Abstract
In response to various pathological stresses, the heart undergoes a pathological remodeling process that is associated with cardiomyocyte hypertrophy. Because cardiac hypertrophy can progress to heart failure, a major cause of lethality worldwide, the intracellular signaling pathways that control cardiomyocyte growth have been the subject of intensive investigation. It has been known for more than a decade that the small molecular weight GTPase RhoA is involved in the signaling pathways leading to cardiomyocyte hypertrophy. Although some of the hypertrophic pathways activated by RhoA have now been identified, the identity of the exchange factors that modulate its activity in cardiomyocytes is currently unknown. In this study, we show that AKAP-Lbc, an A-kinase anchoring protein (AKAP) with an intrinsic Rho-specific guanine nucleotide exchange factor activity, is critical for activating RhoA and transducing hypertrophic signals downstream of α1-adrenergic receptors (ARs). In particular, our results indicate that suppression of AKAP-Lbc expression by infecting rat neonatal ventricular cardiomyocytes with lentiviruses encoding AKAP-Lbc-specific short hairpin RNAs strongly reduces both α1-AR-mediated RhoA activation and hypertrophic responses. Interestingly, α1-ARs promote AKAP-Lbc activation via a pathway that requires the α subunit of the heterotrimeric G protein G12. These findings identify AKAP-Lbc as the first Rho-guanine nucleotide exchange factor (GEF) involved in the signaling pathways leading to cardiomyocytes hypertrophy.
Keywords: cardiac hypertrophy, Rho GTPase, G protein-coupled receptor
Ventricular myocyte hypertrophy is an adaptive growth response to a stress placed on the heart promoting an increase in cardiac contractility. It is associated with a nonmitotic growth of cardiomyocytes, increased myofibrillar organization, and up-regulation of specific subsets of “fetal” genes that are normally expressed during embryonic life (1). Because hypertrophy can often progress to heart failure, a major cause of morbidity and mortality worldwide, many efforts have been made during recent years to define the molecular players involved in this pathological process.
In this respect, several lines of evidence collected over more then 10 years indicate that adrenergic transmission mediated by α1-adrenergic receptors (ARs) can initiate signaling pathways that control cardiomyocyte hypertrophy both in vitro and in vivo (2–4). α1-ARs are seven transmembrane domain receptors that can couple to and activate heterotrimeric G proteins of the Gq and G12/G13 family (5). Although most of the studies have focused on the role of the α subunit of Gq in mediating the effects of α1-ARs on cardiomyocyte hypertrophy, recent evidence now suggests that Gα12 and Gα13 also contribute importantly to the growth responses induced by these receptors (5). In fact, it has been shown that α1-ARs, by means of the stimulation of the α subunits of G12 and G13, can promote the activation of the GTPase RhoA (5). In cardiomyocytes, this small molecular weight GTP-binding protein promotes the activation of different effector kinases, including Rho kinase (5, 6), protein kinase N (PKN) (7), and stress-activated protein (SAP) kinases (8), which control the transcription of genes involved in cardiomyocyte hypertrophy.
At the cellular level, the activation of Rho is controlled by Dbl family guanine nucleotide exchange factors (GEFs), which all share a Dbl homology (DH) domain and an adjacent pleckstrin homology (PH) domain (9). The DH domain is responsible for the guanine nucleotide exchange activity, whereas the PH domain controls the subcellular localization of the GEF or contributes to the binding pocket for Rho-GTPases (10).
Recently, we identified an exchange factor expressed in the heart, termed AKAP-Lbc, which functions as GEF for RhoA as well as an A-kinase anchoring protein (AKAP) (11, 12). Interestingly, AKAP-Lbc is regulated in a bidirectional manner by signals that activate or deactivate its Rho-GEF activity. Activation of AKAP-Lbc occurs in response to agonists that stimulate G proteins coupled receptors linked to the heterotrimeric G protein G12 (11), whereas inactivation occurs through a mechanism that requires phosphorylation of AKAP-Lbc by anchored PKA and subsequent recruitment of the regulatory protein 14-3-3 (13).
Although the implication of RhoA in the hypertrophic pathways activated by the α1-AR is known by more than a decade (14), the identity of the Rho-GEFs that mediate cardiomyocyte hypertrophy has remained elusive mainly because of the unavailability of reagents capable of inhibiting the function of exchange factors in a specific manner. In the present study, we used a lentivirus-based strategy to deliver AKAP-Lbc-specific short hairpin (sh) RNAs into primary cultures of rat neonatal ventricular cardiomyocytes (NVMs). Using this approach, we could demonstrate that AKAP-Lbc plays a key role in mediating α1-AR-induced hypertrophic responses. In particular, we found that AKAP-Lbc participates in a transduction pathway activated by the α1-AR that includes Gα12, AKAP-Lbc, and RhoA that promotes cardiomyocyte hypertrophy. Therefore, our findings identify AKAP-Lbc as a Rho-GEF crucially involved in the transduction pathways associated to cardiomyocyte hypertrophy.
Results
α1-AR Stimulation Up-Regulates AKAP-Lbc Expression in Cardiomyocytes.
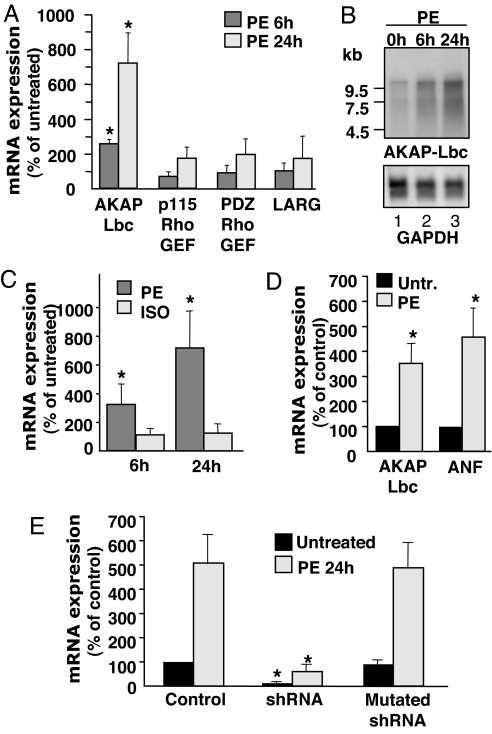
Several lines of evidence demonstrate that RhoA plays an important role in mediating the hypertrophic responses to α1-AR agonists in rat NVMs (5, 14), thus raising the question of which cardiac Rho-GEF could mediate receptor-induced RhoA activation. Interestingly, we found that primary cultures of rat NVMs express several Rho selective exchange factors including LARG, PDZ-Rho-GEF, p115 Rho-GEF, and AKAP-Lbc that are known to be activated by G protein-coupled receptors (GPCRs) [supporting information (SI) Fig. 5] (11, 15–18). We initially determined by real-time quantitative PCR whether the expression of these exchange factors could be modulated in response to the hypertrophic stimulation of cardiomyocytes with phenylephrine (PE). Interestingly, we found that treatment of NVMs for 24 h with 10−4 M PE could increase AKAP-Lbc mRNA expression by >7-fold without significantly affecting the mRNA expression of the other exchange factors (Fig. 1A). Up-regulation of AKAP-Lbc expression was already detectable 6 h after PE treatment as assessed by Northern blot and by real-time PCR (Fig. 1 B Upper and C), suggesting that that the stimulation of AKAP-Lbc expression precedes the phenotypic appearance of the cardiomyocyte hypertrophy, which is detectable 24 h after PE treatment. Moreover, stimulation of NVMs for 24 h with 10−4 M of isoproterenol did not induce any detectable increase in the expression of AKAP-Lbc suggesting that AKAP-Lbc up-regulation is selectively induced in response to the chronic activation of α1-ARs but not β-ARs (Fig. 1C).
Fig. 1.
PE selectively up-regulates AKAP-Lbc expression in cardiomyocytes. (A) Real-time PCR analysis of the mRNA expression of various Rho-GEFs was performed on total RNA samples extracted from rat NVMs that were left untreated or that were stimulated for 24 h with 10−4 M PE. (B) Northern blots were prepared with 20 μg of total RNAs extracted from rat NVMs that were left untreated or were stimulated for 6 h and 24 h with 10−4 M PE. Blots were hybridized with cDNA probes corresponding to the first 500 nucleotides of the rat AKAP-Lbc. (C) Real-time PCR analysis of AKAP-Lbc mRNA expression was performed on total RNA samples extracted from rat NVMs that were left untreated or were stimulated for 6 h and 24 h with 10−4 M PE or isoproterenol. (D) Real-time PCR analysis of AKAP-Lbc and ANF mRNA expression was performed on total RNA samples extracted from C57B6 mice that were left untreated or infused for 14 days with 100 μg/kg/day of PE. (E) shRNA-mediated knockdown of AKAP-Lbc expression. NVMs were infected with lentiviruses encoding GFP (control), or lentiviruses encoding both GFP and wild-type or mutated AKAP-Lbc shRNAs at a moi of 50. Seventy-two hours after infection cells were incubated for 24 h with or without 10−4 M PE. RNAs were extracted and AKAP-Lbc expression analyzed by real-time PCR. Results are the mean ± SE of three independent experiments.
We then examined whether AKAP-Lbc was also up-regulated in vivo during pathological cardiac hypertrophy. To address this issue we analyzed AKAP-Lbc expression in the left ventricular tissue from mice that were subjected to a chronic infusion of PE (100 μg·kg−1·day−1) for a period of 14 days (19). In agreement with previous reports, this chronic PE treatment increased the cardiac weight index by 21% (SI Fig. 6). Interestingly, we found that ventricular expression of AKAP-Lbc and that of the hypertrophic marker atrial natriuretic factor (ANF) were increased in response to PE infusion by 3.5- and 4.6-fold, respectively (Fig. 1D). Altogether, these findings raise the intriguing hypothesis that AKAP-Lbc could participate in the early molecular events that promote PE-induced cardiomyocyte hypertrophy.
Silencing AKAP-Lbc Expression in Rat NVMs Using Lentivirus-Encoded Short Hairpin (sh) RNAs.
To examine the potential implication of AKAP-Lbc in α1-AR-induced cardiomyocyte hypertrophy, we developed a lentivirus-based strategy to deliver shRNAs specific to AKAP-Lbc into rat NVMs. To allow a direct visualization of the infected cells, recombinant lentiviruses were engineered to express the GFP in addition to the specific shRNAs. To investigate the role of AKAP-Lbc in α1-AR-induced hypertrophy we generated the following lentiviruses: a control virus expressing only GFP, a virus encoding AKAP-Lbc-specific shRNA and virus encoding a mutated AKAP-Lbc shRNA. By infecting of rat NVMs with these lentiviruses using a multiplicity of infection (moi) of 50, we could reach infection rates that ranged from 89% to 93% (SI Fig. 7).
Silencing efficiency was evaluated 96 h after infection in cardiomyocytes that were incubated for 24 h in the absence or presence of 10−4 M PE by measuring the expression of AKAP-Lbc mRNA by real-time PCR. AKAP-Lbc silencing was assessed by using this technique because our anti-AKAP-Lbc antibodies recognize only the human but not the rodent forms of AKAP-Lbc. As shown in Fig. 1E, infection of cardiomyocytes with lentiviruses expressing AKAP-Lbc-specific shRNAs resulted in a 87% down-regulation of AKAP-Lbc mRNA expression either under basal conditions or after PE treatment as compared with cells infected with control lentiviruses expressing only GFP. In contrast, the mutated AKAP-Lbc shRNA had no effect on expression AKAP-Lbc levels. Importantly, the AKAP-Lbc shRNA did not alter the mRNA expression of p115-Rho-EGF, PDZ-Rho-GEF, and LARG (results not shown), suggesting that it represents a valuable specific tool for inhibiting the expression of AKAP-Lbc in rat NVMs.
AKAP-Lbc Is Involved in α1-AR-Mediated Rho Activation in Cardiomyocytes.
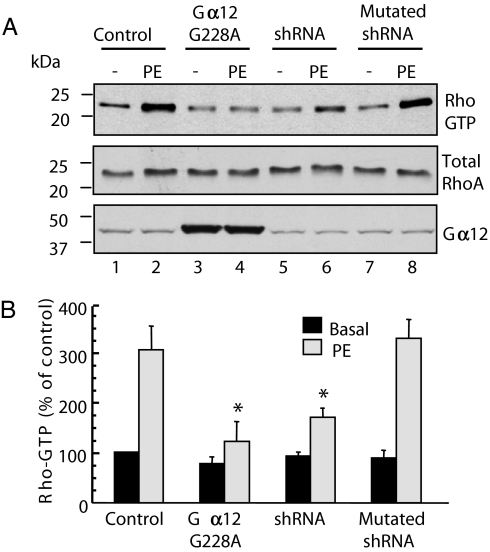
Recent evidence suggests that, in rat NVMs, α1-ARs can induce Rho activation via Gα12 (5). In agreement with these findings, we found that infection of rat NVMs with lentiviruses encoding the dominant negative mutant of Gα12 (Gα12 G228A) (20) (SI Fig. 7) inhibited by 79% the activation of RhoA induced by PE as assessed by using the Rhotekin pulldown assay (Fig. 2A Top, lanes 3 and 4, and B). Based on these results and on our previous findings showing that AKAP-Lbc is a Gα12-activated Rho-GEF (11), we tested the hypothesis that AKAP-Lbc could mediate the activation of RhoA downstream of α1-ARs. To address this point, we assessed the impact of silencing AKAP-Lbc expression in rat NVMs on the ability of PE to induce RhoA activation. Interestingly, infection of rat NVMs with lentiviruses encoding AKAP-Lbc-specific shRNA impaired by 64% the ability of PE to induce the formation of active GTP-bound RhoA as compared with cells infected with control lentiviruses or lentiviruses expressing the mutated AKAP-Lbc shRNA (Fig. 2 A Top and B).
Fig. 2.
Silencing of AKAP-Lbc expression inhibits α1-AR-mediated Rho activation in cardiomyocytes. (A) Rat NVMs were infected with lentiviruses encoding GFP (control), both GFP and wild-type or mutated AKAP-Lbc shRNAs, or the Gα12 G228A mutant at a moi of 50. Seventy-two hours after infection, cells were incubated for 15 min with or without 10−4 M PE. Cell lysates were incubated with GST-RBD beads. The bound RhoA was detected with a monoclonal anti-RhoA antibody (Top). The relative amount of total RhoA and Gα12 G228A mutant in the cell lysates were assessed by using a monoclonal antibody against RhoA (Middle) and a polyclonal antibody against Gα12 (Bottom), respectively. (B) Quantitative analysis of the GTP-RhoA associated with RBD beads was obtained by densitometry. The amount of RhoA bound to RBD was normalized to the RhoA content of cell extracts. Results are expressed as mean ± SE of four independent experiments. Statistical significance was analyzed by paired Student's test. ∗, P < 0.05 as compared with Rho-GTP levels measured in PE stimulated cardiomyocytes infected with control lentiviruses.
The residual effect of PE on the activation of RhoA observed in silenced cardiomyocytes might be due an incomplete down-regulation of the expression of AKAP-Lbc (Fig. 1E). Alternatively, one could hypothesize that an additional Rho-GEF that might mediate part of the activation of Rho in response to the stimulation of α1-ARs. Collectively, these findings strongly suggest that, in rat NVMs, Gα12 and AKAP-Lbc are crucially involved in the activation of Rho in response to α1-AR agonists.
Both α1A- and α1B-AR Subtypes Can Activate Rho via a Gα12-AKAP-Lbc Pathway.
Rat NVMs express α1a- and α1b-AR subtypes, both of which have been shown to participate in the generation of hypertrophic signals (4). To determine whether both these α1-AR subtypes can mediate Rho activation via AKAP-Lbc, we determined whether AKAP-Lbc silencing could impair the formation of Rho-GTP induced by the overexpressed α1a- or α1b-ARs in HEK-293 cells. Infection of the HEK-293 cells with lentiviruses encoding shRNAs specific to the human form of AKAP-Lbc reduced the expression of the anchoring protein to undetectable levels (SI Fig. 8 A and B, AKAP-Lbc panel, lanes 5 and 6) and impaired the ability of α1a- and α1b-ARs to induce the formation of Rho-GTP in the presence of PE by 55% and 78%, respectively (SI Fig. 8 A and B Top, lane 6). No inhibition of Rho activation was observed in cells infected with control lentiviruses encoding the mutated AKAP-Lbc shRNA (SI Fig. 8 A and B Top, lanes 9 and 10). Interestingly this inhibitory effect was totally reversed when HEK-293 cells were transfected with a silencing resistant mutant of AKAP-Lbc (SI Fig. 8 A and B Top, lanes 7 and 8) suggesting that the inhibition of RhoA activation was strictly dependent on reduced AKAP-Lbc expression and not due to an off-target effect. Collectively, these results strongly suggest that both α1a- and α1b-AR subtypes activate RhoA via AKAP-Lbc.
To provide direct evidence that α1a- and α1b-AR subtypes activate AKAP-Lbc, we determined whether the stimulation of these receptors by PE could enhance the interaction between AKAP-Lbc and endogenous RhoA. Importantly, we have previously shown that the formation AKAP-Lbc-RhoA complexes reflects the activation state of AKAP-Lbc (21). Serum starved HEK-293 cells expressing Flag-tagged AKAP-Lbc in the absence or presence of the α1a- and α1b-AR subtypes were stimulated for 15 min with PE. AKAP-Lbc was then immunoprecipitated by using anti-Flag antibodies and the presence of associated RhoA determined by immunoblot. Activation of both α1a- and α1b-AR subtypes induced a significant increase in the amount of RhoA associated with AKAP-Lbc (SI Fig. 9 Top, lanes 5 and 6 and 9 and 10). This effect was impaired when cells were cotransfected with the dominant negative mutant of Gα12 (20) suggesting that both α1-AR subtypes induce AKAP-Lbc activation via Gα12 (SI Fig. 9 Top, lanes 7 and 8 and 11 and 12).
AKAP-Lbc Mediates α1-AR Induced Cardiomyocyte Hypertrophy.
Based on the above results, we investigated the possibility that the Gα12-AKAP-Lbc-RhoA signaling cascade could mediate α1-AR-induced cardiomyocyte hypertrophy. To address this hypothesis, we measured the effect of inhibiting AKAP-Lbc expression in rat NVMs on the hypertrophic responses induced by α1-ARs.
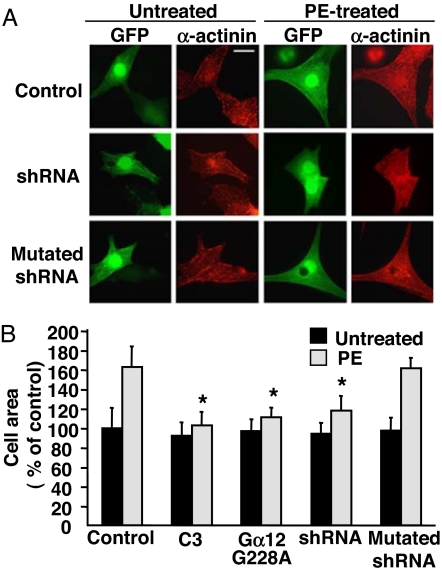
Induction of cardiomyocyte hypertrophy was initially assessed by measuring cell size as well as sarcomere assembly and reorganization. In NVMs infected with control lentiviruses or lentiviruses encoding mutated AKAP-Lbc shRNAs, treatment with PE for 24 h induced a 60–63% increase in cell size and led to the assembly of highly ordered sarcomeres as visualized by staining cardiomyocytes by using anti-α-actinin antibodies (Fig. 3). Interestingly, these effects were profoundly impaired in cardiomyocytes expressing AKAP-Lbc shRNAs, in which PE treatment could only induce a 23% increase in cell size and a modest reorganization of the sarcomeres (Fig. 3).
Fig. 3.
Silencing of AKAP-Lbc expression inhibits α1-AR-mediated cardiomyocytes hypertrophy. (A) Rat NVMs were infected with lentiviruses encoding GFP (control), both GFP and wild-type or mutated AKAP-Lbc shRNAs, or the Gα12 G228A mutant at a moi of 50. Seventy-two hours after infection, cells were incubated for 24 h in the absence or presence of 10−4 M PE. Cells infected with control lentiviruses were treated with or without 2 μg/ml of C3 botulinum toxin before the incubation with PE. Cells were then fixed, permeabilized, and incubated with anti-α-actinin monoclonal antibodies as well as rhodamine-conjugated anti-mouse secondary antibodies. GFP expression was visualized directly by fluorescent excitation at 490 nm. (B) Mean cell surface area (± SE) of cardiomyocytes infected and treated as indicated in A. The cell surface area was determined on a total of 150 GFP-positive cardiomyocytes derived from five independent experiments by using the Image J software. Statistical significance was analyzed by paired Student's test. ∗, P < 0.05 as compared with the cell surface area measured in PE stimulated cardiomyocytes infected with control lentiviruses.
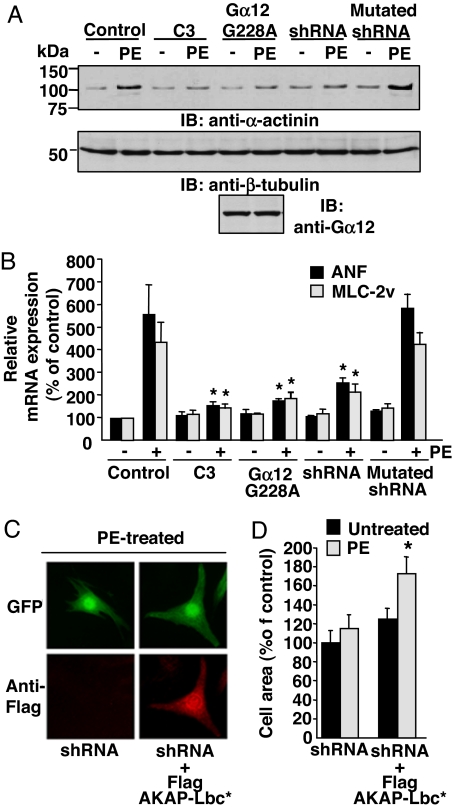
Next, we determined whether silencing the expression of the anchoring protein could also affect the PE-mediated induction of hypertrophic genes including α-actinin, skeletal α-actin and ANF. In agreement with our results showing that AKAP-Lbc mediates hypertrophic cardiomyocyte growth, we could show that suppression of AKAP-Lbc expression strongly inhibits PE-mediated induction of α-actinin, as assessed by immunoblot (Fig. 4A Upper, lanes 7 and 8), as well as of ANF and skeletal α-actin, as assessed by real-time PCR (Fig. 4B).
Fig. 4.
Silencing of AKAP-Lbc expression inhibits α1-AR-mediated induction of hypertrophic markers. (A) Cardiomyocytes were infected and treated with PE as indicated in Fig. 3. The relative expression of α-actinin, β-tubulin, and Gα12 G228A mutant in the cell lysates was assessed by using a monoclonal antibody against α-actinin (Top), β-tubulin (Middle), and a polyclonal antibody against Gα12 (Bottom), respectively. (B) Real-time PCR analysis of ANF and skeletal α-actin (Ska) mRNA expression was performed on total RNA samples extracted from rat ventricular cardiomyocytes that were infected and treated with PE as indicated in Fig. 3. All results are expressed as mean ± SE of three independent experiments. Statistical significance was analyzed by paired Student's test. ∗, P < 0.05 as compared with ANF or Ska levels measured in PE-stimulated cells infected with control lentiviruses. (C and D) Cardiomyocytes were infected with lentiviruses expressing AKAP-Lbc shRNAs and subsequently transfected with the empty Flag vector or with the cDNA encoding the Flag-tagged silencing resistant mutant of AKAP-Lbc. Forty-eight hours after transfection, cells were incubated for 24 h in the absence or presence of PE. Cells were then fixed, permeabilized, and incubated with anti-Flag monoclonal antibodies as well as rhodamine-conjugated anti-mouse secondary antibodies to detect the expression of the Flag-AKAP-Lbc mutant. GFP expression was visualized directly by fluorescent excitation at 490 nm. The mean cell surface area (±SE) of cardiomyocytes was determined as indicated in Fig. 3B. Statistical significance was analyzed by paired Student's test. ∗, P < 0.05 as compared with the cell surface area measured in PE-stimulated cardiomyocytes infected with lentiviruses encoding AKAP-Lbc shRNAs.
A similar inhibition of α1-AR-activated hypertrophic responses was observed after impairing the function of endogenous Rho by using the C3 botulinum toxin (Figs. 3 and 4) or after inhibiting Gα12 by infecting cells with lentiviruses expressing the dominant negative mutant of Gα12 (Figs. 3 and 4). These findings are consistent with the idea that the Gα12-AKAP-Lbc RhoA pathway is involved in α1-AR-induced cardiomyocytes hypertrophy.
To determine the influence of the activation state of AKAP-Lbc on its ability to induce cardiomyocytes hypertrophy, we compared the basal growth response induced by AKAP-Lbc-GFP or by a GFP-tagged constitutively active truncated form of AKAP-Lbc missing the N-terminal inhibitory region (21). We found that whereas AKAP-Lbc-GFP does not promote any significant increase in cardiomyocytes size in rat NVM under basal conditions, a strong hypertrophic response can be observed in cardiomyocytes expressing the constitutively active form(SI Fig. 10). These results are in agreement with our previous findings showing that AKAP-Lbc displays a weak basal Rho-GEF activity (11), and they suggest that AKAP-Lbc can promote cardiomyocyte hypertrophy only when activated.
To determine whether the impairment of PE-induced hypertrophy induced by the AKAP-Lbc shRNAs was strictly related to the inhibition of AKAP-Lbc expression and not to the nonspecific down-regulation of additional signaling components, we determined whether reexpressing AKAP-Lbc in silenced cardiomyocytes could restore the hypertrophic response to PE. Rat NVMs were infected by using lentiviruses encoding AKAP-Lbc shRNAs and subsequently transfected with the cDNA encoding a Flag-tagged silencing resistant mutant of AKAP-Lbc (Fig. 4 C and D). Interestingly, we found that reexpression of recombinant AKAP-Lbc completely restored PE-induced hypertrophy (Fig. 4 C and D), confirming the hypothesis that AKAP-Lbc is an important mediator of the hypertrophic responses activated by the α1-ARs in cardiomyocytes.
We finally assessed whether AKAP-Lbc could also contribute to the hypertrophic responses initiated by other GPCR-agonists, such as angiotensin II (Ang-II) and endothelin 1 (ET-1), that can promote RhoA activation in cardiomyocytes (8, 22). As shown in SI Fig. 11 rat NVMs infected with lentiviruses encoding AKAP-Lbc shRNAs displayed a 35–40% inhibition of Ang-II- or ET-1-induced ANF expression as compared with cardiomyocytes infected with mutated shRNAs. These results suggest that, whereas AKAP-Lbc seems to be primarily involved in the hypertrophic pathways activated by PE, it might also partially contribute to the growth pathways initiated by Ang-II and ET-1.
Discussion
The implication of the Rho signaling pathway in cardiomyocyte hypertrophy has been largely investigated primarily by using the primary cultures of rat NVMs as a model system. Whereas many hypertrophic pathways activated by RhoA have been identified, the signaling molecules involved in the transduction of signals from membrane receptors to RhoA have been only partially elucidated.
In the present study, we demonstrate that the Rho-specific exchange factor AKAP-Lbc is crucially involved in the hypertrophic pathway activated by the α1-ARs in primary cultures of rat NVMs. In particular, our results indicate that suppression of AKAP-Lbc expression not only reduces PE-indicated RhoA activation but also impairs α1-AR-mediated cardiomyocyte hypertrophy. Interestingly, the Rho-GEF activity of AKAP-Lbc is stimulated by both α1A- and α1B-AR subtypes and is mediated by the α subunit of the heterotrimeric G protein G12. Overall, these findings identify AKAP-Lbc as a critical Rho-GEF involved in the activation of hypertrophic signaling pathways in response to PE. The fact that AKAP-Lbc is partially contributes to the of the hypertrophic responses induced by Ang-II and ET-1 suggests that this Rho-GEF might play some more general roles in the growth responses activated by GPCRs coupled to the Rho pathway.
For many years it has been assumed that, in cardiomyocytes, α1-ARs activate RhoA only via a Gαq-dependent pathway (14). This conclusion was drawn mainly on the basis of the observations that α1-ARs are preferentially coupled to Gαq and that overexpression a constitutively activated form of Gαq in cardiomyocytes induces Rho activation (14, 23). This idea is now challenged by the recent studies from the Kurose group, who have shown that, in cardiomyocytes, α1-ARs receptors can mediate RhoA activation mainly via Gα12 and Gα13 (5). Our current results showing that the overexpression of a dominant negative mutant of Gα12 strongly reduces the activation of RhoA by PE in rat NVMs support these findings and underline the importance of the Gα12-RhoA pathway in the induction of cardiomyocyte hypertrophy.
So far, only a few Rho-GEFs have been identified in cardiomyocytes. Recently, two sarcomere-associated Rho-GEFs, termed obscurin and p63Rho-GEF, have been proposed to control the organization of the sarcomeric cytoskeleton in rat NVMs (24, 25). Interestingly, p63Rho-GEF was shown to be activated by Gαq (but not by Gα12 or Gα13) in HEK-293 cells (26). However, it is currently unknown whether Gq-coupled GPCRs can promote the activation of this exchange factor in cardiomyocytes.
We have shown that rat NVMs, in addition to AKAP-Lbc, also express the Gα12/Gα13-activated exchange factors p115-Rho-GEF, PDZ-Rho-GEF, and LARG. Whereas our studies indicate that their expression in cardiomyocytes is not significantly increased after the chronic stimulation of α1-ARs, it will be interesting to determine whether these Rho-GEFs could be regulated by other Gα12/Gα13-coupled GPCRs, including Ang-II, ET-1, or lysophosphatidic acid (LPA) receptors, which are known to activate RhoA and induce hypertrophy in cardiomyocytes. Interestingly, studies performed in HeLa cells, indicate that silencing the expression of PDZ-Rho-GEF by using specific si-RNAs abolishes the Rho response induced by LPA receptors (27). However, whether this Rho-GEF acts downstream of LPA receptors also in cardiomyocytes remains to be elucidated.
In addition to the Gα12-AKAP-Lbc-Rho pathway described here, other signaling cascades activated by Gαq have been shown to play a major role in the hypertrophic responses induced by α1-ARs. In fact, it has been shown that Gαq, by means of the activation of phospholipase C, the production of inositol-3,4,5-triphosphate, and the mobilization of calcium from intracellular stores, regulates the function of calcineurin, a serine/threonine phosphatase that controls the activity of the hypertrophic transcription factor NFAT (28). Alternatively, Gαq can promote cardiomyocytes growth by means of the activation of mitogen activated protein kinase signaling cascades (29) or through the stimulation of a protein kinase C (PKC)–protein kinase D (PKD) pathway, which enhances hypertrophic gene transcription by means of the regulation of chromatin-modifying enzymes (30). Interestingly, inhibition of either of these pathways strongly impairs α1-AR-induced cardiomyocyte hypertrophy suggesting that, taken individually, Gα12 and Gαq-mediated transduction cascades are necessary but not sufficient to mediate the growth response to α1-AR agonists.
It has been recently shown that AKAP-Lbc, in addition of activating RhoA, can also assemble a signaling complex that is required for PKD activation (31). Because PKD controls transduction pathways that regulate the transcription hypertrophic of genes, it would be interesting to determine whether AKAP-Lbc can promote cardiomyocyte hypertrophy also through the regulation of this kinase.
In conclusion, the implications of our findings are twofold. Firstly, they identify AKAP-Lbc as the first Rho-GEF involved in the signaling pathways leading to cardiomyocytes hypertrophy. Secondly, they identify cardiomyocyte hypertrophy as the first pathophysiological response linked to AKAP-Lbc activity.
Materials and Methods
Standard methods discussing the generation of expression constructs, cell culture and transfection, Northern blot experiments, the purification of recombinant proteins in bacteria, immunoprecipitation experiments, Rhotekin pulldown assays, SDS/PAGE and Western blotting, and immunofluorescence microscopy are described in SI Materials and Methods.
Production of Lentiviruses.
VSV-G pseudotyped lentiviruses were produced by cotransfecting 293-T cells with 20 μg of the pSD28-GFP or pAB286.1 vectors (32) containing the AKAP-Lbc shRNA cassette, 15 μg of pCMVDR8.91 (33), and 5 μg of pMD2.VSVG (33) by using the calcium phosphate method. Culture medium was replaced by serum-free DMEM at 12 h after transfection. Cell supernatants were collected 48 h later, filtered through a 0.45-μm filter unit, concentrated by using Centricon-Plus-70 MW 100,000 columns (Millipore), resuspended in PBS and reconcentrated by using Centricon-20 columns (Millipore, Bedford, MA). Virus titers were determined by infecting 293-T cells by using serial dilutions of the viral stocks and by scoring either the number of GFP-positive cells (at 72 h after infection) or puromycin-resistant clones (at 6 days after infection). Titers determined by using these methods were between 7 × 108 and 1.2 × 109 transducing units (TU)/ml for viruses generated from pSD28 vectors and between 3 × 108 and 7 × 108 TU/ml for viruses generated from pAB286.1 vectors.
Lentiviral Infection.
HEK-293 cells were infected at 60% confluency by using pAB286.1-based lentiviruses encoding wild-type or mutated AKAP-Lbc shRNAs at a moi of 10 in the presence of 8 μg/ml of polybrene. Two days after infection, puromycin was added to the culture medium at a final concentration of 2 μg/ml. After 4 days of selection, puromycin-resistant cells were collected an amplified in selective medium containing puromycin at a final concentration of 2 μg/ml.
Rat neonatal ventricular cardiomyocytes were infected 24 h after plating by using pSD28-based lentiviruses encoding wild type or mutated AKAP-Lbc shRNAs or the Gα12 G228A mutant at a moi of 50 in maintenance medium containing 5% horse serum and 8 μg/ml of polybrene. Twenty-four hours after infection, cardiomyocytes were incubated in maintenance medium for an additional 48 h. For rescue experiments, cardiomyocytes were transfected 24 h after infection with 4 μg of the cDNA encoding the silencing-resistant Flag-tagged AKAP-Lbc mutant by using Lipofectamine 2000. Transfection was performed in maintenance medium containing 5% horse serum in the absence of antibiotics for a period of 2 h. Cells were then incubated in maintenance medium for an additional 48 h.
Chronic Phenylehrine Infusion.
Phenylehrine (100 μg·kg−1·day−1 in 0.2% ascorbic acid) was infused for 14 days through osmotic minipumps with a mean fill volume of 200 μl (Alzet model 2002, Alza Corp) that were implanted s.c. in the back of 12-week-old C57B6 male mice. Previous findings have shown that this dose of PE does not elevate blood pressure but is able to induce ventricular hypertrophy (19). After the treatments, the animals were killed, and total mRNA was extracted from heart ventricles.
Real-Time PCR.
Determination of the mRNAs levels of AKAP-Lbc, p115-Rho-GEF, PDZ-Rho-GEF, LARG, ANF, and skeletal α-actin in rat neonatal cardiomyocytes and in C57B6 mouse ventricles was carried out by real-time RT-PCR analysis by using a LightCycler Instrument (Roche Applied Science). Total mRNA was extracted from rat cardiomyocytes or from the heart ventricles of C57B6 mice, and single-strand cDNA was synthesized from 2.5 μg of total RNA by using random hexamers (Applied Biosystems) and SuperScript II reverse transcriptase (Invitrogen). RT-PCR reactions were prepared by using a LightCycler kit (Eurogentec, Belgium) in a final volume of 20 μl containing 125 ng of reverse-transcribed total RNA and 0.5 μl of SYBR Green in the presence of the following forward and reverse primers described in SI Material and Methods. Glyceraldehyde-3-phosphate dehydrogenase mRNA was used as invariant internal control. The relative amount of all mRNAs was calculated by using the LightCycler analysis software Version 3.5.
Supplementary Material
Acknowledgments
This work was supported by Fonds National Suisse de la Recherche Scientifique Grants 3100A0-109440 (to D.D.) and 3100A0-100703 (to S.C.) and by a grant of the Novartis Foundation (to D.D.).
Abbreviations
- AR
adrenergic receptor
- GEF
guanine nucleotide exchange factor
- AKAP
A-kinase anchoring protein
- shRNA
short hairpin RNA
- NVM
neonatal ventricular cardiomyocyte
- GPCR
G protein-coupled receptor
- PE
phenylephrine
- ANF
atrial natriuretic factor
- moi
multiplicity of infection
- Ang-II
angiotensin II
- ET-1
endothelin 1
- LPA
lysophasphatidic acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701099104/DC1.
References
- 1.Frey N, Katus HA, Olson EN, Hill JA. Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 2.Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, Lefkowitz RJ. Proc Natl Acad Sci USA. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowlton KU, Rockman HA, Itani M, Vovan A, Seidman CE, Chien KR. J Clin Invest. 1995;96:1311–1318. doi: 10.1172/JCI118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, Wada T, Nagao T, Kurose H. Circ Res. 2002;91:961–969. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- 6.Yanazume T, Hasegawa K, Wada H, Morimoto T, Abe M, Kawamura T, Sasayama S. J Biol Chem. 2002;277:8618–8625. doi: 10.1074/jbc.M107924200. [DOI] [PubMed] [Google Scholar]
- 7.Morissette MR, Sah VP, Glembotski CC, Brown JH. Am J Physiol Heart Circ Physiol. 2000;278:H1769–H1774. doi: 10.1152/ajpheart.2000.278.6.H1769. [DOI] [PubMed] [Google Scholar]
- 8.Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M. Genes Dev. 2001;15:2702–2719. doi: 10.1101/gad.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerione RA, Zheng Y. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 10.Bi F, Debreceni B, Zhu K, Salani B, Eva A, Zheng Y. Mol Cell Biol. 2001;21:1463–1474. doi: 10.1128/MCB.21.5.1463-1474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diviani D, Soderling J, Scott JD. J Biol Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 12.Wong W, Scott JD. Nat. Rev. Mol Cell Biol. 2004;12:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 13.Diviani D, Abuin L, Cotecchia S, Pansier L. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sah VP, Hoshijima M, Chien KR, Brown JH. J Biol Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 15.Booden MA, Siderovski DP, Der CJ. Mol Cell Biol. 2002;22:4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, Nakamura S, Mano H, Kozasa T. Proc Natl Acad Sci USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 18.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 19.Vecchione C, Fratta L, Rizzoni D, Notte A, Poulet R, Porteri E, Frati G, Guelfi D, Trimarco V, Mulvany MJ, et al. Circulation. 2002;105:1700–1707. doi: 10.1161/01.cir.0000012750.08480.55. [DOI] [PubMed] [Google Scholar]
- 20.Gohla A, Offermanns S, Wilkie TM, Schultz G. J Biol Chem. 1999;274:17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- 21.Baisamy L, Jurisch N, Diviani D. J Biol Chem. 2005;280:15405–15412. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, et al. J Biol Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 23.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., II Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young P, Ehler E, Gautel M. J Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souchet M, Portales-Casamar E, Mazurais D, Schmidt S, Leger I, Javre JL, Robert P, Berrebi-Bertrand I, Bril A, Gout B, et al. J Cell Sci. 2002;115:629–640. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- 26.Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T. J Biol Chem. 2005;280:11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. J Biol Chem. 2004;279:28831–28834. doi: 10.1074/jbc.C400105200. [DOI] [PubMed] [Google Scholar]
- 28.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorburn J, Xu S, Thorburn A. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. Mol Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Nat Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 33.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.