 and 48 hours with either FGF15 or FGF8. While FGF8 induced an approximately 1.7-fold increase in the mitotic index (assessed by comparing the ratio of PH3+/total cells in FGF8-treated and untreated cortical cultures), FGF15 caused an approximately 0.25-fold reduction in the mitotic index (Figure 7e–j, and quantification in 7k). This result is consistent with the increased proliferation of the cortical progenitors we observed in the Fgf15 mutants, and may reflect the distinct effects of FGF15 on the phosphorylation of ERK (42/44), AKT, GSK3 and S6. We did not test these cultures for apoptosis; however, we have not observed a change in the number of apoptotic cells in the Fgf15 mutants (data not shown). A more detailed analysis is required to determine the role of FGF15 on neural progenitor apoptosis.
Discussion
Here we present the first evidence that Fgf15(19), another Fgf expressed in the rostral patterning center, has telencephalic functions that oppose Fgf8 and Fgf17. These dichotomous phenotypes include effects on proliferation and on the expression of genes regulating the rostro-caudal patterning of the telencephalon. In addition, FGF15 promotes the neuronal differentiation of cortical progenitor cells. Therefore, we propose that FGF15 is a secreted negative modulator of FGF8/17 signaling outputs. Below, we discuss the ramifications of this property and insights into the potential mechanisms by which FGF15 regulates the development of the neocortex and may modulate FGF8/17 functions.
Roles of FGF15, FGF8 and SHH in establishing and maintaining the rostral patterning center
The vertebrate rostral patterning center expresses multiple Fgf genes: 3, 8, 15, 17 and 18 [9-14,16,19,67]. It is unknown what induces expression of the earliest Fgf (Fgf8) in the anterior neural ridge. However, Fgf8 is positively upstream of Fgf17 and Fgf18 (Additional file 1q-r') [17], whereas it appears to repress Fgf15 in the rostral midline (Figure 1c, d). In the Fgf8Null/Neosevere hypomorph, Fgf15 expression remains strong, albeit in a reduced area (Figure 1c, d), perhaps secondary to the reduced proliferation in this region [22]. On the other hand, induction/maintenance of Fgf15 expression clearly depends on SHH function (Additional file 2) [6,20,35], as does maintenance of Fgf8 expression [68]. Fgf15 does not appear to have a major role in regulating expression of Spry2, an intracellular antagonist of FGF8 signaling (Figures 1g, j and 6a–l). On the other hand, there is maybe a subtle increase in Fgf8 expression in the Fgf15 mutant (Figure 1g, h); a more quantitative analysis will be needed to verify this observation. Thus, the rostral patterning center has parallel signaling capabilities, one dominated by FGF8 and the other dominated by FGF15, which depends on SHH and not FGF8 (see schema in Figure 8). Currently, functions for FGF3 and FGF18 in the mammalian rostral patterning center are not known, whereas FGF3 and FGF8 have redundant functions in zebrafish [67].
Figure 8.
Model of genetic interactions upstream and downstream of Fgf15 and Fgf8 within embryonic telencephalon. Shh promotes Fgf15 expression (Addition file 2) [6,20,35], and maintains Fgf8 expression [68]. Fgf8 is required for Shh induction [22]. Fgf15 activates, whereas Fgf8 represses, expression of CoupTF1 (among other genes), which represses proliferation and promotes differentiation and caudal fate [37,38].
Patterning the rostroventral telencephalon: FGF15 and FGF8/17 are competing signals
FGF8 and FGF17 promote rostral telencephalic fate, in part by repressing expression of CoupTF1, Fgfr3 and Emx2, and promoting expression of Er81, Erm, Pea3, Mest and Sp8 [12,17,21,22,24,25,41]. The dorsal patterning center, through its expression of BMPs and Wnts [12,69], can, in principle, repress the rostral patterning center. BMPs can repress Fgf8 expression [68]; BMP and Wnt positively regulate Emx2 expression [70] and reduced Emx2 leads to expansion of Fgf expression [1,2,17,69]. However, currently, in vivo genetic studies have principally linked Wnt signaling to hippocampal specification [1,2] and BMP signaling to choroids plexus specification [71,72], so it remains unclear to what extent the dorsal patterning center participates in neocortical patterning.
Here we show that FGF15 functions, at least in part, to repress rostral telencephalic fate, through promoting CoupTF1 and repressing Mest and Sp8 (Figure 2). Thus, FGF15, in conjunction with the putative role of the dorsal patterning center, participates in repressing the size, and perhaps the nature, of the rostral telencephalon.
FGF8 is also essential for inducing the ventral telencephalon, through promoting expression of Nkx2.1 and Shh [22,73]. While Fgf15 is expressed more ventrally than Fgf8 within the rostral patterning center (septum) (this paper) [17], to date we have detected only mild subpallial or septal hypoplasia (Additional files 3 and 4; data not shown). On the other hand, reduced Fgf19 expression in zebrafish (homologue of mouse Fgf15), results in greatly reduce expression of subcortical molecular markers [34], perhaps suggesting divergent functions for these genes in fish and mammals. In the mouse Fgf15 mutant, there are reduced numbers of cortical GABAergic interneurons in the dorsomedial cortex (Additional file 4); at this point we can not distinguish whether this is a defect secondary to the abnormal cortical environment, or secondary to abnormal subpallial development (cortical interneurons are generated in the basal ganglia anlage).
Where and when does FGF15 affect telencephalic development?
We propose that Fgf15 expression in the rostral patterning center has a profound role in telencephalic patterning during early neurulation based on the reduction of CoupTF1 expression at E9.5 (Figure 1e, f). At this stage, the distribution of Fgf15 mRNA is in a rostrocaudal gradient whose pattern is roughly complementary to the expression of CoupTF1 mRNA (Figure 1a, c; Additional file 1a–d). Assuming that the RNA and protein distributions are similar at E9.5, then FGF15 secretion could promote CoupTF1 expression in the most rostral domain. At later stages, Fgf15 expression in the septum and CoupTF1 expression in the cortex are far apart; therefore, it seems unlikely that loss of Fgf15 from the septum at these stages contributes to CoupTF1 reduction, especially in the dorsolateral cortex. However, Fgf15 is also expressed at the PSB, and in the CGE (Additional file 1g–j); Fgf15 from those sites could contribute to maintaining CoupTF1 cortical expression at later developmental stages. This suggests that FGF15 could play a key role in the putative patterning center at the PSB. This is the first evidence for a role of a secreted factor expressed in this putative patterning center [7,8]. Fgf15 is also expressed in the lateral and medial ganglionic eminences (LGE/MGE) and the thalamus; currently, we have not detected phenotypes in these structures.
Establishing the balance between proliferation and differentiation: distinct roles of FGF15(19) in fish and mammals
The most overt phenotype in the Fgf15 mutants was reduced neurogenesis and increased proliferation in the cortex (Figures 3 and 4). Consistent with this, in vitro cultures of cortical progenitors with recombinant FGF15 have reduced neuronal proliferation (Figure 7). These data differ from the results obtained by reducing expression of the zebrafish Fgf15 homologue (Fgf19) with antisense oligonucleotides (morpholinos) [34]. Whereas reduced Fgf19 expression led to a decrease in the number of proliferating cells in the embryonic fish brain, Fgf15-/- mice showed increased proliferation. These divergent phenotypes could be explained by the different methodologies to reduce gene expression (constitutive deletion mutation versus morpholino), different compensatory responses, or distinct functions of mouse Fgf15 and zebrafish Fgf19. It is conceivable that the Fgf15 deletion produces an amino-terminal fragment with altered signaling properties; however, the deletion does remove exon 3, which encodes the residues predicted to bind heparin sulfate proteoglycans and FGF receptors [32]. Future studies with a Fgf19 zebrafish mutant will help resolve whether zebrafish Fgf19 and mouse Fgf15 indeed have divergent functions that may contribute to the divergent morphogenesis of the fish and mammalian telencephalons.
Opposite roles of FGF15 and FGF8 in regulating the balance between proliferation and differentiation through controlling CoupTF1 levels
We suggest that an important aspect of Fgf15's functions is to promote expression of the CoupTF1 transcription factor, because the cortical phenotypes observed through changing Fgf15's expression levels mirror those observed when altering CoupTF1 dosage. Increased CoupTF1 represses proliferation and promotes neurogenesis, whereas loss of CoupTF1 promotes proliferation and represses neurogenesis [38]. As loss of Fgf15 expression results in decreased CoupTF1 expression as early as E9.5 (Figure 1), we suggest that the Fgf15 mutant phenotype is caused, in part, by reduced CoupTF1 levels. However, because the neurogenesis phenotype in Fgf15 mutants is more severe than that of the CoupTF1 mutants, other factors must contribute.
Unlike FGF15, FGF8 represses CoupTF1 [21,22]. Furthermore, FGF8 promotes proliferation in the developing telencephalon [22] (Figure 7), as does FGF2 [14,60,74,75]. Thus, while the in vivo functions of certain FGFs (FGF2 and FGF8) promote proliferation in the developing telencephalon, FGF15 has the opposite role. Therefore, FGF15 and FGF8 provide opposing signals from the rostral patterning center that control the balance of proliferation and differentiation. One can imagine how differential modulation of these signals during development, disease, or evolution will have profound effects on cortical size, thickness and regional fate.
Interplay between FGF15 and FGF8/17 signaling: complementary effects on Spry and Fgf receptor expression
FGF15 and FGF8/17 are believed to signal through several receptor tyrosine kinases (FGFR1-4) that are negatively modulated by several mechanisms. Spry1-4 encode FGF-induced cytoplasmic repressor of FGF-signaling [53,76]. While Spry expression is clearly reduced in the Fgf8 mutant forebrain [17,22], we did not detect a reduction in Spry expression in the rostral patterning center/septum of the Fgf15 mutants (Figures 1i, j and 6e–l). Fgfr3 appears to have atypical signaling outputs. In fact, Fgfr3 mutant mice have increased proliferation and reduced differentiation of chondrocytes [77,78], and pancreatic cells [79]. Fgf8 mutants have increased Fgfr3 expression [21], whereas the Fgf15 mutants show the opposite phenotype (Figure 6). Thus, we propose that FGF15 is a secreted modulator of several cellular processes that are promoted by FGF8.
Distinct effects of FGF15 and FGF8 on signalling pathways
Towards elucidating the differential effects of reducing FGF15 and FGF8, we compared the effects of recombinant FGF15/19 and FGF8 on the levels of phosphorylated forms of ERK (42/44), AKT, S6 and GSK3 in primary cultures made from E12.5 mouse cortex (Figure 7). We demonstrated that while FGF15 phosphorylation of ERK kinase (42/44) was transient (approximately 15 minutes), FGF8 phosphorylation of ERK was sustained over the time of the experiment (1 hour). The duration of FGF-signaling is associated with distinct cellular responses [80]. For example, in fibroblasts, sustained activation of ERK correlates with S phase entry [81-84], while in PC12 cells, sustained, but not transient, activation of ERK induces differentiation into sympathetic-like neurons [85-88].
FGF15 and FGF8 also had distinct effects on the levels of pAKT and pS6. While FGF8 increased the levels of both pAKT and pS6 phosphorylation, FGF15 did not increase pAKT, and appeared to reduce pS6. The differences observed in the phosphorylation of the effector kinases of the FGF signaling pathway may contribute to the opposite phenotype of the FGF15 and FGF8 mutants observed in vivo and in vitro.
The simplest model to account for the signaling differences between FGF8 and FGF15 would be that they differentially activate/repress FGF, or other, receptors. While studies have established the effects of distinct ligands and receptor combinations on mitogenesis and in vitro binding [14,55,57,89], definitive elucidation of the in vivo biochemistry is lacking, particularly for FGF15. Although FGF15/19 preferentially binds FGFR4 [33,55-57], this receptor does not appear to be expressed in the forebrain until approximately E14.5 (Additional file 6). In addition, FGF15/19 binds Klotho-β, which acts as a co-receptor. We have not observed any expression of Klotho-β in the forebrain at E12.5 and E14.5 (data not shown). Therefore, it is likely that FGF15 functions by regulating other FGF receptors. Therefore, additional analysis is needed to establish how FGF8 and FGF15 signal to elucidate how they differentially regulate the balance between proliferation and differentiation.
Conclusion
We provide novel evidence that FGF15 and FGF8 have opposite functions in mouse forebrain development. In the cortex, FGF15 suppresses proliferation and promotes differentiation, expression of CoupTF1 and caudoventral fate. Furthermore, using primary cultures and recombinant proteins, we demonstrate that FGF15 and FGF8 differentially phosphorylate ERK (p42/44), AKT and S6. Finally, we show that FGF15 blocks neural proliferation in these cortical cultures.
Materials and methods
Mice
The Fgf15-/- [32], Fgf8Null/+ and Fgf8Neo/+ [22], Shh± [90], BAT-gal [52] and RARE-LacZ [47] strains were maintained on a mixed C57BL6/CD1 genetic background. Screening of the mutant alleles was performed by PCR genotyping as described previously [22,32,90]. Noon on the day of the vaginal plug was considered as E0.5. Mouse colonies were maintained at the University of California, San Francisco, in accordance with National Institutes of Health and UCSF guidelines.
Histology
Pregnant females were deeply anesthetized with CO2 and sacrificed by cervical dislocation. The embryos were dissected and the brains were fixed by immersion in 4% paraformaldehyde in phosphate buffered saline. The tissue was cryoprotected by immersion in 30% sucrose/phosphate buffered saline, embedded in OCT (Tissue-Tek, Sakura Finetek, Torrance, CA, USA), and cryostat sectioned (10–20 μm).
Immunofluorescence on cryostat sections was performed as previously described [22]. The antibodies used were as follows: mouse anti-Tuj1 (1:1,000; Covance, Princeton, NJ, USA), rabbit anti-PH3 (1:200; Upstate/Millipore, Billerica, MA, USA), mouse anti-BrdU (1:100; Becton Dickinson, Franklin Lakes, NJ, USA), rat anti-BrdU (1:100; Abcam, Cambridge, MA, USA). Goat anti-rabbit, goat anti-mouse and goat anti-rat secondary antibodies, conjugated with either Alexa 488 or Alexa 594 (1:300, Molecular Probes/Invitrogen, Carlsbad, CA, USA), were used at a dilution of 1:300.
In situ RNA hybridization on cryostat sections was performed as previously described [22]. Comparison of gene expression changes between brains of different genotypes was performed by matching the planes of section to the best of our abilities, using multiple anatomical features. Whenever possible, this was performed for multiple planes of section for each gene, and from at least two brains for each genotype.
Cell cycle analysis
For the cell cycle kinetic analysis, a single injection of IdU (50 μg/g; Sigma, St Louis, MO, USA) was administered to pregnant females, carrying either E12.5 or E14.5 embryos, at T = 0. This was followed at T = 1.5 hours by a single injection of BrdU (50 μg/g; Sigma). Mice were sacrificed at T = 2 hours.
For the quantification of the numbers of cells exiting the cell cycle (Q fraction), IdU was administered as a single injection at T = 0. This was followed at T = 1.5 hours by sequential injections of BrdU every 3 hours, for a total of 15 hours. Mice were sacrificed at 0.5 hours after the last injection.
For the calculation of BrdU/IdU labeling index, we sampled two 100 μm bins spaced 200 μm apart in the ventro-lateral region of the cortex (rectangles in Figure 4 and Additional file 5). Images were acquired using a confocal microscope (Radiance 2000, Bio-Rad, Hercules, CA, USA) with a 20× or a 40× objective. BrdU/IdU positive cells (red channel) and BrdU positive cells (red channel) were counted together with the total number of cells for each bin (calculated by the number of Hoechst labeled nuclei). The experimenter was blind during sampling, image analysis, data collection and statistical analysis. Digitized images were imported into Phostoshop CS3 (Adobe) for counting. Statistical analysis was performed with SPSS (SPSS) and data plotted using Excel (Microsoft) and the significance level was taken as p < 0.05.
A total of three cases were counted in sections spaced evenly through the rostrocaudal cortex. The cell cycle length was calculated using the paradigm described in [91].
FGF15 adenovirus preparation and FGF15 protein purification
The adenovirus containing the Fgf15 coding region with a (His)6 tag was the gift of Dr Steven Kliewer (UT Southwestern Medical Center, Dallas, TX, USA). The FGF15 adenovirus was grown in HEK293 cells (ATCC: CRL 1573) and titrated onto the same cells. 1.2 × 107 COS7 cells (ATCC: CRL 1673) were plated in T150 flasks (6 flasks for each protein preparation). The cells were infected with adenovirus at a multiplicity of infection of 2 for 2 hours in Dulbecco's modified Eagle's medium (DMEM)/2% fetal calf serum. After the infection, the medium was changed with DMEM without fetal calf serum. Three days post-infection the supernatant was collected, equilibrated with a 10× solution of 500 mM Tris/HCl pH8, 1 M NaCl, 100 mM Imidazole, centrifuged 20 minutes at 10,000 g, and passed through a 0.8 ml nickel agarose column (Ni-NTA Agarose, Quiagen, Valencia, CA, USA). The column was washed with 25 column volumes of 50 mM Tris/HCl pH 8, 0.25 M NaCl, 0.1% NP-40, 10 mM Imidazole, and then with 25 column volumes of 20 mM Tris/HCl pH 8, 0.25 M NaCl, 10 mM Imidazole. The His-tagged FGF15 protein was eluted in 20 mM Tris/HCl pH 8, 0.25 M NaCl, 0.5 M Imidazole. The eluate was passed through a PD MidiTrap G-25 desalting column (GE Healthcare, Piscataway, NJ, USA) following the manufacturer's protocol, eluted in 10 mM Tris/HCl pH 7.5, 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, and finally concentrated with the Amicon Ultra-4 3 k centrifugal filter device (Millipore, Billerica, MA, USA). The purity of the protein preparation was assessed by Coomassie Blue staining and by immunoblotting (western blot) using an anti-FGF15 polyclonal antibody (SC 16816, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Primary cell culture and treatment with recombinant FGFs
Wild-type CD1 brains (E12.5) were used to prepare primary cortical cultures. The brains were removed in ice-cold Hank's solution (HBSS; Invitrogen, Carlsbad, CA, USA), and the cortices dissected, after removing the external membranes. The samples were mechanically dissociated in DMEM/10% fetal bovine serum (FBS; Invitrogen) and the single cell suspensions were plated at a density of 2.5 × 105 cells per cm2 in two-well chamber slide (Lab-Tek, Nalgene, Rochester, NY, USA). We were careful to plate the cells at the same density in all of the wells. After 16 hours the medium was replaced with DMEM/5% FBS and finally changed with DMEM/0.5% FBS after 24 hours. The cells were starved in DMEM/0.5% FBS for 24 hours before the treatment with the recombinant proteins. The mouse FGF15 recombinant protein was added to the cells at a concentration of 50 ng/ml in DMEM/0.5% FBS/5 μg/ml Heparin. This protein concentration corresponds to 1.7 pmol/ml for FGF2 and 2 pmol/ml for FGF15 and FGF8. The same protocol was used for human FGF2, FGF8 and FGF19 recombinant proteins (Fitzgerald Industries International, RDI, Concord, MA, USA).
For counting the cultured cells, we used an unbiased method that gave every cell an equal chance of being sampled. We defined a systematic series of fields of view in the culture area to cover the surface of the well. The experimenter was blind to sample identity during sampling, image analysis, data collection and statistical analysis. Images were acquired on a fluorescence microscope with a 20× objective. We sampled at least two different wells for each time point obtained from four independent experiments. Digitized images were imported into Phostoshop CS3 (Adobe) for counting. Statistical analysis was performed with SPSS (SPSS) and data plotted using Excel (Microsoft) and the significance level was taken as p < 0.05.
Immunoblotting
Cells from primary E12.5 cortical cultures were lysed in radioimmunoprecipitation buffer (RIPA: 50 mM Tris/HCl pH 7.5, 150 mM NaCl, 0.5% sodium deoxycolate, 0.1% SDS, 1% NP-40) supplemented with protease inhibitors (Protease Inhibitor Cocktail, Roche, Indianapolis, IN, USA) and phosphatase inhibitors (Phosphatase Inhibitor Mix 1 and 2, Sigma). Protein samples (10 μg each) were resolved on polyacrylamide gels and transferred to nitrocellulose membranes by electroblotting. Membranes were pre-incubated in 5% nonfat dry milk in Tris buffered solution (TBS)/0.1% Tween-20 (TBST) and then incubated O/N with the primary antibodies in TBST/5% bovine serum albumin in TBST. The primary antibodies were: rabbit-anti phospho-p44/42 (Thr202/Tyr204) MAPK (1:1,000; Cell Signaling, Boston, MA, USA), rabbit anti-phospho-Akt (Ser 473) (1:500; Cell Signaling), rabbit anti-phospho-GSK3α/β (Ser 21/9) (1:1,000; Cell Signaling), rabbit anti-phospho-S6 ribosomal protein (Ser235/236) (1:1,000; Cell Signaling), and mouse anti-α-tubulin (1:10,000; Sigma). Membranes were probed with the appropriate goat horseradish peroxidase-conjugated antibodies (1:500; Biorad, Hercules, CA, USA) and developed using ECL reagents (GE Healthcare). Each experiment was repeated at least three times. Quantification of optical density was done using NIH ImageJ software; the intensity of the bands was normalized with respect to the intensity of the α-Tubulin band. The numbers indicated in Figure 7 and Additional file 7 represent the fold-induction, or reduction, from the time point before FGF addition (T0).
Abbreviations
BMP: bone morphogenetic protein; BrdU: bromo-deoxyuridine; CGE: caudal ganglionic eminence; DMEM: Dulbecco's modified Eagle's medium; E: embryonic day; FBS: fetal bovine serum; FGF: fibroblast growth factor; FGFR: FGF receptor; IdU: iodo-deoxyuridine; MAPK: mitogen activated protein kinase; PH3: phospho-histone H3; PSB: pallium-subpallium boundary; SHH: sonic hedgehog; SVZ: subventricular zone; VZ: ventricular zone.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
UB conceived of the studies, designed and carried out the experiments, analyzed and interpreted the data, and drafted the paper. IC helped with the in vitro cultures and data analysis. JL helped with the in situ RNA hybridization. CM provided the Fgf15 mouse strain. JLRR conceived of the studies, coordinated them, analyzed and interpreted the data, and drafted the paper. All authors read and approved the final manuscript.
Supplementary Material
Fgf15 and Fgf17 expression in Fgf8Null/Neo mutants and Fgf17 expression in Fgf15-/- mutants. (a-p') In situ hybridization of Fgf15 in Fgf8Null/Neo embryos. E9.5: wild type (a-d); mutant (a'-d'); (a"-d", a"'-d"') show higher magnification images; horizontal sections. E12.5: wild type (e-j); mutant (e'-j'); coronal sections. E14.5: wild type (k-p); mutant (k'-p'); coronal sections. (q-r') In situ hybridization of Fgf17 in Fgf8Null/Neo embryos at E12.5: wild type (q, r); mutant (q', r'); coronal sections. (s-t') In situ hybridization of Fgf17 in Fgf15-/-embryos. E14.5: wild type (s, t); mutant (s', t'); coronal sections. Bar in (a, e, k) is 200 μm.
Fgf15 and the SHH pathway. (a-p') Analysis of Fgf15 expression in the Shh mutant. E12.5: Shh± (a-o); Shh-/- mutant (a'-o'); coronal sections. E14.5: Shh± (b-p); Shh-/- mutant (b'-p'); coronal sections. (q-r') Expression of SHH downstream effector Gli3 in the Fgf15 mutants in coronal sections at E12.5 (q wild type, q' Fgf15-/-mutant) and E14.5 (r wild type, r' Fgf15-/- mutant). Bar in (a) and (b) is 200 μm.
Fgf15 mutant embryos morphology. The morphology of Fgf15-/- mutant embryos was compared to the wild type. (a, b) Examples of lateral views of Fgf15 mutant and wild-type embryos at E10.5. -(c, d) Comparison of dorsal views of Fgf15 mutant and wild-type dissected brains at E14.5. (e, f) β-III-Tubulin immunostaining on coronal hemisections of E14.5 brains.
Analysis of markers of the basal ganglia and basal ganglia-derived cortical interneurons in the Fgf15 mutants. (a-j) In situ hybridization on coronal sections at E14.5: Gad67 (a-d), Reelin (e, f), Nkx2.1 (g, h), Pax6 (i, j). Ncx, neocortex; Pcx, piriform cortex; Se, septum; St, striatum. Arrows in (a, b) indicate reduced cortical interneurons in the dorsomedial cortex; arrowheads in (a, b) indicate suggestive evidence for increased Gad67 expression in the deep tangential migration. Arrows in (c, d) show reduced thickness in the mantle zone of the ventral septum and piriform cortex. Bar in (a, c) is 200 μm.
Analysis of proliferation and cell cycle in the Fgf15 mutants. (a-c) The number of progenitors undergoing mitosis at E12.5 was determined by PH3 immunofluorescence (n = 3; p = 0.0024, Student's t-test). (d-f) the analysis of the cell cycle length was performed at E12.5 by double labeling with IdU and BrdU.
Analysis of FGF signaling components in the Fgf15 mutants. In situ hybridization on coronal sections. (a-d') Fgfr1 at E12.5 (a, b), and at E14.5 (c, d); (c', d') show higher magnification images. (e-h') Fgfr2 at E12.5 (e, f), and at E14.5 (g, h); (g', h') show higher magnification images. (i-l) Fgfr3 at E12.5 (i, j), and at E14.5 (k, l)). (m-n) Fgfr4 at E14.5. CP: cortical plate. Bar in (a) is 200 μm.
Analysis of the downstream cytoplasmic effectors induced by FGF2. (a, b): comparison of phosphorylation of four proteins that are modified in response to FGF2. E12.5 primary cortical cultures were starved for 24 hours and treated with recombinant FGF2. Cell lysates were analyzed after 0, 5, 15, 30 and 60 minutes by immunoblotting to detect phosphorylated forms of pERK 42/44 (a, top panel), pAKT (a, middle panel), pS6 (b, top panel), and pGSK3 (b, middle panel). The α-Tubulin antibody (a, b, bottom panels) was used for normalization. Analysis of the phosphorylation of pERK (42/44) induced by FGF8 and FGF15 at 5 ng/ml (c and d, respectively, top panels). The α-Tubulin antibody was used for normalization (c, d, bottom panels). The numbers under the bands indicate the fold-induction, or reduction, with respect to T0.
Acknowledgments
Acknowledgements
This work was supported by the Human Frontier Science Program (UB), NARSAD (UB), Autism Speaks (JLRR, UB), Nina Ireland (JLRR), and NIH grants to JLRR: R01 NS34661 and K05 MH065670. We thank Renee Hoch and Andrea Faedo for critical reading of the manuscript, Steven Kliewer and Robert D Gerard UT Southwestern for kindly providing us with the adenovirus over-expressing Fgf15, and Susan Mansour for sending us the Fgf15 mutant mice.
Contributor Information
Ugo Borello, Email: ugo.borello@ucsf.edu.
Inma Cobos, Email: inma.cobos@icrea.es.
Jason E Long, Email: jason.long@mac.com.
Cornelis Murre, Email: cmurre@ucsd.edu.
John LR Rubenstein, Email: john.rubenstein@ucsf.edu.
References
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Hayhurst M, Marks ME, Kulessa H, Hogan BL, McConnell SK. BMP ligands act redundantly to pattern the dorsal telencephalic midline. Genesis. 2003;35:214–219. doi: 10.1002/gene.10183. [DOI] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Bachler M, Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100:313–316. doi: 10.1016/s0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Hashemi R, Brulet P, Martinez S. Analysis of Fgf15 expression pattern in the mouse neural tube. Brain Res Bull. 2002;57:297–299. doi: 10.1016/s0361-9230(01)00717-1. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Martinez S. Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev Dyn. 2007;236:2285–2297. doi: 10.1002/dvdy.21237. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17 and Emx2. J Comp Neurol. 2008 doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno L, Brulet P, Martinez S. Study of Fgf15 gene expression in developing mouse brain. Gene Expr Patterns. 2003;3:473–481. doi: 10.1016/s1567-133x(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ashigaki S, Takamatsu M, Suzuki-Migishima R, Ohbayashi N, Itoh N, Takada S, Tanabe Y. Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci. 2004;24:8711–8719. doi: 10.1523/JNEUROSCI.3070-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc Natl Acad Sci USA. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Houart C, Picker A, Reifers F, Macdonald R, Barth A, Griffin K, Brand M, Wilson SW. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Popovici C, Roubin R, Coulier F, Birnbaum D. An evolutionary history of the FGF superfamily. Bioessays. 2005;27:849–857. doi: 10.1002/bies.20261. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Vincentz JW, McWhirter JR, Murre C, Baldini A, Furuta Y. Fgf15 is required for proper morphogenesis of the mouse cardiac outflow tract. Genesis. 2005;41:192–201. doi: 10.1002/gene.20114. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269:264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm. 2008;5:42–48. doi: 10.1021/mp700105z. [DOI] [PubMed] [Google Scholar]
- Miyake A, Nakayama Y, Konishi M, Itoh N. Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev Biol. 2005;288:259–275. doi: 10.1016/j.ydbio.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, McMahon AP. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development. 2002;129:4807–4819. doi: 10.1242/dev.129.20.4807. [DOI] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24:847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Srubek Tomassy G, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-Catenin signaling. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff E, Houben R, Loffler S, Troppmair J, Lee JE, Rapp UR. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16:211–216. doi: 10.1038/sj.onc.1201520. [DOI] [PubMed] [Google Scholar]
- Nanba D, Inoue H, Shigemi Y, Shirakata Y, Hashimoto K, Higashiyama S. An intermediary role of proHB-EGF shedding in growth factor-induced c-Myc gene expression. J Cell Physiol. 2008;214:465–473. doi: 10.1002/jcp.21233. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Hebert JM, Thammongkol U, Smith J, Nisbet G, Surani MA, McConnell SK, Livesey FJ. Genomic characterisation of a Fgf-regulated gradient-based neocortical protomap. Development. 2005;132:3947–3961. doi: 10.1242/dev.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Takahashi T, Nowakowski RS. The G1 restriction point as critical regulator of neocortical neuronogenesis. Neurochem Res. 1999;24:497–506. doi: 10.1023/a:1022579712262. [DOI] [PubMed] [Google Scholar]
- Tarui T, Takahashi T, Nowakowski RS, Hayes NL, Bhide PG, Caviness VS. Overexpression of p27 Kip 1, probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb Cortex. 2005;15:1343–1355. doi: 10.1093/cercor/bhi017. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309:285–297. doi: 10.1016/j.ydbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Harmer NJ, Pellegrini L, Chirgadze D, Fernandez-Recio J, Blundell TL. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry. 2004;43:629–640. doi: 10.1021/bi035320k. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Gutin G, Bhatnagar L, Remedios R, Hebert JM. Development of midline cell types and commissural axon tracts requires Fgfr1 in the cerebrum. Dev Biol. 2006;289:141–151. doi: 10.1016/j.ydbio.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Rhee J, Lyn-Cook R. Fibroblast growth factor signaling regulates growth and morphogenesis at multiple steps during brain development. Curr Top Dev Biol. 1999;46:179–200. doi: 10.1016/s0070-2153(08)60329-4. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3 – an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- Patel S, Doble B, Woodgett JR. Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem Soc Trans. 2004;32:803–808. doi: 10.1042/BST0320803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell. 2003;12:271–280. doi: 10.1016/j.molcel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Stewart MJ, Thomas G. Mitogenesis and protein synthesis: a role for ribosomal protein S6 phosphorylation? Bioessays. 1994;16:809–815. doi: 10.1002/bies.950161107. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Unique and combinatorial functions of Fgf3 and Fgf8 during zebrafish forebrain development. Development. 2003;130:4337–4349. doi: 10.1242/dev.00660. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud-Dabernat S, Kritzik M, Kayali AG, Zhang YQ, Liu G, Ungles C, Sarvetnick N. FGFR3 is a negative regulator of the expansion of pancreatic epithelial cells. Diabetes. 2007;56:96–106. doi: 10.2337/db05-1073. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Balmanno K, Cook SJ. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene. 1999;18:3085–3097. doi: 10.1038/sj.onc.1202647. [DOI] [PubMed] [Google Scholar]
- Cook SJ, McCormick F. Kinetic and biochemical correlation between sustained p44ERK1 (44 kDa extracellular signal-regulated kinase 1) activation and lysophosphatidic acid-stimulated DNA synthesis in Rat-1 cells. Biochem J. 1996;320:237–245. doi: 10.1042/bj3200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Yung Y, Walker JL, Assoian RK. ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets. Mol Biol Cell. 2007;18:1457–1463. doi: 10.1091/mbc.E06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LE, Johnson GL. The beta-PDGF receptor induces neuronal differentiation of PC12 cells. Mol Biol Cell. 1992;3:545–553. doi: 10.1091/mbc.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. 1993;268:9803–9810. [PubMed] [Google Scholar]
- Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20:185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Exploiting the dynamics of S-phase tracers in developing brain: interkinetic nuclear migration for cells entering versus leaving the S-phase. Dev Neurosci. 2000;22:44–55. doi: 10.1159/000017426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fgf15 and Fgf17 expression in Fgf8Null/Neo mutants and Fgf17 expression in Fgf15-/- mutants. (a-p') In situ hybridization of Fgf15 in Fgf8Null/Neo embryos. E9.5: wild type (a-d); mutant (a'-d'); (a"-d", a"'-d"') show higher magnification images; horizontal sections. E12.5: wild type (e-j); mutant (e'-j'); coronal sections. E14.5: wild type (k-p); mutant (k'-p'); coronal sections. (q-r') In situ hybridization of Fgf17 in Fgf8Null/Neo embryos at E12.5: wild type (q, r); mutant (q', r'); coronal sections. (s-t') In situ hybridization of Fgf17 in Fgf15-/-embryos. E14.5: wild type (s, t); mutant (s', t'); coronal sections. Bar in (a, e, k) is 200 μm.
Fgf15 and the SHH pathway. (a-p') Analysis of Fgf15 expression in the Shh mutant. E12.5: Shh± (a-o); Shh-/- mutant (a'-o'); coronal sections. E14.5: Shh± (b-p); Shh-/- mutant (b'-p'); coronal sections. (q-r') Expression of SHH downstream effector Gli3 in the Fgf15 mutants in coronal sections at E12.5 (q wild type, q' Fgf15-/-mutant) and E14.5 (r wild type, r' Fgf15-/- mutant). Bar in (a) and (b) is 200 μm.
Fgf15 mutant embryos morphology. The morphology of Fgf15-/- mutant embryos was compared to the wild type. (a, b) Examples of lateral views of Fgf15 mutant and wild-type embryos at E10.5. -(c, d) Comparison of dorsal views of Fgf15 mutant and wild-type dissected brains at E14.5. (e, f) β-III-Tubulin immunostaining on coronal hemisections of E14.5 brains.
Analysis of markers of the basal ganglia and basal ganglia-derived cortical interneurons in the Fgf15 mutants. (a-j) In situ hybridization on coronal sections at E14.5: Gad67 (a-d), Reelin (e, f), Nkx2.1 (g, h), Pax6 (i, j). Ncx, neocortex; Pcx, piriform cortex; Se, septum; St, striatum. Arrows in (a, b) indicate reduced cortical interneurons in the dorsomedial cortex; arrowheads in (a, b) indicate suggestive evidence for increased Gad67 expression in the deep tangential migration. Arrows in (c, d) show reduced thickness in the mantle zone of the ventral septum and piriform cortex. Bar in (a, c) is 200 μm.
Analysis of proliferation and cell cycle in the Fgf15 mutants. (a-c) The number of progenitors undergoing mitosis at E12.5 was determined by PH3 immunofluorescence (n = 3; p = 0.0024, Student's t-test). (d-f) the analysis of the cell cycle length was performed at E12.5 by double labeling with IdU and BrdU.
Analysis of FGF signaling components in the Fgf15 mutants. In situ hybridization on coronal sections. (a-d') Fgfr1 at E12.5 (a, b), and at E14.5 (c, d); (c', d') show higher magnification images. (e-h') Fgfr2 at E12.5 (e, f), and at E14.5 (g, h); (g', h') show higher magnification images. (i-l) Fgfr3 at E12.5 (i, j), and at E14.5 (k, l)). (m-n) Fgfr4 at E14.5. CP: cortical plate. Bar in (a) is 200 μm.
Analysis of the downstream cytoplasmic effectors induced by FGF2. (a, b): comparison of phosphorylation of four proteins that are modified in response to FGF2. E12.5 primary cortical cultures were starved for 24 hours and treated with recombinant FGF2. Cell lysates were analyzed after 0, 5, 15, 30 and 60 minutes by immunoblotting to detect phosphorylated forms of pERK 42/44 (a, top panel), pAKT (a, middle panel), pS6 (b, top panel), and pGSK3 (b, middle panel). The α-Tubulin antibody (a, b, bottom panels) was used for normalization. Analysis of the phosphorylation of pERK (42/44) induced by FGF8 and FGF15 at 5 ng/ml (c and d, respectively, top panels). The α-Tubulin antibody was used for normalization (c, d, bottom panels). The numbers under the bands indicate the fold-induction, or reduction, with respect to T0.