Abstract
The maintenance of centromeric heterochromatin in fission yeast relies on the RNA interference-dependent complexes RITS (RNA-induced transcriptional silencing complex) and RDRC (RNA-directed RNA polymerase complex), which cooperate in a positive feedback loop to recruit high levels of histone H3 K9 methyltransferase activity to centromeres and to promote the assembly and maintenance of centromeric heterochromatin. However, it is unclear how these complexes are targeted to chromatin. RITS comprises Chp1, which binds K9-methylated histone H3; Ago1, which binds short interfering (siRNAs); the adaptor protein Tas3, which links Ago1 to Chp1; and centromeric siRNAs. We have generated mutants in RITS to determine the contribution of the two potential chromatin-targeting proteins Chp1 and Ago1 to the centromeric recruitment of RITS. Mutations in Tas3 that disrupt Ago1 binding are permissive for RITS recruitment and maintain centromeric heterochromatin, but the role of Tas3's interaction with Chp1 is unknown. Here, we define the Chp1 interaction domain of Tas3. A strain expressing a tas3 mutant that cannot bind Chp1 (Tas3Δ10-24) failed to maintain centromeric heterochromatin, with a loss of centromeric siRNAs, a failure to recruit RITS and RDRC to centromeres, and high levels of chromosome loss. These findings suggest a pivotal role for Chp1 and its association with Tas3 for the recruitment of RITS, RDRC, and histone H3 K9 methyltransferase activity to centromeres.
In eukaryotes, the assembly of chromosomal domains into heterochromatic structures contributes to the maintenance of genomic integrity. In particular, heterochromatin at telomeres prevents homologous recombination and is required for telomeric length regulation (6), and heterochromatin at centromeres contributes to the accuracy of chromosome transmission during cell division (40). Posttranslational modifications of histone tails play a crucial role in the assembly of higher-order chromatin structures, and heterochromatin is characterized by histone hypoacetylation and an enrichment of methyl marks on histones associated with repression. These are evolutionarily conserved marks, and both the enzymes that produce these modifications and the proteins that recognize them are highly conserved from fission yeast to humans (25). The methylation on lysine 9 (K9) of histone H3 is a key event in the assembly of heterochromatin (4, 26, 41). In the fission yeast Schizosaccharomyces pombe, a single methyltransferase, Clr4, directs all methylation of K9 on histone H3 (50), whereas in mammals, several proteins, including Clr4's homologs, the Suv39 proteins, can methylate K9. These enzymes produce the methyl-marked histone (H3K9Me2/3) that is bound by the chromodomain of proteins such as HP1 and its fission yeast homolog, Swi6. The binding of Swi6/HP1 to H3K9Me2 is necessary for the loading of cohesin onto heterochromatic regions (5); thus, Swi6/HP1 and Clr4/Suv39 are crucial for the assembly of higher-order heterochromatin and for the maintenance of cohesion between the centromeres of sister chromatids.
S. pombe has several heterochromatic loci, including telomeres, centromeres, and the mating-type locus. The heterochromatin that assembles on these regions is similar, although the details of its assembly differ. Fission yeast centromeres range from 35 to 110 kb in length and are composed of a central domain on which the kinetochore assembles flanked by outer-repeat sequences, which are coated in heterochromatin that resembles the pericentromeric heterochromatin of mammals (40). At the mating-type locus and subtelomeric regions, there are sequences that share homology with the centromeric repeats (15, 18, 29).
Originally it was thought that fission yeast centromeres were transcriptionally inert, as a marker gene inserted within a centromeric sequence exhibited classical position-effect variegation (1, 2). This silencing was thought to reflect the spreading of heterochromatin over the gene, blocking the access of RNA polymerase II. However, recently it has been shown that centromeres are transcribed in both fission yeast and mammals (21, 27, 33, 49), and, ironically, that this transcription is required for the formation of heterochromatin by the cellular RNA interference (RNAi) apparatus (8, 14, 23, 49).
Fission yeast has a stripped-down RNAi machinery, with single genes encoding the key enzymes (49). Dicer (Dcr1) is the conserved RNase that cleaves double stranded RNA (dsRNA), and Argonaute (Ago1) is the conserved effector protein that binds short interfering RNA (siRNA) and mediates the sequence-specific destruction or inactivation of target (homologous) RNA (9, 19, 28, 44) and RNA-dependent RNA polymerase (Rdp1), which is required in Caenorhabditis elegans, plants, and fungi for the production of dsRNA to mediate the RNAi response (13, 30, 32, 43). Fission yeast that are defective for RNAi show an accumulation of centromeric transcripts, a loss of centromeric siRNAs, and a significant decrease in the levels of H3K9Me2 at centromeres (48, 49). Similar phenotypes are seen for mutants of Chp1, a chromodomain protein that localizes to heterochromatin (10, 36, 38, 39, 42). Chp1 was purified as a component of the RITS (RNA-induced transcriptional silencing complex) along with Ago1, centromeric siRNAs, and an uncharacterized protein, Tas3 (48). RITS weakly associates with a second RNAi effector complex, RDRC (RNA-dependent RNA polymerase complex), which includes Rdp1 (32). The localization of the two complexes is codependent and dependent on both Dcr1 and Clr4.
In the current model for centromeric heterochromatin assembly, RNA polymerase II preferentially transcribes one strand of centromeric sequence to form the short-lived pre-siRNA (14, 23, 49). Rdp1 uses this transcript as the template to synthesize dsRNA, which then is processed by Dicer to form siRNAs. These double-stranded siRNAs eventually are bound by RITS, and following the cleavage of the passenger strand (9), single-stranded siRNA is thought to program the sequence-dependent cleavage of nascent centromeric transcripts by Ago1 (19). The association of RITS and RDRC with centromeres thus triggers an RNAi-dependent positive feedback loop that amplifies centromeric transcripts in order to silence them and, through an unknown mechanism, promotes the recruitment of high levels of Clr4 methyltransferase activity to centromeres (32, 38, 45, 48). High levels of H3K9Me2 allow the maintenance of heterochromatin through the recruitment of other chromodomain proteins, such as Swi6, which are necessary for the spreading of heterochromatin (16).
There is considerable debate over how RITS is recruited to centromeres. RITS targeting initially was thought to occur via the sequence-dependent siRNA-mediated targeting of Ago1 to nascent transcripts or centromeric DNAs. Support for this model came from the loss of the association of RITS with centromeres in dcr1 null cells that lack siRNAs (48). However, these cells exhibit reduced levels of H3K9Me2 at centromeres (42, 49), which impacts the ability of RITS to associate via Chp1's chromodomain binding to H3K9Me2/3 (38). Support for the role of the Chp1 chromodomain in targeting the RITS complex comes from mutational studies, although such experiments are similarly complicated by their effects on siRNA metabolism and centromeric H3K9Me2 levels (35, 39).
We have sought to identify how RITS is recruited to centromeres. Previously, we showed that Chp1 and Tas3 bind directly to each other and that this interaction occurs independently of Ago1 (39). In addition, Tas3 binds to Ago1, and this interaction occurs independently of Chp1 (37). Thus, Tas3 appears to form a bridge between the two components of the complex (Chp1 and Ago1), which could mediate the association of RITS with chromatin. In this work, we demonstrate that the binding of Tas3 to Chp1 is essential for the function of RITS at centromeres. We mapped the Chp1 binding surface on Tas3 and generated an N-terminal deletion mutant of Tas3 that prevents its association with Chp1. This mutant Tas3 maintains an association with Ago1 but exhibits a profound disruption of centromeric function, including the loss of the production of centromeric siRNAs and a reduction in centromeric H3K9Me2 levels. This phenotype contrasts strongly with that of the maintenance of centromeric heterochromatin seen in cells bearing a Tas3-Chp1 subcomplex that cannot bind Ago1 (37), in which both siRNA production and H3K9Me2 levels at centromeres are unaffected. Thus, Chp1's interaction with Tas3-Ago1 is crucial for the association of RITS and RDRC with centromeres, strongly suggesting that Chp1 recruits RITS to centromeric chromatin. In addition, we demonstrate that heterochromatin at the mating-type locus and telomeres is differentially affected by the loss of the Tas3-Chp1 association and that Tas3 and Chp1 may perform important roles at telomeres outside the context of RITS.
MATERIALS AND METHODS
Media and chemicals.
Fission yeast were maintained on rich medium (YES) or on pombe minimal medium with glutamate (PMG) with appropriate supplements if nutritional selection was necessary (31). All chemicals were obtained from Sigma unless otherwise indicated.
Strain generation.
Strains used in this study are listed in Table 1. The chp1Δ his3+, ago1Δ ura4+, clr4Δ LEU2, clr4Δ kanR, rik1Δ LEU2, tas3Δ ura4+, dcr1Δ kanR, and tas3Δ kanR null alleles and the chp1-myc6+-, tas3-TAP+-, and Flag3-ago1+-tagged alleles have been described previously (36-39). The tas3Δ10-24-TAP allele was generated in a two-step process. Nucleotides 27 to 69 of the tas3-TAP open reading frame were replaced with the ura4+ marker gene by transforming Tas3-TAP cells by electroporation with a PCR product containing ura4+ flanked by ∼80 bases of homology to sequences flanking nucleotides 27 to 69 of tas3. ura4+ transformants were selected by growth on medium lacking uracil, and the proper integration of ura4+ into tas3-TAP was checked by PCR. The removal of ura4+ and the generation of tas3Δ10-24-TAP were achieved by retransformation with a chimeric PCR product and selection for growth of cells on medium containing 5-fluoro-orotic acid (5-FOA). PCR and DNA sequencing were used to confirm the generation of this allele. The non-epitope-tagged tas3Δ10-24 allele was generated similarly, using a wild-type strain (PY 42) for the targeting of tas3+. The rdp1-Flag3 allele was generated by the transformation of PY 42 cells with a chimeric PCR product amplified from JP-931 using primers with homology to sequences flanking the stop codon of Rdp1 and that amplify the Flag3 and Kanr sequences from JP-931.
TABLE 1.
List of strains used in this study
| Strain | Genotype |
|---|---|
| PY 42 | h−ura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 1064 | h−tas3::tas3-TAP-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 1210 | h−tas3::tas3-TAP-kanRchp1Δ::his3+ otr1R(dg-glu)SphI::ura4+ ura4-DS/E ade6-210 leu1-32 arg3Δ? his3Δ? |
| PY 1571 | h−tas3::tas3Δ10-24-TAP-kanRura4-DS/E ade6-210 leu1-32 arg3Δ his3Δ |
| PY 1080 | h−tas3::tas3-TAP-kanRchp1::chp1-myc6-LEU2 ura4-D18 ade6-210 leu1-32 arg3Δ? his3Δ? |
| PY 1621 | h−tas3::tas3Δ10-24-TAP-kanRchp1::chp1-myc6-LEU2 ura4-D18 ade6-210 leu1-32 arg3Δ? his3Δ? |
| PY 1813 | h+chp1::chp1-myc6-LEU2 ago1::Flag3ago1-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 1849 | h+chp1::chp1-myc6-LEU2 ago1::Flag3-ago1-kanRtas3::tas3-TAP-kanRura4-D18 ade6-210 leu1-32 |
| PY 1908 | h?chp1::chp1-myc6-LEU2 ago1::Flag3-ago1-kanRtas3::tas3Δ10-24-TAP-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 14 | h−R.int-(cnt1)NcoI::ura4+ ura4-DS/E ade6-210 leu1-32 |
| PY 30 | h+otr1R(dg-glu)SphI::ura4+ ura4-DS/E ade6-210 leu1-32 |
| PY 1402 | h−otr1R(dg-glu)SphI::ura4+ ura4-DS/E tas3Δ::kanRade6-210 leu1-32 |
| PY 1640 | h+otr1R(dg-glu)SphI::ura4+ ura4-DS/E tas3::tas3-TAP-kanRade6-210 leu1-32 his3Δ |
| PY 1620 | h+otr1R(dg-glu)SphI::ura4+ ura4-DS/E tas3::tas3Δ10-24-TAP-kanRade6-210 leu1-32 arg3Δ his3Δ |
| PY 1798 | h−clr4Δ::kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 901 | h−ago1Δ::ura4+ ura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 938 | h−tas3Δ::ura4+ ura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 90 | h−chp1Δ::his3+ ura4-DS/E ade6-210 leu1-32 |
| PY 1550 | h−dcr1Δ::kanRura4-DS/E ade6-210 leu1-32 |
| PY 2038 | h−rdp1::rdp1-Flag3-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 2028 | h−tas3::tas3-TAP-kanRrdp1::rdp1-Flag3-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 3383 | h−tas3::tas3Δ10-24-TAP-kanRrdp1::rdp1-Flag3-kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 3249 | h−tas3::tas3-TAP-kanRrdp1::rdp1-Flag3-kanRclr4Δ::kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 863 | h−tas3::tas3-myc13-kanRura4-DS/E ade6-210 leu1-32 arg3Δ? his3Δ? |
| PY 12 | h− (= 972) |
| PY 261 | h90mat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 |
| PY 1157 | clr4Δ::LEU2 mat3-M(RV)::ade6 ura4-DS/E or ura4-D18 ade6-DN/N leu1-32 |
| PY 260 | h90chp1Δ::ura4+ mat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 |
| PY 1127 | h90ago1Δ::ura4+ mat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 his1-102? his3Δ? |
| PY 2270 | h?tas3Δ::ura4+ mat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 |
| PY 2271 | h?tas3::tas3-TAP-kanRmat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 |
| PY 2272 | h?tas3::tas3Δ10-24-TAP-kanRmat3-M(RV)::ade6 ura4-D18 ade6-DN/N leu1-32 |
| PY 2829 | h?clr4Δ::kanR::JP1084(HpaI)clr4+-his3+tas3::tas3Δ10-24-TAP-kanRmat3-M(RV)::ade6 ura4-DS/E or ura4-D18 ade6-DN/N leu1-32 |
| PY 2830 | h?clr4Δ::kanR::JP1084(HpaI)clr4+-his3+tas3::tas3Δ10-24-TAP-kanRmat3-M(RV)::ade6 ura4-DS/E or ura4-D18 ade6-DN/N leu1-32 |
| PY 2831 | h?clr4Δ::kanR::JP1084(HpaI)clr4+-his3+tas3::tas3-TAP-kanRmat3-M(RV)::ade6 ura4-D18 or ura4-DS/E ade6-DN/N leu1-32 |
| PY 2832 | h?clr4Δ::kanR::JP1084(HpaI)clr4+-his3+tas3::tas3-TAP-kanRmat3-M(RV)::ade6 ura4-D18 or ura4-DS/E ade6-DN/N leu1-32 |
| PY 2610 | h−chp1Δ::his3+ tas3Δ::kanRura4-DS/E or ura4-D18 ade6-210 leu1-32 his3? |
| PY 2613 | h−chp1Δ::his3+ tas3Δ::kanRura4-DS/E or ura4-D18 ade6-210 leu1-32 his3? |
| PY 2208 | h−tas3::tas3-TAP-kanRchp1Δ::his3+ ura4-DS/E ade6-210 leu1-32 his3? |
| PY 2174 | h−tas3::tas3Δ10-24-TAP-kanRchp1Δ::his3+ ura4-DS/E ade6-210 leu1-32 his3? |
| PY 3435, PY 3436 | h−tas3::tas3Δ10-24clr4Δ::kanRura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 3438, PY 3439 | h−clr4Δ::kanR::JP1084(HpaI)clr4+-his3+ura4-D18 or ura4-DS/E ade6-210 leu1-32 arg3Δ his3Δ |
| PY 3441 to PY 3445 | h−tas3::tas3Δ10-24clr4Δ::kanR::JP1084(HpaI)clr4+-his3+ura4-D18 ade6-210 leu1-32 arg3Δ his3Δ |
| PY 3506 | h−tas3::tas3Δ10-24ura4-DSE ade6-210 leu1-32 his3? |
DNA constructs.
Yeast two-hybrid vectors were generated as described previously (39) by PCR amplification of domains of the Tas3 and Chp1 open reading frames and by subcloning them into pGBKT7 (Chp1) and pGADT7 (Tas3) vectors (Clontech). The vector for the reintegration of clr4+ (JP-1084) was previously described and allows the reintegration of a genomic clone of clr4+ (under the control of its endogenous regulatory sequences) into clr4 null cells (37). JP-931 (for C-terminal Flag-tagging Rdp1) was derived from pFA6a-GFP(S65T)-KanMX-6 (3) after the release of green fluorescent protein (GFP) sequences by digestion with PacI and AscI and their replacement with annealed oligonucleotides that bear PacI- and AscI-compatible ends and that encode a 3× Flag epitope sequence (M2) followed by a stop codon.
Protein interaction assays. (i) Yeast two-hybrid assay.
Budding yeast manipulations were performed as previously described (39). The interaction of proteins resulted in the activation of three integrated GAL4-dependent reporters and was detected by the growth of yeast on selective medium and the production of blue coloration due to the metabolism of X-α-galactose (X-α-Gal) present in the medium. Protein truncations were designed to minimize structural disruptions based on information from secondary-structure predictions. The expression of all constructs was confirmed by Western blot analysis of cell extracts using antibodies directed against Gal4 activation domain (GAD) fusions with Tas3 (anti-hemagglutinin; 12CA5) or Gal4 DNA binding domain (GBD) fusions with Chp1 (anti-myc; 9E10).
(ii) Fission yeast.
Cell extracts were prepared by grinding cells in liquid N2 in a pestle and mortar as previously described but using 2.4 × 109 cells per ml of extraction buffer for each immunoprecipitation (39). Western blots were probed with M2 anti-Flag antibody (Sigma), anti-myc antibody (9E10; Roche), or anti-tandem affinity purification tag (TAP) antibody (rabbit anti-immunoglobulin G [IgG] horseradish peroxidase; Sigma).
Real-time PCR. (i) Transcript analysis.
cDNA was generated by the random priming of total cellular RNA, which was prepared as previously described (37). The detection of transcripts by real-time PCR used primers targeting the dh region of the centromere (JPO-769 and JPO-770) that are predicted to amplify from six copies of the repeat within the genome of the h− strains used for these analyses. Primers for alcohol dehydrogenase (adh1) were used as the euchromatic normalization control (JPO-793 and JPO-794). Centromeric transcripts from dg (PCR amplicon B) were measured using JPO-986 (GATACTGATAATATTGAGATCCACAGCAC) and JPO-987 (GCGATGCCAAACAACAATATTG). Telomeric transcripts were detected using primers targeting the telomeric sequence from within the helicase domain of tlh1+ and tlh2+ (JPO-816, CGTTTTTGATACCGGCGC; JPO-819, TTGCCGTAACGACATCATGG) and do not amplify from centromere homologous sequences present elsewhere in tlh1 and tlh2.
Real-time PCR was performed from duplicate cDNA samples, and cDNA was generated from two or three biological duplicates. The comparative threshold cycle, or ΔΔCT, method was used for the analysis of transcript levels (37). This method relies on the use of primer sets that amplify with 100% efficiency, such that the titration of template material in PCRs yields perfect standard curves (with a slope of −3.3 ± 0.1 and an R2 coefficient of determination of 0.99) when the CT is plotted against the concentration of the template. The linear range of amplification for each set of primers was verified, and experiments were performed within this range. The use of such primer sets allows for the comparative analysis of the CTs for samples of interest relative to the CTs of wild-type controls. The averages of these experiments are shown, with error bars representing the standard errors of the means.
(ii) ChIP.
Chromatin immunoprecipitation (ChIP) experiments were performed and analyzed as described previously (37) using primers from the dh region of the centromere (JPO-769 and JPO-770), the dg region of the centromere (JPO-986 and JPO-987), adh1 (JPO-793 and JPO-794), and telomeres (JPO-816 and JPO-819). Experiments were repeated at least three times, and the averages from these experiments (with standard errors of the means) are shown. A second fixation step was used for anti-Rdp1-Flag ChIPs (see Fig. 4E), with the fixation of cells for 30 min in dimethyl adipimidate (5 mM in phosphate-buffered saline; Pierce) after the cells had been washed following formaldehyde fixation. Fixation was stopped by the addition of glycine.
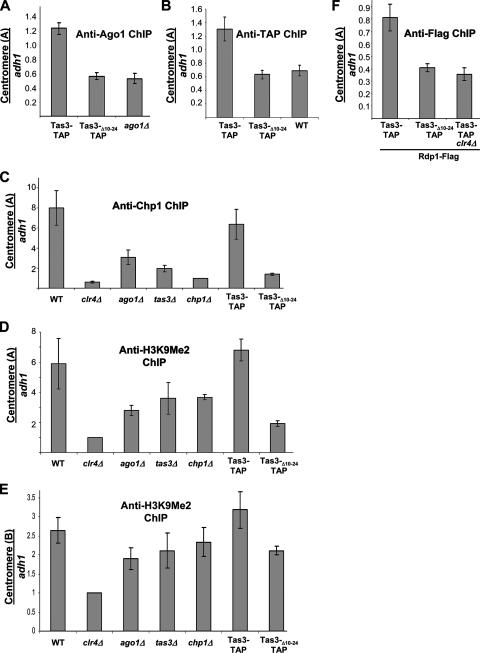
FIG. 4.
ChIP analysis of the centromeric association of heterochromatin components in mutant cell backgrounds. (A) Real-time PCR analysis of chromatin immunoprecipitated with anti-Ago1 antibody to assess Ago1's association with the outer repeats of the centromere (site A in dh) relative to that of an adh1 euchromatic control. The strains used were PY 1064, PY 1571, and PY 901. (B) ChIP analysis of the association of TAP-tagged proteins with centromeric repeats relative to association at adh1 using IgG Sepharose beads. The strains used were PY 1064, PY 1571, and PY 42. (C) Real-time PCR analysis of ChIP with anti-Chp1 antibodies to assess Chp1's association with dh sequences relative to association with adh1 in mutant backgrounds. Values are shown as the change (n-fold) in enrichment above that of the association of Chp1 in a chp1 null background. The strains analyzed in panels C and D were PY 42, PY 1798, PY 901, PY 938, PY 90, PY 1064, and PY 1571. (D) Real-time PCR analysis of ChIP with anti-H3K9Me2 antibody to assess H3K9Me2 enrichment at centromeric sequences within dh (site A) relative to that of an adh1 euchromatic control. Immunoprecipitation was performed with anti-H3K9Me2 antibody on a clr4 null strain (second lane), and this served as a normalization control for the other samples. (E) Real-time PCR analyses of ChIPs with anti-H3K9Me2 antibody to assess the enrichment of centromeric dg sequences (site B) in the samples analyzed in panel D. (F) ChIP analysis of the association of Rdp1-Flag3 with centromeric repeats relative to association with adh1. Strains were tested for the association of Rdp1 with centromeric dh sequences relative to that at adh1 by ChIP with anti-Flag antibody. The strains used in the analysis were PY 2028, PY 3383, and PY 3249.
siRNA analyses were performed as described previously (37) for dh siRNAs. dg siRNAs were detected by hybridization with a probe generated by amplification with JPO-566 (TGGTAATACGTACTAGCTCTCG) and JPO-567 (AACTAATTCATGGTGATTGAT).
Cell growth assays.
Cells were grown in YES at 25°C to 5 × 106 cells/ml, washed in PMG with no supplements, and spotted onto PMG complete, PMG-uracil, or PMG supplemented with 2 mg 5-FOA/ml. Serial (1:5) dilutions of fission yeast were made, and a total of 1.2 × 104 cells were contained in the first spot. Plates were grown for 5 days at 25°C. To assess the maintenance of silencing at the mat2/mat3 locus, serial (1:5) dilutions of cells were grown overnight in YES medium at 25°C to 5 × 106 cells/ml and washed in PMG with no supplements, spotted onto PMG medium containing reduced adenine or no adenine, and incubated for 5 days at 25°C. A total of 1.2 × 104 cells were contained in the first spot.
Immunofluorescence.
Immunofluorescence and cytological chromosome missegregation assays were performed as described previously (39). Anti-tubulin antibody (a kind gift of K. Gull) was used at a 1/30 dilution and was revealed with fluorescein isothiocyanate-conjugated anti-mouse antibody (Jackson Immunologicals) diluted to 1:100. 4′,6′-Diamidino-2-phenylindole (DAPI) was used to identify nuclei.
RESULTS
Defining the interaction domain between Tas3 and Chp1.
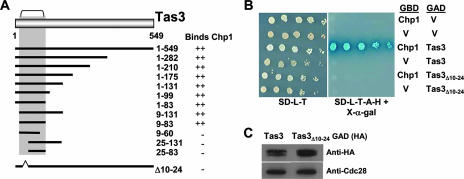
Our previous studies indicated that a Tas3-Chp1 subcomplex was sufficient for the maintenance of centromeric heterochromatin (37). To test the importance of Tas3's association with Chp1 for the recruitment of RITS to heterochromatin, we sought to generate a mutant of Tas3 that specifically abrogated its interaction with Chp1. To identify the minimal sequences of Tas3 required for interaction with Chp1, truncations of Tas3 were tested for their interactions with full-length Chp1 in a GAL4 reporter two-hybrid assay in budding yeast. These analyses identified residues 9 to 83 of Tas3 as necessary and sufficient for interaction with Chp1 and are summarized in Fig. 1A and Fig. S1 in the supplemental material.
FIG. 1.
Association between Tas3 and Chp1 is dependent on residues 10 to 24 of Tas3. (A) Summary of two-hybrid interaction data for the binding of truncated Tas3 proteins to full-length Chp1. (B) Two-hybrid analysis of Chp1-GBD or GBD alone (V) binding to Tas3-GAD or GAD alone (V). Growth on synthetic media lacking leucine and tryptophan (SD-L-T) selects for the GAD and GBD vectors, and growth on synthetic media lacking leucine, tryptophan, histidine, and adenine (SD-L-T-H-A) plus X-α-Gal demonstrates the interaction between GAD and GBD fusion proteins. (C) Western blot analysis of the steady-state expression levels of Tas3-GAD and Tas3Δ10-24-GAD fusion proteins in AH109 budding yeast compared to that of Cdc28 as a loading control.
To further define the Tas3-Chp1 interface, small internal deletions removing regions of predicted secondary structure were made within the Chp1 interaction domain of Tas3, and the effect of these mutations on Tas3's interaction with Chp1 was assessed by a two-hybrid assay (Fig. 1B). A mutant of Tas3 lacking the first predicted alpha helix (residues 10 to 24) failed to interact with Chp1. This loss of interaction was not caused by the instability of the mutant protein, as its steady-state expression level was similar to that of the wild type (Fig. 1C).
The Tas3Δ10-24 mutant cannot associate with Chp1 in fission yeast.
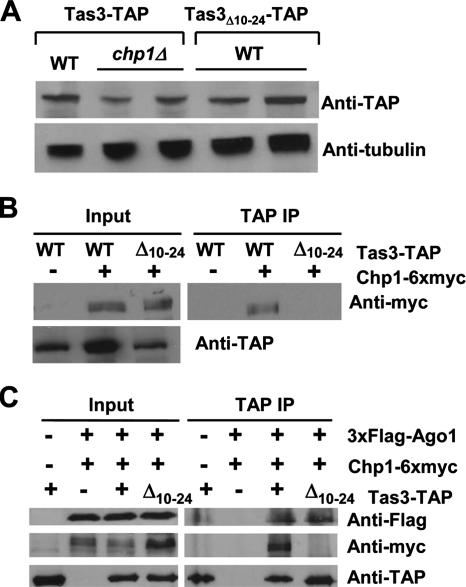
Tas3 is fully functional when the genomic locus is tagged at the C terminus with 13 myc epitopes, but the Tas3-13xmyc protein is unstable in the absence of Chp1 (39). A C-terminal fusion of tas3+ to the TAP tag (Tas3-TAP) is similarly fully competent for centromere function but is stably expressed in the absence of Chp1 (Fig. 2A, Table 2). As antibodies against the endogenous Tas3 protein are not yet available, the genomic tas3-TAP allele was used to investigate the role of the Chp1-Tas3 interaction in the function of the RITS complex.
FIG. 2.
Tas3Δ10-24-TAP cannot associate with Chp1 but coimmunoprecipitates Flag3-Ago1 from fission yeast whole-cell extracts. (A) Western blot analysis of the tas3-TAP and tas3Δ10-24-TAP alleles in an otherwise wild-type or chp1 null background relative to the expression of the α-tubulin loading control in fission yeast whole-cell extracts. The strains used for this analysis were PY 1064, PY 1210, and PY 1571. (B) Chp1-6xmyc coimmunoprecipitates with Tas3-TAP but not Tas3Δ10-24-TAP from fission yeast. Whole-cell extracts (input) were prepared from fission yeast expressing Chp1-myc6 and either Tas3-TAP or Tas3Δ10-24-TAP. Following IgG Sepharose immunoprecipitation of TAP-tagged proteins, immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide electrophoresis and were subjected to Western blotting with anti-myc antibodies to detect the presence of Chp1-myc6. The strains used for this analysis were PY 1064, PY 1080, and PY 1621. (C) Immunopurification of Tas3Δ10-24-TAP complexes from cells coexpressing chp1-myc6 and Flag3-ago1 alleles reveals the copurification of Ago1 but not Chp1 with Tas3 lacking residues 10 to 24. Immunoprecipitates were subjected to Western blotting with antibodies against the myc, Flag, and TAP (IgG) tags. The strains used were PY 1064, PY 1813, PY 1849, and PY 1908.
TABLE 2.
Inability of the Tas3Δ10-24-TAP mutant to interact with Chp1 results in chromosome missegregation
| Background strain | No. of anaphase cells screened | No. (%) of cells with lagging chromosomesa |
|---|---|---|
| Tas3-myc13 | 105 | 2 (2) |
| Tas3-TAP | 202 | 2 (<1) |
| Tas3Δ10-24-TAP | 193 | 51 (26.4) |
| tas3 null | 223 | 52 (23.3) |
| chp1 null | 139 | 37 (26.6) |
| Wild type | 104 | 1 (<1) |
The frequency of lagging chromosomes on late-anaphase spindles (>5 μm) was determined by staining them with anti-α-tubulin antibodies and using DAPI to visualize DNA. The strains used were PY 863, PY 1064, PY 1571, PY 938, PY 90, and PY 42.
The deletion of residues 10 to 24 from the genomic tas3-TAP allele (tas3Δ10-24-TAP) yielded a mutant protein that was expressed at levels similar to that of Tas3-TAP (Fig. 2A). To determine if the loss of residues 10 to 24 from Tas3-TAP blocked binding to Chp1, the wild-type and mutant tas3-TAP alleles were introduced into strains bearing the chp1-6xmyc allele. Immunoprecipitation experiments demonstrated that Tas3-TAP efficiently precipitated Chp1-6xmyc, whereas Tas3Δ10-24-TAP did not coimmunoprecipitate Chp1-6xmyc (Fig. 2B). Thus, the removal of the first predicted alpha helix from Tas3 (residues 10 to 24) prevented Tas3's interaction with Chp1 in fission yeast.
Tas3Δ10-24 associates with Ago1 independently of Chp1.
We recently demonstrated that Tas3 binds Ago1 through a conserved GW-rich domain localized between residues 200 and 310 of Tas3 (37). To address whether the removal of residues 10 to 24 of Tas3 prevented its association with Ago1, a fully functional 3xFlag-ago1 allele was introduced into strains expressing wild-type or mutant Tas3-TAP and Chp1-6xmyc, and the association between these Tas3-TAP proteins and Ago1 was tested by immunoprecipitation. Interestingly, the Tas3Δ10-24-TAP protein efficiently immunoprecipitated 3xFlag-Ago1, suggesting that Tas3 still binds to Ago1 even when the binding to Chp1 is abolished (Fig. 2C). These experiments suggest that Ago1 and Chp1 do not physically interact but that their association is dependent on Tas3. This was further tested by a two-hybrid assay, but we failed to see an association between Chp1 and Ago1 (see Fig. S2 in the supplemental material). This may be a limitation of the two-hybrid technique, since we also were unable to detect an association between Tas3 and Ago1 in two-hybrid assays (see Fig. S2 in the supplemental material). Thus, Tas3 can serve as an adapter protein or scaffold to coordinately bind to both Ago1 and Chp1 in fission yeast to facilitate RITS function.
Loss of interaction between Chp1 and Tas3-Ago1 perturbs centromeric heterochromatin.
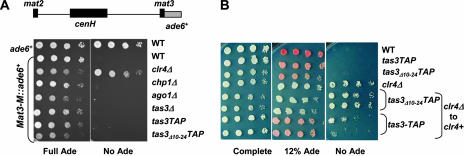
To assess whether the loss of the Chp1 association with the Tas3-Ago1 subcomplex affected the maintenance of centromeric heterochromatin, we first monitored the expression of a centromeric transgene (cen::ura4+) in comparative growth assays. The cen::ura4+ transgene is located within the dg region of the outer repeats of the centromere (Fig. 3A) at a site known to be efficiently silenced in wild-type cells by both transcriptional and posttranscriptional silencing mechanisms [strain FY 648 in reference 2; otr1R(dg-glu)SphI::ura4+] (7, 8, 19). Cells bearing the tas3-TAP allele silenced cen::ura4+ expression, allowing growth on media containing 5-FOA, a drug that is toxic to cells expressing ura4+. In contrast, cells bearing the tas3Δ10-24-TAP allele, like those with a deletion of tas3, failed to grow on 5-FOA, indicating that cen::ura4+ is expressed and that centromeric heterochromatin is disrupted when the Tas3-Chp1 interaction is abrogated (Fig. 3B).
FIG. 3.
Tas3-Chp1 interaction is required for the maintenance of the silencing of centromeric heterochromatin. (A) Cartoon illustrating the layout of centromere 1 and the position of the cen::ura4+ reporter transgene used in these analyses [at otr1R(dg-glu)SphI::ura4+]. The lower panel is a magnification of the otr1R repeats, highlighting the position of sites A and B in dh and dg, respectively, that are monitored in real-time PCR assays, the probes used for the siRNA analyses from dg and dh (si dg and si dh, respectively), and the position of the nearest gene (rad50+) at the right of centromere 1. Some restriction enzyme sites are marked (N, NcoI; S, SphI; H, HindIII; and X, XhoI). Vertical bars within imr1 (innermost repeat) sequences represent clustered tRNA genes. (B) Comparative growth assay of serially diluted strains bearing the centromeric cen::ura4+ marker within the dg region of centromere 1 or a euchromatic insertion of ura4+ (R.Int::ura4+) and assessed for growth on PMG complete medium, PMG medium lacking uracil (−URA), or PMG complete medium supplemented with 5-FOA (FOA). Cells were wild type (WT), were null for tas3 (tas3Δ), or bore genomic tas3-TAP or tas3Δ10-24-TAP alleles. The strains used were PY 14, PY 30, PY 1402, PY 1640, and PY 1620. (C) Real-time PCR analysis of the relative transcription of centromeric sequences at position A within the dh repeat (see panel A) compared to that of adh1 in random primed cDNA samples generated from the indicated strains. Ratios of centromeric to adh1 transcript levels were normalized to 1 for wild-type strains (WT) and represent mean values for duplicate biological samples, with error bars representing the standard errors of the means. The strains used for this analysis were PY 42, PY 1798, PY 901, PY 938, PY 90, PY 1064, and PY 1571. (D) Analysis of relative transcript levels from centromeric dg sequences at position B within the dg repeat (see panel A) in the RNA samples analyzed in panel C. (E) siRNAs are not produced in Tas3Δ10-24 mutant cells. Small RNAs purified from total cellular RNA were hybridized with dh centromeric probes to reveal siRNAs from the dh region of the centromere, and to probes directed against the small RNA, SnoR69 as the loading control. The strains used were PY 1064, PY 1571, PY 1550, and PY 938. (F) Small RNAs purified from total RNA were analyzed with a dg centromeric probe to reveal siRNAs from the dg region of the centromere, and to SnoR69 as the loading control. The strains used were PY 1064, PY 1571, and PY 938. Excess lanes were removed from the image of the blot, as indicated by the black bar.
The disruption of the silencing of the centromeric transgene is an indirect readout of a failure in the maintenance of centromeric heterochromatin. This may be caused by an accumulation of centromeric transcripts following the failure of the Ago1-mediated destruction of the transcripts or of their Dicer-mediated processing into siRNAs. Alternatively, the disruption of the transcription of centromeric sequences can perturb the formation of centromeric heterochromatin without the accumulation of centromeric transcripts (14). To distinguish between these possibilities, quantitative real-time PCR was performed on cDNA derived from strains lacking centromeric transgenes to monitor the levels of endogenous centromeric transcripts generated from the dh region of the centromere (using primers that amplify site A) (Fig. 3A). Silencing in this region of the centromere is attributed to cotranscriptional gene-silencing processes, with Ago1 mediating the destruction of centromeric transcripts (8, 19). The level of centromeric transcripts in wild-type cells relative to that of adh1 expression was normalized to 1, and as expected we saw an accumulation of high levels of centromeric transcripts in cells lacking the Clr4 histone H3 K9 methyltransferase activity (∼50-fold increase), with an intermediate accumulation (∼15- to 30-fold) in cells lacking either Tas3 or Chp1 (Fig. 3C). Cells bearing tas3Δ10-24-TAP accumulated transcripts to about 30-fold the levels found in tas3-TAP cells, which is consistent with the disruption of the Tas3-Chp1 interaction causing a defect in the processing of centromeric transcripts but not in their production. We also analyzed transcript levels at the dg region of the centromere (Fig. 3A), in which silencing is attributable to both transcriptional gene-silencing and cotranscriptional gene-silencing processes (8, 19). Transcripts from the dg region accumulated to 60- to 90-fold higher levels in clr4Δ, ago1Δ, chp1Δ, and tas3Δ cells than in wild-type cells and were 80-fold higher in tas3Δ10-24-TAP cells than in the wild type (Fig. 3D).
Disruption of Chp1-Tas3 association causes a defect in siRNA production.
To determine whether the accumulation of centromeric transcripts was attributable to a defect in the Dicer-mediated processing of these transcripts, small RNAs were isolated from tas3Δ10-24-TAP cells and assessed for the presence of centromeric siRNAs. siRNAs from both the dh and dg regions of the centromere were detected in isolates from Tas3-TAP cells but not from Tas3Δ10-24-TAP cells (Fig. 3E and F), indicating that the loss of the Chp1-Tas3 interaction causes a defect in the processing of centromeric transcripts into siRNAs.
Disruption of Chp1's association with Tas3-Ago1 prevents both RITS and RDRC association with centromeres and causes a reduction in centromeric H3K9Me2.
The accumulation of centromeric transcripts and the loss of siRNAs indicate a failure in the recruitment of RITS and RDRC to centromeres. ChIP assays demonstrated that Ago1 efficiently interacted with the dh region of centromeres in tas3-TAP cells but did not do so in cells expressing tas3Δ10-24-TAP, suggesting that the Chp1-Tas3 interaction is required to recruit the Tas3-Ago1 subcomplex to the dh region of centromeres (Fig. 4A). Similarly, ChIP with IgG Sepharose beads to purify TAP-tagged proteins showed that Tas3 efficiently bound to dh centromeric sequences in Tas3-TAP but not Tas3Δ10-24-TAP cells (Fig. 4B). Centromeric sequences also were efficiently immunoprecipitated by antibodies directed against Chp1 from cells expressing tas3-TAP but not from cells expressing tas3Δ10-24-TAP or strains deleted for tas3 or clr4 (Fig. 4C), demonstrating that the loss of Tas3-Chp1 interaction results in the loss of Chp1 association with the dh region of centromeres. One possible cause for the loss of Chp1 association is reduced levels of H3K9Me2 (38). Consistently with this possibility, ChIP clearly demonstrated that tas3Δ10-24-TAP mutants showed reduced H3K9Me2 levels at dh centromeric sequences compared to those of tas3-TAP cells (Fig. 4D). In contrast, only a minor reduction in centromeric H3K9Me2 levels was seen at centromeric dg repeat sequences in ago1Δ, tas3Δ, chp1Δ, and tas3Δ10-24-TAP mutants (Fig. 4E).
We tested whether the loss of RITS from centromeres disrupted the recruitment of RDRC. A fully functional C-terminal Flag3-tagged Rdp1 allele (see Table S1 in the supplemental material) was introduced into cells expressing tas3-TAP or tas3Δ10-24-TAP. Clr4 is required for the association of RDRC and RITS with chromatin (32, 48); thus, we also introduced tas3-TAP and rdp1-Flag alleles into a clr4 null background to serve as a negative control. ChIP performed with anti-Flag antibodies demonstrated that Rdp1-Flag associated with centromeric dh sequences in the tas3-TAP clr4+ background but did not do so in clr4 null cells or in the tas3Δ10-24 TAP clr4+ background (Fig. 4F). Thus, RDRC is not recruited to centromeres in cells expressing tas3Δ10-24-TAP.
Loss of association between Chp1 and Tas3-Ago1 causes genomic instability.
The disruption of centromeric heterochromatin usually results in defective cohesion between sister chromatids and elevated rates of chromosome missegregation. We therefore monitored the efficiency of chromosome segregation in anaphase cells of the tas3Δ10-24-TAP mutant by immunofluorescence. Whereas cells expressing tas3-TAP rarely gave rise to chromosome missegregation events (<1%), cells bearing tas3Δ10-24-TAP exhibited high levels of chromosome missegregation (26%), similarly to what was seen for tas3Δ or chp1Δ null cells (Table 2).
Disruption of Tas3-Chp1 association does not impact heterochromatin maintenance but perturbs its establishment at the mating-type locus.
Components of RITS also localize to other sites of heterochromatin in fission yeast, such as the telomeres and mating-type locus (10, 39, 42). Interestingly, Tas3 and Chp1 can associate with these loci independently of the RNAi pathway (39). The loss of RNAi components or Chp1 has little effect on the maintenance of silencing of transgenes inserted at the mating-type locus (17, 39, 42, 47). To assess if the loss of Chp1-Tas3 interaction disrupted mat2/mat3 silencing, the tas3Δ10-24-TAP mutation was introduced into cells bearing an ade6+ transgene inserted at mat3 (46) (Fig. 5A). This transgene is efficiently silenced in wild-type cells, but this repression is alleviated upon the deletion of clr4. Similarly to our results with chp1 null and ago1 null cells, no alleviation of silencing of the transgene was observed in tas3 null cells or in cells bearing tas3Δ10-24-TAP. Thus, silencing at the mating-type locus appears to be independent of RITS components and of the RNAi pathway, most likely because redundant RNAi-independent pathways can maintain heterochromatin at mat2/mat3 (17, 20, 24, 46).
FIG. 5.
Tas3Δ10-24-TAP impacts the establishment, but not maintenance, of heterochromatin at the mating-type locus. (A) Cartoon of the mat2/mat3 locus showing the localization of the ade6+ reporter gene inserted at mat3 (EcoRV). Shown are serial-dilution growth assays of cells that were wild type for ade6+ or that carried a wild-type ade6 allele at mat3 (mat3M::ade6+) in different genetic backgrounds and spotted onto PMG complete medium supplemented with adenine (Full Ade) or without adenine (No Ade). The strains analyzed were PY 12, PY 261, PY 1157, PY 260, PY 1127, PY 2270, PY 2271, and PY 2272. (B) Serial dilution assay of cells bearing the mat3M::ade6+ reporter spotted onto PMG complete medium, PMG medium supplemented with a low level of adenine (12% Ade), or medium containing no adenine (No Ade). The strains analyzed were PY 261, PY 2271, PY 2272, PY 1157, PY 2829, PY 2830, PY 2831, and PY 2832, with the last four being clr4 null strains into which clr4+ was reintegrated to allow the monitoring of the establishment of heterochromatin.
Chp1 is important for the establishment of heterochromatin at the mating-type locus (42). To test whether Chp1's role in the establishment of heterochromatin at mat2/mat3 relies on its association with Tas3, we asked if tas3Δ10-24-TAP expression blocked the establishment of heterochromatin at mat2/mat3 after the removal and reintroduction of clr4+ (Fig. 5B). Cells lacking clr4 express mat3M::ade6 and grow on medium lacking adenine. On the reintegration of clr4+ (under its native regulatory sequences) into clr4 null tas3+-TAP cells, silencing was efficiently reestablished, with the repression of ade6+ expression, the red coloration of colonies, and the inhibition of growth on medium lacking adenine. In contrast, silencing was not reestablished in tas3Δ10-24-TAP cells, and cells grew on medium lacking adenine in a manner similar to that of the clr4 null cells. Thus, the association between Chp1 and Tas3 is required for the efficient establishment of heterochromatin at the mating-type locus.
Loss of Tas3 or Chp1, but not disruption of the RNAi pathway, promotes accumulation of telomeric transcripts.
Heterochromatin also assembles in the subtelomeric regions of fission yeast chromosomes, where it extends for ∼50 kb (22). Silencing in these regions depends on Clr4, Swi6, and Taz1, and the recruitment of Clr4 to these sequences is mediated at least in part by Taz1 binding to telomeric repeats at the end of the chromosome and via RITS associating with centromere homologous sequences located within the open reading frame of subtelomeric helicase genes (12, 22, 29, 34). Similarly to the mating-type locus, the maintenance of silencing at telomeres depends on redundant RNAi-dependent and RNAi-independent mechanisms; thus, the removal of genes encoding RNAi components has little impact on the maintenance of the silencing of telomeric transcripts or of reporters inserted within subtelomeric loci (7, 17, 18, 22).
We tested whether telomeric transcripts accumulated in the tas3Δ10-24-TAP mutant by monitoring transcripts of subtelomeric tlh genes using primers that recognize the helicase domain. tlh transcripts accumulated in chp1 null strains as seen previously (18) and in cells deficient for tas3 (Fig. 6A) but did not accumulate in either the tas3Δ10-24-TAP mutant or in ago1 null cells. Taken together, these results suggest that RITS plays little role in the maintenance of the silencing of subtelomeric helicase genes but that Tas3 and Chp1 contribute, independently of each other, to the suppression of transcript accumulation from subtelomeric genes. If Tas3 and Chp1 affect distinct pathways, cells that are mutant for both tas3 and chp1 may further accumulate subtelomeric transcripts. Surprisingly, however, tas3Δ chp1Δ double mutants showed no further accumulation of tlh transcripts (Fig. 6B), suggesting that Tas3 and Chp1 act through a common or redundant pathway. We also analyzed telomere length regulation in these strains (see Fig. S3 in the supplemental material), but we found that of the strains tested (including tas3 and chp1 nulls and the double null), only the clr4 deletion had an impact on telomere repeat length, with a slight lengthening of the average size of the telomeric repeats compared to that of wild-type cells.
FIG. 6.
Chp1 and Tas3, but not the RITS, are required for the maintenance of the silencing of telomeric transcripts. (A) Cartoon showing the location of subtelomeric tlh+ genes (black) and the position of the PCR amplicon A within the helicase domain (gray), which is separate from the centromere homology region (striped). Also shown is the real-time PCR analysis of the accumulation of subtelomeric transcripts (measured at position A in tlh+ genes) relative to that of adh1+ control euchromatic transcripts in cDNA prepared from strains of different genetic backgrounds. The strains analyzed were PY 42, PY 1798, PY 901, PY 938, PY 90, PY 1064, and PY 1571. (B) Real-time PCR analysis of tlh transcript accumulation in double-null mutants of chp1 and tas3, performed as described for panel A, using strains PY 42, PY 1798, PY 938, PY 90, PY 2610, and PY 2613. (C) ChIP analysis of Chp1 association with telomeric sites relative to that of adh1, performed with anti-Chp1 antibodies and measured by real-time PCR. The same strains that were used for panel A were used for this analysis. (D) ChIP analysis of H3K9Me2 levels at subtelomeric sites (designated A) relative to levels at adh1 in PY 1064, PY 1571, and PY 1798. (E) ChIP analysis of Tas3-TAP and Tas3Δ10-24-TAP recruitment to tlh sequences relative to levels at adh1, performed with anti-TAP antibodies and measured by real-time PCR. Experiments were performed on strains lacking a TAP tag on Tas3 (tas3+), tas3-TAP, and tas3Δ10-24-TAP, each in both chp1+ and chp1Δ backgrounds (PY 42, PY 1064, PY 1571, PY 2208, PY 2174, and PY 90). (F) The Chp1-Tas3 complex is required for the establishment of telomeric heterochromatin. Shown is a real-time PCR analysis of tlh transcript accumulation compared to that of the euchromatic control adh1 transcripts in cDNA generated from PY 42, PY 1798, PY 3435, PY 3436, PY 3438, PY 3439, PY 3441, PY 3442, PY 3444, and PY 3445. (G) Real-time PCR analysis of subtelomeric transcripts in strains expressing untagged Tas3Δ10-24 demonstrates tlh transcript accumulation. tlh transcripts were compared to euchromatic adh1 control transcripts in cDNA generated from strains PY 42, PY 1798, PY 938, PY 90, and PY 3506. (H) Real-time PCR analysis of transcripts from the dh region of the centromeric repeats relative to those of adh1 control transcripts in the samples analyzed in panel G. wt, wild type.
We performed ChIP to assess Chp1's association with telomeres in different mutant backgrounds (Fig. 6C). The telomeric association of Chp1 was somewhat reduced in cells bearing TAP-tagged alleles of Tas3, suggesting that the epitope tag on Tas3 interferes (directly or indirectly) with Chp1 recruitment, although no such defect is seen at centromeres (Fig. 4C). However, given this caveat, there was no further reduction of Chp1 association with telomeres in tas3Δ10-24-TAP cells compared to that of tas3-TAP cells. In addition, Chp1's association with telomeres was maintained in tas3 null cells and, as noted previously (39), was increased in ago1 null cells compared to that of tas3-TAP cells. Not surprisingly, since Chp1 binds H3K9Me2, ChIP revealed that telomeric H3K9Me2 is maintained in tas3Δ10-24-TAP mutants at levels similar to those of tas3-TAP cells (Fig. 6D). Thus, the maintenance of telomeric heterochromatin in tas3Δ10-24-TAP cells may be due to H3K9Me2 recruited via RNAi-independent mechanisms. This contrasts with the situation for centromeric dh sequences, for which the H3K9Me2 levels and Chp1 association are reduced in cells expressing tas3Δ10-24 TAP.
We also analyzed the association of Tas3 with telomeres (Fig. 6E). Whereas Tas3-TAP associated with tlh sequences, Tas3Δ10-24-TAP did not. Consistently with this, the Tas3-TAP association with tlh was wholly dependent on Chp1. These results provide an explanation for our failure to detect further accumulation of tlh transcripts on combining tas3 and chp1 null mutations (Fig. 6B), since Tas3 is not associated with telomeres in chp1 null cells. However, it is difficult to explain why telomeric transcripts do not accumulate in tas3Δ10-24-TAP cells, since Tas3Δ10-24-TAP does not associate with telomeres, especially given that tlh transcripts accumulate in cells lacking tas3+. This difference in phenotype could result from our comparison of strains lacking Tas3 altogether (tas3 null) to strains in which the Tas3Δ10-24-TAP protein is expressed but not localized at telomeres.
We next tested whether the establishment of the silencing of tlh genes was perturbed by the tas3Δ10-24-TAP mutation. Chp1 is required for the efficient establishment of telomeric heterochromatin (42), but the use of the tas3Δ10-24-TAP mutant allows us to discern if this requirement comes from Chp1's role in RITS or if Chp1 plays an RITS-independent role in the establishment of telomeric heterochromatin, which would be similar to its RITS-independent role in the silencing of tlh transcripts (Fig. 6A). However, we found that the TAP tag on tas3+ prevented the efficient establishment of telomeric heterochromatin in clr4 null cells following the reintroduction of clr4+. We regenerated the tas3Δ10-24 allele in a non-epitope-tagged tas3+ locus and asked whether tlh transcripts were suppressed following the removal and reintroduction of clr4+. The loss of association between Tas3 and Chp1 prevented the efficient silencing of tlh transcripts following the reintroduction of clr4+ (Fig. 6F).
Following our demonstration that the TAP tag on Tas3 precluded the establishment of heterochromatin at telomeres, we questioned whether the TAP tag influences the stability of the Tas3 protein. In particular, we were interested in determining whether Tas3 normally is unstable in cells lacking Chp1, as we have reported previously for the myc-tagged Tas3 protein (39). If so, we expect that the Tas3Δ10-24 mutation will result in an unstable protein, since the association between Tas3 and Chp1 is lost in this mutant background. Unfortunately, there are no antibodies available against Tas3, so we could not directly assess this question. Instead, we asked whether telomeric transcript regulation differs in strains expressing the untagged Tas3 Δ10-24 protein compared to that of strains expressing the TAP-tagged protein. We found that in strains expressing the untagged Tas3Δ10-24 protein, telomeric transcripts accumulated to levels similar to that of tas3 null cells (Fig. 6G). This result suggests, therefore, that the presence of the TAP tag on the Tas3Δ10-24 protein alters the behavior of this mutant protein at telomeres.
Given the different results that we obtained for telomeric transcript regulation in comparisons of TAP-tagged to untagged Tas3Δ10-24 strains, we reanalyzed the centromeric dh transcript accumulation in untagged tas3Δ10-24 cells and found that centromeric transcripts accumulated to levels similar to those of tas3 null cells and tas3Δ10-24-TAP cells (Fig. 6H).
DISCUSSION
Chp1-Tas3 interaction is important for centromeric heterochromatin integrity.
The data that we present clearly demonstrate that Tas3 is a critical component of RITS and that the association between Tas3 and Chp1 is essential for the maintenance of centromeric heterochromatin. Tas3 binds to Chp1 through its N-terminal domain, a region that is distinct from the GW-rich region of Tas3 that is important for association with Ago1 (37). Indeed, a mutant Tas3 (lacking residues 10 to 24) that is unable to associate with Chp1 retains the association with Ago1. This Tas3Δ10-24-TAP mutant cannot support the recruitment of RITS or RDRC to centromeres. Since Dcr1 physically associates with RDRC (11), Dcr1 recruitment to centromeres likely also is lost in the Tas3Δ10-24-TAP cells. These mutant cells accumulate centromeric transcripts, fail to generate centromeric siRNAs, and cannot recruit the high levels of H3K9-methyltransferase activity that are required at centromeres to maintain centromeric heterochromatin.
Our demonstration that the separation of Chp1 from the Tas3-Ago1 subcomplex disrupts centromeric heterochromatin contrasts strongly with the lack of a centromeric phenotype when Ago1 is separated from the Chp1-Tas3 subcomplex (37). In these cells, the levels of H3K9Me2 at centromeres are maintained, allowing the recruitment of Chp1-Tas3, and siRNAs are produced, promoting the recruitment of Ago1 independently of Tas3-Chp1. Thus, our data strongly support the idea that the recruitment of RITS to centromeres relies on Chp1-mediated targeting to chromatin. The recruitment of Chp1 to chromatin depends on the integrity of the chromodomain (35, 39). The chromodomain of Chp1 can bind H3K9Me3 peptides in vitro (38, 42), and the pattern of localization of Chp1 overlaps with that of H3K9Me2 and is dependent on the activity of the Clr4 H3K9Me2 transferase (10, 36, 39, 42). Cells deficient in the RNAi pathway show a reduction, but not a complete loss, of H3K9Me2 at centromeres (42, 49), demonstrating that, as was the case for telomeres and the mating-type locus, redundant RNAi-dependent and RNAi-independent mechanisms promote the recruitment of Clr4 to centromeres (Fig. 7). For heterochromatin establishment, we suggest that RNAi-independent H3K9Me2 initially recruits Chp1 and RITS to centromeres (37), which in turn recruit RDRC and Dcr1 activities (11, 32). RDRC then generates dsRNA from centromeric transcripts, which is cleaved by Dcr1 to yield siRNAs that ultimately are incorporated into RITS and may function together with Ago1 to suppress the accumulation of nascent transcripts through sequence-directed cleavage activity (9, 19). The association of RITS and RDRC with centromeres facilitates the recruitment (through an as-yet-unidentified mechanism) of high levels of Clr4 activity, resulting in the further recruitment of Chp1 and RITS in an RNAi-dependent positive feedback loop (45), which serves to amplify the H3K9Me2 signal to support the maintenance of centromeric heterochromatin. The loss of the binding of Chp1 to Tas3-Ago1 is sufficient to break this feedback loop, resulting in the loss of recruitment of Tas3-Ago1 to centromeres, the loss of the association of RDRC, a failure to generate siRNAs, reduced levels of H3K9Me2 at centromeres, and, thus, the loss of centromeric recruitment of Chp1.
FIG. 7.
Model for the role of Chp1-Tas3 interaction in the maintenance of centromeric heterochromatin. We propose the following steps. (1) Low levels of Clr4 are recruited to centromeres via an RNAi-independent mechanism and provide H3K9Me2. (2) The H3K9Me2 is bound by Chp1 in the context of RITS (green). (3) The centromeric association of RITS recruits Rdp1 and RDRC (brown). (4) Rdp1 uses the single-stranded pre-siRNA polymerase II transcript as the template for the synthesis of dsRNA. (5) Dcr1 cleaves the dsRNA to generate siRNAs. (6) The siRNAs eventually are loaded onto Ago1 in RITS and allow the Ago1-mediated cleavage of nascent centromeric transcripts, which contributes to centromeric silencing (not shown). (7) The centromeric association of RITS and RDRC facilitates the recruitment of further Clr4, which promotes the accumulation of high levels of H3K9Me2 at centromeres, enhancing the recruitment of RITS and RDRC in an RNAi-dependent positive feedback loop to allow the robust assembly of centromeric heterochromatin. In strains bearing the Tas3Δ10-24 mutant (lower panel), this RNAi-dependent positive feedback loop is disengaged, since the loss of the Chp1-Tas3 association disrupts the recruitment of RITS and RDRC to centromeres, causing a failure in the production of siRNAs and the recruitment of only low levels of H3K9Me2 to centromeres, levels that are insufficient to support the maintenance of centromeric heterochromatin.
At telomeres and the mating-type locus, heterochromatin assembly relies on both RNAi-dependent and RNAi-independent mechanisms (17, 18, 20, 22, 24, 39). However, at these loci the loss of the RNAi pathway has little impact on H3K9Me2 levels, whereas at centromeres the loss of the RNAi-dependent positive feedback loop reduces H3K9Me2 levels below the critical threshold that can support the maintenance of heterochromatin. The RNAi-dependent positive feedback loop therefore is more critical to the maintenance of heterochromatin at centromeres than at other loci, suggesting that the RNAi-independent recruitment of Clr4 to centromeres is inefficient or that heterochromatin-destabilizing activities (such as the activity of transcriptional promoters) are more pronounced at centromeres.
Role of Tas3 and Chp1 at mat2/mat3 and subtelomeres.
Our studies of cells bearing Tas3Δ10-24 have illuminated differences in the regulation of distinct heterochromatic loci. We demonstrate that at the mating-type locus, the Chp1-Tas3 association is not required for the maintenance of heterochromatin but is required for its establishment. While it was known that Chp1 plays a key role in the establishment of heterochromatin at the mating-type locus (42), through studying heterochromatin establishment in the Tas3Δ10-24-TAP mutant we have demonstrated that it is likely the disruption of RITS, and not the loss of an RITS-independent Chp1 function, that impacts heterochromatin establishment. At telomeres, we see yet a different pattern of regulation. Here, we show that although the silencing of transcripts from the subtelomeric helicase genes is maintained following the disruption of RITS function in ago1-deficient cells, tlh transcripts accumulate upon the deletion of chp1, as seen previously (18), in tas3 null cells and in the tas3Δ10-24 mutant. These results suggest that Tas3 and Chp1 function in a subcomplex that is independent of Ago1 (and therefore of RITS) to regulate tlh transcript levels. We saw no further accumulation of telomeric transcripts in tas3 chp1 double null cells (Fig. 6B) compared to that of chp1 null cells, which can be explained either by the epistasis of the two gene products, which is consistent with their association, or because Tas3 is unstable or not able to associate with telomeres in chp1-deficient cells (Fig. 6E). We note, however, that tlh transcripts always accumulate to a greater extent in chp1 null cells than in tas3 null cells, suggesting that Chp1 plays roles at telomeres independently of its association with Tas3. In contrast, Tas3 localization to telomeres is absolutely dependent on Chp1.
Interestingly, it has previously been reported that the deletion of chp1 does not prevent the silencing of telomeric reporter genes and does not affect the levels of H3K9Me2 or Swi6 association with the helicase genes (10, 22, 47). In addition, only transcripts from the forward (or sense) strand accumulate in chp1 null cells rather than transcripts from both strands, as is seen in clr4 null backgrounds (18). Thus, it is possible that the accumulation of tlh transcripts in tas3- and chp1-deficient cells and in the tas3Δ10-24 mutant is not caused by a disruption of subtelomeric heterochromatin but by a defect in the processing of tlh transcripts, similarly to that reported recently for cells mutant in cid14 or components of the exosome complex (7), in which the Swi6 and Chp1 association with tlh genes is unperturbed even though tlh transcripts accumulate. Thus, Tas3 and Chp1 may be important for the proper recruitment of RNAi-independent exosome activities to modulate the posttranscriptional degradation of subtelomeric tlh gene transcripts. Alternatively, tlh transcript levels may simply accumulate following the increased transcription of these genes in tas3 or chp1 mutant backgrounds despite the maintenance of heterochromatin at this locus.
Supplementary Material
Acknowledgments
We thank Keith Gull for anti-TAT antibody and Jurg Bahler for pFA6a-GFP (S65T)-KanMX6. Thanks go to Lawryn Kasper for advice on real-time PCR analyses, to Evan Parganas for help with figure preparation, and to Leemor Joshua-Tor, Paul Brindle, Mark Bix, Sreenath Shanker, Helen Beere, Paul Ney, Tom Lawrence, Doug Green, and Jim Ihle for their support and encouragement.
This work was supported by the Cancer Center (CORE) support grant CA21765 and by the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital.
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allshire, R. C., J. P. Javerzat, N. J. Redhead, and G. Cranston. 1994. Position effect variegation at fission yeast centromeres. Cell 76157-169. [DOI] [PubMed] [Google Scholar]
- 2.Allshire, R. C., E. R. Nimmo, K. Ekwall, J. P. Javerzat, and G. Cranston. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9218-233. [DOI] [PubMed] [Google Scholar]
- 3.Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14943-951. [DOI] [PubMed] [Google Scholar]
- 4.Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410120-124. [DOI] [PubMed] [Google Scholar]
- 5.Bernard, P., and R. Allshire. 2002. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 12419-424. [DOI] [PubMed] [Google Scholar]
- 6.Blasco, M. A. 2007. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 8299-309. [DOI] [PubMed] [Google Scholar]
- 7.Bühler, M., W. Haas, S. P. Gygi, and D. Moazed. 2007. RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 129707-721. [DOI] [PubMed] [Google Scholar]
- 8.Bühler, M., A. Verdel, and D. Moazed. 2006. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 125873-886. [DOI] [PubMed] [Google Scholar]
- 9.Buker, S. M., T. Iida, M. Buhler, J. Villen, S. P. Gygi, J. Nakayama, and D. Moazed. 2007. Two different Argonaute complexes are required for siRNA generation and heterochromatin assembly in fission yeast. Nat. Struct. Mol. Biol. 14200-207. [DOI] [PubMed] [Google Scholar]
- 10.Cam, H. P., T. Sugiyama, E. S. Chen, X. Chen, P. C. FitzGerald, and S. I. Grewal. 2005. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 37809-819. [DOI] [PubMed] [Google Scholar]
- 11.Colmenares, S. U., S. M. Buker, M. Buhler, M. Dlakic, and D. Moazed. 2007. Coupling of double-stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol. Cell 27449-461. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, J. P., E. R. Nimmo, R. C. Allshire, and T. R. Cech. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385744-747. [DOI] [PubMed] [Google Scholar]
- 13.Dalmay, T., A. Hamilton, S. Rudd, S. Angell, and D. C. Baulcombe. 2000. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101543-553. [DOI] [PubMed] [Google Scholar]
- 14.Djupedal, I., M. Portoso, H. Spahr, C. Bonilla, C. M. Gustafsson, R. C. Allshire, and K. Ekwall. 2005. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev. 192301-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewal, S. I., and A. J. Klar. 1997. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics 1461221-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grewal, S. I., and D. Moazed. 2003. Heterochromatin and epigenetic control of gene expression. Science 301798-802. [DOI] [PubMed] [Google Scholar]
- 17.Hall, I. M., G. D. Shankaranarayana, K. Noma, N. Ayoub, A. Cohen, and S. I. Grewal. 2002. Establishment and maintenance of a heterochromatin domain. Science 2972232-2237. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, K. R., P. T. Ibarra, and G. Thon. 2006. Evolutionary-conserved telomere-linked helicase genes of fission yeast are repressed by silencing factors, RNAi components and the telomere-binding protein Taz1. Nucleic Acids Res. 3478-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvine, D. V., M. Zaratiegui, N. H. Tolia, D. B. Goto, D. H. Chitwood, M. W. Vaughn, L. Joshua-Tor, and R. A. Martienssen. 2006. Argonaute slicing is required for heterochromatic silencing and spreading. Science 3131134-1137. [DOI] [PubMed] [Google Scholar]
- 20.Jia, S., K. Noma, and S. I. Grewal. 2004. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 3041971-1976. [DOI] [PubMed] [Google Scholar]
- 21.Kanellopoulou, C., S. A. Muljo, A. L. Kung, S. Ganesan, R. Drapkin, T. Jenuwein, D. M. Livingston, and K. Rajewsky. 2005. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19489-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanoh, J., M. Sadaie, T. Urano, and F. Ishikawa. 2005. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr. Biol. 151808-1819. [DOI] [PubMed] [Google Scholar]
- 23.Kato, H., D. B. Goto, R. A. Martienssen, T. Urano, K. Furukawa, and Y. Murakami. 2005. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 309467-469. [DOI] [PubMed] [Google Scholar]
- 24.Kim, H. S., E. S. Choi, J. A. Shin, Y. K. Jang, and S. D. Park. 2004. Regulation of Swi6/HP1-dependent heterochromatin assembly by cooperation of components of the mitogen-activated protein kinase pathway and a histone deacetylase Clr6. J. Biol. Chem. 27942850-42859. [DOI] [PubMed] [Google Scholar]
- 25.Kouzarides, T. 2007. Chromatin modifications and their function. Cell 128693-705. [DOI] [PubMed] [Google Scholar]
- 26.Lachner, M., D. O'Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410116-120. [DOI] [PubMed] [Google Scholar]
- 27.Lehnertz, B., Y. Ueda, A. A. Derijck, U. Braunschweig, L. Perez-Burgos, S. Kubicek, T. Chen, E. Li, T. Jenuwein, and A. H. Peters. 2003. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 131192-1200. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J., M. A. Carmell, F. V. Rivas, C. G. Marsden, J. M. Thomson, J. J. Song, S. M. Hammond, L. Joshua-Tor, and G. J. Hannon. 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science 3051437-1441. [DOI] [PubMed] [Google Scholar]
- 29.Mandell, J. G., J. Bahler, T. A. Volpe, R. A. Martienssen, and T. R. Cech. 2005. Global expression changes resulting from loss of telomeric DNA in fission yeast. Genome Biol. 6R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mello, C. C., and D. Conte, Jr. 2004. Revealing the world of RNA interference. Nature 431338-342. [DOI] [PubMed] [Google Scholar]
- 31.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194795-823. [DOI] [PubMed] [Google Scholar]
- 32.Motamedi, M. R., A. Verdel, S. U. Colmenares, S. A. Gerber, S. P. Gygi, and D. Moazed. 2004. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119789-802. [DOI] [PubMed] [Google Scholar]
- 33.Murchison, E. P., J. F. Partridge, O. H. Tam, S. Cheloufi, and G. J. Hannon. 2005. Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl. Acad. Sci. USA 10212135-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nimmo, E. R., A. L. Pidoux, P. E. Perry, and R. C. Allshire. 1998. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature 392825-828. [DOI] [PubMed] [Google Scholar]
- 35.Noma, K., T. Sugiyama, H. Cam, A. Verdel, M. Zofall, S. Jia, D. Moazed, and S. I. Grewal. 2004. RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat. Genet. 361174-1180. [DOI] [PubMed] [Google Scholar]
- 36.Partridge, J. F., B. Borgstrom, and R. C. Allshire. 2000. Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev. 14783-791. [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge, J. F., J. L. Debeauchamp, A. M. Kosinski, D. L. Ulrich, M. J. Hadler, and V. J. Noffsinger. 2007. Functional separation of the requirements for establishment and maintenance of centromeric heterochromatin. Mol. Cell 26593-602. [DOI] [PubMed] [Google Scholar]
- 38.Partridge, J. F., K. S. Scott, A. J. Bannister, T. Kouzarides, and R. C. Allshire. 2002. Cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr. Biol. 121652-1660. [DOI] [PubMed] [Google Scholar]
- 39.Petrie, V. J., J. D. Wuitschick, C. D. Givens, A. M. Kosinski, and J. F. Partridge. 2005. RNA interference (RNAi)-dependent and RNAi-independent association of the Chp1 chromodomain protein with distinct heterochromatic loci in fission yeast. Mol. Cell. Biol. 252331-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pidoux, A. L., and R. C. Allshire. 2005. The role of heterochromatin in centromere function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406593-599. [DOI] [PubMed] [Google Scholar]
- 42.Sadaie, M., T. Iida, T. Urano, and J. Nakayama. 2004. A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J. 233825-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sijen, T., J. Fleenor, F. Simmer, K. L. Thijssen, S. Parrish, L. Timmons, R. H. Plasterk, and A. Fire. 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107465-476. [DOI] [PubMed] [Google Scholar]
- 44.Song, J. J., S. K. Smith, G. J. Hannon, and L. Joshua-Tor. 2004. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 3051434-1437. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama, T., H. Cam, A. Verdel, D. Moazed, and S. I. Grewal. 2005. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl. Acad. Sci. USA 102152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thon, G., K. P. Bjerling, and I. S. Nielsen. 1999. Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151945-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thon, G., and J. Verhein-Hansen. 2000. Four chromo-domain proteins of Schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 155551-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdel, A., S. Jia, S. Gerber, T. Sugiyama, S. Gygi, S. I. Grewal, and D. Moazed. 2004. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303672-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe, T. A., C. Kidner, I. M. Hall, G. Teng, S. I. Grewal, and R. A. Martienssen. 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 2971833-1837. [DOI] [PubMed] [Google Scholar]
- 50.Yamada, T., W. Fischle, T. Sugiyama, C. D. Allis, and S. I. Grewal. 2005. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol. Cell 20173-185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.