Abstract
Severe acute respiratory syndrome (SARS) is an acute respiratory disease with significant morbidity and mortality. While its clinical manifestations have been extensively studied, its pathogenesis is not yet fully understood. A limited number of autopsy studies have revealed that the lungs and the immune system are the organs that sustain the most severe damage. Other organs affected include the kidneys, brain, digestive tract, heart, liver, thyroid gland and urogenital tract. The primary target cells are pneumocytes and enterocytes, both cell types abundantly expressing angiotensin-converting enzyme 2 which is the main SARS-CoV receptor. Other cell types infected include the epithelial cells of renal tubules, cerebral neurons, and immune cells. The pathology of this disease results from both direct and indirect injury. Direct injury is caused by infection of the target cells by the virus. Indirect injury mainly results from immune responses, circulatory dysfunction, and hypoxia. In this review, we summarize the major pathological findings at the gross, cellular and molecular levels and discuss the various possible mechanisms that may contribute to the pathogenesis of SARS. The implications of the proposed pathogenesis for prevention, diagnosis and therapy of the disease are discussed.
Keywords: SARS, Pathology, Immune system, Virus, Lung
1. Introduction
Severe acute respiratory syndrome (SARS) is an acute respiratory disease with significant morbidity and mortality. It first emerged in China's Guangdong province in late 2002, and then spread to countries in Southeast Asia and throughout the world. The outbreak resulted in 8096 probable cases of SARS with a mortality rate of 9.6% (WHO website).
SARS is a disease consisting of two phases, including a prodromal influenza-like period characterized by myalgia, malaise, chills and fever, followed by the onset of respiratory and gastrointestinal symptoms. Eight to nine days after the onset of fever, approximately 20% of all patients deteriorate clinically and develop respiratory insufficiency, in some cases resulting in death (Lee et al., 2003, Liu et al., 2004a, Liu et al., 2004b, HKU/UCH SARS Study Group, 2003, Rainer et al., 2004a, Rainer et al., 2004b). The laboratory findings characteristically include lymphopenia, thrombocytopenia, elevated lactate dehydrogenase (Lee et al., 2003, Liu et al., 2004a, Liu et al., 2004b, HKU/UCH SARS Study Group, 2003, Wong et al., 2003), and elevated liver enzymes (Chan et al., 2005, Yang et al., 2005a, Yang et al., 2005b). The majority of patients admitted to the hospital show pulmonary X-rays abnormalities varying from bilateral interstitial infiltrates to focal consolidation (Lee et al., 2003, Liu et al., 2004a, Liu et al., 2004b, HKU/UCH SARS Study Group, 2003). The pathogen causing SARS has been identified as a novel coronavirus, SARS-CoV, and the genome of this virus has been sequenced (Drosten et al., 2003, Fouchier et al., 2003, Ksiazek et al., 2003, Kuiken et al., 2003). The epidemiology, clinical manifestations, laboratory findings and radiological features of this disease have all been studied in detail. However, its pathogenesis is still not fully understood.
This review summarizes the major pathological findings at the gross, cellular and molecular levels, which have been reported in hitherto published postmortem studies of SARS patients including a previous publication on multiple organ infection in this disease. In addition, we discuss the various known mechanisms that may contribute to the pathogenesis of SARS including the one described by our group. The implications of such mechanisms for the prevention, diagnosis and therapy of this disease are discussed.
2. Pathological changes
2.1. Respiratory system
The lungs of autopsied SARS patients were on average heavier than normal lungs (Gu et al., 2005). Severe lung consolidation, edema with pleural effusion and hepatization have been found on gross examination (Ding et al., 2003, Gu et al., 2005, Nicholls et al., 2003, Tse et al., 2004). Focal hemorrhage and mucopurulent material in the tracheobronchial tree have been seen in some SARS patients (Ding et al., 2003, Nicholls et al., 2003).
Microscopic examination has typically demonstrated diffused alveolar damage (DAD) (Cheung et al., 2004, Ding et al., 2003, Franks et al., 2003, Gu et al., 2005, Hwang et al., 2005, Lee et al., 2003). According to the morphological changes observed, most authors have subclassified lung lesions in SARS into two or three consecutive phases, which correlate with the duration of illness. These phases consist of an acute exudative inflammatory phase, a fibrous proliferative phase, and a final fibrotic stage, although there is considerable overlap in histological findings among these phases. Findings characteristic of all three phases have been observed in the same lung tissue specimen, and evidence of the first phase with ongoing acute injury has been found in most cases even up to 108 days after onset of the disease (Hwang et al., 2005). The acute phase is characterized by extensive hyaline membrane formation, edema, alveolar hemorrhage and fibrin exudation in alveolar spaces (Cheung et al., 2004, Franks et al., 2003, Hwang et al., 2005, Lee et al., 2003, Nicholls et al., 2003). In the acute phase both loss of cilia of bronchiolar epithelial cells, and bronchiolar epithelial denudation have been described, reflecting severe bronchiolar damage (Franks et al., 2003). The proliferative phase is characterized by widening of septae, pneumocyte hyperplasia and organizing fibromyxoid and cellular exudates (Cheung et al., 2004, Franks et al., 2003, Lee et al., 2003). In the final phase, septal and alveolar fibrosis are found (Cheung et al., 2004), and the extent of fibrosis is positively correlated with the duration of the disease (Hwang et al., 2005, Tse et al., 2004).
In addition to the histological changes of the recognized phases of pulmonary pathology, many other pathological changes have been described. These include desquamation of epithelial cells (Ding et al., 2003, Franks et al., 2003, Gu et al., 2005, Lang et al., 2003, Nicholls et al., 2003), squamous metaplasia (Franks et al., 2003, Hwang et al., 2005, Nicholls et al., 2003), vascular injury including small vessel vasculitis and vascular edema (Ding et al., 2003, Gu et al., 2005, Hwang et al., 2005), and BOOP-like lesions such as proliferation of fibrogranulation tissue in small airways and alveolar spaces located subpleurally (Tse et al., 2004). Vascular fibrin thrombi and pulmonary infarcts have also been identified in several cases (Ding et al., 2003, Hwang et al., 2005, Lang et al., 2003, Nicholls et al., 2003). Hwang et al. (2005) have reported an acute fibrinous and organizing pneumonia pattern, characterized by intra-alveolar fibrin balls and organizing pneumonia. However, the clinical significance of this assortment of findings has not yet been determined.
Several SARS autopsies have demonstrated a secondary bronchopneumonia, especially in cases where the duration of the disease has been greater than 10 days (Franks et al., 2003, Hwang et al., 2005). The pathogens identified in the lungs include: Pseudomonas aeruginosa, Staphylococcus aureus, Mucor sp., Aspergillus sp., and Cytomegalovirus (Franks et al., 2003, Hwang et al., 2005, Xu et al., 2005).
Some have suggested that a paucity of pulmonary inflammatory infiltrates may be a main pathological feature of SARS (Gu et al., 2005, Tse et al., 2004). Mixed cellular infiltrates have been found in lung interstitial tissue and alveoli. In the majority of lung specimens they consisted of macrophages and lymphocytes (Cheung et al., 2004, Ding et al., 2003, Lang et al., 2003, Nicholls et al., 2003; Tse et al., 2003), although in some specimens macrophages appeared to be the predominant cells (Franks et al., 2003, Ksiazek et al., 2003). Most of the infiltrated lymphocytes have been identified as CD3 positive T cells, some as B cells (CD20 positive) with NK cells (CD56 positive) largely lacking (Tse et al., 2004).
Several authors have reported the presence of atypical, enlarged pneumocytes, characterized by large nuclei, prominent nucleoli, and amphophilic granular cytoplasm (Cheung et al., 2004, Franks et al., 2003, Nicholls et al., 2003, Tse et al., 2004). In addition, large syncytial cells with multiple nuclei have also been reported in most cases (Cheung et al., 2004, Ding et al., 2003, Gu et al., 2005, Lee et al., 2003, Ksiazek et al., 2003, Tse et al., 2004). Immunohistochemistry has identified such multinucleated cells as macrophages and/or epithelial cells (Franks et al., 2003, Nicholls et al., 2003, Hwang et al., 2005). However, the presence of multinucleated cells is not considered to be a specific feature of SARS, since pneumonias caused by other viruses may also result in the appearance of multinucleated cells. SARS viral genome has been detected by ISH in such multinucleated macrophages and epithelial cells (Gu et al., 2005).
Reverse transcriptase polymerase chain reaction (RT-PCR) performed on postmortem lung tissue samples has typically detected SARS-CoV RNA (Cheung et al., 2003; Chow et al., 2004, Ding et al., 2004, Franks et al., 2003, Nicholls et al., 2003). In one case, high viral loads have been found even up to the 8th week after onset of the disease (Farcas et al., 2005). In situ hybridization has demonstrated the presence of SARS-CoV gene fragments in type II pneumocytes, macrophages/monocytes (Chow et al., 2004, Ding et al., 2004, Gu et al., 2005, Shieh et al., 2005, Ye et al., in press) and in T lymphocytes (Gu et al., 2005, Ye et al., in press). The ISH signal localizes to the cytoplasm (Gu et al., 2005, To et al., 2004, Ye et al., in press). Two research groups have also detected the viral genome in both tracheal and bronchiolar epithelial cells as well (Ding et al., 2004, Gu et al., 2005, Ye et al., in press). In data unpublished at this time, there is also evidence of infection of endothelial cells and fibroblasts in the lung. Coronaviral-like particles, and in some cases coronavirus nucleocapsid inclusions, have been observed by electron microscopic (EM) examination in pneumocytes and macrophages (Cheung et al., 2004, Ding et al., 2003, Gu et al., 2005, Shieh et al., 2005, Tse et al., 2004). The identity of these particles was confirmed by immunogold labeling (Gu et al., 2005, Shieh et al., 2005).
Some authors have noted that the pathological features as described above, are not unique to SARS and that diffused alveolar damage may also be caused by several other agents, such as bacteria, oxygen toxicity, trauma and drugs (Hwang et al., 2005, Ksiazek et al., 2003). Therefore, differentiation between cases of SARS and non-SARS pneumonia may not be definitively based on the features of pulmonary histopathology without confirmation by additional tests such as in situ hybridization, RT-PCR, and virus isolation. Nevertheless, certain additional features which are described below, although not specific for SARS infection, may be of help in distinguishing diffused alveolar damage caused by SARS-CoV from others caused by other agents. First, consolidation, edema and hyaline membrane formation have appeared more prominently in SARS cases than in non-SARS cases (Gu et al., 2005), although some authors have attributed this observation to differences in disease duration (Hwang et al., 2005). Second, the inflammatory cell infiltrates of SARS pneumonia are characteristically paucicellular or even absent, in contrast to pneumonias caused by other agents. Third, extensive vascular endothelial injury and damage to epithelial cells were found to be conspicuous features in SARS.
In addition, certain extra-pulmonary disease features may be helpful in confirming suspected SARS cases. First, SARS is often complicated by infection and damage to multiple organs. Second, the immune system injury plays a central role in SARS, and histopathological confirmation of immune injury can be found in destruction of lymphoid tissue in the spleen, lymph nodes and the intestines. No other type of pneumonia has been previously found to have the capacity to cause such broad organ damage and to cause such profound immune system dysfunction (Gu et al., 2005, To et al., 2004).
Ng et al. (2006) have compared the histopathological features of SARS with those of avian influenza (H5N1). Both SARS and human influenza (H5N1) are acute infectious diseases that target pneumocytes, and both diseases are characterized by diffused alveolar damage in the lungs. However, compared with avian influenza (H5N1), the progression of diffused alveolar damage in SARS has been found to be less fulminant. The organizing phase of both SARS and avian influenza (H5N1) are characterized by pulmonary fibrosis, though avian influenza (H5N1) is less fibrocellular and, in contrast to SARS, shows no evidence of BOOP-like organization (Ng et al., 2006).
2.2. Immune system
SARS has been found to have a profoundly adverse effect on the immune system. Hemorrhagic necrosis is usually evident in lymph nodes and spleen (Ding et al., 2003, Lang et al., 2003). In some lymph nodes, especially in the pulmonary hilar lymph nodes, there is destruction of germinal centers, with evidence of cellular infiltrates in the remaining sinuses (Ding et al., 2003, Lang et al., 2003). Splenic white pulp atrophy, depletion of both T and B lymphocytes, and disappearance of germinal centers have all been found in many cases (Gu et al., 2005, Lang et al., 2003, Nicholls et al., 2003, Tse et al., 2004, Wong et al., 2003, Zhan et al., in press). The numbers of peri-arterial lymphatic sheaths are decreased and the splenic capsule shows evidence of shrinkage (Wong et al., 2003, Zhan et al., in press). In addition, massive hemorrhage and necrosis have been found in the red pulp of the spleen. Peyer's patches are also affected, with both CD20 positive B cells and CD3 positive T cells decreased (Shi et al., 2005). RT-PCR has demonstrated high viral loads in both the spleen and lymph nodes (Farcas et al., 2004). With respect to the immune cells, lymphopenia, in particular T cell lymphopenia, has been observed in the majority of SARS patients. Both CD4 and CD8 cell counts have shown a significant decrease, while the CD4/CD8-ratios remained normal. In one series the absolute counts of lymphocyte subsets of SARS patients were listed as follows: T cells (0.60 ± 0.33) × 109 L−1, CD4 positive T cells (0.27 ± 0.15) × 109 L−1, CD8 positive T cells (0.29 ± 0.20) × 109 L−1, B cells (0.21 ± 0.18) × 109 L−1, NK cells (0.17 ± 0.10) × 109 L−1 (Cui et al., 2003). The T cells counts have generally reached a nadir approximately 2 weeks after disease onset (Cui et al., 2003, Gu et al., 2005, He et al., 2005, Li et al., 2004, Wong et al., 2003).
In our research on SARS and the immune system (Gu et al., 2005), EM and in situ hybridization have respectively shown viral particles and genomic sequences in both monocytes and lymphocytes in blood samples of SARS patients, implying that SARS-CoV is capable of infecting both cell types. Approximately 50% of lymphocytes and 30% of monocytes in the circulation were infected by SARS-CoV. A great majority of the infected lymphocytes have been identified as T cells by immunohistochemistry, with the remainder of infected cells comprising both B cells and NK cells. By EM, it has been demonstrated that viral particles tend to form clusters and were located within or near the endoplasmic reticulum. In addition, in autopsy cases, all types of immune cells in the spleen show a significant decrease in number, except that the numbers of macrophages decrease to a lesser extent. However, the average size of macrophages appeared to be increased indicating possible activation (Zhan et al., in press). In autopsies, both EM and ISH have confirmed the infection of substantial numbers of macrophages and lymphocytes in the circulating blood, spleen, and lymph nodes (Gu et al., 2005).
SARS-CoV infection also affects the lymphoid component of the intestine. Excessive atrophy of submucosal lymphoid tissue has been observed, and in some cases only the depleted stromal framework of the follicular structure can be found. Such atrophy is demonstrated in the submucosal tissue of the pharynx and in the Peyer's patches of the ileum, jejunum and appendix (Shi et al., 2005). The follicles, germinal centers of the follicles, and thymus-dependent areas have all been found destroyed (Gu et al., 2005; Shi et al., 2004). EM and in situ hybridization have identified viral particles and viral genome in lymphocytes both in the intestinal mucosa and in Peyer's patches (Gu et al., 2005; Shi et al., 2004).
2.3. Bone marrow
In some SARS cases hemophagocytosis has been detected in bone marrow biopsies (SARS Research Group, 2004, Ng et al., 2006), though this finding was not present in other cases (Wong et al., 2003). No instances of hypoplastic bone marrow have been reported (Wong et al., 2003). RT-PCR and ISH have been both negative for SARS virus on bone marrow specimens (Ding et al., 2004; SARS Research Group, 2004, To et al., 2004).
2.4. Digestive tract
In general, no changes have been evident on gross examination of the digestive system. In some confirmed SARS cases, mild diffused inflammation of the digestive tract, together with autolytic changes have been reported (Gu et al., 2005, Shi et al., 2005, To et al., 2004), whereas other authors have reported no histological abnormalities (Lang et al., 2003, Leung et al., 2003). The most prominent histopathological change, which has been reported in the digestive tract, is atrophy of the submucosal lymphoid tissues (Shi et al., 2005), which is described in detail in Section 2.2 of this review. The epithelial cells of the mucosa of both the small and the large intestines have been confirmed to be infected by SARS-CoV through in situ hybridization, fluorescence in situ hybridization, RT-PCR and EM (Ding et al., 2004, Farcas et al., 2005, Gu et al., 2005, Leung et al., 2003, To et al., 2004). This finding is consistent with reports of viral shedding in stool samples detected by RT-PCR and viral isolation (Chan et al., 2004, Cheng et al., 2004, Liu et al., 2004a, Liu et al., 2004b, HKU/UCH SARS Study Group, 2003). No histological abnormalities have been detected in the tissues of the esophagus or the stomach (Gu et al., 2005).
2.5. Liver
Liver tissues of SARS autopsies have shown numerous hepatocyte mitoses, balloon degeneration of hepatocytes and mild to moderate lymphocytic infiltrates (Chau et al., 2004, Lang et al., 2003). Fatty degeneration and central lobular necrosis have also been reported (Ding et al., 2003, Gu et al., 2005, Lang et al., 2003, Shi et al., 2005). Apoptosis was evident in all three SARS cases reported by Chau et al. (2004). In several cases, RT-PCR has confirmed SARS-infection of hepatocytes (Chau et al., 2004, Farcas et al., 2005), whereas the viral genome and virus particles have not been detected by ISH and EM (Chow et al., 2004, Gu et al., 2005, To et al., 2004).
2.6. Urinary system
The kidneys of some SARS autopsies have been found to be focally hemorrhagic and have shown various degrees of acute tubular necrosis. No glomerular pathology or cellular infiltrates have been found in the kidneys. Non-specific features such as benign hypertensive nephrosclerosis and autolysis have been present in many cases (Chu et al., 2005, Gu et al., 2005, Lang et al., 2003). Both in situ hybridization and EM have detected viral sequences and particles in the epithelial cells of the distal renal tubules (Ding et al., 2004, Gu et al., 2005). The detection of the virus in the distal tubules may also explain the presence of viral RNA and isolation of the SARS-CoV in urinary samples (Chan et al., 2004, Cheng et al., 2004, Gu et al., 2005, HKU/UCH SARS Study Group, 2003).
2.7. The nervous system
Histological examination of brain tissue specimens has shown degeneration and necrosis of neurons, edema, extensive glial cell hyperplasia, and cellular infiltrates (Ding et al., 2003, Gu et al., 2005, Xu et al., 2005). The infiltrates consist of microglia/resident macrophages and CD3 positive T lymphocytes (Ding et al., 2003, Xu et al., 2005). By means of EM and in situ hybridization, viral particles and viral genome sequences have been detected in the cytoplasm of neurons of the brain, mainly in the hypothalamus and the cortex (Gu et al., 2005, Xu et al., 2005), indicating that the virus can cross the blood–brain barrier. The exact route by which SARS-Co infection of the brain takes place is not known. Infected monocytes/macrophages migrating across the blood–brain barrier may be involved in a similar manner as with HIV-1 (Albright et al., 2003). In addition, interaction of SARS-CoV with microvascular endothelial cells, the principal cells forming the blood–brain barrier, could also facilitate viral entry into the brain. Infection of neurons may provide an explanation for the neurological and psychological symptoms frequently observed in SARS patients.
2.8. The endocrine system
Thyroid glands obtained from five SARS patients showed extensive damage to the follicular epithelium, with large numbers of cells exfoliated in the follicle, and loss of parafollicular cells. The follicular architecture was prominently affected, showing follicular distortion and collapse. Terminal deoxynucleotidyl transferase-mediated dUPT nick end labeling (TUNEL) assays have shown many cells in the thyroid undergoing apoptosis (unpublished data), but viral genomic sequences were absent from thyroid cells evaluated by ISH (Ding et al., 2004, Gu et al., 2005).
Vasculitis of small veins in the adrenal gland and the presence of SARS-CoV have been reported by some authors (Ding et al., 2003, Ding et al., 2004), but this feature has not been confirmed by others (Gu et al., 2005).
2.9. The reproductive system
SARS also affects the reproductive system. The testes of SARS patients have displayed widespread destruction of germ cells, few or no spermatozoa in the seminiferous tubules, thickened basement membranes, and infiltration by mainly lymphocytes and macrophages. However, in situ hybridization and EM have failed to detect SARS viral particles and genomic sequences in the testes (Ding et al., 2004, Gu et al., 2005, Xu et al., 2006).
2.10. Other organs
Focal myocyte necrosis in skeletal muscle has been reported in some SARS autopsy cases, but no viral particle or viral sequence has been detected by viral culture, EM or in situ hybridization, respectively (Chow et al., 2004, Ding et al., 2004, Leung et al., 2005, To et al., 2004; Tse et al., 2003). In one publication the presence of SARS-CoV has been reported in sweat glands (Ding et al., 2004). No specific pathological changes have been observed in the heart or the pancreas (Ding et al., 2003, Gu et al., 2005, Lang et al., 2003, To et al., 2004).
3. Pathogenesis of SARS
During the SARS epidemic in 2002 and 2003, more than 8000 people were infected by SARS-CoV globally. SARS-CoV was isolated from Himalayan palm civets found in a live-animal market in Guangdong, China. The full-length genome sequences had 99.8% homology to the SARS-CoV genome found in humans, which indicates a route of interspecies transmission. Many other animals have also been found to host or to be infected by the virus (Guan et al., 2003). Recently, bats were reported as natural carriers of SARS virus (Li et al., 2005). There is certain evidence suggesting that SARS-CoV spreads via droplet and contact transmission and via fecal–oral route (Muller and McGeer, 2006, Wang et al., 2005). Through these routes, SARS-CoV can spread/transmit from animal to human or from human to human.
The pathogenesis of SARS appears to be multifactorial and complex. The most plausible and possible mechanism appears to consist of a direct injury to the target cells by the virus and an indirect injury mediated by subsequent immune system dysfunction.
SARS-CoV has been found to infect the immune system, involving circulating immune cells, lymph nodes and spleen, in addition to injuring pneumocytes. T lymphocytes and macrophages/monocytes are the key immune cells that are infected by SARS-CoV. Severe damage to the splenic white pulp has been demonstrated, which is accompanied by a marked decrease in numbers of splenic immune cells. In addition, epithelial cells both of the intestines and distal renal tubules, together with neurons in the central nervous system are infected by SARS-CoV. Furthermore, limited SARS-CoV infection of the endothelial cells and fibroblasts in the lungs has been identified.
Based on these findings the following pathogenetic mechanism may be postulated. By means of droplet inhalation, SARS-CoV reaches the respiratory tract and invades the epithelial cells of the trachea, bronchi, bronchioles and alveoli, as supported by the presence of viral genomic sequences in such cells. Virus infection and replication in target cells cause direct damages to the respiratory tract. Local inflammatory changes destroy the integrity of the blood–gas barrier and increase the permeability of the capillary blood vessels. Exudation of fibrin results in the formation of hyaline membranes. The infection and associated inflammation bring about acute injury of type II alveolar cells, decreasing the secretion of alveolar surfactant resulting in alveolar collapse. At the same time, SARS-CoV infects resident and circulating immune cells. The infected immune cells include mainly macrophages and T cells. The observed infection and destruction of immune cells is consistent with the fact that lymphopenia is almost always found in SARS patients. Circulating immune cells disseminate the virus to other organs, including the spleen and the lymph nodes. The destruction of immune cells together with extensive damage to the splenic white pulp results in immunodeficiency. A weakened immune defense exacerbates the infection and replication of the virus in the lungs and viral damage to the respiratory alveoli, resulting in respiratory distress. The degree of such immune deficiency as determined by the peripheral lymphocyte count may predict both the severity and outcome of the disease. The clinical observation that the extent of T cell decrease seems related to the severity of the disease (He et al., 2005), lends support to this supposition. In a similar manner, SARS patients with a co-morbid condition such as diabetes mellitus or chronic hepatitis with a compromised immune function have been found to have adverse disease outcomes (Chan et al., 2003, HKU/UCH SARS Study Group, 2003). Fig. 1 diagrammatically illustrates the proposed mechanism including the role of infected immune cells in the pathogenesis of SARS. SARS resembles AIDS with respect to the fact that both are viral diseases that result in immunodeficiency. However, SARS infects various immune cells and progresses rapidly whereas HIV mainly attacks CD4 lymphocytes and is slowly progressive. It should be pointed out that SARS-CoV infections show differing degrees of severity in different cases. Only the most severe cases demonstrate the full range of pathological changes that have been described in autopsy findings.
Fig. 1.
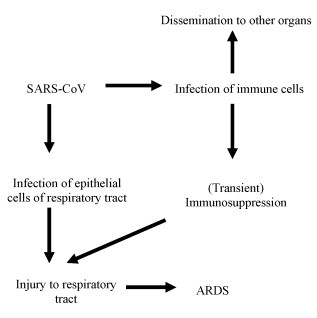
Diagram depicting the role of infected immune cells in the pathogenesis of SARS. Other etiological factors involved are not included in this diagram.
Several other viral infectious diseases, such as measles, Ebola virus infections, and respiratory syncytial virus infections, are also associated with severe lymphopenia (Geisbert and Jahrling, 2004; Schneider-Schaulies et al., 2001, Openshaw, 2002). The etiological factors accounting for lymphopenia in these diseases, however, appear to differ from those in SARS. Direct infection of lymphocytes is not seen in RSV or Ebola virus infections, and only in a very small proportion of peripheral blood cells in measles (Geisbert and Jahrling, 2004, Schneider-Schaulies et al., 2001, Openshaw, 2002). As to these infections, direct infection therefore seems to play a minor role in the depletion of lymphocytes.
Many investigators have speculated that host immune overreaction may play a role in the pathogenesis of SARS. It has been suggested that SARS-CoV infection may cause hyperinduction of the immune system, and increased levels of cytokines and chemokines have indeed been found in SARS patients (Ward et al., 2005), although not consistently. In vitro studies have shown that SARS-CoV infection of both macrophages and dendritic cells may cause cytokine production (Tseng et al., 2005). Through in vitro experiments it has been demonstrated that macrophages, dendritic cell and other cell lines infected by SARS-CoV, induced upregulation of certain chemokines (Cheung et al., 2005, Law et al., 2005a, Law et al., 2005b, Yen et al., 2006). Hyperinduction of cytokines and chemokines may result in exacerbation of a local inflammatory reaction through attraction of inflammatory cells.
However, some authors have suggested that these findings are not sufficiently conclusive and have expressed doubt as to the relevance of cytokines in the process described above (Gu et al., 2005) particularly as no consistent cytokine profile has been observed in SARS patients.
Deficiencies in the innate immune response may also affect the pathogenesis of SARS. Deficiency of mannose binding lectin, a key component of the innate immune system, has been detected in several SARS patients. Such a deficiency may increase susceptibility for SARS infection (Ip et al., 2005). In addition, in vitro experiments have shown little or no interferon response induced by SARS-CoV infection of macrophages and dendritic cells (Cheung et al., 2005, Law et al., 2005a, Law et al., 2005b). Such a minimal interferon response may also result in a substantial shortfall of the innate immune response.
Auto-immune responses may also be involved in the pathogenesis of SARS. In vitro experiments have shown that auto-antibodies obtained from sera of SARS patients induce cytotoxicity against epithelial and endothelial cells (Lin et al., 2005, Yang et al., 2005a, Yang et al., 2005b).
Angiotensin-converting enzyme 2 (ACE2) has been identified as the primary functional receptor for SARS-CoV (Li et al., 2003, Kuba et al., 2005). ACE2 is expressed in the epithelial cells of the lungs and small intestines, as well as in the endothelial and smooth muscle cells of several other organs (Hamming et al., 2004). ACE2 is not only a SARS-CoV receptor, but also a key component in the pathogenesis of SARS-related lung injury. Based on animal experiments, ACE2 may protect against respiratory failure, and down regulation of ACE2 may cause acute lung injury (Imai et al., 2005, Kuba et al., 2006). ACE2 is a negative regulator of the Renin Angiotensin System (RAS) and, as such, has a negative effect on the formation of Angiotensin II. Angiotensin II appears to be one of the elements of the RAS that contributes to exacerbation of acute lung injury (Imai et al., 2005). With respect to SARS-related lung injury, binding of SARS-CoV Spike proteins to ACE2 has been found to reduce ACE2 expression, thus inducing acute lung edema (Kuba et al., 2005).
Local viral replication may also play a significant role in the pathogenesis of SARS. As mentioned previously, the severity of cell damage differs in the various organs, with the lungs and the immune system generally sustaining the most severe injury. The distribution of ACE2, the functional SARS-CoV receptor, partially accounts for such organ-to-organ differences. However, this does not explain how certain cell types, such as lymphocytes, fibroblasts, and colonic intestinal epithelial cells, which do not express ACE2, are affected by SARS-CoV (Gu et al., 2005, Hamming et al., 2004). In contrast, only few vascular endothelial cells, although these cells substantially express ACE2, show infection by SARS-CoV (unpublished data). Both L-SIGN (CLEC4M) and DC-SIGN (CD209L) have been reported to be additional receptors for SARS-CoV (Jeffers et al., 2004, Simmons et al., 2005), though they are much less efficient. In vitro experiments have demonstrated that homozygous L-SIGN plays a protective role against SARS-CoV infection (Chan et al., 2006). Nevertheless, the precise role of both L-SIGN and DC-SIGN needs to be further clarified.
In addition, there is evidence that apoptosis plays a role in the pathogenesis of SARS. In vitro experiments indicate that overexpression of certain non-structural proteins may induce apoptosis in several cell types (Fielding et al., 2004, Law et al., 2005a, Law et al., 2005b, Tan et al., 2004, Yuan et al., 2005). As further evidence for this mechanism, apoptotic cells were found in the liver and the thyroid tissue samples of SARS patients (Chau et al., 2004; unpublished data).
Finally, genetics have been suggested to affect the pathogenesis of SARS. Certain HLA haplotypes are associated with a higher susceptibility to SARS infection (Ip et al., 2005, Ng et al., 2004). In contrast, L-SIGN homozygote individuals have a lower susceptibility to SARS infection (Chan et al., 2006).
4. Implications and future directions
The proposed mechanisms of SARS have significant implications for the prevention, diagnosis, therapy and future research on this newly emerged disease. A better understanding of the distribution of target cells and viral concentration will provide a guide for accurate and early detection of the virus. The status of the immune function should be a key parameter in gauging the treatment of SARS infection. The state of the immune system at different stages of the disease gives clues as to how immune suppressive, immune supportive and steroid therapy may best be administered in treating the disease, and may also help to determine the timing and the dosage of the treatment. Due to the lack of a good understanding of the pathogenesis and the role of immunopathology during the SARS epidemic, the administration of steroids in treating SARS was a controversial topic, and the outcome of such treatment was not uniformly beneficial. The wide spread distribution of the virus in the body and the high concentration of the virus in the blood and leukocytes imply that the virus may be transmissible through blood and body fluid. The detection of virus in the intestine, the kidney, the stool and the urine indicate that the contaminated stool and urine may also be a vehicle of viral transmission. The development of vaccine to SARS depends on the establishment of appropriate animal models. As immune dysfunction and immune pathology play pivotal roles in the pathogenesis of SARS, the measurement of immune indices should be important parameters to be monitored in evaluating animal models and effectiveness of any vaccine under investigation. Unfortunately, this important fact has to date been neglected by investigators developing vaccines to SARS. Infections of the central nervous system and the pathology of other organs should also be taken into consideration when conducting the follow up examinations of recovered SARS patients.
Many questions with respect to the pathogenesis of SARS still remain unanswered and demand further investigation. In particular, investigation of the role played in the interaction between SARS-CoV and the target cells by other receptors or cofactors, in addition to the SARS-CoV receptors that have already been identified is warranted. Furthermore, the exact role of immune cells needs to be further clarified. The role of chemokines and cytokines and their interaction with immune cells must also be further addressed. The role and mechanism of genetic mutations of the virus in causing the disease and in eventual termination of the epidemic are still poorly understood.
Acknowledgements
This review was supported by China National 863 project No. 2003AA 208107, and The Ministry of Education, China, No.104184.
References
- Albright V.A., Soldan S.S., Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J. Neurovirol. 2003;9(2):222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Chan H.L., Kwan A.C., To K.F., Lai S.T., Chan P.K., Leung W.K., Lee N., Wu A., Sung J.J. Clinical significance of hepatic derangement in severe acute respiratory syndrome. World J. Gastroenterol. 2005;11(14):2148–2153. doi: 10.3748/wjg.v11.i14.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.W., Ng C.K., Chan Y.H., Mok T.Y., Lee S., Chua S.Y., Law W.L., Lee M.P., Li P.C. Short-term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):689–699. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Poon L.L., Cheng V.C., Guan Y., Hung I.F., Kong J., Yam L.Y., Seto W.H., Yuen K.Y., Peiris J.S. Detection of SARS coronavirus in patients with suspected SARS. Emerg. Infect. Dis. 2004;10(2):294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V.S., Chan K.Y., Chen Y., Poon L.L., Cheung A.N., Zheng B., Chan K.H., Mak W., Ngan H.Y., Xu X., Screaton G., Tam P.K., Austyn J.M., Chan L.C., Yip S.P., Peiris M., Khoo U.S., Lin C.L. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat. Genet. 2006;38(1):38–46. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau T.N., Lee K.C., Yao H., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C., Choi K.W., Tso Y.K., Lau T., Lai S.T., Lai C.L. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung O.Y., Chan J.W., Ng C.K., Koo C.K. The spectrum of pathological changes in severe acute respiratory syndrome (SARS) Histopathology. 2004;45(2):119–124. doi: 10.1111/j.1365-2559.2004.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K.C., Hsiao C.H., Lin T.Y., Chen C.L., Chiou S.H. Detection of severe acute respiratory syndrome-associated coronavirus in pneumocytes of the lung. Am. J. Clin. Pathol. 2004;121(4):574–580. doi: 10.1309/C0EDU0RAQBTXBHCE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W., Lai T.S., Tong K.L., Lai K.N. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Fan Y., Wu W., Zhang F., Wang J.Y., Ni A.P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2003;37(6):857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Farcas G.A., Poutanen S.M., Mazzulli T., Willey B.M., Butany J., Asa S.L., Faure P., Akhavan P., Low D.E., Kain K.C. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J. Infect. Dis. 2005;191(2):193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding B.C., Tan Y.J., Shuo S., Tan T.H., Ooi E.E., Lim S.G., Hong W., Goh P.Y. Characterization of a unique group-specific protein (U122) of the severe acute respiratory syndrome coronavirus. J. Virol. 2004;78(14):7311–7318. doi: 10.1128/JVI.78.14.7311-7318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stohr K., Osterhaus A.D. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423(6937):240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., Selbs E., McEvoy C.P., Hayden C.D., Fukuoka J., Taubenberger J.K., Travis W.D. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Jahrling P.B. Exotic emerging viral diseases: progress and challenges. Nat. Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., Dwyer D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005;9(6):323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARS Research Group of National Taiwan University College of Medicine, National Taiwan University Hospital Patient data, early SARS epidemic Taiwan. Emerg. Infect. Dis. 2004;10(3):489–493. doi: 10.3201/eid1003.030571. [DOI] [PubMed] [Google Scholar]
- Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., L’Asa S., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U.S.A. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Z.W., Zhang L.J., Zhang S.J., Meng X., Li J.Q., Song C.Z., Sun L., Zhou Y.S., Dwyer D.E. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35(6):526–531(a). doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S., Lau Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law P.T., Wong C.H., Au T.C., Chuck C.P., Kong S.K., Chan P.K., To K.F., Lo A.W., Chan J.Y., Suen Y.K., Chan H.Y., Fung K.P., Waye M.M., Sung J.J., Lo Y.M., Tsui S.K. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J. Gen. Virol. 2005;86:1921–1930. doi: 10.1099/vir.0.80813-0. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T.W., Wong K.S., Hui A.C., To K.F., Lai S.T., Ng W.F., Ng H.K. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch. Neurol. 2005;62(7):1113–1117. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- Li M.H., Li X.H., Li X.W., Ma L., Yi W., Jiang Y.Y., Dong J.P., Li W.L. Difference and significance of T-lymphocyte subsets in differential diagnosis between severe acute respiratory syndrome and common atypical pneumonia. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2004;18(2):137–141. [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronavirus. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lin Y.S., Lin C.F., Fang Y.T., Kuo Y.M., Liao P.C., Yeh T.M., Hwa K.Y., Shieh C.C., Yen J.H., Wang H.J., Su I.J., Lei H.Y. Antibody to severe acute respiratory syndrome (SARS)-associated coronavirus spike protein domain 2 cross-reacts with lung epithelial cells and causes cytotoxicity. Clin. Exp. Immunol. 2005;141(3):500–508. doi: 10.1111/j.1365-2249.2005.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.L., Lu Y.T., Peng M.J., Chen P.J., Lin R.L., Wu C.L., Kuo H.T. Clinical and laboratory features of severe acute respiratory syndrome vis-à-vis onset of fever. Chest. 2004;126(2):509–516. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tang F., Fontanet A., Zhan L., Zhao Q.M., Zhang P.H., Wu X.M., Zuo S.Q., Baril L., Vabret A., Xin Z.T., Shao Y.M., Yang H., Cao W.C. Long-term SARS coronavirus excretion from patient cohort, China. Emerg. Infect Dis. 2004;10(10):1841–1843. doi: 10.3201/eid1010.040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.P., McGeer A. Febrile respiratory illness in the intensive care unit setting: an infection control perspective. Curr. Opin. Crit. Care. 2006;12(1):37–42. doi: 10.1097/01.ccx.0000198056.58083.a1. [DOI] [PubMed] [Google Scholar]
- Ng M.H., Lau K.M., Li L., Cheng S.H., Chan W.Y., Hui P.K., Zee B., Leung C.B., Sung J.J. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J. Infect. Dis. 2004;190(3):515–518. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W.F., To K.F., Lam W.W., Ng T.K., Lee K.C. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum. Pathol. 2006;37(4):381–390. doi: 10.1016/j.humpath.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P.J. Potential therapeutic implications of new insights into respiratory syncytial virus disease. Respir. Res. 2002;1(Suppl.):S15–S20. doi: 10.1186/rr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer T.H., Chan P.K., Ip M., Lee N., Hui D.S., Smit D., Wu A., Ahuja A.T., Tam J.S., Sung J.J., Cameron P. The spectrum of severe acute respiratory syndrome-associated coronavirus infection. Ann. Intern. Med. 2004;140(8):614–619. doi: 10.7326/0003-4819-140-8-200404200-00008. [DOI] [PubMed] [Google Scholar]
- Rainer T.H., Chan P.K., Ip M., Lee N., Hui D.S., Smit D., Wu A., Ahuja A.T., Tam J.S., Sung J.J., Cameron P. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest. 2004;126(2):509–517. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies J., ter Meulen V., Schneider-Schaulies S. Measles virus interactions with cellular receptors: consequences for viral pathogenesis. J. Neurovirol. 2001;7(5):391–399. doi: 10.1080/135502801753170246. [DOI] [PubMed] [Google Scholar]
- Shi X., Gong E., Gao D., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Wu B., Fang W., Liao S., Wang S., Xie Z., Lu M., Hou L., Zhong H., Shao H., Li N., Liu C., Pei F., Yang J., Wang Y., Han Z., Shi X., Zhang Q., You J., Zhu X., Gu J. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am. J. Gastroenterol. 2005;100(1):169–176. doi: 10.1111/j.1572-0241.2005.40377.x. [DOI] [PubMed] [Google Scholar]
- Shieh W.J., Hsiao C.H., Paddock C.D., Guarner J., Goldsmith C.S., Tatti K., Packard M., Mueller L., Wu M.Z., Rollin P., Su I.J., Zaki S.R. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum. Pathol. 2005;36(3):303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U.S.A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.J., Fielding B.C., Goh P.Y., Shen S., Tan T.H., Lim S.G., Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J. Virol. 2004;78:14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.F., Tong J.H., Chan P.K., Au F.W., Chim S.S., Chan K.C., Cheung J.L., Liu E.Y., Tse G.M., Lo A.W., Lo Y.M., Ng H.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in situ hybridization study of fatal cases. J. Pathol. 2004;202(2):157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse G.M., To K.F., Chan P.K., Lo A.W., Ng K.C., Wu A., Lee N., Wong H.C., Mak S.M., Chan K.F., Hui D.S., Sung J.J., Ng H.K. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174(12):7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Y.H., Wu H.H., Chao F.H., Li J.W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. Virol. Methods. 2005;128(1–2):156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S.E., Loutfy M.R., Blatt L.M., Siminovitch K.A., Chen J., Hinek A., Wolff B., Pham D.H., Deif H., LaMere E.A., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Fung L., Dennis J.W., Lai E.K., Fish E.N. Dynamic changes in clinical features and cytokine/chemokine responses in SARS patients treated with interferon alfacon-1 plus corticosteroids. Antivir. Ther. 2005;10(2):263–275. [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K., Chan P.K., Ng M.H., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO website: http://www.who.int/csr/sars/country/table2004_04_21/en/.
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., Ding Y., Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., Peh S., Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol. Reprod. 2006;74(2):410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.H., Huang Y.H., Chuang Y.H., Peng C.M., Wang L.C., Lin Y.T., Chiang B.L. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J. Med. Virol. 2005;77(1):1–7. doi: 10.1002/jmv.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Xu M., Yi J.Q., Jia W.D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobil. Pancreat. Dis. Int. 2005;4(1):60–63. [PubMed] [Google Scholar]
- Ye, J., Zhang, B., Xu, J., Chang, Q., McNutt, M.A., Korteweg, C., Gong, E., Gu, J. Molecular pathology in the lungs of SARS patients. Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed]
- Yen Y.T., Liao F., Hsiao C.H., Kao C.L., Chen Y.C., Wu-Hsieh B.A. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J. Virol. 2006;80(6):2684–2693. doi: 10.1128/JVI.80.6.2684-2693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Shan Y., Zhao Z., Chen J., Cong Y. G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells. Virol. J. 2005;2:66. doi: 10.1186/1743-422X-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, J., Deng, R., Tang, J., Zhang, B., Tang, Y., Wang, J.K., Li, F., Anderson, V.M., McNutt, M.A., Gu, J. The spleen as a target in Severe Acute Respiratory Syndrome (SARS). FASEB J. in press. [DOI] [PubMed]


