Abstract
Lipid metabolism is a continuum from emulsification and uptake of lipids in the intestine to cellular uptake and transport to compartments such as mitochondria. Whether fats are shuttled into lipid droplets in adipose tissue or oxidized in mitochondria and peroxisomes depends on metabolic substrate availability, energy balance and endocrine signaling of the organism. Several members of the nuclear hormone receptor superfamily are lipid-sensing factors that affect all aspects of lipid metabolism. The physiologic actions of glandular hormones (e.g. thyroid, mineralocorticoid and glucocorticoid), vitamins (e.g. vitamins A and D) and reproductive hormones (e.g. progesterone, estrogen and testosterone) and their cognate receptors are well established. The peroxisome proliferator activated receptors (PPARs) and Liver X receptors (LXRs), acting in concert with PPARγ Coactivator 1α (PGC-1α), have been shown to regulate insulin sensitivity and lipid handling. These receptors are the focus of intense pharmacologic studies to expand the armamentarium of small molecule ligands to treat diabetes and the metabolic syndrome (hypertension, insulin resistance, hyperglycemia, dyslipidemia, and obesity). Recently, additional partners of PGC-1α have moved to the forefront of metabolic research, the Estrogen-related Receptors (ERRs). Although no endogenous ligands for these receptors have been identified, phenotypic analyses of knockout mouse models demonstrate an important role for these molecules in substrate sensing and handling as well as mitochondrial function.
Keywords: Estrogen-related receptor, Peroxisome-proliferator associated receptor, liver, X receptor, mitochondria, lipid metabolism
Nuclear Hormone Receptors
Nuclear hormone receptors (NRs) generally function as ligand-activated transcription factors that regulate the expression of specific genes related to reproduction, development and metabolism. Conserved functional domains of NRs, such as the DNA binding domain (DBD), ligand binding domain (LBD), N- and C-terminal transcriptional activation domains (AF-1 and AF-2, respectively) define this class of transcription factors (Evans 1988). Their disparate physiologic roles are determined by diversity in both ligand and DNA binding specificities, as well as in specific interactions with co-activator and co-repressor molecules that combinatorially mediate transcription (Glass and Rosenfeld 2000; Kressler et al. 2002). Models of transcriptional activity are dominated by conformational changes in chromatin that alter access of permissive factors to promoter regions and subsequently nucleate transcriptional machinery (Chen et al. 2001). NRs classically affect these processes as cis-acting ligand-responsive switches to facilitate nucleation of factors to enhance or repress transcription. However, evidence suggests that in the absence of ligand, transcriptionally inactive NRs are not passive and may act in trans to sequester factors involved in other transcriptional pathways—expanding the physiologic role of NRs beyond either chromatin or ligand binding (Reichardt et al. 1998; Lee et al. 2003; Ogawa et al. 2004).
To date, 48 and 49 members of the NR superfamily have been identified in humans and mice, respectively (Mangelsdorf et al. 1995; Bookout et al. 2006). The first receptors to be cloned, the endocrine nuclear hormone receptors, were discovered in an effort to define the mechanism of action of known hormones, such as: the amino-acid-derived thyroid hormone; the steroidal glucocorticoid, mineralocorticoid, and sex hormones; and the vitamins A and D. Several of these endocrine receptors, e.g. thyroid (TR), estrogen, (ER) and Vitamin D (VDR) receptors have established or emerging roles for influencing metabolic and mitochondrial function, reviewed elsewhere (Weitzel et al. 2003; Chen et al. 2005; Roy et al. 2007). Subsequent to the molecular identification of classical endocrine receptors, additional members of the nuclear hormone receptor superfamily, so called “adopted orphan receptors”, were discovered to respond with low-affinity to physiologic ligands derived from dietary and metabolic sources, such as bile salts, fatty acids and eicasanoids, present in concentrations (micromolar) orders of magnitude higher than classic endocrine hormones (nanomolar). Both the endocrine and adopted orphan receptors may have evolved from a phylogenetically ancient transcriptional regulator that underwent multiple duplications and divergences. As a result, the NR superfamily is divided into two broad groups: 1) the transcription factors for which physiologic ligand-dependent activities have been identified; and 2) factors for which physiologic ligands have yet to be identified: the orphan nuclear receptors (Escriva et al. 2004). Unlike the endocrine receptors that generally translocate from the cytosol to the nucleus upon ligand binding, the orphan and adopted orphan receptors tend to be constitutively nuclear. Because most receptors appear to be physically capable of binding small molecules and profoundly influence metabolic function, there has been great interest from both biomedical research and the pharmaceutical industry to control their function in treating endocrine and metabolic diseases (Chawla et al. 2001).
Metabolic Control by NRs
Today one in three adults in the US is obese—an increase of 75% over the last 25 years (Flegal et al. 2002). In addition metabolic disorders associated with obesity have risen, such as hypertension, dyslipidemia, insulin resistance and glucose intolerance that are components of the metabolic syndrome. These endocrine disturbances increase cardiovascular risk and all-cause mortality by 6-7% (Ford 2005). Over the next fifty years, life expectancy may level off or decline due to the health burden of obesity-related illness presenting in younger populations, as well (Olshansky et al. 2005). To address these impacts, public health awareness of meal choices and portions, and physical exercise are foremost. However, for those already affected and refractory to diet and exercise changes, pharmacologic modification of metabolism provides an opportunity to prevent mortality and morbidity associated with diabetes, cardiovascular disease and the metabolic syndrome (Evans et al. 2004; Barish et al. 2006).
While the metabolic syndrome is multifactorial, insulin resistance may be at its core (Eckel et al. 2005; Grundy 2005). Insulin resistance is associated with altered glucose production in liver, elevated blood glucose and insulin, and reduced glucose uptake in fat and muscle by insulin. Less well appreciated are the derangements in lipids that occur. Free-fatty acids (FFAs) are elevated in circulation and are taken up by insulin target tissues where they may directly interfere with insulin signaling (Medina-Gomez et al. 2007). Furthermore, the adipose depots elaborate inflammatory adipokines and cytokines that negatively impact insulin sensitivity and systemic metabolism (Kershaw and Flier 2004).
The increased prevalence of obesity and associated metabolic syndrome has driven intense interest in nuclear receptors that can favorably alter body composition, lipid profiles and insulin sensitivity. At the forefront of pharmacologic treatments of metabolic syndrome are the PPARs that serve as ligands for broadly used fibrates and thiazolidinediones, as well as promising pre-clinical compounds. In addition, LXRs and the orphan receptors ERRs appear to be viable targets for metabolic disorders. However the interactions of these molecules and the side effects of their pharmacologic activation are complex and will require additional explanation before long term treatments are implemented. This is highlighted in chronic treatments that influence inflammation which may produce untoward consequences as seen with glucocorticoids, COX inhibitors (e.g. rofecoxib/Vioxx) and possibly rosiglitazone (Avandia; Lago et al. 2007).
PGC-1α
Transcription factors, including nuclear receptors, are highly dependent on coactivator molecules to affect transcriptional control of physiologic processes. Mitochondrial biogenesis and respiration are highly dependent on the transcriptional coactivators, PGC-1α and β, and their interacting factors (Puigserver et al. 1998; Wu et al. 1999; Lehman et al. 2000; Lin et al. 2002; Kelly and Scarpulla 2004; Arany et al. 2007; Sonoda et al. 2007). In addition, many physiologic, hormonal and dietary cues are transduced into adaptive protein changes via the transcriptional coactivator PGC-1α. The activities are most notable in highly metabolic tissues, such as brown adipose tissue, heart, skeletal muscle, liver and the CNS, where PGC-1a regulates the central characteristics of these differentiated tissues: thermogenesis, contractile force, oxidative fiber types, gluconeogenesis and beta-oxidation, and emerging roles in the CNS such as ROS adaptation and apoptosis. (Puigserver et al. 1998; Herzig et al. 2001; Lin et al. 2002; Puigserver et al. 2003; Rhee et al. 2003; Yoon et al. 2003; Arany et al. 2005; St-Pierre et al. 2006; Handschin et al. 2007). While named for its interaction with PPARγ, many of these PGC-1-dependent adaptations appear to be largely dependent on ERRs and other metabolic transcription factors that are independent of PPAR/RXR (Puigserver et al. 2003; Rhee et al. 2003; Schilling et al. 2006; Alaynick et al. 2007; Huss et al. 2007; Villena et al. 2007).
PPARs
There are three isoforms of PPAR: PPAR, PPAR and PPAR. Expression of PPAR is greatest in tissues with active metabolism, such as BAT, liver, striated muscle, and kidney (Bookout et al. 2006). PPAR has a very broad expression pattern that made identifying its role more difficult. PPAR is highly expressed in fat, colon, placenta and macrophage (Rosen and Spiegelman 2000; Barish et al. 2005). Importantly, the PPARs act as obligate heterodimers with RXR to bind PPAR response elements consisting of direct repeats of AGGTCA separated by one nucleotide (DR1). PPARs have a large, promiscuous ligand binding pocket and in addition, are subject to RXR ligand activation by 9-cis-retinoic acid and synthetic agonists (Nolte et al. 1998; Xu et al. 1999).
Among the adopted orphan receptors, the most clinical knowledge has been gained about the physiology and mechanistic action of PPARs (Berger and Moller 2002). Currently, two PPAR agonist classes are in widespread use, the thiazolidinediones (TZDs) which activate PPAR and increase insulin sensitivity, and the fibrates (gemfibrozil, fenofibrate) which activate PPAR to reduce hepatic triglyceride production and increase hepatic fatty acid oxidation (Harris and Kletzien 1994; Forman et al. 1995; Lehmann et al. 1995; Willson et al. 1996a). In vivo, PPAR and ligands appear to be naturally occurring fatty acids and eicosanoid arachidonic acid metabolites (Forman et al. 1997). Naturally occurring PPAR ligands appear to be nitrolinoleic acid and possibly prostaglandin J2 (Kliewer et al. 1995; Willson et al. 1996b; Schopfer et al. 2005). As these compounds are active at relatively high concentrations, some debate exists as to identity of bone fide PPAR ligands.
PPAR
PPAR controls the expression of several genes involved in fatty acid metabolism from transport across the cell membrane, intracellular binding (liver FABP), formation of acyl-CoA (FASN), to mitochondrial and peroxisomal β-oxidation, as well as microsomal ω-oxidation (ACOX, CYP4A1 and CYP4A6 genes, ACADM, and HMGCS1 and 2) (Desvergne and Wahli 1999). During a fast, when increased utilization of free fatty occurs, PPARα expression and activity promotes increased β-oxidation. In fact, while PPARα mice are relatively healthy when fed ad libitum, they have very poor tolerance for fasting and develop hypoglycemia, hypoketonemia and hypothermia (Kersten et al. 1999; Leone et al. 1999). In skeletal muscle, loss of PPARα is relatively mild, suggesting PPARδ and perhaps other factors may compensate (Muoio et al. 2002). However, PPARα is induced after exercise in human—as are some of its target genes (Horowitz et al. 2000). In the heart, which derives 70% of its energy from lipid in the adult, PPARα expression correlates with the fetal to adult transition, as does PGC-1α (Lehman and Kelly 2002). Furthermore, expression of PPARα is downregulated during pathological cardiac hypertrophy when a fetal metabolic preference for carbohydrate is reiterated and fatty acid oxidation declines (Barger and Kelly 2000).
PPAR
While loss of PPARγ results in a developmental elimination of adipose tissue in mouse, its role in mature adipocytes is less clear, although it is essential for adipocyte survival (Barak et al. 1999; He et al. 2003). Not only a repository of triglycerides, adipose-derived endocrine signals have profound global actions on metabolism by altering insulin sensitivity across tissues. Two isoforms of PPARγ, PPARγ1 (colon, retina, spleen, hematopoietic cells, liver, and skeletal muscle) and PPARγ2 (adipose) can respond to the same signals and activate the same target genes: aP2, LPL, ACS and CD36 (Rosen and Spiegelman 2001; Allen et al. 2006). The importance of PPARγ outside the adipose tissue is highlighted by mice with skeletal muscle-specific loss of PPARγ that had 80% reductions in insulin-stimulated glucose disposal rates that did not improve with TZD treatment (Hevener et al. 2003). Other muscle-specific studies, with different PPARγ mutations, showed some TZD sensitivity (Norris et al. 2003).
PPAR
The actions of PPARδ appear to be more similar to PPARα than PPARγ in favoring the oxidation of fats. In cell culture, UCP2, UCP3, H-FABP, FAT/CD36, LPL, ACS and CPT1 were demonstrated to be PPARδ target genes (Muoio et al. 2002; Dressel et al. 2003). Overexpression of PPARδ in fat produced a lean phenotype due to increased oxidation of fats in adipocytes and resulted in white adipose tissue (WAT) that took on some brown adipose tissue (BAT) characteristics (Wang et al. 2003) Cardiac metabolism is dependent on PPARδ and cardiomyocyte specific loss of PPARδ in mouse results in cardiomyopathy and reduced expression of β-oxidation genes (Cheng et al. 2004). Consistently, ligand treatment (GW610742X) helped preserve β-oxidation in a rat model of congestive heart failure (Jucker et al. 2007). Overexpression of VP16-PPARδ in skeletal muscle has profound effects, converting untrained animals into “marathon mice” with a substantial conversion of muscle to a slow-twitch form (Wang et al. 2004). While genetic introduction of VP16-PPARδ presents an extreme case, some components of this phenotype are reiterated by ligand treatments and exercise that hint at effective treatments for metabolic syndrome mediated by PPARδ (Tanaka et al. 2003; Barish et al. 2006). For instance, patients with poor responsiveness to exercise and dietary changes may benefit from a ‘jump start’ in the form of PPAR agonists.
LXRs
Two additional RXR partners that can alter lipid handling are the receptors, LXR and LXR. Expression of LXR is greatest in fat, liver and macrophage (Repa and Mangelsdorf 2000; Bookout et al. 2006). In contrast, LXR is widely expressed. In vivo, LXRs are activated by physiologic concentrations of 22(R)-, 24(S)-, and 27-sterol metabolites in addition to 24(S),25-hydroxycholesterol ligands (Janowski et al. 1996; Lehmann et al. 1997; Fu et al. 2001). Constitutively nuclear LXR/RXR partners bind LXR response elements of two AGGTCA repeats separated by four nucleotides (DR-4).
Several target genes for LXR, many of which are involved in cholesterol and fatty acid metabolism indicate that LXRs serve as global cholesterol sensors (Peet et al. 1998; Tontonoz and Mangelsdorf 2003). This is dramatically illustrated by CYP7A1, the rate limiting enzyme of bile synthesis in the liver, which is an LXR target gene. LXR-knockout mice have reduced Cyp7a1 expression and develop massive hepatic accumulations of cholesterol when challenged with a high-cholesterol diet (Peet et al. 1998). In contrast, LXR mice are relatively normal. Yet double knockout mice (LXR -/-, -/-) have a greater accumulation of cholesterol, suggesting some functional overlap (Alberti et al. 2001). Pharmacologic activation of LXRs activates lipogenic genes, sterol regulatory element binding protein 1c, SREBP-1c; fatty acid synthase, FAS; and steroyl-CoA desaturase 1, SCD-1—and elevates both hepatic and plasma triglyceride levels (Peet et al. 1998; Schultz et al. 2000).
RXRs
Because RXRs form obligate heterodimers with PPARs, LXRs, and other NRs, they have far reaching actions. There are three isoforms of RXR: RXRα, RXRβ, and RXRγ (Bookout et al. 2006). RXRα is broadly expressed with highest expression in the liver. RXRβ is also broadly expressed with relatively low expression in liver, while RXRγ has a more limited distribution with highest expression in striated muscle. Pharmacologic activation of RXRs by small molecule ligands or rexinoids may provide another avenue of intervention in the metabolic diseases of diabetes and obesity (Claudel et al. 2001; Desreumaux et al. 2001; Shulman and Mangelsdorf 2005). While rexinoids can mimic some of the ligand-dependent actions of their partners (e.g. PPARγ agonists pioglitazone and rosiglitazone) by increasing insulin sensitivity, they suppress the actions of thyroid hormone, increase triglycerides and decrease, rather than increase, body and fat mass (Mukherjee et al. 1997; Liu et al. 2002; Ferre 2004; Haffner et al. 2004; Ogilvie et al. 2004; Li et al. 2005). For example, treatment with the PPARγ-agonist, rosiglitazone, downregulated TNFα and upregulated GLUT4, MCP-1, SCD1 and CD36, while the rexinoid LG268 increased TNFα and had no effect or suppressed other genes in mouse (Singh Ahuja et al. 2001).
ERRs
The first orphan receptors to be cloned, ERRα and ERRβ, were joined years later by the identification of ERRγ (Giguere et al. 1988; Hong et al. 1999). These receptors all share an extended half-site motif TCAAGGTCA and can bind as monomers, dimers or heterodimers (Dufour et al. 2007). Because these receptors have very small ligand biding pockets, endogenous ligand discovery remains unlikely, although synthetic ligands have been developed and ERRα is blocked by diethylstilbestrol, while ERRβ and γ are blocked by tamoxifen (Coward et al. 2001; Greschik et al. 2002; Willy et al. 2004). The activity of ERRs appears to be constitutive, with the ligand binding pocket stably arranged in an active conformation without ligand (Xie et al. 1999; Greschik et al. 2002; Greschik et al. 2004). Because ERRα is widely expressed in adult tissues, especially in tissues that utilize or can utilize fatty acid β-oxidation, many studies have addressed its role in cellular energetics (Luo et al. 2003). Loss of ERRβ results in placental defects and mid-gestational death of embryos, despite relatively limited expression in adult (Luo et al. 1997). ERRγ is highly expressed in tissues with high metabolic activity (e.g. heart, kidney, slow-twitch muscle, BAT and CNS) and loss of this receptor in mouse results in neonatal death, presumably due to motor defects that prevent feeding (Alaynick et al. 2007; Dufour et al. 2007).
ERRα
ERRα is involved in many aspects of lipid metabolism, likely by mediating actions of PGC-1α and -β (Kamei et al. 2003; Schreiber et al. 2003; Schreiber et al. 2004; Rodriguez-Calvo et al. 2006; Sonoda et al. 2007). In the mouse intestine, ERRα controls the ApoA-IV promoter and loss results in reduced uptake of lipids (Luo et al. 2003; Carrier et al. 2004). At the cellular level, defects in oxidative metabolism have been seen in ERRα null intestine, skeletal muscle, heart, and BAT (Carrier et al. 2004; Rodriguez-Calvo et al. 2006; Dufour et al. 2007; Huss et al. 2007; Villena et al. 2007). Several target genes have been identified by these studies including Ckmt2, Mcad, Sdha and Sdhb (Dufour et al. 2007).
ERRβ
Little is known about the physiologic role of ERRβ due to the confounds of mid-gestational lethality and placental defects. Tetraploid rescue experiments have produced adult animals with severe motor defects (Mitsunaga et al. 2004). Use of a floxed ERRβ allele and Sox2∷Cre (which is not expressed in extraembryonic tissues) allowed the Mendelian generation of adult animals that displayed defects in inner ear morphology and endolymph formation (Chen and Nathans 2007). Perhaps the most surprising aspect of these and other nuclear receptor knockout mice is that despite similar DNA element affinities and coactivator interactions, in vivo compensation appears to be limited or absent. PPARγ, ERRγ and ERRβ all have lethal phenotypes that cannot be corrected by the remaining isoforms. However, it remains possible that pharmacologic perturbation of one receptor could be used to address processes controlled by related receptors. Given the roles of ERRα and ERRγ in mitochondrial and lipid metabolism and similarity of ERRs, ERRβ may play a yet unappreciated role in metabolic function.
ERRγ
ERRγ expression is linked with tissues with high metabolic activities, and loss of this receptor is detrimental in tissues examined to date. In the heart, loss of ERRγ appears to prevent a perinatal transition from carbohydrate-based fetal metabolism to lipid-predominant adult metabolism (Alaynick et al. 2007). Furthermore, it appears that ERRα and ERRγ act in concert to direct the oxidative metabolic program in heart (Dufour et al. 2007). Surprisingly, while loss of ERRα is relatively mild, loss of ERRγ results in reduced ventricular mass and increased mitochondrial DNA, even in heterozygous animals (Alaynick et al. 2007). Myocyte specific elimination of ERRγ may bypass the neonatal lethality of these animals and allow examination of ERRγ-dependent changes in the adult heart and skeletal muscles. To what extent ERRγ bears the transcriptional signaling of PGC-1α in a given tissue, relative to other ERRs and PPARs, awaits further study, as well.
Conclusions
The complex actions of nuclear receptors in regulating lipid metabolism can appear counterintuitive or deceptively mild when studied in knockout mice. The phenotype is not simply loss of the receptor, however, but reflects the genome-wide compensatory reaction to that loss—especially in cases where very similar receptors remain. What occurs may represent a skewed stoichiometric ratio of target genes. As a consequence, levels of key enzymes and signaling molecules may exceed homeostatic safety margins where mild physiologic challenges or developmental transitions produce decompensation (Alaynick et al. 2007; Huss et al. 2007). Much remains to be learned about the actions of nuclear receptors from conditional loss of receptors by genetic methods as well as combinatorial uses of ligands to ‘dial-in’ metabolic treatments (Lalloyer et al. 2006; Schug et al. 2007).
The control of mitochondrial biogenesis and substrate utilization by these receptors is a complex issue as well. By mediating the action of PGC-1α and β, the PPARs and ERRs have global influences on mitochondria, increasing their numbers and oxidative capacities. How these receptors act combinatorially in specific cell types at particular times remains to be determined. It does appear however that each of these receptors controls a characteristic suite of genes that determine the genetic composition and metabolic function of mitochondria.
While diet and exercise often provide the best controls of metabolism, individuals who have pathologically, genetically, or behaviorally dysregulated metabolic function may benefit from treatments that activate nuclear receptor mediated metabolic pathways. By improving insulin sensitivity and lipid profiles, or synergistically improving exercise benefits, individuals may be better capable of avoiding a downward spiral of insulin-resistance and lipid accumulation.
Figure 1. The Nuclear Receptor Superfamily.
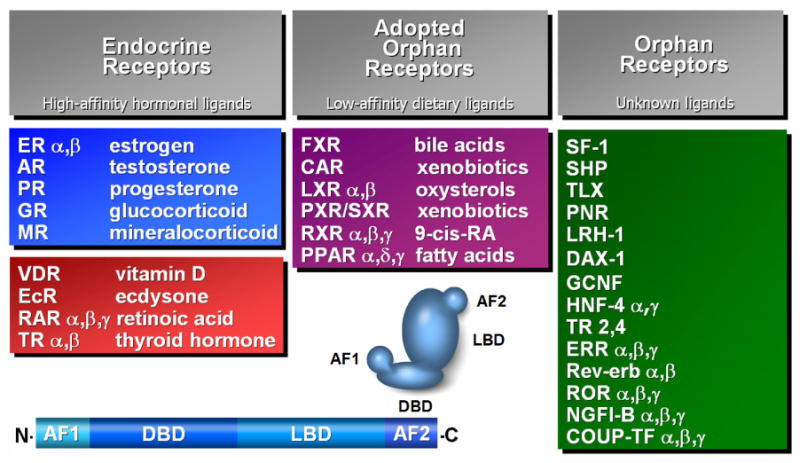
Nuclear hormone receptors can be divided into Endocrine, Adopted Orphan and Orphan subfamilies. The Endocrine receptors have high affinity ligands that are present in nanomolar concentrations, while Adopted orphan receptors have ligands present in the micromolar range. No physiologic ligands have been identified for the orphan receptors. Nuclear receptors share a common arrangement of an amino terminal Activation Function 1 (AF1), a DNA-Binding Domain (DBD), a Ligand Binding Domain (LBD) and a carboxy-terminal Activation Function 2 (AF2). Adapted from Chawla et al. 2001.
Figure 2. Nuclear Receptor Regulation of Metabolic Enzymes.
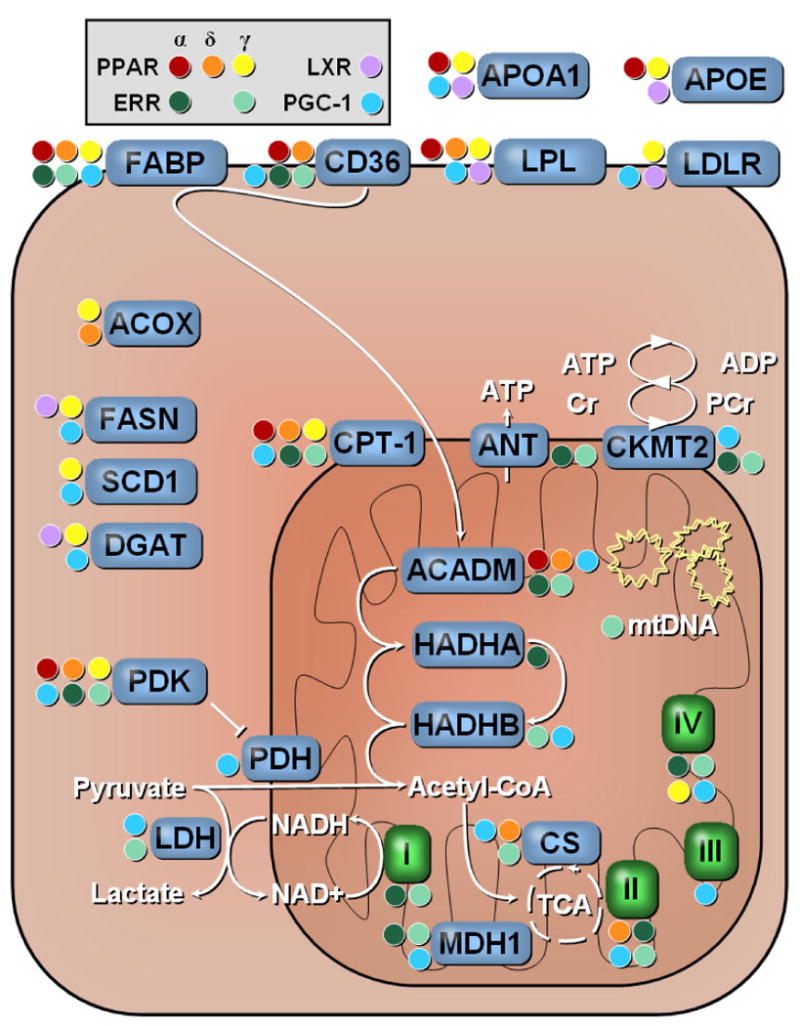
The PPARs, ERRs and LXRs can influence the expression of several genes involved in lipid metabolism. Regulated processes include lipoprotein metabolism, fatty acid uptake, shuttling into mitochondria and fatty acid oxidation. Color-coded circles indicate regulation by a given receptor. I-IV, Electron transport chain complexes; ACADM, acyl-Coenzyme A dehydrogenase, C-4 to C-12 straight chain; ACOX, acyl-Coenzyme A oxidase; ANT, adenine nucleotide translocator; APOA1, apolipoprotein A-I; APOE, apolipoprotein E; CD36, scavenger receptor class B; CKMT2, creatine kinase, mitochondrial 2; CPT-1, carnitine palmitoyltransferase; CS, citrate synthase; DGAT, diacylglycerol O-acyltransferase; FABP, fatty-acid binding protein; FASN, fatty-acid synthase; HADHA, trifunctional protein, alpha subunit; HADHB, trifunctional protein, beta subunit; LDH, lactate dehydrogenase; LDLR, low density lipoprotein receptor; LPL, lipoprotein lipase; MDH1, malate dehydrogenase; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase. Adapted from Alaynick et al. 2007.
Acknowledgments
I would like to thank Ronald Evans and members of the Evans lab, Grant Barish, Michael Downes and Benjamin Gallarda, for valuable comments and critical reading of the manuscript. I apologize to authors and relevant publications which were not cited in this manuscript due to size constraints.
Abbreviations
- ACADM
acyl-Coenzyme A dehydrogenase, C-4 to C-12 straight chain
- ACOX
acyl-Coenzyme A oxidase
- ANT
adenine nucleotide translocator
- APOA1
apolipoprotein A-I
- APOE
apolipoprotein E
- CD36
scavenger receptor class B
- CKMT2
creatine kinase, mitochondrial 2
- CPT-1
carnitine palmitoyltransferase
- CS
citrate synthase
- DGAT
diacylglycerol O-acyltransferase
- ERR
estrogen-related receptor
- FABP
fatty acid binding protein
- FASN
fatty-acid synthase
- FFA
free-fatty acid
- HADHA
trifunctional protein, alpha subunit
- HADHB
trifunctional protein, beta subunit
- LDH
lactate dehydrogenase
- LDLR
low density lipoprotein receptor
- LPL
lipoprotein lipase
- LXR
liver X receptor
- MDH1
malate dehydrogenase
- PCG
PPAR gamma coactivator
- PPAR
peroxisome proliferator associated receptor
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6(1):13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest. 2001;107(5):565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T, Zhang F, Moodie SA, Clemens LE, Smith A, Gregoire F, Bell A, Muscat GE, Gustafson TA. Halofenate is a selective peroxisome proliferator-activated receptor gamma modulator with antidiabetic activity. Diabetes. 2006;55(9):2523–2533. doi: 10.2337/db06-0618. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1(4):259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10(6):238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19(10):2466–2477. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116(3):590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier JC, Deblois G, Champigny C, Levy E, Giguere V. Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem. 2004;279(50):52052–52058. doi: 10.1074/jbc.M410337200. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chen H, Tini M, Evans RM. HATs on and beyond chromatin. Curr Opin Cell Biol. 2001;13(2):218–224. doi: 10.1016/s0955-0674(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Nathans J. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13(3):325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746(1):1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10(11):1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- Claudel T, Leibowitz MD, Fievet C, Tailleux A, Wagner B, Repa JJ, Torpier G, Lobaccaro JM, Paterniti JR, Mangelsdorf DJ, Heyman RA, Auwerx J. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A. 2001;98(5):2610–2615. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci U S A. 2001;98(15):8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K, Derijard B, Desvergne B, Wahli W, Chambon P, Leibowitz MD, Colombel JF, Auwerx J. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med. 2001;193(7):827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17(12):2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5(5):345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53 1:S43–50. doi: 10.2337/diabetes.53.2007.s43. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276(42):38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121–141. [PubMed] [Google Scholar]
- Greschik H, Flaig R, Renaud JP, Moras D. Structural basis for the deactivation of the estrogen-related receptor gamma by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity. J Biol Chem. 2004;279(32):33639–33646. doi: 10.1074/jbc.M402195200. [DOI] [PubMed] [Google Scholar]
- Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, Renaud JP. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell. 2002;9(2):303–313. doi: 10.1016/s1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Point: the metabolic syndrome still lives. Clin Chem. 2005;51(8):1352–1354. doi: 10.1373/clinchem.2005.050989. [DOI] [PubMed] [Google Scholar]
- Haffner CD, Lenhard JM, Miller AB, McDougald DL, Dwornik K, Ittoop OR, Gampe RT, Jr, Xu HE, Blanchard S, Montana VG, Consler TG, Bledsoe RK, Ayscue A, Croom D. Structure-based design of potent retinoid X receptor alpha agonists. J Med Chem. 2004;47(8):2010–2029. doi: 10.1021/jm030565g. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance and myopathy in PGC-1alpha muscle-specific knockout animals. J Biol Chem. 2007 doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Harris PK, Kletzien RF. Localization of a pioglitazone response element in the adipocyte fatty acid-binding protein gene. Mol Pharmacol. 1994;45(3):439–445. [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9(12):1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274(32):22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279(2):E348–355. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6(1):25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Jucker BM, Doe CP, Schnackenberg CG, Olzinski AR, Maniscalco K, Williams C, Hu TC, Lenhard SC, Costell M, Bernard R, Sarov-Blat L, Steplewski K, Willette RN. PPARdelta activation normalizes cardiac substrate metabolism and reduces right ventricular hypertrophy in congestive heart failure. J Cardiovasc Pharmacol. 2007;50(1):25–34. doi: 10.1097/FJC.0b013e31804b4163. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A. 2003;100(21):12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103(11):1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kressler D, Schreiber SN, Knutti D, Kralli A. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor alpha. J Biol Chem. 2002;277(16):13918–13925. doi: 10.1074/jbc.M201134200. [DOI] [PubMed] [Google Scholar]
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Fievet C, Lestavel S, Torpier G, van der Veen J, Touche V, Bultel S, Yous S, Kuipers F, Paumelle R, Fruchart JC, Staels B, Tailleux A. The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2006;26(12):2731–2737. doi: 10.1161/01.ATV.0000248101.93488.84. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302(5644):453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106(7):847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29(4):339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272(6):3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96(13):7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hansen PA, Xi L, Chandraratna RA, Burant CF. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J Biol Chem. 2005;280(46):38317–38327. doi: 10.1074/jbc.M505853200. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu S, Ogilvie KM, Klausing K, Lawson MA, Jolley D, Li D, Bilakovics J, Pascual B, Hein N, Urcan M, Leibowitz MD. Mechanism of selective retinoid X receptor agonist-induced hypothyroidism in the rat. Endocrinology. 2002;143(8):2880–2885. doi: 10.1210/endo.143.8.8930. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388(6644):778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol. 2003;23(22):7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppanen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4):e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga K, Araki K, Mizusaki H, Morohashi K, Haruna K, Nakagata N, Giguere V, Yamamura K, Abe K. Loss of PGC-specific expression of the orphan nuclear receptor ERR-beta results in reduction of germ cell number in mouse embryos. Mech Dev. 2004;121(3):237–246. doi: 10.1016/j.mod.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386(6623):407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem. 2002;277(29):26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395(6698):137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112(4):608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose DW, Johnson RS, Rosenfeld MG, Glass CK. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101(40):14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Saladin R, Nagy TR, Urcan MS, Heyman RA, Leibowitz MD. Activation of the retinoid X receptor suppresses appetite in the rat. Endocrinology. 2004;145(2):565–573. doi: 10.1210/en.2003-0907. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci U S A. 2003;100(7):4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Calvo R, Jove M, Coll T, Camins A, Sanchez RM, Alegret M, Merlos M, Pallas M, Laguna JC, Vazquez-Carrera M. PGC-1beta down-regulation is associated with reduced ERRalpha activity and MCAD expression in skeletal muscle of senescence-accelerated mice. J Gerontol A Biol Sci Med Sci. 2006;61(8):773–780. doi: 10.1093/gerona/61.8.773. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Roy D, Felty Q, Narayan S, Jayakar P. Signature of mitochondria of steroidal hormones-dependent normal and cancer cells: potential molecular targets for cancer therapy. Front Biosci. 2007;12:154–173. doi: 10.2741/2056. [DOI] [PubMed] [Google Scholar]
- Schilling MM, Oeser JK, Boustead JN, Flemming BP, O'Brien RM. Gluconeogenesis: re-evaluating the FOXO1-PGC-1alpha connection. Nature. 2006;443(7111):E10–11. doi: 10.1038/nature05288. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101(17):6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J Biol Chem. 2003;278(11):9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14(22):2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman AI, Mangelsdorf DJ. Retinoid x receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353(6):604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- Singh Ahuja H, Liu S, Crombie DL, Boehm M, Leibowitz MD, Heyman RA, Depre C, Nagy L, Tontonoz P, Davies PJ. Differential effects of rexinoids and thiazolidinediones on metabolic gene expression in diabetic rodents. Mol Pharmacol. 2001;59(4):765–773. doi: 10.1124/mol.59.4.765. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104(12):5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100(26):15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17(6):985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A. 2007;104(4):1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol. 2003;88(1):121–128. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996a;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- Willson TM, Lehmann JM, Kliewer SA. Discovery of ligands for the nuclear peroxisome proliferator-activated receptors. Ann N Y Acad Sci. 1996b;804:276–283. doi: 10.1111/j.1749-6632.1996.tb18622.x. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Murray IR, Qian J, Busch BB, Stevens WC, Jr, Martin R, Mohan R, Zhou S, Ordentlich P, Wei P, Sapp DW, Horlick RA, Heyman RA, Schulman IG. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc Natl Acad Sci U S A. 2004;101(24):8912–8917. doi: 10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol. 1999;13(12):2151–2162. doi: 10.1210/mend.13.12.0381. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3(3):397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev Cell. 2003;5(1):73–83. doi: 10.1016/s1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]


