Abstract
Animal models, by definition, are an approximation of reality, and their use in developing anti-cancer drugs is controversial. Positive retrospective clinical correlations have been identified with several animal models, in addition to limitations and a need for improvement. Model inadequacies include experimental designs that do not incorporate biological concepts, drug pharmacology, or toxicity. Ascites models have been found to identify drugs active against rapidly dividing tumors; however, neither ascitic nor transplantable subcutaneous tumors are predictive of activity for solid tumors. In contrast, primary human tumor xenografts have identified responsive tumor histiotypes if relevant pharmacodynamic and toxicological parameters were considered. Murine toxicology studies are also fundamental because they identify safe starting doses for phase I protocols. We recommend that future studies incorporate orthotopic and spontaneous metastasis models (syngeneic and xenogenic) because they incorporate microenvironmental interactions, in addition to confirmatory autochthonous models and/or genetically engineered models, for molecular therapeutics. Collectively, murine models are critical in drug development, but require a rational and hierarchical approach beginning with toxicology and pharmacology studies, progressing to human primary tumors to identify therapeutic targets and models of metastatic disease from resected orthotopic, primary tumors to compare drugs using rigorous, clinically relevant outcome parameters.
Animal models are critical for the development of novel therapeutics; however, we have been minimally successful in decreasing the age-adjusted death rate for cancer compared with cardiac disease. In 2003, for the first time since 1930 when epidemiological records were initiated, fewer people (<85 years old) died of cardiac disease as compared with cancer.1 This historic change was attributable to a 60, 70, and 0% decrease in mortality by heart disease, stroke, and cancer, respectively. Thus, it is warranted to review the approaches and tumor models used in the identification and development of new anti-cancer therapeutics. Tumor initiation, progression, and metastasis is a complex, multifactorial process that selects tumor variants from a heterogeneous primary tumor.2,3 Therapeutic intervention is also a selective pressure that can result in tumor cell populations refractory to specific drugs.4 Therefore, to model and study tumor biology and drug activity, the selection of clinically relevant animal and tumor models is critical.
Originally, drug screens used leukemic cell lines that, when injected intraperitoneally (i.p.) resulted in tumor ascites. These tumor models were successful in identifying active therapeutics against leukemias and some lymphomas; however, they were inadequate for the identification of therapeutics against solid tumors.5,6,7 Subsequent studies using ectopically implanted syngeneic or human tumor cell lines were found to be ineffective for identifying therapeutically active drugs. In contrast, primary human tumors can predict responsive tumor histiotypes for targeting in phase I/II studies.8,9 Rodent toxicological studies have reliably identified safe initial doses for phase I studies; nonetheless, we have not yet identified animal models that can predict the extent of clinical efficacy. Recent studies have suggested that animal models using orthotopically implanted, syngeneic tumors are more predictive of responses than ectopic tumors.10,11,12,13 There has also been interest in genetically engineered models (GEMs), in part, because of their orthotopic primary tumors and relevance to molecular therapeutics.14 However, GEMs use artificial promoters that can influence the affected cell type, vary expression based on the genetic background,15 and decrease cellular heterogeneity, which in turn can affect tumor progression and metastasis. Furthermore, GEMs may not be universally relevant, ie, only 30% of breast cancer patients with human epidermal growth factor receptor 2 (HER2/neu) mutation can be modeled with HER2/neu transgenic mice. Critical to studies of therapeutic intervention using animal models is the incorporation of pharmacological and toxicological considerations, as well as the mechanistic concepts of tumor induction, progression, and metastasis. We suggest that the cycles of enthusiasm and pessimism for tumor models7 may have become overly negative and that an assessment of clinically useful correlations provided by animal models and their rational utilization is needed.
History of Tumor Models
The origin of our failure to identify drugs that have increased clinical activity is multifactorial and includes, but is not limited to, differences in efficacy of drugs in mice versus humans. Toxicity issues are also a common source of drug failure and are associated with the use of models selected for ease of modeling and a high incidence of positive responses. Many commonly used solid tumor models are biased toward false-positive results because they are selected based on ease of use, sensitivity to therapeutics, rapid growth, and other attributes that facilitate studies, but not clinical correlations. These deficiencies can be reduced by strict attention to proper design and conduct of efficacy studies, as well as the incorporation of a rational design based on an understanding of tumor biology, variant selection, and surrogate endpoints and the integration of testing strategies that reflect clinical tumor biology (Table 1). The history of animal models in the development of cancer drugs has been previously discussed5,6,7,9,16,17; however, few of these reviews have incorporated recommendations for future approaches.
Table 1.
Animal Model Attributes versus Clinical Situation
| Attribute | Murine model | Clinical situation |
|---|---|---|
| Drug administered at the MTD versus LD | MTD | MTD-LD |
| Tumor implant site | Ectopic | Orthotopic |
| Duration of cell cycle | Short cell cycle | Long cell cycle |
| Antigenicity | High antigenicity | Low antigenicity |
| Site of therapeutic target | Primary tumor | Metastatic tumors |
| Treatment protocol 1 | Single cycle of therapy | Multiple cycles of therapy |
| Treatment protocol 2 | Monotherapy | Polychemotherapy |
| Treatment protocol 3 | Single therapeutic approach, occasionally with surgery | Multi-modality including surgery, hormonal, immune, and molecular therapy |
| Treatment protocol 4 | 1 to 3 days of therapy | 1 to 2 weeks of therapy |
| Treatment protocol 5 | Single cycle of therapy | Multiple cycles of therapy |
| Pharmacokinetic consideration | Push delivery | Infusion of drug if it has a short half life |
| Tumor burden | Minimal tumor burden | Locally advanced or systemic disease |
| Duration of tumor presence before diagnosis | Days to weeks | Years to decades |
Clinical and model attributes that need to be considered in the design of screening and developmental strategies and protocols. MTD, maximum tolerated dose; LD, lethal dose.
Ascites Tumors
In 1955, it was suggested that a correlation existed between efficacy against transplanted tumors and clinical activity.18 This stimulated the National Cancer Institute (NCI) to launch an anti-cancer drug screening program using three murine models.19 Over time, the number of tumors studied was reduced, and by 1968, drugs were screened only against the L1210 leukemia cell line. The clinical response to chemotherapy by human leukemias and lymphomas significantly improved during this developmental period,20,21 whereas the treatment of most solid tumors did not. Further, concerns were identified regarding reliance on a single leukemia tumor model because this could preferentially select for drugs targeting rapidly growing tumors.22 Therefore, the B16 melanoma and Lewis lung carcinoma mouse models were incorporated into the screening program in 1972.23,24
Solid Human and Murine Tumors
In 1976, the NCI’s Division of Cancer Treatment initiated the use of a new tumor panel incorporating transplantable solid human tumors that were representative of the major histological types of cancer. The panel consisted of human tumors of the breast, colon, and lung, in addition to the murine L1210 leukemia and B16 melanoma syngeneic models.22 These syngeneic models involved inoculation of tumor cells by i.p., subcutaneous (s.c.), or intravenous (i.v.) routes, whereas human tumor xenografts were grown under the renal subcapsule.25 Although the subrenal capsule assay is labor intensive, it provides a rapid evaluation of drug activity.26 Subsequent analysis of this strategy (1976 to 1982) revealed that the mouse-human tumor panel identified an anti-tumor agent (taxol) that would have been missed by the L1210 model.22 Furthermore, ∼30% of compounds found to be active in at least one human tumor xenograft were not identified by the syngeneic models. Therefore, it was concluded that the mouse-human tumor panel may be successful in identifying new drugs for clinical studies, but with a low correlation between preclinical and clinical efficacy.27 Despite their inherent deficiencies, transplantable tumors remain valuable because they provide an evaluation within the context of an intact immune system and host stroma and extracellular matrix.
Sequential Tumor Model
Based on these results, the NCI implemented a sequential screening strategy in 1982, whereby a potential drug was examined using progressively more rigorous models. Initially, drugs were examined against the P388 leukemia as a prescreen, followed by studies with a panel of murine tumor models (MX-1, B16, M5076, and L1210), resulting in the identification of a large number of potentially active compounds.22 Drugs active in the primary tumor panel were advanced to a secondary screen using compound-orientated tumors based on the properties of each drug and experience in the primary tumor panel. However, a retrospective analysis of preclinical-clinical efficacy28 did not demonstrate a correlation based on tumor histiotype. This was perceived to be attributable to an experimental design that limited tumors to one mouse and one human tumor for each of the three major histiotypes. Thus, it was concluded that a model system composed of several tumors with the same histiotype might better predict a clinical response against a specific tumor histiotype.28
Human Tumor Stem Cell (HTSC) Assay/Clonogenic Assay
A HTSC assay29,30 was developed in the early 1980s to determine whether a model system incorporating multiple tumors of the same histiotype could predict a clinical response for a specific tumor histiotype.22 The HTSC assay was disease-orientated using soft agar colony growth of freshly explanted human tissue with outcomes based on growth inhibition. Salmon et al29 compared in vitro results with the clinical responses of myeloma and ovarian cancer patients and found correlations based on sensitivity and resistance. The HTSC assay was suggested to be appropriate for drug screening based on feasibility, validity, and potential to identify new anti-tumor agents.31 Initial studies examined established chemotherapeutic agents and found that most drugs were active with the exception of drugs requiring systemic activation. Clinically ineffective agents were also found to be negative with 97% accuracy, an observation confirmed by other reports.8,32,33,34,35
However, the HTSC assay8,36 is limited because of the low plating efficiency of most solid tumors and the poor availably of tumor tissue. Thus, only breast, colorectal, kidney, lung, melanoma, and ovarian tumors have been found to provide sufficient cells for evaluation, although strategies have been suggested to improve the growth rates of primary tumor tissues.8 Furthermore, although these models predict responsive histiotypes, no clinical analysis of individualized therapy has demonstrated a significant increase in survival compared with empirically determined standard treatment; therefore, the HTSC assay has not found a role in the individualization of patient therapy.8
In Vitro Human Tumor Cell Line Screen
In 1990, a study using human tumor cell lines for large-scale drug screening was initiated.37 This in vitro human tumor cell line screen shifted from being a compound-orientated to a disease-orientated screening strategy. The initial panel incorporated 60 different human tumor cell lines, resulting in the use of multiple tumors with the same histiotype. Toward the end of the decade, an in vitro prescreen was introduced using three cell lines: MCF-7 (breast carcinoma), NCI-H460 (lung carcinoma), and SF-268 (glioma). The rationale for this prescreen was that it could remove inactive compounds from unnecessary and costly full-scale evaluation. In a study by the NCI of Canada Clinical Trial Group,17 an in vitro cell line model for non-small cell lung cancer was shown to be predictive for phase II activity. This observation was also confirmed in a human xenograft model for non-small cell lung cancer, but not with breast or colon cancer. In addition, a mouse allograft model was found not to be predictive for any histiotype.17 Studies using the panel of 60 human tumor cell lines to assess mechanisms of action (MOAs) supported the concept that pharmacokinetic (PK) and pharmacodynamic parameters must be considered.38,39,40,41 This is critical because the failure of drugs in the clinic41,42 is often associated with a poor PK profile or drug toxicity.40 Indeed, primary human tumor xenografts can be predictive of clinical cytotoxic therapy for a given tumor histiotype provided that clinically relevant, pharmacological dosing parameters are used.9,39,40,41,42 It is noted that human tumor cell lines, in contrast to primary human tumor cells, have generally been cultured for years, losing much of their heterogeneity. This has resulted in undifferentiated tumors lacking the histology and cellular architecture characteristic of the modeled human tumor. Thus, administration of clinically relevant drug doses to animals with s.c. xenografts results in response patterns similar to those observed with the human tumor histiotype and the same drugs.40 These studies emphasize the need to determine the exposure levels required for anti-tumor activity with the intention that unnecessary toxicity is avoided in phase I clinical trials. Further, they suggest that primary human tumor xenografts can be used to identify responsive human histiotypes.
Screening Using Human Tumor Xenografts in Immunodeficient Mice
Many of the initial reports using malignant human tumors showed that they did not metastasize in nude mouse, which cast doubts on the validity of this model. It is now clear that tumor metastasis depends on intrinsic tumor cell properties and host factors, the experimental technique(s) used, as well as the origin, health, and maintenance of the immune-deficient animal. Today, we know that human neoplasms can be studied in immune-deficient mice; however, clinical relevance is obtained only if careful attention is paid to the experimental conditions. The neoplasms must be free of mouse pathogens, and the mice must be kept in specific pathogen-free conditions. Careful consideration must be given to the anatomical site of implantation because the metastatic potential of human tumor cells is dependent on both intrinsic properties of the tumor cells and host factors, which can differ between tissues and organs. These studies require nude (athymic) or severe combined immunodeficient (SCID) mice that are T- and B-cell-deficient, allowing the engraftment of human tumor cells. However, innate immunity, particularly natural killer (NK) cells, can limit tumor growth and prevent metastasis in nude mice.43 Mice with the nude mutation, although T-cell-deficient, have a compensatory increase in innate immunity, most notably increased NK activity and tumoricidal macrophages. The beige mutation (murine homolog of Chediak-Higashi syndrome) results in a delay in NK activation, but not the loss of NK cell function.44 Thus, the NK cells lack secondary granules and have a delayed killing ability. Further, nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice can be humanized (rendered chimeric) with the injection of human peripheral blood (PB) or bone marrow (BM) cells,45 resulting in a somewhat more relevant microenvironment.
Pharmacological and toxicological parameters have been studied using s.c. tumor xenograft in nude mice, and efficacy defined by a delay in tumor growth, with body weight loss, and mortality as parameters of toxicity. Several reports support the use of this model,46,47 including a clinical response comparison study that used a large panel of xenografts derived from patient biopsies.8,48 This study observed a correlation with clinical outcome for both tumor resistance (97%) and sensitivity (90%). It was concluded that if primary tumors are used, xenograft models can predict clinical activity similar to the clonogenic assay. In contrast, a retrospective NCI study evaluated 39 drugs using transplantable human tumor cell lines and compared them to phase II clinical results.9 In these studies, in vivo xenograft activity did not correlate with activity against the same human tumor histology. However, drugs that were active against one third of xenografts correlated with clinical activity.9 A comparison of these two large studies9,48 suggests that ex vivo studies using primary tumors may be predictive of clinical activity, whereas studies using human cell lines are not. One may conclude that the s.c. injection of xenograft cell lines may have value in the development of cytotoxic drugs8,9,39,49; however, these seem to be poorly predictive of a specific histological response. It is noted that a recent review of the athymic nude mouse model using human xenografts39 suggested that an outcome focused on tumor growth rate or cytostasis may be more predictive of clinical activity than tumor shrinkage (cytotoxicity). Regardless of these correlations, murine xenograft models are not ideal for cancer drug development. In addition to the points discussed above, these models lack human stroma and immune cells, which are important to the metastatic process.50
Humanized Mice
The term humanized mice has been used to describe numerous animal models, including immunodeficient mice reconstituted with human stem cells or lymphocytes.51,52 This approach has also been combined with the transplantation of human thymi and/or BM before stem cell injection to provide a human stromal environment. These humanized mice are used to study graft-versus-host disease and solid organ transplantation. Immunologically humanized mice have also been immunized to induce human hybridomas and to study T-cell responses against tumors and viruses. In these models, humanized mice are ones injected with stem cells and then immunized or immune-deficient mice humanized by the injection of T cells from immunized patients/donors. They can then be challenged with human tumor xenografts or viruses to study the effect of immunity on tumor/viral growth.53 Indeed, this approach provides an ethical and cost-effective strategy to test vaccine efficacy. Humanized mice transplanted with fragments of human organs are also used to study the role of interactions between xenogenic human stroma and tumors in tumor progression and metastasis.
Another definition of humanized mice involves the insertion of a human gene into the mouse genome.54 Such GEMs are used to study species-associated differences in phenotypes, including responses to drugs or tumor antigens (Ags).55 Models to study drug responses include GEM mice expressing human cytochrome p450 genes54 that allow the in vivo analysis of cytochrome P450 metabolism of endogenous and exogenous chemicals, including xenobiotics. One example of these studies is the analysis of cytochrome P450 2E1 (CYP2E1) expression on cisplatin-induced hepatotoxicity, using mice with induced or steady-state CYP2E1 levels and a comparison to knockout and CYP2E1-humanized mice.56 Human tumor Ags have also been expressed in GEMs, rendering them tolerant to human Ags and providing a model to study vaccine responses in the presence of immunological tolerance.57 Traditional murine vaccination models may recognize human tumor Ags as foreign, providing overly optimistic results. Thus, a humanized model with the human tumor Ag provides a more relevant tolerant host. Furthermore, wild-type mice do not express class I and II human leukocyte antigens (HLAs), and as such, relevant HLA Ag processing does not occur. However, GEMs that express HLA-Ags in the mouse thymus can be used to support the selection of T cells and recognize relevant antigenic epitopes. These GEMs, after human stem cell engraftment, can potentially allow accurate modeling of T-cell responses via the expressed HLA-Ag.
In summary, humanized mouse models have made tremendous progress since their development almost 20 years ago.56 However, a number of practical limitations still limit their use as rigorous paradigms of the human system. Additional work and validation remain before they can be routinely and confidentially used in drug development.
Orthotopic Tumor Models
Clinical observations have suggested that the organ environment can influence the response of tumors to chemotherapy. For example, in women with breast cancer, lymph node and skin metastases are more sensitive to chemotherapeutic intervention than metastases in either the lung or bone.58 Likewise, orthotopic implantation of human tumor cells from surgical specimens into nude mice is mandatory for an accurate analysis of tumor growth and metastasis. This has been shown with colon carcinomas (into the wall of the colon), renal cell cancers (into the kidney), melanomas (into the skin), mammary carcinomas (into the mammary fat pad), bladder carcinomas (into the bladder wall), prostate carcinoma (into the prostate), pancreatic carcinoma (into the pancreas), and lung cancer (into the bronchi). Orthotopic implantation results in rapid growth of local tumors and in several tumor models, distant metastasis. There is also a striking, site-specific variation in response to chemotherapy. In one study,59 colon carcinoma cells were implanted into different anatomical locations of nude mice using the highly metastatic KM12L4a human colon carcinoma cell line. In this study, mice were injected in the subcutis (ectopic site), spleen (leading to experimental liver metastasis), or cecum (growth at the orthotopic site). Tumor-bearing mice were treated with doxorubicin and subsequently evaluated for responses. Tumors grown within the s.c. tissue showed an 80% inhibition of growth after two i.v. injections of doxorubicin (10 mg/kg), compared with ∼40% inhibition of the intracecal tumors and less than 10% inhibition of lesions in the liver.59
Anti-cancer drugs are commonly screened using panels of human tumor xenografts implanted s.c. in nude mice. However, as discussed above, s.c. tumor models are not representative of the primary tumor site.60 In addition, clinically we treat well-established and frequently advanced metastatic disease, whereas conventional s.c. xenograft models are of recent origin (1 to 14 days) and rarely have metastatic disease.40 Thus, orthotopic tumor models seem to be a better model to assess the morphology and the growth characteristics of clinical disease10,11,12,61 and to be more representative of a primary tumor with respect to tumor site and metastasis.13 One of the obvious advantages of orthotopic models is that targeting processes involved in local invasion (eg, angiogenesis) can be undertaken at a more clinically relevant site.60 Since the early studies showing orthotopic transplantation of colon tumors and metastasis to the liver,62 tumor xenografts have been grown orthotopically in mice. Whether preclinical models are representative of clinical disease (eg, orthotopic/metastatic models) and should replace traditional s.c. nonmetastatic xenografts12,40 remains an unanswered, yet critical, question. Clearly, the poor predictive power of our current models support the use of alternative models and approaches.7 However, despite the clinical relevance of orthotopic models, their utilization is hindered by a need for a high level of technical skill, time, and cost. Therapeutic efficacy is also more difficult to assess with orthotopic models in contrast to the relative ease of s.c. tumor measurements.60 Clearly, murine tumors in intact synergic animals have significant advantage beyond expense as the model of a more clinically relevant host environment.
GEMs
Throughout the past 20 years, GEMs have contributed to our understanding of the molecular pathways responsible for the initiation, progression, and metastasis of cancer cells and have extended our knowledge of the mechanistic role that oncogenes and tumor suppressor genes have in these processes. In addition, studies with GEMs have improved our understanding of the role genes and their mutated counterparts have in tumorigenesis, as well as the cooperation of individual mutations in tumor development. The initial GEMs were murine models that overexpressed viral and cellular oncogenes.63 Subsequent studies used genes targeted to mouse embryonic stem cells, providing oncogene-bearing transgenic mice (knockin) or loss of function, ie, gene knockout mice. In addition to the use of transgene overexpression models, conditional strategies have been developed that allow controlled gene expression in both a tissue- and temporal-specific manner.64 Thus, tet-regulated65 or CRE-inducible alleles can regulate the timing, duration, and tissue compartment of gene expression or inactivation. Furthermore, these technologies can be combined, resulting in GEMs with specific cancers that overexpress or lack genes of interest in all cells or in a specific tissue compartment and/or developmental stage. These approaches have significantly contributed to our understanding of cancer pathogenesis and may ultimately help in the identification of anti-neoplastic drugs.
Although the use of GEMs in drug development has not been validated against drugs with efficacy in the corresponding human tumor, studies with several have suggested potential utility. Retinoic acid has shown activity in GEMs of acute promyelocytic leukemia,66 and Imatinib, a BCR-ABL inhibitor that is active against chronic myelogenous leukemia, has been shown to limit the development of BCR-ABL mutations in P190BCR-ABL GEM mice.67 One model used in the development of the cyclooxygenase (COX-) 2 inhibitor, celecoxib, is the multiple intestinal neoplasia (Min) mouse that was created by germ line mutagenesis. This resulted in a point mutation in the Apc tumor suppressor gene and a high frequency of intestinal tumors.68 The outcomes from this GEM are similar to patients who have familial adenomatous polyposis and a high frequency of intestinal tumors. Overall, GEMs have seldom been used to test novel anti-cancer therapeutics with the goal of accurately predicting clinical responses.69,70 The few studies that have compared GEMs using clinically effective agents have not been encouraging.66,71,72,73 Thus, despite their mechanistic promise, transgenic mouse models have not yet demonstrated a role in drug discovery.
GEMs have been primarily used to study specific therapeutic questions relevant to the affected gene and to study interactions between tumor cells and their microenvironment. They are potentially more representative of specific human tumor histiotypes than transplanted xenografts because of their in situ and autochthonous origin.70 However, GEMs have limitations, including expense, time commitment, intellectual property restrictions,74 and species-specific differences, resulting in different mutant phenotypes in man and mouse.75 Further, no one transgenic model is representative of all of the different forms of even one tumor histiotype; just as one human tumor cannot represent another human tumor of the same histiotype. In GEMs, transgenes are driven by artificial promoters, which may influence the cell type affected. In addition, the genetic background can affect transgene expression. Thus, mice carrying a mutation in the Apc gene express different lesions dependent on the genetic background.15 In this case, the predisposing mutation is identical, but the outcome, including rapidity of lesion development (months to years), type of lesion (hyperplasia to metastatic tumors), and tumor histiotype (mammary, colon cancers, and lymphomas), are affected by genetic background. Furthermore, by their very nature, GEMs do not incorporate the heterogeneity inherent to tumor initiation, progression, and metastasis,16 and systemic disease is rarely observed in GEMs.76,77
Autochthonous Tumor Models
Autochthonous tumors include spontaneously occurring tumors and chemical, viral, or physical carcinogen-induced tumors and are believed to model human tumors more closely than transplanted tumors. Advantages of autochthonous tumors include orthotopic growth, tumor histology devoid of transplantation introduced changes, and metastasis via lymphatic and vascular vessels surrounding and within the primary tumor.78 Despite such positive properties, autochthonous tumor models have not been widely used as an animal model for drug development. Autochthonous tumor models have an inherent variability in the time to and frequency of tumor induction, number of tumor(s) induced, and thus the number of animals required for a study.79 It is noted that all of these suboptimal attributes are similar to these also found with GEMs (Table 2). Thus, time frames of several months to a year for a single experiment, as opposed to weeks with transplanted xenograft models, are required.78,79,80 Thus, autochthonous tumor models are best reserved for confirmation studies,78 although in the postgenome era, autochthonous models have to an extent been replaced by GEMs. A recent study that compared outcomes from autochthonous models and GEMs with a meta-analysis of clinical outcomes81 found that carcinogen-induced tumors correlated best with clinical responses. These intervention studies used aspirin, β-carotene, calcium, and wheat bran to treat recurrent colon adenoma in human volunteers compared with chemoprevention studies with carcinogen-induced intestinal tumors in rats and large intestinal polyp induction in Min (Apc+/−) mice. The final meta-analyses included 6714 volunteers, 3911 rats, and 458 mice. These studies showed that therapeutic responses in carcinogen-induced rat tumors predicted clinical responses for aspirin, calcium, and carotene and were compatible for wheat bran with the Min mouse models. Results from the transgenic Min model were consistent with human responders for aspirin but were discordant for calcium and wheat bran. These results suggest that the carcinogen-induced tumor models may be more predictive for human activity as compared with GEMs. Regardless of the differences between the rodent models, they both provided correlation for chemopreventive activity, clinically.
Table 2.
Comparative Clinical Relevance of Model
| Transgenic tumors | Orthotopic tumors | Ectopic tumors | ||
|---|---|---|---|---|
| Histologically similar to human tumors | > | Often histologically similar to human tumors | = | Often histologically similar to human tumors |
| Generally low immunogenic | > | Low to highly immunogenic | = | Low to highly immunogenic |
| Tumors arise in a stochastic manner, but from a common molecular event | < | Highly heterogeneous | = | Highly heterogeneous |
| The use of a strong promoter results in transgenic overexpression that is not expected clinically | < | Highly heterogeneous | = | Highly heterogeneous |
| Metastatic distribution often parallels that observed clinically | = | Metastatic distribution often parallels human distribution | > | Metastases occur in the lungs, rarely at other sites |
| Metastasis occurs infrequently | < | Metastasis occurs frequently | = | Gross metastasis occurs infrequently |
| One can study chemoprevention | > | One can study prophylaxis | = | One can study prophylaxis |
| May need to screen for expression if homozygous lethality, or administer inducers for conditional expression | < | Fully inbred mouse strains | = | Fully inbred mouse strains |
| Time for tumor induction and development is long, often requiring a year or more | < | If one is studying metastasis protocols, often requires 3 to 4 months | = | If one is studying metastasis protocols, often requires 3 to 4 months |
| Transgenic mouse strains are often established in outbred mice, requiring inbreeding, and can result in strain dependant activities | < | Syngenic mouse strains, but tumor to tumor variation in response occurs | = | Syngenic mouse strains, but tumor to tumor variation in response occurs |
| Expensive, based on labor and housing | < | Labor intensive, but relatively short housing duration | > | Inexpensive based on labor and housing duration |
| Relevant host immune cell infiltration and tumor microenvironment | > | Relevant host immune cell infiltration | > | Irrelevant host infiltration and tumor microenvironment |
| Multiple primary tumors precludes surgical resection | = | Allows surgical resection of primary tumor | > | Allows surgical resection of primary tumor, but in general it is minimally invasive |
| Difficult to vary therapeutic protocol relative to tumor burden | < | One can vary therapeutic schedule relative to tumor burden | = | One can vary therapeutic schedule relative to tumor burden |
| Autochthonous tumor models have proven more predictive then transgenic models | > | Little information available regarding prediction of clinical response | < | High frequency of false positive responses observed relative to clinical response |
Various in vivo and ex vivo outcome measures that are used in animal models. Many of these measures are can also be extended.
A comparison of attributes of transgenic, orthotopic transplanted, and entopic transplanted tumors and the relevance of these parameters to clinical reality. The symbols used are greater than (>), lesser than (<), and equal to (=).
Outcome Criteria for Animal Tumor Models
Inarguably, our ultimate goal is to cure patients of all of their tumors. However, the realistic goal in clinical oncology is to improve survival and quality of life and prevent recurrent disease.82 Rigorous criteria are required for animal models to predict clinical efficacy. This contrasts with the convenient tumor models5 that often use repeated measures of tumor burden as the easiest measure of efficacy. Somewhat better is the assessment of the number of experimental metastases after therapy. In reality, endpoints need to be matched to tumor type (solid, leukemia, or metastatic), study context, implantation site accessibility, type of implantation, and therapeutic drug class. The simplistic criteria often used in mouse models do not match the challenging criteria of clinical or pathological complete responses, and this contributes to the conflicting opinions about the relevance of mouse models. Therefore, other metrics, including survival and evaluation of immunomodulation, angiogenesis, spontaneous metastasis, histopathology, and immunohistochemistry, may need to be considered as part of the study design. Despite the technical difficulty, labor intensity, and expense commonly cited as limitations for detailed examinations, the utility of mouse models is improved by implementing multiple rigorous endpoints (Table 3).
Table 3.
Measurements of Outcomes in Animal Models
| Endpoint | Comment |
|---|---|
| In vivo | |
| Tumor onset | Time to palpable tumor mass of predetermined size |
| Tumor growth rate | Assessment of tumor volume throughout time |
| Number of tumor-bearing animals | Frequency of cure |
| Tumor burden in vivo at set time | Weight of tumor or organ with metastases |
| Tumor growth delay | Volume estimated (mm3) two-dimensional measurement |
| Delay of time for tumor to reach specific volume | |
| Tumor cell kill | Log10 total tumor cell kill |
| Net log10 tumor cell kill | |
| Incidence of metastasis | Gross count (lungs) |
| Cell count, resistance, florescence, 125IUdR uptake... | |
| Survival—life span | Increase in median survival time |
| Survival— number alive | Percent cure at predefined time |
| Ex vivo | |
| Gross pathology | Ulceration/central necrosis, |
| invasion or tissue distribution and gross lesions | |
| Metastasis… | |
| Angiogenesis | |
| Histopathology | H&E staining |
| Morphometrics | |
| Inflammatory cell infiltration | |
| Mitotic index, cellular apoptosis | |
| Immunohistochemistry | T cell, macrophage, and DC infiltration |
| Angiogenesis and lymphoangiogenesis | |
| Tumor cell apoptosis | |
| Enzyme and cytokine levels | |
| Molecular pathology | Cytokines/chemokines or enzymes in serum or qRT-PCR of tumor, blood, spleen |
| Hematology | Complete blood count, platelets, spleen, marrow |
| Blood/spleen/marrow/thymus differential | |
| Immunology | Phenotype spleen, blood, tumor-infiltrating nonparenchymal cells and their function including qRT-PCR |
Tumor prophylactic models, wherein the drug is administered before tumor challenge, are clinically not realistic. Likewise, models that measure tumor growth delay, ie, the measurement of time required to reach a predetermined median tumor volume, has not been shown to predict survival in mice, much less humans. Treatment of mice bearing gross tumors typically assess the therapeutic response based on slowed tumor growth kinetics as opposed to tumor regression. In contrast, the response evaluation criteria in solid tumors criteria classically used for evaluating efficacy in human clinical trials requires at least 50% shrinkage in tumor size to be considered a response. Thus, common outcome criteria for animal models and humans are disparate. Clinically, and in rodent studies, survival provides a rigorous and consistent endpoint for the evaluation of treatment efficacy. However, preclinical studies that monitor survival at the termination of therapy are inadequate because of the lack of follow-up after treatment. Survival must be followed after treatment to assess the complete life expectancy to and if tumor regrowth occurs. Despite a delay in tumor growth throughout the treatment period, a rebound effect after treatment can occur and is indicative of lower overall efficacy. It should be noted that a chronic slowness of tumor growth rate, ie, cytostasis, can be considered a relevant outcome as compared with tumor regression if our goal is to delay tumor progression.
Absorption, Distribution, Metabolism, and Excretion (ADME) and Toxicology
The value of a preclinical tumor model depends on its ability to reflect a clinical process or predict a clinical response. However, the clinical relevance of a tumor model requires that it be studied within the context of a mechanistic hypothesis that incorporates clinically relevant outcome parameters. In addition to assessing anti-tumor activity, preclinical animal models need to provide information on pathology, toxicity, and ADME. Although the ideal tumor model does not yet exist, appropriate development and implementation can provide insight into carcinogenesis, angiogenesis, tumor progression, metastasis, and therapeutic response. As discussed above, xenograft tumor models can effectively predict responsive tumor histiotype(s); however, these models need to incorporate a pharmacological and toxicological foundation to be successful. In addition, animal models can be used to resolve a specific experimental question that can be appropriately translated into clinical trials.
Freireich et al83 showed that rodents, as well as other species, could reliably provide a safe starting dose for phase I studies. They evaluated the results from 18 drugs in human and six different animal species. They concluded that on a mg/kg basis, the maximum tolerated dose (MTD) in human is 1/12 the LD10 in mice and 1/7 the LD10 in rats. This difference is the same as the factor required to convert from mg/kg to mg/m2 skin surface area. Using this approach and two different rodent toxicity studies, 50 new anti-cancer therapies were safely introduced into clinical testing.84 Therefore, animal models can successfully predict a safe starting dose for phase I studies, as well as quantitatively and qualitatively predict human toxicology.85 The MTD that is the lethal dose for 10% of mice (MTD/LD10) has been shown to be associated with the maximum administered dose and clinical dose-limiting toxicity. Thus, in phase I studies in which the starting dose was a dose one-tenth the mouse MTD/LD10 (mg/m2), it was found to be safe for all of the 25 drugs that were investigated. The one toxicity parameter that was an exception was nausea and vomiting, which cannot be assessed in rodents. In this study, dose-limiting toxicities were accurately predicted by murine studies for 7/7 hematological and 3/3 neurological dose-limiting toxicities.
In addition to a quantitative determination of anti-tumor activity, responsive preclinical tumor models can also be used to assess preliminary ADME information and toxicity. Traditionally, toxicity and ADME information is obtained as the last step in the development of a drug, frequently resulting in drug loss to development late in the process. However, if preclinical models are initially used to obtain PK and MTD data, valid preclinical pharmacology and efficacy studies can be undertaken facilitating clinical translation.
Rational Development of Animal Model(s)
Before clinical testing, a new drug or drug formulation should demonstrate an improved safety and/or efficacy profile compared with current therapeutics in animal models. The comparison should incorporate rigorous animal models and not be based on highly responsive model(s), such as ones with a rapid outcome that are convenient or with which the investigator is familiar. Furthermore, tumor and animal models should meet specific biological criteria, including heterogeneity, appropriate histology, metastatic propensity, and appropriate genetic criteria depending on the targeted drug mechanism, limited immunogenicity, and potentially etiology (Table 1). Last, the model should have the potential to provide a correlation between therapeutic model outcome and clinical activity, optimally with previous documentation of relevance between mice and humans.76,86,87
Before undertaking efficacy studies, base line PK and toxicity data are needed, including an initial analysis of cellular/organ toxicity. If during PK studies a half-life >12 hours is observed, administration protocols other then daily injection may be appropriate. Equally, a brief half-life (5 to 20 minutes) would suggest multiple daily injections such that a slow release formulation or administration by continuous infusion might also be required. It should be noted that the toxicity and therapeutic profile can differ significantly between push and continuous infusion. Thus, PK studies can help focus the initial dose finding and toxicological studies. In the initial study(s), five dose escalations should be used to include an expected no effect dose and a 10% lethal dose (LD-10). Weight should also be monitored, and if a cohort loses >30% weight, this dose can be identified as the MTD. In general, most drugs have an MOA and toxicity profile that builds on prior drugs such that the pharmacology and toxicity may be predicted. The route of administration should also be identified before the assessment of toxicity, although most drugs will be initially administered i.v. Weight loss typically parallels toxicity; however, monitored leucopenia may prove to be a sensitive measure of toxicity.
The assessment of organ toxicity should include animal necropsies at multiple time points after drug administration and include, but not necessarily be limited to, two time points, including 24 to 72 hours and 7 to 14 days after completion of drug administration. Target organ analysis must include hematopoietic toxicity (PB, BM, and spleen cellularity), major organ toxicity (lungs, liver, gastrointestinal, and renal) and other targets as appropriate based on the drug profile. Although not a classic toxicity analysis, this preliminary profile will confirm the MTD, organ toxicity targets, and the potential recovery time from toxicity. The latter is critical for insight into the timing of multiple therapy cycles.
A target tumor histiotype responsive to the therapeutic under study can be provisionally identified based on studies using primary human tumor cells. However, these studies require an understanding of the achievable blood serum levels (Cmax) and the toxicity limitation. Thus, information obtained from the PK analyses and the MTD studies is needed to design in vitro and in vivo studies using primary human tumor cells. The identification of a responsive human tumor histiotype can then be used to guide the selection of murine tumors for efficacy analysis. Importantly, the tumor model used must incorporate the complexities associated with tumor development and progression, as well as clinically accepted therapeutic protocols. Indeed, inappropriate models and drug dosages frequently result in false-positive or -negative results. Multiple animal models using the targeted tumor histiotype need to be examined. Transplantable tumor models should incorporate orthotopic primary tumors, surgical resection, and subsequent therapy of metastatic disease. If warranted, confirmation models (autochthonous and/or genetically engineered) should be used to identify the optimal routes, schedules, doses, and duration of therapeutic administration. These efficacy studies can also be used to identify potential surrogate endpoints, including histopathological endpoints to confirm the expected therapeutic target and to help refine dose scheduling relative to growth and cell loss fractions. Thus, one uses a hierarchal approach ending with rigorous autochthonous models and/or GEMs. Last, it should be noted that a number of drugs have shown different toxicity in normal and tumor-bearing patients; therefore, confirming toxicity studies in animals with tumor burdens may be warranted. Investigational new drug applications are strengthened by conducting additional safety studies in tumor-bearing animals.
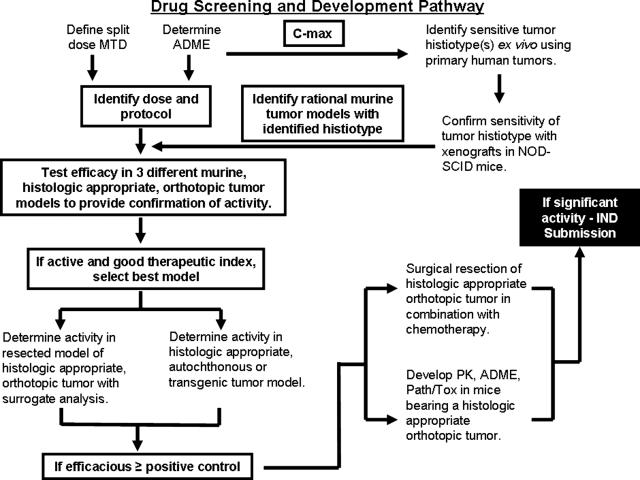
This approach to the identification and development of new drugs is predicated on a rational preclinical cascade culminating in the demonstration of in vivo proof of principle efficacy using appropriate animal models (Table 3). The proposed pathway follows the outline identified in Figure 1. Note that dosage translation from mouse to human needs to reflect skin surface area rather than body weight. In addition, the route of the administration can be critical such that i.p. injections, commonly used in rodent studies, are not used clinically outside of rabies vaccines and the treatment of ovarian cancer. Furthermore, clinically many of the anti-tumor drugs induce tissue damage requiring i.v. administration, although in rodents they may be administered by s.c. or i.p. administration.
Figure 1.
A graphic overview of recommended steps in a screening and developmental strategy for novel therapeutics. This flow chart would be modified based on the characteristic of the therapeutic under development and would change for targeted molecular therapeutics, immunotherapeutics, and hormonal therapeutics.
Inarguably, animal models cannot be used to model all human therapeutics because of the dichotomy in murine and human receptors; however, murine models can generally assess their pharmacology and toxicology. In addition, there are differences in the physiology of murine models and humans such that different enzymes may have varying levels of prominence in human disease. Overall, rodent models have been shown to address mechanisms of toxicology, pharmacology, and efficacy. However, in the instances when a protein sequence is poorly conserved, the human homolog may not be appropriate for preclinical toxicology and pharmacology. Thus, a murine homolog may be active, but at a different IC-50 such that a murine homolog may be better used for pharmacological and toxicological studies. Although of lesser concern with traditional chemotherapy drugs obtained by medicinal chemistry, biotherapeutics such as interferon-α and granulocyte macrophage colony stimulating factor have used the murine homolog for toxicological and pharmacological studies.
Summary
Translation of a therapeutic into the clinic requires evidence of efficacy and safety as compared with the standard of care. Furthermore, one needs to use animal models that parallel the biological, genetic, etiological, immunological, and therapeutic properties of human cancer. To assess clinical relevance, initial studies need to be based on preliminary pathology and toxicity and ADME studies to establish drug dose and use a split dose protocol. In these basic studies, therapeutic targets can be identified using primary human tumor cell models. The establishment of therapeutic efficacy requires a hierarchical approach beginning with orthotopic primary tumors, progressing to the models in which the orthotopic primary tumor is resected before therapy initiation, and then finishing with confirmation in autochthonous models or GEMs. The orthotopic and autochthonous models or GEMs incorporate homeostatic mechanisms in the organ microenvironment that regulates tumor cell growth and survival. Efficacy needs to be justified based on predefined and rigorous outcome criteria and in many instances, surrogate studies are justified. Ideally once efficacy has been demonstrated, additional pathology and toxicity studies and PK/ADME should be initiated in tumor-bearing animals, incorporating histopathology, blood chemistry, and biodistribution analysis. The surrogate parameters could include tumor histopathology and immunohistochemistry (angiogenesis, lymphangiogenesis, tumor cell apoptosis, and infiltrating cellular phenotype).
Although this approach may seem exhaustive, it is relatively rapid and of minimal expense as compared with the failure of a candidate drug in phase II or III clinical studies. Traditional murine tumor models result in a high frequency of false positive(s) as rapidly growing murine tumors are cured, whereas slower growing human tumors progress. We suggest, therefore, that a hierarchal strategy using rigorous outcome measures and continuous improvements in stringency and consistency provides an approach with the potential to facilitate the identification and aid in the development of anti-cancer agents.
Acknowledgments
We thank Ms. Kirsten Stites for critical review and editing of the manuscript.
Footnotes
Address reprint requests to James E. Talmadge, Ph.D., University of Nebraska Medical Center, 987660 Nebraska Medical Center, Omaha, NE, 68198-7660. E-mail: jtalmadg@unmc.edu.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Wolman SR, Fidler IJ. Evidence for the clonal origin of spontaneous metastases. Science. 1982;217:361–363. doi: 10.1126/science.6953592. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Fidler IJ. Cancer metastasis is selective or random depending on the parent tumour population. Nature. 1982;297:593–594. doi: 10.1038/297593a0. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Benedict K, Madsen J, Fidler IJ. Development of biological diversity and susceptibility to chemotherapy in murine cancer metastases. Cancer Res. 1984;44:3801–3805. [PubMed] [Google Scholar]
- Schuh JC. Trials, tribulations, and trends in tumor modeling in mice. Toxicol Pathol. 2004;32(Suppl 1):53–66. doi: 10.1080/01926230490424770. [DOI] [PubMed] [Google Scholar]
- Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005;11:971–981. [PubMed] [Google Scholar]
- Schein PS, Scheffler B. Barriers to efficient development of cancer therapeutics. Clin Cancer Res. 2006;12:3243–3248. doi: 10.1158/1078-0432.CCR-06-0329. [DOI] [PubMed] [Google Scholar]
- Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40:802–820. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10:229–243. doi: 10.1007/BF00050794. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Fertile seed and rich soil: the development of clinically relevant models of human cancer by surgical orthotopic implantation of intact tissues. Teicher BA, editor. Totowa: Humana Press Inc.,; Anticancer Drug Development GuidePreclinical Screening, Clinical Trials, and Approval. 1997:pp 127–144. [Google Scholar]
- Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1999;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- Dexter DL, Diamond M, Creveling J, Chen SF. Chemotherapy of mammary carcinomas arising in ras transgenic mice. Invest New Drugs. 1993;11:161–168. doi: 10.1007/BF00874150. [DOI] [PubMed] [Google Scholar]
- Moser AR, Hegge LF, Cardiff RD. Genetic background affects susceptibility to mammary hyperplasias and carcinomas in Apc(min)/+ mice. Cancer Res. 2001;61:3480–3485. [PubMed] [Google Scholar]
- Stringer JR, Larson JS, Fischer JM, Medvedovic M, Hersh MN, Boivin GP, Stringer SL. Modeling variation in tumors in vivo. Proc Natl Acad Sci USA. 2005;102:2408–2413. doi: 10.1073/pnas.0401340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- Gellhorn A, Hirschberg E. Investigation of diverse systems for cancer chemotherapy screening. Cancer Res. 1955;15(Suppl 3):1–125. [PubMed] [Google Scholar]
- Goldin A, Venditti JM, Kline I, Mantel N. Evaluation of anti-leukemic agents employing advanced leukemia L1210 in mice. Cancer Res. 1959;19:429–466. [Google Scholar]
- DeVita VT, Schein PS. The use of drugs in combination for the treatment of cancer: rationale and results. N Engl J Med. 1973;288:998–1006. doi: 10.1056/NEJM197305102881905. [DOI] [PubMed] [Google Scholar]
- Zubrod C. Chemical control of cancer. Proc Natl Acad Sci USA. 1972;69:1042–1047. [Google Scholar]
- Venditti JM, Wesley RA, Plowman J. Current NCI preclinical antitumor screening in vivo: results of tumor panel screening, 1976–1982, and future directions. Adv Pharmacol Chemother. 1984;20:1–20. doi: 10.1016/s1054-3589(08)60263-x. [DOI] [PubMed] [Google Scholar]
- Geran R, Greenberg N, MacDonald M, Schumacher A. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep. 1972;3:1–13. [Google Scholar]
- Ovejera A, Johnson R, Goldin A. Growth characteristics and chemotherapeutic response of intravenously implanted Lewis lung carcinoma. Cancer Chemother Rep. 1975;5:111–125. [Google Scholar]
- Bogden A. A rapid screening method for testing chemotherapeutic agents against human tumour xenografts. Houchens DOA, editor. New York: Gustav Fischer,; Proceedings of the Symposium of the Use of Athymic (Nude) Mice in Cancer Research. 1978:pp 231–250. [Google Scholar]
- Bogden AE. The subrenal capsule assay: biological properties and testing capability. Fiebig HH, Burger BA, editors. Basel,: Karger,; Relevance of tumour models for anticancer drug development. Contributions to Oncology. 1999:pp 89–99. [Google Scholar]
- Staquet MJ, Byar DP, Green SB, Rozencweig M. Clinical predictivity of transplantable tumor systems in the selection of new drugs for solid tumors: rationale for a three-stage strategy. Cancer Treat Rep. 1983;67:753–765. [PubMed] [Google Scholar]
- Venditti JM. Preclinical drug development: rationale and methods. Semin Oncol. 1981;8:349–361. [PubMed] [Google Scholar]
- Salmon SE, Hamburger AW, Soehnlen B, Durie BG, Alberts DS, Moon TE. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978;298:1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Harris GJ, Johnson G, Glaubiger D. Initial experience with the human tumor stem cell assay system: potential and problems. Prog Clin Biol Res. 1980;48:113–124. [PubMed] [Google Scholar]
- Shoemaker RH, Wolpert-DeFilippes MK, Kern DH, Lieber MM, Makuch RW, Melnick NR, Miller WT, Salmon SE, Simon RM, Venditti JM. Application of a human tumor colony-forming assay to new drug screening. Cancer Res. 1985;45:2145–2153. [PubMed] [Google Scholar]
- Bertelsen CA, Sondak VK, Mann BD, Korn EL, Kern DH. Chemosensitivity testing of human solid tumors. A review of 1582 assays with 258 clinical correlations. Cancer. 1984;53:1240–1245. doi: 10.1002/1097-0142(19840315)53:6<1240::aid-cncr2820530604>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Salmon SE, Alberts DS, Durie BG, Meyskens FL, Jones SE, Soehnlen B, Chen HS, Moon T. Clinical correlations of drug sensitivity in the human tumor stem cell assay. Recent Results Cancer Res. 1980;74:300–305. doi: 10.1007/978-3-642-81488-4_36. [DOI] [PubMed] [Google Scholar]
- Tveit KM, Fodstad O, Lotsberg J, Vaage S, Pihl A. Colony growth and chemosensitivity in vitro of human melanoma biopsies. Relationship to clinical parameters. Int J Cancer. 1982;29:533–538. doi: 10.1002/ijc.2910290508. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Casper J, Bradley E, Sandbach J, Jones D, Makuch R. Association between human tumor colony-forming assay results and response of an individual patient’s tumor to chemotherapy. Am J Med. 1981;70:1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Selby P, Buick RN, Tannock I. A critical appraisal of the “human tumor stem-cell assay.”. N Engl J Med. 1983;308:129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- Sausville EA, Feigal E. Evolving approaches to cancer drug discovery and development at the National Cancer Institute, USA. Ann Oncol. 1999;10:1287–1291. doi: 10.1023/a:1008333901925. [DOI] [PubMed] [Google Scholar]
- Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2:S134–S139. [PubMed] [Google Scholar]
- Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Takimoto CH. Why drugs fail: of mice and men revisited. Clin Cancer Res. 2001;7:229–230. [PubMed] [Google Scholar]
- Habu S, Fukui H, Shimamura K, Kasai M, Nagai Y, Okumura K, Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981;127:34–38. [PubMed] [Google Scholar]
- Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284:622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transpl Oncol. 2006;8:318–329. doi: 10.1007/s12094-006-0177-7. [DOI] [PubMed] [Google Scholar]
- Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66:3355–3358. doi: 10.1158/0008-5472.CAN-05-3827. [DOI] [PubMed] [Google Scholar]
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. [DOI] [PubMed] [Google Scholar]
- Scholz CC, Berger DP, Winterhalter BR, Henss H, Fiebig HH. Correlation of drug response in patients and in the clonogenic assay with solid human tumour xenografts. Eur J Cancer. 1990;26:901–905. doi: 10.1016/0277-5379(90)90196-z. [DOI] [PubMed] [Google Scholar]
- Steel GG, Courtenay VD, Peckham MJ. The response to chemotherapy of a variety of human tumour xenografts. Br J Cancer. 1983;47:1–13. doi: 10.1038/bjc.1983.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002;16:471–477. [PubMed] [Google Scholar]
- Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, Jagannath S, Dhodapkar MV. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Yacoub-Youssef H, Marcheix B. Reconstitution of a human immune system in immunodeficient mice: models of human alloreaction in vivo. Tissue Antigens. 2005;66:73–82. doi: 10.1111/j.1399-0039.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Yu AM. Cytochrome P450 and xenobiotic receptor humanized mice. Annu Rev Pharmacol Toxicol. 2006;46:41–64. doi: 10.1146/annurev.pharmtox.45.120403.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, Walther B. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Macchiarini F, Manz MG, Palucka AK, Shultz LD. Humanized mice: are we there yet? J Exp Med. 2005;202:1307–1311. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelli MG, Rosso R, Garattini S. Selective chemotherapy in relation to the site of tumor transplantation. Int J Cancer. 1967;2:421–424. doi: 10.1002/ijc.2910020503. [DOI] [PubMed] [Google Scholar]
- Wilmanns C, Fan D, O’Brian C, Radinsky R, Bucana C, Tsan R, Fidler I. Modulation of doxorubicin sensitivity and level of P-glycoprotein expression in human colon carcinoma cells by ectopic and orthotopic environments in nude mice. Int J Oncol. 1993;3:413–422. doi: 10.3892/ijo.3.3.413. [DOI] [PubMed] [Google Scholar]
- Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004;40:852–857. doi: 10.1016/j.ejca.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5:29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- Tan MH, Holyoke ED, Goldrosen MH. Murine colon adenocarcinoma: syngeneic orthotopic transplantation and subsequent hepatic metastases. J Natl Cancer Inst. 1977;59:1537–1544. doi: 10.1093/jnci/59.5.1537. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Messing A, Van DT, Levine AJ, Palmiter RD. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego EM, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci USA. 2000;97:10173–10178. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain J, Saksena A, Laneuville P. The kinase inhibitor STI571 reverses the Bcr-Abl induced point mutation frequencies observed in pre-leukemic P190(Bcr-Abl) transgenic mice. Leuk Res. 2002;26:1011–1016. doi: 10.1016/s0145-2126(01)00181-3. [DOI] [PubMed] [Google Scholar]
- Shoemaker AR, Gould KA, Luongo C, Moser AR, Dove WF. Studies of neoplasia in the Min mouse. Biochim Biophys Acta. 1997;1332:F25–F48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- Macleod KF, Jacks T. Insights into cancer from transgenic mouse models. J Pathol. 1999;187:43–60. doi: 10.1002/(SICI)1096-9896(199901)187:1<43::AID-PATH246>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- Basu GD, Pathangey LB, Tinder TL, Lagioia M, Gendler SJ, Mukherjee P. Cyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancer. Mol Cancer Res. 2004;2:632–642. [PubMed] [Google Scholar]
- Lallemand-Breitenbach V, Guillemin MC, Janin A, Daniel MT, Degos L, Kogan SC, Bishop JM, de The H. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearss DJ, Subler MA, Hundley JE, Troyer DA, Salinas RA, Windle JJ. Genetic determinants of response to chemotherapy in transgenic mouse mammary and salivary tumors. Oncogene. 2000;19:1114–1122. doi: 10.1038/sj.onc.1203275. [DOI] [PubMed] [Google Scholar]
- Weiss B, Shannon K. Mouse cancer models as a platform for performing preclinical therapeutic trials. Curr Opin Genet Dev. 2003;13:84–89. doi: 10.1016/s0959-437x(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Jacks T. Tumor suppressor gene mutations in mice. Annu Rev Genet. 1996;30:603–636. doi: 10.1146/annurev.genet.30.1.603. [DOI] [PubMed] [Google Scholar]
- Hann B, Balmain A. Building ‘validated’ mouse models of human cancer. Curr Opin Cell Biol. 2001;13:778–784. doi: 10.1016/s0955-0674(00)00283-0. [DOI] [PubMed] [Google Scholar]
- Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Berger M. Is there a relevance for anticancer drug development. Fiebig HH, Burger BA, editors. Basel,: Karger,; Relevance of Tumour Models for Anticancer Drug Development. Contributions to Oncology. 1999:pp 15–27. [Google Scholar]
- Talmadge JE, Lenz BF, Klabansky R, Simon R, Riggs C, Guo S, Oldham RK, Fidler IJ. Therapy of autochthonous skin cancers in mice with intravenously injected liposomes containing muramyltripeptide. Cancer Res. 1986;46:1160–1163. [PubMed] [Google Scholar]
- Schwartz B, Birk Y, Raz A, Madar Z. Nutritional-pharmacological combinations—a novel approach to reducing colon cancer incidence. Eur J Nutr. 2004;43:221–229. doi: 10.1007/s00394-004-0462-6. [DOI] [PubMed] [Google Scholar]
- Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–1922. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Schipper H, Goh CR, Wang TL. Shifting the cancer paradigm: must we kill to cure? J Clin Oncol. 1995;13:801–807. doi: 10.1200/JCO.1995.13.4.801. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- Burtles SS, Newell DR, Henrar RE, Connors TA. Revisions of general guidelines for the preclinical toxicology of new cytotoxic anticancer agents in Europe. The Cancer Research Campaign (CRC) Phase I/II Clinical Trials Committee and the European Organization for Research and Treatment of Cancer (EORTC) New Drug Development Office. Eur J Cancer. 1995;31A:408–410. doi: 10.1016/0959-8049(94)00483-l. [DOI] [PubMed] [Google Scholar]
- Newell DR, Burtles SS, Fox BW, Jodrell DI, Connors TA. Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br J Cancer. 1999;81:760–768. doi: 10.1038/sj.bjc.6690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A. Cancer as a complex genetic trait: tumor susceptibility in humans and mouse models. Cell. 2002;108:145–152. doi: 10.1016/s0092-8674(02)00622-0. [DOI] [PubMed] [Google Scholar]
- Siemann DW. Satisfactory and unsatisfactory tumor models factors influencing the selection of a tumor model for experimental evaluation. Kallman RF, editor. Elmsford: Pergamon Books, Inc.,; Rodent Tumor Models in Experimental Cancer Therapy. 1987 [Google Scholar]