Abstract
Upon viral infection, the major defense mounted by the host immune system is activation of the interferon (IFN)-mediated antiviral pathway that is mediated by IFN regulatory factors (IRFs). In order to complete their life cycle, viruses must modulate the host IFN-mediated immune response. Kaposi's sarcoma-associated herpesvirus (KSHV), a human tumor-inducing herpesvirus, has developed a unique mechanism for antagonizing cellular IFN-mediated antiviral activity by incorporating viral homologs of the cellular IRFs, called vIRFs. Here, we report a novel immune evasion mechanism of KSHV vIRF3 to block cellular IRF7-mediated innate immunity in response to viral infection. KSHV vIRF3 specifically interacts with either the DNA binding domain or the central IRF association domain of IRF7, and this interaction leads to the inhibition of IRF7 DNA binding activity and, therefore, suppression of alpha interferon (IFN-α) production and IFN-mediated immunity. Remarkably, the central 40 amino acids of vIRF3, containing the double α helix motifs, are sufficient not only for binding to IRF7, but also for inhibiting IRF7 DNA binding activity. Consequently, the expression of the double α helix motif-containing peptide effectively suppresses IRF7-mediated IFN-α production. This demonstrates a remarkably efficient means of viral avoidance of host antiviral activity.
Interferon (IFN) regulatory factors (IRFs) are a growing family of transcription factors that have been implicated in antiviral defense and immune regulation (15, 19, 31, 39, 42). This family is mainly defined by a highly conserved amino-terminal DNA binding domain (DBD) characterized by a repeat containing five tryptophan residues (16, 19, 42). Two closely related members of this family, IRF3 and IRF7, have been identified as direct transducers of virus-mediated signaling in the induction of type I IFN (alpha/beta interferon [IFN-α/β]). Upon viral infection, both IRF3 and IRF7 undergo virus infection-induced serine phosphorylation within a carboxy-terminal regulatory domain (RED), a modification that stimulates protein dimerization, nuclear retention, and interaction with transcriptional coactivators, resulting in the activation of a robust response comprised of a broader spectrum of IFN isotypes (26). Gene targeting shows that both IRF3 and IRF7 are essential for the induction of IFN-α/β genes via the virus-activated classical pathway (36). In addition, IRF7, but not IRF3, is also required for both the systemic production of IFN in innate immunity and the local action of IFN from plasmacytoid dendritic cells in adaptive immunity (16).
Most viruses have evolved immune evasion strategies to protect themselves against host IFN responses, elaborating viral proteins as a counterdefense against the host IFN defenses (13, 14). These strategies include blocking of IFN signaling by downregulation of JAK-STAT signal molecule basal levels, suppression of particular molecule modification, and prevention of molecule translocation (12, 14, 18, 40). Herpesviruses have also been shown to antagonize IFN responses through numerous mechanisms. Herpes simplex virus, a prototype alphaherpesvirus, encodes at least two modulators of the IFN response: US11 and ICP34.5, targeting a similar IFN response pathway, the double-stranded RNA-dependent protein kinase PKR pathway (5-7, 33). Epstein-Barr virus also blocks the antiviral IFN response through downregulation of IFN-γ receptor gene expression by BZLF1, an immediate-early gene product (29). Kaposi's sarcoma-associated herpesvirus (KSHV) has developed a unique mechanism for antagonizing cellular IFN-mediated antiviral activity by incorporating viral homologs to several cellular regulatory genes into its genome, including viral homologs of the IRFs (vIRFs) (14). vIRF1 has been shown to downregulate IFN- and IRF-mediated transcriptional activation by interacting with cellular transcriptional coactivators (20, 21, 34). In addition, vIRF2 represses IFN signaling mediated by IRF1 and IRF3; however, the specific mechanism has not been illustrated (11). Furthermore, KSHV immediate-early open reading frame 45 (ORF45) tegument protein interacts with IRF7, leading to inhibition of its phosphorylation and nuclear accumulation and, therefore, inhibition of virus-mediated activation of IFN gene expression (43). This indicates that KSHV deregulates IFN-mediated innate immunity in various ways to establish and/or maintain persistent infection.
KSHV vIRF3, also called latency-associated nuclear antigen 2 (LANA2), which is expressed in KSHV-infected hematopoietic tissues, but not Kaposi's sarcoma lesions, has been shown to affect p53-mediated apoptosis (35), PKR-mediated translational control (8), and NF-κB activation (37). In this report, we show that KSHV vIRF3 interacts with cellular IRF7 and that this interaction specifically suppresses IRF7 DNA binding activity and, therefore, inhibits the expression of the IFN-α gene upon virus infection. Remarkably, the central 40 amino acids (aa) of vIRF3, containing the double helix motifs, are not only required, but also sufficient, for interacting with and inhibiting IRF7 activity. This suggests that KSHV vIRF3 suppresses both IFN-mediated and tumor suppressor-mediated host surveillance, which ensures a comprehensive avoidance of host antiviral activity.
MATERIALS AND METHODS
Cell culture, cytokine treatment, and transfection.
BCBL1 cells were grown in RPMI 1640 (Gibco-BRL) with 10% fetal calf serum (FCS) (Gibco-BRL). The inducible vIRF3 and control BCBL1 cell lines were established by using TREx BCBL1 cells and pcDNA5/FRT/TO vector (Invitrogen). The detailed procedure was described elsewhere (30); the cells were grown in RPMI 1640 with 10% FCS with hygromycin (Invitrogen). 293T cells were grown in Dulbecco's modified Eagle's medium (Gibco-BRL) with 10% FCS and 1% penicillin-streptomycin (Gibco-BRL). Treatments of cells with IFN were performed with 1,000 U of IFN-β (Sigma) per ml. For transient assay, each plasmid DNA was transfected into 293T cells using Transfectin (Bio-Rad) or calcium phosphate (BD Biosciences).
Plasmid construction.
The KSHV vIRF3 cDNA that was kindly provided by Yuan Chang was subsequently amplified by PCR using a 5′ primer that corresponds to the amino-terminal sequence and a 3′ primer that corresponds to the carboxy-terminal sequence. Primers used for PCR contained AflII and NotI recognition sequences for subsequent cloning. Amplified DNA was ligated into the AflII and NotI cloning sites of the pcDNA5/FRT/TO vector (Invitrogen), with Myc tagging at the carboxy terminus. All vIRF3-mutant constructs used in this study were generated by using an oligonucleotide-directed mutagenesis kit (Stratagene) and completely sequenced to verify the presence of the mutation. Each was subcloned into either the pEBG glutathione S-transferase (GST) expression vector or the pEFIRES-P expression vector. IKKɛ and TBK1 were kindly provided by J. Hiscott and T. Maniatis.
Immunoblotting and immunoprecipitation.
Transfected cells were harvested and resuspended with lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail [Roche]), followed by incubation at 4°C for 10 min and centrifugation at 12,000 rpm for 5 min. The supernatants were precleared with protein A/G agarose beads (Santa Cruz Biotech) and incubated with a specific antibody for 2 h, followed by the addition of protein A/G agarose beads and incubation for 1 h. In the case of the GST pull-down assay, glutathione Sepharose 4B (Amersham) was added to the precleared supernatants and incubated at 4°C for 3 h. The beads were washed three times vigorously with washing buffer (50 mM Tris, pH 8.0, 300 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail [Roche]). Immune complexes were resuspended with sodium dodecyl sulfate (SDS) sample buffer (Sigma), separated by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to a polyvinylidene difluoride membrane (Roche). The membrane was blocked with phosphate-buffered saline (PBS) containing 5% skim milk for 30 min, incubated with primary antibody for 1 h, washed three times with PBS-Tween 20, incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, and washed three times with PBS-Tween 20. Specific signals were detected with an enhanced chemiluminescence system (Pierce) and ECL machine (LAS-3000; Fuji). The primary antibodies were purchased from the following sources: FLAG (M2), cMyc, V5, and GST antibodies from Sigma; IRF7 antibody from Santa Cruz; and vIRF3 antibody from Novus.
Yeast two-hybrid screen.
Yeast transformation with library cDNA was performed by a method previously described (9). Yeast strain AH109 bearing the Gal4-vIRF3 fusion gene plasmid was grown overnight in synthetic dropout Trp medium to a density of approximately 107 cells/ml and then diluted in 1 liter of warmed yeast extract-peptone-dextrose to an optical density at 600 nm of 0.2 to 0.3 and grown to exponential stage. The cells were harvested and washed with 100 ml of water twice and with Tris-EDTA (Clontech, CA) once. The pellet was resuspended in 8 ml of 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 0.1 M lithium acetate (LiOAc). The suspension was mixed with 1 mg of transforming DNA and 20 mg of single-stranded salmon sperm DNA, after which 60 ml of a solution of 40% polyethylene glycol 4000 in Tris-EDTA-LiOAc was added and mixed thoroughly, followed by incubation at 30°C with agitation for 30 min. After a heat pulse at 42°C for 15 min, the cells were pelleted, washed with 50 ml of Tris-EDTA, and plated on selective medium. Library screening and recovery of plasmids were performed according to the manufacturer's instructions (Clontech, CA).
Luciferase reporter assay.
293T cells were seeded onto 24-well plates with 105 cells/well 24 h before transfection. The cells were transfected with a luciferase reporter plasmid and a control renilla luciferase plasmid, pRL-SV40 (Promega). At 24 h after transfection, the cells were treated with IFN-β or infected with Sendai virus for the times indicated in the figure legends. The luciferase activity was measured with a luminometer, using a dual luciferase assay kit (Promega, Madison, WI), and normalized to renilla luciferase activity to measure the transfection efficiency.
Real-time quantitative RT-PCR (qRT-PCR).
293T cells were transfected with IRF7 alone or with vIRF3. At 24 h posttransfection, the cells were infected with Sendai virus for 12 h. Total RNAs were extracted by using an RNeasy plus kit (QIAGEN). Total RNA levels of the samples were checked with a spectrophotometer and made up to equal amounts by dilution. The reverse transcription (RT) (28) reaction was performed by using a First Strand cDNA kit (Invitrogen) using 10 μg of total RNA and random hexamers. Real-time PCR was executed on a MyIQ system (Bio-Rad) using MyIQ SYBR green II master mix (Bio-Rad). The efficiencies of each primer set were calculated from twofold serial dilution curves, and the relative amounts of mRNA were calculated by Pfaffle's method (32). The primer sets used were as follows: IFNa1 (5′-GGA CCT TGA TGC TCC TGG CAC AAA-3′ and 5′-TCT AGG AGG TCC TCA TCC CAA GCA-3′), IFNa4 (5′-GAG GGC CTT GAT ACT CCT GGC ACA-3′ and 5′-TCT AGG AGG CTC TGT TCC CAA GCA-3′), IFNa6 (5′-AGA TTT CCC CAG GAG GAG TTT GAT-3′ and 5′-TCT CTG TCA GGT AGA GAG TGA TTC-3′), and bActin (5′-TGG ACA TCC GCA AAG ACC TG-3′ and 5′-CCG ATC CAC ACG GAG TAC TT-3′). To check for contamination with genomic DNA, the same reaction was performed for each sample simultaneously without RT and the amplified products were checked by using conventional electrophoresis.
Electromobility shift assay (EMSA).
Cells were washed with PBS and lysed in lysis buffer (10 mM Tris-Cl [pH 8.0], 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.5% NP-40, protease inhibitor cocktail [Roche]) 48 h after the transfection. Equivalent amounts of whole cell extract were assayed for IRF7 binding by gel shift analysis using a biotin-labeled double-stranded oligonucleotide corresponding to the PRDI-PRDIII region of the IFN-β promoter (5′-GAA AAC TGA AAG GGA GAA GTG AAA GTG-3′). The binding mixture contained 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 50 mM NaCl, 2 mM dithiothreitol, 5% glycerol, 0.5% NP-40, 10 μg of bovine serum albumin per ml, and 62.5 μg of poly(dI-dC) per ml was added to reduce nonspecific binding. The total volume of the reaction mixture with the whole cell extract (20 μg) was 20 μl. After 20 min of incubation with the probe, extracts were loaded on a DNA retardation gel (Novex) prepared in 0.5× Tris-borate-EDTA. After 1 h at 100 V, the gel was transferred to nylon (Pierce) membrane. The membrane was cross-linked with UV (GS Gene Linker; Bio-Rad) and processed according to the manufacturer's protocol (chemiluminescent nucleic acid detection module; Pierce) to detect biotin signals. To demonstrate the specificity of protein-DNA complex formation, a 1,000-fold molar excess of unlabeled oligonucleotides or the antibody to IRF7 was added to the binding mixture before the labeled probe was added to the binding reaction mixtures.
RESULTS
Interaction of vIRF3 with a cellular IRF7.
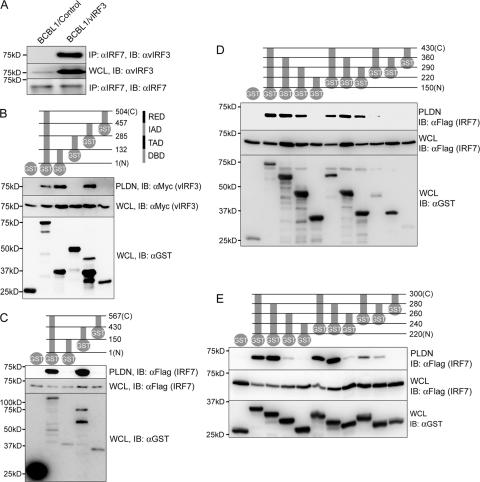
To investigate the role of vIRF3 in the virus-host interaction, we employed the yeast two-hybrid screen to identify cellular targets. A DNA fragment containing aa 1 to 430 of vIRF3 was fused in frame to the GAL4 DBD for use as bait to screen the target cDNA library from Epstein-Barr virus-transformed human B-lymphocytes. DNA sequence analysis of positive clones showed that four vIRF3-interacting clones contained two different-sized fragments of cellular IRF7, starting at the aa 24 residue and the aa 163 residue. To confirm the interaction between vIRF3 and IRF7, KSHV-infected BCBL-1 primary effusion lymphoma cells were used to express vIRF3 in a tetracycline-inducible manner. Coimmunoprecipitation showed that the interaction of vIRF3 with endogenous IRF7 was readily detected in KSHV-infected BCBL-1 cells (Fig. 1A, lane 2). IRF7 consists of an N-terminal tryptophan repeat-containing DBD, a transactivation domain (TAD), an IRF association domain (IAD), and a C-terminal RED (22). The GST-IRF7 mammalian fusion proteins containing each domain of IRF7 were used for mammalian GST pull-down assay. This showed that either the N-terminal DBD or central IAD of IRF7 was not only required, but also sufficient, for interacting with vIRF3 (Fig. 1B, lanes 3 and 5). In contrast, the GST protein alone, GST-TAD, and GST-RED of IRF7 were not capable of interacting with vIRF3 under the same conditions (Fig. 1B, lanes 1, 4 and 6).
FIG. 1.
Interaction of vIRF3 with IRF7. (A) Interaction between endogenous IRF7 and vIRF3 in KSHV-infected BCBL1 cells. TREx BCBL1/control and TREx BCBL1/vIRF3 cells were stimulated with doxycycline for 12 h and, subsequently, with IFN-β for 12 h to induce the expression of vIRF3 and IRF7, respectively. Whole cell lysates (WCL) were used for immunoprecipitation with an anti-IRF7 antibody, followed by immunoblotting with an anti-vIRF3 antibody. (B) vIRF3 binds to the two independent domains of IRF7. 293T cells were transfected with Myc-tagged vIRF3 and GST-fused IRF7 mammalian expression plasmids, as indicated to the right at the top; the numbers indicate the amino acid positions of the IRF7 domains. After 48 h, WCL were used for GST pull-down assay as described in Materials and Methods, followed by immunoblotting with an anti-Myc antibody (bottom). WCL were also used for immunoblotting with anti-Myc and anti-GST antibodies. (C, D, and E) Mapping of IRF7 binding regions in vIRF3. 293T cell were transfected with Flag-tagged IRF7 and GST-fused vIRF3 fragment expression plasmids as depicted at the top of each figure. The numbers on the right side indicate the amino acid positions in vIRF3. After 48 h, WCL were used for GST pull-down assay, followed by immunoblotting with anti-Flag antibody. WCL were used for immunoblotting with anti-Flag and GST antibodies. Molecular size markers are on the left of all panels. PLDN, GST pull-down assay; IP, immunoprecipitation; IB, immunoblotting.
To define the region of vIRF3 required for IRF7 interaction, 293T cells cotransfected with GST-vIRF3 mammalian fusion proteins and Flag-tagged IRF7 were used for GST pull-down assay. The assay results showed that aa 150 to 430 of vIRF3 were sufficient for interacting with Flag-IRF7 (Fig. 1C, lane 4). A series of deletion mutations of GST-vIRF3 showed that the central 40 aa (aa 240 to 280) of vIRF3 bound efficiently to Flag-IRF7 (Fig. 1D and E). Interestingly, the two short sequences of vIRF3 containing 20 aa (aa 240 to 260 and aa 260 to 280) were also capable of binding to IRF7, while their binding affinities were significantly lower than that of the sequence containing 40 aa (aa 240 to 280) (Fig. 1E). These results indicate that KSHV vIRF3 specifically interacts with cellular IRF7 through its central 40-aa sequence.
The putative double α helix motifs of vIRF3 are sufficient to interact with IRF7.
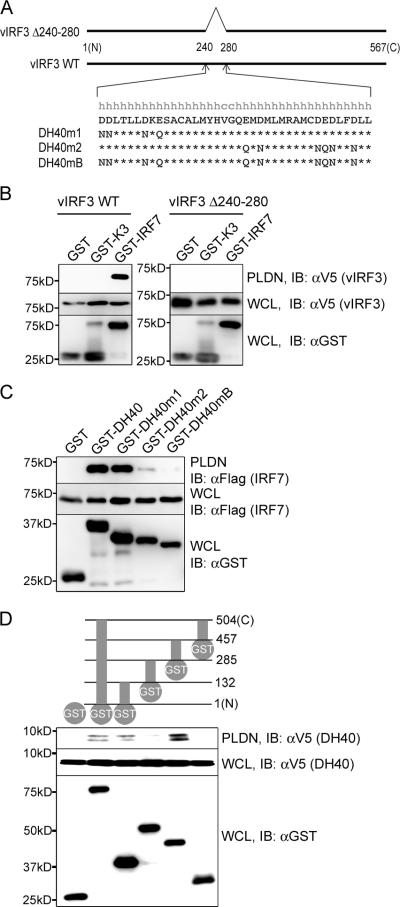
The secondary structure prediction reveals the presence of the double α helix motifs within the 40-aa sequence (aa 240 to 280) of vIRF3 (Fig. 2A). To test their role in IRF7 binding, the mammalian GST-vIRF3 Δ240-280 mutant (vIRF3 Δ240-280) carrying the deletion of both α helix motifs and the wild-type GST-vIRF3 (WT vIRF3) were compared for their IRF7 binding abilities. A mammalian GST-K3 fusion containing the KSHV K3 immune modulatory protein was included as a negative control. This showed that deletion of the double α helix motifs completely abolished vIRF3's ability to bind to IRF7 (Fig. 2B). As described above, both the GST-vIRF3 240-260 and the GST-vIRF3 260-280 mutant, carrying the first or second α helix motif, respectively, showed considerable reductions in IRF7 binding activity (Fig. 1E, lanes 8 and 10). To further delineate the role of vIRF3 double α helix motifs in IRF7 binding, the negatively charged aspartate and glutamate in the helix motifs were replaced with asparagine and glutamine, respectively. GST-vIRF3 mutants containing these replacements in the first (GST-DH40m1) or second (GST-DH40m2) or both α motifs (GST-DH40mB) were then compared with GST-DH40 containing WT vIRF3 aa 240 to 280 for their IRF7 binding abilities (Fig. 2C). GST-DH40m1 bound to IRF7 as efficiently as WT GST-DH40, whereas GST-DH40m2 showed a considerable reduction of IRF7 binding activity. In addition, GST-DH40mB displayed almost complete loss of IRF7 binding ability. Finally, as seen with full-length vIRF3 (Fig. 1B), the V5-tagged central double α helix motifs interacted sufficiently for binding with either GST-IRF7-DBD or GST-IRF7-IAD, whereas it did not with GST, GST-IRF7-TAD, or GST-IRF7-RED under the same conditions (Fig. 2D). These results indicate that the central 40 aa of vIRF3 containing the double α helix motifs are sufficient for IRF7 binding, that the second α helix motif appears to play a more important role in IRF7 binding than the first α helix motif, and that either the N-terminal DBD or central IAD of IRF7 is sufficient for binding to the central double α helix motifs of vIRF3.
FIG. 2.
The central 40 aa (aa 240-280) of vIRF3 are sufficient to bind IRF7. (A) Schematic diagram of the central double helix motifs of vIRF3 and its mutants. The vIRF3 Δ240-280 mutant carried the deletion of aa 240 to 280 that contain the putative helix-turn-helix structure. Aspartic acids (D) and glutamic acids (E) in the double helix motif of vIRF3 were replaced with asparagines (N) and glutamines (Q), respectively. The DH40m1, DH40m2, and DH40mB mutants carried the replacements at the first helix motif, the second helix motif, and both helix motifs, respectively. (B) Deletion mutation of the putative double helix motifs of vIRF3 abolishes IRF7 interaction. 293T cells were transfected with GST or the GST-IRF7 expression vector together with the V5-tagged WT vIRF3 or vIRF3 Δ240-280 mutant. At 48 h, whole cell lysates (WCL) were used for GST pull-down assay, followed by immunoblotting with anti-V5 antibody. WCL were also used for immunoblotting with anti-V5 and anti-GST antibodies to show the levels of expression of the proteins. The GST-K3 vector containing the KSHV K3 gene was used for a negative control. (C) The second helix motif of vIRF3 plays a more important role in IRF7 binding than the first helix motif. At 48 h after transfection of 293T cells with Flag-tagged IRF7 together with the GST, GST-DH40, GST-DH40m1, GST-DH40m2, or GST-DH40mB expression vector, WCL were used for GST pull-down assay, followed by immunoblotting with anti-Flag antibody. WCL were also used for immunoblotting with anti-Flag and anti-GST antibodies to show the levels of expression of the proteins. (D) The 40-aa fragment of vIRF3 binds independently to either the DBD or the IAD of IRF7. At 48 h after transfection of 293T cells with V5-DH40 (vIRF3 240-280) together with the GST, GST-WT IRF7, or GST-IRF7 truncation-mutant expression vector, WCL were used for GST pull-down assay, followed by immunoblotting with anti-V5 antibody. WCL were also used for immunoblotting with anti-V5 and anti-GST antibodies to show the levels of expression of the proteins. Molecular size markers are on the left of all gels. PLDN, GST pull-down assay; IB, immunoblotting.
vIRF3 interaction inhibits the virus-induced activation of IRF7 activity.
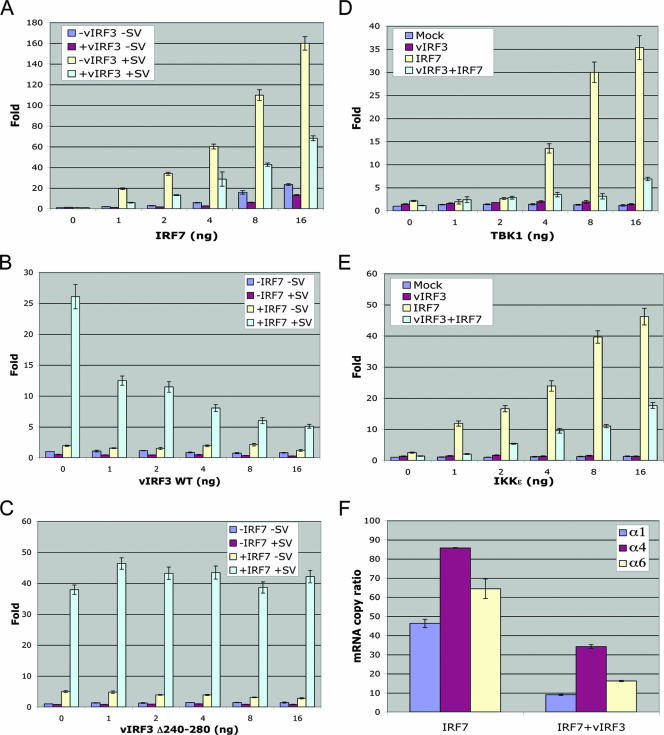
Upon viral infection, IRF7 undergoes virus infection-induced serine phosphorylation within a carboxy-terminal RED, a modification that stimulates protein dimerization, nuclear retention, and interaction with transcriptional coactivators, resulting in the activation of a robust response comprised of a broader spectrum of IFN isotypes (26, 27). To investigate the effect of vIRF3 interaction on IRF7 function, we measured the IFN-α6 promoter activity that is primarily regulated by IRF7. As previously shown (1), IRF7 markedly activated IFN-α6 promoter activity upon Sendai virus infection (Fig. 3A), whereas vIRF3 expression considerably suppressed this IRF7 activity (Fig. 3B), in a dose-dependent manner. By contrast, the vIRF3 Δ240-280 mutant that no longer interacted with IRF7 showed no effect on the IRF7 transcriptional activity under the same conditions (Fig. 3C). As previously shown (38), the expression of the TBK1 and IKKɛ kinases that phosphorylate the carboxy-terminal RED of IRF7 led to the activation of IRF7 transcriptional activity, resulting in a dramatic increase in IFN-α6 promoter activity (Fig. 3D and E). However, vIRF3 coexpression effectively suppressed the TBK1 or IKKɛ kinase-mediated activation of IRF7 transcriptional activity (Fig. 3D and E). Finally, we measured the increases in the levels of endogenous IFN-α1, -α4, and -α6 mRNAs induced by Sendai virus infection in the presence or absence of vIRF3 expression. Correlated with its activity in suppressing IFN-α promoter activity, vIRF3 expression led to considerable reductions in endogenous IFN-α1, -α4, and -α6 mRNA levels, of four- to fivefold (Fig. 3F). The validity of the real-time qRT-PCR results was confirmed by conventional electrophoresis (data not shown). These results collectively indicate that vIRF3 efficiently suppresses IRF7 transcriptional activity in an interaction-dependent manner.
FIG. 3.
vIRF3 inhibits the IRF7-mediated activation of IFN promoter activity. Amounts of 1 × 105 293T cells were seeded into 24-well plates 24 h prior to transfection and transfected with the pGL3-based IFN-α6 promoter construct (100 ng) and pRL-SV40 (10 ng). The total amounts of transfected DNA were adjusted to 200 ng by adjusting the empty pcDNA5 vector. The transfection and the luciferase assay were performed as described in Materials and Methods. (A) 293T cells were transfected with increasing amounts of IRF7 with or without 10 ng of vIRF3. At 24 h posttransfection, the cells were stimulated with 25 hemagglutination (HA) units of Sendai virus for 12 h. (B and C) 293T cells were transfected with increasing amounts of WT vIRF3 (B) or vIRF3 Δ240-280 (C) with or without IRF7 (1 ng). At 24 h posttransfection, the cells were stimulated with 25 HA units of Sendai virus for 12 h. (D and E) 293T cells were transfected with increasing amounts of TBK1 (D) or IKKɛ (E) in combination with vIRF3 (10 ng) and IRF7 (1 ng). (F) Amounts of 1 × 105 293T cells were seeded into 6-well plates and subsequently transfected with IRF7 (4 ng) with or without vIRF3, followed by stimulation with 100 HA units of Sendai virus for 12 h. Real-time qRT-PCR was performed as described in Materials and Methods.
vIRF3 interaction inhibits the DNA binding activity of IRF7.
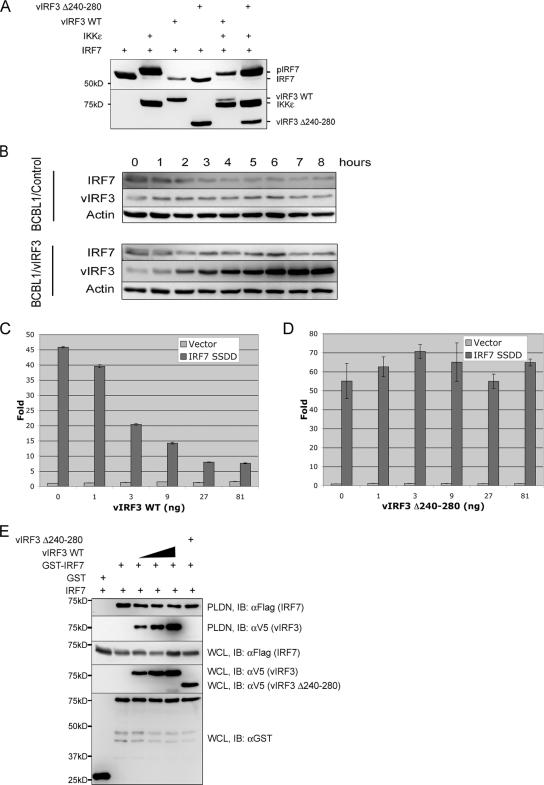
In order to detail the molecular mechanism of vIRF3-mediated inhibition of IRF7 transcription activity, we assessed each step of the IRF7 activation process: phosphorylation, dimerization, and DNA binding activity. As previously shown (4), IKKɛ or TBK coexpression led to the virtually complete shift of IRF7 to the slowly migrating phosphorylated form in SDS-PAGE (Fig. 4A). We found that neither WT vIRF3 nor the vIRF3 Δ240-280 mutant showed any effect on IKKɛ-mediated migration retardation of IRF7, indicating that the vIRF3 interaction has no role in the IKKɛ-mediated IRF7 phosphorylation (Fig. 4A). In addition, vIRF3 expression in KSHV-infected BCBL-1 cells did not alter the endogenous IRF7 protein level at a detectable level (Fig. 4B). The mutant with mutations of the C-terminal 477 and 479 serine residues of IRF7 to negatively charged glutamic acids, called IRF7 S477D/S479D, has been shown to mimic the TBK- or IKKɛ-mediated phosphorylation, resulting in the constitutive activation of IRF7 transcriptional activity (22). As seen with WT IRF7, IRF7 S477D/S479D's transcriptional activity was effectively suppressed by vIRF3 in a dosage-dependent manner, whereas it was not by the vIRF3 Δ240-280 mutant (Fig. 4C and D). Furthermore, the homodimerization of IRF7 was not detectably altered by either WT vIRF3 or vIRF3 Δ240-280 (Fig. 4E). These results indicate that vIRF3 interaction does not affect IRF7 phosphorylation and dimerization.
FIG. 4.
vIRF3 interaction shows no effect on phosphorylation, protein level, or dimerization of IRF7. (A) vIRF3 does not affect IRF7 phosphorylation. At 48 h posttransfection with V5-IRF7, Flag-IKKɛ and/or Flag-vIRF3 expression vectors in various combinations as indicated at the top and 293T whole cell lysates (WCL) were separated through SDS-PAGE, followed by immunoblotting with anti-IRF7 or anti-Flag antibody. pIRF7 indicates the phosphorylated form of IRF7. (B) vIRF3 does not affect IRF7 protein level. TREx BCBL1/control and TREx BCBL1/vIRF3 cells were stimulated with doxycycline for the indicated times. WCL were used for immunoblotting with anti-IRF7, anti-vIRF3, and antiactin antibodies. (C and D) WT vIRF3, but not the vIRF3 Δ240-280 mutant, inhibits the transcriptional activity of constitutively active IRF7 S477D/S479D (IRF7 SSDD). Amounts of 1 × 105 293T cells were seeded into 24-well plates 24 h prior to transfection and transfected with the pGL3-IFN-α6 promoter construct (100 ng) and IRF7 S477D/S479D (1 ng) with increasing amounts of WT vIRF3 or the vIRF3 Δ240-280 mutant. The levels of luciferase were measured at 36 h posttransfection. (E) vIRF3 does not affect IRF7 dimerization. At 48 h posttransfection with GST-IRF7 and Flag-IRF7, together with increasing amounts of WT vIRF3 or the vIRF3 Δ240-280 mutant, WCL were used for GST pull-down assay, followed by immunoblotting with anti-Flag antibody or anti-V5 antibody to detect IRF7 homodimerization and IRF7-vIRF3 interaction, respectively. WCL were also used for immunoblotting with anti-GST, Flag, and V5 antibodies to detect the levels of expression of each protein. Molecular size markers are on the left of panels A and E. PLDN, GST pull-down assay; IB, immunoblotting.
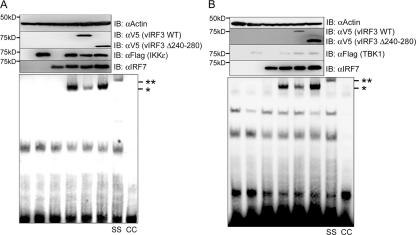
Finally, vIRF3's effect on IRF7 DNA binding activity was examined by EMSA, using extracts derived from 293T cells transfected with expression vectors that included Flag-IRF7, IKKɛ, TBK1, vIRF3, or the vIRF3 Δ240-280 mutant. The strong DNA binding activity of IRF7 was identified upon coexpression with IKKɛ or TBK1 (Fig. 5A and B). However, this DNA binding activity was markedly reduced by vIRF3 expression but not by the expression of the vIRF3 Δ240-280 mutant (Fig. 5A and B). The IRF7 DNA binding activity was specific, because the slowly migrating band was not identified in the absence of IKKɛ or TBK1 coexpression (Fig. 5A and B). Furthermore, the supershift analysis with a specific anti-IRF7 antibody demonstrated that this protein-DNA complex contained IRF7 (Fig. 5A and B). These results indicate that vIRF3 specifically inhibits the DNA binding ability of IRF7 in an interaction-dependent manner.
FIG. 5.
vIRF3 interaction inhibits the DNA binding activity of IRF7. 293T cells were transfected with IRF7 (no tag), V5-vIRF3, V5-vIRF3 Δ240-280, Flag-IKKɛ (A) or Flag-TBK1 (B) in various combinations, as indicated. At 48 h posttransfection, cell lysates were subjected to EMSA as described in Materials and Methods (bottom panels). The same samples were also used for immunoblotting (IB) with anti-V5, anti-Flag, and anti-IRF7 antibodies to show the levels of expression of the proteins (top). *, position of the shifted probe containing IRF7 protein; **, position of the supershifted (SS) probe containing anti-IRF7 antibody. The specificity of IRF7 DNA binding activity was tested by competition with the cold probe (CC). Molecular size markers are on the left of each panel.
The central double helix motifs of vIRF3 are sufficient to inhibit IRF7 DNA binding activity and, thereby, its functional activity.
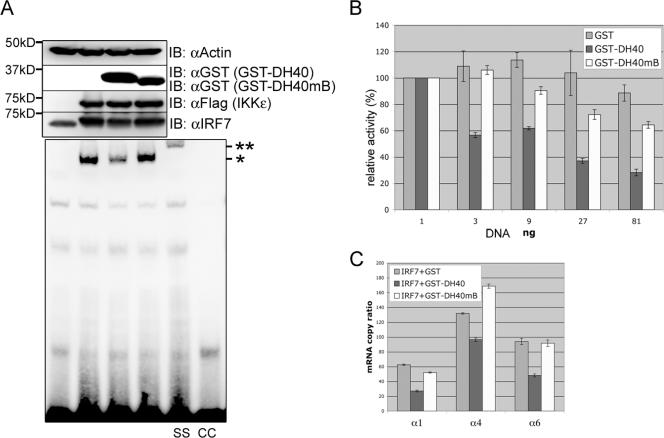
We investigated whether the central 40-aa sequence (aa 240 to 280) of vIRF3 containing the putative double helix motifs was sufficient to inhibit the DNA binding activity of IRF7 as seen with full-length vIRF3. At 48 h after transfection with IRF7, IKKɛ, GST-DH40, or GST-DH40mB, 293T cell lysates were subjected to EMSA. The IKKɛ-induced DNA binding activity of IRF7 was considerably suppressed by GST-DH40, whereas it was not affected by the GST-DH40mB mutant (Fig. 6A). Supershift analysis demonstrated the specificity of IRF7 DNA binding in EMSA (Fig. 6A). Luciferase assay also showed that GST-DH40, but not GST-DH40mB, effectively suppressed the IRF7-induced activation of IFN-α6 promoter activity in a dose-dependent manner (Fig. 6B). Finally, we used a real-time qRT-PCR to measure the increases in levels of endogenous IFN-α1, -α4, and -α6 mRNA upon Sendai virus infection. Correlated with its activity in suppressing IFN-α promoter activity, GST-DH40 expression led to a detectable reduction in virus-induced endogenous IFN-α1, -α4, and -α6 mRNA levels, by twofold, whereas GST-DH40mB showed no effect under the same conditions (Fig. 6C). The validity of the real-time qRT-PCR results was confirmed by conventional electrophoresis (data not shown). These results collectively indicate that the central double helix motifs of vIRF3 are sufficient not only for binding to IRF7, but also for inhibiting its transcriptional activity.
FIG. 6.
The central double helix motifs of vIRF3 are sufficient to bind to and inhibit IRF7. (A) The central double helix motifs of vIRF3 are sufficient to inhibit the DNA binding activity of IRF7. 293T cells were transfected with IRF7 (no tag), GST-DH40, GST-DH40mB, and/or Flag-IKKɛ in various combinations, as indicated. At 48 h posttransfection, whole cell lysates were subjected to EMSA as described in Materials and Methods. The same samples were also used for immunoblotting (IB) with anti-GST, anti-Flag, and anti-IRF7 antibodies to show the levels of expression of the proteins (top). Molecular size markers are on the left. *, position of the shifted probe containing IRF7 protein; **, position of the supershifted (SS) probe containing anti-IRF7 antibody. The specificity of IRF7 DNA binding activity was tested by competition with the cold probe (CC). (B) The central double helix motifs of vIRF3 are sufficient to inhibit IRF7 transcription factor activity. 293T cells were transfected with the pGL3-IFN-α6 promoter construct (100 ng) and IRF7 (1 ng) together with increasing amounts of GST, GST-DH40, or GST-DH40mB. At 24 h posttransfection, the cells were stimulated with 25 hemagglutination (HA) units of Sendai virus for 12 h. Luciferase assay was performed as described in Materials and Methods, and the percent inhibition was calculated by comparison with cells transfected with the GST expression vector. (C) The central double helix motifs of vIRF3 are sufficient to suppress IFN-α1, IFN-α4, and IFN-α6 mRNA induction upon Sendai virus infection. Amounts of 1 × 105 293T cells were seeded into 6-well plates and subsequently transfected with IRF7 (4 ng) along with GST, GST-DH40, or GST-DH40mB, followed by stimulation with 100 HA units of Sendai virus for 12 h. Real-time qRT-PCR was performed as described in Materials and Methods.
DISCUSSION
IFN is a mediator of the front-line defense against microbe infections. To replicate in host cells, viruses thus have to downregulate IFN signaling to complete their life cycles. KSHV has a unique cluster of four vIRFs (vIRF1 to 4) that display homology with cellular IRFs in their tryptophan repeats. Previous studies have shown that vIRF1 inhibits IFN signaling by blocking the cellular IRF3 recruitment of the CBP/p300 coactivator (20, 21). In addition, vIRF2 represses IFN signaling mediated by IRF1 and IRF3; however, the specific mechanism has not been illustrated (11). Here, we demonstrate that KSHV vIRF3 specifically interacts with either the DBD or the IAD of IRF7 and that this interaction leads to the inhibition of IRF7 DNA binding activity and, thereby, suppression of IFN-α production and IFN-mediated immunity. Remarkably, the central 40 aa of vIRF3 containing the double α helix motifs are sufficient not only for binding to IRF7, but also for inhibiting IRF7's DNA binding activity, resulting in the effective suppression of IRF7-mediated IFN-α production. With a significant disparity to our observation, however, Lubyova et al. (24) have previously shown that the KSHV vIRF3 activates IFN signal transduction by interacting with cellular IRF3 and IRF7. This previous study showed that vIRF3 directly interacts with cellular IRF3, IRF7, and the transcriptional coactivator CBP/p300 and that these interactions stimulate the IRF3- and IRF7-mediated activation of type I IFN (IFN-α and IFN-β) genes (24). However, several issues regarding this previous study should be of concern. First of all, despite the authors' conclusion, the vIRF3-mediated activation of IFN promoter activity was only 1.5- to threefold, considered to be extremely minimal, based on the highly sensitive reporter assay. This study also showed that Sendai virus infection activated the IRF7-mediated IFN promoter activity by 1.5- to twofold, which is also a striking contrast to the results of our and other studies that showed more than 50- to 100-fold activation (17, 20, 21). Finally, to our surprise, the same authors have also reported in a separate publication (25) that vIRF3 functions as a dominant-negative factor of both IRF3 and IRF7 and inhibits the virus-mediated transcriptional activity of the IFN promoter, showing conflicting conclusions of their own. While it is difficult to speculate on the discrepancy in the role of KSHV vIRF3 in IFN signal transduction, our study unambiguously demonstrates that vIRF3 interacts with IRF7 in yeast and mammalian cells and that this interaction leads to the suppression of IRF7 transcription activity induced by virus infection, as well as by the TBK1/IKKɛ kinases.
Secondary-structure prediction reveals the presence of the double α helix motifs within the 40-aa sequence (aa 240 to 280) of vIRF3 (Fig. 2A). Interestingly, these double α helix motifs of vIRF3 are sufficient to bind to IRF7, which leads to the suppression of its transcriptional activity. The helix-turn-helix structure has been shown to mediate DNA binding, as well as protein-protein interactions (3, 10). For example, the DBDs of AraC family proteins contain two sets of helix-turn-helix motifs, which insert into two adjacent segments of the major groove of the target DNA (28). Another example is the HIF-1α that is a member of the basic helix-loop-helix-Per/Arnt/Sim (bHLH-PAS) transcription factor family and interacts with HIF-1α/ARNT via the bHLH and part of the PAS domain (2, 17). Latent cytoplasmic IRF7 is activated through the phosphorylation of specific serine residues located in its C-terminal end upon virus infection (22). This virus-induced phosphorylation of IRF7 appears to relieve an intramolecular association between two autoinhibitory domains and unmask the N-terminal DBD and C-terminal IAD, resulting in the formation of homodimers through the IAD. IRF7 dimers subsequently translocate from the cytoplasm to the nucleus and stimulate DNA binding and transcriptional activities (reviewed in references 16, 19, and 42). We showed that the central double α helix motifs of vIRF3 were sufficient for an independent binding to the IRF7 DBD or IAD. In addition, we found that the acidic residues in the double helix motifs of vIRF3 were important for IRF7 binding (Fig. 2) and that the synthetic peptide containing the double helix motifs of vIRF3 bound strongly to the bacterially purified IAD of IRF7 in vitro (unpublished results). Since vIRF3 nuclear protein shows no effect on IRF7 phosphorylation and dimerization, its interaction with the DBD and IAD of IRF7 may physically put each domain close together, which mimics the closed, inactive form of IRF7 and thereby inhibits its transcriptional activity. In addition, it should be noted that the N-terminal, V5-tagged IRF7 showed significant migration retardation upon IKKɛ-mediated phosphorylation (Fig. 4A), whereas IRF7 without tagging showed only a minimal level of migration retardation under the same conditions (Fig. 5). This indicates that tagging may influence IRF7 structure to be more sensitive to phosphorylation, while it does not affect IRF7 functional activity. Thus, further detailed structural analysis should facilitate our understanding of the molecular mechanism of vIRF3-mediated inhibition of IRF7.
IRF7 is a master regulator of innate immunity and also plays an important role in the transition from innate immunity to acquired immunity (16). Due to its essential role in host immunity, numerous viruses have been shown to have various strategies to downregulate IRF7 activation. The ICP0s encoded by herpes simplex virus and bovine herpesvirus 1 inhibit the phosphorylation step by TBK1 and IKKɛ (23). A KSHV immediate-early lytic protein encoded by ORF45 that is incorporated into the virus particle as a tegument protein binds to IRF7 and blocks its phosphorylation and accumulation in the nucleus, resulting in a blockage of IFN-α and IFN-β transcription in response to viral infection (43). In addition, the KSHV RTA immediate-early lytic nuclear transcription factor acts as a ubiquitin E3 ligase to promote the ubiquitination and degradation of IRF7 protein in a proteasome-dependent fashion (41). Our present study adds vIRF3 to the expanding family of viral proteins that inhibit cellular IRF7 transcriptional activity by virtue of protein-protein interactions. Our results and those of others indicate that KSHV employs the ORF45 and RTA immediate-early lytic proteins and the vIRF3 latent protein to target the multiple steps of the IRF7 activation process, which leads to the comprehensive downregulation of IRF7 transcription factor activity during the entire life cycle of KSHV. Additionally, vIRF3 has been also shown to block PKR-mediated apoptosis, but not oligoadenylate pathway-mediated apoptosis (8). Therefore, the manipulation of the modification, stability, and function of IRF7 by the KSHV gene products provides a novel regulatory strategy for circumventing the innate immune defense system.
Acknowledgments
We especially thank J. Hiscott, Y. Chang, and T. Maniatis for providing reagents.
This work was partly supported by U.S. Public Health Service grants CA106156, CA82057, CA91819, and RR00168 and Korea Research Foundation grant KRF-2005-214-C00104.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Au, W. C., Y. Su, N. B. Raj, and P. M. Pitha. 1993. Virus-mediated induction of interferon A gene requires cooperation between multiple binding factors in the interferon alpha promoter region. J. Biol. Chem. 268:24032-24040. [PubMed] [Google Scholar]
- 2.Bardos, J. I., and M. Ashcroft. 2005. Negative and positive regulation of HIF-1: a complex network. Biochim. Biophys. Acta 1755:107-120. [DOI] [PubMed] [Google Scholar]
- 3.Baxevanis, A. D., and C. R. Vinson. 1993. Interactions of coiled coils in transcription factors: where is the specificity? Curr. Opin. Genet. Dev. 3:278-285. [DOI] [PubMed] [Google Scholar]
- 4.Caillaud, A., A. G. Hovanessian, D. E. Levy, and I. J. Marie. 2005. Regulatory serine residues mediate phosphorylation-dependent and phosphorylation-independent activation of interferon regulatory factor 7. J. Biol. Chem. 280:17671-17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, G., M. E. Brett, and B. He. 2002. Signals that dictate nuclear, nucleolar, and cytoplasmic shuttling of the γ134.5 protein of herpes simplex virus type 1. J. Virol. 76:9434-9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5-mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteban, M., M. A. Garcia, E. Domingo-Gil, J. Arroyo, C. Nombela, and C. Rivas. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84:1463-1470. [DOI] [PubMed] [Google Scholar]
- 9.Feng, P., J. Park, B. S. Lee, S. H. Lee, R. J. Bram, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J. Virol. 76:11491-11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong, J. H., A. E. Keating, and M. Singh. 2004. Predicting specificity in bZIP coiled-coil protein interactions. Genome Biol. 5:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuld, S., C. Cunningham, K. Klucher, A. J. Davison, and D. J. Blackbourn. 2006. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 80:3092-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 14.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 15.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 16.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, B. H., E. Rue, G. L. Wang, R. Roe, and G. L. Semenza. 1996. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271:17771-17778. [DOI] [PubMed] [Google Scholar]
- 18.Kerr, I. M., A. P. Costa-Pereira, B. F. Lillemeier, and B. Strobl. 2003. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 546:1-5. [DOI] [PubMed] [Google Scholar]
- 19.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22:87-93. [DOI] [PubMed] [Google Scholar]
- 20.Li, M., B. Damania, X. Alvarez, V. Ogryzko, K. Ozato, and J. U. Jung. 2000. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell. Biol. 20:8254-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 22.Lin, R., Y. Mamane, and J. Hiscott. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 275:34320-34327. [DOI] [PubMed] [Google Scholar]
- 23.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubyova, B., M. J. Kellum, A. J. Frisancho, and P. M. Pitha. 2004. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 279:7643-7654. [DOI] [PubMed] [Google Scholar]
- 25.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marie, I., E. Smith, A. Prakash, and D. E. Levy. 2000. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol. Cell. Biol. 20:8803-8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 29.Morrison, T. E., A. Mauser, A. Wong, J. P. Ting, and S. C. Kenney. 2001. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura, H., M. Lu, Y. Gwack, J. Souvlis, S. L. Zeichner, and J. U. Jung. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen, H., J. Hiscott, and P. M. Pitha. 1997. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 8:293-312. [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pozharskaya, V. P., L. L. Weakland, J. C. Zimring, L. T. Krug, E. R. Unger, A. Neisch, H. Joshi, N. Inoue, and M. K. Offermann. 2004. Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells. J. Virol. 78:6621-6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 37.Seo, T., J. Park, C. Lim, and J. Choe. 2004. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi's sarcoma-associated herpesvirus. Oncogene 23:6146-6155. [DOI] [PubMed] [Google Scholar]
- 38.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 39.Solis, M., D. Goubau, R. Romieu-Mourez, P. Genin, A. Civas, and J. Hiscott. 2006. Distinct functions of IRF-3 and IRF-7 in IFN-alpha gene regulation and control of anti-tumor activity in primary macrophages. Biochem. Pharmacol. 72:1469-1476. [DOI] [PubMed] [Google Scholar]
- 40.Yokota, S., N. Yokosawa, T. Kubota, T. Suzutani, I. Yoshida, S. Miura, K. Jimbow, and N. Fujii. 2001. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology 286:119-124. [DOI] [PubMed] [Google Scholar]
- 41.Yu, Y., S. E. Wang, and G. S. Hayward. 2005. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity 22:59-70. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, L., and J. S. Pagano. 2002. Structure and function of IRF-7. J. Interferon Cytokine Res. 22:95-101. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]