Abstract
The murine cytomegalovirus (MCMV) M33 gene is conserved among all betaherpesviruses and encodes a homologue of seven-transmembrane receptors (7TMR) with the capacity for constitutive signaling. Previous studies have demonstrated that M33 is important for MCMV dissemination to or replication within the salivary glands. In this study, we probed N- and C-terminal regions of M33 as well as known 7TMR signature motifs in transmembrane (TM) II and TM III to determine the impact on cell surface expression, constitutive signaling, and in vivo phenotype. The region between amino acids R340 and A353 of the C terminus was found to be important for CREB- and NFAT-mediated signaling, although not essential for phosphatidylinositol turnover. Tagging or truncation of the N terminus of M33 resulted in loss of cell surface expression. Within TM II, an F79D mutation abolished constitutive signaling, demonstrating a role, as in other cellular and viral 7TMR, of TM II in receptor activation. In TM III, the arginine (but not the asparagine) residue of the NRY motif (the counterpart of the common DRY motif in cellular 7TMR) was found to be essential for constitutive signaling. Selected mutations incorporated into recombinant MCMV showed that disruption of constitutive signaling for a viral 7TMR homologue resulted in a reduced capacity to disseminate to or replicate in the salivary glands. In addition, HCMV UL33 was found to partially compensate for the lack of M33 in vivo, suggesting conserved biological roles of the UL33 gene family.
Human cytomegalovirus (HCMV) is a betaherpesvirus that establishes life-long latency within the host following primary infection. In the immunocompetent host, infection with HCMV is generally asymptomatic, with persistent virus shedding, particularly in the saliva. Reactivation can occur following immunosuppression or immunodeficiency, and under these circumstances, HCMV can be associated with various pathological conditions, including atherosclerosis, pneumonia, retinitis, colitis, and graft rejection. In addition, congenital HCMV infection can cause severe neurological disease in the immunologically immature host, producing hearing loss, visual impairment, and mental retardation (18). Although antiviral therapies have led to a significant decrease in HCMV-associated disease, viral drug resistance, drug toxicity, and immune reconstitution inflammatory syndrome result in HCMV remaining a major problem worldwide (17, 30, 49).
The CMVs are noted for the expression of multiple immunomodulatory factors, including several sequence homologues of cellular genes, which are likely to contribute to the ability of the virus to persist in the immunocompetent host (reviewed in reference 40). Of the sequence homologues, the chemokines and chemokine receptors are a common feature throughout the beta- and gammaherpesvirus families, suggesting pivotal roles for these genes in contributing to immune evasion, virus dissemination, virus replication, or a combination of these functions (15, 53, 63). Unlike most cellular chemokine receptors, a number of the viral homologues signal constitutively and bind a broad spectrum of ligands (52, 53, 66). In the case of the human herpesvirus 8 (HHV-8) chemokine receptor, ORF74, its constitutive activity has been associated with pathological properties in vivo (33).
Within the HCMV genome, the US28, US27, UL33, and UL78 genes are homologous to cellular chemokine receptors (13). Of these, US28 has been the most extensively studied. Ligand-independent activation of phospholipase C (PLC), NF-kB, NFAT, and CREB signaling by US28 has been reported in addition to ligand-independent endocytosis and recycling of the receptor (23, 37, 67). Constitutive activity of US28 may be regulated by the binding of chemokines (37). A role for US28 has been demonstrated in the migration of HCMV-infected smooth muscle cells, and hence US28 has been implicated in the development of HCMV-associated atherosclerosis (62).
Whereas homologues of US28 and US27 have been identified only in primate CMVs closely related to HCMV, homologues of UL33 and UL78 are conserved throughout the betaherpesvirus family. In the case of UL33, homologues include the M33 and R33 genes of MCMV and rat CMV (RCMV), respectively, as well as the U12 counterparts in HHV-6A/B and HHV-7 (13, 28, 43, 48, 65). Consistent with their homology to CC chemokine receptors, there is evidence that certain UL33 family members are functionally active in response to CC chemokine ligands. The HHV-6B and HHV-7 U12 genes bind CC chemokines, with subsequent mobilization of calcium from intracellular stores (35).
Unlike cellular CC chemokine receptors, UL33, M33, and R33 demonstrate constitutive activity in multiple signaling pathways (10, 32, 68). Studies of MCMV demonstrated that although M33 is dispensable for virus replication in vitro, the absence of M33 renders MCMV unable to replicate in, or disseminate to, the salivary glands following infection of mice (16). Similar results were obtained in RCMV infection studies of R33 function (4). In addition, both M33 and R33 have been shown to play a role in virus-induced smooth-muscle cell migration in vivo and, thus, may contribute to the vascular sclerosis that is observed during CMV infections (38, 61). Although chemokine binding to the CMV UL33 family has not been directly demonstrated, recent studies have shown migration of cells, transduced via an adenovirus vector expressing M33, through activation of Rac1 and extracellular signal-related kinase 1 and 2 following exposure to murine RANTES (38).
While a common feature of the virally encoded chemokine receptors is their constitutive signaling, little is known of the residues or domains that confer constitutive activity. In this article we report a mutational analysis of M33 to determine amino acid residues critical for cellular localization, constitutive signaling, and MCMV replication/dissemination in vivo. A variety of mutations, including deletions at the N and C termini and point mutations in transmembrane (TM) domains II and III, were generated and assessed for cellular expression, PLC signaling, and activation of downstream CREB- and NFAT-mediated transcription. Selected mutations were incorporated into recombinant MCMV to determine the effects upon virus replication in vivo. Furthermore, the ability of UL33 to functionally substitute for M33 in vivo was examined. In addition to determining the functional importance of specific domains/residues for M33, these studies provide the first evidence that disruption of signaling for a viral G protein-coupled receptor homologue results in an attenuated phenotype during in vivo infection.
MATERIALS AND METHODS
PCR and cloning.
Primers used for mutagenesis and cloning are shown in Table 1, and the locations of restriction sites are shown in Fig. 1. The M33 and UL33 sequences were derived from the HCMV K181 (Perth) and AD169 strains, respectively. Mutagenesis was achieved by either the megaprimer or overlap extension PCR methods (34, 54). All constructs were verified by sequencing prior to use.
TABLE 1.
PCR primers used in this study for the generation of wt, tagged, and mutated M33 fragments
| Primer namea | Sequence (5′-3′)b | Orientation | Restriction site(s)c | PCR methodd |
|---|---|---|---|---|
| M33Ecof | TTCGAATTCGGGTCGAGATGGACGTC | + | EcoRI | Standard |
| M33Ecor | CGGAATTCTCACTGGGGCGGAGGAGCGCT | − | EcoRI | Standard |
| HA-M33f | CCGCGAATTCGGATGGACGTCCTTTTGGGC | + | EcoRI | Standard |
| HA-M33r | CGGGAATTCTCACTGGGGCGGAGGAGC | − | EcoRI | Standard |
| UL33BamHindf | CGGGATCCAAGCTTATGGACACCATCATCCAC | + | (BamHI)/HindIII | Standard |
| UL33Hincr | CGGTCGACCCCGCTGAGGTTATG | − | HincII | Standard |
| EGFPF(ΔATG)f | CGCACGTGAGCAAGGGCGAGG | + | PmlI | Standard |
| EGFPr | GCTCTAGAGCGGCCGCTTACTTGTACAGCTCGTCCAT | − | (XbaI)/NotI | Standard |
| M33ND10f | CGGGATCCGTGGACGTCCTTTTGGGCCGG | + | EcoRI | Standard |
| M33CD8r | CGGGAATTCCGCGGCCGCTCACCTCTGCAGCTCCGAACGCGAGC | − | EcoRI(NotI) | Standard |
| M33CΔ24r | CGGGAATTCCGCGGCCGCTCATGCCAGCTGGCGCGCGGCGCGGTG | − | EcoRI/(NotI) | Standard |
| M33CΔ38r | CGGGAATTCCGCGGCCGCTCACACCCCAGCGCGCTCCTGAAGCATGC | − | EcoRI/(NotI) | Standard |
| M33CΔ57r | GGAATTCGCGGCCGCTCATTGCATGAAGCGTTTG | − | EcoRI/(NotI) | Standard |
| M33CΔ8xstopr | CCGGAATTCGGGCCCACCTCTGCAGCTCCGAACG | − | (EcoRI)/ApaI | Standard |
| M33CΔ24xstopr | CCGGAATTCGGGCCCTTGCCAGCTGGCGCGCGGC | − | (EcoRI)/ApaI | Standard |
| M33CΔ38xstopr | CCGGAATTCGGGCCCACACCCCAGCGCGCTCCTG | − | (EcoRI)/ApaI | Standard |
| M33CΔ57xstopr | CCGGAATTCGGGCCCATTGCATGAAGCGTTTGTTGAAG | − | (EcoRI)/ApaI | Standard |
| M33Y78Af | CATGACCAACCTGGCCTTTGCGAACCTG | + | Megaprimer | |
| M33F79Df | GACCAACCTGTACGATGCGAACCTGTTG | + | Megaprimer | |
| M33A80Df | CAACCTGTACTTTGACAACCTGTTGACGG | + | Megaprimer | |
| M33N81Df | CTGTACTTTGCGGACCTGTTGACG | + | Megaprimer | |
| M33L82Df | CTGTACTTTGCGAACGACTTGACGGTGACGATG | + | Megaprimer | |
| M33N130Df | TCGGTGGACAGGTATCGAGTGATC | + | SOE | |
| M33N130Dr | CTCGATACCTGTCCACCGAGATC | − | SOE | |
| M33R131Qf | CAGTATCGAGTGATCCACCAGAG | + | SOE | |
| M33R131Qr | CTGGTTCACCGAGATCAGCGCC | − | SOE | |
| vrM33D24f | CGCCAGCTGGCATGATCAGGGACGCTGACGCCAAGC | + | BclI | SOE |
| vrM33D24r | GCGTCCCTGATCATGCCAGCTGGCGCGCG | − | BclI | SOE |
| vrM33D38f | GCTGGGGTGTGATCGCCCTCGAC | + | SOE | |
| vrM33D38r | CGAGGGCGATCACACCCCAGCGCG | − | SOE | |
| vrUL33Hindf | CCGAAGCTTGAGATGGACACCATCATCCACAAC | + | HindIII | Standard |
| vrUL33NotXbar | GCTCTAGAGCGGCCGCTCAGACCCCGCTGAGGTTATG | − | NotI/(XbaI) | Standard |
Primers with vr prefixes were used solely for the generation of virus recombinants.
The locations of start and stop codons are underlined, where appropriate. The locations of point mutations are shown in bold.
Introduced restriction sites are shown; those in parenthesis were not used for cloning purposes in this study.
FIG. 1.
Genetic organization of the M33 region of the MCMV genome (BglII fragment) showing the location of restriction enzyme sites used for cloning in this study (not to scale). The HindIII site was introduced by site-directed mutagenesis.
Plasmids for signaling assays.
The M33 cDNA sequence (i.e., lacking the 5′ intron) in pTEJ (a kind gift of M. Waldhoer, The Panum Institute, Copenhagen, Denmark) was reamplified and cloned into the EcoRI site of pcDNA3 (Invitrogen) to generate pcDNA3/M33. The cDNA of UL33 was cloned into the EcoRI site of pcDNA3 to generate pcDNA3/UL33. The various modified forms of M33 were generated by PCR-mutagenesis and cloning of either the full M33 cassette into pcDNA3 or mutated fragments of M33 into pcDNA3/M33. For N-terminal truncation, pcDNA3/M33ΔN10 was generated by amplification of M33 lacking the initial ATG, such that translation would initiate at the next available ATG a further nine amino acids downstream. For C-terminal truncations, pcDNA3/M33ΔC57, pcDNA3/M33ΔC38, pcDNA3/M33ΔC24, and pcDNA3/M33ΔC8 were generated by amplification of M33 incorporating a stop codon prior to the final 57, 38, 24, and 8 C-terminal amino acids, respectively.
Plasmids for fluorescence imaging studies.
Hemagglutinin (HA) and c-myc tagging of the N terminus of M33 was achieved by PCR amplification of M33 lacking the initiator ATG (from pcDNA3/M33) which was cloned into the EcoRI site of pCMV-HA or pCMV-myc, respectively (BD Biosciences, Oxford, United Kingdom). A pTEJ-based plasmid containing M33 fused in frame with enhanced green fluorescent protein (EGFP) at the C terminus was kindly provided by M. Waldhoer, The Panum Institute, Copenhagen, Denmark. The M33-EGFP cassette from this plasmid was PCR amplified and cloned into pcDNA3 to generate pcDNA3/M33-GFP. All mutant M33 constructs (described above in “Plasmids for signaling assays”) were fused with EGFP at the C terminus to facilitate protein localization studies in transfected cells. Mutations upstream of the PfiMI site (position 890 within the M33 coding sequence (Fig. 1) were subcloned from the mutant pcDNA3/M33 plasmids as PfiMI/HindIII fragments and introduced into PfiMI/HindIII-digested pcDNA3/M33-GFP. The C-terminal deletion mutants were PCR amplified as full-length cassettes, using primers that incorporated an EcoRI upstream of the start codon and an ApaI site at the position of the 5′ truncation, and cloned in frame into EcoRI/ApaI-digested pEGFP-N1 (BD Biosciences, Oxford, United Kingdom). For the generation of UL33-GFP, a three-way ligation was performed between HindIII/NotI-digested pcDNA3, a UL33 fragment that had been amplified from pcDNA/UL33 with 5′ HindIII and 3′ HincII restriction sites (the latter blunt site replacing the stop codon), and an EGFP fragment amplified from p-EGPF-N1 (Clontech) with a 5′ PmlI restriction site (concomitantly removing the initiating methionine) and a 3′ NotI site.
Plasmids for preparation of MCMV recombinants.
The recombination plasmid pING/M33, comprising an MCMV BglII fragment [between 39.4 and 44.4 kb of the MCMV K181 (Perth) genomic sequence] that contains the M33 sequence and approximately 5 kb of flanking sequence, has been described previously (16). A second recombination plasmid, designated pGEM/M33, was generated by cloning the above BglII fragment into the PvuII site of pGEM 4(Z). To facilitate the introduction of N-terminal mutations of M33, the pGEM/M33 plasmid was subsequently modified by the introduction of a unique HindIII site upstream of the M33 start codon to produce pGEM/hM33. The generation of 24- and 38-residue C-terminal truncations of M33 was achieved by the introduction of in-frame stop codons at appropriate sites within M33 while retaining M33 sequences downstream of the stop codon. The modified fragments were subsequently cloned into pING/M33 as BstEII/NotI fragments to produce pING/M33ΔC24 and pING/M33ΔC38, respectively. M33 TM III point mutations N130D and R131Q were introduced into pGEM/hM33 by subcloning BstEII/NsiI fragments from their respective pcDNA3 signaling plasmids (described above) into pGEM/hM33. The M33 TM II F79D mutation was cloned from pcDNA3/M33(F79D) as a HindIII/BstEII fragment into pGEM/hM33. GFP-tagged wild-type (wt) and mutated M33 were introduced into pING/M33 via shuttling of a BstEII/NotI fragment from the relevant GFP-tagged expression plasmid (described above). Replacement of M33 with untagged UL33 was achieved by amplification, with 5′ HindIII and 3′ NotI sites, from plasmids derived from either genomic DNA (intron plus) or cDNA (intron minus). The UL33 cassettes were then inserted between the HindIII/NotI sites of pGEM/hM33 to produce plasmids pGEM/UL33 and pGEM/UL33Δi, respectively. Replacement of the wt M33 sequence (intron plus) with M33 lacking the intron was achieved by amplification of M33 (with 5′ HindIII and 3′ NotI sites) from pcDNA3/M33 and cloning into the HindIII/NotI sites of pGEM/hM33 to produce plasmid pGEM/M33Δi.
Purification of expression plasmids.
Plasmids used in transfection experiments were purified using Qiagen EndoFree plasmid kits to an optical density ratio (260 nm:280 nm) of greater than 1.8 prior to use.
Luciferase reporter assays.
HEK-293 cells were grown in Dulbecco's modified Eagle's medium (DMEM)-GlutaMAX (Invitrogen) supplemented with 10% heat inactivated fetal calf serum, 180 U/ml penicillin, and 45 μg/ml streptomycin at 37°C and 5% CO2. Cells were seeded (5 × 104 cells/well) in 96-well black and white isoplates (PerkinElmer Life and Analytical Sciences) using medium without antibiotics. One day after seeding, cells were cotransfected with various concentrations of pcDNA3-based receptor plasmid and either 50 ng of pNFAT-Luc (Pathdetect cis-reporting system) or 50 ng of pFR-Luc and 6 ng of pFA2-CREB (Pathdetect trans-reporting system; both from Stratagene). Transfections were carried out using OptiMEM and Lipofectamine 2000 reagent according to the manufacturer's guidelines (Invitrogen). The medium was replaced with DMEM-GlutaMAX containing 0.5% heat-inactivated fetal calf serum without antibiotics at 5 h posttransfection. After a 24-h incubation, the cells were washed with phosphate-buffered saline (PBS), and an equal volume of LucLite was added (PerkinElmer). Luminescence was measured for 5 s at 22°C, following 10 min of dark adaptation on a TopCount microplate scintillation and luminescence counter.
Phosphatidylinositol turnover assay.
HEK-293 cells were seeded (3.5 × 104 cells/well) in poly-l-lysine-coated 96-well plates and cultured overnight in growth medium (DMEM containing 10% FBS, 180 U/ml penicillin, and 45 U/ml streptomycin) at 37°C and 10% CO2. The cells were cotransfected the following day with receptor constructs at 3, 6, or 10 ng/well, and the promiscuous Gα subunit GαΔ6qi4myr ((kindly provided by Evi Konstenis, 7TM-Pharma, Denmark) at 10 ng/well using the serum-free medium OptiMEM and Lipofectamine 2000 at 10 μl/ml. At 5 h posttransfection the cells were transferred to normal growth medium. Twenty-four hours later the cells were labeled with 5 μCi of [myo-3H]inositol per well and incubated overnight at 37°C. The following day, the cells were washed once in assay buffer (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, and CaCl2) and incubated for 90 min at 37°C in assay buffer containing 10 mM LiCl. Subsequently, the cells were incubated on ice in cold formic acid (10 mM) for 30 min. A total of 20 μl of the lysis solution was subsequently transferred to a new white 96-well plate and mixed with 80 μl of freshly diluted scintillation proximity assay YSi beads at 12.5 mg/ml. The plates were vigorously shaken at room temperature for 30 min, centrifuged at 1,500 rpm for 5 min, left overnight at room temperature, and counted the following day using a TopCounter (Packard) after a final round of centrifugation.
Quantitation of expression in transfected cells.
HEK-293 cells were transfected with various amounts (6.25 to 200 ng/well) of expression plasmids for GFP-tagged (C terminus) wt and mutated M33, using the methods described above (luciferase reporter assays) with the following modifications. Cells were seeded into 96-well optical bottom black-walled plates (Fluorcarbon/Black; Nunc). At 24 h posttransfection, cells were washed three times in Hanks balanced salt solution supplemented with Ca2+ and Mg2+ salts (HBSS plus CaCl2 and MgCl2; Invitrogen). Fluorescence was quantified using a POLARstar fluorimeter (BMG Labtech), with excitation (485 nm) and measurement of emission (520 nm) via the base of the plate.
Quantitation of expression in infected cells.
Mouse embryonic fibroblasts (MEFs), cultured in minimal essential medium-GlutaMAX supplemented with 10% fetal calf serum, 180 U/ml penicillin, and 45 U/ml streptomycin (Invitrogen), were seeded at 4 × 104 cells per well in 96-well optical bottom black-walled plates (Fluorcarbon/Black; Nunc). After overnight growth, cultures were infected at a high multiplicity of infection (3 PFU/cell) with either K181 or recombinant MCMV expressing GFP-tagged M33 (wt or mutated). At 15 h postinfection, the culture medium was removed, and cells were washed (two times) using PBS and fixed in 2% paraformaldehyde-PBS for 15 min at room temperature. Cells were then washed (three times) with PBS and permeabilized using 0.2% Triton X-100-PBS for 15 min at room temperature. Cells were processed for immunofluorescence as follows (room temperature throughout): wash (twice in PBS), block (5% normal goat serum [NGS]-PBS for 30 min), wash (one time in PBS), primary antibody (1/1,000 rabbit anti-GFP [Abcam AB290] in 1% NGS-PBS for 1 h), wash (three times in PBS), secondary antibody (1/1,000 goat anti-rabbit Alexa Fluor 488 conjugate [Molecular Probes A-11034] in 1% NGS-PBS for 1 h), and wash (three times in PBS). Fluorescence was quantified by fluorimeter as described above.
Fluorescent imaging.
HEK-293 cells (106 cells/well in six-well trays; Corning) were grown in antibiotic-free DMEM-GlutaMAX supplemented with 8% fetal calf serum for 24 h prior to transfection with 2 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 6 h after transfection, cells were trypsinized and distributed evenly across three wells of glass coverslip cultures in 24-well trays (Corning). Following a further 18-h incubation at 37°C, coverslips were processed for confocal microscopy, using HBSS plus CaCl2 and MgCl2 (Invitrogen) for all wash and incubation steps, as follows. Cells were washed (two times) and then incubated (for 10 min at 37°C) with Image-iT LIVE plasma membrane and a nuclear labeling kit (Invitrogen), using 200 μl of labeling solution (5 μg/ml Alexa Fluor 594 wheat germ agglutinin) per coverslip. Cells were then washed (two times) and fixed using 2% paraformaldehyde-HBSS for 15 min at 37°C. Fixed cells were washed (three times), and coverslips were mounted onto glass slides using ProLong Gold Antifade reagent (Invitrogen). Slides were examined using a Leica TCS SP2 confocal microscope fitted with a 63× objective, and images were acquired with the Leica confocal software using a zoom factor of ×2.5. Images were imported into Adobe Photoshop CS2, version 9, and overlays of the GFP and Alexa Fluor 594 images were produced.
Generation of recombinant virus.
Purified genomic DNA was obtained from MEFs infected with KΔ33BT2 recombinant virus, which contains the E. coli lacZ cassette within the second exon of M33 (15). Accordingly, this parent virus exhibits blue plaques upon staining with 5-bromo-4-chloro-3-inolyl-β-d-galactopyranoside. Transfection of MEFs was performed using the calcium phosphate method as previously described (16). Recombinant virus progeny were selected by the presence of “white” plaques. Recombination frequencies were of the order of 0.5 to 4%. Recombinant viruses were plaque purified at least twice and verified by sequencing across the M33 region. As a control, five independent transfections were performed between KΔ33BT2 genomic DNA and the wt recombination plasmid pING/M33 to establish whether “repair” of the M33 region conferred wt properties of the resulting recombinant progeny in each case.
Growth of MCMV recombinants in vitro.
Following several rounds of plaque purification, working stocks of MCMV recombinants were prepared, and titers were determined by plaque assay on MEFs. Multistep growth of each of the recombinants in NIH-3T3 cells was compared with that of wt MCMV over a 5-day period. Subconfluent cultures of NIH-3T3 cells were prepared in six-well dishes and infected with each of the MCMV recombinants or wt MCMV at a multiplicity of infection (MOI) of 0.01. Infected culture medium (cell free) was harvested daily for 5 days and stored at −80°C until quantification by plaque assay on MEFs. Values shown are the average from two independent cultures for each virus.
Growth of MCMV recombinants in vivo.
In the first set of experiments (“low dose”), groups of five weanling BALB/c female mice were inoculated intraperitoneally with 5 × 103 PFU of either wt MCMV or recombinants. Seventeen days postinoculation, the mice were euthanized, and the virus titer from the pooled salivary glands was determined by plaque assay on MEFs. A second experiment (“high dose”) was performed in the same manner, except a higher virus dose was used as the inoculum (4 × 104 PFU), and the titers from the salivary glands were assessed from individual mice. Results are expressed as the total number of PFU recovered per salivary gland.
Western blotting.
COS-7 cells (2 × 105 cells/well in six-well trays) were seeded at 37°C and 10% CO2 in minimal essential medium-GlutaMAX supplemented with 10% fetal calf serum (antibiotic free) prior to transfection with 1 μg/well of plasmid DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's guidelines. Untransfected cells were used as controls. At 24 h posttransfection, cells (harvested from two wells for each sample) were washed twice with PBS and lysed with 200 μl of ice-cold nonionic solubilization buffer (20 mM Tris-HCl, 150 mM NaCl, 1% sodium deoxycholate, 1% NP-40, 2 mM EDTA, and 10 mM iodoacetamide, supplemented with 1 protease inhibitor cocktail tablet/10 ml [Roche] prior to use). Samples were centrifuged at 17,900 × g for 15 min at 4°C, and the supernatants were discarded. Pellets were lysed in 50 μl of the above solubilization buffer that had been supplemented with 0.1% sodium dodecyl sulfate (SDS). Samples were centrifuged at 17,900 × g for 15 min at 4°C to remove insoluble material. Soluble lysates were mixed 1:1 with 2× reducing sample buffer (125 mM Tris-HCl, 20% glycerol, 4% SDS, 0.2% bromophenol blue, and 200 mM dithiothreitol) and loaded directly (without heating) onto 10% polyacrylamide gels, using 16 μl of diluted lysate (derived from 6.4 × 104 cells) per lane. Duplicate gels were stained with Coomassie for total protein detection. Following SDS-polyacrylamide gel electrophoresis, protein was transferred to nitrocellulose, blocked with PBS-0.05% Tween 20 (PBS-T) containing 5% bovine serum albumin (BSA), and probed with a mouse anti-GFP monoclonal antibody (monoclonal antibody JL8; BD Living Colors). Following extensive washing with PBS-T, membranes were probed with a horseradish peroxidase-conjugated secondary antibody (Dako) in PBS-T containing 5% bovine serum albumin and again washed thoroughly in PBS-T, and bound antibody was visualized with an enhanced chemiluminescence kit (Amersham Biosciences). Protein separation was visualized with rainbow prestained markers (Bio-Rad); biotinylated protein markers (Bio-Rad) were additionally used for marker visualization on ECL Hyperfilm.
Statistics.
For CREB and NFAT signaling assays, wt and mutant M33 samples were tested alongside the pcDNA3 empty vector control in quadruplicate in each assay. Assays were repeated two to four times, giving 8 to 16 sets of data in each case. For phosphatidylinositol (PI) turnover assays, both groups were tested in triplicate, with assays repeated three times, for a total of nine sets of data. Within each assay, raw data were normalized as a percentage of the negative control (100 × S/C, where S is the value of the sample and C is the mean value of the vector control samples). The transformed values were then used to calculate the overall means and standard errors for each sample from the combined values of the repeated assays, expressed as a percentage of pcDNA3. For determination of expression levels in transfected cells by fluorimetry, samples were tested in quadruplicate and analyzed as above (n = 4). For determination of expression levels in infected cells by fluorimetry, six replicates were used (n = 6), and results were normalized to K181-infected (GFP negative) control wells. Graphing and statistical tests (analysis of variance with Bonferroni posttests for comparison of wt M33 with mutants) were performed using GraphPad Prism, version 5.00, for Windows (www.graphpad.com).
RESULTS
Cell surface expression and signaling properties of wt M33 and UL33.
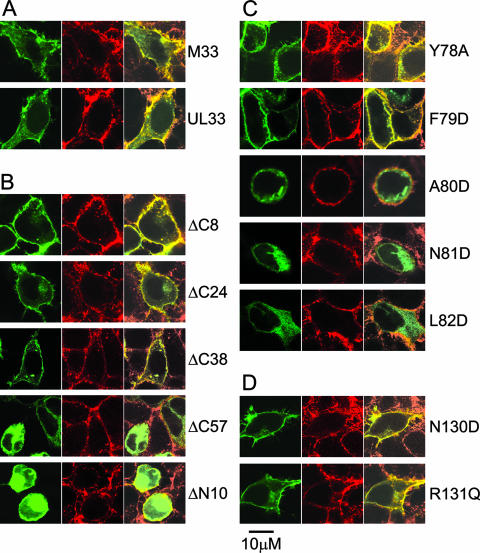
It has been demonstrated previously that C-terminally tagged M33 and the HCMV homologue, UL33, are expressed at the cell surface and are constitutively active (24, 68). In the absence of available antiserum to M33, both N-terminal (c-myc and HA) and C-terminal (EGFP) tagging strategies were assessed to determine the optimal method for the detection of wt M33 at the cell surface, to which subsequent M33 mutants could be compared. Cellular expression of wt M33 in HEK-293 cells was examined by confocal microscopy, using wheat germ agglutinin as a marker for cell surface expression; wt UL33 was included as a comparison. Both wt M33-GFP and wt UL33-GFP were clearly detected at the cell surface as well as in the perinuclear Golgi region and small vesicular structures (see Fig. 3), consistent with previous studies. In contrast, the HA and c-myc N-terminal tags of wt M33 each failed to be detected at the cell surface, with concomitant loss of constitutive signaling (data not shown), suggesting that the N-terminal region of M33 is important for translocation of the protein to the cell surface. Consequently, C-terminal EGFP tagging was used to compare the distribution of M33 mutants with that of wt M33.
FIG. 3.
Expression characteristics of wt M33, wt UL33, and M33 mutants. HEK-293 cells were transiently transfected with plasmids expressing GFP-tagged wt M33 or wt UL33 (A), the C- or N-terminal truncation M33 mutants (B), the TM II M33 mutants (C), or the TM III M33 mutants (D). Fluorescence was visualized by confocal microscopy at 24 h posttransfection with the cell surface shown by the reactivity of Alexa Fluor 594-conjugated wheat germ agglutinin (red, middle panels), the distribution of the M33 constructs shown by EGFP fluorescence (green, left panels), and the colocalization of the expressed M33 constructs with the cell surface (yellow, right panels). Scale bar, 10 μm.
Cell surface expression and signaling properties of M33 mutants.
To identify regions of critical importance to M33 expression and signaling, N- and C-terminal truncations, as well as TM II and TM III point mutations were generated as summarized in Fig. 2, and the mutants were examined for cellular expression and constitutive CRE- and NFAT-mediated signaling in HEK-293 cells.
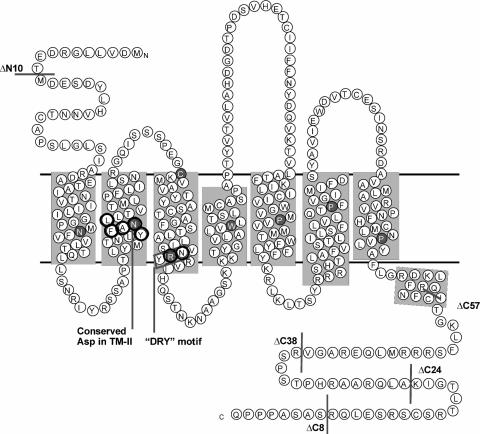
FIG. 2.
Serpentine model of MCMV M33 indicating positions of mutations. The predicted topology of M33 within the cell membrane is shown, with shading indicating TMs I to VII and the eighth predicted α-helix. The M33 sequence shown is of the K181 (Perth) strain used in this study (GenPept accession number CAP08079). This sequence differs by two conservative amino acid substitutions relative to the prototypic Smith strain (GenPept accession number Q83207), namely L108 → M and M319 → L. N- and C-terminal truncation mutants characterized in this study are indicated with gray bars; point mutations are circled in black. The location of the 7TMR-conserved DRY motif (NRY in M33) is indicated by the box. Positions of residues conserved among rhodopsin-like 7TMR are indicated as white letters within gray circles.
N- and C-terminal truncation mutants of M33.
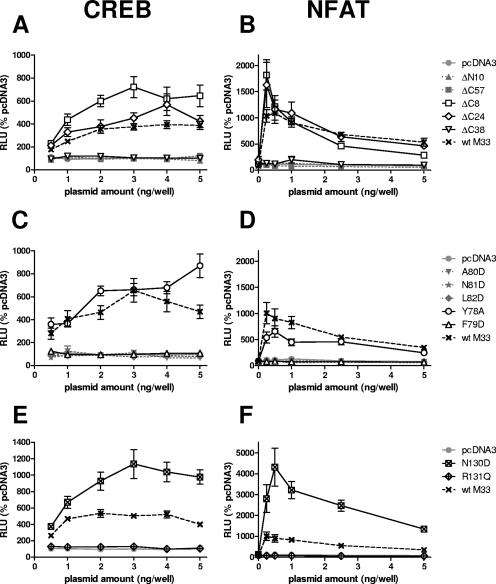
While the N termini of seven-transmembrane receptors (7TMR) are not generally implicated in the regulation of constitutive 7TMR signaling per se, signaling could potentially be modulated via their interaction with cognate ligands. Thus, for M33, it was predicted that little or no effect in constitutive signaling in vitro would result following mutation of the N terminus. Nevertheless, if M33 signaling in vivo was regulated by ligand interaction, then such a mutation may exhibit an in vivo phenotype. A deletion of 10 amino acids from the N terminus (M33ΔN10) prevented detection of the receptor at the cell surface (Fig. 3B) and ablated activation of CRE- and NFAT-mediated transcription (Fig. 4A and B). Taken together with the observations of disrupted cellular distribution and signaling by the N-terminally tagged (HA or c-myc) M33 mutants, these data suggest that the N terminus is required for correct subcellular trafficking of M33.
FIG. 4.
CREB-mediated transcription (A, C, and E) and NFAT-mediated transcription (B, D, and F) stimulated by wt M33 and mutant derivatives. HEK-293 cells were transiently transfected with either the C- or N-terminal M33 truncation mutants (A and B), the TM II M33 mutants (C and D), or the TM III M33 mutants (E and F) at the various doses shown, together with luciferase reporter constructs. Results are expressed as relative light units (RLU) compared to the negative control plasmid pcDNA3. wt M33 was included as a reference in each assay. Mutants which were negative for cell surface distribution are shown as gray symbols with dotted lines, and mutants which were positive for cell surface distribution are shown as black open or crossed symbols with solid lines; the symbols are identified on the figure. The mean and standard error are shown (n = 8 to 16).
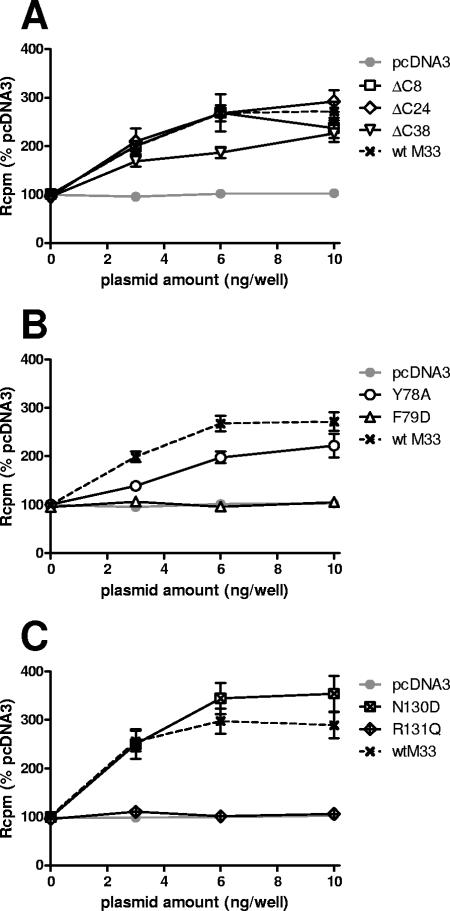
Regulation of G protein coupling and cell surface expression have been ascribed to the C termini of numerous 7TMR. Thus, we examined how progressive C-terminal deletions of M33 affected signaling, expression, and in vivo function. Truncations of 8, 24, 38, and 57 amino acids (designated M33ΔC8, M33ΔC24, M33ΔC38, and M33ΔC57, respectively) were generated. Of these truncation mutants, M33ΔC8, M33ΔC24 and M33ΔC38 (lacking amino acids 370 to 377, 354 to 377, and 340 to 377, respectively) were all localized at the cell surface, but there was no detectable cell surface expression for the mutant with the largest deletion (amino acids 321 to 377), M33ΔC57 (Fig. 3B). M33ΔC8 and M33ΔC24 were constitutively active in CREB and NFAT signal transduction pathways (Fig. 4A and B). Notably, M33ΔC8 stimulated CRE-mediated transcription that was between 70 to 130% higher than wt M33 at all doses examined (the differences were statistically significant, with a P value of <0.001 between 1 to 5 ng/well). However, CRE- and NFAT-mediated transcription was undetectable for M33ΔC38 despite its localization at the cell surface. As expected, there was no constitutive activation for M33ΔC57. These data indicate that while the final 38 amino acids (340 to 377) of the M33 C terminus are dispensable for cell surface expression, the region between amino acids 340 to 353 of M33 (present in M33ΔC24 but deleted in M33ΔC38) is important for constitutive CRE- and NFAT-mediated signaling.
M33 TM II point mutations.
Within the second predicted TM domain of the 7TMR family is a highly conserved, positively charged aspartic acid located at II:10, according to the numbering system introduced by Baldwin (1) and modified by Schwartz (58). In many 7TMR this residue has been shown to be important for either ligand binding or signal transduction or both. In addition, it has a major role in the sensitivity of some 7TMR to sodium ion regulation of ligand binding (11, 20). ORF74 from HHV-8 lacks this conserved acidic residue, and it was recently demonstrated that the introduction of aspartic acid at this locus resulted in loss of constitutive activity but retention of its ability to be stimulated by agonist (50). M33 also lacks an acidic amino acid within TM II, and therefore several residues in this region were altered to aspartic acid to assess the effect on receptor function. These included N81 [M33(N81D); II:10], which is the residue most likely to correspond to the conserved Asp determined by alignment with other 7TMR, and the surrounding residues, F79 [M33(F79D); II:08], A80 [M33(A80D); II:09], and L82 [M33(L82D); II:11]. In addition, residue Y78 (II:07) was mutated to alanine M33(Y78A) as alanine is much more commonly found in 7TMR at this position. Of the TM II mutants, only M33(Y78A) and M33(F79D) could be detected at the cell periphery (Fig. 3C). The three other mutants that were not detected at the cell surface were negative for constitutive signaling (Fig. 4C and D). M33(Y78A) retained constitutive activation of both CRE- and NFAT-mediated transcription. In contrast, despite its detection at the cell surface, M33(F79D) did not stimulate either signaling pathway (Fig. 4C and D).
Mutations of the M33 NRY motif.
An amino acid triplet motif, (D/E)RY, is found at the cytoplasmic end of TM III of most 7TMR. This sequence plays an important role in 7TMR activation. Of these residues, the arginine is the most highly conserved, and mutations of this residue generally confer a loss of G protein-mediated signaling (2, 44, 57, 71). In contrast, mutational analysis of the D/E residue, whereby the acidic residue is replaced by a neutral residue, has revealed a gain of constitutive activity for several receptors (42, 47, 57). UL33 has the prototypic DRY motif, whereas the equivalent motif within R33 and M33 is NRY. To assess the effects of conversion of this motif to the conventional DRY or modification of the arginine in M33, both N130D and R131Q mutations were made. Expression of both mutant receptors was detected at the cell surface similar to wt M33 (Fig. 3D); however, only M33(N130D) was constitutively active (Fig. 4E and F). This mutant activated CRE-mediated signaling at a level 60 to 200% greater than wt M33 levels (P < 0.05, 1 to 5 ng), whereas a much higher increase from 200 to 440% was observed through NFAT signaling (P < 0.05, 0.25 to 5 ng/well).
PLC activity.
The mutant receptors that retained cell surface distribution were further assessed for membrane-associated PLC activation by measurement of PI turnover across a plasmid dose range. In agreement with previous reports (60, 68), constitutive activity was observed for wt M33 (Fig. 5). All mutants which were positive for CREB and NFAT signaling[M33ΔC8, M33ΔC24, M33(Y78A), and M33(N130D)] were positive for PI turnover (Fig. 5), although in the case of (Y78A)M33the level of signaling was somewhat reduced (30 to 60% lower than wt M33; P < 0.01, 3 to 10 ng/well). However, while no CREB- or NFAT-mediated signaling was detected for the M33ΔC38 mutant, there was activation of PLC (Fig. 5A), albeit at 30 to 50% reduced levels compared with wt M33 (the difference was statistically significant only at 6 ng; P < 0.001). The other two mutants that were negative for CREB- and NFAT-mediated signaling [M33(F79D) and M33(R131Q)] were also negative for IP turnover (Fig. 5B and C). Therefore, the effect most mutants had on PI turnover was similar to its effect on the downstream CREB and NFAT signal transduction pathways.
FIG. 5.
Activation of PLC by cell surface-expressed receptors. HEK-293 cells were transiently transfected with selected M33 mutants (cell surface expressed), alongside wt M33 and pcDNA3. Measurements of [myo-3H]inositol phosphorylation stimulated by the various constructs are expressed as relative cpm (Rcpm) compared to pcDNA3. The mean and standard error are shown (n = 9).
Detection of wt M33 and mutated derivatives by Western blotting.
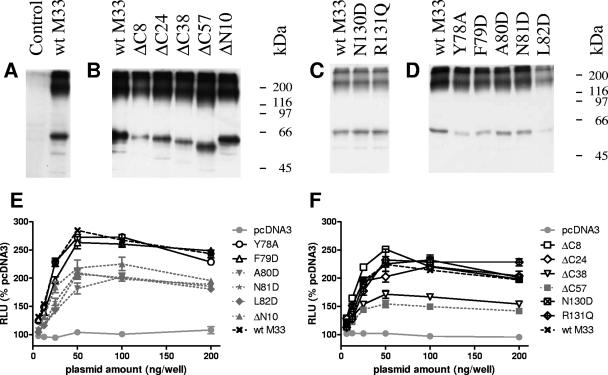
To determine whether the M33 mutants were aberrantly expressed compared with wt M33, lysates from transfected COS-7 cells were examined by Western blotting, using an anti-GFP monoclonal antibody (JL8; Clontech). Due to the number of constructs examined, the transfections and Western blotting were carried out in batches on separate occasions, with wt M33 included each time as a control. Typical results are shown in Fig. 6A to D. The GFP-tagged wt M33 protein was detected as a major band running slightly lower than the predicted mass of 69 kDa. Slight anomalies in migration from the predicted size may have been due to the fact that samples were not heated prior to loading to prevent M33 aggregation. Point mutants of M33 displayed the same mobility as wt M33, while truncations conferred minor changes in mobility, consistent with the sizes of deletion. Higher forms of each of the proteins were also detected, which is consistent with oligomer formation. These studies confirmed that expression of wt M33 and the mutated derivatives was at approximately the expected mass and suggest that the mutations did not affect oligomerization of the receptors. For wt M33 and the various mutants, a minor, lower-molecular-weight band was also detected, which may be a breakdown product or otherwise truncated form of the full-length proteins.
FIG. 6.
Western blotting and total protein expression analysis of GFP-tagged wt M33 and mutated M33 derivatives. (A to D) Western blotting samples were prepared from COS-7 cell lysates 24 h posttransfection and immunoblotted using an anti-EGFP monoclonal antibody. Blots were derived from separate experiments, with wt M33-transfected cells included on each occasion for comparison purposes. (E and F) Total protein expression. HEK-293 cells were transfected with the various GFP-tagged mutants, alongside wt M33 and pcDNA3. Mutants which were negative for cell surface distribution are shown as gray symbols with dotted lines, and mutants which were positive for cell surface distribution are shown as black open or crossed symbols with solid lines; the symbols are specified in the figure. GFP fluorescence was determined and is expressed as relative light units (RLU) compared with the negative control (pcDNA3). The mean and standard error are shown (n = 4).
Quantitation of expression of wt M33 and mutated derivatives by fluorimetry.
In order to determine the relative levels of protein expression for the various mutants, relevant to the signaling assays, HEK-293 cells were transfected with GFP-tagged mutant and wt M33, and total protein levels were determined via GFP fluorescence (Fig. 6E and F) All of the mutants which were negative for cell surface expression were found to have reduced levels of GFP fluorescence (generally 30 to 60% of wt M33 levels; P < 0.05 for all cell surface negative mutants from 12.5 to 200 ng/well). The remaining mutants were expressed at levels similar to wt M33 across the range of DNA doses tested, with the exception of M33ΔC38, which was consistently lower (approximately 60% wt M33 levels; P < 0.05, 25 to 200 ng/well).
In vitro and in vivo growth properties of MCMVs bearing M33 mutations.
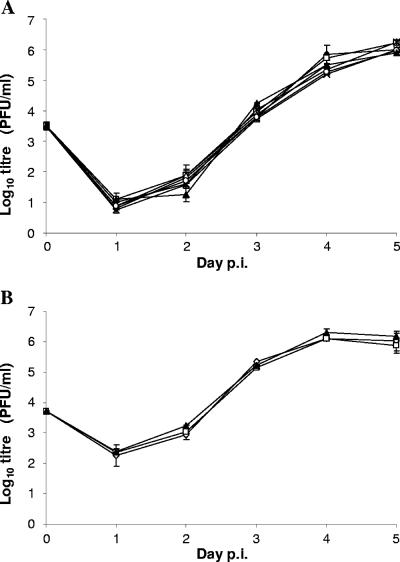
A number of the M33 mutants were selected for the generation of MCMV recombinants. Prior to investigations of in vivo replication, each of the recombinants was assessed for replication in vitro. Multistep growth analysis revealed that there was no significant difference between the recombinants and wt in their ability to replicate in NIH-3T3 cells (Fig. 7). At various occasions during the course of construction and in vivo assay of mutant viruses, the ability of wt M33 to restore the wt M33 phenotype from the lacZ-disrupted M33 parental virus was assessed. Thus, recombinant progeny from five independent transfections that resulted in repair of the disrupted M33 region were evaluated for their ability to disseminate to the salivary glands in weanling BALB/c mice. Titers of M33 revertants were compared with those of M33-disrupted parent virus (KΔ33BT2) and wt MCMV [K181 (Perth) strain] (Table 2). While the K181 virus strain replicated to high levels in the salivary glands, virus was not detected in mice infected with the KΔ33BT2 parent recombinant, consistent with previous studies (16). In contrast, all MCMV “wt-reconstituted” revertants grew to levels similar to wt K181, demonstrating that restoration of the wt phenotype upon repair of M33 was reproducible. The large difference in titer of MCMV between wt and the M33 deletion mutant enabled us to determine whether selected M33 recombinants resulted in full or partial recovery of the wt phenotype for salivary gland replication.
FIG. 7.
Multistep in vitro growth curve of wt MCMV and M33 mutants. Subconfluent cultures of NIH-3T3 cells were prepared in six-well dishes and infected with each of the MCMV recombinants or wt MCMV at an MOI of 0.01. Infected culture medium (cell free) was harvested daily for 5 days and stored at −80°C until quantification by plaque assay on MEFs. The mean and standard errors are shown (duplicate cultures were titrated for each virus). Panels A and B comprise two separate experiments, characterizing different sets of viruses. In panel A, the symbols are as follows: filled triangles, wt MCMV; open circles, M33Δi; open triangles, M33ΔC24; crosses, M33ΔC38; filled diamonds, M33(F79D); stars, M33(N130D); and open squares, UL33. In panel B the wt MCMV is represented by filled triangles, and two independent M33(R131Q) transfectants are represented by open diamonds and open squares. p.i., postinfection.
TABLE 2.
Detection of wt MCMV and M33 recombinants in mouse salivary glands
| MCMV constructa | In vitro signaling by pathwayb
|
In vivo titer (log10 PFU/s.g.) ± SDc
|
|||
|---|---|---|---|---|---|
| CREB | NFAT | PLC | Low dosed | High dosee | |
| wt M33 | ++ | ++ | ++ | 6.2, 5.9 | 5.8 ± 0.2 |
| ΔM33BT2 | − | − | − | <1.0 | <1.0 |
| M33 Revertant | ++ | ++ | ++ | 5.5, 5.9, 5.7, 6.1, 5.8 | ND |
| M33Δi | ++ | ++ | ++ | 4.6, 4.8 | ND |
| Μ33ΔC24 | ++ | ++ | ++ | 5.0 | 5.9 ± 0.2 |
| Μ33ΔC38 | − | − | + | 2.8, 1.7 | 4.4 ± 0.1 |
| M33(F79D) | − | − | − | <1.0, <1.0 | <1.0, <1.0 |
| M33(N130D) | +++ | +++ | ++ | 5.3 | 5.8 ± 0.2 |
| M33(R131Q) | − | − | − | ND | <1.0, <1.0 |
| UL33 | ** | ** | ** | 2.4 | ND |
| UL33ΔI | ** | ** | ** | <1.0 | ND |
M33 revertant and mutated virus constructs were all derived from KΔM33BT2 (M33 disrupted with lacZ, described previously [16]).
Predicted signaling properties of the MCMV recombinants for each of the three pathways are indicated as either negative (−), reduced (+), similar (++), or increased (+++) compared to wt M33. UL33 signaling by each of these pathways, which is not equivalent to M33, is indicated (**).
Mean virus titers recovered from the salivary glands (s.g.) 17 days postinfection Where more than one titer is presented for a given recombinant virus, this corresponds to the analysis of an independent virus recombinant(s). ND, not done.
Mice were inoculated with 5 × 103 PFU intraperitoneally of either wt MCMV or mutated M33 derivatives; titers were derived from a pool of five mice that were titrated in triplicate. The standard deviation of each of the samples (as a measure of the variation of the plaque assay) was <5% of the mean value and is not shown.
Mice were inoculated with 4 × 104 PFU intraperitoneally of either wt MCMV or mutated M33 derivatives; titers were derived from the average of five individually processed mouse salivary glands, and the standard deviation (SD) for each group is shown.
The mutations incorporated into MCMV recombinants were as follows: (i) C-terminal truncations of 24 and 38 amino acids; (ii) point substitutions of TM II F79D, TM III N130D, and TM III R131Q; and (iii) replacement of M33 with UL33. For the F79D mutant, the intron near the 5′ end of the M33 coding sequence was also lacking. In order to determine whether loss of the intron had a major effect upon virus replication, a recombinant with the intron removed but no other mutations of M33 was also examined. All recombinant viruses were sequenced across the entire M33 coding region to confirm the introduction of the mutation prior to conducting in vivo infections (data not shown). In addition, virus recovered from the salivary glands (where detected) was also sequenced to confirm the presence of the relevant mutation (data not shown).
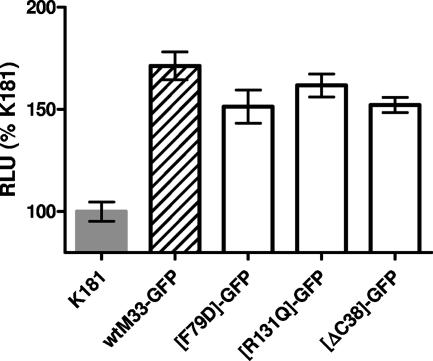
The titers of viruses recovered from the salivary glands following a series of intraperitoneal infection experiments, using either a high- or low-dose inoculum, are shown in Table 2. Following infection at the lower dose (5 × 103 PFU/mouse), the intronless, control recombinant (M33Δi) rescued salivary gland replication but yielded a salivary gland titer approximately 10-fold lower than that of the revertants that retained the intron. This indicates that retention of the M33 intron sequence is required for full salivary gland virulence, but loss of the intron results in a relatively minor attenuation. Whether this effect is mediated via transcriptional regulation of M33 expression or some other mechanism is not known. The C-terminal truncation mutants had displayed similar cell surface distribution but markedly different signaling properties. Whereas M33ΔC24 (signaling similar to wt M33) grew to levels in the salivary glands that were up to 10-fold lower than wt MCMV, the replication of the ΔC38 truncation mutant (reduced signaling compared with wt) was reduced approximately 1,000-fold. Similar to the truncation mutants, the point mutants had retained cell surface expression but showed markedly different signaling properties. Whereas the M33(N130D) mutant (signaling increased compared to wt M33) replicated to wt MCMV levels in the salivary glands, the M33(F79D) mutation (negative for signaling) was negative for salivary gland replication. Following infection with an eightfold higher dose (4 × 104 PFU/mouse), the level of M33ΔC38 virus recovered from the salivary glands increased to a level almost 100-fold higher than that recovered following the lower-dose inoculation. Even at this increased dose, there was a highly statistically significant difference between the M33ΔC38 titers and those of wt MCMV (P < 0.001; unpaired two-tailed Student's t test). In contrast, both signaling-inactive mutants, M33(F79D) and M33(R131Q), failed to yield detectable virus in the salivary glands at the higher dose. wt MCMV, M33ΔC24, and the M33(N130D) viruses gave modest increases (<10-fold) in salivary gland titer, compared with the lower-dose infection, suggesting that a maximum obtainable level of virus replication had been reached. There were no statistically significant differences between the titers of M33ΔC24 or M33(N130D) and wt MCMV (P > 0.1, Student's t test). In order to determine whether the three mutations resulting in in vivo attenuation were associated with reduced expression levels of M33 in the context of virus-infected cells, recombinant MCMV expressing GFP-tagged derivatives of each of the mutants (F79D, R131Q and ΔC38) were generated. M33 is expressed at a relatively low level in MCMV-infected cells, so M33-GFP expression was quantitated by fluorimetry following immunofluorescent staining with a polyclonal anti-GFP rabbit antibody. MEFs were infected at high multiplicity (MOI of 3 PFU/cell), and expression of M33-GFP in replicated cultures (n = 6) was determined at 15 h postinfection (Fig. 8). Expression levels of each of the mutated M33-GFP proteins were similar to that of wt M33-GFP, with no statistically significant differences between the mean levels (P > 0.05). Taken together, these data suggest that disruption of constitutive signaling is associated with an attenuated phenotype in vivo.
FIG. 8.
Expression of GFP-tagged wt M33 and mutated derivatives by recombinant MCMV. Subconfluent monolayers of MEFs were prepared in 96-well trays and infected at an MOI of 3 with MCMV recombinants expressing GFP-tagged wt M33 or selected mutants (F79D, R131Q, or ΔC38). K181 (Perth) was included as a GFP-negative control. At 15 h postinfection, M33-GFP expression was quantitated by immunofluorescence using a rabbit polyclonal anti-GFP antiserum, expressed as relative light units (RLU) compared with the negative control (K181). The mean and standard error are shown (n = 6).
The UL33 substitution mutant enabled assessment of the ability of UL33 to functionally substitute for M33 in vivo. Full replacement of M33 with UL33 (including the endogenous intron of UL33) resulted in low, but detectable virus replication in the salivary glands, suggesting that UL33 can provide partial compensation for the absence of M33. This compensation was ablated in the UL33 virus recombinant with the intron removed.
DISCUSSION
In this study, we have identified specific regions of M33 that are critical for either its cellular localization or constitutive signaling. Many virus-encoded chemokine receptors have been shown to signal constitutively through a broad range of transcription factors (10, 32, 68). Signaling pathways in close proximity to the M33 receptor at the cell surface (PLCβ activation measured by PI turnover) as well as signaling mediators downstream (transcription factors CREB and NFAT measured by gene-dose luciferase reporter systems) were constitutively activated by M33. While CREB activates a broad range of cellular processes, NFAT is most commonly associated with regulation of components of the immune and vascular systems. Thus, M33 has the capacity for multifaceted mechanisms of cellular and viral activation, possibly through a number of Gα subunits and in a wide range of cell types.
Many 7TMR, including chemokine receptors, have been reported to form dimers and oligomers, although the functional relevance of higher-molecular-weight forms is controversial (reviewed by in references 7 and 12) Notably, all the mutant proteins were capable of forming dimers (and possibly higher-order structures) similar to the wt M33-GFP protein. Hence the lack of cell surface expression by certain mutants was not correlated with an aberrant M33 protein species or an inability to oligomerize. It was found that deletions at the either the N or C terminus did not result in disruption of oligomer formation, consistent with studies of other 7TMR, indicating that it is commonly the TM domains that form the dimerization interface (7, 22).
Unlike most cellular 7TMR, the detection of both UL33 and M33 at the cell surface under steady-state conditions was relatively low, with considerable intracellular localization being observed. The effects of M33 mutations on cell surface expression in HEK-293 cells were evaluated by confocal microscopy. While this method did not quantify the level of M33 protein at the cell surface, it was able to discern the mutations that fully ablated cell surface expression. Quantitation of M33 protein expression in HEK-293 cells demonstrated that all of the mutants which were deficient in cell surface expression had lower (between 30 to 60%) total expression than wt M33 across a range of DNA amounts. This may have resulted from an increased rate of degradation of intracellularly retained M33 mutants, for example, via quality control mechanisms operating in the endoplasmic reticulum (ER) which target misfolded proteins, including G protein-coupled receptor (19), but we have not investigated this further. Of the few mutants for which cell surface expression appeared normal but the mutation disrupted constitutive signaling [i.e., M33(R131Q), M33(F79D), and M33ΔC38], only M33ΔC38 was found to have reduced total protein expression (approximately 60% of wt M33). Interpretation of the results for this mutant regarding signaling must therefore take into account its reduced expression level. For the other two mutants, it is possible that the loss of signaling was due to a reduction in the relative level of cell surface expression, such that signaling was not detectable even at the highest plasmid dose used. We consider this unlikely, however, since the qualitative assessment by confocal microscopy did not indicate a deficiency in cell surface expression, and for M33(R131Q) the apparent intensity of cell surface staining was consistently higher than that of wt M33.
N-terminal tags including HA, FLAG, and CD4 have been used successfully by ourselves and others to detect a number of viral 7TMR homologues (25, 39). However, N-terminally tagged (HA or c-myc) versions of M33 or deletion of 10 amino acids from the N terminus resulted in ablation of cell surface expression and constitutive signaling, indicating that M33 subcellular localization is adversely affected by modifications of the N terminus. These results are consistent with a recent study which reported that deletion of the first 10 amino acids of M33 resulted in intracellular retention and loss of PLC-mediated signaling (60). Similar observations were made for the HCMV homologue UL33, where deletion of 22 amino acids from the N terminus resulted in loss of both cell surface expression and constitutive signaling (10).
The effect of deletions of increasing length from the M33 C terminus was investigated. The C termini of 7TMR have been shown to be important for G protein coupling and/or regulation of expression, signaling, and endocytosis. A study of C-terminal deletions for several cellular 7TMR demonstrated variable effects on cell surface expression. Thus, whereas sequential deletions of the CCR5 C terminus resulted in progressive loss of cell surface expression, associated with ER retention, deletion of the entire intracellular C terminus of CCR2B (the closest sequence homologue to CCR5) had no effect (64). Studies of the C terminus of US28 indicated that it was not required for signaling but mediated constitutive endocytosis. Thus, deletion of the intracellular C-terminal domain (amino acids 300 to 354) dramatically increased the level of cell surface expression (via reduced endocytosis) and resulted in enhanced signaling (67). For M33, C-terminal truncations of 8, 24, and 38 amino acids did not abolish cell surface expression, whereas deletion of a further 19 amino acids resulted in a lack of detectable cell surface expression, with concomitant intracellular accumulation. These results are very similar to those obtained by Gruijthuijsen and colleagues in studies of R33, where deletion of 44 amino acids from the C terminus did not affect cell surface expression or constitutive signaling, whereas deletion of 61 amino acids resulted in an inactive R33 which was retained intracellularly (31). The M33ΔC57 mutant is deleted of both an RR (R329/R330) motif and upstream cysteine residue (C321) that are conserved in the CMV UL33 family. Positively charged residues in the C terminus of CCR5 have been shown to be important for cell surface expression (64), and the RR motif of R33 has been shown to be important for correct cell surface expression of R33 (31). Mutation of cysteine residues within the C terminus for other 7TMR, including chemokine receptors, has also resulted in their ER retention (46). C-terminal cysteine residues are potential sites for palmitoylation, a common feature of 7TMR, which may result in the formation of a fourth intracellular loop through insertion of the cysteine residue into the membrane (6, 41). Accordingly, the loss of both the RR motif and conserved cysteine is likely to have contributed to the loss of cell surface expression for M33ΔC57.
As expected from the lack of cell surface expression, M33ΔC57 was negative for constitutive signaling. In contrast, the M33ΔC8 and M33ΔC24 mutants were competent for signaling in all assays. Despite being positive for cell-surface expression, M33ΔC38 was negative for NFAT and CRE-mediated signaling but retained PLC signaling, albeit at somewhat reduced levels (up to 50% lower than wt M33). The reduced level of total protein expression of M33ΔC38 may have contributed to the effects upon signaling. In the case of PLC, the reduction in signaling is approximately proportional to the reduced expression level, suggesting that the reduced protein levels may have been a major factor resulting in reduced PLC signaling. In the case of CREB and NFAT, signaling was completely ablated across at least a 10-fold range of DNA amounts, which suggests that other effects in addition to the reduced level of expression are likely to have played a role. It has been demonstrated for M33 that PLC signaling is mediated via coupling to Gq/11 proteins (60). Previous studies of R33 had demonstrated activation of PLC predominantly via coupling with Gq/11 and repression of CRE via coupling with Gi/0 (10). Furthermore, an R33 mutant deficient in Gq/11 coupling but retaining Gi/0-mediated signaling was produced, demonstrating that these two activities were at least in part controlled independently. By analogy, the differential effects observed for M33ΔC38 upon NFAT and CRE versus PLC signaling may be explained by the requirement of the region between residues 340 and 353 for interaction with a subset of the G proteins which contribute to M33 constitutive signaling, but this hypothesis has yet to be tested.
Within the second predicted TM domain of the 7TMR superfamily is a highly conserved aspartic acid residue that has a role in the regulation of signal transduction and/or ligand binding in many cellular receptors. It has been suggested, from computer modeling and experimental data that the TM II aspartate (II:10) interacts with a highly conserved asparagine residue within each of the TM regions I and VII (20, 45, 70). Further, it has been suggested that these residues form a “polar pocket” which assists in “burying” the conserved arginine residue of the TM III DRY motif. This interaction is proposed to stabilize the receptor in an inactive state with activation occurring through a shift of the arginine residue out of the pocket (2, 3, 20, 45, 56).
In contrast to many cellular 7TMR, M33 and other members of the UL33 family do not contain an acidic residue at the comparable site within the TM II domain. Potentially, this may contribute to the constitutive signaling activity observed for UL33 family members, by destabilizing the proposed polar pocket interaction. The predicted topology of TM II of M33 suggests that N81 corresponds to the conserved aspartate, and so this and flanking residues were mutated. Substitution of aspartate for residues A80, N81, and L82 resulted in ablation of cell surface expression and signaling activity. In contrast, although M33(F79D) showed normal protein expression levels and cell surface distribution, signaling was abolished. In a similar study of ORF74 TM II, replacement of selected residues of the ORF74 TM II domain with aspartate also resulted in mutants that no longer possessed constitutive activity. It therefore appears that the TM II domain is important for constitutive signaling in both ORF74 and M33, possibly by shifting the equilibrium of the receptor toward an active state. Similarly, for the cellular chemokine receptors CCR2 and CCR5, TM II has been shown to be important for receptor activation (29). The observation that ORF74 with the mutation L91D (L91D-ORF74) and L94D-ORF74 could mediate signaling in the presence of an agonist (50) demonstrated that these mutated receptors could still be shifted to an active state. Recently murine RANTES was proposed as a candidate ligand for M33 (38), and thus it would be of interest to see if the M33(F79D) receptor was capable of agonist-induced activation upon binding of murine RANTES or other potential ligands.
The function of the DRY motif located within the cytoplasmic region of TM III has been the focus of much attention as it is highly conserved within the class I family of 7TMR. Studies of several different 7TMR have implicated this motif in either the regulation of receptor conformation states and/or mediation of G protein activation (reviewed in reference 27). Mutational studies of the D and R residues of this motif have confirmed their importance, but whereas in some receptors mutation away from the consensus resulted in loss of signaling (9, 36, 57, 69), in others it promoted gain of constitutive signaling (3, 8, 14, 21, 55, 59). Recent studies of the ORF74 7TMR of equine herpesvirus 2 provided the first example of a naturally occurring 7TMR which signals both constitutively and in an agonist-dependent manner, despite lacking the DRY motif. Interestingly, mutation of the equine herpesvirus 2 ORF74 DTW motif to DRY resulted in a reduction of constitutive signaling with maintenance of agonist induced signaling (51).
Whereas UL33 encodes the consensus DRY motif, both M33 and R33 encode a variant NRY motif. Mutation of the arginine residue at position 131 to neutral glutamine in M33(R131Q) abolished constitutive activity, without any detected reduction in protein expression level or cell surface distribution. This result is in agreement with a recent study of M33 with an R131A substitution, which was found to abolish constitutive PLC-mediated signaling (60), and similar to that obtained for R33, where replacement of the arginine by alanine resulted in loss of signaling (31). In contrast, mutation of asparagine at position 130 to aspartate [M33(N130D)] led to an increase in constitutive signaling, particularly of NFAT-mediated transcription. Thus, the atypical asparagine residue is not required for constitutive signaling of M33. This result is similar to that observed for R33, where an N130D substitution did not affect constitutive signaling, although in this case no increase in signaling was seen (31).
CMV infection in vivo is highly cell associated, with leukocytes and endothelial cells implicated as the predominant vehicles for dissemination from the blood to host tissues. A hallmark feature of the CMVs is their salivary gland tropism. Previous studies have indicated an important role for M33 and R33 in the dissemination to and/or replication in salivary glands of MCMV and RCMV, respectively (4, 16). In this study, there is evidence that disruption of constitutive signaling of M33 is accompanied by loss of biological activity in vivo. Mutants M33(F79D), M33ΔC38, and M33(R131Q) were disrupted for signaling but retained cell surface expression, although in the case of M33ΔC38 a somewhat reduced level of total protein expression in transfected HEK-293 cells was observed. Analysis of MCMV recombinants expressing GFP-tagged M33 derivatives demonstrated that the levels of expression for each of these mutants were similar to that of wt M33 in infected murine fibroblasts in tissue culture, confirming that the mutations did not adversely affect expression in the context of virus-infected cells. Recombinant MCMV expressing each of these mutations was attenuated for salivary gland replication. However, whereas M33(F79D) and M33(R131Q) mimicked the M33 null mutant, with no detectable infectious virus in the salivary glands, the M33ΔC38 recombinant virus was recovered, albeit at reduced titers. These in vivo results correlated with the in vitro signaling characteristics, since M33(F79D) and M33(R131Q) were negative in all signaling assays whereas M33ΔC38 was negative for NFAT and CREB signaling assays but retained (albeit reduced) PLC signaling activity. Interestingly, dissemination to the salivary glands in mice infected with the M33ΔC38 recombinant was markedly more efficient following an increased virus inoculum, suggesting that the defect in dissemination in the signaling-disrupted mutants may be present early after infection when the initial pool of cells that are responsible for the spread of MCMV in vivo are infected. Importantly, recombinants with other mutations which did not adversely affect constitutive signaling [M33ΔC24 and M33(N130D)] replicated to nearly normal levels.
G protein-mediated signaling molecules have been shown to be important for the initiation and maintenance of the cellular chemotactic response, as well as for the leukocyte adhesion and transendothelial migration—a process that is disrupted when the leukocytes and/or the endothelium is infected with HCMV in vitro (5, 26). Both M33 and R33 have been shown to promote the mobilization of smooth-muscle cells in vitro (38, 61), suggesting that they have the capacity to subvert normal cellular trafficking. In the context of an MCMV infection, the possibility exists that signaling by M33 promotes the trafficking of cells important for MCMV dissemination, and experiments to address this hypothesis are in progress using marker-tagged MCMVs expressing either wt or inactive mutants of M33. Signaling by M33 may also initiate an intracellular environment that is favorable for replication in permissive cells that are critical players in dissemination. While our studies did not detect a phenotype for M33 in fibroblasts in vitro, it is possible that M33 might be required for efficient replication in cell types that play a role in MCMV dissemination in vivo.
Viral 7TMR have attracted attention as potential antiviral drug targets. The data presented here provide the first evidence that disruption of signaling is accompanied by attenuation in vivo, supporting the proposal that drug-induced suppression of viral 7TMR signaling may have therapeutic potential. Interestingly, UL33 was found to partially compensate for the lack of M33 in vivo. This observation provides supporting evidence for conserved biological roles of the UL33 gene family for CMVs and may facilitate future studies of anti-UL33 drugs in vivo.
Acknowledgments
This work was supported by the Wellcome Trust (United Kingdom), the National Health and Medical Research Council of Australia, and Queensland Health.
The technical assistance of Grace Chojnowski, Katja Fischer, Simone Reynolds, Lisbet Elbak, Randi Thøgersen, and Inger Smith Simonsen and the statistical advice of William Henley are gratefully acknowledged.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Baldwin, J. M. 1993. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 121693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballesteros, J., S. Kitanovic, F. Guarnieri, P. Davies, B. J. Fromme, K. Konvicka, L. Chi, R. P. Millar, J. S. Davidson, H. Weinstein, and S. C. Sealfon. 1998. Functional microdomains in G protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J. Biol. Chem. 27310445-10453. [DOI] [PubMed] [Google Scholar]
- 3.Ballesteros, J. A., A. D. Jensen, G. Liapakis, S. G. Rasmussen, L. Shi, U. Gether, and J. A. Javitch. 2001. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 27629171-29177. [DOI] [PubMed] [Google Scholar]
- 4.Beisser, P. S., C. Vink, J. G. Van Dam, G. Grauls, S. J. Vanherle, and C. A. Bruggeman. 1998. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J. Virol. 722352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentz, G. L., M. Jarquin-Pardo, G. Chan, M. S. Smith, C. Sinzger, and A. D. Yurochko. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 8011539-11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanpain, C., V. Wittamer, J. M. Vanderwinden, A. Boom, B. Renneboog, B. Lee, E. Le Poul, L. El Asmar, C. Govaerts, G. Vassart, R. W. Doms, and M. Parmentier. 2001. Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J. Biol. Chem. 27623795-23804. [DOI] [PubMed] [Google Scholar]
- 7.Bulenger, S., S. Marullo, and M. Bouvier. 2005. Emerging role of homo- and heterodimerization in G protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 26131-137. [DOI] [PubMed] [Google Scholar]
- 8.Burger, M., J. A. Burger, R. C. Hoch, Z. Oades, H. Takamori, and I. U. Schraufstatter. 1999. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 1632017-2022. [PubMed] [Google Scholar]
- 9.Capra, V., A. Veltri, C. Foglia, L. Crimaldi, A. Habib, M. Parenti, and G. E. Rovati. 2004. Mutational analysis of the highly conserved ERY motif of the thromboxane A2 receptor: alternative role in G protein-coupled receptor signaling. Mol. Pharmacol. 66880-889. [DOI] [PubMed] [Google Scholar]
- 10.Casarosa, P., Y. K. Gruijthuijsen, D. Michel, P. S. Beisser, J. Holl, C. P. Fitzsimons, D. Verzijl, C. A. Bruggeman, T. Mertens, R. Leurs, C. Vink, and M. J. Smit. 2003. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J. Biol. Chem. 27850010-50023. [DOI] [PubMed] [Google Scholar]
- 11.Ceresa, B. P., and L. E. Limbird. 1994. Mutation of an aspartate residue highly conserved among G protein-coupled receptors results in nonreciprocal disruption of alpha 2-adrenergic receptor-G protein interactions. A negative charge at amino acid residue 79 forecasts alpha 2A-adrenergic receptor sensitivity to allosteric modulation by monovalent cations and fully effective receptor/G protein coupling. J. Biol. Chem. 26929557-29564. [PubMed] [Google Scholar]
- 12.Chabre, M., and M. le Maire. 2005. Monomeric G protein-coupled receptor as a functional unit. Biochemistry 449395-9403. [DOI] [PubMed] [Google Scholar]
- 13.Chee, M. S., S. C. Satchwell, E. Preddie, K. M. Weston, and B. G. Barrell. 1990. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature 344774-777. [DOI] [PubMed] [Google Scholar]
- 14.Chen, A., Z. G. Gao, D. Barak, B. T. Liang, and K. A. Jacobson. 2001. Constitutive activation of A(3) adenosine receptors by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 284596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couty, J. P., and M. C. Gershengorn. 2005. G protein-coupled receptors encoded by human herpesviruses. Trends Pharmacol. Sci. 26405-411. [DOI] [PubMed] [Google Scholar]
- 16.Davis-Poynter, N. J., D. M. Lynch, H. Vally, G. R. Shellam, W. D. Rawlinson, B. G. Barrell, and H. E. Farrell. 1997. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J. Virol. 711521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deayton, J. R. 2001. Changing trends in cytomegalovirus disease in HIV-infected patients. Herpes 837-40. [PubMed] [Google Scholar]
- 18.de Jong, M. D., G. J. Galasso, B. Gazzard, P. D. Griffiths, D. A. Jabs, E. R. Kern, and S. A. Spector. 1998. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 39141-162. [DOI] [PubMed] [Google Scholar]
- 19.Dong, C., C. M. Filipeanu, M. T. Duvernay, and G. Wu. 2007. Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 1768853-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly, D., S. Maudsley, J. P. Gent, R. N. Moser, C. R. Hurrell, and J. B. Findlay. 1999. Conserved polar residues in the transmembrane domain of the human tachykinin NK2 receptor: functional roles and structural implications. Biochem. J. 33955-61. [PMC free article] [PubMed] [Google Scholar]
- 21.Favre, N., F. Fanelli, M. Missotten, A. Nichols, J. Wilson, M. di Tiani, C. Rommel, and A. Scheer. 2005. The DRY motif as a molecular switch of the human oxytocin receptor. Biochemistry 449990-10008. [DOI] [PubMed] [Google Scholar]
- 22.Filizola, M., and H. Weinstein. 2005. The study of G protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2722926-2938. [DOI] [PubMed] [Google Scholar]
- 23.Fraile-Ramos, A., T. N. Kledal, A. Pelchen-Matthews, K. Bowers, T. W. Schwartz, and M. Marsh. 2001. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 121737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraile-Ramos, A., T. A. Kohout, M. Waldhoer, and M. Marsh. 2003. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic 4243-253. [DOI] [PubMed] [Google Scholar]
- 25.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3218-232. [DOI] [PubMed] [Google Scholar]
- 26.Frascaroli, G., S. Varani, B. Moepps, C. Sinzger, M. P. Landini, and T. Mertens. 2006. Human cytomegalovirus subverts the functions of monocytes, impairing chemokine-mediated migration and leukocyte recruitment. J. Virol. 807578-7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gether, U., F. Asmar, A. K. Meinild, and S. G. Rasmussen. 2002. Structural basis for activation of G protein-coupled receptors. Pharmacol. Toxicol. 91304-312. [DOI] [PubMed] [Google Scholar]
- 28.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 20929-51. [DOI] [PubMed] [Google Scholar]
- 29.Govaerts, C., C. Blanpain, X. Deupi, S. Ballet, J. A. Ballesteros, S. J. Wodak, G. Vassart, L. Pardo, and M. Parmentier. 2001. The TXP motif in the second transmembrane helix of CCR5. A structural determinant of chemokine-induced activation. J. Biol. Chem. 27613217-13225. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths, P. D. 2002. Tomorrow's challenges for herpesvirus management: potential applications of valacyclovir. J. Infect. Dis. 186(Suppl. 1)S131—S137. [DOI] [PubMed] [Google Scholar]
- 31.Gruijthuijsen, Y. K., E. V. Beuken, M. J. Smit, R. Leurs, C. A. Bruggeman, and C. Vink. 2004. Mutational analysis of the R33-encoded G protein-coupled receptor of rat cytomegalovirus: identification of amino acid residues critical for cellular localization and ligand-independent signalling. J. Gen. Virol. 85897-909. [DOI] [PubMed] [Google Scholar]
- 32.Gruijthuijsen, Y. K., P. Casarosa, S. J. Kaptein, J. L. Broers, R. Leurs, C. A. Bruggeman, M. J. Smit, and C. Vink. 2002. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J. Virol. 761328-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holst, P. J., M. M. Rosenkilde, D. Manfra, S. C. Chen, M. T. Wiekowski, B. Holst, F. Cifire, M. Lipp, T. W. Schwartz, and S. A. Lira. 2001. Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J. Clin. Investig. 1081789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 7761-68. [DOI] [PubMed] [Google Scholar]
- 35.Isegawa, Y., Z. Ping, K. Nakano, N. Sugimoto, and K. Yamanishi. 1998. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J. Virol. 726104-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, Z. L., C. A. Curtis, P. G. Jones, J. Pavia, and E. C. Hulme. 1997. The role of the aspartate-arginine-tyrosine triad in the m1 muscarinic receptor: mutations of aspartate 122 and tyrosine 124 decrease receptor expression but do not abolish signaling. Mol. Pharmacol. 51234-241. [DOI] [PubMed] [Google Scholar]
- 37.McLean, K. A., P. J. Holst, L. Martini, T. W. Schwartz, and M. M. Rosenkilde. 2004. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology 325241-251. [DOI] [PubMed] [Google Scholar]
- 38.Melnychuk, R. M., P. Smith, C. N. Kreklywich, F. Ruchti, J. Vomaske, L. Hall, L. Loh, J. A. Nelson, S. L. Orloff, and D. N. Streblow. 2005. Mouse cytomegalovirus M33 is necessary and sufficient in virus-induced vascular smooth muscle cell migration. J. Virol. 7910788-10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, W. E., D. A. Houtz, C. D. Nelson, P. E. Kolattukudy, and R. J. Lefkowitz. 2003. G protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 27821663-21671. [DOI] [PubMed] [Google Scholar]
- 40.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10332-339. [DOI] [PubMed] [Google Scholar]
- 41.Morello, J. P., and M. Bouvier. 1996. Palmitoylation: a post-translational modification that regulates signalling from G protein coupled receptors. Biochem. Cell Biol. 74449-457. [DOI] [PubMed] [Google Scholar]
- 42.Morin, D., N. Cotte, M. N. Balestre, B. Mouillac, M. Manning, C. Breton, and C. Barberis. 1998. The D136A mutation of the V2 vasopressin receptor induces a constitutive activity which permits discrimination between antagonists with partial agonist and inverse agonist activities. FEBS Lett. 441470-475. [DOI] [PubMed] [Google Scholar]
- 43.Nicholas, J. 1996. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J. Virol. 705975-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira, L., A. C. Paiva, C. Sander, and G. Vriend. 1994. A common step for signal transduction in G protein-coupled receptors. Trends Pharmacol. Sci. 15170-172. [DOI] [PubMed] [Google Scholar]
- 45.Perlman, J. H., A. O. Colson, W. Wang, K. Bence, R. Osman, and M. C. Gershengorn. 1997. Interactions between conserved residues in transmembrane helices 1, 2, and 7 of the thyrotropin-releasing hormone receptor. J. Biol. Chem. 27211937-11942. [DOI] [PubMed] [Google Scholar]
- 46.Qanbar, R., and M. Bouvier. 2003. Role of palmitoylation/depalmitoylation reactions in G protein-coupled receptor function. Pharmacol. Ther. 971-33. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen, S. G., A. D. Jensen, G. Liapakis, P. Ghanouni, J. A. Javitch, and U. Gether. 1999. Mutation of a highly conserved aspartic acid in the beta2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol. Pharmacol. 56175-184. [DOI] [PubMed] [Google Scholar]
- 48.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 708833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razonable, R. R., and C. V. Paya. 2003. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes 1060-65. [PubMed] [Google Scholar]
- 50.Rosenkilde, M. M., T. N. Kledal, P. J. Holst, and T. W. Schwartz. 2000. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. J. Biol. Chem. 27526309-26315. [DOI] [PubMed] [Google Scholar]
- 51.Rosenkilde, M. M., T. N. Kledal, and T. W. Schwartz. 2005. High constitutive activity of a virus-encoded seven transmembrane receptor in the absence of the conserved DRY motif (Asp-Arg-Tyr) in transmembrane helix 3. Mol. Pharmacol. 6811-19. [DOI] [PubMed] [Google Scholar]
- 52.Rosenkilde, M. M., K. A. McLean, P. J. Holst, and T. W. Schwartz. 2004. The CXC chemokine receptor encoded by herpesvirus saimiri, ECRF3, shows ligand-regulated signaling through Gi, Gq, and G12/13 proteins but constitutive signaling only through Gi and G12/13 proteins. J. Biol. Chem. 27932524-32533. [DOI] [PubMed] [Google Scholar]
- 53.Rosenkilde, M. M., M. Waldhoer, H. R. Luttichau, and T. W. Schwartz. 2001. Virally encoded 7TM receptors. Oncogene 201582-1593. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8404-407. [PubMed] [Google Scholar]
- 55.Scheer, A., T. Costa, F. Fanelli, P. G. De Benedetti, S. Mhaouty-Kodja, L. Abuin, M. Nenniger-Tosato, and S. Cotecchia. 2000. Mutational analysis of the highly conserved arginine within the Glu/Asp-Arg-Tyr motif of the alpha(1b)-adrenergic receptor: effects on receptor isomerization and activation. Mol. Pharmacol. 57219-231. [PubMed] [Google Scholar]
- 56.Scheer, A., F. Fanelli, T. Costa, P. G. De Benedetti, and S. Cotecchia. 1997. The activation process of the alpha1B-adrenergic receptor: potential role of protonation and hydrophobicity of a highly conserved aspartate. Proc. Natl. Acad. Sci. USA 94808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheer, A., F. Fanelli, T. Costa, P. G. De Benedetti, and S. Cotecchia. 1996. Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J. 153566-3578. [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz, T. W. 1994. Locating ligand-binding sites in 7TM receptors by protein engineering. Curr. Opin. Biotechnol. 5434-444. [DOI] [PubMed] [Google Scholar]
- 59.Seibold, A., M. Dagarag, and M. Birnbaumer. 1998. Mutations of the DRY motif that preserve beta 2-adrenoceptor coupling. Receptors Channels 5375-385. [PubMed] [Google Scholar]
- 60.Sherrill, J. D., and W. E. Miller. 2006. G protein-coupled receptor (GPCR) kinase 2 regulates agonist-independent Gq/11 signaling from the mouse cytomegalovirus GPCR M33. J. Biol. Chem. 28139796-39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Streblow, D. N., C. N. Kreklywich, P. Smith, J. L. Soule, C. Meyer, M. Yin, P. Beisser, C. Vink, J. A. Nelson, and S. L. Orloff. 2005. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am. J. Transplant. 5436-442. [DOI] [PubMed] [Google Scholar]
- 62.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99511-520. [DOI] [PubMed] [Google Scholar]
- 63.van Cleef, K. W., M. J. Smit, C. A. Bruggeman, and C. Vink. 2006. Cytomegalovirus-encoded homologs of G protein-coupled receptors and chemokines. J. Clin. Virol. 35343-348. [DOI] [PubMed] [Google Scholar]
- 64.Venkatesan, S., A. Petrovic, M. Locati, Y. O. Kim, D. Weissman, and P. M. Murphy. 2001. A membrane-proximal basic domain and cysteine cluster in the C-terminal tail of CCR5 constitute a bipartite motif critical for cell surface expression. J. Biol. Chem. 27640133-40145. [DOI] [PubMed] [Google Scholar]
- 65.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 747656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vischer, H. F., R. Leurs, and M. J. Smit. 2006. HCMV-encoded G protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol. Sci. 2756-63. [DOI] [PubMed] [Google Scholar]
- 67.Waldhoer, M., P. Casarosa, M. M. Rosenkilde, M. J. Smit, R. Leurs, J. L. Whistler, and T. W. Schwartz. 2003. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J. Biol. Chem. 27819473-19482. [DOI] [PubMed] [Google Scholar]
- 68.Waldhoer, M., T. N. Kledal, H. Farrell, and T. W. Schwartz. 2002. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J. Virol. 768161-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wess, J. 1998. Molecular basis of receptor/G protein-coupling selectivity. Pharmacol. Ther. 80231-264. [DOI] [PubMed] [Google Scholar]
- 70.Wilson, M. H., H. A. Highfield, and L. E. Limbird. 2001. The role of a conserved inter-transmembrane domain interface in regulating alpha(2a)-adrenergic receptor conformational stability and cell-surface turnover. Mol. Pharmacol. 59929-938. [DOI] [PubMed] [Google Scholar]
- 71.Zhu, S. Z., S. Z. Wang, J. Hu, and E. E. el-Fakahany. 1994. An arginine residue conserved in most G protein-coupled receptors is essential for the function of the m1 muscarinic receptor. Mol. Pharmacol. 45517-523. [PubMed] [Google Scholar]