Abstract
Agrin is believed to be a factor used by motoneurons to direct acetylcholine receptor (AChR) clustering at the neuromuscular junction. However, exactly how agrin mediates this effect remains unclear. Here we demonstrate that the β-catenin interacts with rapsyn, a molecule key for AChR clustering. Agrin stimulation increases the association of β-catenin with surface AChRs. Suppression of β-catenin expression inhibited agrin-induced AChR clustering, suggesting a necessary role of β-catenin in this event. The β-catenin action did not appear to require the function of T-cell factors (TCFs), suggesting a mechanism independent of TCF-mediated transcription. In contrast, prevention of β-catenin from interacting with α-catenin attenuated agrin-induced AChR clustering. These results suggest that β-catenin may serve as a link between AChRs and α-catenin-associated cytoskeleton, revealing a novel function of β-catenin in synaptogenesis.
Keywords: β-catenin, rapsyn, interaction, AChR, cluster, α-catenin
Introduction
The neuromuscular junction (NMJ) is a peripheral cholinergic synapse that conveys signals from motoneurons to muscle cells. It has served as an informative model of synaptogenesis because of its simplicity and easy accessibility (Sanes and Lichtman, 1999). During development, acetylcholine receptors (AChRs) aggregate at the postjunctional membrane in response to factors released from motoneurons including agrin (McMahan et al., 1992; Sanes and Lichtman, 1999). Agrin activates the muscle-specific tyrosine kinase (MuSK) and induces AChR clusters in cultured muscle cells. Mice deficient in agrin or MuSK do not form the NMJ (DeChiara et al., 1996; Gautam et al., 1996). A third protein that is essential for NMJ formation is rapsyn, an intracellular molecule (Burden et al., 1983; LaRochelle and Froehner, 1986; Gautam et al., 1995; Moransard et al., 2003). This protein is associated with AChRs and is thought to link AChRs to actin cytoskeleton (Dai et al., 2000). Interference of actin cytoskeleton attenuates AChR clustering (Bloch, 1986; Dai et al., 2000). However, how rapsyn couples the AChR to the actin cytoskeleton remains unclear. In this study, we demonstrate that rapsyn interacts with β-catenin. Suppression of β-catenin expression or inhibition of its interaction with α-catenin inhibited agrin-induced AChR clustering. Our results identify a role of β-catenin in regulating AChR cluster formation by agrin.
Materials and Methods
DNA constructs and antibodies.
Wnt, DKK1, and Fz-Fc constructs were described previously (Semenov et al., 2001; Zhang et al., 2006). Stabilized β-catenin (β-cat*) was a gift from Dr. X. Yu (Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China) (Yu and Malenka, 2003). β-Catenin-AA was generated by using the Quick Change mutagenesis kit (Stratagene, La Jolla, CA). β-Catenin short hairpin RNA (shRNA) constructs were generated by using the BLOCK-iT U6 RNAi Entry Vector kit (Invitrogen, Carlsbad, CA). The sequences of shRNA primers were as follows: for shRNA–β-cat811, 5′-CACCGCACCATGCAGAATACAAATGCGAACATTTGTATTCTGCATGGTGC (sense) and 5′-AAAAGCACCATGCAGAATACAAATGTTCGCATTTGTATTCTGCATGGTGC (antisense); for shRNA–β-cat1196, 5′-CACCGGACCTACACTTATGAGAAGCCGAAGCTTCTCATAAGTGTAGGTCC (sense) and 5′-AAAAGGACCTACACTTATGAGAAGCTTCGGCTTCTCATAAGTGTAGGTCC (antisense); for shRNA–β-cat2405, 5′-CACCGGACCCTATGATGGAGCATGACGAATCATGCTCCATCATAGGGTCC (sense) and 5′-AAAAGGACCCTATGATGGAGCATGATTCGTCATGCTCCATCATAGGGTCC (antisense). The numbers in the names of shRNA constructs indicate the targeting nucleotide sequences. To generate refractory β-catenin expression vectors, site-directed mutations were made in green fluorescent protein (GFP)–β-cat* on corresponding nucleotides underlined in the sense primers described above. For RF811, CACC were mutated to TACT; for RF1196, TTAT were mutated to GTAC. These mutations were silent and thus did not change the amino acid sequences of β-catenin. Antibodies were from the following companies: β-catenin (catalog #610154; BD Biosciences, San Jose, CA), α-catenin (catalog #610194; BD Biosciences), GFP (catalog #TP401; Torrey Pines Biolabs, Houston, TX), Flag M2 (catalog #F3165; Sigma, St. Louis, MO), HA 12CA5 (catalog #ab16918; Abcam, Cambridge, MA), Fc (catalog #209-005-098; Jackson ImmunoResearch, West Grove, PA), glyceraldehyde-3-phosphate dehydrogenase (catalog #MAB374; Millipore, Temecula, CA), and AChR α-subunit (AChRα; mAb35; gift from Dr. R. Rotundo, University of Miami, Miami, FL). Anti-rapsyn antibody was generated in rabbits with glutathione S-transferase (GST)–rapsyn.
Cell culture and transfection.
C2C12 cells were maintained as undifferentiated myoblasts in DMEM with high glucose supplemented with 20% fetal bovine serum, 0.5% chicken embryo extract, and 2 mm l-glutamine. Fusion of myoblasts into myotubes was induced by culturing in the differentiation medium (DM): DMEM supplemented with 5% horse serum and 2 mm l-glutamine. Rapsyn−/− (clone 11-7) and control (clone 12-10) muscle cells were generated from rapsyn null mutant mice (Apel et al., 1997) and, as gift from Dr. C. Fuhrer, were cultured as described previously (Fuhrer et al., 1999). A concentration of 10 ng/ml agrin was used for stimulation for 16 h unless indicated otherwise. C2C12 myoblasts were transfected using lipofectamine 2000 (catalog #11668-019; Invitrogen) or Nucleofector kit V (catalog #VCO-1001; Amaxa, Gaithersburg, MD) according to the manufacturer's instructions. Cells were switched to the DM 24 h later.
Protein interaction assays.
Yeast two hybridization was performed as described previously (Huang et al., 2000). To assay the direct interaction, [35S]-labeled β-catenin was generated by in vitro translation using the T7/SP6 Coupled Reticulocyte Lysate System (Promega, Madison, WI) and incubated with GST–rapsyn. Bound β-catenin was visualized by autoradiogram (Luo et al., 2002).
Isolation of surface AChR and its associated proteins.
Live myotubes were incubated with 300 nm soluble biotinylated α-bungarotoxin (biotin-αBTX) for 2 h at 4°C. After washing, cells were lysed in the extraction buffer containing 0.5% Lubrol-PX, 75 mm KCl, 50 mm Tris-HCl, pH 7.4, 2 mm CaCl2, 4 mm MgCl2, 20% glycerol, and 1 mm DTT. Lysates were incubated with streptavidin-coupled agarose beads (Invitrogen) for 6 h at 4°C and washed extensively with the extraction buffer, except Lubrol-PX was 0.1%. Beads-associated proteins were subjected to immunoblotting.
AChR cluster assays.
C2C12 cells plated on coverslips were transfected with or without indicated constructs. Fully differentiated myotubes were treated with agrin (C4,8) to induce AChR clusters (Luo et al., 2002). After fixation in 2% paraformaldehyde for 30 min, cells were incubated with 50 nm rhodamine-conjugated α-bungarotoxin (R-BTX) (Invitrogen) for 60 min to label AChR. Coverslips were mounted in VectaSheild (Vector Laboratories, Burlingame, CA) and viewed under a Zeiss (Thornwood, NY) epifluoresence microscope. Images were collected with Axiovision 3.1 software. AChR clusters with diameters or a longer axis ≥4 μm were scored.
Results
β-Catenin associates with the AChR complex through rapsyn
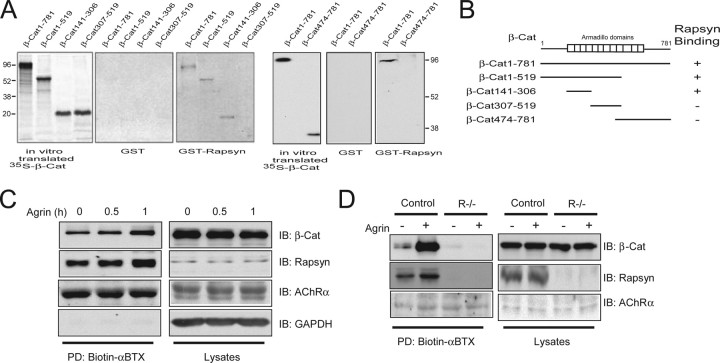
To understand mechanisms of how rapsyn mediates AChR clustering, we screened for rapsyn-interacting proteins by yeast two-hybrid screens and identified β-catenin using full-length rapsyn as bait (Z. Hall, personal communication). The interaction between β-catenin and rapsyn was specific because rapsyn did not bind to the intracellular domain of ErbB4, an irrelevant kinase and various control proteins (data not shown). To determine whether rapsyn interacts directly with β-catenin, not via a third component, and to identify the domain in β-catenin necessary for binding, recombinant β-catenin proteins were generated by in vitro translation. [35S]-labeled proteins were incubated with GST-rapsyn immobilized on agarose beads. GST–rapsyn, but not GST alone, bound to β-catenin (Fig. 1 A). The C-terminal region (amino acids 474–781) including armadillo domains 9–12 did not bind to β-catenin; nor did the armadillo domains 5–9, suggesting that the interacting domain for rapsyn is localized in the N-terminal region (Fig. 1 A, right). In agreement, a recombinant protein containing armadillo domains 1–4 was sufficient to interact with rapsyn (Fig. 1 A,B). Thus β-catenin interacts directly with rapsyn via armadillo domains 1–4.
Figure 1.
β-Catenin associates with the AChR complex through rapsyn. A, Direct interaction of β-catenin with rapsyn. [35S]-labeled β-catenin proteins were incubated with GST alone or GST–rapsyn immobilized on beads. Bound [35S]-β-catenin proteins were visualized by autoradiogram. Molecular weight markers are in kilodaltons. B, Schematic diagram of β-catenin constructs and binding activity to rapsyn. C, Association of β-catenin with surface AChR in muscle cells. Live myotubes, treated with agrin for indicated times, were incubated with biotin-αBTX to label surface AChR. Lysates were incubated with streptavidin-coupled agarose beads, and bead-associated proteins were subjected to immunoblotting with antibodies against AChRα, β-catenin, and rapsyn. Lysates (5% of input) were blotted to indicate equal amounts of inputs. D, β-Catenin association with AChR requires rapsyn. Control or rapsyn mutant (R−/−) myotubes were stimulated without or with agrin for 12 h. Proteins associated with surface AChRs were isolated as in C and probed with indicated antibodies. Lysates (5% of input) were blotted to indicate equal amounts of inputs. PD, Pull down; IB, immunoblotting; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Because rapsyn is associated with AChRs, we reasoned that via interacting with rapsyn, β-catenin may be in the AChR complex in muscle cells. To test this hypothesis, live C2C12 myotubes were incubated with biotin-αBTX to label surface AChRs, which were then isolated by streptavidin-coupled agarose beads. AChRs and associated proteins were revealed by immunoblotting with antibodies against the AChRα, rapsyn, and β-catenin. As shown in Figure 1 C, rapsyn was detectable in the AChR complex, and such interaction was increased by agrin, indicating that agrin regulates the rapsyn–AChR interaction in agreement with a previous report (Moransard et al., 2003). Interestingly, β-catenin coprecipitated with biotinylated AChRs, suggesting that it associates with the AChR complex. Such association appeared to be specific because it did not occur with streptavidin–agarose beads or Sepharose 4B beads incubated with biotinylated lysates (data not shown). Moreover, the β-catenin–AChR association was increased by agrin, in a time course similar to that of the rapsyn–AChR interaction, suggesting that the interaction may be stimulated by agrin.
We next determined whether β-catenin association to the AChR complex requires rapsyn. Muscle cells derived from rapsyn−/− mice (clone 11-7) are deficient in rapsyn and do not form AChR clusters in response to agrin (Apel et al., 1997; Fuhrer et al., 1999). As shown in Figure 1 D, rapsyn as well as β-catenin became associated with the AChR complex in agrin-stimulated control muscle cells (clone 12-10) derived from heterozygous littermates. In contrast, however, β-catenin was barely detectable in the AChR complex in rapsyn−/− myotubes (Fig. 1 D). Note that the amounts of β-catenin were similar in control and rapsyn−/− myotube lysates. These results demonstrate the dependence of the β-catenin–AChR association on rapsyn, suggesting that rapsyn may recruit β-catenin to the AChR complex in a manner that can be regulated by agrin. Immunohistochemical analysis showed that β-catenin was enriched at the NMJ but also present in the sarcolemma of muscle fibers (data not shown).
Suppression of β-catenin expression reduced AChR clusters in agrin-stimulated muscle cells
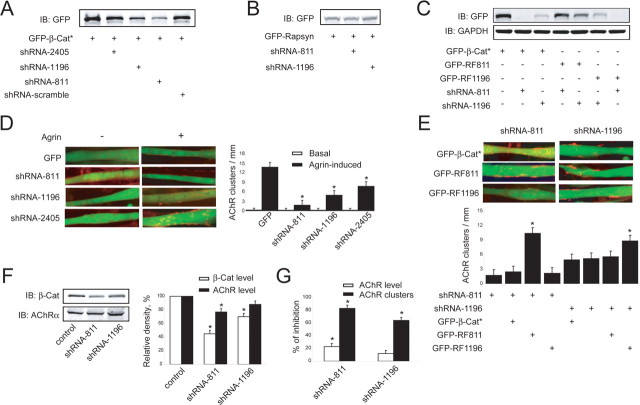
To determine whether β-catenin plays a role in AChR clustering, a DNA-based shRNA approach was used to suppress β-catenin expression in C2C12 muscle cells. Three shRNA–β-catenin constructs were tested for effectiveness to inhibit β-catenin expression (Fig. 2 A). shRNA–β-Cat811 (shRNA-811) was most effective in inhibiting expression of cotransfected β-catenin in COS cells, followed by shRNA-1196 and shRNA-2405. As a control, a shRNA construct containing the scrambled sequence had no effect. Moreover, shRNA-811 and shRNA-1196 had no effect on expression of rapsyn (Fig. 2 B). To further demonstrate the specificity of the shRNA constructs, we generated GFP–RF811 and GFP–RF1196 that had silent mutations (without changing the amino acid sequence) that avoid targeting by respective shRNAs. As shown in Figure 2 C, expression of GFP–RF811 and GFP–RF1196 could not suppressed by shRNA-811 and shRNA-1196, respectively. These results suggest that the shRNA constructs inhibit expression of β-catenin via specific targeting sequences. Next, shRNA constructs were introduced into C2C12 myoblasts together with a plasmid encoding enhanced GFP (pEGFP) to label transfected cells. Resulting myotubes were stained with R-BTX and analyzed for AChR clusters in response to agrin. Agrin induced AChR clusters in control pEGFP-expressing myotubes (Fig. 2 D). In contrast, the number of clusters was reduced in myotubes cotransfected with shRNA–β-catenin constructs. The inhibitory effect correlated positively with that on β-catenin expression, with shRNA-811 being most potent, followed by shRNA-1196 and shRNA-2405 (Fig. 2 A,D). Notably, the inhibitory effect of β-catenin shRNAs on AChR clustering was rescued by respective refractory β-catenin mutants (Fig. 2 E).
Figure 2.
β-Catenin is required for agrin-induced AChR clusters. A, B, β-Catenin shRNAs suppress expression of β-catenin but not rapsyn. COS7 cells were cotransfected with stable GFP–β-catenin (GFP-β-cat*) (A) or GFP-rapsyn (B) with β-catenin shRNA-811, -1196, -2405, or control shRNA-scramble. Lysates of transfectants were subjected to immunoblotting using anti-GFP antibody. C, Refractory GFP-RF811 and GFP-RF1196 were resistant to respective β-catenin shRNA constructs. COS7 cells were cotransfected with GFP-β-cat* or refractory constructs with shRNA-811 or -1196. Lysates of transfectants were subjected to immunoblotting using antibodies against GFP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). D, Agrin-induced AChR clustering was inhibited by β-catenin shRNAs. C2C12 myoblasts were transfected with pEGFP alone or together with individual shRNA constructs (pEGFP:shRNA, 1:20). Transfected myotubes, labeled by GFP, were stimulated without or with agrin and scored for AChR clusters. Images of representative experiments that were repeated five times with similar results are shown. Histograms (right) show quantification of AChR clusters in each transfection. Open bars, Without agrin; filled bars, agrin stimulated. E, Rescue of β-catenin shRNA-mediated inhibition by refractory constructs. C2C12 myoblasts were transfected with pEGFP together with indicated shRNA and β-catenin expression constructs (pEGFP:β-catenin:shRNA, 1:10:10). AChR clusters were assayed as in D. Histograms (bottom) show quantification of AChR clusters in each transfection. F, β-Catenin shRNA effect on AChR expression. C2C12 myoblasts were transfected by nucleofection with pEGFP alone or together with shRNA constructs. Eight hours after transfection, cells were switched to the DM. Myotube lysates were subjected to immunoblotting using antibodies against β-catenin and AChRα. Band density was analyzed by NIH Image. Data shown in the histograms are mean ± SEM with control as 100% (n = 3). G, Comparison of β-catenin shRNA effects on AChR levels and clusters. Data on AChR levels are from F, whereas data on AChR clusters are from D. All quantitative data are shown as mean ± SEM. *p < 0.01. IB, Immunoblotting.
To determine whether the reduction in AChR clusters was attributable to possible changes in AChR expression, C2C12 myoblasts were transfected by a nucleofection method with high efficiency. As shown in Figure 2 F, shRNA-1196 reduced β-catenin expression but had little effect on AChR levels. However, shRNA-811, which was more potent in inhibiting β-catenin expression, caused a mild but significant decrease in AChR levels. These results could suggest a role of β-catenin in regulating AChR expression. Figure 2 G compares the inhibitory effects of the two shRNA constructs on AChR expression and on cluster formation. shRNA-1196 inhibited agrin-induced AChR clustering by 60% without significantly altering AChR levels. When β-catenin levels were further reduced by shRNA-811, AChR expression was compromised. Together, these results suggest that β-catenin may be necessary for agrin-induced AChR aggregation.
T-cell factor transcription activity was dispensable for AChR clustering
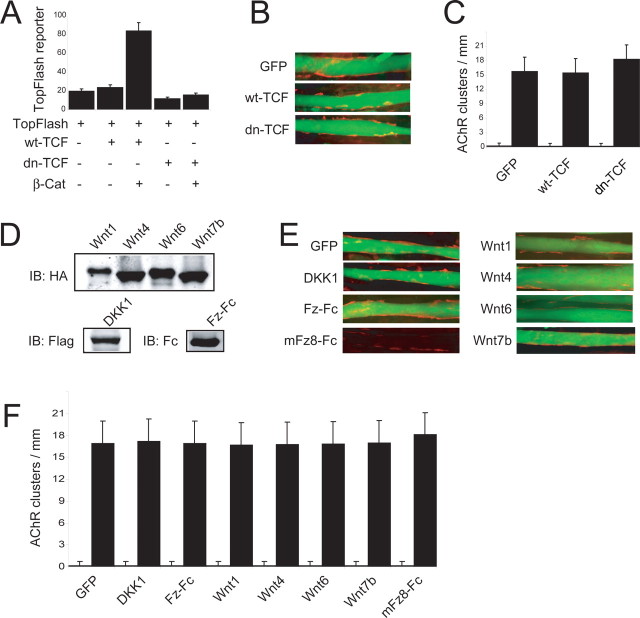
β-Catenin is a protein with multiple functions (Yap et al., 1997). It serves as a transcription cofactor by interacting with T-cell factor (TCF)/LEF1 to regulate gene expression in the canonical Wnt pathway (Behrens et al., 1996). To investigate whether β-catenin-mediated transcription is necessary for AChR clustering, we explored the consequence of suppressing the function of TCF/LEF1. The N terminus of TCF/LEF1 binds to β-catenin (Molenaar et al., 1996). TCF/LEF1 deletion mutants without the N terminus are unable to associate with β-catenin and thus function as a dominant-negative (dn) mutant (Molenaar et al., 1996). Expression of dn-TCF4 blocked β-catenin induction of the TopFlash reporter (Fig. 3 A) (Korinek et al., 1997). However, dn-TCF4 had little effect on basal- or agrin-induced AChR clustering (Fig. 3 B,C), which argues against the involvement of TCF/LEF1-mediated transcription in AChR cluster formation.
Figure 3.
β-Catenin transcription activity is dispensable in regulation of AChR clustering. A, Inhibition of TopFlash reporter activity by dn-TCF4. COS7 cells were transfected with TopFlash with or without β-catenin and wild-type (wt)- or dn-TCF. pRL-TK was cotransfected as control of transfection efficiency and sample handling. The ratio was 10:10:1 for TopFlash:TCF contructs:pRL-TK. Relative luciferase activities (firefly/Renila; mean ± SEM) from a representative experiment in duplicates, which was repeated three times with similar results, are shown. B, Normal agrin-induced AChR clusters in C2C12 cells expressing dn-TCF4. C2C12 myoblasts were transfected with pEGFP, dn-TCF4, or wt-TCF4 in a ratio of 1:20 (pEGFP:TCF4 constructs). AChR clusters were assayed as in Figure 2 D. C, Quantitative analysis of data in B. Data were shown as mean ± SEM (n = 30). Open bars, Without agrin; filled bars, agrin stimulated. D, Expression of Wnt, DKK, and Fz-Fc constructs in C2C12 cells. C2C12 myoblasts were transfected with pFLAG-DKK1, hIgG-mFz8CRD, pKH3-Wnt1, pKH3-Wnt4, pKH3-Wnt6, or pKH3-Wnt7b. Expression in resulting myotubes was analyzed by immunoblotting with indicated antibodies. E, Normal AChR clustering by agrin in C2C12 cells expressing Wnt signaling molecules. C2C12 myoblasts were transfected with pEGFP alone or together with indicated constructs in a ration of 1:20. AChR clusters were assayed as in Figure 2 D. F, Quantitative analysis of data in B. Data were shown as mean ± SEM (n = 30). Open bars, Without agrin; filled bars, agrin stimulated. IB, Immunoblotting.
Several components of the Wnt signaling pathway including Dishevelled (Dvl) and adenomatous polyposis coli (APC) are implicated in AChR cluster formation (Luo et al., 2002; Wang et al., 2003). To investigate whether Wnt signaling is necessary for AChR clustering, we studied effects of Wnt signaling inhibitors and activators on AChR clusters. Wnt signaling can be blocked by overexpression of DKK1 or Frizzled-Fc fusion protein, or by application of the soluble Frizzled-Fc fragment in the medium (Deardorff et al., 1998; Glinka et al., 1998). To determine whether Wnt signaling is necessary for AChR clustering, C2C12 muscle cells were treated with mFz8-Fc or transfected with indicated Wnt agonists and antagonists (Fig. 3 D). As shown in Figure 3, E and F, basal- and agrin-induced AChR clusters did not appear to be affected by the treatment or transfection compared with nontreated cells or cells expressing GFP alone. These results are in line with the notion that Wnt signaling may have a limited role in AChR cluster formation.
β-Catenin regulates AChR clustering through interaction with α-catenin
β-Catenin is a cytoplasmic protein of the adherence complex (Yap et al., 1997). It binds to the cytoplasmic domain of cadherins, the transmembrane components of the adherens junction, and, at the same time, α-catenin to regulate actin cytoskeleton (Drees et al., 2005). In light of the findings that β-catenin interacts with rapsyn and is necessary for AChR clustering, we hypothesized that β-catenin may bridge rapsyn to α-catenin to regulate actin cytoskeleton. To test this hypothesis, we determined whether α-catenin was present in the complex of surface AChRs in C2C12 myotubes. As shown in Figure 4 A (left), α-catenin was detectable in the AChR complex and, as observed with β-catenin, the amount of AChR-associated α-catenin was increased in C2C12 myotubes stimulated with agrin. Next, we investigated whether the β-catenin interaction with α-catenin is involved in AChR cluster formation. The motif in β-catenin necessary for interaction with α-catenin is mapped to the N-terminal region. Thr-120 and Val-122 are two key amino acid residues in β-catenin to interact with α-catenin (Aberle et al., 1996). Mutation of the two residues to alanines (as in β-catenin-AA) prevents β-catenin from binding to α-catenin (Aberle et al., 1996) (data not shown). As shown in Figure 4 A, expression of β-catenin-AA prevented endogenous α-catenin from associating with surface AChRs, even in the presence of agrin, suggesting that α-catenin association to the AChR complex was dependent on interacting with β-catenin. Accordingly, expression of β-catenin-AA attenuated agrin-stimulated AChR clustering (Fig. 4 B). Together, these results suggest a necessary role of α-catenin in AChR clustering, probably by interacting with β-catenin.
Figure 4.
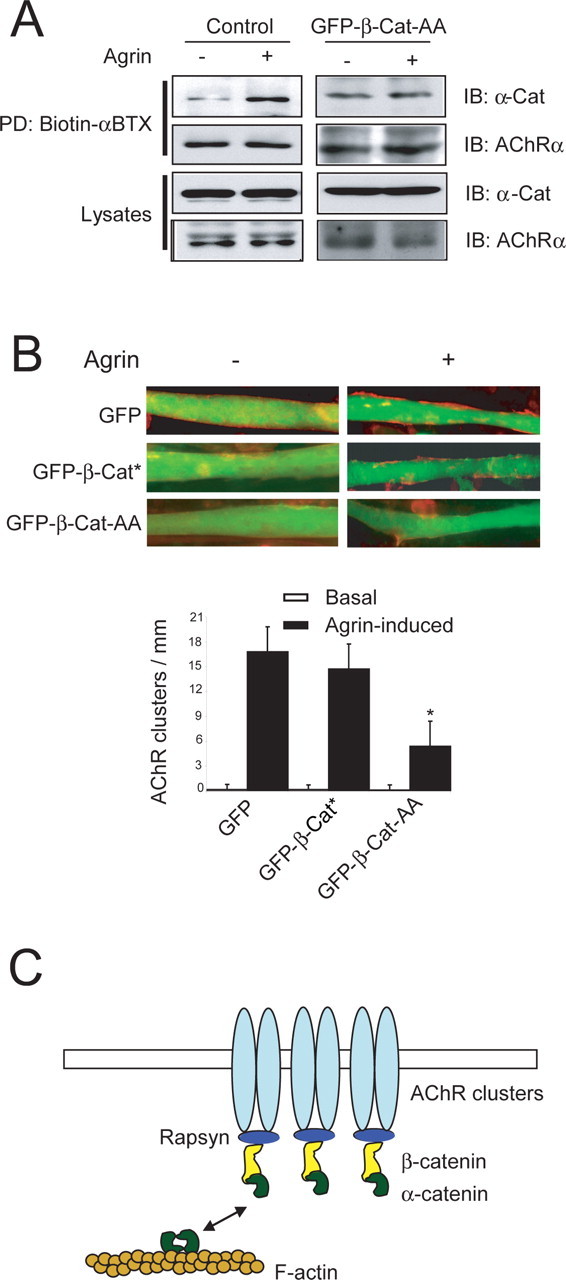
β-Catenin regulation of AChR clustering requires interaction with α-catenin. A, Association of α-catenin with surface AChR complex in muscle cells. Surface AChR was labeled and purified from control C2C12 myotubes (left) or those differentiated from cells nucleofected with GFP–β-catenin-AA (GFP-β-Cat-AA; right), as described in Figure 1 C. AChR-associated proteins were analyzed by immunoblotting with antibodies against AChRα and α-catenin. Lysates (5% of input) were also subjected to immunoblotting to indicate equal amounts of inputs. B, Expression of β-catenin-AA inhibits AChR clustering. C2C12 myoblasts were transfected with GFP–β-cat* or GFP–β-Cat-AA. AChR clusters were induced and scored as in Figure 2 D. Images of representative experiments that were repeated five times with similar results, are shown. Histograms show quantification of AChR clusters (mean ± SEM; n = 30; *p < 0.01). C, Working model. Via interacting with rapsyn and α-catenin, β-catenin may link the AChR to the cytoskeleton. β-Catenin may also regulate expression of synaptic proteins including the AChR. PD, Pull down; IB, immunoblotting.
Discussion
The present study reveals a potentially novel mechanism that regulates AChR cluster formation. First, we showed that β-catenin interacts directly with rapsyn and thus associates with surface AChR. The association of β-catenin with AChR is upregulated by agrin. Second, suppression of β-catenin expression attenuates agrin-induced AChR clustering in muscle cells, suggesting a necessary role in this process. We also demonstrate that AChR clustering does not require the transcription activity of TCF/LEF1 factors. Rather, the interaction of β-catenin with α-catenin appeared to be necessary. These results corroborate that β-catenin, functioning downstream of rapsyn, is necessary for AChR cluster formation, probably by regulating the cytoskeleton in a manner dependent on α-catenin (Fig. 4 C).
Wnt signaling has been shown to mediate or regulate presynaptic and postsynaptic differentiation of glutamatergic NMJs in Drosophila (Hall et al., 2000; Mathew et al., 2005). At mammalian CNS synapses, β-catenin has been implicated in vesicle localization at presynaptic terminals (Bamji et al., 2003), and neural activity induces the redistribution of β-catenin into dendritic spines to influence synaptic size and strength (Murase et al., 2002; Yu and Malenka, 2003). Recent studies suggest that Wnt signaling molecules including Dvl and APC may regulate AChR cluster formation. Dvl, an adapter protein downstream of the Wnt receptor Frizzled, acts by interacting with both MuSK and Pak1, whereas APC, a protein that regulates β-catenin stability, binds to the AChR β-subunit (Luo et al., 2002; Wang et al., 2003). Moreover, APC may play a role in organizing neuronal AChR clusters in chick ciliary ganglion neurons (Temburni et al., 2004). Together with results of this study, these observations suggest that Wnt signaling components may be involved in mammalian NMJ formation, although the role of each component appears to be distinct from those in classic Wnt pathways.
Footnotes
This work was supported in part by grants from the National Institutes of Health (L.M., W.-C.X.) and the Muscular Dystrophy Association (L.M.). We thank Drs. Richard Rotundo, Xian Yu, Xi He, and Christian Fuhrer for providing reagents. We are grateful to Dr. Zach Hall for sharing unpublished results and to members of the Mei laboratory for discussion. We declare no competing interests.
References
- Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J Biol Chem. 1996;271:1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bloch RJ. Actin at receptor-rich domains of isolated acetylcholine receptor clusters. J Cell Biol. 1986;102:1447–1458. doi: 10.1083/jcb.102.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden SJ, DePalma RL, Gottesman GS. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Dai Z, Luo X, Xie H, Peng HB. The actin-driven movement and formation of acetylcholine receptor clusters. J Cell Biol. 2000;150:1321–1334. doi: 10.1083/jcb.150.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Conrad LJ, Klein PS. Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development. 1998;125:2687–2700. doi: 10.1242/dev.125.14.2687. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer C, Gautam M, Sugiyama JE, Hall ZW. Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J Neurosci. 1999;19:6405–6416. doi: 10.1523/JNEUROSCI.19-15-06405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cells. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- LaRochelle WJ, Froehner SC. Determination of the tissue distributions and relative concentrations of the postsynaptic 43-kDa protein and the acetylcholine receptor in Torpedo. J Biol Chem. 1986;261:5270–5274. [PubMed] [Google Scholar]
- Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan UJ, Horton SE, Werle MJ, Honig LS, Kroger S, Ruegg MA, Escher G. Agrin isoforms and their role in synaptogenesis [review] Curr Opin Cell Biol. 1992;4:869–874. doi: 10.1016/0955-0674(92)90113-q. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Moransard M, Borges LS, Willmann R, Marangi PA, Brenner HR, Ferns MJ, Fuhrer C. Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J Biol Chem. 2003;278:7350–7359. doi: 10.1074/jbc.M210865200. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vetebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. The Wnt antagonist Dickkopf1 is a ligand for low density lipoprotein receptor related proteins. Curr Biol. 2001;26:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Temburni MK, Rosenberg MM, Pathak N, McConnell R, Jacob MH. Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J Neurosci. 2004;24:6776–6784. doi: 10.1523/JNEUROSCI.1826-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang ZZ. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, Liu C. Novel cross talk of Kruppel-like factor 4 and |beta}-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]