Abstract
Background
Merozoite surface protein (MSP) 5 is a candidate antigen for a malaria vaccine. In cross-sectional and longitudinal studies, we measured MSP5 antibody responses in Papuans with acute Plasmodium falciparum malaria, Plasmodium vivax malaria, and mixed P. falciparum and P. vivax malaria and in those with past exposure.
Methods
Enzyme-linked immunosorbant assay (ELISA) was used to quantitate antibody responses to P. falciparum MSP5 (PfMSP5) and P. vivax MSP5 (PvMSP5) in 82 subjects with P. falciparum infection, 86 subjects with P. vivax infection, 85 subjects with mixed infection, and 87 asymptomatic individuals. Longitudinal responses through day 28 were tested in 20 persons. Cross-reactivity was tested by competition ELISA.
Results
PfMSP5 or PvMSP5 immunoglobulin (Ig) G was detected in 39%–52% of subjects, and IgM was detected in 44%–72%. IgG responses were distributed equally between IgG3 and IgG1 for PfMSP5 but were predominantly IgG3 for PvMSP5. Although IgG responses were generally specific for PfMSP5 or PvMSP5, cross-species reactivity was found in 7 of 107 dual-positive responders. No significant difference was seen in the magnitude, frequency, or subclass of PfMSP5 or PvMSP5 IgG antibodies between groups. There was no significant association between antibody responses and therapeutic response.
Conclusion
PfMSP5 and PvMSP5 were frequently recognized by short-lived, species-specific antibodies. Although infrequent, the cross-reactive MSP5 antibodies indicate that an appropriately formulated vaccine may elicit and/or enhance cross-species recognition, which may be very useful in areas where both parasites are endemic.
Revised estimates indicate that the global burden of Plasmodium falciparum malaria has been significantly underestimated, with at least 0.5 billion clinical cases each year and a death toll that may exceed 2 million people annually [1]. Recent data indicate that the global burden of the other major Plasmodium species that causes human disease, Plasmodium vivax, has also been underestimated, with up to 390 million cases per year [2] and an association with significant morbidity and mortality in areas of emerging chloroquine resistance [3]. Although the development of vaccines against P. falciparum remains a high priority, the public health importance of P. vivax vaccine development is increasingly being recognized. Vaccines that confer protection against both species are needed for the 2.5 billion people resident in areas of mixed endemicity [3]. A vaccine candidate antigen currently being assessed for trial in humans is merozoite surface protein (MSP) 5. MSP5 is relatively conserved between P. falciparum isolates [4, 5], is highly polymorphic in P. vivax [6], and is 72.2% homologous within the epidermal growth factor (EGF)–like domain between P. falciparum and P. vivax [7]. The relatively conserved nature of the MSP5 EGF-like domain suggests that immune responses targeting this region may confer cross-species protection. MSP5 is expressed not only on merozoites but also on sporozoites and infected hepatocytes [8] and is therefore a potential target for a combined liver- and blood-stage vaccine.
Because little information on MSP5 antibody responses in humans naturally exposed to Plasmodium species is available, we investigated the magnitude, frequency, induction, and boosting pattern of humoral responses to P. falciparum MSP5 (PfMSP5) and P. vivax MSP5 (PvMSP5) in a region with mixed endemicity of P. falciparum and P. vivax. Furthermore, competition ELISAs were used to determine the potential for cross-reactivity between antibodies reactive to PfMSP5 and PvMSP5. To our knowledge, this study is the first to compare recognition of PfMSP5 and PvMSP5 in humans. In a longitudinal clinical follow-up study, we investigated the association between PfMSP5 antibody responses and protection from recurrent Plasmodium infection. We describe the detection of MSP5 antibody responses that were predominantly species specific, with a different isotype-induction pattern between PfMSP5 and PvMSP5, and examine the association between MSP5 antibody responses and the recurrence of infection.
METHODS
Study subjects and samples
Patients were recruited in Timika, a lowland region of Papua, Indonesia, that has endemic unstable transmission of both P. falciparum and P. vivax, a similar ratio of P. falciparum to P. vivax (57:43) year-round, and an annual malaria incidence of 938 cases per 1000 person-years [9]. Subjects were enrolled in trials of chloroquine and sulfadoxine-pyrimethamine therapy or artemisinin combination therapy (ACT) after providing informed consent [9]. Papuans with fever or a history of fever within 48 h of enrollment, with no alternative cause of fever identified, and with microscope-identified P. falciparum (82 subjects), P. vivax (86 subjects), or mixed P. falciparum and P. vivax infection (85 subjects) were included in this study. Eighty-seven Plasmodium-exposed healthy adults who had resided in Timika for at least 2 years and who had not had fever or symptoms of malaria during the preceding 2 weeks were also evaluated as exposed asymptomatic control subjects. Venous blood was collected at the time of presentation and, for the groups with malaria, at ~7 and 28 days after treatment. Plasma was separated and cryopreserved in Timika and transported to the Menzies School of Health Research for analysis. Plasma samples from 36 anonymous healthy Australian blood donors (provided by the Australian Red Cross Blood Service) were tested as negative controls. The study was approved by the ethics committees of the National Institute of Health Research and Development, Ministry of Health, Jakarta, Indonesia; the Menzies School of Health Research; and the Australian Red Cross Blood Service.
MSP5 antigen
Recombinant PfMSP5 was expressed and purified as described elsewhere [10, 11]. Recombinant PvMSP5 protein of the Salvador I isolate was expressed from Escherichia coli BL21 (DE3) strain (Novagen) containing a pTrcHis-A recombinant plasmid [7] by induction with 2 mmol/L isopropyl-β-D-thiogalactopyranoside (Progen Industries). Lysate was first passed through a metal affinity resin column (Amersham) for binding of the hexahistidine-tagged protein, in accordance with the manufacturer’s instructions, and the eluate was then subjected to ion-exchange chromatography on a Sepharose column (GE Healthcare), in which proteins were eluted on a linear gradient of sodium chloride. Analysis of purified protein by SDS-PAGE and Western blotting using anti-His antibody (Roche) and rabbit anti-PvMSP5 polyclonal serum identified a 62-kDa protein band corresponding to PvMSP5 (data not shown). E. coli host cell protein content was determined using an immunoenzymometric assay (Cygnus Technologies) and was found to be 0.004% of total protein content. In the total IgG ELISA, PfMSP5 was used at 1 μg/mL and PvMSP5 at 0.25 μg/mL, and both were used at 0.75 μg/mL in the competition ELISA.
MSP5 ELISA
Nunc plates coated with MSP5 protein were blocked with 5% skim milk in PBS containing 0.05% Tween (Sigma) (PBS-T) and washed with PBS-T. Then, 50 μL of plasma, diluted 1:800 in PBS-T, was added to the plate, and the assay was incubated for 1 h. Anti–human total IgG horseradish peroxidase (HRP; 1:2000 dilution; Zymed), anti–human total IgM (1:2000 dilution; Chemicon), or a 1:500 dilution of anti–human IgG1, IgG2, IgG3, and IgG4 (Zymed; clones HP6069, HP6014, HP6047, and HP6025) was added, and the assay was incubated for 1 h. The assay was developed using tetramethyl benzidine solution (Zymed), the color reaction was stopped with 1 mol/L hydrochloric acid, and the plate was read at 450 nm. The binding of antibodies in plasma from 36 unexposed Australian blood donors was used to define a cutoff (mean optical density plus 3 SDs) for positive and negative responses to each antigen. ChromPure human IgG and IgM (myeloma) whole molecules (Jackson ImmunoResearch Laboratories) were used as standards for quantitation of antibody responses, after dilution in PBS to 0–300 ng/mL for IgG or 0–125 ng/mL for IgM. Our cutoffs for positive responses were ≥23 μg/mL PfMSP5 and 24.8 μg/mL PvMSP5 for IgG and 7.9 μg/mL PfMSP5 and 7.2 μg/mL PvMSP5 for IgM.
Competition ELISA
First, 120 μL of plasma (diluted 1:400 in PBS-T) was incubated with serial dilutions of PfMSP5 and PvMSP5, respectively, at 0.02–10 μg/mL in separate 96-well round-bottom tissue culture plates (Becton Dickinson) and incubated at 37°C for 2 h. Then, 50 μL from each well was transferred into Nunc Maxisorb plates coated with 0.75 μg/mL PfMSP5 and PvMSP5, respectively, after blocking with 5% skim milk in PBS-T and incubated for 1 h at room temperature. The ELISA was performed as described above, using anti–human total IgG HRP (1:2000 dilution; Zymed). IgG responses were considered to be cross-reactive if preincubation with PfMSP5 resulted in reduced antibody reactivity in the PvMSP5 ELISA, and vice versa.
Statistical analysis
Statistics were calculated using GraphPad Prism software (version 5; GraphPad) and SPSS for Windows (version 15; SPSS). Fisher’s exact test was used to compare proportions of responders between 2 groups. Antibody titers were compared between groups by the nonparametric Mann-Whitney U test. Spearman’s rank correlation was used to compare antibody titers and parasitemia or age. The significance level used was α = .05 for all statistical tests. The therapeutic response was defined on the basis of the cumulative incidence on day 42, calculated by the Kaplan-Meier method. The risk of treatment failure was compared by the Mantel-Haenszel log-rank test and the hazard ratio presented. In multivariate analysis, any variable found to be significantly associated with the dependent variable in univariate analysis was entered into a Cox regression model, and the model was constructed using all factors.
RESULTS
Frequent recognition of MSP5
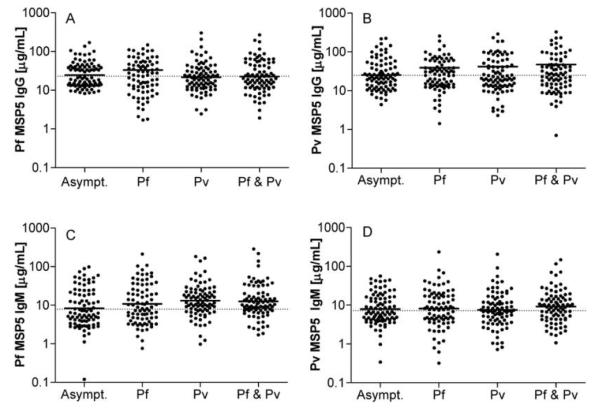
Plasma was available from 253 Papuans treated for malaria (82 with P. falciparum infection, 86 with P. vivax infection, and 85 with mixed P. falciparum and P. vivax infection) and from 87 Papuan control subjects without malaria symptoms (table 1). Both PfMSP5 and PvMSP5 were frequently recognized by both IgG and IgM antibodies from Papuans. The overall prevalence of IgG responses was similar across groups with asymptomatic or symptomatic infection, with 39%–50% of subjects seropositive for PfMSP5 and 42%–52% seropositive for PvMSP5 (table 2). High antibody levels, with mean IgG responses of 32–35 μg/mL for PfMSP5 and 39–47 μg/mL for PvMSP5, were found in each group, similar to the levels of antibody induced in the clinical trial of another malaria vaccine candidate, MSP142 (mean IgG level, 48.3 μg/mL) [12]. There was no significant difference in the magnitude of IgG responses to PfMSP5 or PvMSP5 between patients with acute symptomatic P. falciparum, P. vivax, or mixed P. vivax and P. falciparum infection and the exposed asymptomatic subjects (figure 1) and no significant association between antibody responses and parasitemia (data not shown).
Table 1. Characteristics of the study cohort.
| Characteristic | Group |
|||
|---|---|---|---|---|
| Exposed asymptomatic (n = 87) |
Acute P. falciparum (n = 82) |
Acute P. vivax (n = 86) |
Acute P. falciparum and P. vivax (n = 85) |
|
| Age, mean (range), years | 27 (18–42) | 24 (3–60) | 21 (4–60) | 20 (3–50) |
| Female/male, no. | 8/79 | 27/55 | 37/49 | 31/54 |
| Temperature ≥37.5°C, % | 0 | 31 | 17 | 38 |
| P. falciparum, mean (range), parasites/μL | 20 (0–1118) | 12,487 (23–132,840) | 0 | 8450 (26–79,200) |
| P. vivax, mean (range), parasites/μL | 13 (0–555) | 0 | 4175 (30–33,000) | 4474 (15–121,000) |
Table 2. Frequency of merozoite surface protein (MSP) 5 recognition among study groups.
| Group | IgG response |
IgM response |
||
|---|---|---|---|---|
| PfMSP5 | PvMSP5 | PfMSP5 | PvMSP5 | |
| Exposed asymptomatic subjects (n = 87) | 40 (46) | 41 (47) | 38 (44) | 40 (46) |
| Subjects with acute infection | ||||
| P. falciparum (n = 82) | 41 (50) | 43 (52) | 44 (54) | 46 (56) |
| P. vivax (n = 86) | 37 (43) | 36 (42) | 61 (71)a | 42 (49) |
| P. falciparum and P. vivax (n = 85) | 33 (39) | 43 (51) | 61 (72)a | 51 (60) |
NOTE. Data are no. (%) of subjects with a positive response. PfMSP5, Plasmodium falciparum MSP5; PvMSP5, Plasmodium vivax MSP5.
P < .05 for the comparison between exposed asymptomatic subjects or those with acute P. falciparum infection and patients with acute P. vivax infection or those with mixed P. falciparum and P. vivax infection (Fisher’s exact test).
Figure 1.
Total plasma IgG specific for merozoite surface protein (MSP) 5 of Plasmodium falciparum (PfMSP5) (A) or MSP5 of Plasmodium vivax (PvMSP5) (B) and IgM specific for PfMSP5 (C) and PvMSP5 (D) in asymptomatic subjects exposed to Plasmodium species (n = 87) and in subjects with acute P. falciparum (Pf; n = 82), acute P. vivax (Pv; n = 86), or acute mixed (Pf & Pv; n = 85) infection. Solid horizontal lines indicate geometric means, and dotted lines indicate cutoffs for positive responses (mean optical density plus 3 SDs for 36 unexposed blood donors).
Mean IgM levels were lower than mean IgG levels for both PfMSP5 (16–23 μg/mL) and PvMSP5 (12–16 μg/mL) across all groups. There was no significant difference in the magnitude of IgM responses between groups with acute symptomatic malaria and exposed asymptomatic individuals (figure 1). Surprisingly, we found that IgM responses to PfMSP5 but not to PvMSP5 were significantly and substantially more frequent in subjects with acute P. vivax or mixed P. falciparum and P. vivax infection (71% and 72%, respectively) than in the exposed asymptomatic control subjects (44%) (P < .05) (table 2), even though the magnitude of IgM responses did not differ between groups.
MSP5 IgG cross-reactivity
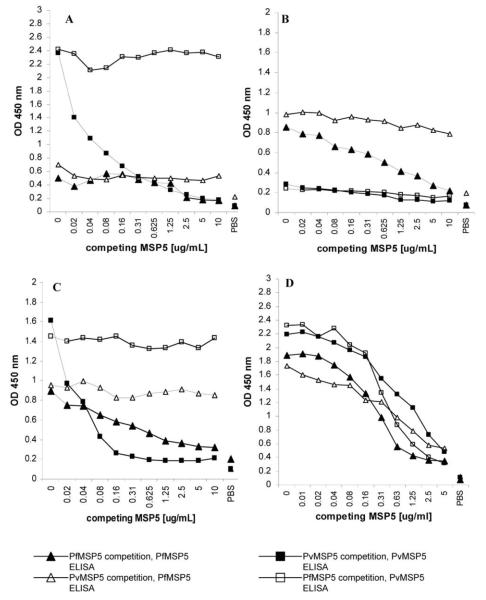
In our cohort of 340 individuals, 69%–71% of subjects with symptomatic infection or asymptomatic exposure who had IgG to MSP5 responded to both PfMSP5 and PvMSP5. Plasma samples from 107 subjects with dual IgG responses were tested for cross-species reactivity by competition ELISA. This involved preincubation of plasma with MSP5 from 1 species at serial protein dilutions followed by ELISA using MSP5 from the other species. Cross-reactive immunoglobulin would bind to MSP5 during the preincubation step and, with increasing concentrations of competing MSP5 protein, would reduce signal in the ELISA. Homologous proteins were used as controls for the assay. Figure 2 shows representative results for samples reactive to either PfMSP5 or PvMSP5 only, reactive to both but with no cross-reactivity, or with cross-recognition of both proteins. We found that 7 (7%) of the 107 subjects tested had cross-reactive IgG responses, indicating cross-species recognition, but the majority of IgG responses recognized PfMSP5 or PvMSP5 exclusively (data not shown). These data show that natural exposure to Plasmodium species induces MSP5 IgG responses that cross-react with P. falciparum and P. vivax, albeit infrequently. The 7 subjects with cross-reactive antibodies were from 3 different cohorts (3 exposed asymptomatic subjects, 3 with mixed infection, and 1 with P. vivax infection).
Figure 2.
IgG cross-reactivity between merozoite surface protein (MSP) 5 of Plasmodium falciparum (PfMSP5) and MSP5 of Plasmodium vivax (PvMSP5). Plasma samples were preincubated with titrating concentrations of PfMSP5 or PvMSP5 before being tested for remaining IgG specific for PfMSP5 (triangles) or PvMSP5 (squares) by ELISA. Black symbols represent preincubation with the same protein as tested in the ELISA, and white symbols represent preincubation with MSP5 from the other species. Results for 4 representative donors are shown: PvMSP5 IgG only (A), PfMSP5 IgG only (B), IgG to both PfMSP5 and PvMSP5 with no cross-reactivity (C), and IgG to both PfMSP5 and PvMSP5 with some cross-reactivity (D).
The IgG subclass of MSP5 humoral responses
Because malaria-specific IgG1 and IgG3 responses have been associated with lower parasitemia and a reduced risk of clinical malaria [13–16], we evaluated all PfMSP5 and PvMSP5 IgG responders to determine the subclass of response by ELISA. Across all groups tested, the majority of anti-PfMSP5 antibodies (53%–69%) were of the IgG1 and IgG3 subclass, with 20%–31% of subjects producing both IgG1 and IgG3 (table 3). PfMSP5-specific IgG2 was detected in 6%–8% of subjects with acute malaria, compared with 23% for exposed subjects without clinical malaria (P = .08; χ2 test). No IgG4 responses were detected in any of the groups.
Table 3. IgG subclass of merozoite surface protein (MSP) 5 humoral responses.
| Group | IgG subclass, % of subjects |
||||
|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | Dual IgG1 and IgG3 |
|
| Plasmodium falciparum MSP5 | |||||
| Exposed asymptomatic (n = 40) | 53 | 23 | 60 | 0 | 20 |
| Acute P. falciparum (n = 41) | 59 | 7 | 68 | 0 | 27 |
| Acute P. vivax (n = 36) | 67 | 8 | 53 | 0 | 22 |
| Acute P. falciparum and P. vivax (n = 32) | 69 | 6 | 56 | 0 | 31 |
| Plasmodium vivax MSP5 | |||||
| Exposed asymptomatic (n = 41) | 24 | 5 | 85 | 0 | 12 |
| Acute P. falciparum (n = 44) | 27 | 7 | 86 | 0 | 14 |
| Acute P. vivax (n = 36) | 50 | 6 | 69 | 0 | 22 |
| Acute P. falciparum and P. vivax (n = 43) | 40 | 0 | 77 | 0 | 16 |
A different distribution pattern of IgG subclass was observed for antibodies to PvMSP5, because IgG3 responses were on average 2.5 times more prevalent than IgG1 responses across all groups. Similar to findings for PfMSP5, 12%–21% of subjects had both IgG1 and IgG3 antibodies (table 3). PvMSP5 IgG2 responses were rare in all groups (0%–7%), including in exposed individuals with no clinical malaria, and no IgG4 was detected. Overall, we found no relationship between the frequency of IgG subclass response and clinical presentation (i.e., asymptomatic or acute). The differing ratios of IgG1 and IgG3 isotype responses to PfMSP5 and PvMSP5 highlight a species-specific difference in the MSP5 antibody response, which occurred irrespective of past exposure or current infection.
MSP5 humoral responses and recurrence of parasitemia
The similar magnitudes of antibody responses to PfMSP5 and PvMSP5 across the different clinical cohorts led us to question whether the MSP5 antibody responses detected during acute infection contribute to parasite clearance and/or impair reinfection. The potential clinical relevance of MSP5 IgM and IgG antibody responses was evaluated in 2 groups: (1) a subset of 26 patients with malaria who received chloroquine and sulfadoxine-pyrimethamine treatment [17] and (2) 191 patients who received ACT [9], all of whom were monitored for up to 45 days after treatment for disease recurrence. Because significant drug resistance to both chloroquine and sulfadoxine-pyrimethamine has emerged [17], whereas ACT clears parasites rapidly [9], the correlation with therapeutic response was assessed separately in both groups. In the 26 patients who received chloroquine and sulfadoxine-pyrimethamine treatment, the magnitude of PfMSP5 IgM and IgG antibody responses were compared between the 15 subjects who successfully cleared P. falciparum (monitored for 20–45 days) and the 11 patients in whom reinfection was detected between days 7 and 31. IgG responses did not differ significantly between the 2 groups (data not shown). IgM responses, however, were significantly stronger in the group that successfully cleared parasites (P = .005; data not shown), but this difference did not remain after controlling for age.
In the second group, a clinical response after antimalarial therapy was documented in 96% of patients (243/253) with symptomatic malaria treated with ACT. Of the 78% of patients (191/243) who received ACT, 47 were infected with P. falciparum, 71 were infected with P. vivax, and 73 were infected with both species. There was no significant relationship between the magnitude of total IgM or IgG antibody levels and the age of the patient or the therapeutic response (data not shown).
Longitudinal analysis of MSP5 IgM and IgG humoral responses
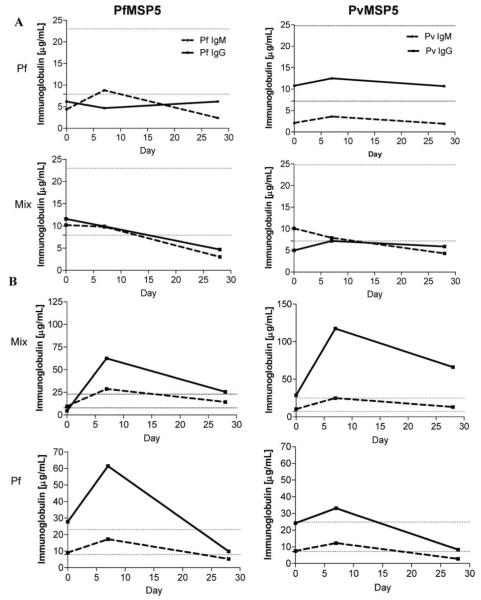
To determine whether IgM and IgG antibodies to PfMSP5 and PvMSP5 were induced and/or boosted during acute malaria and/or after recovery, we compared the magnitude of antibody responses detected at treatment with those detected 7 and 28 days later. The pattern of total antibody responsiveness was monitored over time in 20 patients with acute uncomplicated P. falciparum, P. vivax, or mixed malaria. Antibody responses to MSP5, when detected, generally increased between the day of presentation and peaked on day 7, in accordance with antibody responses to other P. falciparum merozoite antigens [18]. In general, 2 separate patterns of responsiveness were observed: (1) IgM but no IgG antibody responses, associated with immune priming (9/20 subjects [45%]); and (2) IgM and IgG antibody responses, representing immune boosting of a secondary or later infection (11/20 subjects [55%]) (figure 3). Surprisingly, in 5 of 9 subjects singly infected with P. falciparum, increased PfMSP5 IgG responses (corresponding to the current infection) and boosted PvMSP5 IgG responses were detected. Induction or boosting of PvMSP5 IgM was observed in 7 of 9 individuals with a diagnosis of P. falciparum infection. The data show that a current Plasmodium infection can prime and/or boost MSP5 antibody responses. The data also suggest an elevation in cross-species MSP5 immune responses. The subclass of MSP5 IgG responses, once induced, appeared to be durable until day 28 after treatment in the 20 subjects with a range of ages (5–60 years) tested, providing no evidence of IgG class switching in the time frame analyzed (data not shown). The MSP5-specific antibodies, whether IgM or IgG, appeared to be short-lived, with their levels declining by day 28.
Figure 3.
Longitudinal merozoite surface protein 5 (MSP) 5 responses. Shown are IgM and IgG antibody responses to Plasmodium falciparum (Pf) MSP5 (PfMSP5) (left panels) and Plasmodium vivax (Pv) MSP5 (PvMSP5) (right panels) in plasma collected on the day of treatment (day 0) and 7 and 28 days later. Subjects had MSP5-specific IgM but no IgG responses (A) or both IgM and IgG responses (B) (4 representative donors are shown). Horizontal dotted lines indicate the thresholds for positive IgM (lower line) and IgG (upper line) responses.
DISCUSSION
Antibodies are widely agreed to play a role in protection from blood-stage malaria [19], but the prevalence of MSP5-specific antibody responses and their contribution to protection is currently unknown. This study identified frequent antibody responses to PfMSP5 and PvMSP5 in Papuans who were acutely infected with P. falciparum, P. vivax, or both or who had past exposure. Surprisingly, there was no difference in the magnitude of MSP5 antibody responses between persons with clinical disease and those with asymptomatic exposure. In general, IgG responses were species specific, with a different reactivity pattern for PfMSP5 (which had an equal induction of IgG3 and IgG1 responses) than for PvMSP5 responses (which were predominantly of the IgG3 subclass). We speculate that differences in IgG isotype induction are related to the polymorphic or conserved nature of the MSP5 antigen of P. falciparum and P. vivax. Because cross-reactive antibodies were uncommon (7/107 subjects), we propose that antibodies predominantly recognize regions with variability between PfMSP5 and PvMSP5. In general, similar patterns of induction and/or boosting of PfMSP5 or PvMSP5 antibody responses were observed during P. falciparum and P. vivax infection.
Several malaria antigens, including MSP119 [20], MSP2 [21], MSP4 [22], MSP6, MSP7 [23], and glycosylphosphatidylinositol [24], are recognized by IgG3 and IgG1 antibodies. We demonstrated that MSP5 induces a similar subclass of antibody responses. According to conservation theory, the IgG subclass may be associated with the degree of conservation of the antigen [23], with conserved antigens preferentially inducing IgG1 and polymorphic antigens preferentially inducing IgG3 [22, 25], particularly if they contain repetitive polymorphic sequences [26]. Because PfMSP5 is one of the most conserved Plasmodium antigens of the merozoite integral membrane proteins [4] and PvMSP5 is less conserved, the data presented in our study support the conservation theory, because PvMSP5 predominantly induced IgG3 antibodies and PfMSP5 induced both IgG3 and IgG1. In our study, the proportions of IgG subclass responses to PfMSP5 and PvMSP5 were similar in the exposed asymptomatic subjects who had resided in Timika for at least 2 years and in the subjects with acute symptomatic infection, suggesting that the isotypes appear relatively stable when induced.
Several studies have associated the subclass of malaria-specific antibody responses with the outcome of malaria. Increased levels of malaria-specific IgG1 and IgG3 responses have been associated with lower parasitemia and reduced risk of clinical malaria [13-16, 21], presumably because IgG1 and IgG3 are key components of Fcγ receptor (FcγR)–mediated effector responses, including antibody-dependent cell-mediated cytotoxicity and antibody-mediated phagocytosis [27, 28]. IgG2 and IgG4 antibodies, on the other hand, are believed to compete with IgG1 and IgG3 for FcγR binding [14]. High levels of anti-malarial IgG4 have indeed been associated with an increased risk of infection, but the role played by IgG2 appears to be more ambiguous, because high levels correlate with a low risk of infection in Burkina Faso [29]. Our study identified PfMSP5 and PvMSP5 IgG responses as being predominantly cytophilic or opsonizing IgG3 and IgG1 with no IgG4, both during acute infection and 28 days after drug therapy. Although the difference was not statistically significant, PfMSP5-specific but not PvMSP5-specific IgG2 was observed more frequently in exposed asymptomatic individuals than in subjects with acute uncomplicated infection. However, we found no significant association between antibody responses and parasitemia, the isotype of responses, or the presence or absence of acute infection, nor was there a significant relationship between antibody responses and therapeutic outcome. In a study in Vietnam, PfMSP4 IgG1 and IgG3 antibodies did not correlate with the absence of parasitemia [22]. Thus, our data are in accord with those of past studies and appear to agree with the current limited PfMSP5 antibody data from malaria-exposed individuals in Vietnam and Papua New Guinea, where responses are reported as being predominantly IgG1 [23].
The longitudinal data acquired in the present study showed that acute infection was able to both induce and boost PfMSP5 and PvMSP5 IgM and IgG immune responses. However, a single malarial infection was not necessarily sufficient to generate IgG responses. When PfMSP5 and PvMSP5 IgG responses were detected, they were frequently boosted on day 7 irrespective of the current infecting Plasmodium species. This result led us to conclude that (1) mixed infections were underdiagnosed by microscopy, as has been reported [30]; (2) boosting generated cross-reactive antibody responses; and/or (3) a shared region between P. falciparum and P. vivax, such as the EGF-like domain of MSP5 or another MSP, nonspecifically boosted the species-specific MSP5 antibody responses. The higher frequency of PfMSP5 IgM responses found in acute P. vivax and mixed infection supports the notion of non–species-specific boosting of antibody responses. The longitudinal data also showed that PfMSP5 and PvMSP5 antibody levels wane over time and generally fall below threshold levels 3–4 weeks after treatment. A short duration of anti-malarial antibody responses has been reported by others [31]. Whether this waning is associated with the short half-life of the antibodies or with dysfunctions in the generation of memory B cell responses, as suggested by Dorfman et al. [32], is not clear. Although beyond the scope of the present study, B cell biology in the context of malarial infection and its potential link to immunological memory responses deserves closer investigation. The short duration of antibody responses may offer an explanation as to why our study found no relationship between the magnitude or frequency of PfMSP5 or PvMSP5 antibody responses and Plasmodium reinfection.
This study has determined that natural exposure to P. vivax and/or P. falciparum frequently elicits MSP5 antibody responses of a high magnitude. Although responses were mostly species specific, the presence of cross-reactive MSP5 antibodies in a minority of subjects indicates that vaccines can be developed to confer cross-species recognition, which may be very useful in areas where both parasites are endemic.
Acknowledgments
We thank Ferryanto Chalfein, Buhari, Prayoga, Rosmini, and Elvi Yoshi for technical and logistical assistance; Hadjar Siswantoro, Alison Ratcliff, and the Mitra Masyarakat Hospital staff for clinical support; Mauritz Okeseray, Erna Tresnaningsih, Jeanne Rini, and Paulus Sugiarto for support; and the staff of the PT Freeport Indonesia Public Health and Malaria Control Department, International SOS, and Lembarga Pengembangan Masyarakat Amungme Kamoro for support and technical assistance in the community-based studies. Cross-checking of slides was done by Ferryanto Chalfein (Timika) and Budi Prasetyorini (National Institute of Health Research and Development, Jakarta). We thank Joseph McDonnell for assistance with statistics. We further acknowledge the support of the Australian Red Cross Blood Service and the National Health and Medical Research Council Program in Malaria.
Financial support: Wellcome Trust (International Collaborative Research Grant [ICRG] GR071614MA to the study and career development award to R.N.P.); National Health and Medical Research Council of Australia (ICRG ID 283321 and program grants 290208 and 323229 to the study, practitioner fellowship to N.M.A., and senior fellowship to M.P.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–36. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(Suppl 6):79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.Polson HE, Conway DJ, Fandeur T, Mercereau-Puijalon O, Longacre S. Gene polymorphism of Plasmodium falciparum merozoite surface proteins 4 and 5. Mol Biochem Parasitol. 2005;142:110–5. doi: 10.1016/j.molbiopara.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Black CG, Wang L, Hibbs AR, Coppel RL. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol Biochem Parasitol. 1999;103:243–50. doi: 10.1016/s0166-6851(99)00134-6. [DOI] [PubMed] [Google Scholar]
- 6.Gomez A, Suarez CF, Martinez P, Saravia C, Patarroyo MA. High polymorphism in Plasmodium vivax merozoite surface protein-5 (MSP5) Parasitology. 2006;133:661–72. doi: 10.1017/S0031182006001168. [DOI] [PubMed] [Google Scholar]
- 7.Black CG, Barnwell JW, Huber CS, Galinski MR, Coppel RL. The Plasmodium vivax homologues of merozoite surface proteins 4 and 5 from Plasmodium falciparum are expressed at different locations in the merozoite. Mol Biochem Parasitol. 2002;120:215–24. doi: 10.1016/s0166-6851(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 8.Bodescot M, Silvie O, Siau A, et al. Transcription status of vaccine candidate genes of Plasmodium falciparum during the hepatic phase of its life cycle. Parasitol Res. 2004;92:449–52. doi: 10.1007/s00436-003-1061-9. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant Plasmodium falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall VM, Tieqiao W, Coppel RL. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol Biochem Parasitol. 1998;94:13–25. doi: 10.1016/s0166-6851(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 11.Black CG, Wang L, Hibbs AR, Werner E, Coppel RL. Identification of the Plasmodium chabaudi homologue of merozoite surface proteins 4 and 5 of Plasmodium falciparum. Infect Immun. 1999;67:2075–81. doi: 10.1128/iai.67.5.2075-2081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon IK, Angov E, Larson D, Heppner DG, Cummings JF, Stewart VA. Characterization of a human reference standard for antibody to Plasmodium falciparum merozoite surface protein 142. Am J Trop Med Hyg. 2005;72:714–8. [PubMed] [Google Scholar]
- 13.Aribot G, Rogier C, Sarthou JL, et al. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa) Am J Trop Med Hyg. 1996;54:449–57. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 14.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–81. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck HP, Felger I, Genton B, et al. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect Immun. 1995;63:596–600. doi: 10.1128/iai.63.2.596-600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun. 2004;72:247–52. doi: 10.1128/IAI.72.1.247-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratcliff A, Siswantoro H, Kenangalem E, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–9. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wipasa J, Xu H, Makobongo M, Gatton M, Stowers A, Good MF. Nature and specificity of the required protective immune response that develops postchallenge in mice vaccinated with the 19-kilodalton fragment of Plasmodium yoelii merozoite surface protein 1. Infect Immun. 2002;70:6013–20. doi: 10.1128/IAI.70.11.6013-6020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braga EM, Barros RM, Reis TA, et al. Association of the IgG response to Plasmodium falciparum merozoite protein (C-terminal 19 kD) with clinical immunity to malaria in the Brazilian Amazon region. Am J Trop Med Hyg. 2002;66:461–6. doi: 10.4269/ajtmh.2002.66.461. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–13. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Richie TL, Stowers A, Nhan DH, Coppel RL. Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect Immun. 2001;69:4390–7. doi: 10.1128/IAI.69.7.4390-4397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Crouch L, Richie TL, Nhan DH, Coppel RL. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 2003;25:403–12. doi: 10.1111/j.1365-3024.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 24.Boutlis CS, Fagan PK, Gowda DC, et al. Immunoglobulin G (IgG) responses to Plasmodium falciparum glycosylphosphatidylinositols are short-lived and predominantly of the IgG3 subclass. J Infect Dis. 2003;187:862–5. doi: 10.1086/367897. [DOI] [PubMed] [Google Scholar]
- 25.Cavanagh DR, Dobano C, Elhassan IM, et al. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect Immun. 2001;69:1207–11. doi: 10.1128/IAI.69.2.1207-1211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tongren JE, Drakeley CJ, McDonald SL, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–64. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garraud O, Perraut R, Riveau G, Nutman TB. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 2003;19:300–4. doi: 10.1016/s1471-4922(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 28.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990;141:529–42. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- 29.Aucan C, Traore Y, Tall F, et al. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68:1252–8. doi: 10.1128/iai.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonma P, Christensen PR, Suwanarusk R, Price RN, Russell B, Lek-Uthai U. Comparison of three molecular methods for the detection and speciation of Plasmodium vivax and Plasmodium falciparum. Malar J. 2007;6:124. doi: 10.1186/1475-2875-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giha HA, Staalsoe T, Dodoo D, et al. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun. 1999;67:4092–8. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorfman JR, Bejon P, Ndungu FM, et al. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;191:1623–30. doi: 10.1086/429671. [DOI] [PubMed] [Google Scholar]