Abstract
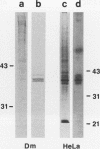
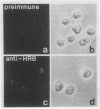
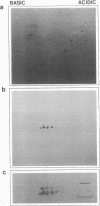
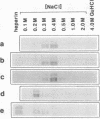
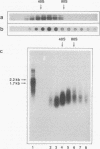
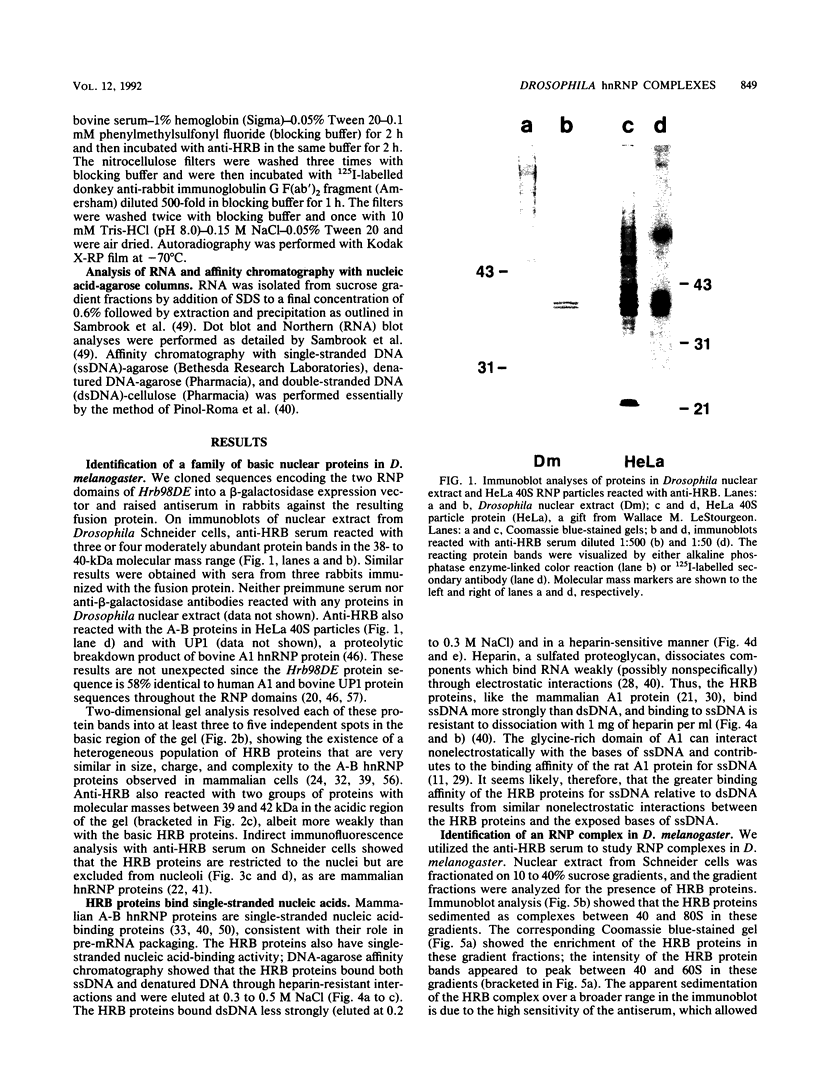
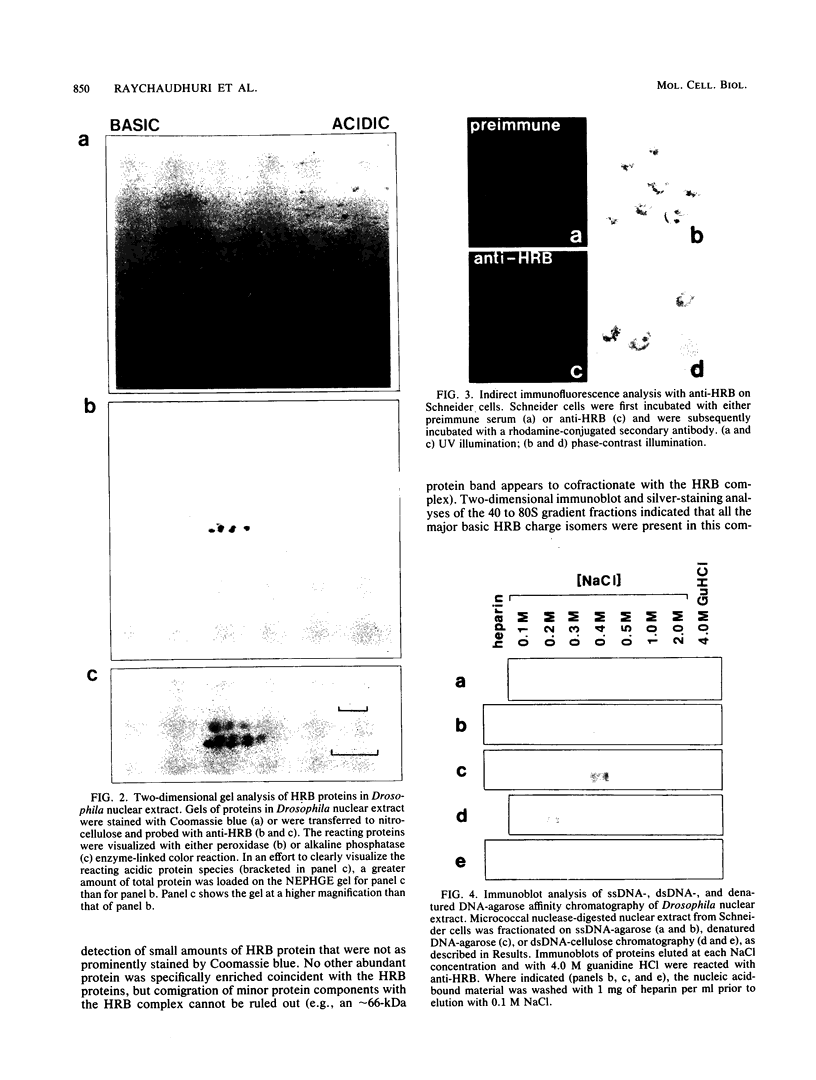
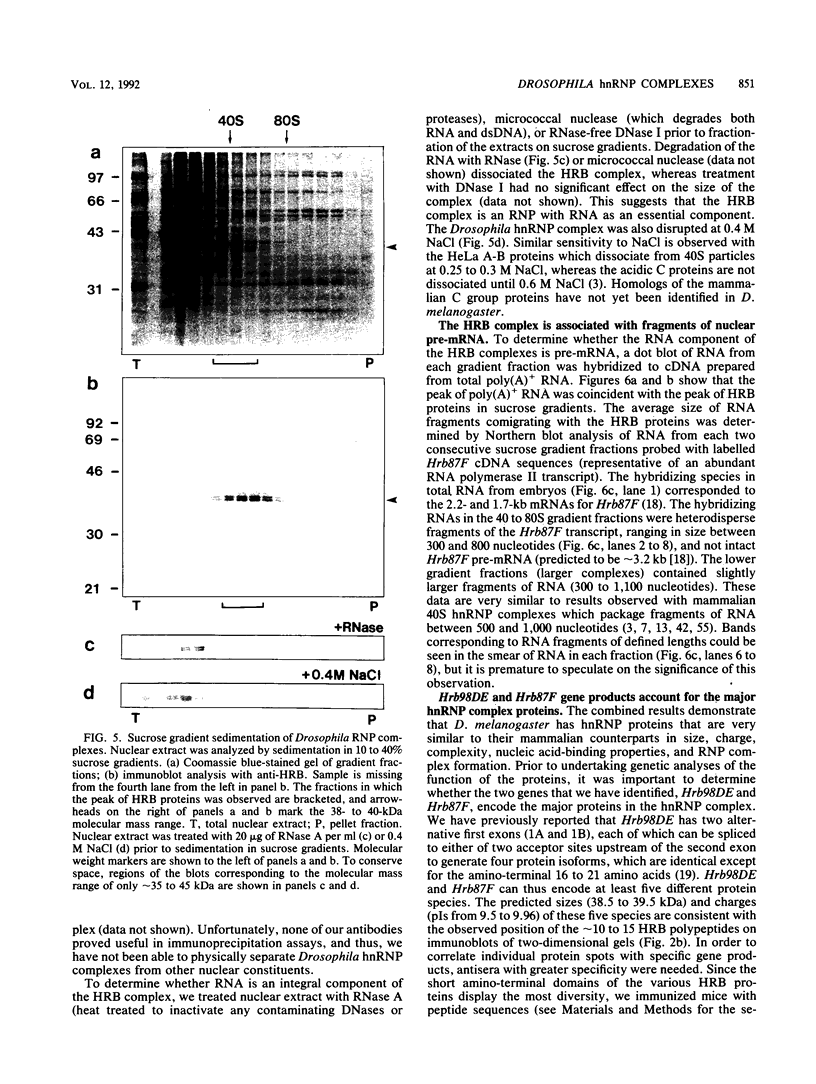
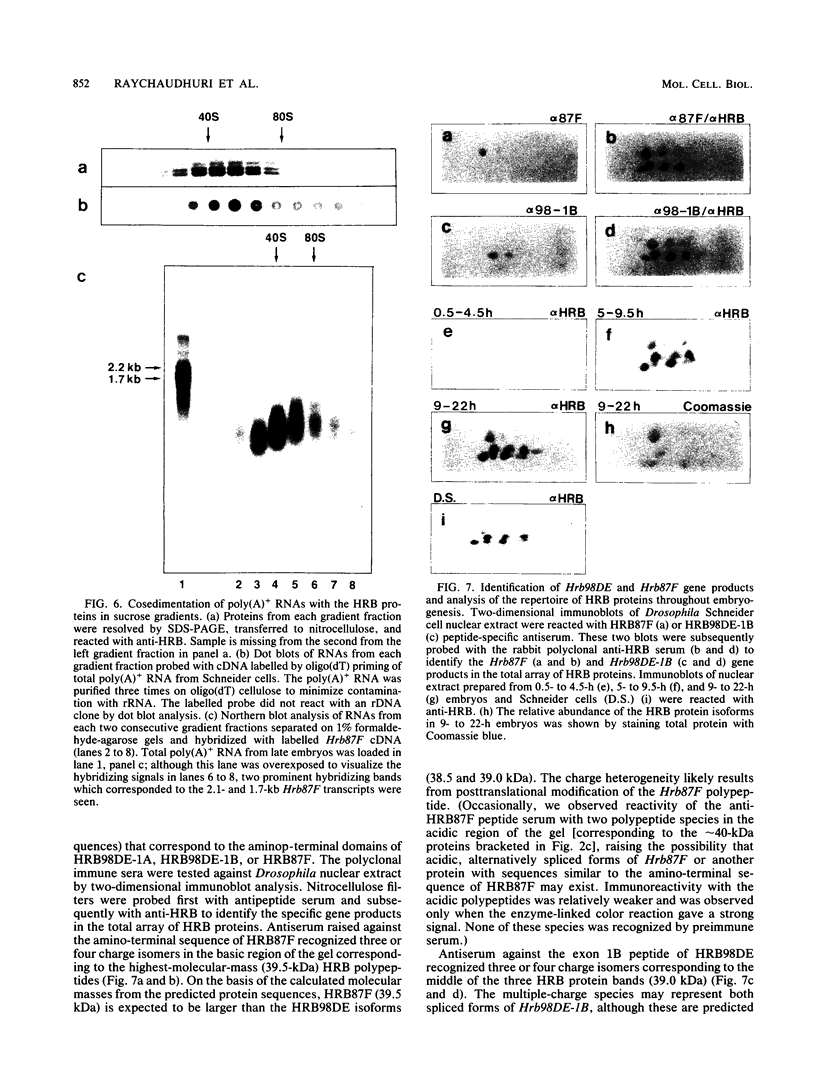
Pre-mRNAs cotranscriptionally associate with a small group of proteins to form heterogeneous nuclear ribonucleoprotein (hnRNP) complexes. We have previously identified two genes in Drosophila melanogaster, Hrb98DE and Hrb87F (i.e., genes at 98DE and 87F encoding putative hnRNA binding proteins), which encode five protein species homologous to the mammalian A-B hnRNP proteins. The studies presented herein show that antibodies against the RNP domains of Hrb98DE reacted with 10 to 15 distinct spots of 38 to 40 kDa in the basic region of two-dimensional gels. These nuclear proteins bound single-stranded nucleic acids and were extracted from Drosophila tissue culture cells as 40 to 80S hnRNP complexes in association with 300 to 800 nucleotide fragments of RNA. The peak of poly(A)+ RNA sequences was coincident with the peak of HRB proteins in sucrose gradients, strongly suggesting that the HRB complexes identified are Drosophila hnRNP complexes. The repertoire of HRB proteins did not change significantly during embryogenesis and was similar to that observed in Drosophila tissue culture cells. Analyses with peptide-specific antisera demonstrated that the major proteins in the hnRNP complex were encoded by the two genes previously identified. Although the Drosophila HRB proteins are only approximately 60% identical throughout the RNP domains to the mammalian A-B hnRNP proteins, features of the basic pre-mRNA packaging mechanism appear to be highly conserved between D. melanogaster and mammals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Biamonti G., Buvoli M., Bassi M. T., Morandi C., Cobianchi F., Riva S. Isolation of an active gene encoding human hnRNP protein A1. Evidence for alternative splicing. J Mol Biol. 1989 Jun 5;207(3):491–503. doi: 10.1016/0022-2836(89)90459-2. [DOI] [PubMed] [Google Scholar]
- Burd C. G., Swanson M. S., Görlach M., Dreyfuss G. Primary structures of the heterogeneous nuclear ribonucleoprotein A2, B1, and C2 proteins: a diversity of RNA binding proteins is generated by small peptide inserts. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9788–9792. doi: 10.1073/pnas.86.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7471–7475. doi: 10.1073/pnas.81.23.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J Cell Biol. 1984 Dec;99(6):1997–1204. doi: 10.1083/jcb.99.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Grabowski P. J., Sharp P. A., Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986 Mar 28;231(4745):1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- Chung S. Y., Wooley J. Set of novel, conserved proteins fold pre-messenger RNA into ribonucleosomes. Proteins. 1986 Nov;1(3):195–210. doi: 10.1002/prot.340010302. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Karpel R. L., Williams K. R., Notario V., Wilson S. H. Mammalian heterogeneous nuclear ribonucleoprotein complex protein A1. Large-scale overproduction in Escherichia coli and cooperative binding to single-stranded nucleic acids. J Biol Chem. 1988 Jan 15;263(2):1063–1071. [PubMed] [Google Scholar]
- Cobianchi F., SenGupta D. N., Zmudzka B. Z., Wilson S. H. Structure of rodent helix-destabilizing protein revealed by cDNA cloning. J Biol Chem. 1986 Mar 15;261(8):3536–3543. [PubMed] [Google Scholar]
- Conway G., Wooley J., Bibring T., LeStourgeon W. M. Ribonucleoproteins package 700 nucleotides of pre-mRNA into a repeating array of regular particles. Mol Cell Biol. 1988 Jul;8(7):2884–2895. doi: 10.1128/mcb.8.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G. Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu Rev Cell Biol. 1986;2:459–498. doi: 10.1146/annurev.cb.02.110186.002331. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Immunoelectron microscope visualization of nuclear ribonucleoprotein antigens within spread transcription complexes. J Cell Biol. 1986 Oct;103(4):1153–1157. doi: 10.1083/jcb.103.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Johnson D., Raychaudhuri G., Beyer A. L. The Drosophila Hrb87F gene encodes a new member of the A and B hnRNP protein group. Nucleic Acids Res. 1991 Jan 11;19(1):25–31. doi: 10.1093/nar/19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Raychaudhuri G., Beyer A. L. The Drosophila Hrb98DE locus encodes four protein isoforms homologous to the A1 protein of mammalian heterogeneous nuclear ribonucleoprotein complexes. Mol Cell Biol. 1990 Jan;10(1):316–323. doi: 10.1128/mcb.10.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Rebbert M. L., Mozer B. A., Forquignon F., Dawid I. B. pen repeat sequences are GGN clusters and encode a glycine-rich domain in a Drosophila cDNA homologous to the rat helix destabilizing protein. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1819–1823. doi: 10.1073/pnas.84.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Jones R. E., Okamura C. S., Martin T. E. Immunofluorescent localization of the proteins of nuclear ribonucleoprotein complexes. J Cell Biol. 1980 Jul;86(1):235–243. doi: 10.1083/jcb.86.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Kay B. K., Sawhney R. K., Wilson S. H. Potential for two isoforms of the A1 ribonucleoprotein in Xenopus laevis. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1367–1371. doi: 10.1073/pnas.87.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986 Sep 12;46(6):845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- Kumar A., Casas-Finet J. R., Luneau C. J., Karpel R. L., Merrill B. M., Williams K. R., Wilson S. H. Mammalian heterogeneous nuclear ribonucleoprotein A1. Nucleic acid binding properties of the COOH-terminal domain. J Biol Chem. 1990 Oct 5;265(28):17094–17100. [PubMed] [Google Scholar]
- Kumar A., Williams K. R., Szer W. Purification and domain structure of core hnRNP proteins A1 and A2 and their relationship to single-stranded DNA-binding proteins. J Biol Chem. 1986 Aug 25;261(24):11266–11273. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leser G. P., Martin T. E. Changes in heterogeneous nuclear RNP core polypeptide complements during the cell cycle. J Cell Biol. 1987 Nov;105(5):2083–2094. doi: 10.1083/jcb.105.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., Stone K. L., Cobianchi F., Wilson S. H., Williams K. R. Phenylalanines that are conserved among several RNA-binding proteins form part of a nucleic acid-binding pocket in the A1 heterogeneous nuclear ribonucleoprotein. J Biol Chem. 1988 Mar 5;263(7):3307–3313. [PubMed] [Google Scholar]
- Moore C. L., Chen J., Whoriskey J. Two proteins crosslinked to RNA containing the adenovirus L3 poly(A) site require the AAUAAA sequence for binding. EMBO J. 1988 Oct;7(10):3159–3169. doi: 10.1002/j.1460-2075.1988.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Dasgupta A. Antibody to a synthetic nonapeptide corresponding to the NH2 terminus of poliovirus genome-linked protein VPg reacts with native VPg and inhibits in vitro replication of poliovirus RNA. J Virol. 1983 Nov;48(2):429–439. doi: 10.1128/jvi.48.2.429-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Peters K. E., Commings D. E. Two-dimensinal gel electrophoresis of rat liver nuclear washes, nuclear matrix, and hnRNA proteins. J Cell Biol. 1980 Jul;86(1):135–155. doi: 10.1083/jcb.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñol-Roma S., Choi Y. D., Matunis M. J., Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988 Feb;2(2):215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S., Swanson M. S., Gall J. G., Dreyfuss G. A novel heterogeneous nuclear RNP protein with a unique distribution on nascent transcripts. J Cell Biol. 1989 Dec;109(6 Pt 1):2575–2587. doi: 10.1083/jcb.109.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman J. M., Martin T. E. Reconstitution of nucleoprotein complexes with mammalian heterogeneous nuclear ribonucleoprotein (hnRNP) core proteins. J Cell Biol. 1983 Jul;97(1):99–111. doi: 10.1083/jcb.97.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Rio D. C. Accurate and efficient pre-mRNA splicing in Drosophila cell-free extracts. Proc Natl Acad Sci U S A. 1988 May;85(9):2904–2908. doi: 10.1073/pnas.85.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Symmons P., Saumweber H., Frasch M. Nonpackaging and packaging proteins of hnRNA in Drosophila melanogaster. Cell. 1983 Jun;33(2):529–541. doi: 10.1016/0092-8674(83)90434-8. [DOI] [PubMed] [Google Scholar]
- Riva S., Morandi C., Tsoulfas P., Pandolfo M., Biamonti G., Merrill B., Williams K. R., Multhaup G., Beyreuther K., Werr H. Mammalian single-stranded DNA binding protein UP I is derived from the hnRNP core protein A1. EMBO J. 1986 Sep;5(9):2267–2273. doi: 10.1002/j.1460-2075.1986.tb04494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Schenkel J., Sekeris C. E., Alonso A., Bautz E. K. RNA-binding properties of hnRNP proteins. Eur J Biochem. 1988 Feb 1;171(3):565–569. doi: 10.1111/j.1432-1033.1988.tb13825.x. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Sierakowska H., Szer W., Furdon P. J., Kole R. Antibodies to hnRNP core proteins inhibit in vitro splicing of human beta-globin pre-mRNA. Nucleic Acids Res. 1986 Jul 11;14(13):5241–5254. doi: 10.1093/nar/14.13.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Glowacka S. K., Szer W. Structure of complexes between a major protein of heterogeneous nuclear ribonucleoprotein particles and polyribonucleotides. J Mol Biol. 1983 Dec 25;171(4):439–455. doi: 10.1016/0022-2836(83)90039-6. [DOI] [PubMed] [Google Scholar]
- Wilk H. E., Werr H., Friedrich D., Kiltz H. H., Schäfer K. P. The core proteins of 35S hnRNP complexes. Characterization of nine different species. Eur J Biochem. 1985 Jan 2;146(1):71–81. doi: 10.1111/j.1432-1033.1985.tb08621.x. [DOI] [PubMed] [Google Scholar]
- Williams K. R., Stone K. L., LoPresti M. B., Merrill B. M., Planck S. R. Amino acid sequence of the UP1 calf thymus helix-destabilizing protein and its homology to an analogous protein from mouse myeloma. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5666–5670. doi: 10.1073/pnas.82.17.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Feig D. I., Shenk T. The C proteins of heterogeneous nuclear ribonucleoprotein complexes interact with RNA sequences downstream of polyadenylation cleavage sites. Mol Cell Biol. 1988 Oct;8(10):4477–4483. doi: 10.1128/mcb.8.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]