Abstract
Purpose
To test induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CRT) or surgery/ radiotherapy (RT) for advanced oropharyngeal cancer and to assess the effect of human papilloma virus (HPV) on response and outcome.
Patients and Methods
Sixty-six patients (51 male; 15 female) with stage III to IV squamous cell carcinoma of the oropharynx (SCCOP) were treated with one cycle of cisplatin (100 mg/m2) or carboplatin (AUC 6) and with fluorouracil (1,000 mg/m2/d for 5 days) to select candidates for CRT. Those achieving a greater than 50% response at the primary tumor received CRT (70 Gy; 35 fractions with concurrent cisplatin 100 mg/m2 or carboplatin (AUC 6) every 21 days for three cycles). Adjuvant paclitaxel was given to patients who were complete histologic responders. Patients with a response of 50% or less underwent definitive surgery and postoperative radiation. Pretreatment biopsies from 42 patients were tested for high-risk HPV.
Results
Fifty-four of 66 patients (81%) had a greater than 50% response after IC. Of these, 53 (98%) received CRT, and 49 (92%) obtained complete histologic response with a 73.4% (47 of 64) rate of organ preservation. The 4-year overall survival (OS) was 70.4%, and the disease-specific survival (DSS) was 75.8% (median follow-up, 64.1 months). HPV16, found in 27 of 42 (64.3%) biopsies, was associated with younger age (median, 55 v 63 years; P = .016), sex (22 of 30 males [73.3%] and five of 12 females [41.7%]; P = .08), and nonsmoking status (P = .037). HPV titer was significantly associated with IC response (P = .001), CRT response (P = .005), OS (P = .007), and DSS (P = .008).
Conclusion
Although the numbers in this study are small, IC followed by CRT is an effective treatment for SCCOP, especially in patients with HPV-positive tumors; however, for patients who do not respond to treatment, alternative treatments must be developed.
INTRODUCTION
The incidence of oral/oropharyngeal tumors is increasing.1 In the United States, 34,360 new instances (24,180 men; 10,180 women) of oral and pharynx cancer are expected in 2007.2 Tobacco and alcohol are the strongest etiologic factors,3,4 but high-risk human papillomavirus (HPV), commonly HPV16, is an emerging etiologic factor in oropharynx carcinomas.5-9 Nonsmokers with SCCOP are 15-fold more likely to be HPV positive than smokers.10 Some studies have indicated a relatively better outcome for HPV-positive patients who have head and neck squamous cell carcinoma (HNSCC), particularly those with tonsillar cancer,11-13 but the reports are not unanimous.14,15
The use of neoadjuvant chemotherapy in HNSCC to reduce tumor burden and to improve outcomes with local treatment modalities has had variable success.16,17 On the basis of provocative findings from the Department of Veterans Affairs Laryngeal Cancer Group Study18 and a University of Michigan Cancer Center clinical trial (UMCC 9520) in patients with advanced laryngeal cancers,19 we designed this study (UMCC 9921) that uses the response to IC to select patients with squamous cell carcinoma of the oropharynx (SCCOP) who were most likely to respond to CRT. We also prospectively looked at the outcomes of patients who were HPV16 positive or HPV16 negative and correlated these findings to smoking status and sex.
PATIENTS AND METHODS
Eligibility Criteria and Patient Population
All patients had histologically confirmed, previously untreated, stage III to IV SCCOP and were candidates for complete surgical resection. Staging included direct laryngoscopy (DL) and CT. Patients with bone destruction or Karnofsky performance status (KPS) less than 60% were ineligible. This protocol was approved by the institutional review board at the University of Michigan with Department of Health and Human Services assurance; all patients gave documented informed consent. The study schema is shown in Figure 1.
Fig 1.
UMCC 9921 study schema. CDDP, cisplatin; FU, fluorouracil; CR, complete response; PR, partial response; XRT, radiation therapy. (*) If the original neck node was > 3 cm, selective neck dissection was performed. (**) If a positive biopsy was obtained, primary site resection with or without neck dissection was performed.
Treatment Plan
Induction Chemotherapy
In patients who had a creatinine clearance (CrCl) of at least 60 mL/min and hearing loss of ≤ 30 dB between 500 and 2000 Hz, cisplatin (100 mg/m2) was administered (day one). Those with a CrCl of 30 to 59 mL/min and hearing loss of greater than 30 dB received carboplatin (AUC 6). Fluorouracil (1,000 mg/m2/d) was administered on days 1 through 5 by continuous infusion.
Tumor Assessment
Bidimensional measurements of the primary tumor by DL with anesthesia were assessed pretreatment and at 3 weeks after IC. Response Evaluation Criteria in Solid Tumors Group criteria20 were not used. Patients with a greater than 50% reduction in the bidimensional product of the primary tumor area were eligible for CRT. Patients who were nonresponders (ie, had ≤ 50% response) underwent definitive surgery and radiation.
Concurrent CRT
Concurrent CRT began within 3 weeks after IC. Patients received cisplatin 100 mg/m2 or carboplatin (AUC 6) on days 1, 22, and 43 concurrent with radiation. The National Cancer Institute Common Toxicity Criteria (version 2) were used for classification of adverse events.21 Radiation was administered once daily for 5 days each week. The dose administered to the gross disease and 1 to 2 cm margins, as defined by clinical examination, head/neck computed tomography, and/or positron emission tomography (PET) scans, was 70 Gy at 2 Gy/fraction. Tissue volumes at risk of harboring subclinical disease, including the bilateral neck, received 59 to 63 Gy at 1.7 to 1.8 Gy/fraction by using intensity-modulated radiation therapy, according to published methods.22,23
Treatment Evaluations and Chemotherapy/Dose Modifications
Before enrollment, history and physical examination, complete blood count (CBC) with differential and platelets, and serum chemistries were obtained. Chemistries and renal function were monitored before each chemo-therapy cycle. A CBC was drawn on day 15 and on day one of each subsequent cycle. Dosage modifications were based on the nadir laboratory values of the preceding cycle. Chemotherapy subsequently was administered if the absolute neutrophil count (ANC) was at least 1,000/μL and the platelet count was greater than 100,000/μL; dosage adjustments were made if the nadir ANC ≤ 500/μL.
During CRT, patients whose CrCl dropped to less than 30 mL/min or who developed grade 2 or greater tinnitus were changed to carboplatin (AUC 6). Cisplatin was held for grade 2 or greater sensory neuropathy until symptoms resolved to less than grade 1; the cisplatin dosage was decreased by 20 mg/m2 for each subsequent event. If grade 3 nausea/vomiting had not resolved by the next cycle, chemotherapy was held.
Tumor Assessment After CRT
Eight weeks after CRT, DL with biopsy was performed. Patients without residual disease at the primary site were eligible for two cycles of adjuvant paclitaxel (175 mg/m2) every 21 days. Paclitaxel was not administered to those with grade 2 or greater sensory neuropathy. Those with biopsy-proven disease at the primary site underwent surgical resection and ipsilateral neck dissections. Resection of primary cancers was dictated by pretreatment tumor extent. Patients with neck nodes greater than 3 cm at the time of initial staging, a palpable neck mass, or neck recurrence during follow-up had selective/modified radical neck dissection.
HPV Testing
Pretreatment biopsies were retrieved from 50 of 66 patients for construction of a tissue microarray (TMA).24 Blocks were not available from 16 patients who had outside biopsies. In 42 of 50 biopsies, sufficient tumor was present for DNA isolation and HPV analysis. There was no residual tumor after TMA construction in eight patient cases.
HPV type and copy number were assessed with a sensitive, specific, quantitative method25 that involved real-time competitive polymerase chain reaction and matrix-assisted laser desorption/ionization-time of flight mass spectroscopy separation of products on a matrix-loaded silicon chip array. Internal standards allow quantification of HPV copies per cellular genome copy. Primers (Appendix Table A1, online only) designed to amplify the E6 region distinguish 13 discrete high-risk HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68).26
Statistical Analysis
The covariates of interest were age, sex, disease site (base of tongue v tonsil), T class, N class, KPS, smoking status (never v past [quit > 6 months ago]) v current smoker), and HPV16 titer. T class, N class, KPS, and smoking status were analyzed as ordinal data. The outcomes of interest were IC response, CRT response, and time to event outcomes (ie, overall survival [OS] and disease-specific survival [DSS]). DSS was defined as the time to death from oropharynx cancer. Patients who were alive at last follow-up or who died as a result of reasons other than SCCOP were censored. Organ preservation was defined as time to local failure; the events included a poor response to IC or CRT and local recurrence.
To evaluate univariate association between two-level variables and response to IC, the Cochran-Armitage trend test was used. To evaluate univariate associations between HPV16 titer and ordinal variables of interest, the Spearman correlation coefficient was used. For HPV16 titer associations with nominal variables, the Wilcoxon rank sum test was employed for two-level variables and the Kruskal-Wallis test was employed for variables with three or more levels. The Kaplan-Meier method and the log-rank test were used to test for differences in the survival functions between strata defined by clinical variables. For descriptive purposes, we show the survival function for responders to IC who are subsequently treated by CRT compared with the survival function for those who do not respond to IC. We did not show a P value for the comparison, because the two groups were not defined at the point of IC treatment. (The intermediate outcome of IC response determines the CRT treatment.)
Cox proportional hazards models were used to relate time to event outcomes to HPV16 titer and to other predictors. For each time to event outcome, three models were constructed: a model with HPV16 titer alone; a model with clinical variables alone; and a model with clinical variables and the HPV16 titer. Models 2 and 3 were used to assess the HPV16 effects beyond the effects of clinical variables. Likelihood ratio statistics were used to compare the models. The power of the test may be limited by the sample size; therefore, a nonsignificant result may be a false negative, but a significant result still holds. All statistical analyses were done using SAS version 9.0 (SAS Institute, Carey, NC).27 A two-tailed P value of .05 or less was considered statistically significant.
RESULTS
Treatment and Outcome
From January 1, 2000 through November 30, 2002, 69 patients with stage III or IV SCCOP were enrolled. One patient refused treatment, one died as a result of acute aspiration, and one had a CrCl value too low to receive chemotherapy; 66 assessable patients remained. Patient characteristics and TNM staging are described in Tables 1 and 2.
Table 1.
Patient Characteristics, Responses to Therapy, and Survival Outcomes
| Analyses |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients by Sex |
Therapy Response |
Survival Outcome |
||||||||
| IC* |
CRT |
OS |
DSS |
|||||||
| Characteristic | Female | Male | Spearman ρ |
P | OR | 95% CL | HR | 95% CL | HR | 95% CL |
| Total No. of patients† | 15 | 51 | .05‡§ | 0.19 | 0.05, 0.65‡ | 4.12 | 1.83, 9.29∥ | 5.17 | 2.1, 12.7∥ | |
| Age, years | 0.08 | .51 | 0.96 | 0.91, 1.03 | 1.04 | 1.0, 1.09 | 1.04 | 1.0, 1.10 | ||
| Median | 61 | 55 | ||||||||
| Range | 50-74 | 37-77 | ||||||||
| KPS¶ | −0.15 | .23 | 1.06 | 0.98, 1.15 | 0.94 | 0.9, 0.99‡ | 0.96 | 0.9, 1.02 | ||
| 80 | 2 | 4 | ||||||||
| 90 | 7 | 6 | ||||||||
| 100 | 6 | 41 | ||||||||
| T class# | 0.31 | .01‡ | 0.58 | 0.30, 1.11 | 1.75 | 1.08, 2.82‡ | 2.26 | 1.3, 4.0∥ | ||
| 1 | 1 | 4 | ||||||||
| 2 | 2 | 15 | ||||||||
| 3 | 5 | 18 | ||||||||
| 4 | 7 | 14 | ||||||||
| N class** | −0.10 | .42 | 1.91 | 1.01, 3.62‡ | 0.70 | 0.45, 1.10 | 0.56 | 0.34, 0.92‡ | ||
| 0 | 6 | 6 | ||||||||
| 1 | 1 | 14 | ||||||||
| 2 | 7 | 26 | ||||||||
| 3 | 1 | 5 | ||||||||
| Smoking status†† | 0.29 | .02‡ | 0.81 | 0.39, 1.71 | 2.08 | 1.17, 3.73‡ | 3.33 | 1.6, 6.9∥ | ||
| Never | 1 | 15 | ||||||||
| Past | 7 | 21 | ||||||||
| Current | 7 | 15 | ||||||||
| HPV titer‡‡ | −0.48 | .001∥ | 1.4 | 1.08, 1.82‡ | 0.81 | 0.70, 0.94‡ | 0.77 | 0.63, 0.93‡ | ||
| Positive | 5 | 22 | ||||||||
| Negative | 7 | 8 | ||||||||
| Unavailable | 3 | 21 | ||||||||
| Primary site§§ | > .99§ | 0.38 | 011, 1.32 | 2.6 | 1.0, 6.5‡ | 3.3 | 1.1, 9.8‡ | |||
| Base of tongue | 11 | 29 | ||||||||
| Tonsil | 4 | 22 | ||||||||
NOTE. Bold text indicates a statistically significant value.
Abbreviations: IC, induction chemotherapy; CRT, concurrent chemotherapy/radiation therapy; OS, overall survival; DSS, disease-specific survival; OR, odds ratio; CL, confidence limit; HR, hazard ratio; KPS, Karnofsky performance score; HPV, human papilloma virus.
IC was analyzed as ordinal data in which the coding for complete response at the primary site, partial (> 50%) response at the primary site, stable disease, and progressive disease are 1, 2, 3, and 4, respectively.
Female sex was associated with a poor outcome.
P < .05.
By Cochran-Armitage trend test.
P < .005.
Lower KPS was associated with a poor outcome.
Higher T class was associated with a poor outcome.
Lower N class was associated with a poor outcome.
Current smoking status was associated with a poor outcome.
HPV-negative status or low HPV copies per cell was associated with a poor outcome.
Base-of-tongue tumor subsite was associated with a poor outcome.
Table 2.
TNM Staging
| N Stage |
|||||
|---|---|---|---|---|---|
| T Stage | 0 | 1 | 2 | 3 | Total |
| 1 | 0 | 2 | 3 | 0 | 5 |
| 2 | 0 | 3 | 12 | 2 | 17 |
| 3 | 5 | 5 | 10 | 3 | 23 |
| 4 | 7 | 5 | 8 | 1 | 21 |
| Total | 12 | 15 | 33 | 6 | 66 |
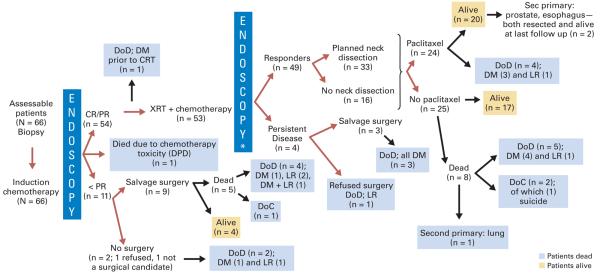
Figure 2 outlines the responses to treatment. Fifty-six of 66 patients received cisplatin; 10 had carboplatin. Nine of 10 patients treated with carboplatin were responders and received CRT. Together, 54 of 66 patients responded to IC, one died as a result of metastatic disease before CRT, and 53 received CRT. Thirty-eight (72%) of 53 patients received all three cycles of chemotherapy during radiation. Neutropenia and thrombocytopenia (in 13 patients) or severe nausea and vomiting (in two patients) limited concurrent cisplatin administration; 13 patients (25%) received two cycles, and two patients (3%) received only one cycle.
Fig 2.
Schema showing the response of patients according to treatment. Endoscopy*, endoscopy with biopsy. DoD, died of disease; Sec, second; DM, distant metastases; CRT, concurrent chemotherapy/radiation therapy; XRT, radiation therapy; LR, local/regional unresectable; CR/PR, complete response/partial response (> 50% response to induction chemotherapy); < PR, stable disease or progressive disease (≤ 50% response to induction chemotherapy); DPD, dihydropyrimidine dehydrogenase deficiency; DoC, died of other causes.
Thirty-three of 49 responders to CRT underwent planned neck dissections; none had residual tumor in the neck. Twenty-four of 49 responders received adjuvant paclitaxel (12 had two cycles and 12 had one cycle); 24 patients with platinum-induced neuropathy received no adjuvant paclitaxel, and one patient refused.
Toxicities and Chemotherapy Compliance
Common grade 2 through 5 toxicities are noted in Table 3. One patient died as a result of febrile neutropenia after fluorouracil treatment and subsequently was shown to have a dihydropyrimidine dehydrogenase deficiency. Five patients were hospitalized for febrile neutropenia—three after IC and two during CRT. Six patients were admitted for dehydration or intractable pain secondary to mucositis—three after IC and three during CRT. Eight patients were given outpatient intravenous fluids for grade 3 nausea/vomiting—three after IC and five during CRT. Twenty-one patients received feeding tubes during treatment—16 for weight loss or aspiration and five prophylactically. Only two patients remained dependent on a gastrostomy tube, and both had surgery at the primary site after CRT. Another late toxicity that occurred after CRT was mandibular osteoradionecrosis (in three patients). Nine patients who received three cycles of cisplatin during CRT developed persistent grade 2 to 3 peripheral neuropathy after one cycle of paclitaxel. Three patients refused the second cycle of paclitaxel. No patients required permanent tracheostomy.
Table 3.
Common Adverse Events
| Adverse Event Grade |
||||||||
|---|---|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
5 |
|||||
| Event by Time of Occurrence | No. | % | No. | % | No. | % | No. | % |
| After induction chemotherapy | ||||||||
| Neutropenia | 8 | 12 | 4 | 6 | 1 | 1 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Leukopenia | 4 | 6 | 0 | 0 | 1 | 1 | 0 | 0 |
| Anemia | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 6 | 9 | 2 | 3 | 0 | 0 | 0 | 0 |
| Mucositis | 20 | 30 | 2 | 3 | 1 | 1 | 0 | 0 |
| Nausea | 7 | 11 | 2 | 3 | 0 | 0 | 0 | 0 |
| Vomiting | 4 | 6 | 1 | 1 | 0 | 0 | 0 | 0 |
| Tinnitus | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensory neuropathy | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| During chemoradiation | ||||||||
| Neutropenia | 1 | 1 | 9 | 14 | 1 | 1 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 |
| Leukopenia | 44 | 66 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 6 | 9 | 1 | 1 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis | 21 | 32 | 42 | 64 | 3 | 4 | 0 | 0 |
| Nausea | 8 | 12 | 2 | 3 | 0 | 0 | 0 | 0 |
| Vomiting | 5 | 8 | 3 | 4 | 0 | 0 | 0 | 0 |
| Sensory neuropathy | 21 | 32 | 3 | 4 | 0 | 0 | 0 | 0 |
| Tinnitus | 5 | 8 | 2 | 3 | 0 | 0 | 0 | 0 |
| Creatinine | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| After paclitaxel | ||||||||
| Neutropenia | 12 | 18 | 4 | 6 | 2 | 3 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Leukopenia | 6 | 9 | 0 | 0 | 1 | 1 | 0 | 0 |
| Anemia | 5 | 7 | 1 | 1 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Sensory neuropathy | 2 | 3 | 7 | 14 | 0 | 0 | 0 | 0 |
During CRT, seven patients developed grade 2 or greater tinnitus; five patients received carboplatin for one cycle, and two patients received carboplatin for two cycles. Twenty-one patients required cisplatin dosage adjustments because of peripheral neuropathy. Of these, two required a second dosage adjustment.
OS and DSS
At 4 years after CRT, 41 (62%) of 66 patients are alive; 25 (38%) of 66 are dead—20 from their primary SCCOP (six with local/regional disease; 13 with distant metastases; and one with both local/regional disease and distant metastases), one from a second primary cancer (lung), one from chemotherapy toxicity, two from other causes, and one from suicide (Fig 2). With a median time to follow-up of 64 months (95% CI, 59 to 72), the 4-year OS was 70.4% (95% CI, 57.6% to 80.0%; Fig 3A), and the 4-year DSS was 75.8% (95% CI, 63.1% to 84.7%; Fig 3B). DSS by response to IC is shown in Appendix Figure A1A (online only). Adjuvant paclitaxel did not provide a survival advantage. (Appendix Fig A1B).
Fig 3.
(A) Overall survival and (B) disease-specific survival plots of patients (n = 66). (C) Disease-specific survival based on sex. (D) Disease-specific survival based on smoking status. (E) Response to induction chemotherapy based on mean human papilloma virus (HPV) titers; blue box, interquartile range (25th to 75th percentile), not including outliers; horizontal black line, median; range bars, range of minimum to maximum observations, not including outliers; red dot, mean; blue dot, outlier. (F) Disease-specific survival according to HPV16 titers. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 1 summarizes the response to therapy and survival on the basis of sex, age, KPS, smoking status, T class, N class, and tumor site. In this cohort, female sex (DSS, P = .0002; Fig 3C), higher T class (DSS, P = .003), lower N class (DSS, P = .03; Appendix Fig A1C), current smoking status (DSS, P = .003; Fig 3D) and base-of-tongue primary site (P = .04; Appendix Fig A1D) were significantly associated with poor DSS. A lower KPS significantly influenced OS (P = .03) but not DSS (P = .16).
Organ Preservation
Eleven of 66 patients were nonresponders to IC; nine received surgery, one refused surgery, and one was excluded for medical reasons. Of four patients with persistent disease after CRT, three had surgery and one refused (Fig 2). Of 66 patients, two died prior to either surgery or chemoradiation, leaving 64 assessable patients. Of these, 49 patients had organ preservation; however, two patients had local recurrence at the primary site, leaving 47 of 64 patients with organ preservation. The Kaplan-Meier estimated organ preservation rate was 73.4% at 7 years. No patients with preserved organs were dependent upon tracheostomy tube or gastrostomy tube.
Eight of nine patients who received only carboplatin during CRT were histologic responders; one later died of lung cancer. The nonresponder underwent salvage surgery and has remained alive. All patients who received one or two cycles of carboplatin during RT were complete histologic responders and remained disease-free.
Outcomes Related to HPV Status
Of the forty-two pretreatment biopsies analyzed, 27 (64%) were positive for high-risk HPV (all HPV16), and 15 were negative. The 27 HPV-positive tumors included 16 (61.5%) of 26 at the base of the tongue and 11 (68.8%) of 16 at the tonsil. The HPV16 viral load varied from less than 2 to 7,875 copies/cell. HPV presence was associated with younger age (median age, 55 v 63 years; P = .016; Appendix Fig A1E) and with nonsmoking status (P = .037; Appendix Fig A1F). A greater proportion of men (22 [73%] of 30) than women (5 [41.7%] of 12; P = .08) were HPV positive. HPV copy number was significantly associated with response to IC (P = .003; Fig 3E), CRT (P = .005), OS (P = .007), and DSS (P = .008; Fig 3F). Twenty-five of 27 HPV-positive patients were responders to IC; 24 were responders to CRT; and 21 (78%) have remained alive with organ preservation (Appendix Fig A2, online only).
Of the 15 HPV-negative patients, 10 responded to IC, and 5 did not. Of these 10 responders, seven responded to CRT; one died of metastasis before CRT. Four of the seven patients have remained alive with preserved organs, and three died; all five nonresponders died (Appendix Fig A2).
Multivariate Analysis
The favorable prognosis of patients with higher HPV titers was maintained after adjusting for sex, smoking status, T class, N class, age, and primary site (OS, P = .008; DSS, P = .004). Although KPS influenced OS, it was not significant in multivariate analysis after other variables were considered.
DISCUSSION
Selection for CRT versus early salvage surgery by using the response to IC is an approach to organ preservation that has shown an improved outcome in advanced larynx cancer.19 In the present study, a similar treatment strategy was used in patients with advanced SCCOP. The organ preservation, DSS, and OS rates compare favorably with historical controls.28,29 Our results are comparable to those obtained by the Southwest Oncology Group trial of 37 patients with stage III to IV base-of-tongue squamous cell carcinoma who were treated with two cycles of cisplatin and fluorouracil that was followed by CRT for those who responded to IC.30 Many trials include multiple sites in the head and neck; however, because site affects outcome, it is important to analyze sites separately. For example, in the Southwest Oncology Group trial,30 base-of-tongue tumors fared better than hypopharynx tumors. Similarly, patients with stage III to IV squamous cell carcinoma of the oral cavity who were treated with the UMCC 9921 protocol had poorer OS, poorer organ preservation,31 and a lower rate of functional swallowing than oropharynx patients who were treated identically. Likewise, patients on UMCC 9921 with base-of-tongue tumors had poorer survival compared with those who had tonsillar tumors on UMCC 9921 or compared with those who had stage III or IV larynx cancers treated on the similar UMCC protocol (9520).19
Other factors that vary by site also affect outcome. In particular, HPV plays an important role in SCCOP.5,6 By using the AttoSense HPV Test (licensed to SensiGen, Ann Arbor, MI) to identify and quantify high-risk HPV viral copy number, we found that 69% of tonsil and 62% of base-of-tongue tumors contained HPV16. High HPV copy number in the pretreatment biopsy was associated strongly with response to IC and CRT and with improved survival. It is not known why patients with HPV-positive tumors fare better in nearly all studies published to date.5,32-35 One explanation is the immune response to viral antigens.36 Another possibility is that the Rb and p53 pathways are compromised but remain intact to retain some function, such that, under the pressure of chemotherapy or radiotherapy, p53-mediated apoptotic pathways may still function. However, much work is needed to test these possibilities.
The use of carboplatin in the metastatic or recurrent setting has inferior response results when compared with cisplatin.37 Although our study of previously untreated tumors was not designed to compare efficacy of the two chemotherapeutic agents, it appears that carboplatin was not inferior to cisplatin in terms of response to IC or organ preservation. Furthermore, during CRT, substitution of carboplatin for cisplatin helped to improve compliance rates with chemotherapy. Seventy percent of our patients received all three cycles of chemotherapy compared with similar regimens that used only cisplatin, in which compliance rates were 50% to 60%.38,39
UMCC 9921 included planned neck dissections for patients with neck nodes greater than 3 cm, but all were negative for residual tumor. On the basis of this and on the value of PET imaging to assess CRT responses in multiple tumor types,40-43 our practice evolved to monitor the neck at 8 to 10 weeks after CRT with computed tomography/PET. PET scans, however, were not required by the protocol. Patients with N0 disease in our study had worse outcome than those with N-positive disease. This was independent of HPV and may be due to a greater incidence of current smokers in the N0 group.
A survival benefit for adjuvant chemotherapy has never been documented.44,45 In UMCC 9921, adjuvant paclitaxel increased permanent sensory neuropathy, was difficult to administer because of cumulative toxicity after cisplatin, and did not result in any survival benefit. Hence, adjuvant paclitaxel was not effective in this design.
In contrast to our expectations from UMCC studies that involved the larynx, salvage surgery for SCCOP was ineffective in all who failed CRT and in five of nine nonresponders to IC. Whether nonresponders to IC would have fared better if they received CRT instead of surgery cannot be assessed from this trial design. Of the 20 patients with recurrent disease, 14 died as a result of distant metastases, which suggests that better systemic therapy is needed. The combination of docetaxel, cisplatin, and fluorouracil (TPF) in IC regimens has demonstrated improved response and OS rates with less toxicity.46-48 It is interesting to speculate whether nonresponders to IC with platinum and fluorouracil, HPV-negative patients, or patients with other unfavorable tumor biomarker profiles would benefit from the addition of a taxane up front.
The factors that govern the response to IC and the subsequent response to CRT are poorly understood. However, it is clear that HPV-positive tumors were most likely to respond to IC, whereas HPV-negative tumors were less likely to respond to IC or had borderline responses. All HPV-negative patients were former or current smokers. For reasons that are not entirely clear, smoking decreases the advantage of having an HPV-positive tumor. Kumar et al.24 examine the role of tumor biomarkers (ie, p53, BCLXL, and EGFR), patient characteristics, and HPV status in response to therapy and survival. They identify marker patterns associated with good response and survival and those associated with treatment failure and poor survival. These markers suggest potential targets for individualized treatment for patients who are unlikely to respond to CRT. It is clear that alternative treatment strategies must be developed for those patients least likely to benefit from IC and CRT or surgery and RT. Finally, the high incidence of high-risk HPV-associated cancers in men suggests that vaccination of all adolescents against this oncogenic risk should be considered.
Acknowledgments
Supported in part by Grants No. R01 DE13346 and P30 DC 05188 from the National Institutes of Health (NIH) NIDCR; Head and Neck Cancer SPORE Grant No. P50 CA97248; Cancer Center Support Grant No. P30 CA46592; and Grants from the state of Michigan.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Footnotes
Presented in part at the 38th Annual Meeting of the American Society of Clinical Oncology, May 18-21, 2002, Orlando, FL; 6th International Conference on Head and Neck Cancer, August 9, 2004, Washington, DC; 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL; American Association for Cancer Research Meeting, April 4, 2006, Washington, DC; and Multidisciplinary Head and Neck Cancer Symposium, January 18-20, 2007, Rancho Mirage, CA.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: David M. Kurnit, SensiGen (U), SensiGen (C) Consultant or Advisory Role: David M. Kurnit, SensiGen (U) Stock Ownership: David M. Kurnit, SensiGen Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: Increasing trends in the US population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Bagnardi V, Blangiardo M, La Vecchia C, et al. Alcohol consumption and the risk of cancer: A meta-analysis. Alcohol Res Health. 2001;25:263–270. [PMC free article] [PubMed] [Google Scholar]
- 4.Lewin F, Norell SE, Johansson H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer. 1998;82:1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Sturgis EM. The role of human papillomavirus in squamous carcinoma of the head and neck. Curr Oncol Rep. 2006;8:130–139. doi: 10.1007/s11912-006-0048-y. [DOI] [PubMed] [Google Scholar]
- 8.Ringstrom E, Peters E, Hasegawa M, et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 9.van Houten VM, Snijders PJ, van den Brekel MW, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93:232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 10.Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: A radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Thompson CH, O'Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–558. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 13.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 14.Badaracco G, Venuti A, Morello R, et al. Human papillomavirus in head and neck carcinomas: Prevalence, physical status and relationship with clinical/pathological parameters. Anticancer Res. 2000;20:1301–1305. [PubMed] [Google Scholar]
- 15.Pintos J, Franco EL, Black MJ, et al. Human papillomavirus and prognoses of patients with cancers of the upper aerodigestive tract. Cancer. 1999;85:1903–1909. doi: 10.1002/(sici)1097-0142(19990501)85:9<1903::aid-cncr4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC) Br J Cancer. 2000;83:1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: A study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86:265–272. doi: 10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 18.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer: The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 19.Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: A new treatment paradigm. J Clin Oncol. 2006;24:593–598. doi: 10.1200/JCO.2005.01.2047. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute . Investigator's Handbook: A Manual for Participants in Clinical Trials of Investigational Agents Sponsored by Division of Cancer Treatment and Diagnosis, National Cancer Institute. National Cancer Institute; Bethesda, MD: 1993. [Google Scholar]
- 22.Eisbruch A, Marsh LH, Martel MK, et al. Comprehensive irradiation of head and neck cancer using conformal multisegmental fields: Assessment of target coverage and noninvolved tissue sparing. Int J Radiat Oncol Biol Phys. 1998;41:559–568. doi: 10.1016/s0360-3016(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 23.Vineberg KA, Eisbruch A, Coselmon MM, et al. Is uniform target dose possible in IMRT plans in the head and neck? Int J Radiat Oncol Biol Phys. 2002;52:1159–1172. doi: 10.1016/s0360-3016(01)02800-0. [DOI] [PubMed] [Google Scholar]
- 24.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2007 doi: 10.1200/JCO.2007.12.7662. 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102:7683–7688. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obiso R, Lorincz A. Digene Corporation. Pharmacogenomics. 2004;5:129–132. doi: 10.1517/phgs.5.1.129.25678. [DOI] [PubMed] [Google Scholar]
- 27.Carey VJ. Using hypertext and the internet for structure and management of observational studies. Stat Med. 1997;16:1667–1682. doi: 10.1002/(sici)1097-0258(19970815)16:15<1667::aid-sim602>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Adelstein DJ, Saxton JP, Lavertu P, et al. Maximizing local control and organ preservation in stage IV squamous cell head and neck cancer with hyperfractionated radiation and concurrent chemo-therapy. J Clin Oncol. 2002;20:1405–1410. doi: 10.1200/JCO.2002.20.5.1405. [DOI] [PubMed] [Google Scholar]
- 29.Garden AS, Harris J, Vokes EE, et al. Preliminary results of Radiation Therapy Oncology Group 9703: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2004;22:2856–2864. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Urba SG, Moon J, Giri PG, et al. Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: A Southwest Oncology Group trial. J Clin Oncol. 2005;23:88–95. doi: 10.1200/JCO.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Urba S, Worden F, Carey T, et al. One cycle of induction chemotherapy (IC) to select for organ preservation for patients (PTS) with advanced squamous carcinoma of the oral cavity (SCCOC) J Clin Oncol. 2005;23:16S. suppl; abstr 5555. [Google Scholar]
- 32.Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck. 2004;26:1–9. doi: 10.1002/hed.10335. [DOI] [PubMed] [Google Scholar]
- 33.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 34.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 35.Sisk EA, Soltys SG, Zhu S, et al. Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck. 2002;24:841–849. doi: 10.1002/hed.10146. [DOI] [PubMed] [Google Scholar]
- 36.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–11155. doi: 10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 37.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: A Southwest Oncology Group study. J Clin Oncol. 1992;10:1245–1251. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 38.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 39.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 40.Brucher BL, Weber W, Bauer M, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: Response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flamen P. Positron emission tomography in colorectal cancer. Best Pract Res Clin Gastroenterol. 2002;16:237–251. doi: 10.1053/bega.2001.0283. [DOI] [PubMed] [Google Scholar]
- 42.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 43.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 44.Bourhis J, Le Maitre A, Baujat B, et al. Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19:188–194. doi: 10.1097/CCO.0b013e3280f01010. [DOI] [PubMed] [Google Scholar]
- 45.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data—MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 46.Calais G, Pointreau Y, Alfonsi CS, et al. Randomized phase III trial comparing induction chemotherapy using cisplatin (P) fluorouracil (F) with or without docetaxel (T) for organ preservation in hypopharynx and larynx cancer: Preliminary results of GORTEC 2000-01. J Clin Oncol. 2006;24:18s. suppl; abstr 5506. [Google Scholar]
- 47.Hitt R, Grau J, Lopez-Pousa A, et al. Randomized phase II/III clinical trial of induction chemotherapy (ICT) with either cisplatin/5-fluorouracil (PF) or docetaxel/cisplatin/5-fluorouracil (TPF) followed by chemoradiotherapy (CRT) vs. CRT alone for patients (pts) with unresectable locally advanced head and neck cancer (LAHNC) J Clin Oncol. 2006;24:18s. suppl; abstr 5515. [Google Scholar]
- 48.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]